1. Introduction
Ebstein's anomaly is a rare congenital heart disease characterized by typical abnormal apical displacement of the septal and posterior leaflets of the tricuspid valve, with an incidence of approximately 1 in 200,000 live births[
1]. Surgical intervention, including tricuspid valve repair or replacement and one-and-a-half or biventricular repair, such as EA anatomical repair[
2] has significantly improved survival and symptoms. However, the long-term prognosis is primarily determined by RV function rather than solely by the severity of tricuspid regurgitation or the technical success of valve surgery. Postoperative assessment of right ventricular function remains challenging due to the complex RV geometry and the presence of an "atrialized" portion[
3].
Conventional echocardiographic parameters, such as tricuspid annular plane systolic excursion (TAPSE) and fractional area change (FAC), are widely used for assessing RV systolic function, However, these measures have limitations[
3] and may not accurately reflect intrinsic myocardial contractility[
4],especially in the context of the highly abnormal RV geometry seen in EA[
5].
Recent advances in echocardiographic myocardial deformation imaging have enabled more sensitive and quantitative assessment of RV mechanics. RV global longitudinal strain (RVGLS) and related parameters (free-wall and septal longitudinal strain) may better reflect intrinsic RV contractile performance and have emerged as independent predictors of adverse outcome in acquired and congenital heart disease.[
6].Recent studies have identified RVGLS as being not only superior to TAPSE and FAC in quantifying RV dysfunction, but also as the strongest independent echocardiographic predictor of adverse outcome in patients with Ebstein’s anomaly and other congenital right heart disease, suggesting the urgent need for regular and sensitive RV functional monitoring during follow-up[
7,
8] Cardiac magnetic resonance (CMR) imaging is considered the gold standard for assessing RV volumes and function due to its ability to provide detailed three-dimensional evaluation of cardiac structures[
9]. However, its routine use is limited by cost, accessibility, and contraindications in certain patient populations. Therefore, there is a need for more readily available and accurate echocardiographic methods to evaluate postoperative RV function in EA patients.
Speckle Tracking Echocardiography (STE) is an ultrasound technique that assesses cardiac function by tracking the movement of speckles within cardiac tissue. By marking speckles in the echocardiographic images, their motion can be tracked and analyzed to obtain myocardial acoustic signals. This allows the derivation of various motion parameters of the myocardium in longitudinal, radial, circumferential, and rotational directions, ultimately enabling the evaluation of cardiac systolic and diastolic functions as well as the movement of the cardiac walls. It provides a more accurate assessment of cardiac function, including both systolic and diastolic performance and the analysis of ventricular wall motion. STE is not affected by the angle of the ultrasound beam, allowing for in-plane tracking of myocardial displacement and quantitative evaluation of global and regional left ventricular systolic function, as well as early detection of reduced left ventricular systolic function[
10,
11]. Compared to Tissue Doppler Echocardiography (TDE), the advantages of STE include independence from the ultrasound beam incidence angle and no need for specialized imaging, as speckle analysis is performed based on conventional B-mode images[
12]. It supports multiparameter measurements, offline analysis, good reproducibility, and low inter-observer variability [
13,
14]. However, it requires high-quality images.
Myocardial strain refers to the degree of deformation that myocardial tissue undergoes during systole and diastole, commonly used to assess myocardial systolic and diastolic functions. Strain rate refers to the speed of myocardial deformation, reflecting the velocity of myocardial contraction and relaxation. Two-dimensional Speckle Tracking Echocardiography (2D-STE) can be used to measure both local and global myocardial strain. It is well known that right ventricular (RV) myocardial fibers are typically divided into three layers: superficial, middle, and deep. The superficial fibers are aligned primarily along the longitudinal axis of the RV, contracting in the direction of the RV’s long axis; the middle fibers are arranged circumferentially, contracting around the RV; and the deep fibers are oriented along the transverse axis of the RV, contracting in the direction of the RV’s short axis. Thus, RV myocardial motion includes longitudinal motion along the long axis, transverse shortening motion, and rotational motion. According to guidelines , RV function assessment commonly uses two parameters: RVGLS and RVFLS. RVGLS is the average of values from all segments of the interventricular septum and free wall. However, as septal myocardial motion is influenced by left ventricular contraction, the accuracy of RVGLS for assessing RV function may be reduced. Therefore, RVFLS is often used to evaluate RV function. Literature reports a large study involving 575 patients with pulmonary hypertension, where free wall strain measured by 2D-STE could predict patient prognosis [
15]. Another study indicated that in heart failure patients with reduced left ventricular ejection fraction but preserved TAPSE, RVFLS provided incremental prognostic information and improved risk stratification[
16]. (
Figure 1.6) Schematic diagram of myocardial strain.
This study aims to evaluate the perioperative changes and clinical utility of RV strain parameters in adult EA patients undergoing tricuspid valve surgery, with emphasis on their predictive value and practical superiority over traditional indices.
2. Materials and Methods
2.1. Study Population
This retrospective study enrolled 46 consecutive patients who underwent biventricular repair for Ebstein’s anomaly at the First Hospital of Tsinghua University between May 2014 and December 2023. Inclusion criteria were as follows: (1) diagnosis of EA and surgical biventricular repair; (2) at least two complete postoperative echocardiographic examinations of adequate quality; and (3) follow-up period of at least 6 months. Exclusion criteria were: (1) poor echocardiographic image quality preventing accurate strain analysis; (2) absence of ECG gating during echocardiography; (3) atrial fibrillation or other obvious irregular heart rhythm; and (4) severe RV enlargement beyond the echocardiographic field of view.
The study was approved by the Institutional Ethics Committee (approval no. 2023-024-01(R), June 3, 2023), and informed consent was obtained from all participants or their legal guardians.
2.2. Echocardiographic Examination
All patients underwent comprehensive transthoracic echocardiography (TTE) using GE Vivid E9 or E95 ultrasound systems (GE Healthcare, Chicago, IL, USA) preoperatively and at 1, 3, 6, and 12 months postoperatively. Conventional parameters, including TAPSE, FAC, and tricuspid annular systolic velocity (S'), were measured according to current guidelines[
17]. RV strain analysis was performed using the EchoPAC workstation (GE Healthcare). RV global longitudinal strain (RVGLS), RV free wall longitudinal strain (RVFLS), and RV septal longitudinal strain (RVSLS) were measured from the RV-focused apical four-chamber view. All measurements were performed by experienced echocardiographers blinded to clinical data.
2.3. Cardiac Magnetic Resonance Imaging
CMR examinations were performed using a Philips 1.5T MR scanner (Philips Healthcare, Best, Netherlands). Right ventricular end-diastolic volume (RVEDV), end-systolic volume (RVESV), and ejection fraction (RVEF) were measured independently by two experienced radiologists using MEDIS and CVI software. Twenty patients had CMR data available within the same follow-up period as their echocardiographic examinations, allowing for direct correlation analysis between the two imaging modalities.
2.4. Patient Grouping
Part1.Patients were classified into three groups according to postoperative cardiac function and echocardiographic characteristics: Group A: NYHA class I-II, mild or no tricuspid regurgitation, normal RV size. Group B: NYHA class I-II, moderate to severe tricuspid regurgitation, enlarged RV. Group C: NYHA class III-IV, moderate to severe tricuspid regurgitation, enlarged RV.( The clinical symptoms of patients in Group A were mild, those in Group B were moderate, and those in Group C were severe).
Part2.During the follow-up period, 13 patients underwent reoperation. Ultrasound parameters from preoperative, early postoperative, and one-month postoperative assessments were compared and analyzed to examine their patterns of change and differences.
Part3.Twenty patients underwent both cardiac ultrasound and CMR examinations within one month, and the CMR-measured RVEF, RVEDV, and RVESV were analyzed for correlation with the ultrasound indices.
2.5. Statistical Analysis
Continuous variables are presented as mean ± standard deviation. Normality was assessed using the Shapiro-Wilk test. For normally distributed data, one-way ANOVA with Tukey's post hoc test was used to compare parameters among the three groups; for non-normally distributed data, the Kruskal-Wallis test with Dunn's post hoc test was used. Paired t-test or Wilcoxon signed-rank test was used to compare parameters before and after reoperation. Pearson or Spearman correlation coefficients were used to analyze the correlation between echocardiographic and CMR parameters. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Baseline Characteristics and Conventional/Strain Echocardiographic Parameters in the Three Groups
The study included 46 patients: 15 in group A, 20 in group B, and 11 in group C. According to the statistical results in
Table 1, there were no statistically significant differences (P > 0.05) among groups A, B, and C in terms of age, gender, and body surface area.
According to the statistical results presented in
Table 2, there were no statistically significant differences (P > 0.05) in TAPSE and S' among groups A, B, and C, indicating that TAPSE and S' could not accurately distinguish between the three groups.The results of the Tukey test revealed a statistically significant difference in FAC between group A and group B (P < 0.05), with group A showing a significantly lower FAC than group B. Similarly, there was a statistically significant difference in FAC between group A and group C (P < 0.05), with group A also exhibiting a lower FAC than group C. However, no statistically significant difference was observed in FAC between group B and group C, indicating no notable distinction between these two groups.
However,
Table 3 shows that the one-way ANOVA results for RVGLS, RVFLS, and RVSLS across groups A, B, and C indicate: the free wall strain differed significantly among the three groups (F=8.45, P<0.05), and the right ventricular strain also showed a statistically significant difference (F=12.48, P<0.05) (
Table 4.5). This suggests that the echocardiographic parameters RVFLS, RVSLS, and RVGLS can accurately assess the differences among the three groups.
Based on the findings from the first part of the study, we concluded that RVGLS, RVFLS, and RVSLS are superior to conventional ultrasound parameters (TAPSE and S') in assessing the severity of the condition in patients after EA surgery. Among the conventional parameters, FAC can also be used to evaluate disease severity in EA patients.
3.2. Echocardiographic Changes in Reoperation Patients
Table 4.
Repeated measures analysis of variance (ANOVA) for preoperative and 1 month postoperative assessments.
Table 4.
Repeated measures analysis of variance (ANOVA) for preoperative and 1 month postoperative assessments.
| Parameter |
Mean Difference |
Standard Error |
P-value |
| TAPSE |
3.39 |
1.43 |
0.11 |
| FAC |
-0.04 |
0.04 |
0.77 |
| RV |
8.84 |
1.98 |
<0.01 |
| RA |
10.39 |
2.07 |
<0.01 |
| RVGLS |
2.13 |
0.71 |
0.03 |
| RVFLS |
1.49 |
0.63 |
0.11 |
Table 5.
Changes in echocardiographic parameters in reoperation patients.
Table 5.
Changes in echocardiographic parameters in reoperation patients.
| Parameter |
Pre-operation |
Early postoperative period |
one month post-operation |
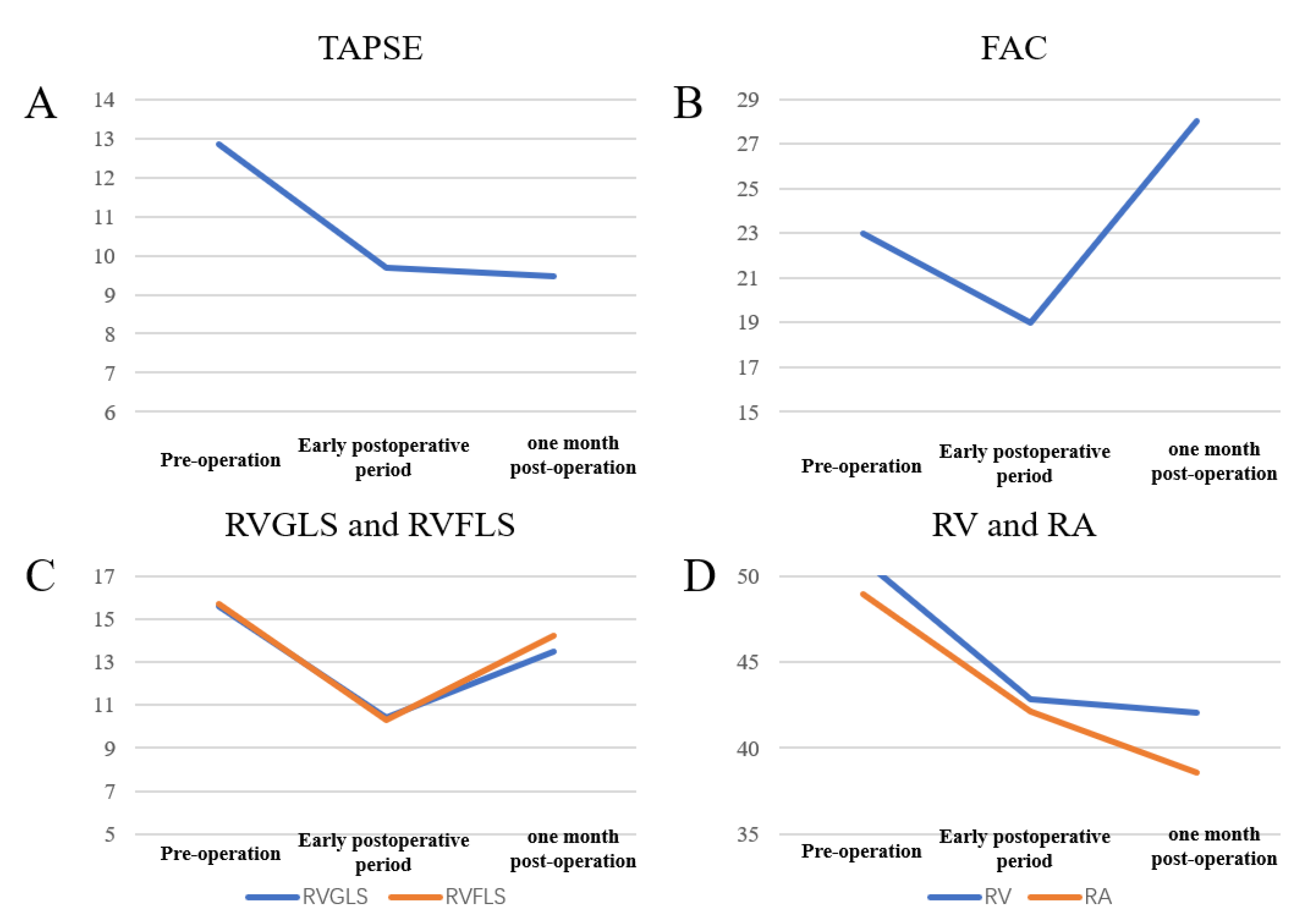
| TAPSE |
12.85±4.06 |
9.69±3.28 |
9.46±4.42 |
| FAC |
0.23±0.10 |
0.19±0.08 |
0.28±0.08 |
| RV |
50.92±9.88 |
42.85±11.73 |
42.08±12.27 |
| RA |
48.92±14.13 |
42.15±15.17 |
38.54±8.88 |
| RVGLS |
15.61±4.03 |
10.39±2.73 |
13.48±2.85 |
| RVFLS |
15.72±3.86 |
10.32±3.67 |
14.23±3.85 |
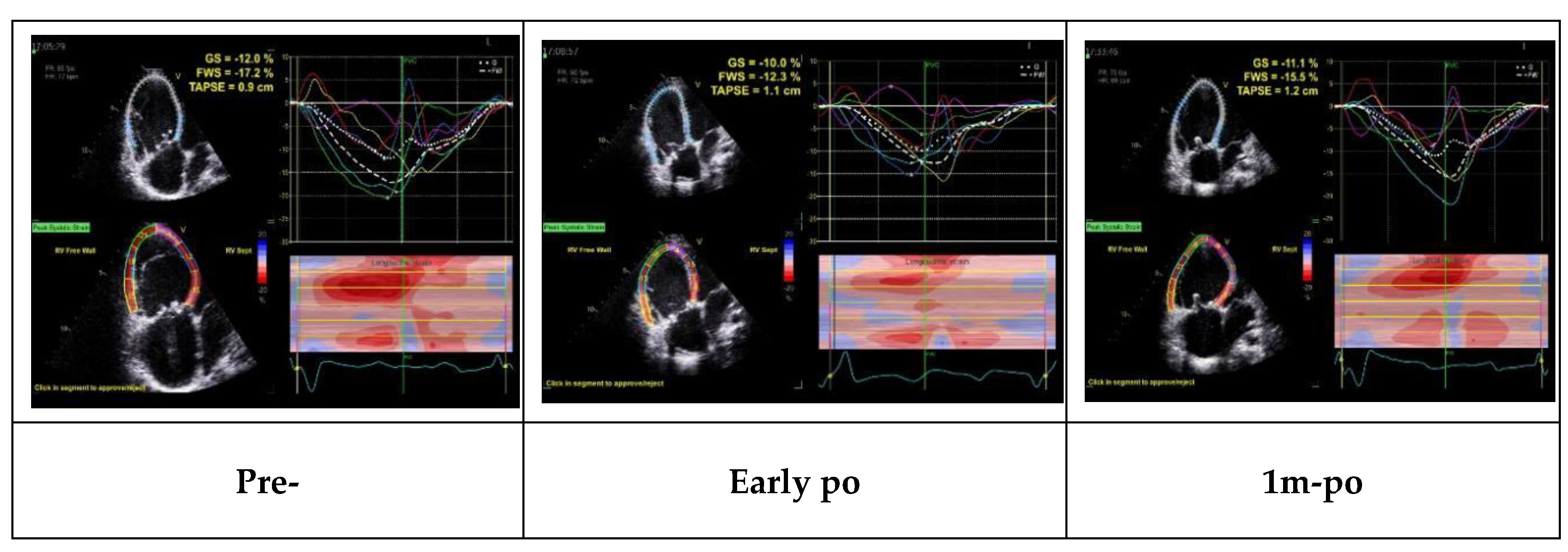
Among the 13 patients who underwent reoperation (8 from group C and 5 from group B), all had significant right atrial and ventricular enlargement with RV dysfunction, including 11 with severe tricuspid regurgitation and 2 with severe stenosis. Surgery included tricuspid valve replacement in 8 patients and repeat repair in 5.
Trends in echocardiographic parameters before, early after, and one month after reoperation are summarized in
Figure 2. FAC, RVGLS, and RVFLS declined early postoperatively but nearly returned to baseline values within one month, whereas TAPSE remained reduced at one month. These patterns demonstrate that strain parameters are more sensitive than conventional indices for detecting early postoperative changes and functional recovery.
Improvements in RV strain paralleled advances in NYHA class and reduced arrhythmia burden. Notably, some patients’ TAPSE and FAC normalized despite persistent impairment of RVGLS and RVFLS, highlighting the greater sensitivity of strain imaging.
3.3. Correlation Analysis Between Echocardiographic and CMR Parameters
The In this study, 20 patients who underwent EA surgery underwent CMR examination. The RVEDV and RVESV data measured by CMR were analyzed, and the average RVEF was calculated. Correlation analyses were then performed between RVEF and the conventional ultrasound parameters TAPSE, FAC, and S', respectively.
For the CMR parameters, we used the Intraclass Correlation Coefficient (ICC) test to assess the consistency of RVEDV and RVESV measurements obtained by two imaging specialists in our hospital using the MEDIS software and CVI software on the 20 post-EA surgery patients.
An Intraclass Correlation Coefficient (ICC) greater than 0.7 indicates a strong correlation. Both ICC values exceeded 0.7, and a Cronbach's α greater than 0.7 suggests good consistency between the measurements taken by the two raters. Therefore, the data obtained by the two imaging specialists using different software are reliable.
Correlation Analysis
The results of correlation analysis between echocardiographic parameters and CMR-derived RVEF, RVEDV, and RVESV are presented in
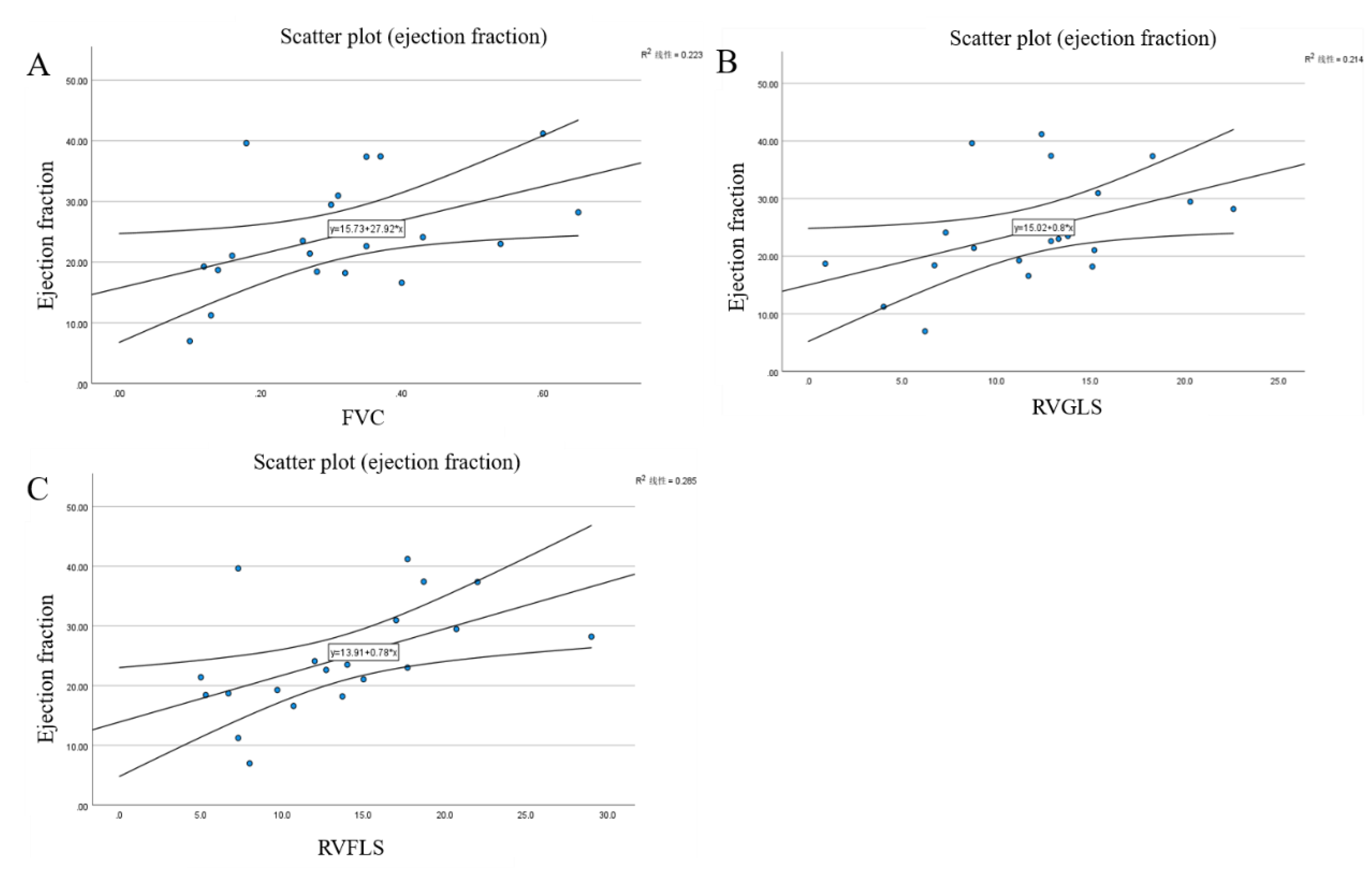
Table 6. RVEF showed moderate correlation with FAC (r=0.47, P=0.04), RVFLS (r=0.53, P=0.02), and RVGLS (r=0.46, P=0.04). RVEDV correlated with RVFLS (r=-0.48, P=0.03), and RVESV was correlated with RVFLS (r=-0.49, P=0.03) and RVGLS (r=-0.45, P=0.04). Scatter plots and linear regression analyses for the most significant correlations are presented in Figure 3, demonstrating the relationships between FAC and RVEF (Figure 3A), RVGLS and RVEF (Figure 3B), and RVFLS and RVEF (
Figure 3C). Notably, RVFLS showed the strongest correlation with CMR-derived RVEF among all echocardiographic parameters.
Based on the findings from the third part of the study, we concluded that the novel ultrasound indices RVGLS and RVFLS demonstrate stronger correlations with RVEF, RVEDV, and RVESV measured by CMR than the conventional indices TAPSE, FAC, and S'.
Table 6.
Intraclass Correlation Coefficient (ICC) between the two raters.
Table 6.
Intraclass Correlation Coefficient (ICC) between the two raters.
| Parameter |
ICC |
Cronbach's |
F-value |
P-value |
| RVEDV |
0.800 |
0.946 |
18.38 |
<0.01 |
| RVESV |
0.834 |
0.955 |
22.38 |
<0.01 |
Table 6.
Correlation of echocardiographic parameters with CMR measurements.
Table 6.
Correlation of echocardiographic parameters with CMR measurements.
| Variable |
RVEF |
RVEDV |
RVESV |
| coef. |
P value |
coef. |
P value |
coef. |
P value |
| TAPSE |
0.23 |
0.33 |
-0.13 |
0.58 |
-0.15 |
0.53 |
| FAC |
0.47 |
0.04 |
-0.35 |
0.13 |
0.03 |
0.91 |
| S’ |
-0.67 |
0.78 |
-0.08 |
0.97 |
-0.38 |
0.10 |
| RVFLS |
0.53 |
0.02 |
-0.48 |
0.03 |
-0.49 |
0.03 |
| RVSLS |
0.25 |
0.28 |
-0.34 |
0.14 |
-0.35 |
0.13 |
| RVGLS |
0.46 |
0.04 |
-0.44 |
0.06 |
-0.45 |
0.04 |
Figure 2A Scatter plot demonstrating the correlation between fractional area change (FAC) and CMR-derived right ventricular ejection fraction (RVEF). The moderate positive correlation (r=0.47, P=0.04) supports the validity of FAC as a measure of RV systolic function.
Figure 2B: Scatter plot showing the correlation between right ventricular global longitudinal strain (RVGLS) and CMR-derived RVEF. The correlation (r=0.46, P=0.04) demonstrates the relationship between strain parameters and gold standard CMR measurements.
Figure 2C: Scatter plot illustrating the correlation between right ventricular free wall longitudinal strain (RVFLS) and CMR-derived RVEF. RVFLS showed the strongest correlation with RVEF (r=0.53, P=0.02) among all echocardiographic parameters.
4. Discussion
Our study demonstrates that echocardiographic strain analysis, particularly RVFLS and RVGLS, provides more sensitive and better evaluation of RV systolic function after biventricular EA repair compared to conventional parameters. The superior discriminatory ability of strain parameters in distinguishing between patient groups of varying severity (as illustrated in Figure.2) may be attributed to their ability to directly reflect myocardial contractility without being influenced by overall cardiac motion[
18]. This advantage was most pronounced in reoperation patients, where conventional indices often lagged behind strain recovery.
Among conventional parameters, only FAC was able to differentiate between severity groups, likely because it accounts for two-dimensional changes in RV area and therefore provides a more comprehensive functional assessment than TAPSE or S'[
12]. However, FAC measurement is still limited by the constraints of two-dimensional imaging and load dependency. Traditional echo parameters TAPSE and S' showed no significant differences among the groups, consistent with previous studies suggesting their limitations in evaluating complex congenital heart disease.
The enhanced sensitivity demonstrated by strain parameters in reoperation patients (Figure.2) highlights their potential value in monitoring early postoperative RV functional changes. Unlike TAPSE, which showed persistent reduction one month after surgery, strain parameters (RVGLS, and RVFLS) demonstrated recovery approaching preoperative levels, suggesting their ability to reflect true myocardial recovery. This could be crucial for guiding postoperative management and enabling timely intervention when necessary.
The moderate to good correlation between strain parameters and CMR measurements (Figure.3) supports the validity of strain analysis in assessing RV function. Specifically, RVFLS showed the strongest correlation with CMR-derived RVEF (r=0.53, Figure 3C), while both RVFLS and RVGLS correlated well with RV volumes. This correlation is stronger than that of conventional parameters, suggesting that strain analysis may provide an RV functional assessment closer to CMR measurements.
The clinical implications of these findings are significant for the management of EA patients after biventricular repair. The superior sensitivity of strain parameters in detecting RV dysfunction could lead to earlier identification of patients at risk for postoperative complications or long-term right heart failure. This early detection could enable more timely interventions and potentially improve long-term outcomes for EA patients post-repair. Furthermore, the strong correlation between strain parameters and CMR measurements suggests that echocardiographic strain analysis could serve as a reliable, more accessible alternative to CMR for routine follow-up of EA patients. This could be particularly beneficial in settings where CMR is not readily available or in patients with contraindications to CMR.
These findings are in line with recent multicenter studies highlighting the prognostic significance of RVGLS in EA, showing its role as the dominant independent predictor of mortality and functional outcome[
7,
8]. Our perioperative data further support the superiority of deformation indices over standard echocardiographic markers for risk stratification and patient management.
Given the technical feasibility and reproducibility of speckle-tracking techniques, routine integration of RV strain analysis into follow-up echocardiography for EA patients appears warranted. Furthermore, strain analysis may be helpful in selecting optimal timing for surgical intervention, especially in ambiguous or borderline cases.
Study Limitations
Limitations include the retrospective single-center design, relatively modest sample size, and potential vendor-related variability in strain measurement. Prospective multicenter studies with longer follow-up are needed to validate these findings and to investigate the potential incremental prognostic value of RV strain imaging in routine clinical practice.
5. Conclusions
RVGLS and RVFLS provide superior sensitivity and clinical insight into RV functional recovery after tricuspid valve surgery in EA compared to conventional indices, either in echo or CMR. Adoption of serial RV strain assessment will improve risk stratification and timing of intervention in this complex patient cohort.
Author Contributions
Conceptualization, Lianyi Wang, qing yu Wu, ming kui Zhang and yong hong Niu; Methodology, zhi qiang MA and jun xiang Pan; Software, jun xiang Pan; Formal analysis, zhi qiang MA; Investigation, Lianyi Wang, qing yu Wu, ming kui Zhang and yong hong Niu; Resources, zhi qiang MA, yong qiang Jin and yong hong Niu; Data curation, zhi qiang MA, Jian Cui and yong qiang Jin; Writing – original draft, zhi qiang MA; Project administration, ming kui Zhang.
Institutional Review Board Statement
The study was approved by the Institutional Ethics Committee (approval no. 2023-024-01(R), June 3, 2023).
Informed Consent Statement
The study was informed consent was obtained from all participants or their legal guardians.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Perspective Statement: Echocardiographic strain analysis offers better evaluation of right ventricular function post-Ebstein's anomaly repair compared to conventional echo parameters. RVFLS and RVGLS are more sensitive in detecting dysfunction, correlate well with CMR, and provide accessible monitoring alternative. This facilitates clinical management through earlier detection of dysfunction and improve patient outcomes.
Abbreviations
The following abbreviations are used in this manuscript:
| CMR |
Cardiac magnetic resonance |
| EA |
Ebstein's anomaly |
| FAC |
Fractional area change |
| RV |
Right ventricle/ventricular |
| RVEF |
Right ventricular ejection fraction |
| RVEDV |
Right ventricular end-diastolic volume |
| RVESV |
Right ventricular end-systolic volume |
| RVFLS |
Right ventricular free wall longitudinal strain |
| RVGLS |
Right ventricular global longitudinal strain |
| RVSLS |
Right ventricular septal longitudinal strain |
| TAPSE |
Tricuspid annular plane systolic excursion |
References
- Attenhofer Jost, CH; Connolly, HM; Dearani, JA; Edwards, WD; Danielson, GK. Ebstein's anomaly. Circulation 2007, 115(2), 277–85. [Google Scholar] [CrossRef]
- Wu, Q; Zhang, X; Zhang, M; et al. Strategy and technique for surgical treatment of Ebstein's anomaly. Chin Med J (Engl) 2024, 137(10), 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Fratz, S; Chung, T; Greil, GF; et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013, 15(1), 51. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V; Lang, RM; Badano, LP; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011, 24(3), 277–313. [Google Scholar] [CrossRef] [PubMed]
- Rudski, LG; Lai, WW; Afilalo, J; et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010, 23(7), 685-713; quiz 786-8. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F; Moon, JC; Bellenger, NG; Smith, GS; Klein, HU; Pennell, DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004, 147(2), 218–23. [Google Scholar] [CrossRef] [PubMed]
- Almeida, ALC; Melo, MDT; Bihan, D; et al. Position Statement on the Use of Myocardial Strain in Cardiology Routines by the Brazilian Society of Cardiology's Department Of Cardiovascular Imaging - 2023. Arq Bras Cardiol;Posicionamento do Departamento de Imagem Cardiovascular da Sociedade Brasileira de Cardiologia sobre o Uso do Strain Miocárdico na Rotina do Cardiologista – 2023 2023, 120(12), e20230646. [Google Scholar] [CrossRef]
- Egbe, AC; Miranda, WR; Jain, CC; et al. Prognostic Performance of Right Ventricular Global Longitudinal Strain Measurements in Patients With Ebstein Anomaly. J Am Coll Cardiol 2023, 82(6), 503–513. [Google Scholar] [CrossRef] [PubMed]
- Lang, RM; Badano, LP; Mor-Avi, V; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015, 28(1), 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Mondillo, Sergio; et al. Speckle-tracking echocardiography: a new technique for assessing myocardial function. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine 2011, vol. 30(1), 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, Victor; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography 2011, vol. 24(3), 277–313. [Google Scholar] [CrossRef] [PubMed]
- Isaaz, K; et al. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. The American journal of cardiology 1989, vol. 64(1), 66–75. [Google Scholar] [CrossRef]
- Miyatake, K; et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. Journal of the American College of Cardiology 1995, vol. 25(3), 717–24. [Google Scholar] [CrossRef]
- Perk, Gila; et al. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography 2007, vol. 20(3), 234–43. [Google Scholar] [CrossRef]
- Fine; Nowell, M; et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation. Cardiovascular imaging 2013, vol. 6(5), 711–21. [Google Scholar] [CrossRef]
- Carluccio; Erberto; et al. Prognostic Value of Right Ventricular Dysfunction in Heart Failure With Reduced Ejection Fraction: Superiority of Lonitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circulation. Cardiovascular imaging 2018, vol. 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Attenhofer Jost, CH; Edmister, WD; Julsrud, PR; et al. Prospective comparison of echocardiography versus cardiac magnetic resonance imaging in patients with Ebstein's anomaly. Int J Cardiovasc Imaging 2012, 28(5), 1147–59. [Google Scholar] [CrossRef] [PubMed]
- Khoo, NS; Young, A; Occleshaw, C; Cowan, B; Zeng, IS; Gentles, TL. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2009, 22(11), 1279–88. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E; Biagioli, P; Alunni, G; et al. Prognostic Value of Right Ventricular Dysfunction in Heart Failure With Reduced Ejection Fraction: Superiority of Longitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circ Cardiovasc Imaging 2018, 11(1), e006894. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).