1. Introduction
Cardiac amyloidosis (CA) is an infiltrative cardiomyopathy caused by extracellular deposition of misfolded amyloid fibrils within the myocardium, leading to progressive ventricular stiffening, diastolic dysfunction, and ultimately heart failure. [
1,
2,
3]. Once considered a rare disease, CA is now increasingly recognized as an underdiagnosed cause of heart failure, particularly heart failure with preserved ejection fraction (HFpEF), owing to advances in imaging techniques and heightened clinical awareness [
4,
5,
6]. The two most common etiologies are transthyretin amyloidosis (ATTR), resulting from deposition of wild-type or mutant transthyretin, and immunoglobulin light-chain amyloidosis (AL), caused by monoclonal plasma cell–derived light chains. AL amyloidosis results from a plasma cell dyscrasia and is characterized by rapid disease progression and high early mortality if untreated. ATTR amyloidosis, caused by misfolded transthyretin protein, occurs either as a hereditary disease due to pathogenic variants or as a wild-type form associated with aging [
7,
8]. Despite important differences in pathogenesis, both forms ultimately converge on a shared phenotype of restrictive cardiomyopathy and progressive myocardial dysfunction. The clinical phenotype of CA overlaps substantially with other forms of hypertrophic or restrictive cardiomyopathy, frequently resulting in delayed diagnosis and advanced disease at presentation. Although noninvasive imaging modalities—including bone-avid scintigraphy, cardiac magnetic resonance, and echocardiographic strain imaging—have transformed diagnostic pathways, they primarily detect established myocardial involvement. Thus, limitations persist in early disease detection, disease monitoring, and risk stratification [
9,
10]. Similarly, contemporary therapies such as transthyretin stabilizers, gene-silencing agents, and plasma cell–directed regimens improve outcomes but are most effective when initiated early. Accordingly, there is a critical unmet need for molecular biomarkers that reflect early disease biology, enable etiologic differentiation, and provide insight into disease activity and therapeutic response.
MicroRNAs (miRNAs) are short, noncoding RNA molecules that regulate gene expression through post-transcriptional repression of target messenger RNAs. By orchestrating complex transcriptional networks, miRNAs play essential roles in cardiovascular development, myocardial remodeling, mitochondrial homeostasis, inflammation, and fibrosis. Dysregulation of miRNA expression has been implicated in a wide spectrum of cardiovascular diseases, including heart failure, hypertrophic cardiomyopathy, and ischemic heart disease. Importantly, miRNAs are detectable in both cardiac tissue and circulation, conferring potential utility as minimally invasive biomarkers [
11,
12,
13].
In recent years, accumulating evidence has linked specific miRNA signatures to the pathophysiology of both ATTR and AL cardiac amyloidosis. These miRNAs modulate key disease processes, including amyloid precursor protein handling, mitochondrial dysfunction, oxidative stress, extracellular matrix remodeling, microvascular dysfunction, and neurocardiac cross-talk. Moreover, distinct miRNA expression profiles appear to differentiate ATTR from AL cardiomyopathy and to correlate with disease severity, functional impairment, and prognosis.
In this review, we provide a comprehensive synthesis of current evidence on the mechanistic roles, diagnostic potential, and therapeutic implications of miRNAs in cardiac amyloidosis. Emphasis is placed on pathways central to heart failure pathophysiology, including mitochondrial energetics, myocardial fibrosis, inflammation, and microvascular dysfunction, as well as emerging RNA-based therapeutic strategies. By integrating molecular insights with clinical relevance, we highlight the potential of miRNA profiling to complement multimodality imaging and advance precision medicine in amyloid-related heart failure.
2. Pathophysiology of Cardiac Amyloidosis
2.1. General Mechanisms of Amyloid-Related Cardiac Dysfunction
CA is characterized by the extracellular deposition of insoluble amyloid fibrils with a β-pleated sheet conformation within the myocardial interstitium. Progressive fibril accumulation disrupts myocardial architecture, increases ventricular stiffness, impairs diastolic filling, alters electrical conduction, and ultimately culminates in restrictive cardiomyopathy [
1,
2,
3]. Amyloid fibrillogenesis is initiated by protein destabilization resulting from inherited mutations in transthyretin (ATTRv), age-related conformational instability of wild-type transthyretin (ATTRwt), or the overproduction of misfolded κ or λ immunoglobulin light chains in plasma cell dyscrasias (AL amyloidosis). These unstable monomers assemble into soluble oligomers and protofibrils before forming mature amyloid fibrils. Increasing evidence indicates that soluble oligomeric species, rather than deposited fibrils, exert the greatest cardiotoxic effects by inducing endothelial dysfunction, oxidative stress, intracellular calcium dysregulation, and cardiomyocyte apoptosis [
4,
5,
6]. The pathological hallmark of CA is diffuse amyloid infiltration of the myocardial extracellular matrix, leading to increased ventricular wall thickness, reduced compliance, and impaired ventricular relaxation. Unlike hypertrophic cardiomyopathy, myocardial thickening in CA reflects infiltrative expansion rather than myocyte hypertrophy, resulting in a restrictive physiology that progressively worsens over time [
7,
14]. This structural remodeling profoundly compromises cardiac filling and output, particularly during exertion.
In AL amyloidosis, circulating monoclonal light chains exert direct cardiotoxic effects independent of fibril deposition, promoting oxidative stress, mitochondrial dysfunction, and cardiomyocyte apoptosis. This dual pathogenic mechanism—amyloid infiltration combined with proteotoxic injury—accounts for the rapid clinical deterioration and poor prognosis commonly observed in AL cardiac amyloidosis [
15,
16]. In contrast, ATTR amyloidosis typically follows a more indolent course, with chronic transthyretin-derived fibril accumulation leading to progressive myocardial stiffening and fibrosis.
Amyloid deposition also disrupts intracellular calcium handling and electromechanical coupling, predisposing patients to atrial and ventricular arrhythmias as well as conduction system disease. Concomitant microvascular dysfunction and endothelial impairment further exacerbate myocardial ischemia and accelerate disease progression [
17,
18]. Fibrotic remodeling represents a key downstream consequence of amyloid infiltration and correlates closely with disease severity and prognosis. Cardiac magnetic resonance imaging has demonstrated that the extent of late gadolinium enhancement reflects both amyloid burden and interstitial fibrosis, underscoring the contribution of secondary remodeling processes to adverse clinical outcomes [
19].
2.2. Transthyretin Cardiac Amyloidosis
Transthyretin (ATTR) amyloidosis results from destabilization of the transthyretin tetramer, leading to dissociation into misfolded monomers that aggregate into insoluble amyloid fibrils. Wild-type ATTR amyloidosis is increasingly recognized as an age-related systemic disorder, predominantly affecting the heart and frequently associated with extracardiac manifestations such as carpal tunnel syndrome, lumbar spinal stenosis, and spontaneous biceps tendon rupture. Cardiac involvement typically evolves gradually and is characterized by progressive myocardial infiltration and restrictive physiology [
20,
21]. Hereditary ATTR amyloidosis arises from pathogenic mutations in the transthyretin gene that promote tetramer instability and influence fibril formation and tissue tropism. Depending on the specific variant, clinical presentation may range from predominantly cardiac to mixed cardiac–neurologic phenotypes [
22]. Key pathophysiologic features of ATTR amyloidosis include progressive extracellular deposition of amyloid fibrils within the myocardial interstitium, accompanied by chronic low-grade inflammation and activation of hypoxia-responsive signaling pathways. These processes contribute to microvascular dysfunction with impaired myocardial perfusion, mitochondrial energetic failure, and sustained metabolic stress. Mitochondrial dysfunction is increasingly recognized as a central mechanism in ATTR cardiomyopathy. Proteomic and transcriptomic studies demonstrate disrupted oxidative phosphorylation, altered mitochondrial dynamics, and impaired bioenergetic reserve, contributing to exercise intolerance and heart failure progression. Importantly, in contrast to AL amyloidosis, ATTR cardiomyopathy is characterized by a relative absence of direct cardiomyocyte toxicity. Instead, myocardial dysfunction arises predominantly from infiltrative burden, metabolic derangements, and microvascular impairment rather than acute cytotoxic injury. [
20,
23,
24].
2.3. Immunoglobulin Light-Chain Cardiac Amyloidosis
Immunoglobulin light-chain (AL) amyloidosis is a rapidly progressive multisystem disease associated with a plasma cell dyscrasia and remains the most aggressive form of cardiac amyloidosis. In contrast to ATTR, myocardial dysfunction in AL amyloidosis is driven not only by amyloid fibril deposition but also by the direct cardiotoxic effects of circulating monoclonal light chains. Experimental and clinical studies have demonstrated that amyloidogenic light chains induce oxidative stress, mitochondrial injury, and cardiomyocyte apoptosis, leading to profound diastolic dysfunction and early hemodynamic compromise. Microvascular dysfunction, endothelial impairment, and nitric oxide depletion further exacerbate myocardial injury. Without prompt treatment, cardiac AL amyloidosis frequently progresses rapidly to advanced heart failure and cardiogenic shock. Compared with ATTR amyloidosis, AL amyloidosis is associated with substantially higher early mortality, often occurring within months of diagnosis if left untreated. Key pathophysiologic features of immunoglobulin light-chain (AL) amyloidosis include severe restrictive diastolic dysfunction driven by myocardial infiltration, coupled with direct light chain–mediated cardiotoxicity that induces oxidative stress, mitochondrial injury, and cardiomyocyte apoptosis. These processes are accompanied by pronounced microvascular dysfunction and endothelial damage, contributing to impaired myocardial perfusion. In the absence of prompt therapy, AL cardiac amyloidosis is characterized by rapid clinical progression and high early mortality [
4].
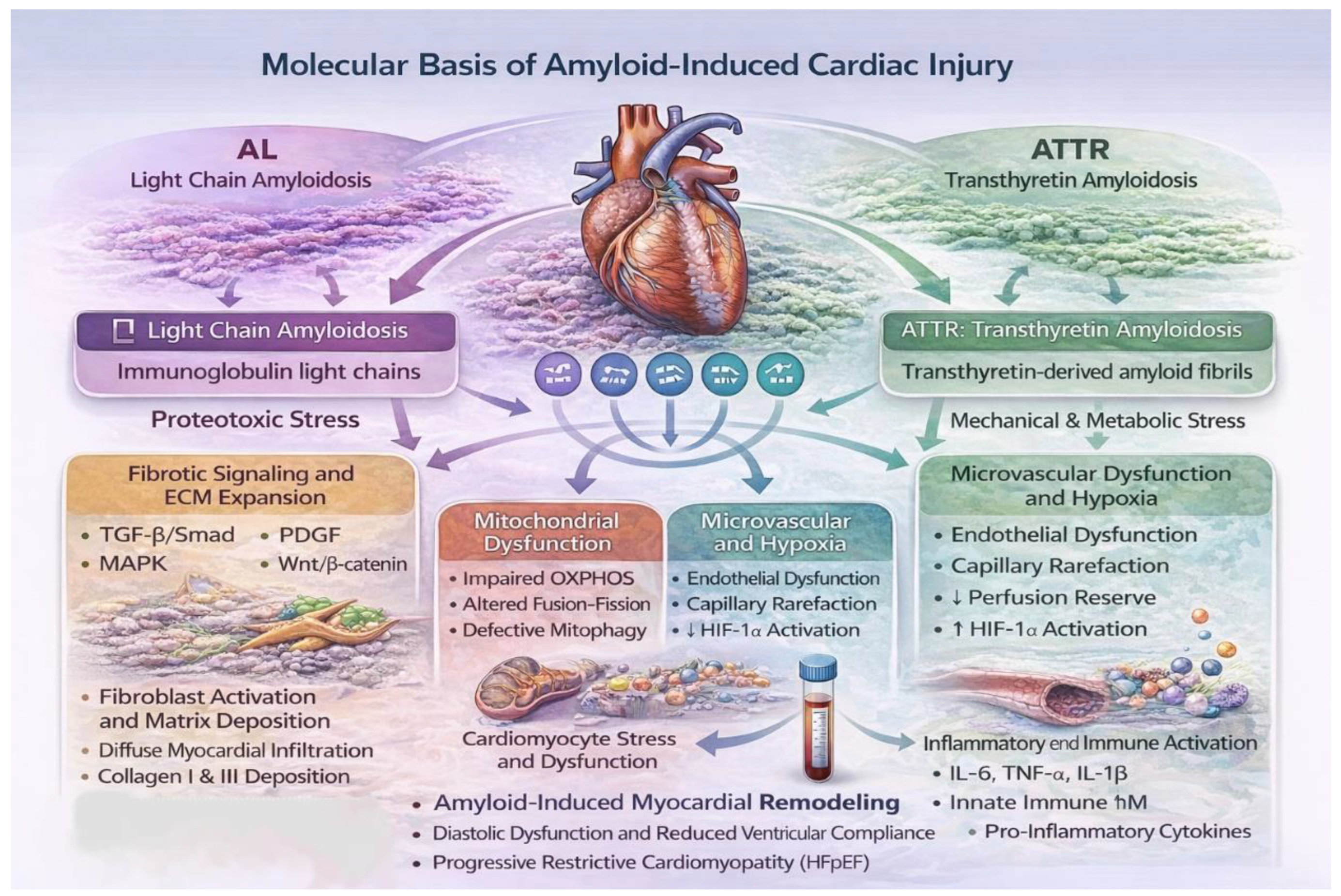
3. Molecular Basis of Amyloid-Induced Cardiac Injury
Cardiac involvement in amyloidosis arises from a multifactorial pathogenic cascade encompassing extracellular amyloid fibril deposition, intracellular stress responses, mitochondrial dysfunction, immune activation, microvascular injury, and progressive disruption of myocardial architecture. Although ATTR and AL amyloidosis converge clinically on a restrictive cardiomyopathy phenotype, their molecular drivers differ substantially. ATTR cardiomyopathy is largely characterized by progressive extracellular infiltration and chronic mechanical and metabolic stress, whereas AL cardiac amyloidosis (AL-CA) is dominated by direct cardiotoxic effects of circulating light chains. These mechanistic distinctions are critical for interpreting disease-specific microRNA dysregulation patterns.
3.1. Extracellular Matrix Remodeling and Fibrosis
Amyloid deposition activates resident cardiac fibroblasts, leading to excessive extracellular matrix (ECM) expansion and diffuse interstitial fibrosis. Central signaling pathways include transforming growth factor-β (TGF-β)/Smad-mediated induction of collagen types I and III, platelet-derived growth factor (PDGF)/ERK-driven fibroblast proliferation, mitogen-activated protein kinase (MAPK) signaling, and Wnt/β-catenin activation, which collectively promote ECM accumulation while suppressing matrix degradation [
11,
25]. MiRNAs act as critical post-transcriptional regulators of these pathways and play a central role in shaping the fibrotic response in amyloid cardiomyopathy (
Figure 1).
3.2. Mitochondrial Dysfunction
Mitochondrial dysfunction has emerged as a core pathogenic mechanism in cardiac amyloidosis, particularly in AL disease but also in ATTR cardiomyopathy. Proteomic and transcriptomic analyses of amyloid-infiltrated myocardium demonstrate widespread disruption of mitochondrial energetics, ultrastructure, and quality-control systems in both subtypes [
16,
26]. Experimental and translational studies reveal impaired oxidative phosphorylation, reduced electron transport chain efficiency, and diminished ATP generation [
26]. Alterations in mitochondrial dynamics, characterized by an imbalance between fission and fusion processes, further contribute to mitochondrial instability and cardiomyocyte dysfunction [
27,
28]. Defective mitochondrial quality control, including suppression of PTEN-induced kinase 1 (PINK1)/Parkin-dependent mitophagy, results in accumulation of damaged mitochondria and heightened cellular stress [
29]. Excess reactive oxygen species production and metabolic inflexibility amplify inflammatory and pro-fibrotic signaling. In parallel, downregulation of key regulators of mitochondrial biogenesis—peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), nuclear respiratory factor-1 (NRF1), and mitochondrial transcription factor A (TFAM)—limits adaptive energetic responses in amyloid cardiomyopathy [
26,
30]. MiRNAs targeting these mitochondrial regulatory networks, including those modulating PGC-1α, dynamin-related protein-1 (DRP1), BCL2 Interacting Protein 3 (BNIP3), and the PINK1/Parkin axis, provide a molecular link between proteotoxic stress, impaired mitochondrial homeostasis, and disease progression [
23,
31] (
Figure 1).
3.3. Microvascular Rarefaction and Hypoxia
Both AL and ATTR amyloidosis impair endothelial function, reduce nitric oxide bioavailability, and promote microvascular rarefaction, thereby exacerbating myocardial hypoxia. Emerging evidence suggests that age-related hypovascularity synergizes with amyloid-induced endothelial dysfunction to activate hypoxia-inducible factor-1α (HIF-1α) signaling and downstream inflammatory cascades, further aggravating myocardial injury [
23,
24] (
Figure 1).
4. MicroRNA Biology and Relevance to Amyloidosis
MicroRNAs are short (18–24 nucleotides), noncoding RNA molecules that regulate post-transcriptional gene expression through sequence-specific binding to complementary regions within target mRNAs, resulting in translational repression or mRNA degradation [
12,
32]. In the cardiovascular system, miRNAs orchestrate complex gene regulatory networks involved in cardiomyocyte survival, fibrosis, inflammation, and vascular homeostasis, processes that are centrally implicated in amyloid cardiomyopathy [
12,
33].
Proteostasis—the balance between protein synthesis, folding, and degradation—is a defining feature of amyloid diseases. Although direct evidence in cardiac amyloidosis remains limited, miRNAs are known regulators of ubiquitin–proteasome activity, autophagy, and endoplasmic reticulum stress responses in cardiovascular disease models [
12,
34]. In addition, miRNAs modulate ECM turnover, mitochondrial function, immune activation, endothelial integrity, and cardiomyocyte hypertrophy, all of which contribute to amyloid-mediated myocardial dysfunction [
11,
33]. Despite the relatively early stage of investigation, circulating miRNA profiling studies in cardiac amyloidosis have identified reproducible disease-associated signatures. Importantly, certain circulating miRNAs are detectable in peripheral blood and show disease-related alterations, supporting their potential role as minimally invasive biomarkers in amyloid cardiomyopathy [
35].
4.1. Differential miRNA Expression in AL vs ATTR Cardiomyopathy
The first systematic blood-based miRNA profiling study in cardiac amyloidosis demonstrated distinct expression patterns compared with healthy controls, with miR-339-3p emerging as a significantly upregulated candidate with discriminatory potential, highlighting the feasibility of miRNA-based diagnostics in this population [
35]. While direct comparative studies between AL and ATTR cardiomyopathy remain limited, fundamental pathophysiological differences between the two entities suggest divergent miRNA involvement.
AL cardiomyopathy is driven by direct light-chain-mediated cardiotoxicity, leading to oxidative stress, apoptosis, and rapid myocardial dysfunction. In contrast, ATTR cardiomyopathy evolves through progressive extracellular amyloid deposition, chronic myocardial stiffening, and fibrotic remodeling [
36,
37]. Extrapolating from broader cardiovascular literature, AL disease is therefore more likely to involve apoptosis- and oxidative stress–related miRNAs, whereas ATTR cardiomyopathy may preferentially engage miRNAs regulating fibroblast activation and ECM remodeling [
12,
33] (
Figure 2).
4.2. Pro-Fibrotic miRNAs in Amyloid Cardiomyopathy
Myocardial fibrosis is a defining feature of ATTR cardiomyopathy and a major determinant of diastolic dysfunction. Among pro-fibrotic miRNAs, miR-21 is a well-established regulator of cardiac fibroblast survival and TGF-β signaling in multiple cardiomyopathies [
38]. Although direct ATTR-specific mechanistic studies are lacking, the pronounced fibrotic phenotype of ATTR cardiomyopathy supports a contributory role for miR-21-mediated pathways.
Additional miRNAs with pro-fibrotic and pro-inflammatory effects, including miR-199a and miR-155, promote fibroblast proliferation and inflammatory–fibrotic signaling in experimental and clinical cardiovascular disease, providing further mechanistic plausibility for their involvement in amyloid-associated myocardial remodeling [
39,
40] (
Figure 3).
4.3. Anti-Fibrotic and ECM-Regulatory miRNAs
The miR-29 family plays a central role in ECM homeostasis by repressing the expression of collagen types I and III and fibrillin [
41]. Downregulation of miR-29 has been consistently linked to pathological myocardial fibrosis across diverse cardiac disease states. In amyloid cardiomyopathy, suppression of miR-29-mediated ECM regulation represents a plausible molecular mechanism contributing to early matrix expansion and increased myocardial stiffness, even before overt systolic dysfunction becomes apparent [
37,
41] (
Figure 3).
4.4. Apoptosis and Cardiomyocyte Stress miRNAs
AL amyloidosis is uniquely characterized by direct light-chain-mediated cardiotoxicity, leading to cardiomyocyte apoptosis and biomarker release independent of amyloid burden. MiRNAs such as miR-34a, miR-320a, and miR-181b have been implicated in cardiomyocyte apoptosis, oxidative stress, and mitochondrial dysfunction in cardiovascular disease and heart failure cohorts [
42,
43,
44]. Although not yet validated specifically in AL amyloidosis, these miRNAs represent biologically plausible mediators of light-chain-induced myocardial injury.
In contrast, ATTR cardiomyopathy is associated with less prominent apoptotic signaling and is dominated by chronic metabolic stress and low-grade inflammation. In this context, miR-146a—a key regulator of innate immune and NF-κB signaling—has been linked to inflammatory and metabolic stress responses and may contribute to ATTR-related myocardial dysfunction [
45].
In addition to apoptosis, amyloid deposition induces direct cardiomyocyte toxicity, resulting in cellular stress, impaired calcium handling, and eventual cell death. Cardiomyocyte-enriched miRNAs such as miR-133 and miR-1 play key roles in regulating hypertrophy, electrical stability, and survival pathways [
33]. Dysregulation of these miRNAs has been associated with myocardial injury and adverse remodeling in heart failure and other cardiomyopathies. Endothelial-associated miRNAs, particularly miR-126, play essential roles in angiogenesis, vascular integrity, and endothelial repair [
46]. Dysregulation of endothelial miRNAs has been demonstrated across cardiovascular disease states and may mechanistically underlie the impaired perfusion reserve observed in amyloid cardiomyopathy (
Figure 3).
5. Circulating miRNAs as Noninvasive Biomarkers
Circulating miRNAs exhibit remarkable stability in peripheral blood, owing to their encapsulation within extracellular vesicles, association with lipoproteins, or binding to RNA-binding proteins [
47]. Their tissue specificity and disease-related expression patterns make them attractive candidates for biomarker development in cardiovascular disorders, including cardiomyopathies and heart failure [
33]. In this context, we have shown that specific circulating miRNAs, including miR-21 and miR-1, are differentially expressed in cardiovascular disease states and correlate with indices of myocardial injury and functional impairment, supporting their potential role as clinically informative blood-based biomarkers [
48,
49].
In CA, dysregulated miRNA expression reflects fundamental pathogenic mechanisms such as amyloid deposition, cardiomyocyte stress, fibrosis, and inflammation. Fibrosis-associated miRNAs, including miR-21 and members of the miR-29 family, have been implicated in extracellular matrix remodeling and myocardial stiffening—key pathological features of amyloid cardiomyopathy [
38,
41]. These circulating miRNAs may provide complementary information to established cardiac biomarkers such as N-terminal pro–B-type natriuretic peptide (NT-proBNP) and cardiac troponins, which reflect hemodynamic stress and myocardial injury but lack disease specificity.
5.1. miRNAs for Differentiating Amyloidosis Subtypes
Distinguishing between light-chain (AL) amyloidosis and ATTR amyloidosis is of paramount clinical importance, as prognosis, therapeutic strategies, and urgency of treatment differ substantially between the two entities [
8]. Emerging evidence suggests that miRNA expression profiles may differ between amyloidosis subtypes, reflecting distinct underlying biological mechanisms. AL amyloidosis, driven by plasma cell dyscrasia and systemic proteotoxicity, has been associated with dysregulation of miRNAs involved in immune responses, apoptosis, and oxidative stress, including miR-150 and miR-155 [
40]. In contrast, ATTR amyloidosis—characterized by chronic myocardial infiltration and progressive fibrosis—appears to be associated with miRNAs linked to extracellular matrix remodeling and cardiomyocyte stress signaling [
7,
41]. A comprehensive profiling study of circulating microRNAs in patients with transthyretin variant amyloidosis (ATTRv) identified substantial dysregulation compared with multiple control groups. Specifically, ATTRv patients exhibited 33 up-regulated and 48 down-regulated miRNAs relative to healthy controls, 9 up-regulated and 30 down-regulated miRNAs compared with patients with Charcot–Marie–Tooth disease (CMT), and 19 up-regulated and 38 down-regulated miRNAs compared with asymptomatic carriers of transthyretin variants (TTRv) [
50]. Subsequent validation analyses highlighted miR-150-5p as a robust biomarker capable of discriminating symptomatic ATTRv patients from asymptomatic TTRv carriers [
47]. Functional studies using Schwann cell culture models demonstrated that miR-150-5p acts as a potent negative regulator of CREB, BDNF, and NGF expression—genes that are also implicated in cardiac dysfunction [
50]. In a study investigating microRNA dysregulation in AL amyloidosis, bone marrow plasma cells (BMPCs) were purified from patients with AL amyloidosis and from control subjects. Comparative microarray analysis revealed that ten miRNAs were up-regulated by more than 1.5-fold in AL amyloidosis. These findings were subsequently validated by stem-loop RT-qPCR for the most markedly up-regulated miRNAs, including miR-148a, miR-26a, and miR-16. Notably, miR-16—previously implicated in other hematopoietic malignancies—was significantly elevated in AL patients at diagnosis and in treated individuals with persistent monoclonal plasma cells in the bone marrow, but not in patients who achieved hematologic remission following therapy. Collectively, these results indicate that miRNA expression is dysregulated in clonal plasma cells in AL amyloidosis and suggest that specific miRNAs may serve as potential biomarkers of disease presence and treatment response [
51]. Although current data are limited and largely exploratory, these findings support the concept that miRNA signatures may assist in subtype discrimination when conventional diagnostic algorithms are inconclusive. A summary of key miRNAs implicated in cardiac amyloidosis, their associated molecular pathways, and the current level of supporting evidence is provided in
Table 1.
5.2. Diagnostic Value in Early and Subclinical Disease
One of the most promising potential applications of miRNAs in cardiac amyloidosis lies in the detection of early or subclinical cardiac involvement. Structural and functional abnormalities often develop gradually and may remain undetected by conventional imaging modalities until advanced disease stages [
6,
19]. In contrast, miRNA dysregulation may occur earlier in the disease course, reflecting molecular alterations that precede overt myocardial dysfunction.
Consistent with this concept, we have shown that alterations in circulating miRNAs associated with myocardial remodeling and vascular dysfunction can be detected in cardiovascular conditions prior to the development of overt structural heart disease, supporting their potential utility as early diagnostic or risk-stratification marker [
48,
49,
52]. These observations align with broader evidence suggesting that stress- and fibrosis-related miRNAs may be altered before significant echocardiographic or magnetic resonance abnormalities become apparent [
39] . Applied to cardiac amyloidosis, these findings indicate that miRNA-based assays could support earlier recognition of cardiac involvement, particularly among individuals at increased risk or in preclinical stages of disease. In clinical practice, circulating miRNAs are unlikely to replace established diagnostic tools but may add incremental value when incorporated into multimodal diagnostic pathways. Integration of miRNA profiling with echocardiography, cardiac magnetic resonance imaging, nuclear scintigraphy, and conventional serum biomarkers could facilitate earlier referral, improve diagnostic confidence, and enable more accurate disease classification and monitoring. [
9,
10]. Such integrative approaches could reduce reliance on invasive endomyocardial biopsy in selected cases and improve diagnostic confidence, particularly in early or atypical presentations.
However, methodological challenges—including variability in miRNA isolation techniques, normalization strategies, and analytical platforms—currently limit direct clinical translation [
47]. Despite growing interest, miRNA-based diagnostics in CA remain at an early stage of development. Most available studies are observational, involve small patient cohorts, and use heterogeneous methodologies. Furthermore, overlap in miRNA dysregulation across different cardiovascular diseases may limit disease specificity when individual miRNAs are considered in isolation [
33]. Future research should prioritize the development and validation of multi-miRNA diagnostic panels, longitudinal studies assessing temporal changes in miRNA expression, and standardized protocols for miRNA quantification. Advances in high-throughput sequencing, bioinformatics, and machine learning are expected to facilitate the identification of robust diagnostic signatures and accelerate the clinical translation of miRNA-based diagnostics in cardiac amyloidosis.
6. Therapeutic Applications of MicroRNAs in Cardiac Amyloidosis
Beyond their diagnostic and prognostic potential, microRNAs have attracted increasing interest as potential therapeutic targets in cardiac amyloidosis. By regulating multiple genes within interconnected signaling pathways, miRNAs occupy a central position in the molecular networks governing amyloid-induced myocardial stress, fibrosis, inflammation, and cardiomyocyte dysfunction. Modulating miRNA activity therefore represents a theoretically attractive strategy to influence disease progression at a post-transcriptional level.
The pathophysiology of CA is multifactorial, involving extracellular amyloid deposition, mechanical myocardial stiffening, proteotoxic stress, oxidative injury, and progressive fibrotic remodeling. Conventional therapies largely target upstream processes—such as suppression of light-chain production in AL amyloidosis or stabilization and silencing of transthyretin in ATTR amyloidosis—while downstream myocardial remodeling often continues despite effective disease-modifying treatment. MiRNAs offer a complementary therapeutic avenue by modulating intracellular responses to amyloid toxicity. Several miRNAs implicated in fibrosis (e.g., miR-21, miR-29), cardiomyocyte survival (e.g., miR-133, miR-1), and inflammatory signaling have been shown to influence pathways relevant to myocardial remodeling and diastolic dysfunction [
33,
38,
39,
41]. Given their pleiotropic effects, therapeutic modulation of miRNAs could theoretically attenuate maladaptive remodeling processes common to both AL and ATT R cardiomyopathy.
Myocardial fibrosis is a central determinant of diastolic dysfunction, arrhythmogenesis, and adverse prognosis in CA. Among the best-studied miRNAs in cardiac fibrosis, miR-21 has been shown to promote fibroblast activation and extracellular matrix deposition through MAP kinase and TGF-β–dependent pathways [
38]. Experimental inhibition of miR-21 has been associated with attenuation of cardiac fibrosis and improved myocardial compliance in preclinical models [
53]. Conversely, members of the miR-29 family function as negative regulators of collagen and extracellular matrix gene expression, and their downregulation has been linked to fibrotic remodeling in multiple cardiac disease states [
41]. Restoring miR-29 expression has demonstrated antifibrotic effects in experimental settings, suggesting potential therapeutic relevance for infiltrative cardiomyopathies characterized by progressive myocardial stiffening.
Although these findings are not specific to amyloidosis, the shared fibrotic phenotype supports cautious extrapolation to CA, particularly in ATTR cardiomyopathy, where fibrosis evolves over a prolonged disease course.
Several platforms for miRNA modulation have been developed, including antisense oligonucleotides (antagomiRs), miRNA mimics, and locked nucleic acid (LNA)–based inhibitors. These approaches have entered clinical testing in noncardiac diseases, demonstrating the feasibility of targeting miRNAs in humans [
54]. However, translation to cardiovascular disease—and particularly to CA—faces substantial challenges. Key obstacles include tissue-specific delivery to the myocardium, off-target effects due to the pleiotropic nature of miRNAs, immune activation, and long-term safety concerns. Furthermore, the advanced age and comorbidity burden typical of patients with ATTR amyloidosis necessitate particularly stringent safety profiles for any novel therapeutic intervention.
Rather than replacing established disease-modifying treatments, miRNA-based therapies—if successfully developed—are more likely to function as adjunctive strategies. In AL amyloidosis, miRNA modulation could potentially complement plasma cell–directed therapies by mitigating downstream myocardial remodeling. In ATTR amyloidosis, miRNA-targeted approaches might synergize with transthyretin stabilizers or gene-silencing therapies by attenuating fibrosis and cardiomyocyte stress [
55,
56].
Such combination strategies align with a precision-medicine framework, in which upstream amyloidogenic processes and downstream myocardial responses are addressed simultaneously.
7. Future Directions and Challenges
Despite growing enthusiasm, several challenges must be addressed before miRNA-based approaches can be integrated into routine clinical practice. Methodological heterogeneity in miRNA detection platforms, normalization strategies, and study design limits comparability across cohorts. Standardization of pre-analytical and analytical workflows is essential to enable validation and regulatory approval [
33,
39]. Additionally, the causal versus associative roles of specific miRNAs in amyloid-related cardiac dysfunction remain incompletely defined. Most available data are observational, underscoring the need for mechanistic studies and prospective clinical trials to clarify therapeutic relevance. Given the systemic nature of amyloidosis, careful consideration must also be given to off-target effects and interactions between cardiac and extracardiac miRNA signaling.
Future research should focus on integrating miRNA profiling with established imaging and biochemical markers to develop multimodal risk stratification strategies. Large, well-characterized cohorts with longitudinal follow-up will be critical to defining clinically meaningful miRNA signatures and thresholds. Advances in delivery technologies and improved understanding of miRNA biology may ultimately enable precision targeting of pathogenic pathways in amyloid-related heart failure.
Ultimately, the clinical integration of miRNA-based approaches will depend on demonstrating clear incremental value over established diagnostic and therapeutic strategies. For diagnostics, this includes improved early detection, subtype differentiation, or risk stratification. For therapeutics, it requires evidence of meaningful clinical benefit, acceptable safety profiles, and feasibility within real-world healthcare settings [
10,
39]. Multidisciplinary collaboration between clinicians, molecular scientists, and data scientists will be essential to translate miRNA research into clinically actionable tools for patients with cardiac amyloidosis.
8. Conclusions
CA represents a complex and heterogeneous cause of heart failure, driven by distinct yet overlapping mechanisms in transthyretin and light-chain disease. MicroRNAs have emerged as central regulators of key pathways underlying amyloid-related myocardial dysfunction, including fibrosis, mitochondrial failure, oxidative stress, inflammation, and microvascular impairment. Accumulating evidence supports the potential of circulating and tissue miRNA signatures to enhance diagnostic accuracy, enable etiologic differentiation, and refine prognostic assessment beyond conventional biomarkers.
As RNA-based therapeutics continue to advance, miRNAs offer a compelling bridge between mechanistic insight and clinical translation in cardiac amyloidosis. Integration of miRNA profiling with contemporary multimodality imaging and disease-specific therapies may facilitate earlier diagnosis and support precision management of amyloid-related heart failure. Continued translational and clinical investigation will be essential to realize the full potential of miRNA-based strategies in this rapidly evolving field.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Gill, SS; Fellin, E; Stampke, L; Zhao, Y; Masri, A. Clinical Clues and Diagnostic Workup of Cardiac Amyloidosis. Methodist Debakey Cardiovasc J 2022, 18(2), 36–46. [Google Scholar] [CrossRef]
- Wechalekar, AD; Gillmore, JD; Hawkins, PN. Systemic amyloidosis. Lancet 2016, 387(10038), 2641–54. [Google Scholar] [CrossRef]
- Buxbaum, JN; Eisenberg, DS; Fändrich, M; McPhail, ED; Merlini, G; Saraiva, MJM; et al. Amyloid nomenclature 2024: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2024, 31(4), 249–56. [Google Scholar] [CrossRef] [PubMed]
- Falk, RH; Alexander, KM; Liao, R; Dorbala, S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol. 2016, 68(12), 1323–41. [Google Scholar] [CrossRef]
- Kittleson, MM; Maurer, MS; Ambardekar, AV; Bullock-Palmer, RP; Chang, PP; Eisen, HJ; et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142(1), e7–e22. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A; Hawkins, PN; Fontana, M. Cardiac amyloidosis. Clin Med (Lond) 2018, 18 (Suppl 2), s30–s5. [Google Scholar] [CrossRef]
- Rapezzi, C; Quarta, CC; Riva, L; Longhi, S; Gallelli, I; Lorenzini, M; et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010, 7(7), 398–408. [Google Scholar] [CrossRef]
- Maurer, MS; Elliott, P; Merlini, G; Shah, SJ; Cruz, MW; Flynn, A; et al. Design and Rationale of the Phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail 2017, 10(6). [Google Scholar] [CrossRef]
- Gillmore, JD; Maurer, MS; Falk, RH; Merlini, G; Damy, T; Dispenzieri, A; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133(24), 2404–12. [Google Scholar] [CrossRef]
- Dorbala, S; Cuddy, S; Falk, RH. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc Imaging 2020, 13(6), 1368–83. [Google Scholar] [CrossRef] [PubMed]
- Thum, T. Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol. 2014, 11(11), 655–63. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J; Thum, T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011, 109(3), 334–47. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G; Bletsa, E; Stampouloglou, PK; Oikonomou, E; Tsigkou, V; Paschou, SA; et al. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. 2020, 61(3), 165–73. [Google Scholar] [CrossRef]
- Merlini, G; Bellotti, V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003, 349(6), 583–96. [Google Scholar] [CrossRef] [PubMed]
- Liao, R; Jain, M; Teller, P; Connors, LH; Ngoy, S; Skinner, M; et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 2001, 104(14), 1594–7. [Google Scholar] [CrossRef]
- Brenner, DA; Jain, M; Pimentel, DR; Wang, B; Connors, LH; Skinner, M; et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004, 94(8), 1008–10. [Google Scholar] [CrossRef]
- Migrino, RQ; Truran, S; Gutterman, DD; Franco, DA; Bright, M; Schlundt, B; et al. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol. 2011, 301(6), H2305–12. [Google Scholar] [CrossRef]
- Dorbala, S; Vangala, D; Bruyere, J, Jr.; Quarta, C; Kruger, J; Padera, R; et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014, 2(4), 358–67. [Google Scholar] [CrossRef]
- Fontana, M; Pica, S; Reant, P; Abdel-Gadir, A; Treibel, TA; Banypersad, SM; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132(16), 1570–9. [Google Scholar] [CrossRef]
- Tschöpe, C; Elsanhoury, A; Kristen, AV. Transthyretin Amyloid Cardiomyopathy-2025 Update: Current Diagnostic Approaches and Emerging Therapeutic Options. J Clin Med. 2025, 14(13). [Google Scholar] [CrossRef]
- Karam, C; Moffit, C; Summers, C; Merkel, MP; Kochman, FM; Weijers, L; et al. The journey to diagnosis of wild-type transthyretin-mediated (ATTRwt) amyloidosis: a path with multisystem involvement. Orphanet J Rare Dis. 2024, 19(1), 419. [Google Scholar] [CrossRef]
- Adams, D; Sekijima, Y; Conceição, I; Waddington-Cruz, M; Polydefkis, M; Echaniz-Laguna, A; et al. Hereditary transthyretin amyloid neuropathies: advances in pathophysiology, biomarkers, and treatment. Lancet Neurol. 2023, 22(11), 1061–74. [Google Scholar] [CrossRef] [PubMed]
- Phua, TJ. Understanding human aging and the fundamental cell signaling link in age-related diseases: the middle-aging hypovascularity hypoxia hypothesis. Front Aging 2023, 4, 1196648. [Google Scholar] [CrossRef]
- Russo, MA; Tomino, C; Vernucci, E; Limana, F; Sansone, L; Frustaci, A; et al. Hypoxia and Inflammation as a Consequence of β-Fibril Accumulation: A Perspective View for New Potential Therapeutic Targets. Oxid Med Cell Longev. 2019, 2019, 7935310. [Google Scholar] [CrossRef]
- Long, M; Cheng, M. Small extracellular vesicles associated miRNA in myocardial fibrosis. Biochem Biophys Res Commun. 2024, 727, 150336. [Google Scholar] [CrossRef]
- Morfino, P; Aimo, A; Franzini, M; Vergaro, G; Castiglione, V; Panichella, G; et al. Pathophysiology of Cardiac Amyloidosis. Heart Fail Clin. 2024, 20(3), 261–70. [Google Scholar] [CrossRef]
- Ong, SB; Kalkhoran, SB; Hernández-Reséndiz, S; Samangouei, P; Ong, SG; Hausenloy, DJ. Mitochondrial-Shaping Proteins in Cardiac Health and Disease - the Long and the Short of It! Cardiovasc Drugs Ther. 2017, 31(1), 87–107. [Google Scholar] [CrossRef]
- Gustafsson, Å B; Dorn, GW, 2nd. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol Rev. 2019, 99(1), 853–92. [Google Scholar] [CrossRef]
- Kubli, DA; Gustafsson, Å B. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012, 111(9), 1208–21. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z; He, H; Lin, J; Hoyer, K; Handschin, C; Toka, O; et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005, 1(4), 259–71. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S; Bhatti, GK; Khullar, N; Bhatti, JS. Targeting Mitochondrial microRNAs in Cardiovascular Pathologies: A New Frontier in Precision Cardiology. Ageing Res Rev 2025, 102920. [Google Scholar] [CrossRef] [PubMed]
- Ha, M; Kim, VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014, 15(8), 509–24. [Google Scholar] [CrossRef]
- Small, EM; Olson, EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469(7330), 336–42. [Google Scholar] [CrossRef]
- Mendell, JT; Olson, EN. MicroRNAs in stress signaling and human disease. Cell. 2012, 148(6), 1172–87. [Google Scholar] [CrossRef]
- Derda, AA; Pfanne, A; Bär, C; Schimmel, K; Kennel, PJ; Xiao, K; et al. Blood-based microRNA profiling in patients with cardiac amyloidosis. PLoS One 2018, 13(10), e0204235. [Google Scholar] [CrossRef]
- Falk, RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005, 112(13), 2047–60. [Google Scholar] [CrossRef]
- Patel, KS; Hawkins, PN. Cardiac amyloidosis: where are we today? J Intern Med. 2015, 278(2), 126–44. [Google Scholar] [CrossRef]
- Thum, T; Gross, C; Fiedler, J; Fischer, T; Kissler, S; Bussen, M; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456(7224), 980–4. [Google Scholar] [CrossRef]
- Tijsen, AJ; Pinto, YM; Creemers, EE. Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res. 2012, 93(4), 573–82. [Google Scholar] [CrossRef] [PubMed]
- O'Connell, RM; Rao, DS; Baltimore, D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, RL; Yu, G; Latimer, PA; Stack, C; Robinson, K; Dalby, CM; et al. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med. 2014, 6(10), 1347–56. [Google Scholar] [CrossRef]
- Boon, RA; Iekushi, K; Lechner, S; Seeger, T; Fischer, A; Heydt, S; et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013, 495(7439), 107–10. [Google Scholar] [CrossRef]
- Tian, ZQ; Jiang, H; Lu, ZB. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia-reperfusion injury by targeting AKIP1. Cell Mol Biol Lett. 2018, 23, 41. [Google Scholar] [CrossRef]
- Yuan, L; Fan, L; Li, Q; Cui, W; Wang, X; Zhang, Z. Inhibition of miR-181b-5p protects cardiomyocytes against ischemia/reperfusion injury by targeting AKT3 and PI3KR3. J Cell Biochem. 2019, 120(12), 19647–59. [Google Scholar] [CrossRef]
- Taganov, KD; Boldin, MP; Chang, KJ; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006, 103(33), 12481–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, S; Aurora, AB; Johnson, BA; Qi, X; McAnally, J; Hill, JA; et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008, 15(2), 261–71. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, A; Lee, I; Hood, L; Galas, D; Wang, K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011, 717(1-2), 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kontaraki, JE; Marketou, ME; Zacharis, EA; Parthenakis, FI; Vardas, PE. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens. 2014, 28(8), 510–6. [Google Scholar] [CrossRef]
- Marketou, M; Kontaraki, J; Papadakis, J; Kochiadakis, G; Vrentzos, G; Maragkoudakis, S; et al. Platelet microRNAs in hypertensive patients with and without cardiovascular disease. J Hum Hypertens. 2019, 33(2), 149–56. [Google Scholar] [CrossRef]
- Vita, GL; Aguennouz, M; Polito, F; Oteri, R; Russo, M; Gentile, L; et al. Circulating microRNAs Profile in Patients With Transthyretin Variant Amyloidosis. Front Mol Neurosci. 2020, 13, 102. [Google Scholar] [CrossRef]
- Weng, L; Spencer, BH; SoohHoo, PT; Connors, LH; O'Hara, CJ; Seldin, DC. Dysregulation of miRNAs in AL amyloidosis. Amyloid 2011, 18(3), 128–35. [Google Scholar] [CrossRef]
- Marketou, M; Kontaraki, J; Patrianakos, A; Kochiadakis, G; Anastasiou, I; Fragkiadakis, K; et al. Peripheral Blood MicroRNAs as Potential Biomarkers of Myocardial Damage in Acute Viral Myocarditis. Genes (Basel) 2021, 12(3). [Google Scholar] [CrossRef] [PubMed]
- Patrick, DM; Montgomery, RL; Qi, X; Obad, S; Kauppinen, S; Hill, JA; et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010, 120(11), 3912–6. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R; Slack, FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017, 16(3), 203–22. [Google Scholar] [CrossRef] [PubMed]
- Adams, D; Gonzalez-Duarte, A; O'Riordan, WD; Yang, CC; Ueda, M; Kristen, AV; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018, 379(1), 11–21. [Google Scholar] [CrossRef]
- Maurer, MS; Schwartz, JH; Gundapaneni, B; Elliott, PM; Merlini, G; Waddington-Cruz, M; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018, 379(11), 1007–16. [Google Scholar] [CrossRef]
Figure 1.
Molecular basis of amyloid-induced cardiac injury. Schematic representation of amyloid-induced molecular injury in cardiac amyloidosis. Deposition of immunoglobulin light-chain (AL) or transthyretin-derived (ATTR) amyloid fibrils disrupts myocardial homeostasis through activation of fibrotic signaling, mitochondrial dysfunction, oxidative stress, microvascular impairment, and inflammatory pathways. These processes are modulated by dysregulated microRNA networks, which contribute to adverse cardiac remodeling, diastolic dysfunction, and progression of amyloid cardiomyopathy.
Figure 1.
Molecular basis of amyloid-induced cardiac injury. Schematic representation of amyloid-induced molecular injury in cardiac amyloidosis. Deposition of immunoglobulin light-chain (AL) or transthyretin-derived (ATTR) amyloid fibrils disrupts myocardial homeostasis through activation of fibrotic signaling, mitochondrial dysfunction, oxidative stress, microvascular impairment, and inflammatory pathways. These processes are modulated by dysregulated microRNA networks, which contribute to adverse cardiac remodeling, diastolic dysfunction, and progression of amyloid cardiomyopathy.
Figure 2.
Differential microRNA expression in AL versus ATTR cardiomyopathy. Overview of disease-specific microRNA (miRNA) signatures in cardiac amyloidosis. Light-chain (AL) cardiomyopathy is associated with miRNAs linked to oxidative stress, apoptosis, and cardiotoxicity, whereas transthyretin (ATTRwt and ATTRv) cardiomyopathy is characterized by miRNAs regulating fibroblast activation and extracellular matrix remodeling. Distinct circulating and tissue miRNA profiles reflect these divergent pathogenic mechanisms and highlight the potential of miRNAs as minimally invasive biomarkers for disease classification and monitoring.
Figure 2.
Differential microRNA expression in AL versus ATTR cardiomyopathy. Overview of disease-specific microRNA (miRNA) signatures in cardiac amyloidosis. Light-chain (AL) cardiomyopathy is associated with miRNAs linked to oxidative stress, apoptosis, and cardiotoxicity, whereas transthyretin (ATTRwt and ATTRv) cardiomyopathy is characterized by miRNAs regulating fibroblast activation and extracellular matrix remodeling. Distinct circulating and tissue miRNA profiles reflect these divergent pathogenic mechanisms and highlight the potential of miRNAs as minimally invasive biomarkers for disease classification and monitoring.
Figure 3.
Mechanistic roles of disease-associated microRNAs in amyloid cardiomyopathy. Schematic summary of key microRNA (miRNA) networks implicated in myocardial injury and remodeling in cardiac amyloidosis. Pro-fibrotic miRNAs, including miR-21, miR-199a, and miR-155, promote fibroblast activation and inflammatory–fibrotic signaling, contributing to extracellular matrix expansion, particularly in transthyretin (ATTR) cardiomyopathy. In contrast, anti-fibrotic and ECM-regulatory miRNAs, most notably the miR-29 family, repress collagen expression and modulate matrix remodeling. In light-chain (AL) amyloidosis, miRNAs associated with cardiomyocyte stress and apoptosis, including miR-34a, miR-320a, and miR-181b, reflect direct light-chain–mediated cardiotoxicity and mitochondrial dysfunction. Cardiomyocyte- and endothelial-enriched miRNAs, such as miR-1, miR-133, miR-126, and miR-146a, regulate electrical stability, survival pathways, angiogenesis, and inflammatory signaling. Together, these miRNA networks link amyloid burden to fibrosis, cellular stress, vascular dysfunction, and progressive cardiac impairment.
Figure 3.
Mechanistic roles of disease-associated microRNAs in amyloid cardiomyopathy. Schematic summary of key microRNA (miRNA) networks implicated in myocardial injury and remodeling in cardiac amyloidosis. Pro-fibrotic miRNAs, including miR-21, miR-199a, and miR-155, promote fibroblast activation and inflammatory–fibrotic signaling, contributing to extracellular matrix expansion, particularly in transthyretin (ATTR) cardiomyopathy. In contrast, anti-fibrotic and ECM-regulatory miRNAs, most notably the miR-29 family, repress collagen expression and modulate matrix remodeling. In light-chain (AL) amyloidosis, miRNAs associated with cardiomyocyte stress and apoptosis, including miR-34a, miR-320a, and miR-181b, reflect direct light-chain–mediated cardiotoxicity and mitochondrial dysfunction. Cardiomyocyte- and endothelial-enriched miRNAs, such as miR-1, miR-133, miR-126, and miR-146a, regulate electrical stability, survival pathways, angiogenesis, and inflammatory signaling. Together, these miRNA networks link amyloid burden to fibrosis, cellular stress, vascular dysfunction, and progressive cardiac impairment.
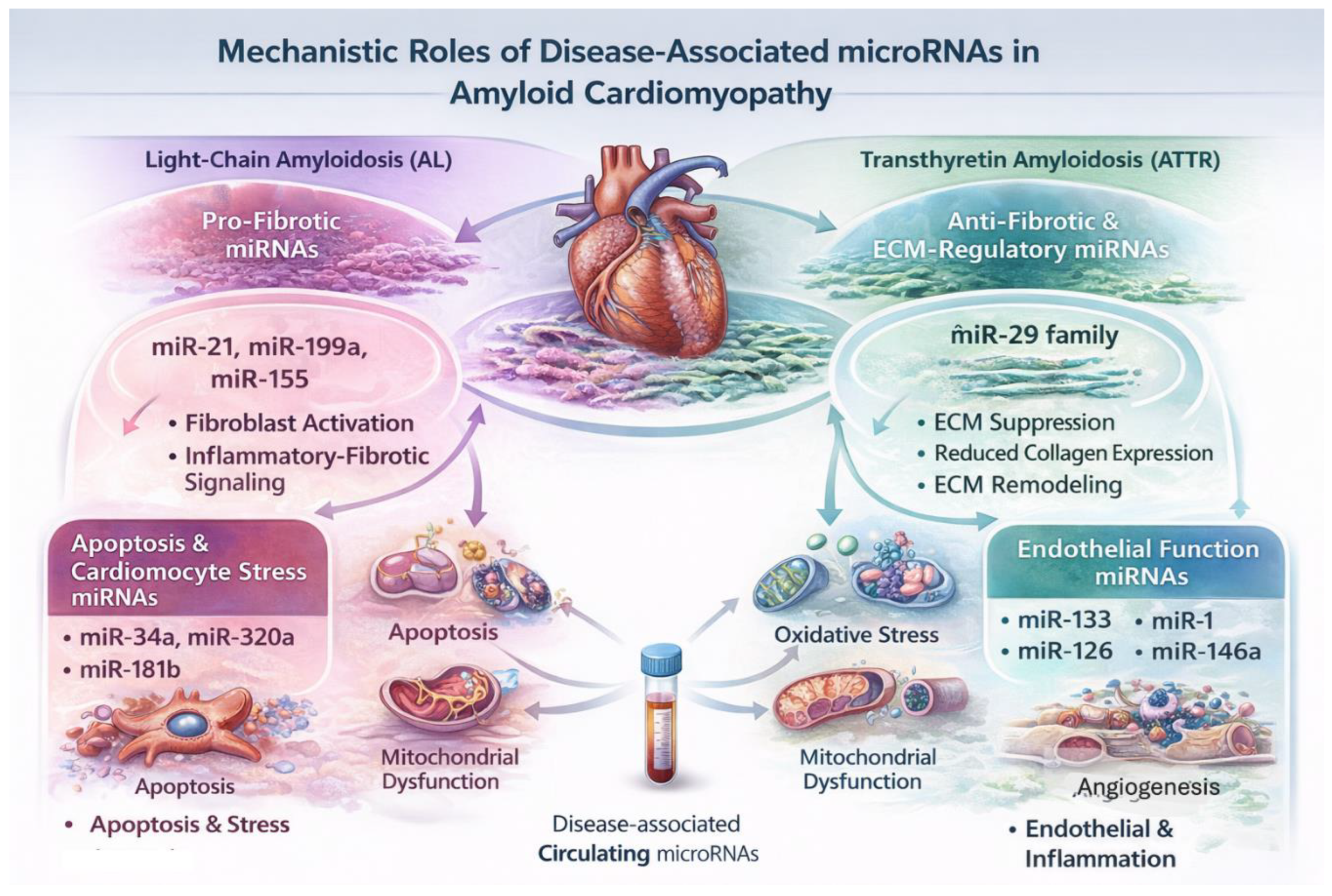
Table 1.
Key microRNAs implicated in cardiac amyloidosis: molecular pathways and level of evidence.
Table 1.
Key microRNAs implicated in cardiac amyloidosis: molecular pathways and level of evidence.
| microRNA |
Primary molecular pathway(s) |
Principal biological effect |
Amyloidosis subtype |
Level of evidence |
Key reference(s) |
| miR-339-3p |
Disease-associated circulating signature |
Diagnostic discrimination |
AL, ATTR |
Human blood profiling |
Derda et al., 2018 [35] |
| miR-150-5p |
CREB, BDNF, NGF regulation |
Differentiates symptomatic ATTRv from asymptomatic carriers; modulates neuro-cardiac signaling |
ATTRv |
Human circulating miRNA profiling + functional validation |
Vita et al., 2020 [50] |
| miR-21 |
TGF-β/Smad signaling, fibroblast activation |
Promotes myocardial fibrosis and ECM expansion |
ATTR (predominant) |
Strong cardiovascular evidence; indirect amyloidosis relevance |
Thum et al., 2008 [38] |
| miR-29 family (miR-29a/b/c) |
ECM regulation (collagen I/III, fibrillin) |
Anti-fibrotic; downregulation promotes myocardial stiffness |
AL, ATTR |
Strong cardiovascular and experimental evidence |
Montgomery et al., 2014 [41] |
| miR-199a |
Fibroblast proliferation, hypertrophic signaling |
Enhances fibrotic remodeling |
ATTR |
Experimental cardiovascular evidence |
Tijsen et al., 2012 [39] |
| miR-155 |
Inflammatory and immune signaling |
Pro-inflammatory and pro-fibrotic effects |
ATTR (putative) |
Indirect cardiovascular evidence |
O’Connell et al., 2012 [40] |
| miR-34a |
Apoptosis, aging-related pathways |
Promotes cardiomyocyte apoptosis |
AL (putative) |
Experimental cardiovascular evidence |
Boon et al., 2013 [42] |
| miR-320a |
Oxidative stress, mitochondrial dysfunction |
Induces cardiomyocyte injury and apoptosis |
AL (putative) |
Experimental cardiovascular evidence |
Tian et al., 2018 [43] |
| miR-181b |
Oxidative stress and inflammatory signaling |
Regulates cardiomyocyte stress responses |
AL (putative) |
Experimental cardiovascular evidence |
Yuan et al., 2019 [44] |
| miR-146a |
NF-κB–mediated innate immune signaling |
Modulates inflammatory stress responses |
ATTR (putative) |
Experimental cardiovascular evidence |
Taganov et al., 2006 [45]] |
| miR-126 |
Endothelial integrity and angiogenesis |
Maintains vascular homeostasis |
AL, ATTR |
Strong endothelial cardiovascular evidence |
Wang et al., 2008 [46] |
| miR-223 |
Inflammation, platelet–endothelial crosstalk |
Modulates vascular inflammation |
AL, ATTR |
Indirect cardiovascular evidence |
O’Connell et al., 2012 [40] |
| miR-148a |
Plasma-cell differentiation, immunoglobulin synthesis |
Upregulated in clonal plasma cells |
AL |
Human bone marrow plasma-cell profiling |
Weng et al., 2011 [51]. |
| miR-26a |
Cell-cycle regulation, plasma-cell survival |
Associated with clonal plasma-cell persistence |
AL |
Human bone marrow plasma-cell profiling |
Weng et al., 2011 [51].] |
| miR-16 |
Apoptosis and hematopoietic regulation |
Elevated in active AL and persistent monoclonal plasma cells; normalizes in remission |
AL |
Human bone marrow plasma-cell profiling |
Weng et al., 2011 [51]. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).