1. Introduction
As of 2021, the Centers for Disease Control estimated that more than 38 million people in the United States were living with diabetes and over 97 million with prediabetes, underscoring the scale of dysglycemia as a public health challenge [
1]. Type 2 Diabetes (T2D) management hinges on daily self-care behaviors, dietary choices, physical activity, medication use, and glucose monitoring, yet many individuals struggle to translate education into sustained behavior change [
2]. Dysregulated eating, emotional eating, and loss of control around highly processed foods are strongly correlated with T2D [
3].
In the face of the growing epidemic of diabetes, the introduction of continuous glucose monitors (CGMs) has changed how these patients view the direct relationship between their food intake and subsequent glucose readings [
4]. Food addiction (FA) has emerged as a construct capturing compulsive, addictive-like responses to ultra-processed foods rich in refined carbohydrates and fats [
5]. Population data indicates that prevalence of FA as measured by the modified Yale Food Addiction Scale (mYFAS) affects approximately 20% of adults, and with rates as high as 30% in T2D [
6]. FA symptoms, including cravings, diminished control, and continued overeating despite negative consequences, are associated with higher BMI, binge-eating pathology, and metabolic risk, and may help explain why some individuals experience disproportionate difficulty adhering to standard dietary recommendations [
3,
5,
6]
. Identifying and targeting FA-related mechanisms in T2D could therefore offer novel pathways to improving both behavioral adherence and glycemic outcomes.
CGMs have transformed how people with diabetes perceive the relationship between their daily choices and glucose fluctuations. Real-time continuous glucose monitoring systems provide near-continuous glycemicdata, visual trend arrows, and summary metrics (e.g., time-in-range, glycemic variability) that can be used to guide both pharmacologic adjustments and lifestyle decisions [
4,
7]. Professional societies now recommend CGMs for selected adults with T2D to improve glycemic control and reduce hypoglycemia [
8,
9,
10,
11]. Recent qualitative and mixed-method studies in adults with T2D report that CGM can increase motivation, confidence in self-management, and perceived control by making glucose responses to meals and activities more visible and personally meaningful. [
12,
13,
14,
15,
16].
Beyond its biomedical utility, CGMs can be conceptualized as a behavioral feedback tool embedded within self-regulation frameworks. Self-regulation and feedback-control frameworks propose that timely, interpretable feedback strengthens the perceived connection between behavior and physiological outcomes, thereby supporting self-monitoring and reinforcing adaptive health behaviors [
17,
18]. Early work has suggested that CGM-guided behavioral education and intermittent CGM use may prompt dietary modifications, increase awareness of physiological responses, and facilitate problem solving around glycemic excursions, including in youth-onset T2D and adults with T2D not on insulin [
19,
20,
21,
22].
Parallel advances in digital health offer additional context. mobile health apps, Short Message service (SMS)-based programs, and wearable activity trackers have been shown to improve self-management behaviors, self-efficacy, and in some cases glycemic outcomes in T2D by providing real-time or near-real-time feedback, goal tracking, and prompts [
23,
24,
25,
26,
27]. For example, diabetes self-management apps and text-messaging interventions have improved HbA1c and self-care behaviors by combining monitoring with tailored feedback and coaching [
23,
24]. Wearable activity trackers similarly leverage immediate feedback, self-monitoring, and goal setting to increase physical activity and support metabolic health [
25,
26] have explicitly examined how CGM, as a glucose-specific feedback modality, may influence addictive-like eating among adults with T2D.
The present mixed-methods study examines how CGM, when integrated into a structured group medical visit (GMV) program, may act as a feedback mechanism that strengthens awareness, accountability, and motivation around dietary behavior in adults with T2D. We assess changes in glycemic outcomes, patient activation, and FA symptoms (using the modified Yale Food Addiction Scale 2.0) and integrate these quantitative improvements with participants’ qualitative accounts of CGM-supported behavior change. By focusing on adults with T2D engaged in CGM-supported GMVs, this study aims to generate preliminary evidence on CGM’s potential to function as a behavioral intervention targeting both metabolic control and addictive-like eating.
2. Materials and Methods
This study was a secondary mixed-methods analysis of a previously implemented group medical visit (GMV) program that integrated CGM as a behavioral feedback tool for adults with T2D [
27]. All participants used Freestyle Libre 2 CGM that is worn on the upper arm. The Freestyle Libre 2 is a 14-day, CGM system measuring glucose every minute, featuring optional real-time alarms, Bluetooth connectivity for smartphone apps, a 40-400 mg/dL range, and water resistance, with a 1-hour warm-up and measures 5mm x 35mm [
28]. The GMV program was delivered over a 14-week period in a primary care setting and consisted of seven sessions, each lasting approximately 120 minutes and scheduled every two weeks. During these sessions, CGM data were reviewed collectively to facilitate goal setting and problem solving related to diet, physical activity, and self-management. A pragmatic mixed-methods approach was adopted, assuming quantitative and qualitative data offer complementary insights into behavioral change mechanisms. This study was approved by the institutional Investigational Review Board Human Protocol: IRB00058951.
The GMV program included seven sessions, each building on CGM feedback to guide learning and behavior change. Session 1 introduced participants to CGM use, nighttime hypoglycemia, and key glucose metrics while encouraging personal reflection through a motivation sheet and goal setting. From session 2 onward, participants’ weekly CGM tracings were reviewed, highlighting stable patterns and troubleshooting glycemic excursions with dietary swaps and physical activity. Session 2 focused on reactive hypoglycemia, individualized CGM targets such as the “No Hitter” goal with 100% time in range, diabetes complications, and the sugar content of common foods. Session 3 emphasized nutrition label reading, hidden sugars, dining strategies, macronutrient education, and the role of insulin resistance in metabolic syndrome. Session 4 shifted to physical activity, with participants writing personalized “exercise prescriptions.” Session 5 addressed the impact of stress and sleep on glucose, exploring mindfulness, stress relief, and sleep optimization. Session 6 introduced intermittent fasting (with a minimum 13-hour fast and optional use of the Zero app for tracking) and reviewed cardiometabolic labs including cholesterol fractions and liver enzymes. Finally, session 7 consolidated all prior content, reviewed participant progress, and provided space for questions and discussion. All sessions were delivered by experienced providers including a certified diabetes care and education specialist and several family physicians board certified in metabolic health and obesity medicine.
2.1. Participants
The program enrolled 16 adults enrolled May 2021 to September 2021. For this analysis, we included participants who had paired CGM summaries and paired psychosocial measures at baseline and follow-up, resulting in an analytic sample of 13 participants. Ten of these participants attended six or more sessions. The inclusion criteria for the original program required participants to be at least 18 years old, have a diagnosis of T2D, be willing to wear a CGM device, and complete baseline assessments.
2.2. Measures
CGM data were collected throughout the 14-week program [
12]. For each participant, summary metrics were calculated for two standardized 14-day windows: Weeks 0–2 (baseline) and Weeks 12-14 (follow-up). These metrics consist of mean glucose in mg/dL, glycemic variability expressed as percent coefficient of variation (%CV), and percent time-in-range (TIR) defined as 70-180 mg/dL. We also recorded the percentage of time the CGM device was active. Summaries of mean glucose, %CV, and TIR were extracted when available for sensitivity analyses.
Psychosocial measures included the modified Yale Food Addiction Scale 2.0 (m YFAS 2.0), which was administered at baseline and follow-up to assess addictive-like eating behaviors [
29]. We computed symptom counts and treated the change in symptom count as a primary psychological outcome. The Patient Activation Measure (PAM-13) was also administered at both time points, and we used both total scores and categorical levels (level 3 versus level 4) for descriptive profiling [
30]. Health-related quality of life was assessed using the SF-12, which provided Physical Component Summary (PCS) and Mental Component Summary (MCS) scores at baseline and follow-up [
31].
Qualitative data was obtained from open-ended survey questions administered at baseline and follow-up. These questions asked participants about their expectations and experiences with CGM, including perceived impacts on eating behavior, awareness, and daily management. These responses were used for thematic analysis and for constructing case vignettes.
2.3. Data Analysis
To standardize interpretation across outcomes, we defined improvement as a decrease in mean glucose and %CV, an increase in TIR, and a reduction in mYFAS 2.0 symptom count. For reporting purposes, we coded these changes so that positive values represented improvement. Changes in PAM, MCS, and PCS were calculated as follow-up minus baseline. We also created attendance strata (six or more sessions versus fewer than six) and defined psycho-behavioral phenotypes by crossing baseline mYFAS 2.0 status (any symptoms versus none) with PAM level (3 versus 4).
Given the small sample and non-normal distributions, we prioritized estimation using bootstrapped confidence intervals and non-parametric effect sizes (Cliff’s δ, Hedges’ g) over null-hypothesis testing [
32,
33,
34,
35,
36]. We examined associations between engagement variables (sessions attended, percent CGM active at baseline and follow-up, and change in percent active) and improvements in psychosocial and CGM outcomes using Spearman’s rank correlation coefficients with bias-corrected and accelerated (BCa) 95% bootstrap confidence intervals with 5,000 resamples. For transparency, we report percentile CIs in-text and provide BCa CIs in Supplementary Tables; both yielded the same inferences. Attendance contrasts were summarized using Cliff’s delta and Hedges’ g with small-sample correction, each accompanied by bootstrap confidence intervals [
37]. Permutation p-values were calculated for mean differences but were reported only for completeness and not emphasized in interpretation. Phenotype profiles were presented as descriptive cell means with bootstrap confidence intervals and explicit sample sizes for transparency. Sensitivity analyses included re-estimating dose–response associations using 90-day CGM summaries and repeating analyses after 5% Winsorization of outcome distributions to assess robustness [
38,
39].
We conducted a reflexive thematic analysis of open-ended responses [
40]. Two analysts independently reviewed responses and developed a codebook that included four themes: awareness from immediate feedback, accountability and engagement, motivation and gamification, and relief from finger-stick burden. Coding was iterative, and discrepancies were resolved through discussion. We created a theme-by-case matrix to summarize the presence or absence of each theme for every participant. For case vignettes, we selected participants whose quotations were particularly rich or illustrative and paired these with their quantitative change profiles.
Quantitative and qualitative findings were integrated through a theme-by-case joint display and case vignettes. Integration occurred at the interpretation and reporting stages, enabling triangulation of mechanisms (themes) with outcome changes (CGM, mYFAS).
Figure 1 illustrates the mixed-methods flow.
3. Results
3.1. Participant Characteristics and Engagement
Of the 16 participants enrolled in the parent program, 13 had paired CGM and psychosocial data and were included in this analysis (participant characteristics are summarized in
Table 1). The median age was 55 years old (25 to 69 years) and there were more males (62.5%) than female participants. The participants consisted of both Black (37.5%) and White individuals. Attendance was high, with a median of six sessions attended. Ten participants attended six or more sessions. The percentage time CGM was active was generally high at both baseline and follow-up.
3.2. Overall Changes from Baseline to Follow-Up
Participants demonstrated clinically meaningful improvements in CGM metrics. Individual pre-to-post changes in CGM and psychosocial measures are presented in
Table 2. On average, mean glucose decreased by approximately 21 mg/dL, glycemic variability decreased by about two percentage points, and time-in-range increased by nearly nine percentage points. Regarding food addiction symptoms based on the mYFAS 2.0, seven participants reported no symptoms at either timepoint, while six participants showed reductions in symptoms severity. Specifically, four participants moved from mild to no symptoms, one from moderate to now symptoms, and one from severe to no symptoms. Across all participants, the average reduction was 1.23 symptoms. Changes in PAM and SF-12 scores varied across individuals and are reported descriptively in the supplementary tables.
3.2.1. Dose-Response Associations
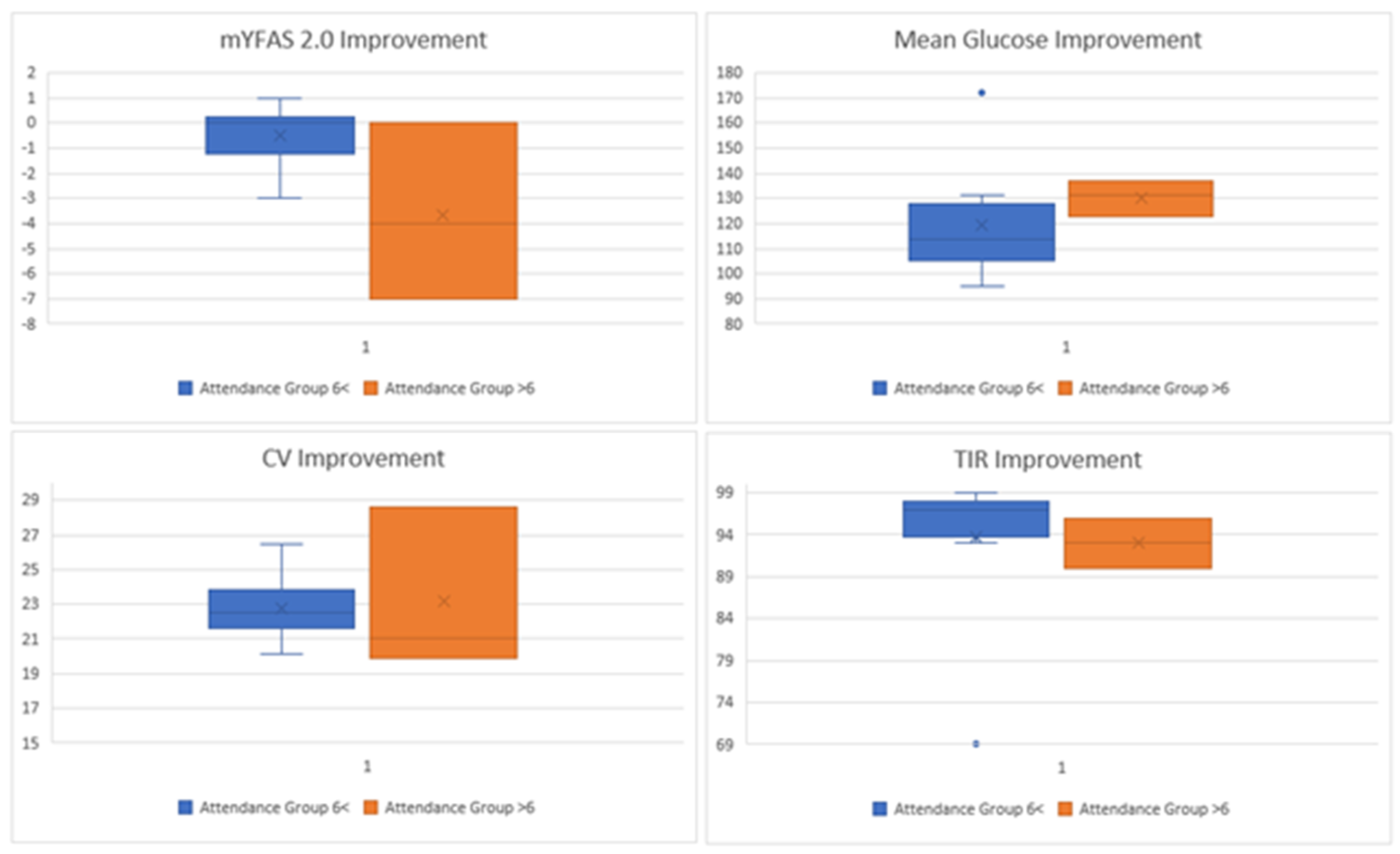
Associations between engagement and outcomes were inconsistent and uncertain. The correlation between sessions attended and improvement in food addiction symptoms was approximately −0.49, with a bootstrap confidence interval ranging from −0.88 to 0.07. The correlation between sessions attended and improvement in time-in-range was approximately −0.43, with a confidence interval from −0.79 to 0.06. Attendance contrasts suggested that participants who attended fewer than six sessions had larger reductions in food addiction symptoms, but this finding is based on only three participants and should be interpreted with caution. Percent CGM active showed no stable association with improvements in psychosocial or CGM outcomes. These findings are presented visually and should be considered exploratory (Exploratory dose-response patterns by session attendance are displayed in
Figure 2, with corresponding effect sizes shown in
Table A1 and
Table A2).
3.3. Psycho-Behavioral Phenotypes
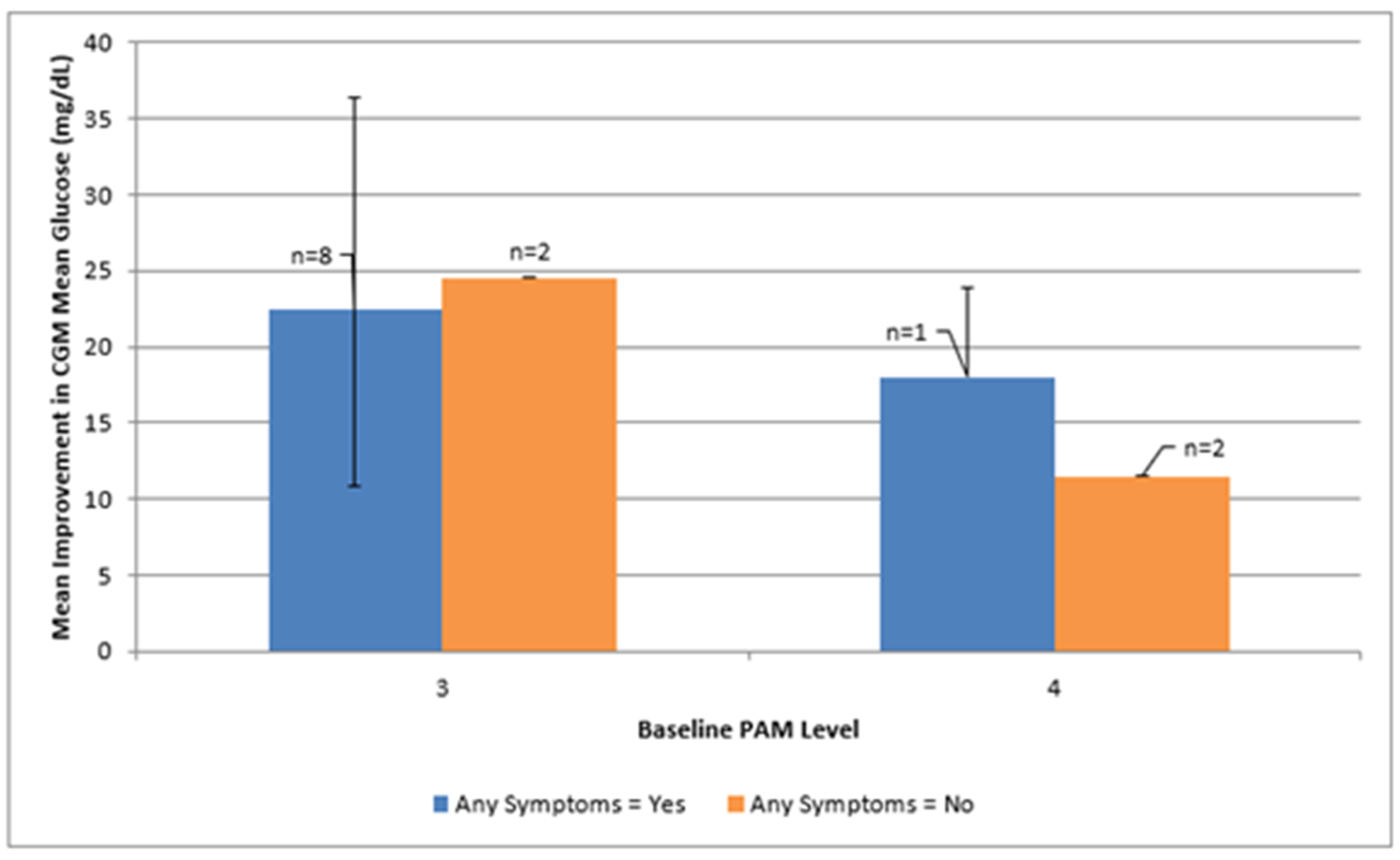
Baseline phenotype cells were unevenly distributed. Mean glycemic improvements across psycho-behavioral phenotypes defined by mYFAS 2.0 symptom burden and PAM level are shown in
Figure 3 and summarized in
Table A3. The mYFAS 2.0 scale includes 11 possible symptoms, with higher scores indicating greater food addition symptom burden. Participants presenting with any food addiction symptoms and PAM level 3 (n = 8) demonstrated average improvements of approximately 22 mg/dL in mean glucose and 12 percentage points in time-in-range, along with an average reduction of about two symptoms on the mYFAS 2.0 scale. Cells with two or fewer participants were too sparse for meaningful interpretation. Effect sizes for food addiction symptoms (mYFAS 2.0 ≥ 1 vs. 0) within PAM levels are provided in the supplementary tables but should be interpreted cautiously.
3.4. Mixed-Methods Case Vignettes
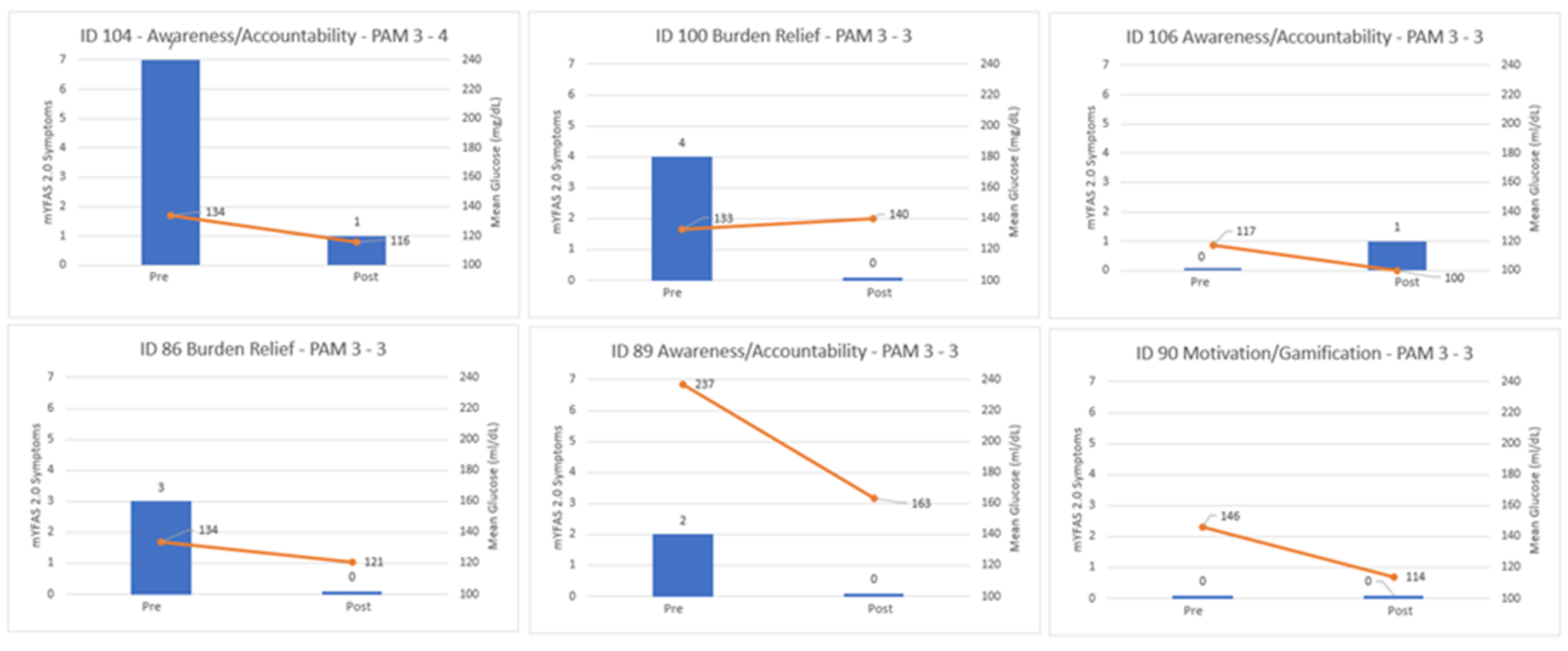
Case vignettes illustrate how psychological themes intersected with objective improvements (see
Figure 1). Participants described the experience as engaging and motivating, reflecting gamification and positive reinforcement dynamics. For example, one participant (ID 104) described the program as “an excellent thing to be a part of… very helpful in monitoring my glucose” and experienced a reduction of seven food addiction symptoms, going from severe food addiction symptoms to none, a decrease of 18 mg/dL in mean glucose, and an increase of 10 percentage points in time-in-range. Another participant (ID 90) stated, “It was sorta fun. It was like a numbers game… I liked doing what it took to drive them down” reflecting motivation and gamification and showed a 32 mg/dL improvement in mean glucose and a 14-point increase in time-in-range. These narratives, combined with quantitative data, underscore the role of awareness, accountability, and motivation in supporting behavior change.
3.5. Sensitivity Analyses
Re-estimating dose–response associations using 90-day CGM summaries and after Winsorization of outcome distributions did not materially change the interpretation. Engagement metrics were not reliably associated with improvements, and descriptive patterns remained consistent.
4. Discussion
The purpose of this study was to explore the psychological and behavioral changes associated with CGM supported GMVs in adults with T2D, with particular attention to behaviors linked to food addiction. Within this small mixed-methods sample, participants demonstrated clinically meaningful improvements in glycemic outcomes over the 14-week program: an average 21 mg/dL reduction in mean glucose, approximately a full-percentage-point decrease in glycemic variability, and a nearly twelve-percentage-point increase in time-in-range (see
Table 2). Regarding food addiction symptoms, seven of the 13 participants reported no symptoms at either baseline or follow-up, while the remaining six demonstrated improvement. Specifically, 4 participants moved from mild to no symptoms, one from moderate to no symptoms, and one from severe to no symptoms, based on mYFAS 2.0 scoring criteria (no food addiction = 1 or fewer symptoms; mild = 2–3; moderate = 4–5; severe = 6 or more). Our findings support a behavioral feedback model in which CGM enhances self-regulation by making the relationship between food intake and physiological response visible. This immediacy may reinforce adaptive behavior through heightened awareness and accountability. Specifically, these findings suggest that integrating real-time CGM feedback into a structured, supportive group setting may promote both metabolic and psychological benefits for adults managing T2D, including reductions in addictive-like eating behaviors among those presenting with such symptoms at baseline.
4.1. Interpretation and Mechanisms of Change
Although engagement metrics such as session attendance and CGM wear percentage did not show consistent dose–response relationships, the mixed-methods analysis revealed psychological mechanisms that appear to drive behavioral change. Qualitative and case-based data emphasize awareness, accountability, motivation and gamification, and relief from finger-stick burden as perceived catalysts of improvement. Participants described how CGM feedback made glucose fluctuations visible and personally relevant transforming diabetes management from an abstract concept into a tangible, interactive process (illustrated in
Figure 1). For some, monitoring became “a numbers game,” where seeing the direct impact of food and activity choices created a sense of mastery and enjoyment.
These mechanisms align with self-regulation and patient activation models, which posit that feedback enhances self-efficacy by strengthening the cognitive link between behavior and outcome [
17,
18]. CGM may function as a real-time self-monitoring tool that provides immediate reinforcement and reduces the delay between action and consequence. The observed improvements in both glucose metrics and food-addiction symptoms suggest that CGM-mediated awareness and accountability may interrupt automatic or compulsive eating patterns by fostering mindful decision-making. This process may be particularly valuable for individuals struggling with the behavioral rigidity, emotional eating, and loss of control that characterize food-addiction-like behaviors.
4.2. Comparison with Prior Literature
Previous studies have established the clinical utility of CGM for improving glycemic control, reducing hypoglycemia, and supporting medication titration in T2D [
41]. Systematic reviews show that when combined with education and clinical follow-up, CGM use in T2D is associated with clinically meaningful reductions in HbA1c and increased time-in-range, even in non-insulin-treated populations [
16,
20]. However, most of this work has emphasized biomedical outcomes rather than the psychological processes that facilitate sustained self-management. Recent studies have begun to address this gap by examining how integrated behavioral interventions, such as therapeutic carbohydrate reduction combined with remote monitoring and behavioral support, can improve food addiction and binge eating symptoms, with 40.7% and 34.7% reductions from baseline, respectively [
41]. Similarly, Unwin et al. (2022) demonstrated that whole-food, low-carbohydrate dietary interventions delivered through group visits can meaningfully reduce symptoms of ultra-processed food addiction even without CGM use [
42]. In contrast, among 138 individuals who participated in 14 weeks of weekly group counseling focused on calorie restriction (1,000-1,200kcal/day) through a meal replacement program combined with up to 175 minutes of weekly physical activity, food addiction symptoms improved by only 13.8% [
43].
The present study extends this literature by explicitly linking CGM use to psychological constructs such as addictive-like eating, patient activation, and perceived behavioral control. Prior research on GMVs has shown that group-based education can improve self-efficacy and diabetes knowledge through peer learning and shared accountability [
44]. By embedding CGM feedback into the GMV format, this study bridges these domains illustrating how social support and behavioral feedback can synergistically promote awareness and self-regulation [
41]. These findings echo prior qualitative work reporting that CGM increases “body literacy” and emotional connection to physiological data, yet the current study uniquely quantifies concurrent improvements in food-addiction symptoms [
7,
13]. This integrated approach broadens the scope of CGM research beyond glucose numbers, emphasizing its psychological and behavioral relevance for complex metabolic conditions.
4.3. Clinical and Practical Implications
From a clinical perspective, the results highlight CGM’s potential to function as both a diagnostic and therapeutic instrument in behavioral diabetes and food addiction care [
45,
46,
47]. Within GMVs, reviewing CGM tracings collectively allows participants to learn from one another’s data, normalize fluctuations, and co-construct behavioral strategies in real time. The emerging themes of awareness and accountability suggest that seeing one’s glucose response to food may counteract the denial or emotional disengagement that often perpetuates maladaptive eating [
48]. Meanwhile, the gamification aspect viewing glucose improvement as a challenge or score to beat may introduce an intrinsically motivating element to self-management that sustains engagement even outside the clinical environment [
49,
50,
51].
Importantly, reductions in food addiction symptom counts among all participants who started with food addiction symptoms indicate that CGM-based behavioral feedback could be leveraged to address addictive-like eating behaviors, an under-recognized contributor to poor diabetes outcomes. Unlike pharmacologic or purely educational interventions, CGM provides continuous experiential learning, transforming self-care into a feedback-driven habit rather than a cognitive task. Integrating such tools into primary-care GMVs may help clinicians target both the metabolic and psychological dimensions of diabetes care simultaneously, fostering more personalized, mechanism-based interventions.
4.4. Theoretical Considerations
The present findings contribute to emerging behavioral models that frame technology-enabled feedback as a mediator of self-regulation [
52,
53]. Specifically, they support a conceptual model in which psychological readiness (as reflected by the Patient Activation Measure) interacts with real-time behavioral feedback (from CGM) to enhance awareness, confidence, and adaptive action. Although the small sample precludes formal moderation testing, descriptive patterns suggest that participants with lower activation (PAM Level 3) and food-addiction symptoms derived particular benefit from CGM feedback. This potential interaction between internal readiness and external cues warrants further investigation as a pathway for tailoring interventions (see
Figure 3).
Furthermore, these findings align with the broader literature on biofeedback, mindfulness, and interoceptive awareness, suggesting that making physiological states visible can transform health information into lived, actionable experiences [
54,
55]. CGM, therefore, may represent a digital health analog to mindfulness training encouraging reflective awareness of internal states, but through data visualization rather than meditation.
4.5. Limitations and Future Directions
Key strengths of this study include its mixed-methods design, which captured both quantitative outcomes and qualitative mechanisms, and its integration of validated psychosocial measures (mYFAS 2.0, PAM-13, SF-12) alongside objective CGM data. The inclusion of patient voice through verbatim quotations and case vignettes provides contextual richness often absent from metabolic studies, illustrating how patients interpret and internalize their data.
However, several limitations must be acknowledged. The small sample size limits statistical power and generalizability. The absence of a control group precludes causal inference, and improvements could partially reflect regression to the mean, increased clinical contact, or social desirability bias. Further, attendance contrasts and correlations were exploratory and unstable, emphasizing estimation rather than hypothesis testing. Additionally, participants willing to wear CGMs and attend GMVs may represent a more activated subset of patients, potentially inflating engagement effects. Thus, findings may not generalize to individuals with lower digital literacy or limited healthcare access. Finally, the short follow-up period prevents evaluation of long-term sustainability of both glycemic and psychosocial improvements.
Future research should confirm these preliminary findings in larger, controlled trials designed to isolate the mechanisms by which CGM influences behavioral change. Longitudinal follow-up is needed to determine whether reductions in food-addiction symptoms translate into sustained improvements in metabolic control and health-related quality of life. Incorporating ecological momentary assessment or digital logging could further elucidate how moment-to-moment feedback shapes food decisions and emotional responses.
Additionally, tailoring interventions by psycho-behavioral phenotype for example, matching CGM feedback intensity or coaching style to baseline activation or food-addiction severity may enhance engagement and efficiency. Finally, qualitative inquiry into how patients interpret CGM data across cultural, literacy, and motivational contexts could guide implementation strategies for diverse populations.
5. Conclusions
This exploratory mixed-methods study suggests that CGM functions not only as a biomedical monitoring tool but as a behavioral feedback system that promotes self-regulation and reduces addictive-like eating in adults with T2D. Integrating CGM into GMVs may operationalize feedback-based learning principles within routine care. Improvements in glucose control occurred alongside reductions in addictive-like eating and increases in awareness and accountability, highlighting CGM’s potential as a feedback-driven behavioral intervention. While preliminary, these findings underscore the importance of integrating psychosocial support and self-regulation mechanisms into diabetes care. By merging objective data with patient reflection, CGM-supported GMVs may help patients move from reactive disease management toward proactive, empowered self-care addressing both the physiological and psychological roots of T2D. Future healthcare delivery models could leverage real-time biofeedback to support psychological readiness and behavioral adherence, improving outcomes across chronic disease management contexts.
Author Contributions
Conceptualization, D.J. and J.K.; methodology, D. J.; software, NA.; validation, D.J., J.K. and L.B.; formal analysis, D.J.; investigation, J.K., L.B., and M.C.; resources, J.K., M.C.; data curation, J.K., L.B., and M.C.; writing—original draft preparation, D.J.; writing—review and editing, L.B., M.C., J.K., and E.S.; visualization, D.J.; supervision, J.K.; project administration, J.K.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Wake Forest University School of Medicine (IRB00058951 and May 1, 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
We would like to thank Abbott Diabetes Care Inc., for providing continuous glucose monitoring supplies for the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CGM |
Continuous Glucose Monitoring |
| FA |
Food Addiction |
| GMV |
Group Medical Visit |
| TIR |
Time in Range |
| mYFAS 2.0 |
modified Yale Food Addiction Scale 2.0 |
| PAM-13 |
Patient Activation Measure 13 |
| PCS |
Physical Component Summary |
| MCS |
Mental Component Summary |
Appendix A
Appendix A.1
Table A1.
Dose-Response Associations Between Engagement and Outcomes.
Table A1.
Dose-Response Associations Between Engagement and Outcomes.
| Predictor |
Outcome |
N |
Spearman ρ
|
95% CI Low |
95% CI High |
| Sessions |
Δ mYFAS 2.0 Symptoms |
13 |
-0.49 |
-0.88 |
+0.07 |
| Sessions |
Δ Mean Glucose |
13 |
-0.12 |
-0.55 |
+0.31 |
| Sessions |
Δ Mean TIR |
13 |
-0.43 |
-.079 |
+0.06 |
| % CGM Active |
Δ mYFAS 2.0 Symptoms |
13 |
-0.31 |
-0.79 |
+0.32 |
| % CGM Active |
Δ Mean Glucose |
13 |
-0.15 |
-0.60 |
+0.43 |
Table A2.
Attendance Contrasts for Improvements in Outcomes.
Table A2.
Attendance Contrasts for Improvements in Outcomes.
| Outcome |
n High |
n Low |
Cliff’s δ |
95% CI Low |
95% CI High |
Hedges’ g
|
| Δ mYFAS 2.0 Symptoms |
10 |
3 |
-0.60 |
-0.95 |
+0.05 |
-0.72 |
| Δ Mean Glucose |
10 |
3 |
-0.10 |
-0.50 |
+0.30 |
-0.15 |
Table A3.
Phenotype Profiles and Effect Sizes.
Table A3.
Phenotype Profiles and Effect Sizes.
| mYFAS 2.0 Any |
PAM Level |
n |
Δ mYFAS 2.0 Symptoms |
Δ Mean Glucose |
Δ %TIR |
| True |
3 |
8 |
+2.13 |
+22.4 |
+12.3 |
| False |
3 |
2 |
-0.5 |
+24.5 |
+9.5 |
| False |
4 |
2 |
-0.5 |
+11.5 |
-1.5 |
| True |
4 |
1 |
+7.0 |
+18.0 |
+10.0 |
References
- CDC. National Diabetes Statistics Report. Diabetes. July 23, 2024. Accessed October 29, 2025. https://www.cdc.gov/diabetes/php/data-research/index.html.
- Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. [CrossRef]
- Horsager C, Bruun JM, Færk E, Hagstrøm S, Lauritsen MB, Østergaard SD. Food addiction is strongly associated with type 2 diabetes. Clin Nutr Edinb Scotl. 2023;42(5):717-721. [CrossRef]
- Willis HJ, Johnson E, JaKa M. A Nutrition-Focused Approach During Continuous Glucose Monitoring Initiation in People With Type 2 Diabetes: Using a Theoretical Framework to Unite Continuous Glucose Monitoring and Food Choices. J Diabetes Sci Technol. 2025;19(5):1400-1406. [CrossRef]
- Gordon EL, Ariel-Donges AH, Bauman V, Merlo LJ. What Is the Evidence for “Food Addiction?” A Systematic Review. Nutrients. 2018;10(4):477. [CrossRef]
- Praxedes DRS, Silva-Júnior AE, Macena ML, et al. Prevalence of food addiction determined by the Yale Food Addiction Scale and associated factors: A systematic review with meta-analysis. Eur Eat Disord Rev J Eat Disord Assoc. 2022;30(2):85-95. [CrossRef]
- Soto-Rodriguez A, Fernández-Conde A, Leirós-Rodríguez R, Opazo ÁT, Martinez-Blanco N. The Experience of Patients with Type 1 Diabetes Mellitus with the Use of Glucose Monitoring Systems: A Qualitative Study. Nurs Rep Pavia Italy. 2025;15(8):294. [CrossRef]
- Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786. [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2025. Diabetes Care. 2024;48(Supplement_1):S128-S145. [CrossRef]
- McCall AL, Lieb DC, Gianchandani R, et al. Management of Individuals With Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2023;108(3):529-562. [CrossRef]
- Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Pland—2022 Update. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2022;28(10):923-1049. [CrossRef]
- Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [CrossRef]
- Lawton J, Blackburn M, Allen J, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18(1):12. [CrossRef]
- Shields S, Thomas R, Durham J, Moran J, Clary J, Ciemins EL. Continuous glucose monitoring among adults with type 2 diabetes receiving noninsulin or basal insulin therapy in primary care. Sci Rep. 2024;14(1):31990. [CrossRef]
- Clark T L, Polonsky WH, Soriano EC. The Potential Impact of Continuous Glucose Monitoring Use on Diabetes-Related Attitudes and Behaviors in Adults with Type 2 Diabetes: A Qualitative Investigation of the Patient Experience | Diabetes Technology & Therapeutics. Diabetes Technol Ther. 2024;26(10):700-708. [CrossRef]
- Jackson MA, Ahmann A, Shah VN. Type 2 Diabetes and the Use of Real-Time Continuous Glucose Monitoring. Diabetes Technol Ther. 2021;23(S1):S-27. [CrossRef]
- Steed L, Barnard M, Hurel S, Jenkins C, Newman S. How does change occur following a theoretically based self-management intervention for type 2 diabetes. Psychol Health Med. 2014;19(5):536-546. [CrossRef]
- Sheehan JL, Greene-Higgs L, Resnicow K, et al. Self-Efficacy, Patient Activation, and the Burden of Inflammatory Bowel Disease on Patients’ Daily Lives. Dig Dis Sci. 2024;69(11):4089-4097. [CrossRef]
- Cox DJ, Taylor AG, Moncrief M, et al. Continuous Glucose Monitoring in the Self-management of Type 2 Diabetes: A Paradigm Shift | Diabetes Care | American Diabetes Association. Diabetes Care. 2016;39(5):e71-e73. [CrossRef]
- Irace C, Avocaro A, Bertuzzi F, et al. Enhancing Type 2 Diabetes Care With CGM Integration: Insights From an Italian Expert Group. Diabetes Metab Res Rev. 2025;41(5):e70059.
- Bendixen BE, Wilhelmsen-Langeland A, Lomborg K, et al. Intermittent Use of Continuous Glucose Monitoring in Type 2 Diabetes Is Preferred: A Qualitative Study of Patients’ Experiences. Sci Diabetes Self-Manag Care. 2025;51(3):323-332. [CrossRef]
- Manfredo J, Lin T, Gupta R, et al. Short-term use of CGM in youth onset type 2 diabetes is associated with behavioral modifications. Front Endocrinol. 2023;14. [CrossRef]
- Desveaux L, Shaw J, Saragosa M, et al. A Mobile App to Improve Self-Management of Individuals With Type 2 Diabetes: Qualitative Realist Evaluation. J Med Internet Res. 2018;20(3):e81. [CrossRef]
- Gerber BS, Biggers A, Tilton JJ, et al. Mobile Health Intervention in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw Open. 2023;6(9):e2333629. [CrossRef]
- Chua REC, Lau Y, Ang WW, Boey AAYF, Lau ST. Effectiveness of wearable technology-based physical activity interventions for adults with type 2 diabetes mellitus: A systematic review and meta-regression. J Diabetes. 2024;16(10):e70002. [CrossRef]
- Laffi A, Persiani M, Piras A, et al. Effectiveness of Wearable Technologies in Supporting Physical Activity and Metabolic Health in Adults with Type 2 Diabetes: A Systematic–Narrative Hybrid Review. Healthcare. 2025;13(19). [CrossRef]
- Buchanan LA, Calkins MW, Boyd CT, et al. Utilization of Continuous Glucose Monitors in a Group Medical Visit Setting. Int J Diabetes Clin Res. 2022;8:164. [CrossRef]
- FreeStyle Libre 2 System | FreeStyle Libre US. Accessed December 23, 2025. https://www.freestyle.abbott/us-en/products/freestyle-libre-2.html.
- Brunault P, Berthoz S, Gearhardt AN, et al. The Modified Yale Food Addiction Scale 2.0: Validation Among Non-Clinical and Clinical French-Speaking Samples and Comparison With the Full Yale Food Addiction Scale 2.0. Front Psychiatry. 2020;11:480671. [CrossRef]
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918-1930. [CrossRef]
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [CrossRef]
- Keselman HJ, Algina J, Lix LM, Wilcox RR, Deering KN. A generally robust approach for testing hypotheses and setting confidence intervals for effect sizes. Psychol Methods. 2008;13(2):110-129. [CrossRef]
- Lovell DP. Null hypothesis significance testing and effect sizes: can we “effect” everything … or … anything? Curr Opin Pharmacol. 2020;51:68-77. [CrossRef]
- Li JCH. Effect size measures in a two-independent-samples case with nonnormal and nonhomogeneous data. Behav Res Methods. 2016;48(4):1560-1574. [CrossRef]
- Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med Off J Soc Acad Emerg Med. 2005;12(4):360-365. [CrossRef]
- Dwivedi AK, Mallawaarachchi I, Alvarado LA. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36(14):2187-2205. [CrossRef]
- Meissel K, Yao ES. Using Cliff’s Delta as a Non-Parametric Effect Size Measure: An Accessible Web App and R Tutorial. Pract Assess Res Eval. 2024;29(1). [CrossRef]
- Herrero P, Alalitei A, Reddy M, Georgiou P, Oliver N. Robust Determination of the Optimal Continuous Glucose Monitoring Length of Intervention to Evaluate Long-Term Glycemic Control. Diabetes Technol Ther. 2021;23(4):314-319. [CrossRef]
- Cichosz SL, Jensen MH, Hejlesen O. Optimal Data Collection Period for Continuous Glucose Monitoring to Assess Long-Term Glycemic Control: Revisited. J Diabetes Sci Technol. 2023;17(3):690-695. [CrossRef]
- Braun V, Clarke V, Hayfield N, Davey L, Jenkinson E. Doing Reflexive Thematic Analysis. In: Bager-Charleson S, McBeath A, eds. Supporting Research in Counselling and Psychotherapy : Qualitative, Quantitative, and Mixed Methods Research. Springer International Publishing; 2022:19-38. [CrossRef]
- Saner E, Kalayjian T, Buchanan L, et al. TOWARD: a metabolic health intervention that improves food addiction and binge eating symptoms. Front Psychiatry. 2025;16:1612551. [CrossRef]
- Unwin J, Delon C, Giæver H, et al. Low carbohydrate and psychoeducational programs show promise for the treatment of ultra-processed food addiction. Front Psychiatry. 2022;13:1005523. [CrossRef]
- Chao AM, Wadden TA, Tronieri JS, et al. Effects of Addictive-Like Eating Behaviors on Weight Loss with Behavioral Obesity Treatment. J Behav Med. 2019;42(2):246-255. [CrossRef]
- Maiorino MI, Signoriello S, Maio A, et al. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2020;43(5):1146-1156. [CrossRef]
- Richardson KM, Jospe MR, Bohlen LC, Crawshaw J, Saleh AA, Schembre SM. The efficacy of using continuous glucose monitoring as a behaviour change tool in populations with and without diabetes: a systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2024;21(1):145. [CrossRef]
- Jospe MR, Richardson KM, Saleh AA, et al. Leveraging continuous glucose monitoring as a catalyst for behaviour change: a scoping review. Int J Behav Nutr Phys Act. 2024;21(1):74. [CrossRef]
- Presseller EK, Velkoff EA, Riddle DR, Liu J, Zhang F, Juarascio AS. Using Continuous Glucose Monitoring to Passively Classify Naturalistic Binge Eating and Vomiting Among Adults With Binge-Spectrum Eating Disorders: A Preliminary Investigation. Int J Eat Disord. 2024;57(11):2285-2291. [CrossRef]
- Rania M, Caroleo M, Carbone EA, et al. Reactive hypoglycemia in binge eating disorder, food addiction, and the comorbid phenotype: unravelling the metabolic drive to disordered eating behaviours. J Eat Disord. 2023;11(1):162. [CrossRef]
- Baranyi R, Willinger R, Lederer N, Walcher F, Grechenig T. DiaBeaThis — A gamified self-tracking portal to support people suffering from diabetes mellitus to control their blood glucose level. In: 2018 IEEE 6th International Conference on Serious Games and Applications for Health (SeGAH). 2018:1-8. [CrossRef]
- AlMarshedi A, Wills G, Ranchhod A. Gamifying Self-Management of Chronic Illnesses: A Mixed-Methods Study. JMIR Serious Games. 2016;4(2):e14. [CrossRef]
- Theng YL, Lee JWY, Patinadan PV, Foo SSB. The Use of Videogames, Gamification, and Virtual Environments in the Self-Management of Diabetes: A Systematic Review of Evidence. Games Health J. 2015;4(5):352-361. [CrossRef]
- Li L, Peng W. Does Health Information Technology Promote Healthy Behaviors? The Mediating Role of Self-Regulation. Health Commun. 2020;35(14):1772-1781. [CrossRef]
- Brohman K, Addas S, Dixon J, Pinsonneault A. Cascading Feedback: A Longitudinal Study of a Feedback Ecosystem for Telemonitoring Patients with Chronic Disease1. Accessed October 29, 2025. [CrossRef]
- Inna K. Mindfulness- and Acceptance-Based Biofeedback. Biofeedback. 2015;43(3):104-110.
- Tanzer M, Bobou M, Koukoutsakis A, et al. Biofeedback and Training of Interoceptive Insight and Metacognitive Efficacy Beliefs to Improve Adaptive Interoception: A Subclinical Randomised Controlled Trial. Psychother Psychosom. Published online June 5, 2025:1-23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).