1. Introduction
Since the onset of the COVID-19 pandemic, it has become increasingly evident that SARS-CoV-2 infection is not limited to the respiratory tract but can lead to multisystem dysfunction, including involvement of the endocrine system. The thyroid gland has emerged as one of the potential targets of the virus, with numerous studies reporting thyroid abnormalities both during the acute phase of infection and in the post-infectious period [
1,
2,
3,
4]. Initially, these manifestations were largely attributed to non-thyroidal illness syndrome (NTIS), a common consequence of severe systemic infections. Subsequently, however, distinct forms of primary thyroid involvement have been described, including subacute thyroiditis (SAT) and autoimmune thyroid diseases (AITD), such as Hashimoto’s thyroiditis and Graves’ disease, occurring de novo or being exacerbated following SARS-CoV-2 infection [
4,
5,
6,
7]. Several pathophysiological mechanisms have been proposed to explain how SARS-CoV-2 may induce thyroid dysfunction both during the acute phase of the disease and in the post-infectious period.
One important mechanism is the direct viral invasion of thyroid tissue. The thyroid gland abundantly expresses the angiotensin-converting enzyme 2 (ACE2) receptor, the main entry point of SARS-CoV-2 into human cells, as well as the transmembrane protease serine 2 (TMPRSS2), which is required for Spike protein activation. This dual expression facilitates viral entry into thyroid follicular cells, and post-mortem studies in patients with severe COVID-19 have demonstrated inflammatory infiltrates and cellular degeneration consistent with direct viral injury [
8,
9].
A second mechanism, frequently implicated in moderate and severe forms of COVID-19, is intense systemic inflammation, commonly referred to as the “cytokine storm.” Elevated concentrations of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), and other proinflammatory mediators can affect the hypothalamic–pituitary–thyroid axis, reducing peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and contributing to TSH suppression, changes characteristic of NTIS [
10,
11]. At the same time, systemic inflammation may exacerbate pre-existing thyroiditis or trigger acute inflammatory processes within the gland, potentially explaining the increased number of SAT cases reported after COVID-19 in prospective and multicenter studies, case series, and clinical reports [
5,
6,
7,
12,
13].
A third major mechanism involves immune dysregulation and post-infectious autoimmunity. SARS-CoV-2 has the potential to induce autoimmune responses through molecular mimicry, nonspecific (“bystander”) activation of autoreactive lymphocytes, or the release of autoantigens from damaged follicular cells [
14,
15]. These mechanisms may promote the development or exacerbation of autoimmune thyroid diseases, including Hashimoto’s thyroiditis and Graves’ disease [
9,
11,
15]. Recent studies have reported increases in anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) antibody titers following SARS-CoV-2 infection, even in previously euthyroid patients, suggesting a triggering role in the initiation of thyroid autoimmunity [
15]. In some cases, this autoimmune response persists and may progress to chronic hypothyroidism. Collectively, these mechanisms contribute to a broad spectrum of post-COVID thyroid manifestations, ranging from transient thyroid function abnormalities to well-defined inflammatory and autoimmune diseases [
11,
12].
Despite the growing body of literature addressing the relationship between thyroid function and SARS-CoV-2 infection, several aspects remain insufficiently clarified. A substantial proportion of published studies are retrospective and involve relatively short follow-up periods, limiting the assessment of the long-term evolution of post-infectious thyroid dysfunction [
4,
11,
12]. Moreover, analyzed cohorts are heterogeneous, including patients with varying disease severity and different screening strategies, resulting in widely variable reported incidence rates [
4,
11]. Uncertainties also persist regarding the relative contribution of viral infection itself, administered treatments, particularly corticosteroids, which may mask manifestations of thyroiditis, or SARS-CoV-2 vaccination, occasionally mentioned as a potential triggering factor [
11].
In this context, we initiated a prospective, single-center study aimed at evaluating the longitudinal evolution of thyroid function in adult patients hospitalized with COVID-19. The study focused on the incidence of subacute and autoimmune thyroiditis during the first 12 months after infection, their relationship with acute disease severity, and the impact of treatments administered during the acute phase [
11,
12]. Through these integrated objectives, the study seeks to provide a comprehensive understanding of post-COVID thyroid involvement and to contribute to the optimization of endocrine monitoring and management strategies during the recovery period.
2. Materials and Methods
2.1. Study Design
The study was designed as a prospective, observational, single-centre investigation conducted at the Brașov Clinical Hospital for Pulmonology and Infectious Diseases, a regional referral centre for the diagnosis and management of COVID-19 patients in central Romania. Patient recruitment took place between December 2022 and December 2023, and each participant was followed for a period of 12 months after the acute phase of SARS-CoV-2 infection.
2.2. Participants, Inclusion and Exclusion Criteria
Adult patients aged 18–80 years with SARS-CoV-2 infection confirmed by RT-PCR or high-sensitivity antigen testing, who were hospitalized at the Brașov Clinical Hospital for Pulmonology and Infectious Diseases, were eligible for inclusion. All participants provided written informed consent and confirmed their availability for scheduled follow-up visits at 4–6 weeks, 3 months, 6 months, and 12 months after the acute infection. Exclusion criteria included age <18 or >80 years; prior or ongoing treatment with interferons, amiodarone, or other medications known to significantly affect thyroid function; chronic corticosteroid or anticoagulant therapy before the COVID-19 episode; known thyroid disease requiring thyroid hormone replacement or antithyroid treatment; and inability to comply with the planned follow-up schedule.
2.3. Data Collection
For each participant, demographic data, relevant comorbidities, thyroid medical history, SARS-CoV-2 vaccination status, severity of acute COVID-19, and treatments administered during the acute phase were recorded. All clinical and paraclinical data were extracted from electronic medical records. The collected variables are summarized in
Table 1.
Table 1.
Variables Collected in the Study.
Table 1.
Variables Collected in the Study.
| Variable Category |
Included Parameters |
| Demographic data |
Age, sex |
| Comorbidities |
Diabetes mellitus, arterial hypertension, chronic kidney disease, cardiovascular diseases, active oncological diagnosis, COPD/bronchial asthma |
| COVID-19 severity |
Clinical form (mild, moderate, severe), oxygen requirement, acute respiratory failure, radiological findings (chest X-ray/pulmonary CT) |
| Acute-phase treatment |
Antivirals (remdesivir, favipiravir, nirmatrelvir/ritonavir, molnupiravir), corticosteroids (dexamethasone, methylprednisolone, etc.), anticoagulants, tocilizumab |
| Inflammatory markers |
ESR, CRP, fibrinogen, ferritin, IL-6 |
| Thyroid function |
TSH, FT4, FT3 |
| Thyroid autoantibodies |
anti-TPO, anti-TG |
| Imaging investigations |
Colour Doppler thyroid ultrasound (baseline, 6 months, and 12 months) |
| Additional factors |
SARS-CoV-2 vaccination status |
2.4. Thyroid Function Assessment and Paraclinical Investigations
Thyroid function was assessed both during hospitalization and throughout the follow-up visits. Laboratory testing included measurements of TSH, FT4, and FT3 using standardized immunochemical assays, as well as determination of thyroid autoantibodies (anti-TPO and anti-TG). The biological evaluation also comprised inflammatory markers (CRP, ESR, fibrinogen, ferritin), hematological parameters, coagulation markers (D-dimer), tissue injury markers (AST, ALT, LDH), and renal function tests (urea and creatinine). Colour Doppler thyroid ultrasound was performed using high-resolution equipment by a physician experienced in thyroid imaging. Thyroid ultrasound examination was performed during hospitalization and systematically repeated at 6 and 12 months, with additional assessments conducted when clinically indicated, based on the presence of suggestive symptoms or paraclinical abnormalities
2.5. Follow-Up Protocol
Patients were followed according to a predefined schedule at 4–6 weeks, 3 months, 6 months, and 12 months after the acute COVID-19 episode. At each visit, a general and endocrine clinical examination was performed, along with repeat thyroid function tests, thyroid autoantibody measurements, and inflammatory markers. Imaging investigations were repeated at 6 and 12 months or earlier when clinically indicated, particularly in the presence of symptoms suggestive of thyroid involvement.
2.6. Operational Definitions and Diagnostic Criteria
The diagnosis of COVID-19 was established based on a positive RT-PCR test or a high-sensitivity antigen test in conjunction with compatible clinical symptoms.
The diagnosis of subacute thyroiditis (SAT) was based on a combination of clinical and paraclinical criteria recognized in the international endocrinology literature. Specifically, SAT was defined by the presence of anterior cervical pain or tenderness, often radiating to the jaw or ear, associated with a systemic inflammatory syndrome characterized by marked elevations of ESR and/or CRP. The endocrine component included transient thyrotoxicosis with suppressed TSH, elevated FT4, and a relatively reduced FT3/FT4 ratio, followed by a reversible hypothyroid phase and subsequent recovery to euthyroidism within several months. Ultrasonographic findings supporting the diagnosis included heterogeneous hypoechoic areas with reduced vascularity, sometimes exhibiting a pseudonodular appearance [
16,
17,
18].
The diagnosis of autoimmune thyroiditis (Hashimoto’s thyroiditis) was based on criteria recommended by the European Thyroid Association and the American Thyroid Association, including the combination of elevated anti-TPO and/or anti-TG titters with a characteristic ultrasound pattern of diffuse hypoechogenicity and heterogeneity, with normal or increased vascularity in early stages, as well as functional changes consistent with subclinical or overt hypothyroidism (elevated TSH with low or normal FT4). In cases with negative autoantibodies, the diagnosis was supported by the characteristic ultrasound appearance and compatible clinical evolution, in accordance with descriptions accepted in international endocrine guidelines [
19,
20].
2.7. Thyroid Laboratory Assessment
Serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), anti-thyroid peroxidase antibodies (anti-TPO), and anti-thyroglobulin antibodies (anti-TG) were measured using a chemiluminescent immunoassay (CLIA) on a fully automated immunoassay analyzer (MAGLUMI X3, Snibe Diagnostic, Shenzhen, China). The reference ranges were as follows: TSH 0.4–4.5 mIU/L, FT4 8.9–17.2 pmol/L, FT3 2.6–6.0 pmol/L, anti-TPO <10 IU/mL, and anti-TG <95 IU/mL. All measurements were performed in the same certified laboratory using standardized procedures and internal and external quality control.
2.8. Study Objectives
The primary aim of this study was to longitudinally characterize thyroid function and structural changes following SARS-CoV-2 infection during the first year following the acute episode. Given the increasing reports of post-COVID thyroid dysfunction, ranging from transient hormonal abnormalities to inflammatory and autoimmune manifestations, we sought to determine the prevalence of these alterations and their temporal evolution in patients without major pre-existing thyroid disease.
In this context, we evaluated the longitudinal dynamics of thyroid parameters (TSH, FT4, FT3, anti-TPO, anti-TG) and documented the incidence of subacute and autoimmune thyroiditis emerging in the post-infectious period, integrating clinical, biochemical, immunological, and ultrasonographic data. We also analyzed the relationship between systemic inflammatory, hepatic, and coagulation-related markers after COVID-19 and thyroid parameters, with the aim of exploring potential shared pathophysiological mechanisms.
An additional objective was to identify factors that may predict the development of thyroid abnormalities over the 12-month follow-up period, with particular attention to early hormonal values and markers of inflammation or coagulation. Furthermore, we investigated the influence of acute disease severity and anti–SARS-CoV-2 vaccination status on the subsequent risk of thyroid involvement.
2.9. Statistical Analysis
Data were analyzed using descriptive statistics (means and standard deviations or medians and interquartile ranges, as appropriate). Comparisons between subgroups were performed using parametric tests (t-test, ANOVA) or non-parametric tests (Mann–Whitney U test, Kruskal–Wallis test), while categorical variables were compared using the χ² test or Fisher’s exact test. Correlations between hormonal parameters, autoantibodies, and inflammatory markers were assessed using Spearman’s correlation coefficient. Longitudinal changes in thyroid parameters were analyzed using mixed-effects models or repeated-measures ANOVA, accounting for within-subject variability over time. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics and R software.
2.10. Ethical Considerations
The study was approved by the Ethics Committee of the Brașov Clinical Hospital of Pulmonology and Infectious Diseases and by the Ethics Committee of “Lucian Blaga” University of Sibiu. All participants provided written informed consent. Data were anonymized and managed in accordance with GDPR regulations and the principles of the Declaration of Helsinki.
3. Results
3.1. Cohort Characteristics
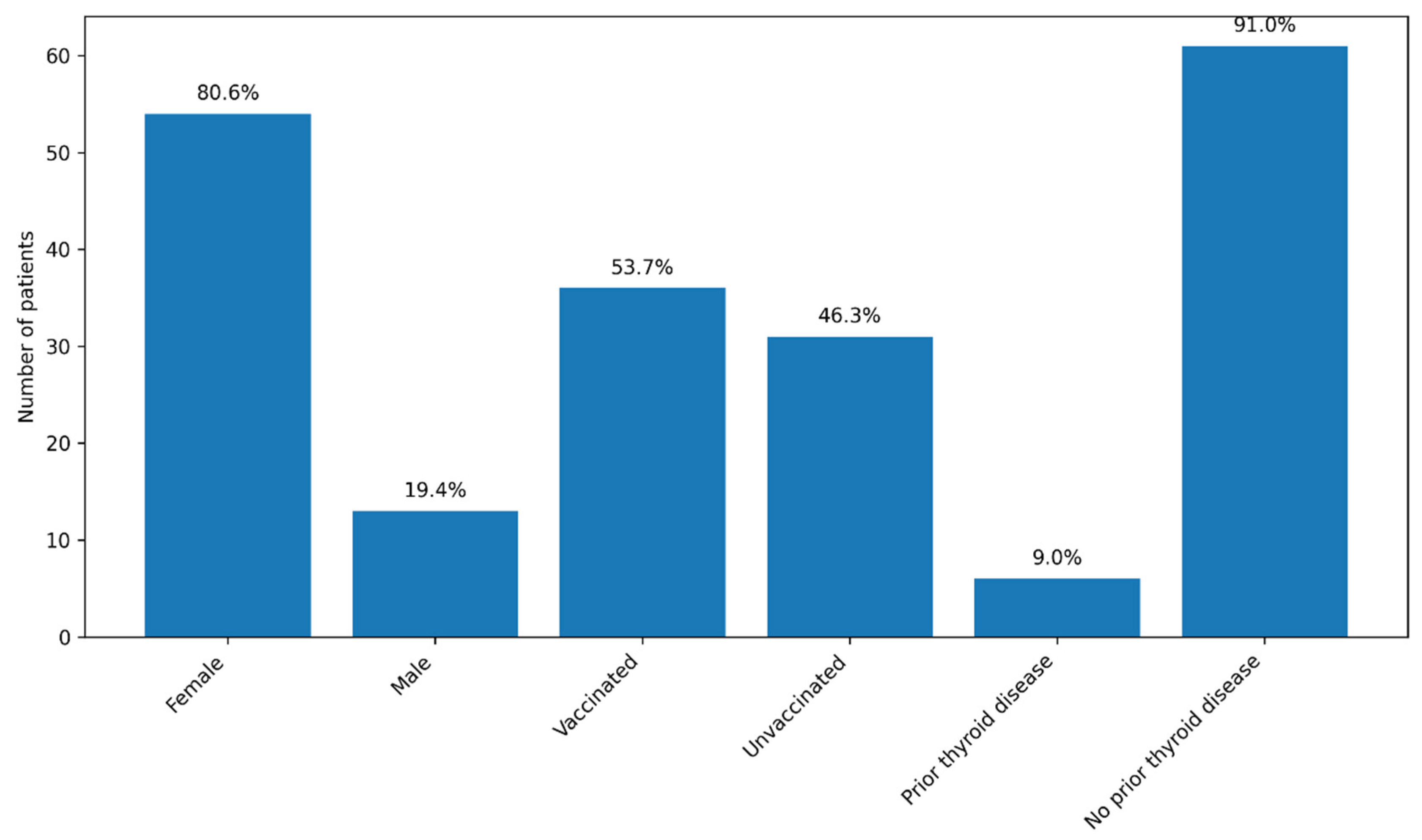
A total of 71 patients were initially enrolled in the study, including 55 women (77.5%) and 16 men (22.5%). Recruitment was conducted continuously between December 2022 and September 2024, with patients enrolled during hospitalization for COVID-19 at the Brașov Clinical Hospital of Pneumology and Infectious Diseases. During the follow-up period, four patients were lost: one female patient died due to an accidental cause, one patient subsequently diagnosed with lung cancer did not attend further visits, and two patients were lost to follow-up after the 3-month evaluation. The final cohort included 67 patients, predominantly female (54 patients; 80.6%). The median age was 54 years (range: 25–78 years).
Approximately half of the patients (36; 53.7%) had been vaccinated against SARS-CoV-2 prior to the index COVID-19 episode, most commonly with the Pfizer–BioNTech vaccine (two or three doses), while three patients had received the Johnson & Johnson vaccine. Most patients (59; 88.1%) had no known history of thyroid disease. Six patients (9%) reported pre-existing thyroid conditions, mainly autoimmune thyroiditis or thyroid nodules; none were receiving thyroid hormone replacement therapy or antithyroid medication at the time of enrolment. No consistent sex-related patterns in thyroid outcomes were evident during follow-up; given the marked female predominance of the cohort, sex was therefore reported descriptively and not included as a primary variable in subsequent analyses.
Figure 1.
Baseline characteristics of the cohort (n = 67). Legend: The graph illustrates the distribution of patients according to sex, SARS-CoV-2 vaccination status, and the presence of known pre-existing thyroid disease. Values are expressed as the number of patients, with the corresponding percentages displayed above each bar. Most patients were female (80.6%), more than half were vaccinated (53.7%), and known pre-existing thyroid disease was uncommon (9.0%).
Figure 1.
Baseline characteristics of the cohort (n = 67). Legend: The graph illustrates the distribution of patients according to sex, SARS-CoV-2 vaccination status, and the presence of known pre-existing thyroid disease. Values are expressed as the number of patients, with the corresponding percentages displayed above each bar. Most patients were female (80.6%), more than half were vaccinated (53.7%), and known pre-existing thyroid disease was uncommon (9.0%).
3.2. Severity of Acute COVID-19 and Administered Treatments
Severe forms of COVID-19 were uncommon in the cohort, with only three patients (4.5%) presenting with severe pulmonary involvement. Eleven patients (16.4%) experienced moderate disease, while nine patients (13.4%) had mild clinical forms. Interstitial radiological changes were relatively frequent, being observed in 34 patients (50.7%), whereas ground-glass opacities were less commonly reported. Nine patients (13.4%) had normal chest radiography or computed tomography findings. Acute respiratory failure was documented in eight patients (11.9%), all of whom required supplemental oxygen therapy; however, none of the patients required non-invasive ventilation.
Table 2.
Clinical Severity and Respiratory/Radiologic Features in the Study Cohort.
Table 2.
Clinical Severity and Respiratory/Radiologic Features in the Study Cohort.
| Severity Category |
Number (%) |
| Severe disease |
3 (4.5%) |
| Moderate disease |
11 (16.4%) |
| Mild disease |
9 (13.4%) |
| Interstitial radiologic changes |
34 (50.7%) |
| Normal imaging |
9 (13.4%) |
| Acute respiratory failure |
8 (11.9%) |
Antiviral therapy was administered in 39 patients (58.2%), most commonly remdesivir (24 patients), followed by favipiravir (7 patients), nirmatrelvir/ritonavir (6 patients), and molnupiravir (2 patients). Systemic corticosteroid therapy was used in 23 patients (34.3%), predominantly dexamethasone. Approximately half of the cohort (34 patients; 50.7%) received prophylactic or therapeutic anticoagulation, mainly with enoxaparin. One patient was treated with tocilizumab.
Table 3.
Treatments Administered During the Acute COVID-19 Phase.
Table 3.
Treatments Administered During the Acute COVID-19 Phase.
| Treatment |
Received n (%) |
Not received n (%) |
| Antiviral therapy (any) |
39 (58.2%) |
28 (41.8%) |
| Remdesivir |
24 (35.8%) |
43 (64.2%) |
| Favipiravir |
7 (10.4%) |
60 (89.6%) |
| Nirmatrelvir/ritonavir |
6 (9.0%) |
61 (91.0%) |
| Molnupiravir |
2 (3.0%) |
65 (97.0%) |
| Corticosteroids (any) |
23 (34.3%) |
44 (65.7%) |
| Anticoagulation |
34 (50.7%) |
33 (49.3%) |
| Tocilizumab |
1 (1.5%) |
66 (98.5%) |
3.3. Correlations Between Inflammatory, Biochemical, and Coagulation Markers at Baseline
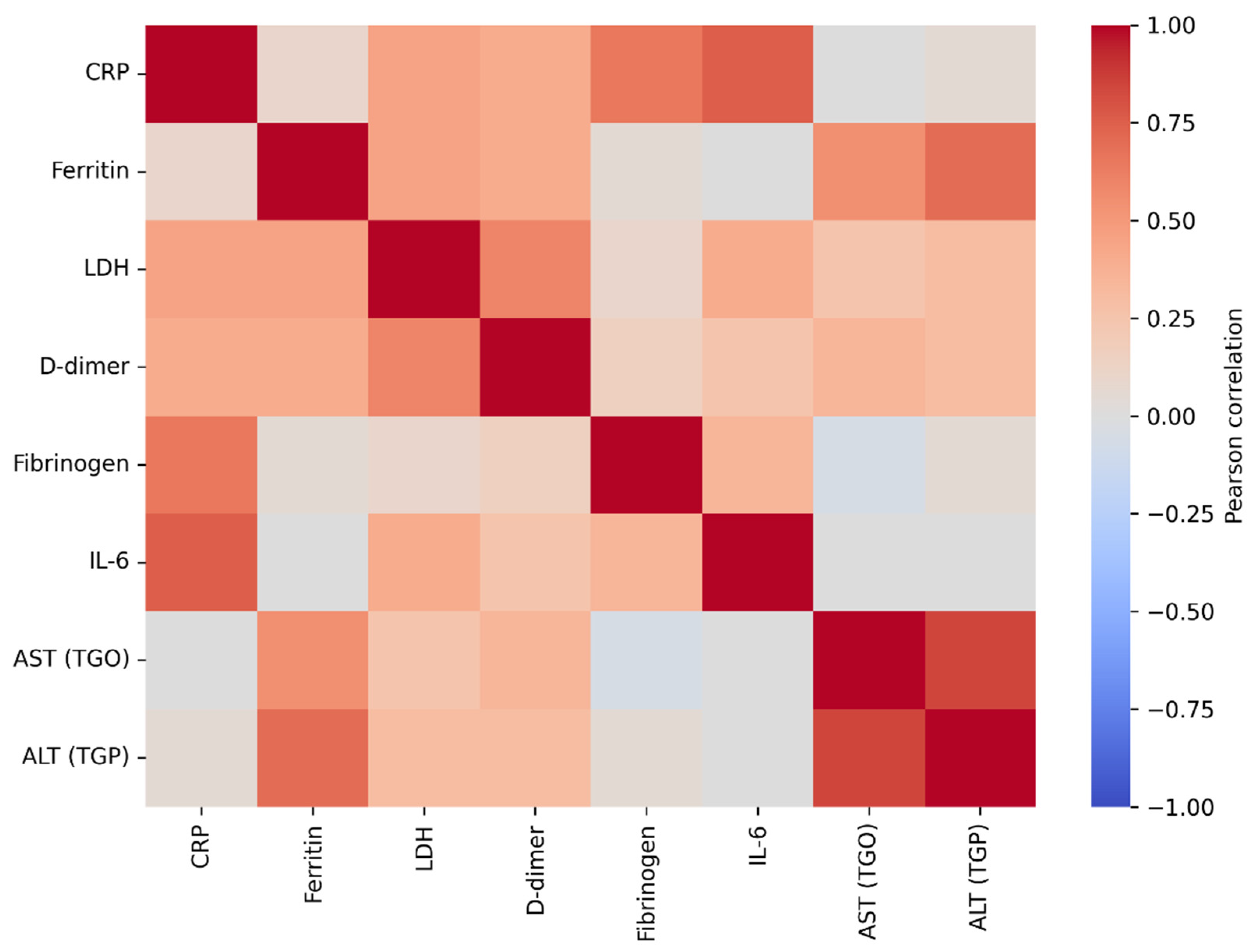
The correlation analysis of baseline laboratory parameters revealed a coherent post–COVID-19 systemic profile, characterized by interrelationships among inflammatory markers, indices of tissue injury, and coagulation activation. Hepatic transaminases (AST and ALT) showed the strongest association (r = 0.894, p < 0.001), consistent with hepatic involvement in the early post-infectious period. Ferritin correlated significantly with ALT (r = 0.739, p < 0.001) and AST (r = 0.598, p < 0.001), in line with its role as an acute-phase reactant and a marker of systemic inflammation. LDH, an indicator of cellular injury that often rises in inflammatory states, was also associated with markers of coagulation activation; specifically, LDH correlated with D-dimer levels (r = 0.639, p < 0.001), suggesting that greater tissue injury may coexist with a prothrombotic milieu. Classical inflammatory markers (ESR, CRP, and fibrinogen) were strongly correlated with one another (r = 0.654–0.671, all p < 0.001), indicating coordinated acute-phase activation at the time of study inclusion.
Overall, this pattern of correlations supports the biological plausibility of the dataset and places thyroid dysfunction within a broader multisystemic context, in which inflammatory, hepatic, and coagulation-related processes are interconnected during post–COVID-19 recovery. This systemic background is relevant for the interpretation of thyroid-related changes observed in subsequent analyses, suggesting that post-viral thyroid dysfunction may represent one component of a more complex, multisystem inflammatory process.
Figure 2.
Pearson correlation heatmap of baseline inflammatory and related laboratory marker. Legend: The figure illustrates the strength and direction of linear associations between inflammatory markers (CRP, ESR, fibrinogen, IL-6) and biochemical parameters (ferritin, LDH, D-dimer, AST, ALT). Red colors indicate positive correlations, whereas blue colors indicate negative correlations, with color intensity reflecting the magnitude of the correlation coefficient (r). Very strong correlations are observed between hepatic transaminases (AST–ALT), as well as between CRP–IL-6, LDH–D-dimer, and ferritin–ALT, suggesting a coherent inflammatory and cytolytic profile at baseline in patients with COVID-19.
Figure 2.
Pearson correlation heatmap of baseline inflammatory and related laboratory marker. Legend: The figure illustrates the strength and direction of linear associations between inflammatory markers (CRP, ESR, fibrinogen, IL-6) and biochemical parameters (ferritin, LDH, D-dimer, AST, ALT). Red colors indicate positive correlations, whereas blue colors indicate negative correlations, with color intensity reflecting the magnitude of the correlation coefficient (r). Very strong correlations are observed between hepatic transaminases (AST–ALT), as well as between CRP–IL-6, LDH–D-dimer, and ferritin–ALT, suggesting a coherent inflammatory and cytolytic profile at baseline in patients with COVID-19.
3.4. Longitudinal Time Course of Thyroid Hormones (TSH, FT4, FT3)
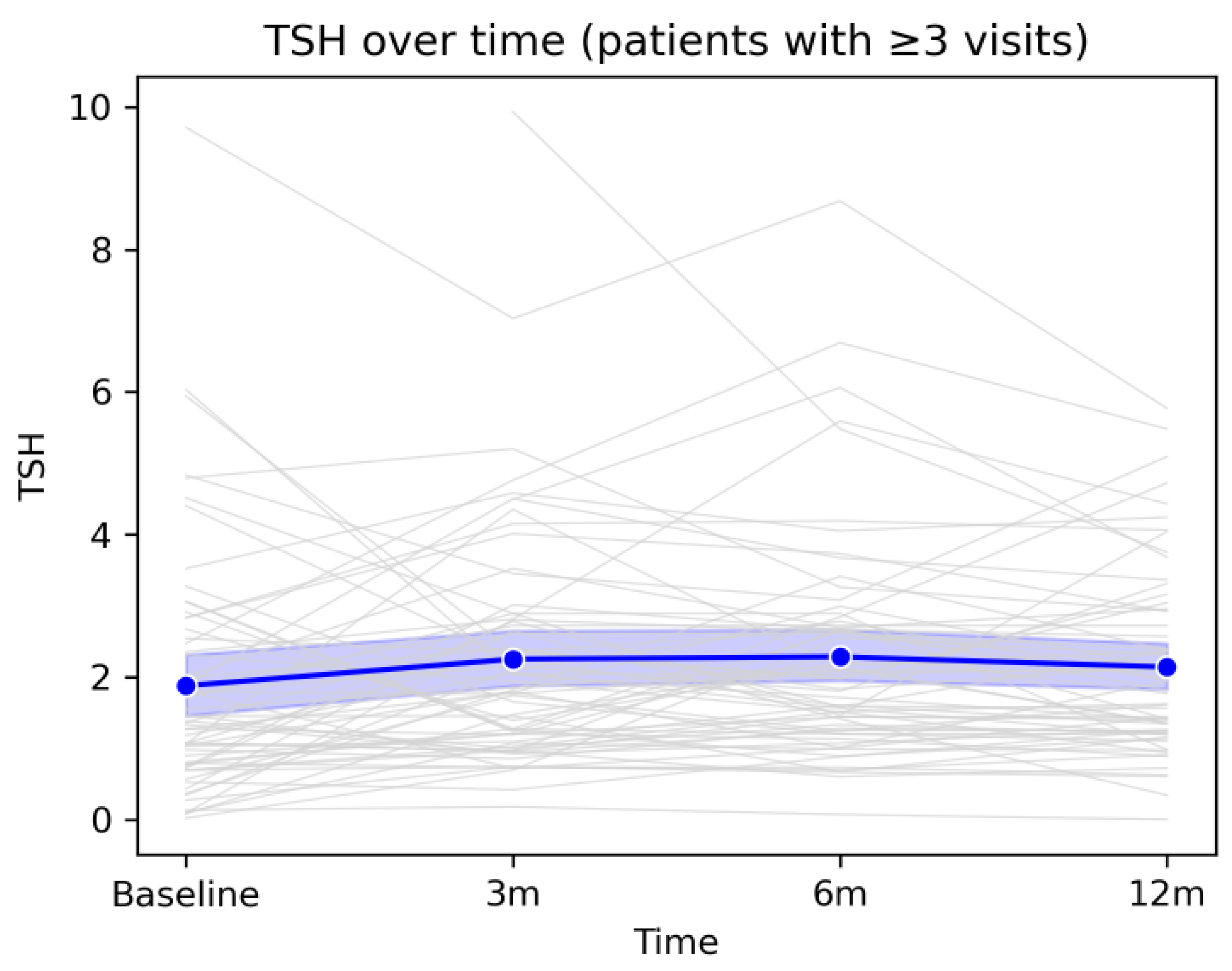
The longitudinal analysis of the three thyroid hormones revealed an overall pattern of gradual stabilization of thyroid function over the 12-month follow-up period after COVID-19 infection. Although individual hormone levels varied considerably among patients, as reflected by divergent individual trajectories, the mean evolution of the cohort followed a coherent and predictable pattern. TSH showed a mild increase at the 3- and 6-month assessments, followed by a tendency to return toward baseline values at 12 months, without major population-level fluctuations. FT4 exhibited a moderate decline during the early post-infectious period, likely reflecting the resolution of inflammatory processes and post-viral adaptive responses, followed by a gradual return toward baseline values by the end of follow-up. Similarly, FT3 showed a slight increase at 3 months, followed by a progressive decrease up to 12 months, suggesting a medium-term metabolic re-equilibration process.Taken together, these trajectories indicate that, although a subset of patients experienced transient hormonal deviations or isolated episodes of thyroid dysfunction, the overall trend was one of spontaneous normalization, without evidence of progression toward persistent or severe thyroid dysfunction at the cohort level. These findings support the concept that post-COVID thyroid alterations, when present, are predominantly mild, dynamic, and self-limited, and are integrated into a broader process of systemic recovery following viral infection.
Overall, mean TSH values remained within the reference range throughout the entire follow-up period, with a slight upward trend between baseline and the 6-month visit, followed by a modest decline at 12 months. Individual fluctuations were frequent but did not follow a uniform pattern indicative of sustained thyroid dysfunction at the group level. This evolution suggests that, despite notable interindividual variability, the average thyroid function of the cohort remained relatively stable during the first year after COVID-19, further supporting the transient nature of most observed thyroid-related changes.
Figure 3.
Longitudinal evolution of TSH levels during 12-month follow-up. Legend: Evolution of TSH values over the 12-month follow-up period in patients with at least three study visits. Grey lines represent individual trajectories, while the blue line depicts the cohort mean with the corresponding 95% confidence interval. A moderate degree of inter-individual variability is observed, alongside an overall tendency toward stabilization of TSH values by the end of the follow-up period.
Figure 3.
Longitudinal evolution of TSH levels during 12-month follow-up. Legend: Evolution of TSH values over the 12-month follow-up period in patients with at least three study visits. Grey lines represent individual trajectories, while the blue line depicts the cohort mean with the corresponding 95% confidence interval. A moderate degree of inter-individual variability is observed, alongside an overall tendency toward stabilization of TSH values by the end of the follow-up period.
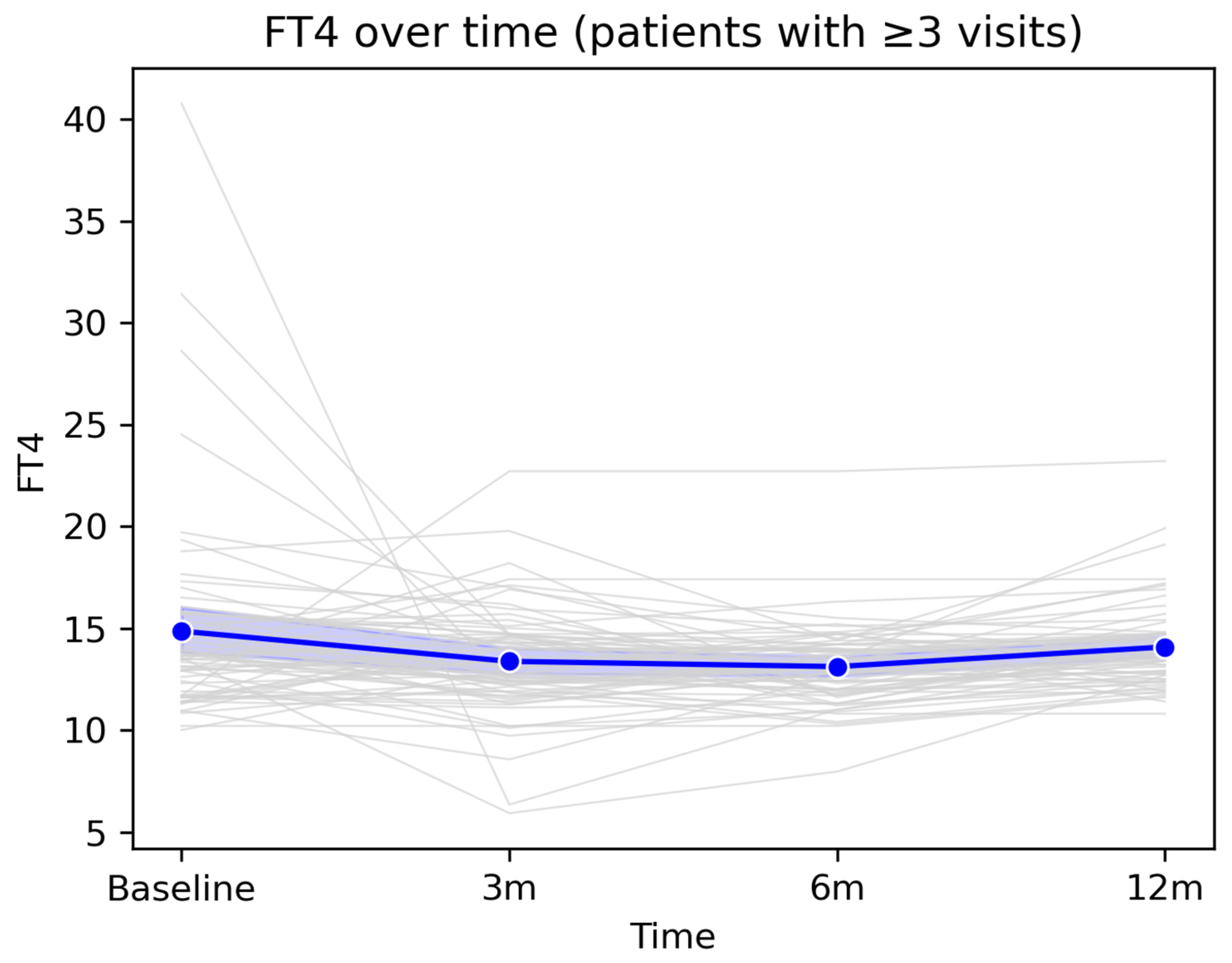
The mean FT4 value showed a slight decrease from baseline to the 3-month visit, followed by relative stabilization at 6 months and a modest return toward baseline values at 12 months. Overall, FT4 levels remained within the reference range at the group level, despite isolated fluctuations observed in some patients. This pattern suggests that most post-COVID changes in FT4 are transient and do not reflect a persistent long-term thyroid dysfunction.
Figure 4.
Longitudinal evolution of FT4 levels during 12-month follow-up. Legend: Longitudinal evolution of FT4 values over the 12-month follow-up period in patients with ≥3 follow-up visits. The figure illustrates the longitudinal dynamics of FT4 values among patients with at least three consecutive measurements available during the 12-month follow-up period. Grey lines represent individual patient trajectories, highlighting inter-individual variability in thyroid function following COVID-19 infection. The blue line indicates the cohort mean at each assessment time point (baseline, 3 months, 6 months, and 12 months), accompanied by the 95% confidence interval, displayed as a semi-transparent blue band.
Figure 4.
Longitudinal evolution of FT4 levels during 12-month follow-up. Legend: Longitudinal evolution of FT4 values over the 12-month follow-up period in patients with ≥3 follow-up visits. The figure illustrates the longitudinal dynamics of FT4 values among patients with at least three consecutive measurements available during the 12-month follow-up period. Grey lines represent individual patient trajectories, highlighting inter-individual variability in thyroid function following COVID-19 infection. The blue line indicates the cohort mean at each assessment time point (baseline, 3 months, 6 months, and 12 months), accompanied by the 95% confidence interval, displayed as a semi-transparent blue band.
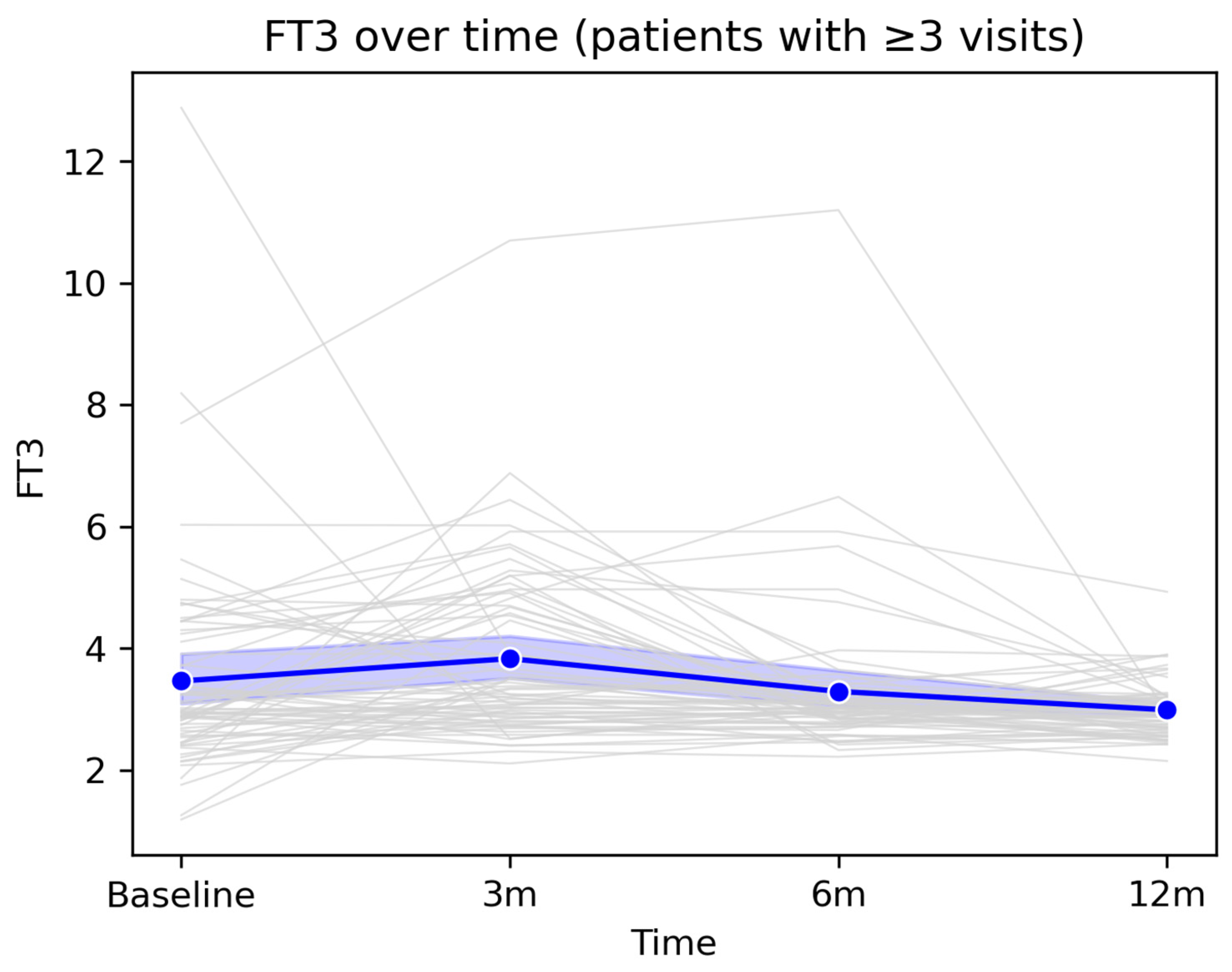
At the population level, FT3 showed a slight increase at the 3-month visit, followed by a gradual decline toward 12 months, with mean values remaining within the physiological range throughout the entire monitoring period. This profile suggests an overall trend toward stabilization and normalization of peripheral thyroid function after the acute infectious episode.
Figure 5.
Longitudinal evolution of FT3 levels during 12-month follow-up. Legend:
Figure 5 illustrates the longitudinal evolution of serum FT3 concentrations among patients with at least three available measurements over the 12-month follow-up period. Grey lines represent individual trajectories, highlighting inter-individual variability, including several markedly elevated baseline values that progressively declined over time. The thick blue line represents the cohort mean, accompanied by the 95% confidence interval (semi-transparent blue band). A slight increase in mean FT3 levels is observed at the 3-month visit, followed by a gradual decline at 6 and 12 months, with values returning close to the reference range.
Figure 5.
Longitudinal evolution of FT3 levels during 12-month follow-up. Legend:
Figure 5 illustrates the longitudinal evolution of serum FT3 concentrations among patients with at least three available measurements over the 12-month follow-up period. Grey lines represent individual trajectories, highlighting inter-individual variability, including several markedly elevated baseline values that progressively declined over time. The thick blue line represents the cohort mean, accompanied by the 95% confidence interval (semi-transparent blue band). A slight increase in mean FT3 levels is observed at the 3-month visit, followed by a gradual decline at 6 and 12 months, with values returning close to the reference range.
3.5. Prevalence and Patterns of TSH Abnormalities
Among the 67 patients who completed the 12-month follow-up and had complete TSH measurements available, 23 patients (34.3%) experienced at least one episode of abnormal TSH, defined as TSH <0.4 mIU/L or >4.5 mIU/L. The remaining 44 patients (65.7%) maintained TSH values within the reference range throughout the entire monitoring period. Patterns of TSH abnormalities were heterogeneous. Some patients exhibited transient episodes of TSH suppression, consistent with post-inflammatory thyrotoxicosis, whereas others developed episodic or persistent elevations in TSH, suggestive of subclinical or early hypothyroidism. Patients with abnormal TSH values were, on average, older, and age showed a modest but statistically significant association with the presence of at least one abnormal TSH value (Welch’s t-test, p = 0.0428).
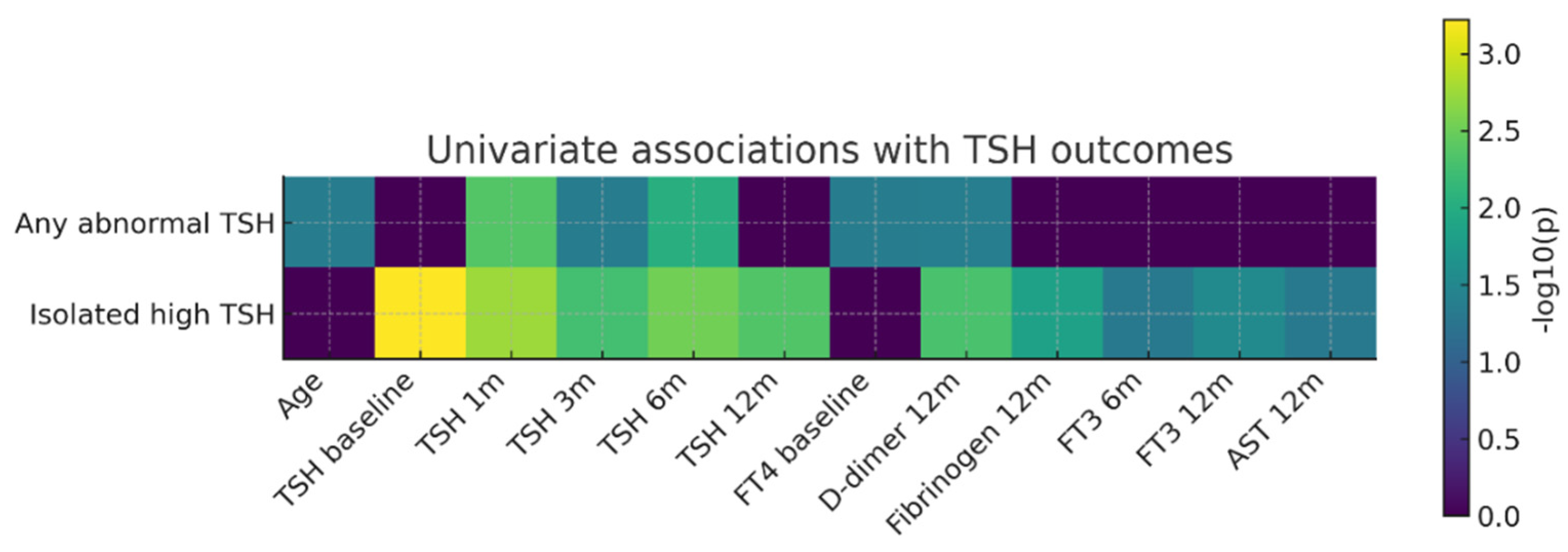
Univariate association analysis (
Figure 6) showed that isolated elevated TSH was most strongly correlated with baseline TSH values and with those obtained at early follow-up visits (1–6 months). In contrast, the composite outcome “any abnormal TSH” was primarily associated with early post-COVID TSH values, baseline FT4 levels, age, and D-dimer concentrations at 12 months. These findings suggest a complex relationship between early post-infectious thyroid regulation and later systemic parameters.
Figure 6.
Heatmap of univariate associations between clinical/paraclinical predictors and thyroid outcomes (TSH). Legend: Heatmap of univariate associations between clinical/paraclinical predictors and thyroid outcomes (TSH). Rows represent the two thyroid outcomes analyzed (“Any abnormal TSH” and “Isolated elevated TSH”), while columns correspond to baseline predictors and variables measured during follow-up (age, TSH values at different time points, baseline FT4, D-dimer levels at 12 months, fibrinogen at 12 months, FT3 at 6 and 12 months, and AST at 12 months). Color intensity reflects the strength of the statistical association expressed as −log10(p), with darker shades indicating smaller p-values (stronger associations), whereas values close to zero (dark purple) indicate the absence of a statistically significant association.
Figure 6.
Heatmap of univariate associations between clinical/paraclinical predictors and thyroid outcomes (TSH). Legend: Heatmap of univariate associations between clinical/paraclinical predictors and thyroid outcomes (TSH). Rows represent the two thyroid outcomes analyzed (“Any abnormal TSH” and “Isolated elevated TSH”), while columns correspond to baseline predictors and variables measured during follow-up (age, TSH values at different time points, baseline FT4, D-dimer levels at 12 months, fibrinogen at 12 months, FT3 at 6 and 12 months, and AST at 12 months). Color intensity reflects the strength of the statistical association expressed as −log10(p), with darker shades indicating smaller p-values (stronger associations), whereas values close to zero (dark purple) indicate the absence of a statistically significant association.
This univariate analysis in a post-COVID cohort highlight that a relevant proportion of patients develop TSH abnormalities within the first year after infection and that early post-infectious TSH values are closely associated with subsequent thyroid function trajectories. Although inflammatory and coagulation markers reflected a persistent pro-inflammatory and pro-thrombotic systemic profile in a subset of patients, their direct associations with thyroid dysfunction were more modest. Overall, these findings suggest that post-COVID thyroid dysregulation is more strongly driven by instability of the hypothalamic–pituitary–thyroid axis than by the intensity of acute systemic inflammation.
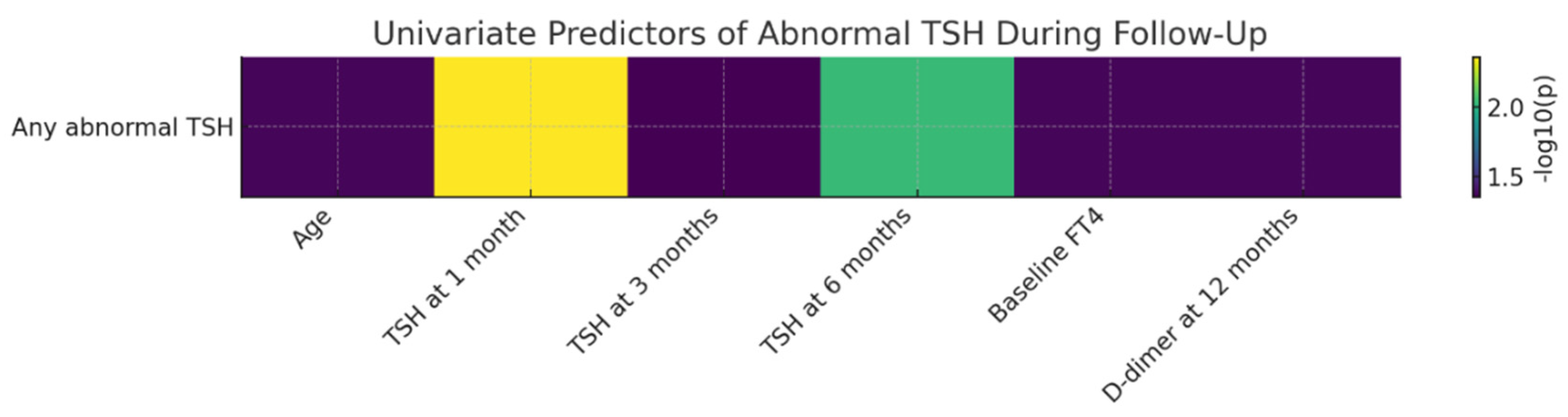
3.6. Predictors of TSH Abnormalities During Follow-Up
Univariate analyses identified several factors associated with the occurrence of TSH abnormalities over the 12-month follow-up period. TSH values measured at early visits (4–6 weeks, 3 months, and 6 months) were significantly higher in patients who subsequently developed persistent or recurrent TSH abnormalities, suggesting that early disturbances of the hypothalamic–pituitary–thyroid axis may anticipate delayed thyroid dysfunction.
Baseline FT4 levels were also significantly associated with the later development of TSH abnormalities (p = 0.0430), indicating that subtle alterations in peripheral thyroid hormone dynamics during the early post-infectious phase may have predictive relevance. In addition, D-dimer levels measured at 12 months were significantly associated with the presence of abnormal TSH values (p = 0.0423), suggesting a potential link between persistent low-grade activation of the coagulation system and instability of thyroid regulation in the post-COVID period.
The figure bellow illustrates, in the form of a heatmap, the strength of univariate associations between the evaluated clinical and paraclinical predictors and the occurrence of abnormal TSH during follow-up. The strongest predictor identified was TSH at the 4–6-week visit, followed by TSH at 6 months. Additional predictors showing moderate associations included baseline FT4 levels, age, and D-dimer concentrations at 12 months.
Figure 7.
Univariate Predictors of Abnormal TSH During 12-Month Follow-Up After SARS-CoV-2 Infection. Legend: Heatmap of univariate associations between clinical/paraclinical predictors and the occurrence of abnormal TSH during the 12-month follow-up period. Values are expressed as −log10(p), allowing visual comparison of the relative relevance of each predictor.
Figure 7.
Univariate Predictors of Abnormal TSH During 12-Month Follow-Up After SARS-CoV-2 Infection. Legend: Heatmap of univariate associations between clinical/paraclinical predictors and the occurrence of abnormal TSH during the 12-month follow-up period. Values are expressed as −log10(p), allowing visual comparison of the relative relevance of each predictor.
The strongest predictor identified was TSH measured at 4–6 weeks, with higher early values being significantly associated with the subsequent development of TSH abnormalities. This finding suggests that early disturbances of hypothalamic–pituitary–thyroid feedback may anticipate delayed thyroid dysfunction. Similarly, TSH levels at 6 months showed a strong association, indicating persistence of thyroid regulatory instability during the subacute post-COVID phase. Additional predictors with a moderate level of association included baseline FT4 levels, age, and D-dimer concentrations at 12 months, all exhibiting −log10(p) values compatible with statistical significance. These results suggest that both early thyroid function parameters and markers of late coagulation activation may contribute to shaping the risk of thyroid dysfunction during long-term recovery after COVID-19. Overall, the heatmap highlights a heterogeneous profile of factors associated with TSH abnormalities, with a clear predominance of early thyroid-related markers and additional associations involving selected systemic parameters, including coagulation markers. This integrated visualization underscores that post-COVID TSH abnormalities are linked to a diverse set of longitudinally assessed clinical and paraclinical variables.
3.7. Longitudinal Thyroid Ultrasound Evolution over 12 Months of Follow-Up
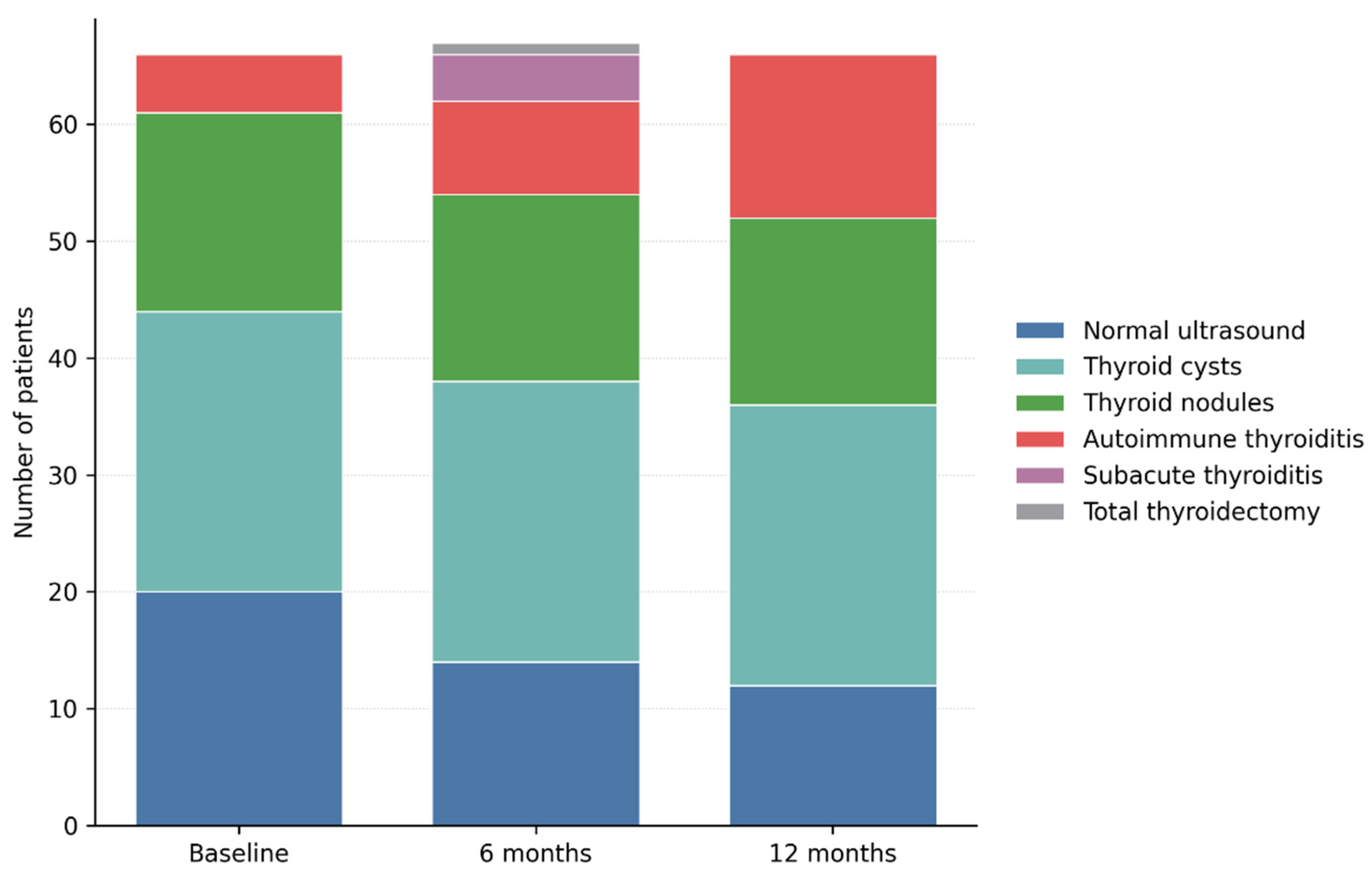
Longitudinal thyroid ultrasound evaluation revealed dynamic structural changes of the thyroid gland throughout the 12-month follow-up period. Compared with baseline, the proportion of patients with a normal thyroid ultrasound pattern progressively decreased, from 29.9% at inclusion (20 patients) to 20.9% at 6 months (14 patients) and 17.9% at 12 months (12 patients), suggesting the de novo development of thyroid structural abnormalities during the post-COVID period.
Through longitudinal ultrasound monitoring, the prevalence of thyroid cysts remained unchanged, being identified in 24 patients at baseline as well as at the 6- and 12-month evaluations. Similarly, thyroid nodules exhibited a stable course, being observed in 17 patients at baseline and in 16 patients at subsequent assessments. No significant changes in lesion size, ultrasound characteristics, or vascular behaviour were documented during follow-up. This stability indicates a benign and non-progressive ultrasound profile of cystic and nodular lesions, without evidence of inflammatory activation associated with SARS-CoV-2 infection.
Figure 8.
Changes in Thyroid Ultrasound Patterns from Baseline to 12 Months after SARS-CoV-2 Infection. Legend: Longitudinal evolution of thyroid ultrasound findings over 12 months of follow-up. Stacked bar chart illustrating the distribution of thyroid ultrasound patterns at baseline, 6 months, and 12 months after SARS-CoV-2 infection. While benign cystic and nodular findings remained largely stable, a progressive increase in autoimmune thyroiditis was observed over time, along with transient cases of subacute thyroiditis. These findings highlight the dynamic and predominantly structural nature of post-COVID thyroid involvement.
Figure 8.
Changes in Thyroid Ultrasound Patterns from Baseline to 12 Months after SARS-CoV-2 Infection. Legend: Longitudinal evolution of thyroid ultrasound findings over 12 months of follow-up. Stacked bar chart illustrating the distribution of thyroid ultrasound patterns at baseline, 6 months, and 12 months after SARS-CoV-2 infection. While benign cystic and nodular findings remained largely stable, a progressive increase in autoimmune thyroiditis was observed over time, along with transient cases of subacute thyroiditis. These findings highlight the dynamic and predominantly structural nature of post-COVID thyroid involvement.
In contrast, ultrasound findings suggestive of autoimmune thyroiditis showed a progressive and clinically relevant increase over time. The number of patients exhibiting a diffusely hypoechoic and heterogeneous thyroid pattern increased from 6 patients at baseline (9.0%) to 8 patients at 6 months (11.9%) and to 14 patients at 12 months (20.9%). This evolution supports the development or progression of structural changes compatible with autoimmune thyroiditis during the post-infectious period, even in the absence of marked functional thyroid abnormalities. Subacute thyroiditis was identified on ultrasound in 4 patients (6.0%) at the 6-month evaluation, characterized by poorly defined hypoechoic areas, reduced vascularity, and a diffuse inflammatory appearance. At the 12-month assessment, no patients exhibited active ultrasound features of subacute thyroiditis, confirming the transient and self-limiting nature of this condition. One patient required total thyroidectomy during follow-up after being diagnosed with papillary thyroid carcinoma at the 6-month evaluation and was therefore classified as post-total thyroidectomy at the 12-month visit.
Overall, longitudinal ultrasound analysis demonstrates a shift from a predominantly normal structural profile, or one characterized by benign, nonspecific lesions toward progressive inflammatory and autoimmune changes. These findings suggest that post-COVID thyroid involvement may manifest as a delayed structural phenotype, detectable on ultrasound before the development of overt hormonal dysfunction.
Table 4.
Longitudinal evolution of thyroid ultrasound findings during the 12-month follow-up after SARS-CoV-2 infection.
Table 4.
Longitudinal evolution of thyroid ultrasound findings during the 12-month follow-up after SARS-CoV-2 infection.
| Ultrasound Pattern |
Baseline (n = 67) |
6 Months (n = 67) |
12 Months (n = 67) |
| Normal ultrasound appearance |
20 |
14 |
12 |
| Thyroid cysts |
24 |
24 |
24 |
| Thyroid nodules |
17 |
16 |
16 |
| Autoimmune thyroiditis |
6 |
8 |
14 |
| Subacute thyroiditis |
0 |
4 |
0 |
| Status post-total thyroidectomy |
0 |
0 |
1 |
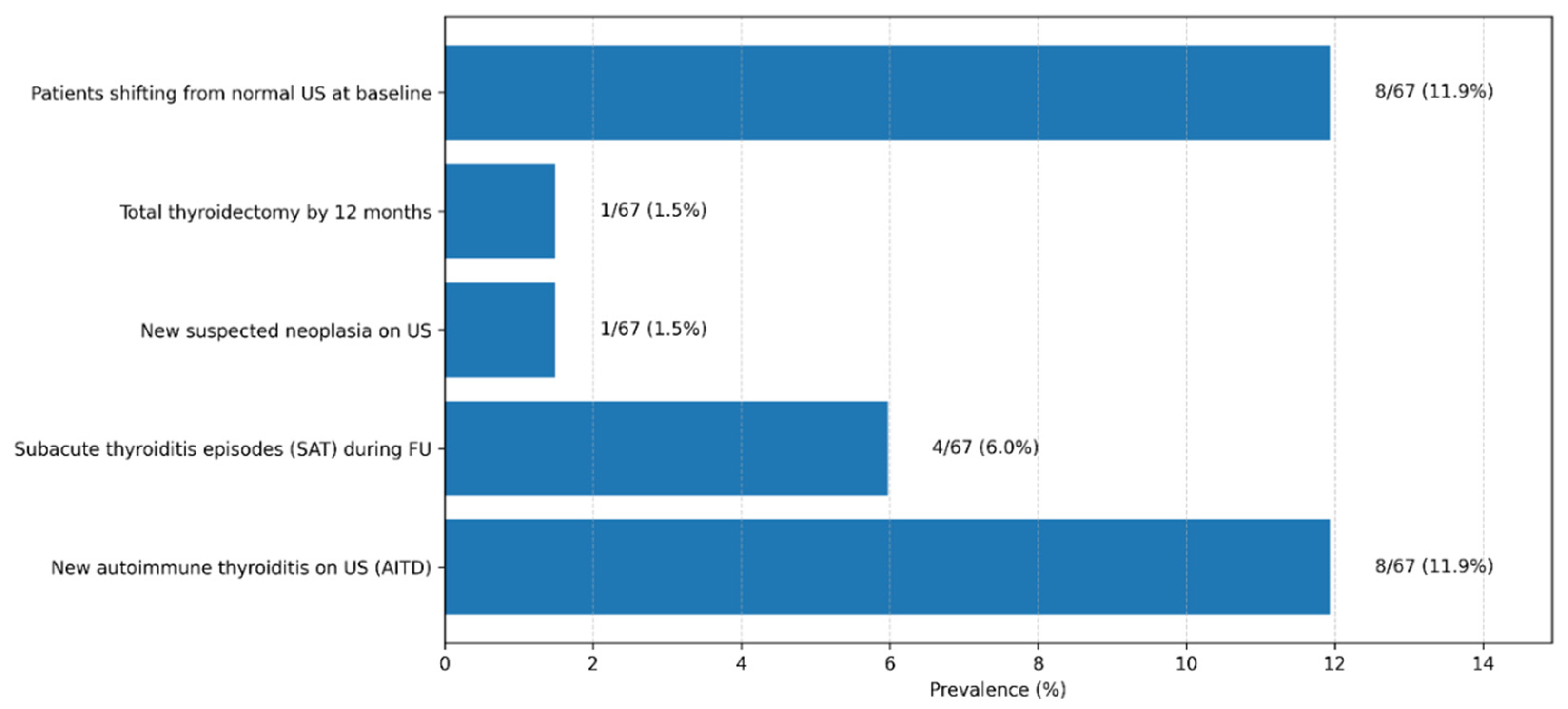
3.8. Prevalence of Thyroid Disorders at 12-Month Follow-Up
In the cohort of 67 patients monitored for 12 months after acute SARS-CoV-2 infection, we identified a substantial proportion of inflammatory and autoimmune thyroid disorders. Overall, despite the predominantly mild or moderate severity of the acute infection, nearly one in five patients (17.9%) developed an inflammatory or autoimmune thyroid condition during the year following infection. Moreover, only approximately 12% of participants remained completely free of biochemical and/or ultrasound thyroid abnormalities throughout follow-up, suggesting that subtle functional or structural thyroid alterations are common after COVID-19, even in patients without prior thyroid disease.
These findings are consistent with emerging data from the international literature and support the concept that SARS-CoV-2 may induce both transient inflammatory thyroid processes and persistent immune autoreactivity. The results underscore the need for careful endocrine follow-up after COVID-19, particularly in patients with persistent symptoms or early detected hormonal abnormalities.
Figure 9.
Thyroid-Related Clinical and Ultrasonographic Outcomes Observed During 12-Month Post–COVID-19 Follow-Up. Legend: The bar chart summarizes the prevalence of key thyroid-related outcomes identified during the 12-month follow-up period in the study cohort (n = 67). Displayed outcomes include the development of new autoimmune thyroiditis on ultrasound (AITD), episodes of subacute thyroiditis (SAT), transition from normal baseline thyroid ultrasound to abnormal findings, newly suspected thyroid neoplasia, and total thyroidectomy performed during follow-up. Values are expressed as absolute numbers and corresponding percentages. Autoimmune thyroiditis was defined based on characteristic ultrasonographic features, with or without associated thyroid autoantibodies.
Figure 9.
Thyroid-Related Clinical and Ultrasonographic Outcomes Observed During 12-Month Post–COVID-19 Follow-Up. Legend: The bar chart summarizes the prevalence of key thyroid-related outcomes identified during the 12-month follow-up period in the study cohort (n = 67). Displayed outcomes include the development of new autoimmune thyroiditis on ultrasound (AITD), episodes of subacute thyroiditis (SAT), transition from normal baseline thyroid ultrasound to abnormal findings, newly suspected thyroid neoplasia, and total thyroidectomy performed during follow-up. Values are expressed as absolute numbers and corresponding percentages. Autoimmune thyroiditis was defined based on characteristic ultrasonographic features, with or without associated thyroid autoantibodies.
3.8.1. Incidence of Subacute Thyroiditis
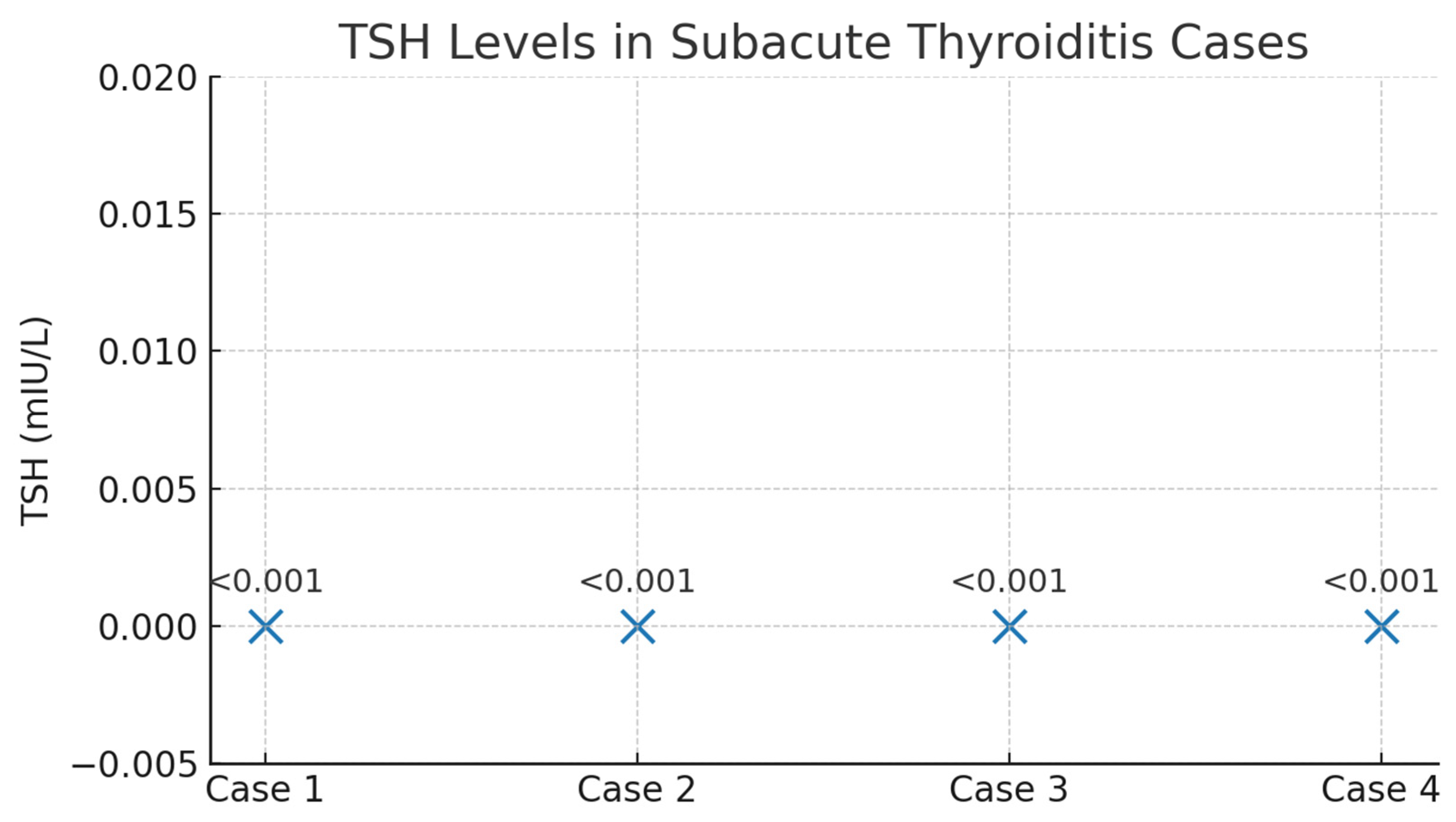
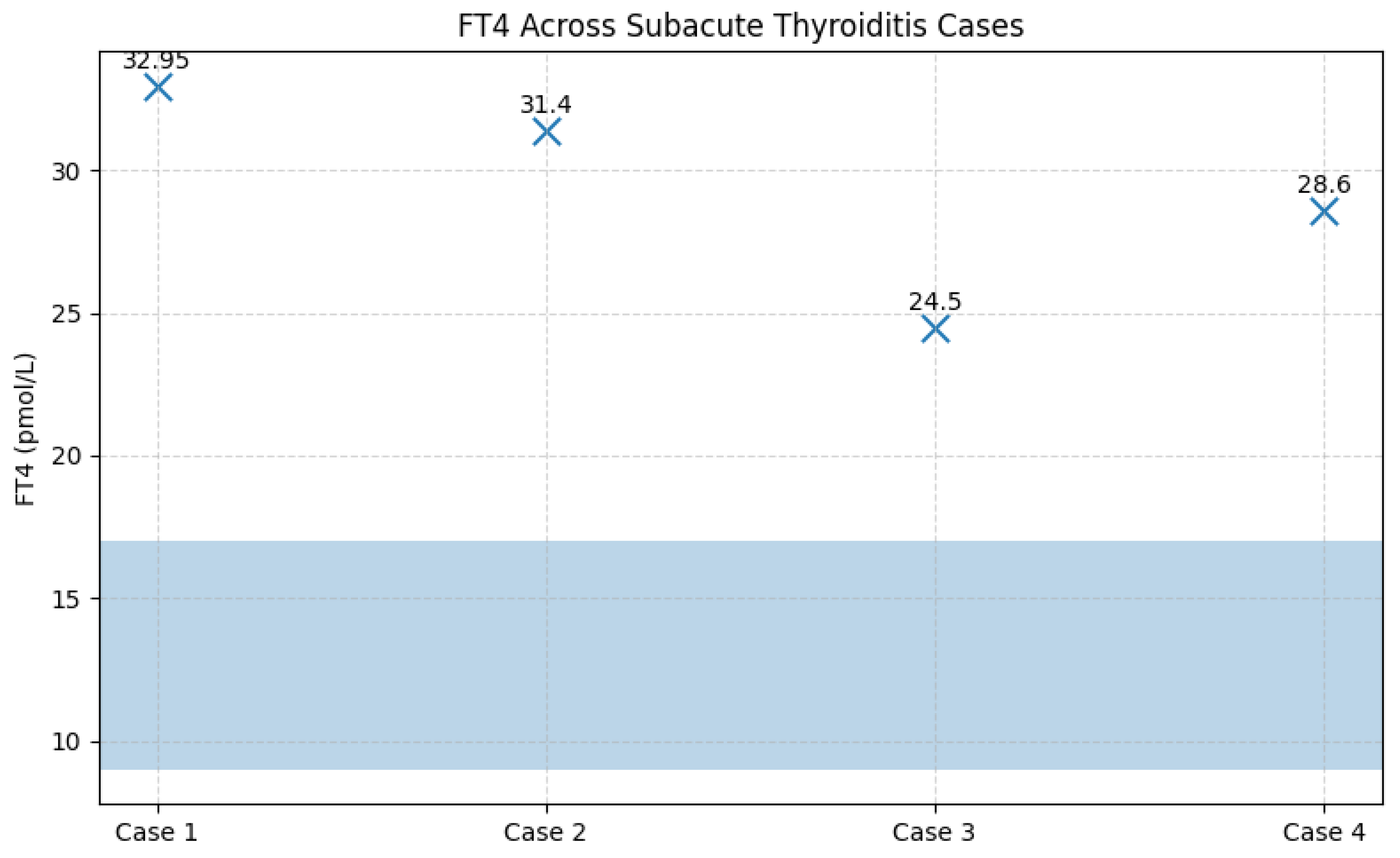
Among the 67 patients prospectively followed for 12 months after acute SARS-CoV-2 infection, four cases of subacute thyroiditis (SAT) were identified, corresponding to an incidence of 6.0%. The cases were evenly distributed by sex (two women and two men), with ages ranging from 40 to 71 years. Symptom onset occurred between 30 and 45 days after the acute COVID-19 episode, a time frame consistent with post-viral inflammatory manifestations. Clinically, all patients presented with persistent fever and anterior neck pain; alternative infectious causes of fever were excluded through standardized evaluation. From a paraclinical perspective, all patients exhibited marked systemic inflammation, including leukocytosis and significantly elevated ESR, fibrinogen, and CRP levels. Thyroid function tests revealed a clear pattern of transient thyrotoxicosis, with profoundly suppressed TSH levels (<0.001 mIU/L; reference range 0.3–4.5 mIU/L) and markedly elevated FT4 concentrations ranging from 24.5 to 32.95 pmol/L (reference range 8.9–17.2 pmol/L). Thyroid autoantibodies, anti-TPO (<30 IU/mL) and anti-thyroglobulin (<95 IU/mL), remained within normal limits in all cases, confirming the non-autoimmune nature of the inflammatory process.
Thyroid ultrasound, performed by an experienced thyroid imaging specialist, demonstrated features characteristic of subacute thyroiditis, including hypoechoic, heterogeneous, poorly vascularized areas, without nodules or ultrasound findings suggestive of autoimmune thyroid disease. These findings supported the diagnosis in accordance with European Thyroid Association criteria.
All patients were treated with corticosteroids, with a rapid clinical response characterized by prompt resolution of neck pain and fever within the first days of therapy. Notably, none of the patients had received corticosteroid treatment during the acute COVID-19 episode, as all had experienced mild forms of the disease. Clinical evolution was favorable: at follow-up (three months for two patients and six months for the remaining two), thyroid function had normalized in all cases, with no requirement for subsequent thyroid hormone replacement therapy. Although a brief transient hypothyroid phase was observed in two patients, this resolved spontaneously, consistent with the classic triphasic course of SAT.
Analysis of vaccination status showed that two affected patients were unvaccinated against COVID-19, while the other two had received two doses of the Pfizer-BioNTech vaccine approximately two years prior to the onset of thyroiditis, an interval that does not support a direct temporal association between vaccination and the inflammatory episode.
Table 5.
Biochemical and Immunological Profile of Patients with Subacute Thyroiditis at Diagnosis.
Table 5.
Biochemical and Immunological Profile of Patients with Subacute Thyroiditis at Diagnosis.
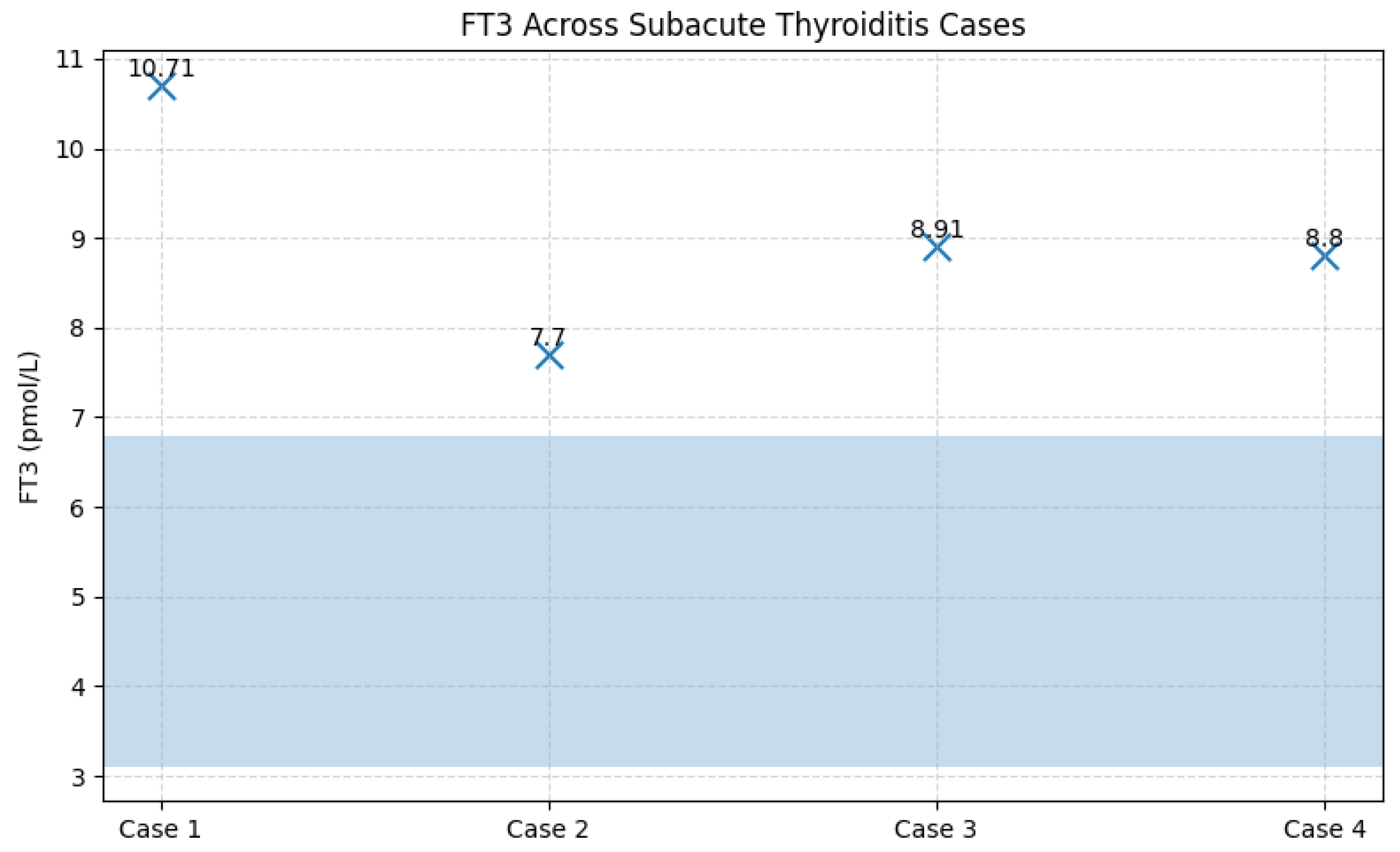
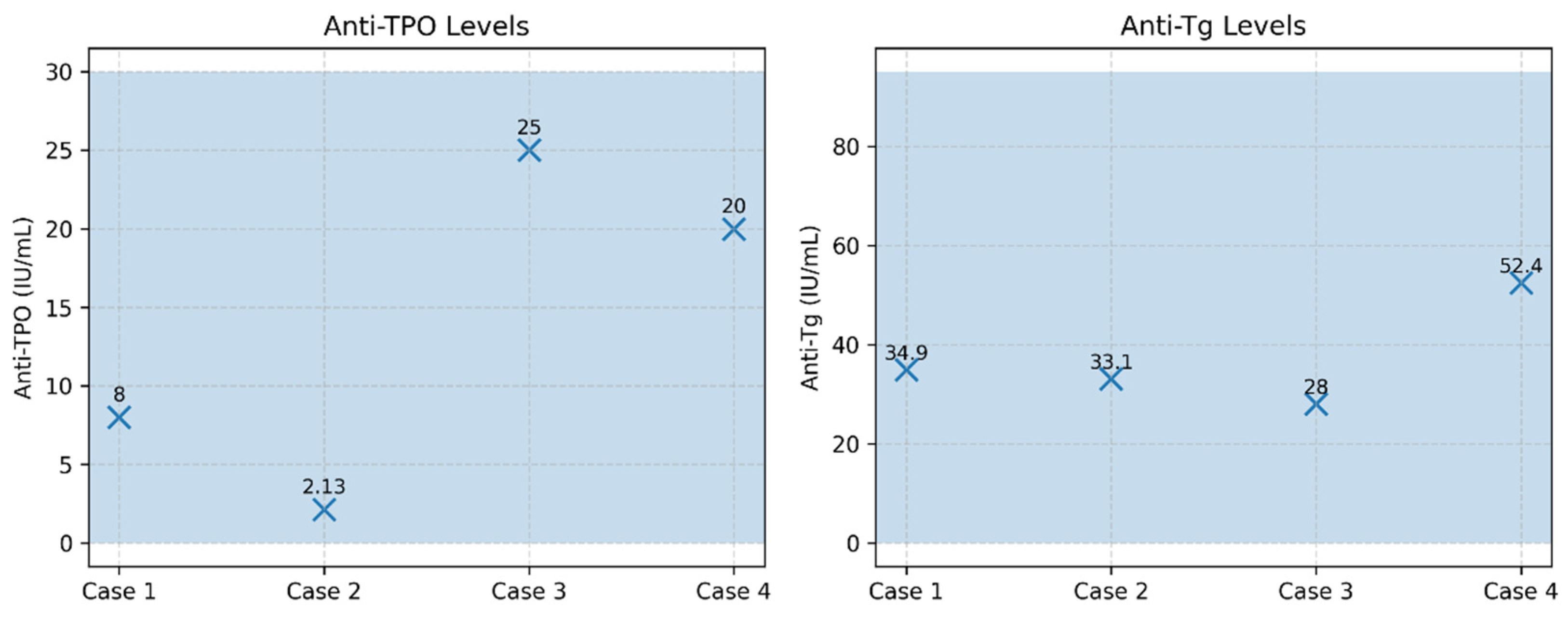
| Case |
Sex |
TSH (mIU/L) |
FT4 (pmol/L) |
FT3 (pmol/L) |
Anti-TPO (IU/mL) |
Anti-Tg (IU/mL) |
| 1 |
F |
<0.001 |
32.9 |
10.7 |
8 |
34.9 |
| 2 |
M |
0.018 |
31.4 |
7.7 |
2.13 |
33.1 |
| 3 |
F |
<0.001 |
24.5 |
8.91 |
25 |
28 |
| 4 |
M |
<0.001 |
28.6 |
8.8 |
20 |
52.4 |
Figure 10.
TSH values in patients with subacute thyroiditis identified during follow-up. Legend: Scatter plot illustrating TSH levels in the four cases of subacute thyroiditis diagnosed during follow-up. All patients exhibited biochemical thyrotoxicosis, with TSH values below the assay detection limit (<0.001 mIU/L). The uniform and profound suppression of TSH reflects the acute destructive process characteristic of subacute thyroiditis.
Figure 10.
TSH values in patients with subacute thyroiditis identified during follow-up. Legend: Scatter plot illustrating TSH levels in the four cases of subacute thyroiditis diagnosed during follow-up. All patients exhibited biochemical thyrotoxicosis, with TSH values below the assay detection limit (<0.001 mIU/L). The uniform and profound suppression of TSH reflects the acute destructive process characteristic of subacute thyroiditis.
Figure 11.
FT4 concentrations in patients with subacute thyroiditis in relation to the reference range. Legend: FT4 values for the four cases of subacute thyroiditis are displayed relative to the reference range (8.9–17.2 pmol/L, highlighted by the shaded blue area). All patients exhibited markedly elevated FT4 concentrations, consistent with the thyrotoxic phase of subacute thyroiditis. Individual FT4 values are indicated above each data point for clarity.
Figure 11.
FT4 concentrations in patients with subacute thyroiditis in relation to the reference range. Legend: FT4 values for the four cases of subacute thyroiditis are displayed relative to the reference range (8.9–17.2 pmol/L, highlighted by the shaded blue area). All patients exhibited markedly elevated FT4 concentrations, consistent with the thyrotoxic phase of subacute thyroiditis. Individual FT4 values are indicated above each data point for clarity.
Figure 12.
FT3 concentrations in patients with subacute thyroiditis in relation to the reference range. Legend: FT3 values are shown relative to the normal reference range (highlighted by the shaded blue area). All four patients exhibited elevated FT3 concentrations, indicating active thyrotoxicosis due to the release of thyroid hormones from the inflamed thyroid gland. Individual FT3 values are displayed numerically on the graph for clarity.
Figure 12.
FT3 concentrations in patients with subacute thyroiditis in relation to the reference range. Legend: FT3 values are shown relative to the normal reference range (highlighted by the shaded blue area). All four patients exhibited elevated FT3 concentrations, indicating active thyrotoxicosis due to the release of thyroid hormones from the inflamed thyroid gland. Individual FT3 values are displayed numerically on the graph for clarity.
Figure 13.
Thyroid autoantibodies (anti-TPO and anti-Tg) in patients with subacute thyroiditis. Legend: Serum anti-TPO (reference <30 IU/mL) and anti-thyroglobulin antibodies (anti-Tg; reference <95 IU/mL) are shown for the four cases of subacute thyroiditis. All patients exhibited antibody levels within the normal reference ranges, a finding characteristic of non-autoimmune subacute thyroiditis. Shaded blue areas indicate the reference intervals for each marker.
Figure 13.
Thyroid autoantibodies (anti-TPO and anti-Tg) in patients with subacute thyroiditis. Legend: Serum anti-TPO (reference <30 IU/mL) and anti-thyroglobulin antibodies (anti-Tg; reference <95 IU/mL) are shown for the four cases of subacute thyroiditis. All patients exhibited antibody levels within the normal reference ranges, a finding characteristic of non-autoimmune subacute thyroiditis. Shaded blue areas indicate the reference intervals for each marker.
3.8.2. Incidence of Autoimmune Thyroiditis
For the analysis of autoimmune thyroiditis incidence, patients with a known history of autoimmune thyroid disease were excluded from this evaluation. At baseline, all patients included in this subgroup had no prior history of autoimmune thyroiditis, and thyroid ultrasound performed at enrolment was normal.
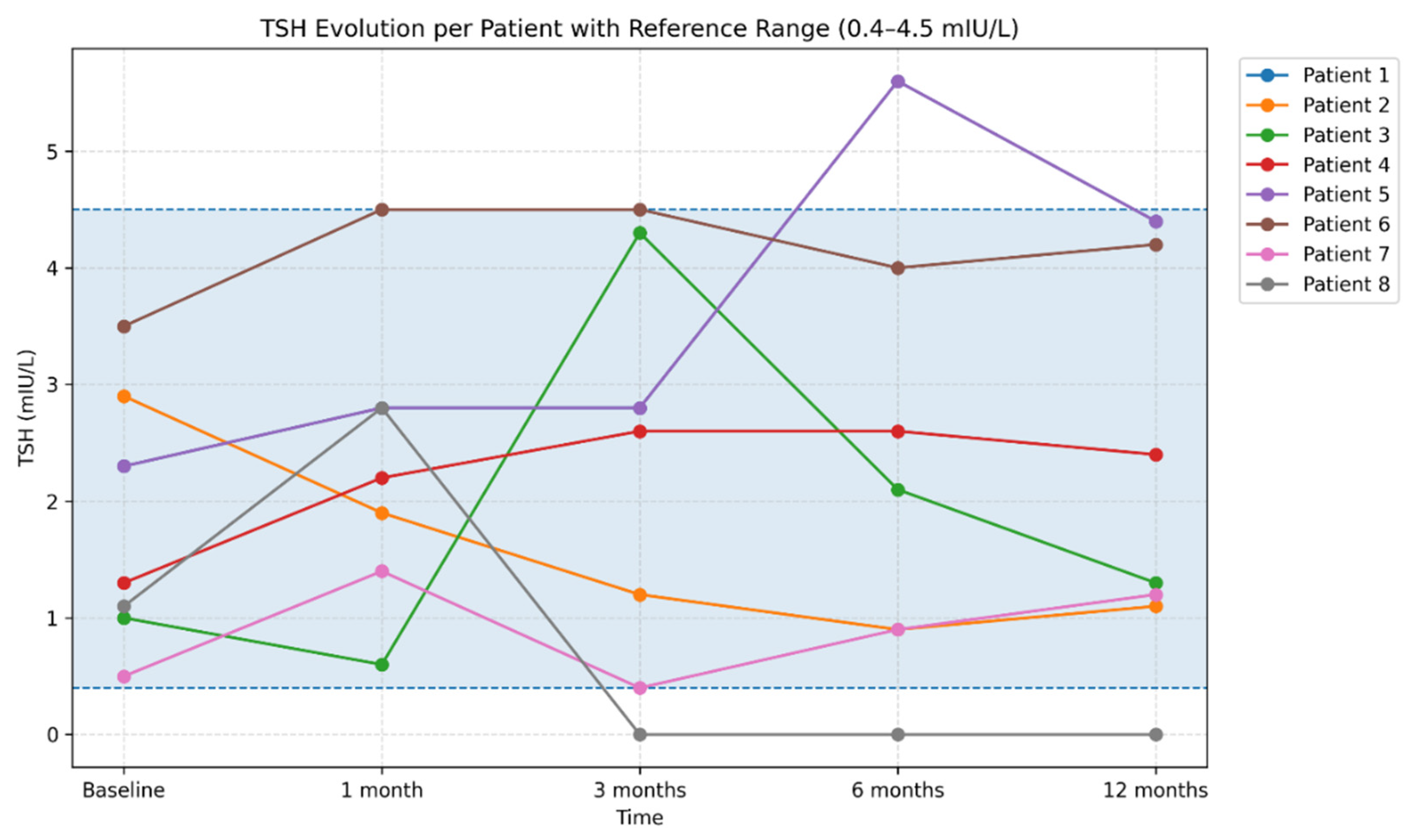
During follow-up, autoimmune thyroiditis was identified in 8 patients (11.9%). In these patients, thyroid ultrasound abnormalities developed over the course of monitoring, becoming evident predominantly at the 6-month evaluation and characterized by a diffuse hypoechoic and heterogeneous echotexture. Assessment of thyroid autoantibodies revealed elevated anti-thyroid peroxidase (anti-TPO) and/or anti-thyroglobulin (anti-Tg) titters in four patients, whereas in the remaining cases ultrasound changes were present in the absence of detectable thyroid autoantibodies. From a functional perspective, patients exhibited either a euthyroid profile or TSH values consistent with subclinical or early hypothyroidism. TSH levels showed a heterogeneous course within this subgroup. Most patients maintained TSH values within the reference range (0.3–4.5 mIU/L) throughout follow-up; however, several patients experienced significant fluctuations during the first months, compatible with transient post-infectious thyroid dysfunction, including one case with marked TSH suppression (<0.001 mIU/L) persisting between 3 and 12 months. In addition, one patient demonstrated progressive increases in TSH at 6 and 12 months, reaching 5.59 mIU/L. Overall, TSH dynamics highlighted substantial interindividual variability over the 12-month follow-up period (
Figure 14).
Figure 14.
Individual trajectories of TSH values over the 12-month follow-up period. Legend: Each line represents an individual patient trajectory, illustrating interindividual variability in thyroid function during the post-COVID period. Both transient TSH fluctuations and stable profiles are observed, reflecting the heterogeneity of thyroid responses following SARS-CoV-2 infection. The shaded area indicates the TSH reference range (0.4–4.5 mIU/L), facilitating the identification of transient or persistent deviations beyond normal limits. Serological analysis demonstrated a variable evolution of thyroid autoantibody titers. In a subset of patients, increases in anti-thyroid peroxidase (anti-TPO) and/or anti-thyroglobulin (anti-Tg) levels were observed during the first months, followed by a tendency toward decline at 6 and 12 months (
Figure 15 and
Figure 16). In other patients, autoantibody titers remained within normal limits or showed only mild elevations throughout follow-up.
Figure 14.
Individual trajectories of TSH values over the 12-month follow-up period. Legend: Each line represents an individual patient trajectory, illustrating interindividual variability in thyroid function during the post-COVID period. Both transient TSH fluctuations and stable profiles are observed, reflecting the heterogeneity of thyroid responses following SARS-CoV-2 infection. The shaded area indicates the TSH reference range (0.4–4.5 mIU/L), facilitating the identification of transient or persistent deviations beyond normal limits. Serological analysis demonstrated a variable evolution of thyroid autoantibody titers. In a subset of patients, increases in anti-thyroid peroxidase (anti-TPO) and/or anti-thyroglobulin (anti-Tg) levels were observed during the first months, followed by a tendency toward decline at 6 and 12 months (
Figure 15 and
Figure 16). In other patients, autoantibody titers remained within normal limits or showed only mild elevations throughout follow-up.
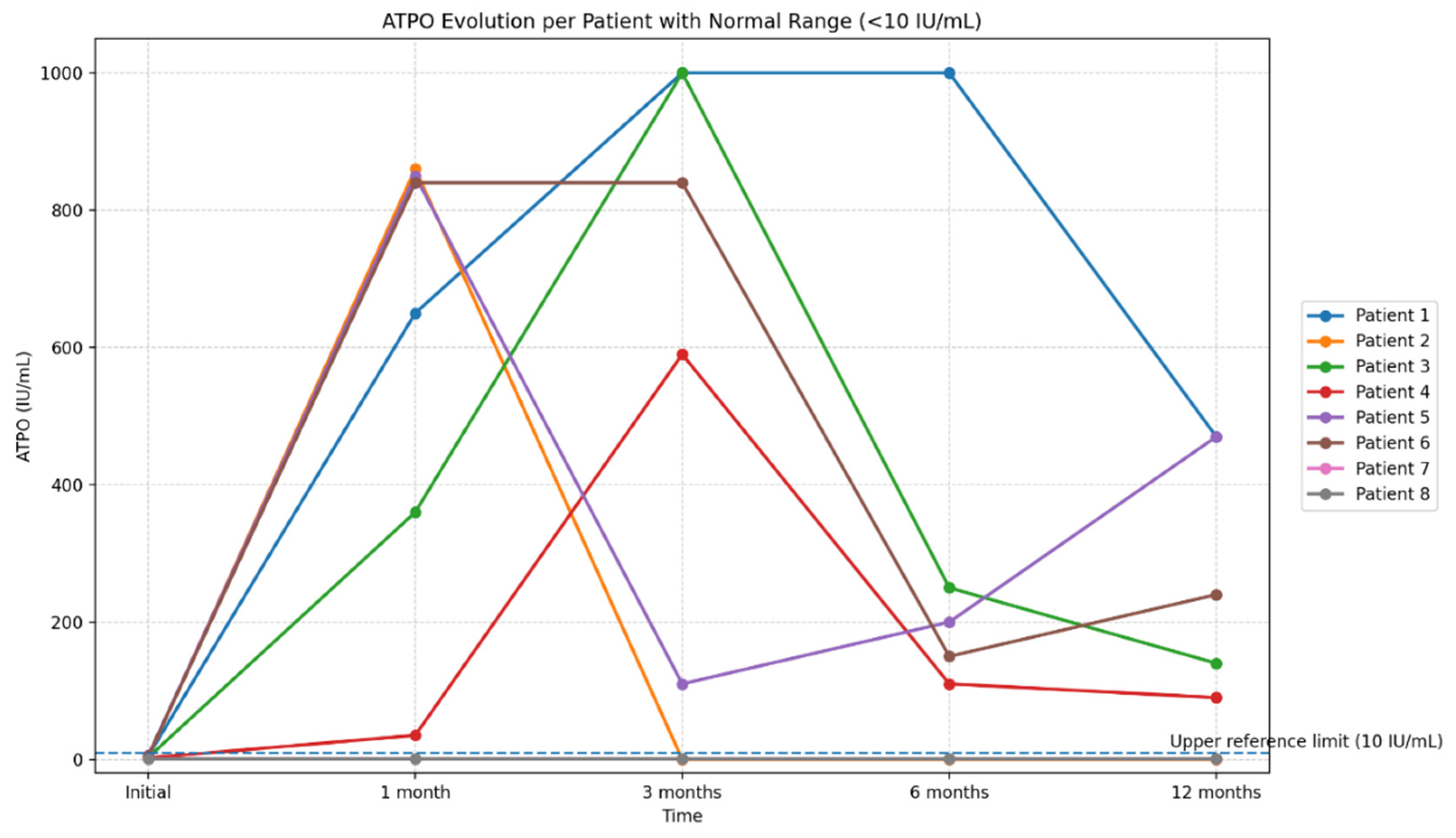
Figure 15.
Longitudinal evolution of anti–thyroid peroxidase antibody (anti-TPO) titers over the 12-month follow-up period. Legend: The figure illustrates the individual trajectories of anti-TPO levels in eight patients with serial measurements obtained at baseline, 1 month, 3 months, 6 months, and 12 months. Each line represents the longitudinal profile of a single patient, highlighting the substantial interindividual variability in autoantibody titers during the post-COVID period. The reference range for anti-TPO (<10 IU/mL) is indicated to facilitate interpretation of deviations from normal values. The graph emphasizes the presence of marked but transient increases in anti-TPO titers in a subset of patients.
Figure 15.
Longitudinal evolution of anti–thyroid peroxidase antibody (anti-TPO) titers over the 12-month follow-up period. Legend: The figure illustrates the individual trajectories of anti-TPO levels in eight patients with serial measurements obtained at baseline, 1 month, 3 months, 6 months, and 12 months. Each line represents the longitudinal profile of a single patient, highlighting the substantial interindividual variability in autoantibody titers during the post-COVID period. The reference range for anti-TPO (<10 IU/mL) is indicated to facilitate interpretation of deviations from normal values. The graph emphasizes the presence of marked but transient increases in anti-TPO titers in a subset of patients.
Figure 16.
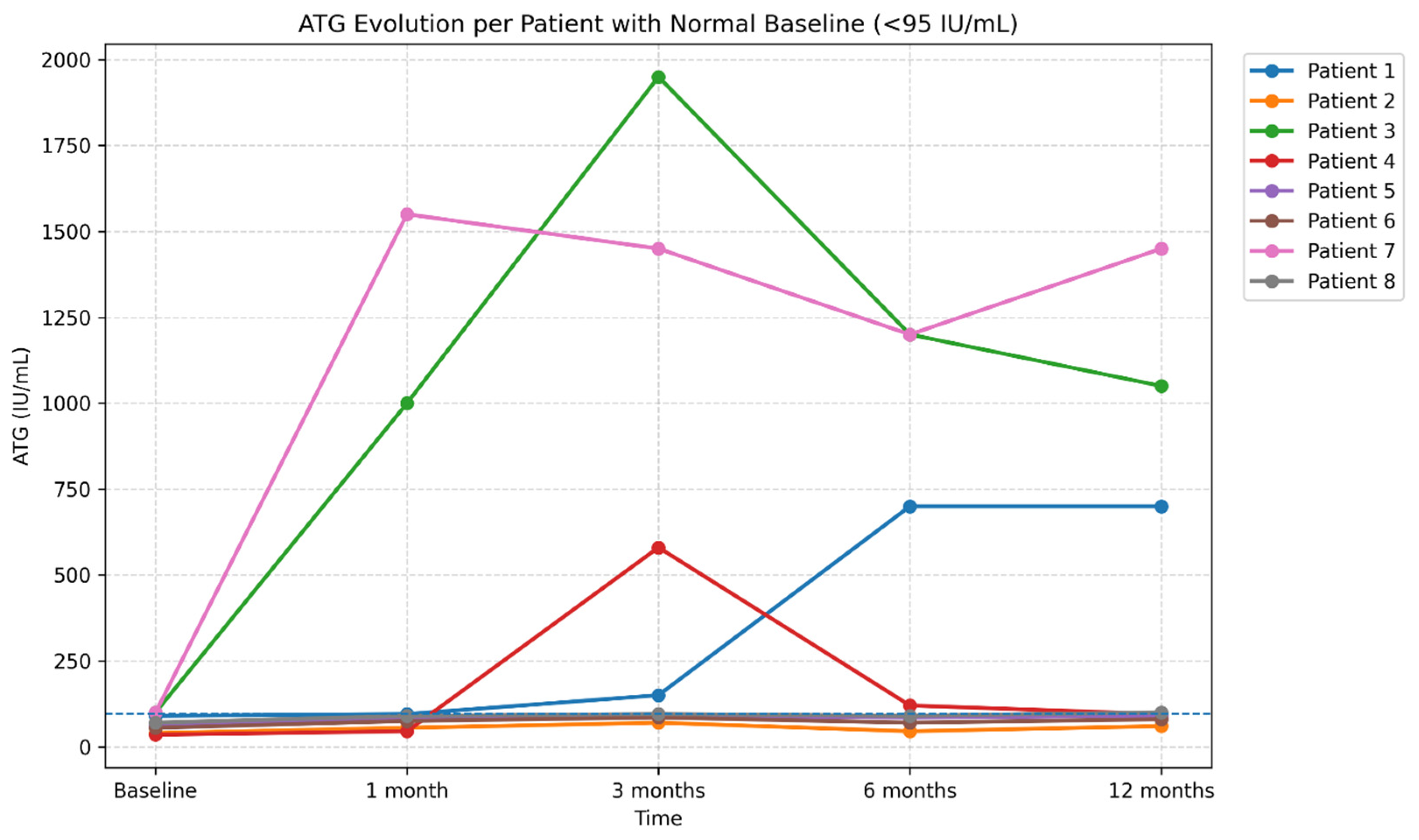
Longitudinal evolution of anti-thyroglobulin antibody (ATG) levels over 12 months of follow-up (reference range <95 IU/mL. Legend: The figure illustrates the individual trajectories of ATG levels in eight patients monitored at baseline, 1 month, 3 months, 6 months, and 12 months. Each line represents one patient, highlighting the substantial interindividual variability of the post-COVID autoimmune response. While most patients exhibited stable or only mildly elevated ATG levels, two cases showed marked increases followed by a subsequent decline, suggesting a transient post-infectious autoimmune response. The reference range (<95 IU/mL) is indicated in the title to facilitate interpretation of elevated values.
Figure 16.
Longitudinal evolution of anti-thyroglobulin antibody (ATG) levels over 12 months of follow-up (reference range <95 IU/mL. Legend: The figure illustrates the individual trajectories of ATG levels in eight patients monitored at baseline, 1 month, 3 months, 6 months, and 12 months. Each line represents one patient, highlighting the substantial interindividual variability of the post-COVID autoimmune response. While most patients exhibited stable or only mildly elevated ATG levels, two cases showed marked increases followed by a subsequent decline, suggesting a transient post-infectious autoimmune response. The reference range (<95 IU/mL) is indicated in the title to facilitate interpretation of elevated values.
Thyroid ultrasound frequently revealed a diffusely heterogeneous echotexture, variable inflammatory changes, as well as the presence of hypoechoic nodular lesions, small bilateral cysts, or nonsuspicious micronodules. In some cases, well-defined nodules with a hypoechoic halo and preserved vascular signal were described.
3.9. Analysis of the Relationship Between Anti–SARS-CoV-2 Vaccination and Thyroid Changes
In the analyzed cohort, 36 patients had a history of anti–SARS-CoV-2 vaccination, predominantly with the Pfizer-BioNTech vaccine, administered in two, three, or four doses, while a small number of patients had received the Johnson & Johnson vaccine.
Thyroid ultrasound examinations demonstrated a wide spectrum of findings, including benign lesions commonly encountered in the general population (thyroid cysts, microcysts, and nodules), features suggestive of autoimmune thyroiditis, and, in a few isolated cases, subacute thyroiditis. In a subset of patients, thyroid ultrasound findings were entirely normal.
The distribution of ultrasound abnormalities did not show clear differences according to the number of vaccine doses administered. Benign lesions were observed both in patients who had received two doses and in those who had received three or four doses, while ultrasound patterns compatible with autoimmune thyroiditis were similarly distributed across these categories. The small number of subacute thyroiditis cases precluded any meaningful epidemiological assessment of an association with vaccination.
Statistical analysis did not reveal significant associations between vaccination status and the occurrence of abnormal TSH values during the 12-month follow-up, nor with elevated titers of antithyroid antibodies (anti-TPO, anti-TG) or structural thyroid changes on follow-up ultrasound (all p > 0.05). Likewise, no significant differences were observed in inflammatory markers (CRP, ferritin, D-dimer, IL-6) according to vaccination status.
Overall, within the analyzed cohort, anti–SARS-CoV-2 vaccination was not associated with detectable structural, functional, or immunological thyroid abnormalities during the follow-up period.
4. Discussion
4.1. Principal Findings and Clinical Relevance
In this prospective, single-center study with a 12-month follow-up period, we identified a substantial burden of thyroid involvement following SARS-CoV-2 infection, even in the context of predominantly mild or moderate acute disease. The most relevant findings include a relatively high incidence of subacute thyroiditis (6.0%) and autoimmune thyroiditis (11.9%), the largely mild and self-limiting clinical course of these conditions, and the observation that only a small proportion of patients remained completely free of biochemical or structural thyroid abnormalities at the end of follow-up. In addition, more than one third of participants (34.3%) experienced at least one episode of abnormal TSH during monitoring, underscoring the high frequency of post-COVID thyroid dysfunction.
The incidence of subacute thyroiditis observed in our cohort exceeds that expected in the general population and is consistent with the growing body of evidence supporting SARS-CoV-2 as a trigger for post-viral thyroid inflammation [
13,
21,
22]. While early reports consisted mainly of case descriptions, subsequent observational studies suggest that the true incidence of subacute thyroiditis after COVID-19 may be underestimated, particularly in patients with mild or atypical symptoms [
23]. The temporal pattern observed in our patients, with symptom onset approximately 4–6 weeks after acute infection, mirrors the latency described for subacute thyroiditis following other viral infections and supports a post-infectious inflammatory mechanism rather than a coincidental association [
24]. The 6% incidence rate is comparable to that reported in other post-pandemic case series and observational studies [
25,
26], reinforcing the role of SARS-CoV-2 as a clinically relevant viral trigger, like other known viral agents.
Autoimmune thyroiditis was identified in nearly 12% of patients, a proportion that, although comparable to the upper range of reported prevalence for subclinical thyroid autoimmunity in the general population, is notable for its de novo occurrence and its clear post-infectious temporal association [
27,
28]. Importantly, in a subset of patients, thyroid autoantibodies appeared de novo during follow-up, suggesting that SARS-CoV-2 infection may actively trigger autoimmune processes rather than merely unmask pre-existing subclinical disease. This observation is consistent with emerging data indicating that COVID-19 may promote autoimmune phenomena through mechanisms such as molecular mimicry, bystander immune activation, epitope spreading, and cytokine-mediated immune dysregulation [
14,
29]. The persistence of thyroid autoantibodies at 12 months in some patients also raises questions regarding the potential long-term endocrine consequences of SARS-CoV-2 infection [
28]. Compared with the general population, in which the prevalence of subclinical thyroid dysfunction is estimated at approximately 4–10%, with values around 8% in the European adult population [
30], the prevalence observed in our cohort is substantially higher. This difference supports the hypothesis that SARS-CoV-2 infection represents an important perturbing factor of thyroid homeostasis [
8,
31]. Our findings are in line with international reports describing heterogeneous thyroid involvement after COVID-19, including episodes of thyrotoxicosis, non-thyroidal illness syndrome (NTIS), subacute thyroiditis, and autoimmune activation [
22,
32].
Despite the relatively high prevalence of thyroid involvement, most disorders identified in our cohort followed a mild clinical course and were frequently transient. Cases of subacute thyroiditis exhibited the classic triphasic evolution and responded well to corticosteroid therapy, in agreement with previous reports [
13,
22]. Similarly, many patients with autoimmune thyroiditis remained euthyroid or developed only subclinical dysfunction during follow-up, suggesting that progression to overt hypothyroidism within the first year after COVID-19 is relatively uncommon [
32,
33].
An important clinically relevant observation is that post-COVID thyroid dysfunction frequently occurred even in patients without severe acute disease. Most participants did not require advanced respiratory support, and thyroid involvement was largely independent of acute disease severity, supporting a predominantly immune-mediated inflammatory mechanism [
28,
33]. The phenomenon of “silent thyroid dysfunction,” characterized by subclinical, often transient and paucisymptomatic hormonal abnormalities, is increasingly recognized in the recent literature and may easily be overlooked in the absence of systematic testing [
34]. Prospective studies have shown that up to one third of patients may develop abnormalities in TSH or thyroid autoantibodies in the months following infection, even after clinically mild COVID-19 [
28].
Overall, the central message of this study is that thyroid involvement represents a frequent, yet often underdiagnosed, component of post-COVID-19 morbidity. Although most abnormalities are mild and self-limiting, their high prevalence justifies increased clinical vigilance and risk-adapted thyroid monitoring after SARS-CoV-2 infection, particularly in patients with persistent symptoms or early abnormalities in thyroid function. These findings add to the growing body of evidence that COVID-19 should be viewed not only as an acute infectious disease, but also as a condition with potential long-term endocrine consequences.
4.2. Incidence of Post-COVID Subacute Thyroiditis: Clinical and Paraclinical Features
In our prospective cohort of patients monitored for 12 months after SARS-CoV-2 infection, subacute thyroiditis (SAT) was identified in 6.0% of participants. This incidence falls within the wide range reported in the international literature, where the frequency of post-COVID SAT varies approximately between 0.5% and 10%, depending on study design, population characteristics, and the intensity of endocrine follow-up [
13,
22,
25,
26,
35]. Higher incidence rates are generally reported in prospective studies or in cohorts with systematic follow-up, suggesting that the true incidence of post-COVID SAT may be underestimated in the absence of active endocrine surveillance.
An important point of concordance with existing data is the typical interval between acute infection and symptom onset, which in our cohort occurred approximately 30–45 days after the acute COVID-19 episode. This temporal delay is similar to that described both in SARS-CoV-2–associated SAT and in classical forms of subacute thyroiditis triggered by other viral infections, such as influenza viruses, Coxsackie viruses, adenoviruses, or Epstein–Barr virus [
24,
25,
26]. This latency supports the hypothesis of a post-viral inflammatory mechanism rather than direct viral invasion of the thyroid parenchyma.
From a clinical and paraclinical perspective, all SAT cases identified in our study exhibited a clinical profile fully consistent with the classical description of this entity. The initial phase was characterized by biochemical thyrotoxicosis, with marked TSH suppression and significant elevations of FT4 and FT3, accompanied by a pronounced systemic inflammatory response (elevated ESR, CRP, and fibrinogen). Thyroid ultrasound examination revealed typical findings, including poorly defined hypoechoic and heterogeneous areas with reduced vascularity, without features suggestive of nodular disease or autoimmune thyroid pathology. This imaging pattern is well documented in SAT and represents an important criterion for differentiating it from other causes of thyrotoxicosis [
13,
26,
35].
A key feature of the SAT cases in our cohort was the absence of thyroid autoantibodies (anti-TPO and anti-thyroglobulin) at all evaluated time points, confirming the non-autoimmune nature of the inflammatory process. This finding is fully consistent with the existing literature, which describes SAT as a destructive, inflammation-mediated thyroiditis, distinct from the autoimmune mechanisms underlying Hashimoto’s thyroiditis or Graves’ disease [
24,
35]. The lack of autoantibodies, together with the characteristic clinical and ultrasonographic evolution, supports the role of SARS-CoV-2 as a trigger of a transient post-viral inflammatory process affecting the thyroid gland.
The subsequent clinical course of patients with SAT in our cohort followed the classic triphasic pattern, with resolution of the thyrotoxic phase, the transient occurrence of a hypothyroid phase in some patients, and eventual normalization of thyroid function. All patients received systemic corticosteroid therapy, with a rapid clinical response and marked improvement in anterior neck pain and systemic symptoms, in accordance with current recommendations and with experiences reported in previous studies [
13,
22,
35]. None of the patients required long-term thyroid hormone replacement, and complete recovery of thyroid function was documented at follow-up evaluations.
Overall, our findings confirm that subacute thyroiditis represents a relevant post-viral manifestation of SARS-CoV-2 infection, with a non-negligible incidence even in cohorts with predominantly mild or moderate forms of COVID-19. The clinical, biochemical, and imaging characteristics, as well as the favorable response to anti-inflammatory treatment, closely resemble those described for classical SAT, further supporting the hypothesis that SARS-CoV-2 acts as a viral trigger capable of inducing transient thyroid inflammation in susceptible individuals.
4.3. Autoimmune Thyroiditis after COVID-19: Trigger or Accelerator of Latent Autoimmunity?
One of the most relevant findings of the present study is the identification of a substantial prevalence of autoimmune thyroiditis (AITD) during the post-COVID follow-up period, affecting 11.9% of patients. This observation raises an important conceptual question: does SARS-CoV-2 infection act as a de novo trigger of thyroid autoimmunity, or rather as an accelerator of a previously latent, subclinical autoimmune process? In our cohort, most patients diagnosed with AITD exhibited persistent elevations of anti-thyroid peroxidase (anti-TPO) and/or anti-thyroglobulin antibodies, in association with diffuse hypoechoic and heterogeneous ultrasound features characteristic of Hashimoto’s thyroiditis. Notably, in a subset of patients, thyroid autoantibodies appeared de novo during follow-up, in the absence of any known thyroid disease history or previously documented antibody positivity. This finding supports the hypothesis that SARS-CoV-2 may play an active role in initiating thyroid autoimmunity, rather than merely unmasking a pre-existing but undiagnosed condition.
The pathophysiological mechanisms through which SARS-CoV-2 infection may promote the development of autoimmunity are likely multifactorial. Existing literature describes several potential pathways, including molecular mimicry between viral antigens and thyroid structures, nonspecific bystander immune activation, epitope spreading, and persistent immune dysregulation mediated by pro-inflammatory cytokines [
14,
29]. In addition, the expression of angiotensin-converting enzyme 2 (ACE2) receptors and the TMPRSS2 protease in thyroid follicular cells suggests an intrinsic susceptibility of the thyroid gland to immune-inflammatory perturbations induced by SARS-CoV-2 [
8].
A clinically important finding in our cohort is the persistence of thyroid autoantibodies at 12 months in a subset of patients. Although most of these individuals remained euthyroid or developed only subclinical thyroid dysfunction, the persistence of autoimmune markers raises concerns regarding the potential risk of progression to overt hypothyroidism over longer follow-up periods. Data from the general population indicate that the presence of anti-TPO antibodies is a strong predictor of progressive thyroid functional decline, particularly in the presence of additional triggering factors [
36,
37].
Our results are consistent with findings from other prospective and observational post-COVID studies, which have reported transient or persistent increases in thyroid autoantibody levels among patients without known pre-existing thyroid disease [
28,
33]. Several longitudinal studies have described de novo appearance or temporary elevations of thyroid autoantibodies in a relevant subset of patients during the months following SARS-CoV-2 infection, including after mild forms of acute COVID-19 [
28]. Although data from prospective and observational studies support an association between SARS-CoV-2 infection and activation of thyroid autoimmunity, dedicated meta-analyses systematically evaluating the incidence and long-term evolution of thyroid autoantibodies after COVID-19 are currently lacking. This gap reflects the methodological heterogeneity of available studies and highlights the need for standardized prospective cohorts with long-term follow-up.
Overall, the findings of the present study support the concept that SARS-CoV-2 infection may function both as a trigger and as an accelerator of thyroid autoimmunity, depending on individual susceptibility. The identification of de novo thyroid autoantibodies and their persistence at 12 months underscore the importance of medium- and long-term endocrine monitoring in patients who develop early functional or immunological thyroid abnormalities after COVID-19. This subgroup of patients may benefit from personalized follow-up strategies, given the potential risk of progression toward clinically overt thyroid dysfunction.
4.4. Longitudinal Thyroid Hormone Dynamics and Transient Dysfunction
The longitudinal analysis of thyroid hormone dynamics in our cohort revealed a complex and heterogeneous post-infectious endocrine response, characterized by early fluctuations in TSH, FT4, and FT3, followed by an overall trend toward stabilization and normalization over the 12-month follow-up period. This pattern is consistent with findings from other longitudinal post-COVID studies, which have described transient alterations in thyroid function during recovery, with progressive return to euthyroidism in the majority of patients [
28,
32,
33,
39]. Although mean thyroid hormone values remained within reference ranges at the cohort level, individual trajectories varied substantially, highlighting significant interindividual heterogeneity in hypothalamic–pituitary–thyroid (HPT) axis responses following SARS-CoV-2 infection [
28,
37].
During the first months after the acute COVID-19-episode, mild fluctuations in TSH were frequently observed, sometimes accompanied by transient reductions in FT3 and, less commonly, subtle variations in FT4. These early changes may reflect an adaptive metabolic response of the thyroid axis to systemic stress and persistent post-viral inflammation, rather than the establishment of primary thyroid dysfunction. In this context, a subset of patients exhibited a hormonal profile characterized by low FT3 with normal or slightly reduced TSH, consistent with non-thyroidal illness syndrome (NTIS) or a prolonged “NTIS-like” pattern, a phenomenon previously described both in severe infections and in COVID-19 [
22,
28,
33,
39]. Mechanistically, this pattern is associated with impaired peripheral conversion of T4 to T3 and alterations in central regulation of the HPT axis under inflammatory conditions [
38].
As follow-up progressed, most patients demonstrated a clear trend toward normalization of hormone levels, with gradual stabilization of TSH and recovery of FT3 and FT4 toward baseline values. This evolution supports the hypothesis that post-COVID thyroid hormone abnormalities are, in most cases, transient and reversible, integrating into a broader process of systemic recovery after viral infection [
12,
33,
37,
39]. Nevertheless, the persistence of interindividual variability up to 12 months suggests that thyroid functional dynamics are influenced by multiple factors, including age, intensity of the inflammatory response, immune status, and individual susceptibility, factors also recognized in the general epidemiology of thyroid disorders [
40,
41].
From a pathophysiological perspective, our data allows the differentiation of at least three distinct patterns of post-COVID thyroid dysfunction. The first corresponds to NTIS-like changes, characterized by transient reductions in FT3 with spontaneous recovery and absence of thyroid autoimmunity or structural abnormalities [
28,
34,
39]. The second pattern reflects transient inflammatory thyroid dysfunction, in which fluctuations in TSH and peripheral hormones are temporally associated with persistent inflammatory markers and resolve as systemic inflammation subsides, as reported in several post-COVID cohorts [
35,
42]. The third and less frequent pattern is suggestive of incipient thyroid autoimmunity, characterized by the appearance or persistence of thyroid autoantibodies and TSH variations that may precede the development of subclinical or overt thyroid dysfunction [
36,
40,
41].
This conceptual stratification has important clinical implications for the interpretation of thyroid function tests in the post-COVID setting. While most patients exhibit mild and self-limited hormonal deviations, a small subset may progress toward persistent thyroid dysfunction, particularly in the context of emerging autoimmunity. Therefore, the longitudinal dynamics of TSH, FT4, and FT3 should be interpreted within an integrative framework that considers temporal evolution, inflammatory context, and immunological status, in order to avoid both overdiagnosis of transient abnormalities and delayed identification of patients at risk for unfavorable endocrine outcomes.
4.5. Systemic Inflammation, Coagulation Markers, and Thyroid Involvement
Although SARS-CoV-2 infection is characterized by a complex systemic inflammatory response, often accompanied by activation of coagulation pathways, the relationship between systemic inflammation and delayed thyroid involvement remains incompletely understood. In our cohort, correlations between classical inflammatory markers (CRP, ESR, fibrinogen, IL-6) and thyroid function parameters were generally weak or absent, despite the presence of a coherent systemic inflammatory profile at baseline. This observation suggests that, in patients with predominantly mild or moderate COVID-19, the mechanisms underlying post-infectious thyroid dysfunction may be at least partially decoupled from the magnitude of acute systemic inflammation [
28,
33,
39].
One possible explanatory model is the existence of a temporal and mechanistic “disconnect” between systemic inflammation and delayed thyroid involvement. While circulating inflammatory markers primarily reflect acute or subacute immune activation, thyroid abnormalities, particularly autoimmune or subclinical forms, may emerge later as a result of persistent, low-grade immunological processes that are no longer captured by standard inflammatory biomarkers. This hypothesis is supported by the observation that a proportion of patients with thyroid abnormalities exhibited relatively normal inflammatory marker levels during follow-up, suggesting that thyroid involvement is not necessarily proportional to the severity of the initial systemic inflammatory response [
37,
39].
Nevertheless, our data points toward a subtle association between thyroid dysfunction and markers of coagulation activation, particularly persistently elevated D-dimer levels at 12 months. Although these associations are modest and exploratory, they raise the hypothesis that persistent microinflammation and post-COVID endothelial dysfunction may indirectly contribute to instability of thyroid regulation. SARS-CoV-2 is known to induce a prothrombotic state and diffuse microvascular injury, and the thyroid gland, owing to its rich vascular supply, may be particularly vulnerable to such subtle mechanisms, even in the absence of overt or intense systemic inflammation [
10,
39,
42].
Compared with studies reporting stronger associations between inflammatory markers and thyroid dysfunction, it is important to note that those investigations generally included patients with severe or critical COVID-19. In such cohorts, elevated IL-6, CRP, and ferritin levels were frequently correlated with thyrotoxicosis, non-thyroidal illness syndrome (NTIS), or marked TSH suppression, reflecting the direct impact of the cytokine storm on the hypothalamic–pituitary–thyroid axis [
1,
2,
28]. In contrast, the population studied here consisted predominantly of patients with non-severe disease, in whom systemic inflammation was milder and more transient, which may explain the lack of robust correlations between inflammatory markers and thyroid parameters.
Overall, our findings suggest that, in non-severe COVID-19, post-infectious thyroid dysfunction is more likely driven by sustained local immunological and tissue-level mechanisms rather than by the magnitude of acute systemic inflammation. This perspective supports the notion that classical inflammatory markers alone are insufficient to identify patients at risk for delayed thyroid involvement and underscores the need for dedicated endocrine evaluation during convalescence, even in the absence of overt biochemical evidence of persistent inflammation [
39,
42].
4.6. Acute COVID-19 Severity and Corticosteroid Exposure in Relation to Post-COVID Thyroid Involvement
In our cohort, post-COVID thyroid involvement was frequently observed in patients who experienced mild or moderate forms of acute infection, supporting the concept that thyroid dysfunction following SARS-CoV-2 infection is not exclusively a complication of severe disease. This finding is consistent with the growing body of literature describing a broad spectrum of thyroid disturbances in COVID-19, ranging from transient NTIS-like alterations to subacute thyroiditis and autoimmune activation, including in patients without critical illness [
11,
13,
28,
39]. Furthermore, within the context of post-COVID condition (long COVID), persistent symptoms may coexist with subtle endocrine abnormalities, and the severity of the acute episode is not always a reliable predictor of medium-term sequelae [
43,
44,
45,
46].
An important consideration in interpreting our findings is the potential role of corticosteroid therapy administered during the acute phase of COVID-19. In clinical practice, systemic corticosteroids are primarily used in patients with hypoxemia or oxygen requirements, in accordance with national treatment protocols and current guidelines, with the aim of mitigating systemic inflammation and immune-mediated tissue damage [
47,
48]. In our cohort, none of the patients who subsequently developed subacute thyroiditis had received corticosteroid therapy prior to the onset of SAT, suggesting that SAT may occur independently of corticosteroid exposure during acute COVID-19 and reinforcing the hypothesis of an autonomous post-viral or post-inflammatory mechanism [
13,
49].
However, this observation does not exclude the possibility that, in patients treated with corticosteroids, the clinical expression of subacute thyroiditis may be attenuated or “masked.” Corticosteroids reduce inflammatory symptoms and acute-phase reactants, potentially diminishing the likelihood that mild SAT is clinically recognized in the immediate post-infectious period. At the same time, corticosteroid therapy can directly influence the hypothalamic–pituitary–thyroid axis and thyroid hormone metabolism, contributing to TSH suppression and/or FT3 alterations in the context of systemic inflammation, thereby complicating the interpretation of early post-COVID thyroid function tests [
11,
39,
50]. From this perspective, the absence of prior corticosteroid exposure in our SAT cases may partly explain their classic and clearly delineated clinical, biochemical, and ultrasonographic presentation, characterized by destructive thyrotoxicosis and overt inflammatory syndrome. Distinguishing between the effects of viral infection itself and those of therapies administered during the acute phase is essential in the analysis of post-COVID thyroid dysfunction. On one hand, experimental and observational data support thyroid susceptibility to SARS-CoV-2–induced perturbations via ACE2-mediated mechanisms, with potential direct or indirect effects on follicular cells and amplification of local immune responses [
8,
51]. On the other hand, systemic inflammation and cytokine-driven pathways can significantly alter thyroid axis set points and peripheral hormone conversion, generating transient patterns that do not necessarily reflect primary thyroid disease [
11,
28,
51]. Consequently, inter-study differences in the reported frequency and patterns of thyroid dysfunction may be influenced not only by disease severity but also by therapeutic strategies employed during the acute phase [
13,
28,
39].
Overall, our data suggest that neither clinical severity of COVID-19 nor exposure to corticosteroid therapy is a prerequisite for the development of post-infectious thyroid involvement. The identification of SAT and autoimmune phenomena in patients with non-severe disease, and the occurrence of all SAT cases in the absence of prior corticosteroid therapy, support a predominant role for post-viral and immuno-inflammatory mechanisms in the pathogenesis of thyroid involvement after SARS-CoV-2 infection. From a practical standpoint, these findings reinforce the rationale for targeted endocrine monitoring even in patients with mild or moderate COVID-19, particularly in the presence of persistent symptoms or early hormonal abnormalities, regardless of acute-phase treatment [
11,
39,
45,
46].
4.7. Thyroid Ultrasound Findings and Subclinical Structural Changes
A distinctive feature of our study design, with direct clinical relevance, is the systematic longitudinal thyroid ultrasound assessment performed at baseline, 6 months, and 12 months after SARS-CoV-2 infection. In the context of COVID-19, thyroid ultrasound represents a sensitive tool for detecting subtle inflammatory or structural changes that may remain undetected when evaluation relies exclusively on serum hormonal parameters. Recent literature highlights the heterogeneous nature of post-COVID thyroid involvement and emphasizes the existence of predominantly structural phenotypes, in which ultrasonographic abnormalities may occur in the absence of overt hormonal dysfunction [
11,
13,
39]. In this regard, ultrasound complements standard endocrine evaluation, particularly during the post-acute phase, which is often characterized by nonspecific symptoms and transient biochemical fluctuations [
43,
44,
45,
46].
At baseline, a relevant proportion of patients exhibited a normal thyroid ultrasound, and the most common findings consisted of benign lesions frequently encountered in the general population, such as simple or multiple cysts and non-suspicious thyroid nodules. However, longitudinal ultrasound analysis revealed a progressive shift in this profile, characterized by a decline in the number of normal examinations at 6 and 12 months and an increase in the proportion of patients with features suggestive of autoimmune thyroiditis. This evolution supports the hypothesis that, in a subset of patients, post-COVID thyroid involvement is not present during the acute or immediate post-infectious phase but develops progressively during convalescence, suggesting a delayed immuno-inflammatory mechanism.
A particularly relevant observation was the dissociation between imaging phenotype and serological markers of autoimmunity. In our cohort, diffuse hypoechoic and heterogeneous ultrasound patterns were identified even in patients with normal or only minimally elevated thyroid autoantibody titers. This finding suggests that, in the post-COVID setting, thyroid inflammation or tissue remodeling may evolve subclinically and may precede the development of serologically detectable autoimmunity. Conceptually, these data align with the continuum model of thyroid autoimmunity, in which inflammatory infiltration and structural changes can appear months or even years before the onset of hormonal dysfunction or significant autoantibody elevation [
36,
40,
52].
Thyroid ultrasound also proved valuable in differentiating etiopathogenic mechanisms of post-COVID thyroid dysfunction. In cases of subacute thyroiditis, the characteristic ultrasound appearance, poorly defined hypoechoic areas with reduced vascularity, supported the diagnosis and allowed distinction from other causes of thyrotoxicosis, such as Graves’ disease or drug-induced thyrotoxicosis [
13,
49,
53]. Complete resolution of these ultrasound abnormalities at 12 months confirmed the transient nature of the destructive inflammatory process and was concordant with the favorable clinical course observed in our cohort.
From a clinical standpoint, these findings have important implications for post-COVID monitoring strategies. An evaluation based solely on TSH, FT4, and FT3 risks underestimating the true prevalence of thyroid involvement, particularly in subclinical forms with predominantly structural expression. Given the frequency of nonspecific post-COVID symptoms, such as fatigue, palpitations, or exercise intolerance, and the absence of persistent hormonal abnormalities in some patients, thyroid ultrasound can contribute to a more accurate characterization of post-infectious endocrine involvement and support individualized follow-up strategies, especially in prospective studies and post-COVID care pathways [
43,
44,
45,
46].
At the same time, the stability over time of cystic and nodular thyroid lesion prevalence observed in our cohort supports the notion that these findings represent benign, incidental changes commonly encountered in the general population and do not appear to be significantly influenced by SARS-CoV-2 infection. Epidemiological data indicate that simple cysts and non-suspicious thyroid nodules are highly prevalent on population-based ultrasound screening, particularly among adults and women, and typically exhibit slow and stable evolution [
36,
40]. The absence of increased prevalence or ultrasound features suggestive of inflammatory activation or structural transformation during follow-up suggests that these findings likely reflect background imaging “noise” rather than a direct consequence of viral infection [
11,
39]. This distinction is essential to avoid overattribution of pre-existing benign lesions to SARS-CoV-2 effects.
In contrast, longitudinal ultrasound analysis demonstrated the progressive emergence of diffuse structural changes suggestive of autoimmune thyroiditis in a subset of patients who were initially euthyroid, autoantibody-negative, and had normal baseline ultrasound examinations. This observation suggests that post-COVID thyroid involvement may, in some cases, have a predominantly tissue-level and subclinical expression that precedes or evolves independently of classical biochemical or immunological alterations.
Thus, the inclusion of longitudinal ultrasound assessment in our protocol demonstrates that post-COVID thyroid involvement is not exclusively a biochemical phenomenon but may include a structural component detectable by imaging and evolving over time. By enabling early identification of tissue remodeling and increasing diagnostic sensitivity for emerging inflammatory or autoimmune phenotypes, thyroid ultrasound adds value to standard endocrine evaluation and contributes to a more comprehensive characterization of the spectrum of thyroid involvement associated with SARS-CoV-2 infection [
8,
11,
13,
39].
4.8. COVID-19 Vaccination and Thyroid Outcomes
In the context of ongoing discussions regarding the safety of anti–SARS-CoV-2 vaccination, assessing the relationship between vaccination status and thyroid involvement has important clinical and public health implications. In our prospective cohort, we found no significant association between COVID-19 vaccination and subsequent thyroid dysfunction, whether functional, autoimmune, or structural. The incidence of hormonal abnormalities, thyroid autoantibodies, and ultrasound findings was comparable between vaccinated and unvaccinated participants, suggesting that vaccination was not a triggering or aggravating factor for post-COVID thyroid involvement in this population.
This finding is relevant for clarifying the conceptual and biological distinction between the effects of SARS-CoV-2 infection and those of immunization. Whereas viral infection is characterized by a complex systemic inflammatory response, polyclonal immune activation, and, in some cases, persistent low-grade inflammation, COVID-19 vaccines induce a controlled, transient, antigen-specific immune response. The available literature supports this distinction, indicating that most thyroid disorders reported after vaccination are rare and sporadic, largely described as case reports or small case series, without robust evidence of causality or an increased population-level risk [
11,
13,
39].
Moreover, observational studies and pharmacovigilance analyses suggest that the incidence of subacute thyroiditis and other thyroid abnormalities following vaccination is substantially lower than that reported after SARS-CoV-2 infection and does not exceed the background prevalence in the general population [
39,
49,
55]. This contrast reinforces the view that the virus—rather than vaccination—represents the main factor implicated in post-COVID thyroid involvement through direct and indirect pathways, including systemic inflammation, endothelial dysfunction, and non-specific immune activation [
13,
56].
The absence of an association between vaccination and thyroid dysfunction in our study is also consistent with endocrine society recommendations and expert statements indicating that COVID-19 vaccination is safe in patients with pre-existing thyroid disease and does not require special endocrine monitoring in the absence of suggestive symptoms [
11,
55]. Clinically, these data are important to avoid misattributing delayed thyroid abnormalities to vaccination, particularly in individuals with prior SARS-CoV-2 infection, where temporal relationships may be misleading.
Overall, our results support a clear and coherent public health message: anti–COVID-19 vaccination is not associated with an increased risk of thyroid dysfunction and should not be considered a limiting factor or require additional precaution in post-COVID follow-up. Instead, SARS-CoV-2 infection remains the principal determinant of the thyroid involvement observed during the post-acute period. This distinction is essential both for clinical practice and for addressing vaccine hesitancy, providing prospective evidence that the benefits of vaccination far outweigh potential endocrine risks, which appear minimal and rare [
11,
13,
39].
4.9. Clinical Implications and Follow-Up Recommendations
The present findings have direct clinical implications for the management of patients during convalescence after SARS-CoV-2 infection and support the need for a risk-adapted approach to post-COVID thyroid monitoring. Although most abnormalities identified in our cohort were mild and transient, the relatively high frequency of thyroid dysfunction and the emergence of de novo autoimmunity in a subset of patients indicate that endocrine evaluation should not be restricted solely to individuals who experienced severe acute disease.
Based on our data, thyroid function monitoring appears particularly justified in patients reporting persistent or non-specific post-COVID symptoms, such as fatigue, palpitations, heat or cold intolerance, unexplained weight loss or gain, or cardiac rhythm disturbances. In addition, older patients and those with TSH values near the upper or lower limits of the reference range during the early post-infectious period represent a higher-risk subgroup for developing persistent subclinical thyroid dysfunction and may benefit from closer follow-up.
Importantly, our findings highlight the clinical relevance of an early thyroid function assessment at 4–6 weeks after acute SARS-CoV-2 infection. At this time point, the acute-phase effects of systemic illness and non-thyroidal illness syndrome are largely attenuated, allowing for the identification of patients with persistent dysregulation of the hypothalamic–pituitary–thyroid axis. In our cohort, TSH values measured at 4–6 weeks emerged as the strongest predictor of subsequent thyroid abnormalities, underscoring the prognostic value of early endocrine evaluation in the post-COVID setting.
A comprehensive reassessment at approximately 3 months post-infection represents an optimal window for confirming the persistence or resolution of early abnormalities, while avoiding overdiagnosis of transient acute-phase changes and delayed recognition of clinically relevant dysfunction. Further reevaluation at 6 months is advisable in patients with abnormal or fluctuating TSH values. Thyroid autoantibody testing and ultrasound examination should be considered in cases of persistent hormonal abnormalities, suggestive imaging findings, or a personal or family history of autoimmune disease.
Thyroid ultrasound adds value beyond serological assessment, particularly in patients with subclinical dysfunction or positive autoantibodies, as it may detect diffuse structural changes compatible with inflammatory or autoimmune processes even in the absence of overt hormonal dysfunction. Incorporating ultrasound into post-COVID evaluation may improve risk stratification and facilitate differentiation between transient functional changes and thyroid conditions with potential for progression.
Taken together, our data support a stepwise, risk-adapted follow-up strategy, consisting of early functional assessment at 4–6 weeks, confirmation at 3 months, reassessment at 6 months in cases of persistent or borderline abnormalities, and extended monitoring up to 12 months in patients with documented autoimmunity, subacute thyroiditis, or marked hormonal fluctuations. Such an approach enables early identification of patients at risk for persistent thyroid dysfunction while avoiding unnecessary investigations in those with fully reversible trajectories.
In conclusion, post-COVID thyroid monitoring should be guided by the patient’s clinical, biochemical, and immunological profile rather than applied universally. Integrating functional testing, autoimmune markers, and—when indicated—thyroid ultrasound may optimize post-infectious endocrine care and help prevent medium- and long-term complications associated with post-COVID thyroid dysfunction.
4.10. Strengths and Limitations
The present study has several strengths that enhance its relevance and robustness within the current literature on post-COVID-19 thyroid involvement. A major strength is its prospective design, which allowed for systematic and standardized follow-up of patients over a 12-month period after SARS-CoV-2 infection. The well-structured protocol, with predefined assessments at successive time points (4-6 weeks, 3 months, 6 months, and 12 months), enabled accurate capture of the temporal dynamics of thyroid function and reduced the risk of selection bias, recall bias, and missing data, common limitations of retrospective studies.
The extended duration of follow-up represents another important strength, providing a longitudinal perspective that is still relatively scarce in post-COVID research. This allowed differentiation between transient post-viral hormonal alterations and persistent or progressive dysfunction, as well as identification of emerging phenomena such as de novo thyroid autoimmunity or subclinical progression toward hypothyroidism.
A distinctive feature of this study is the multimodal assessment of thyroid involvement, integrating hormonal parameters (TSH, FT4, FT3), immunological markers (anti-TPO and anti-thyroglobulin antibodies), and systematic thyroid ultrasound examination, including Doppler evaluation. This integrated approach enabled detection of subtle or subclinical thyroid phenotypes that might have remained undetected without imaging or immunological assessment and contributed to a more refined characterization of the spectrum of post-COVID thyroid dysfunction. Furthermore, the systematic inclusion of inflammatory, metabolic, and coagulation markers (CRP, ESR, fibrinogen, IL-6, D-dimer, ferritin, LDH, and transaminases) provided additional pathophysiological context for interpreting endocrine changes and allowed exploratory analysis of the relationship between persistent systemic inflammation and thyroid instability. The single-center design, with clearly defined inclusion criteria and a uniform diagnostic and monitoring strategy, minimized inter-institutional variability and enhanced internal consistency.
Nevertheless, the results should be interpreted considering several limitations. The relatively small sample size limits statistical power, particularly for exploratory analyses and for identifying robust associations between inflammatory markers and thyroid dysfunction and precluded the construction of complex multivariable models controlling for multiple confounders simultaneously. In addition, the single-center nature of the study may limit the generalizability of the findings to other populations or healthcare settings with different patient profiles and clinical practices. Another limitation is the absence of a non-COVID control group, which restricts the ability to definitively attribute all observed changes exclusively to SARS-CoV-2 infection. However, the increased prevalence of thyroid dysfunction and de novo autoimmunity compared with epidemiological data from the general population supports the clinical relevance of the findings. The potential influence of uncontrolled factors, such as pandemic-related stress, seasonal variation in thyroid function, or concomitant treatments during the acute phase, cannot be entirely excluded.
Despite these limitations, the prospective design, long-term follow-up, and detailed, integrated endocrine evaluation supports the robustness of the conclusions and position this study as a meaningful contribution to understanding the medium-term endocrine impact of SARS-CoV-2 infection.
4.11. Future Directions
The findings of this study highlight the need for further research to fully elucidate the medium- and long-term impact of SARS-CoV-2 infection on thyroid function and structure. First, large, multicenter studies are required to allow external validation of our findings and to improve generalizability across populations with different demographic, genetic, and clinical characteristics.
Second, the integration of advanced immunological analyses could substantially enhance understanding of the pathophysiological mechanisms underlying post-COVID thyroid dysfunction. Detailed assessment of cytokine profiles, lymphocyte subpopulations, and markers of immune activation may help distinguish transient post-viral inflammation from incipient thyroid autoimmunity and persistent immune dysregulation.
Another important area for future investigation is the impact of SARS-CoV-2 reinfections and repeated viral antigen exposure on the hypothalamic–pituitary–thyroid axis. It remains unclear whether reinfections amplify the risk of thyroid dysfunction, accelerate latent autoimmunity, or affect long-term thyroid stability in patients with pre-existing abnormalities.
Finally, extending follow-up beyond 24 months is essential to determine whether persistent thyroid autoantibodies identified after COVID-19 are associated with an increased risk of overt hypothyroidism or chronic thyroid disease. Such long-term studies could inform personalized monitoring strategies and contribute to the development of evidence-based clinical recommendations for endocrine management in post-COVID patients.