Submitted:
15 December 2025
Posted:
16 December 2025
You are already at the latest version
Abstract
Keywords:
Introduction
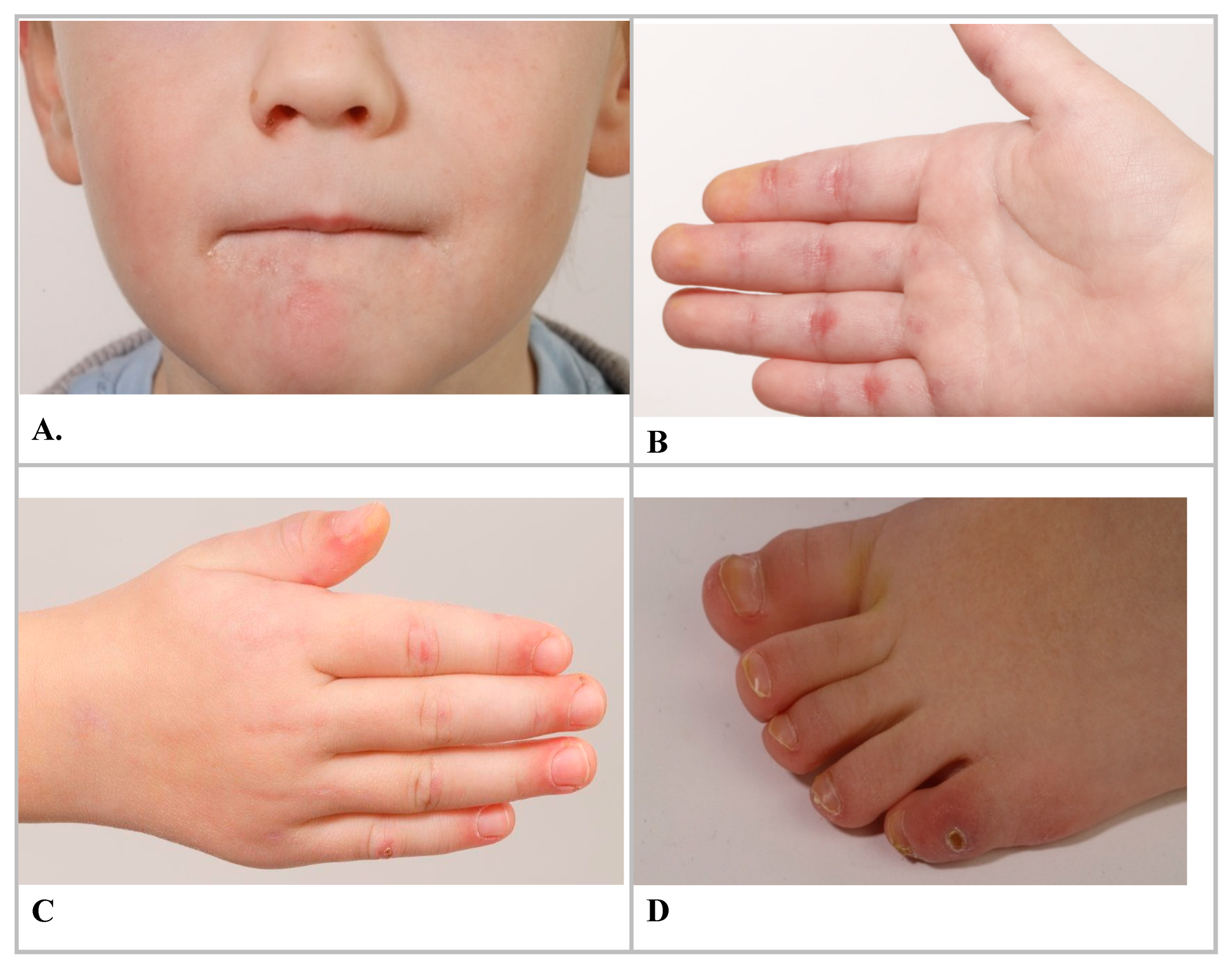
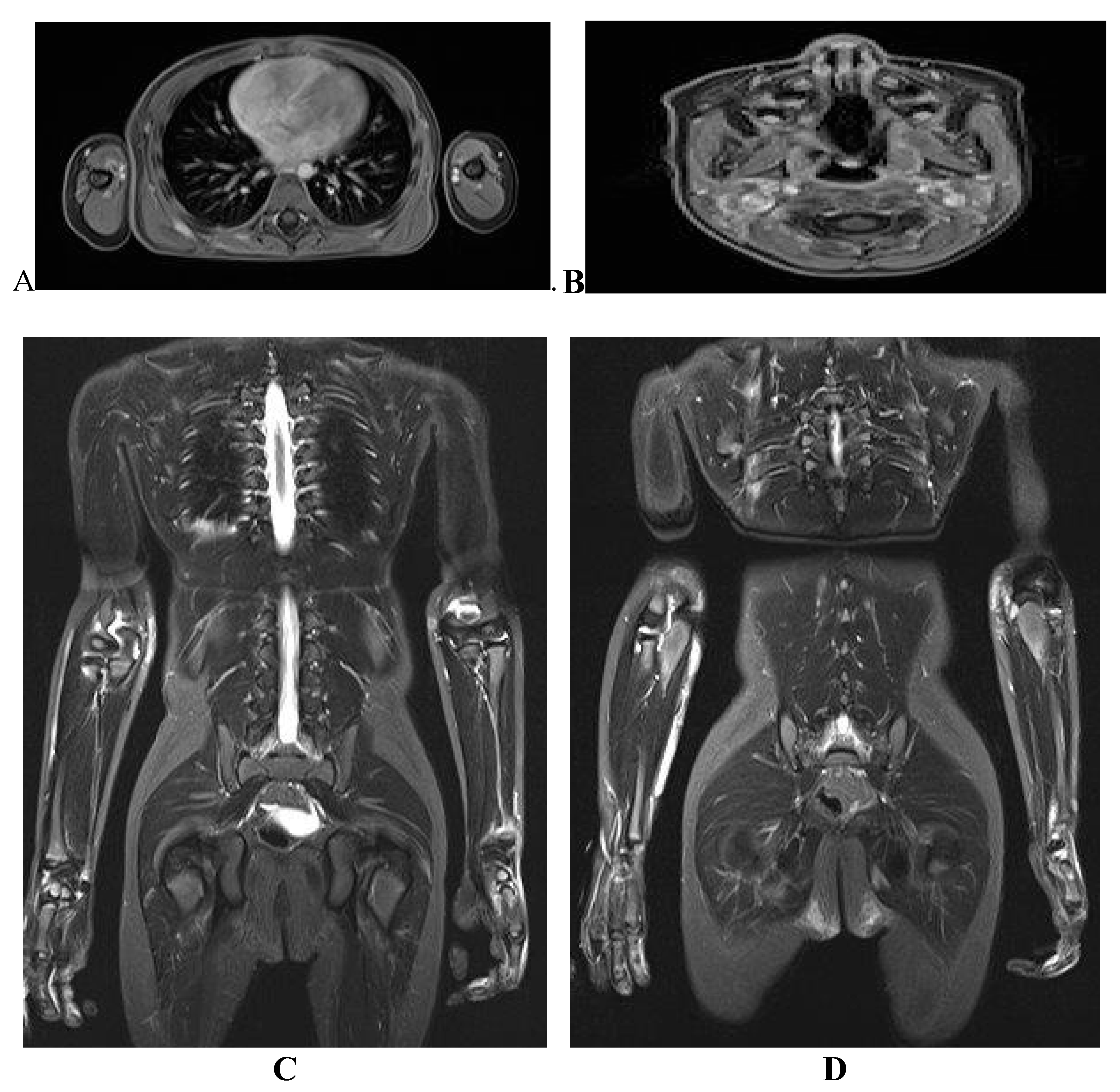
Case Report
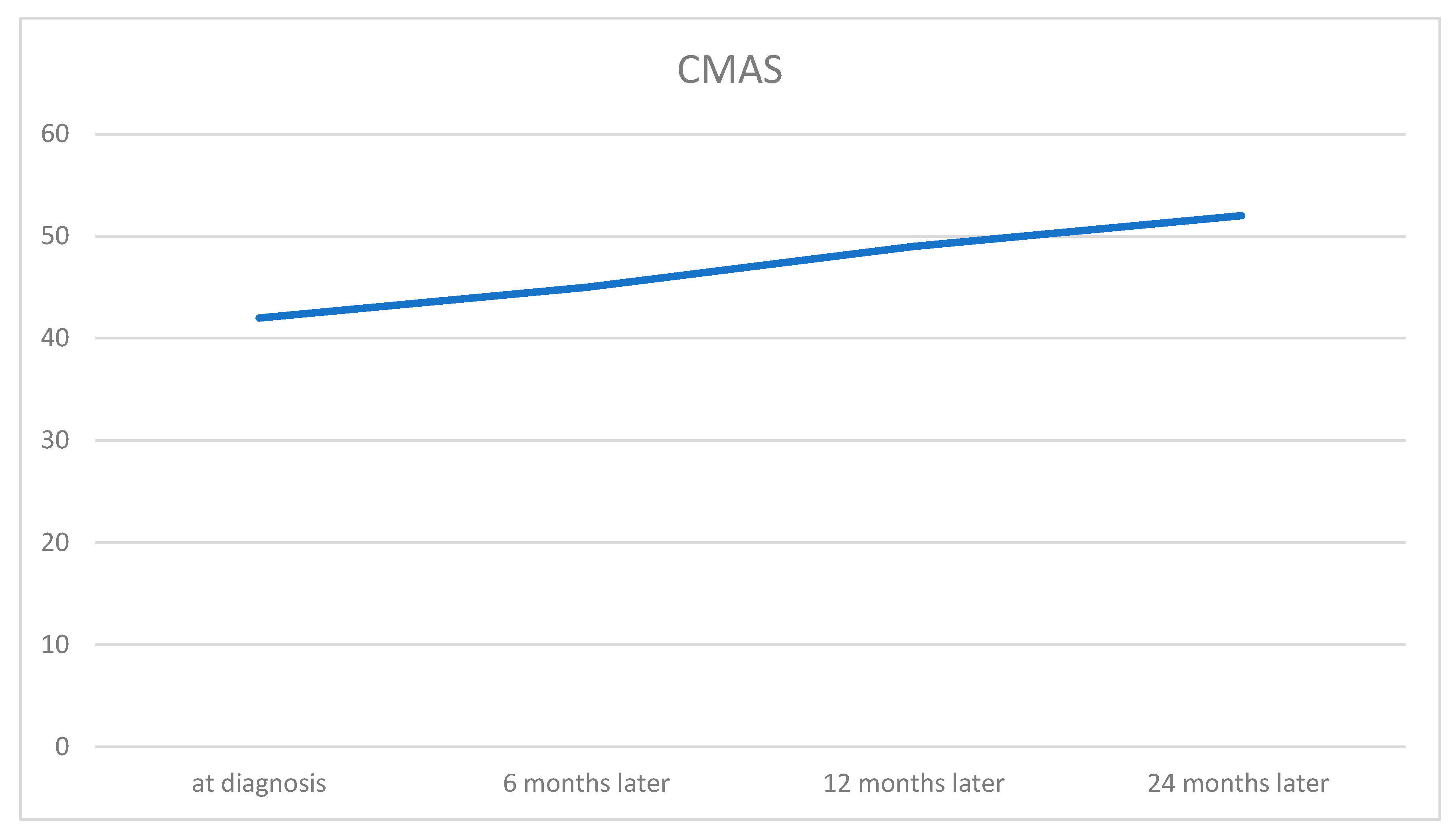
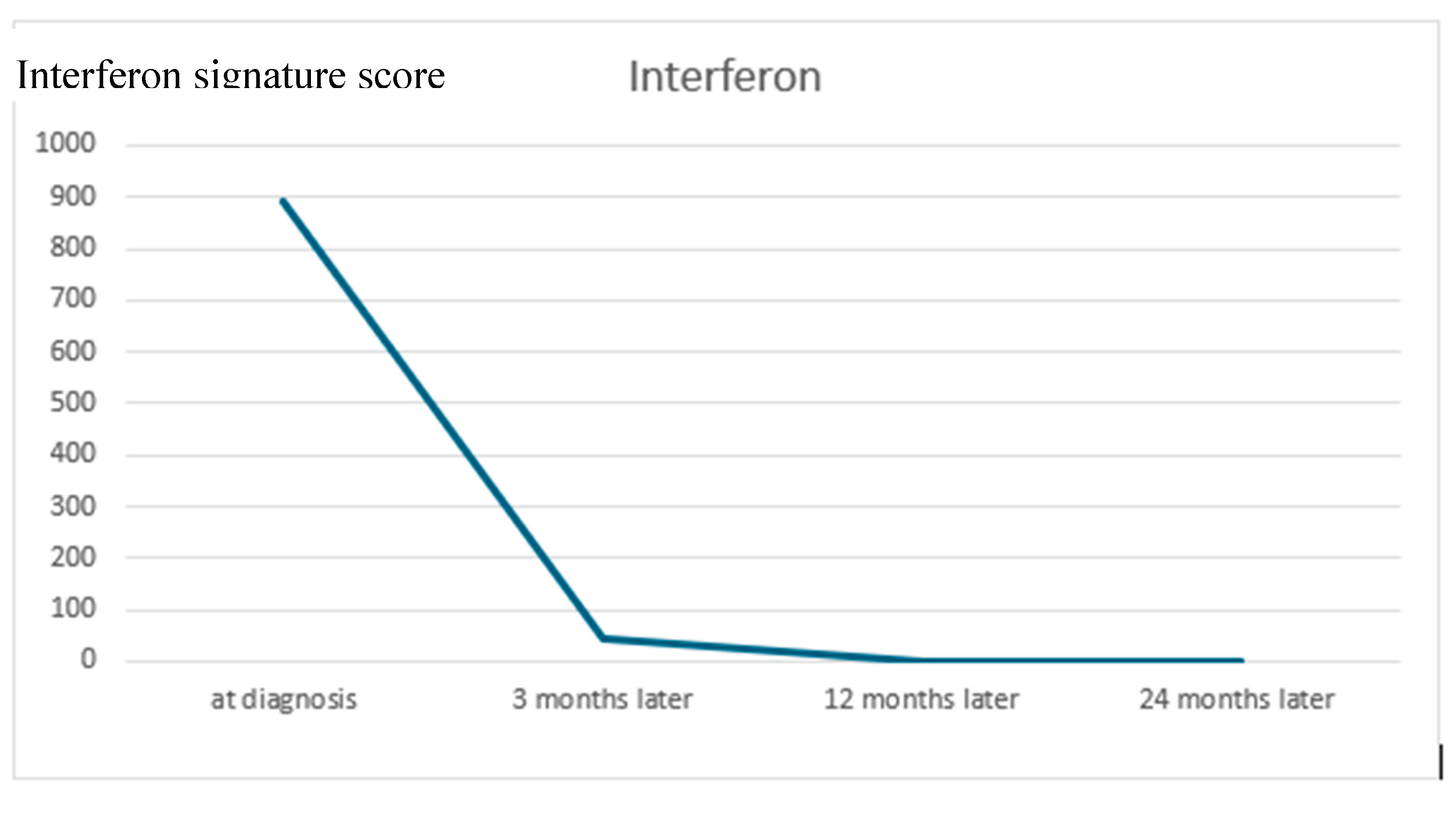
Discussion
Conclusion
Funding
Authors' contributions
Institutional Review Board approval
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marie, I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012, 14(3), 275–85. [Google Scholar] [CrossRef]
- Cassidy JT, P.R.; Laxer, RM; Lindsley, CB. Textbook of pediatric rheumatology E-Book; 2010. [Google Scholar]
- Baechler, E.C.; et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007, 13(1-2), 59–68. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019, 93(12), e1193–e1204. [Google Scholar] [CrossRef]
- Wallwork, R.S.; Paik, J.J.; Kim, H. Current evidence for janus kinase inhibitors in adult and juvenile dermatomyositis and key comparisons. Expert Opin Pharmacother 2024, 25(12), 1625–1645. [Google Scholar] [CrossRef] [PubMed]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Mammen, A.L. Inhibiting interferon pathways in dermatomyositis: rationale and preliminary evidence. Curr Treatm Opt Rheumatol 2021, 7(3), 258–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; et al. Risk factors for mortality in anti-MDA5 antibody-positive dermatomyositis with interstitial lung disease: a systematic review and meta-analysis. Front Immunol 2025, 16, 1628748. [Google Scholar] [CrossRef]
- Li, X.; et al. Roles of biomarkers in anti-MDA5-positive dermatomyositis, associated interstitial lung disease, and rapidly progressive interstitial lung disease. J Clin Lab Anal 2022, 36(11), p. e24726. [Google Scholar] [CrossRef]
- Huber, A.M.; et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children's Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res (Hoboken) 2010, 62(2), 219–25. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, F.A.; et al. A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain 2018, 141(11), p. e80. [Google Scholar] [CrossRef]
- Hinze, C.H.; et al. Development of practice and consensus-based strategies including a treat-to-target approach for the management of moderate and severe juvenile dermatomyositis in Germany and Austria. Pediatr Rheumatol Online J 2018, 16(1), p. 40. [Google Scholar] [CrossRef]
- Strauss, T.; et al. Rapid and sustained response to JAK inhibition in a child with severe MDA5 + juvenile dermatomyositis. Pediatr Rheumatol Online J 2023, 21(1), p. 104. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C. Juvenile dermatomyositis-what's new? Z Rheumatol 2019, 78(7), 627–635. [Google Scholar] [CrossRef]
- Sherman, M.A.; et al. Approach to Janus kinase inhibition for juvenile dermatomyositis among CARRA and PReS providers. Rheumatology (Oxford) 2025, 64(8), 4732–4737. [Google Scholar] [CrossRef]
- Ladislau, L.; et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 2018, 141(6), 1609–1621. [Google Scholar] [CrossRef]
- Huard, C.; et al. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon beta in dermatomyositis. Br J Dermatol 2017, 176(5), 1224–1230. [Google Scholar] [CrossRef]
- Veldkamp, S.R.; et al. Targeting interferon responses in juvenile dermatomyositis: Siglec-1 as an in vitro biomarker for JAK inhibitor efficacy. Rheumatology (Oxford) 2025, 64(9), 5132–5141. [Google Scholar] [CrossRef] [PubMed]
- Heinen, A.; et al. Interferon signature guiding therapeutic decision making: ruxolitinib as first-line therapy for severe juvenile dermatomyositis? Rheumatology (Oxford) 2021, 60(4), e136–e138. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; et al. Long-term follow-up of Janus-kinase inhibitor and novel active disease biomarker in juvenile dermatomyositis. Rheumatology (Oxford) 2023, 62(3), 1227–1237. [Google Scholar] [CrossRef]
- Ding, Y.; et al. Janus kinase inhibitor significantly improved rash and muscle strength in juvenile dermatomyositis. Ann Rheum Dis 2021, 80(4), 543–545. [Google Scholar] [CrossRef] [PubMed]
- Chan Ng, P.L.P.; et al. Janus kinase inhibition in induction treatment of anti-MDA5 juvenile dermatomyositis-associated rapidly progressive interstitial lung disease. Int J Rheum Dis 2022, 25(2), 228–231. [Google Scholar] [CrossRef]
- Kaplan, M.M.; et al. Cardiac involvement in a case of juvenile dermatomyositis with positive anti-melanoma differentiation associated protein 5 antibody. Int J Rheum Dis 2023, 26(8), 1582–1585. [Google Scholar] [CrossRef]
- Agud-Dios, M.; et al. Juvenile dermatomyositis-associated calcinosis successfully treated with combined immunosuppressive, bisphosphonate, oral baricitinib and physical therapy. Dermatol Ther 2022, 35(12), p. e15960. [Google Scholar] [CrossRef]
- Mastrolia, M.V.; et al. Efficacy of Janus kinase inhibitor baricitinib in the treatment of refractory juvenile dermatomyositis complicated by calcinosis. Clin Exp Rheumatol 2023, 41(2), 402–403. [Google Scholar] [CrossRef] [PubMed]
- Kostik, M.M.; et al. The Safety and Efficacy of Tofacitinib in 24 Cases of Pediatric Rheumatic Diseases: Single Centre Experience. Front Pediatr 2022, 10, 820586. [Google Scholar] [CrossRef] [PubMed]
- Le Voyer, T.; et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: a monocentric retrospective study. Rheumatology (Oxford) 2021, 60(12), 5801–5808. [Google Scholar] [CrossRef]
- Quintana-Ortega, C.; et al. Colchicine as rescue treatment in two pediatric patients with chronic recurrent multifocal osteomyelitis (CRMO). Mod Rheumatol Case Rep 2023, 7(1), 215–218. [Google Scholar] [CrossRef]
- Bellutti Enders, F.; et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis 2017, 76(2), 329–340. [Google Scholar] [CrossRef]
- Papadopoulou, C.; et al. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 2019, 142(3), p. e8. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.; et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain 2019, 142(11), p. e59. [Google Scholar] [CrossRef]
- Melki, I.; et al. Anti-MDA5 juvenile idiopathic inflammatory myopathy: a specific subgroup defined by differentially enhanced interferon-alpha signalling. Rheumatology (Oxford) 2020, 59(8), 1927–1937. [Google Scholar] [CrossRef]
- Sozeri, B.; Demir, F. A striking treatment option for recalcitrant calcinosis in juvenile dermatomyositis: tofacitinib citrate. Rheumatology (Oxford) 2020, 59(12), e140–e141. [Google Scholar] [CrossRef]
- Kim, H.; et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis 2021, 80(3), 406–408. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; et al. Progressive, refractory macrophage activation syndrome as the initial presentation of anti-MDA5 antibody positive juvenile dermatomyositis: a case report and literature review. Pediatr Rheumatol Online J 2022, 20(1), p. 16. [Google Scholar] [CrossRef]
- Wang, Y.T.; et al. Anti-nuclear matrix protein 2+ juvenile dermatomyositis with severe skin ulcer and infection: A case report and literature review. World J Clin Cases 2022, 10(11), 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; et al. Efficiency of tofacitinib in refractory interstitial lung disease among anti-MDA5 positive juvenile dermatomyositis patients. Ann Rheum Dis 2023, 82(11), 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; et al. Janus kinase inhibitor, tofacitinib, in refractory juvenile dermatomyositis: a retrospective multi-central study in China. Arthritis Res Ther 2023, 25(1), 204. [Google Scholar] [CrossRef]
- Yu, Z.; et al. Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: a pilot study and literature review. Rheumatology (Oxford) 2021, 60(4), 1700–1707. [Google Scholar] [CrossRef]
- Xiangyuan, C.; et al. Juvenile dermatomyositis complications: navigating gastrointestinal perforations and treatment challenges, a case report. Front Pediatr 2024, 12, 1419355. [Google Scholar] [CrossRef] [PubMed]
- Kinkor, M.; et al. 14-month-old female with anti-MDA5 juvenile dermatomyositis complicated by liver disease: a case report. Pediatr Rheumatol Online J 2024, 22(1), p. 86. [Google Scholar] [CrossRef]
- Huang, B.; Huang, W.; Hao, S. Cutaneous ulceration in juvenile dermatomyositis with anti-melanoma differentiation-associated gene 5. Pediatr Dermatol 2024, 41(2), 342–343. [Google Scholar] [CrossRef]
- Bader-Meunier, B.; et al. Myositis-specific autoantibody subtypes are associated with response to Janus kinase inhibitors in patients with juvenile dermatomyositis. Rheumatology (Oxford) 2025, 64(10), 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Arguelles Balas, D.; et al. Tofacitinib monotherapy as maintenance treatment in juvenile dermatomyositis: a case report. RMD Open 2025, 11(2). [Google Scholar] [CrossRef] [PubMed]
| Study | Author(s) | Year | Patient Characteristics | Treatment | Efficacy | Safety |
|---|---|---|---|---|---|---|
| [10] | Aeschlimann et al. | 2018 | 13-year-old female, refractory JDM, anti-NXP2+ | Ruxolitinib | Sustained remission, improved muscle strength, reduced inflammation | No major adverse events |
| [29] | Papadopoulou et al. | 2019 | 11-year-old, refractory JDM | Baricitinib | Strength, skin disease improved; steroid tapering | No adverse events, relapse after stopping |
| [30] | Sabbagh et al. | 2019 |
Anti-MDA5+ JDM, refractory | Tofacitinib | Muscle, skin, lung function improved | No adverse events |
| [31] | Melki et al. | 2020 | JIIM patients with/without anti-MDA5, 4 cases | Various therapies, 4 patients Ruxolitinib | Required for remission in severe skin cases | Not specified |
| [32] | Sozeri & Demir | 2020 | 2 pediatric patients (JDM, refractory calcinosis) | Tofacitinib | Complete resolution of calcinosis in one patient, moderate improvement in the other | Not mentioned |
| [21] | Chan Ng et al. | 2021 | Pediatric JDM, rapidly progressive ILD | Tofacitinib | Remission achieved, ILD biomarkers improved | No adverse events |
| [20] | Ding et al. | 2021 | 25 JDM patients (mean age 7.2±4.0) | Tofacitinib (n=7), Ruxolitinib (n=18) | Rash resolved (96%), muscle strength improved (40%) | No major adverse events |
| [18] | Heinen et al. | 2021 | 14-year-old male, NXP2+ JDM | Ruxolitinib | Improved strength, reduced inflammation | No major adverse events |
| [33] | Kim et al. | 2021 | 4 refractory JDM cases (ages 5.8–20.7) | Baricitinib | Strength, corticosteroid tapering improved | Not specified |
| [27] | Quintana-Ortega et al. | 2022 | 11-year-old, anti-MDA5+ JDM, ILD | Tofacitinib | No response | Fatal SARS-CoV-2 complication |
| [23] | Agud-Dios et al. | 2022 | 5-year-old male, JDM, calcinosis, contractures | Baricitinib | Improved muscle strength, calcinosis, mobility | No major adverse events |
| [25] | Kostik et al. | 2022 | 2 JDM patients (6-month follow-up) | Tofacitinib | One complete, one partial response | Lymphadenitis |
| [26] | Le Voyer et al. | 2022 | 10 JDM cases (9 refractory, 1 new-onset) | Ruxolitinib (n=7), Baricitinib (n=3) | 5 achieved inactive disease, steroids reduced | Herpes zoster, skin abscesses |
| [34] | Stewart et al. | 2022 | 1 pediatric patient (JDM, MAS as initial manifestation) | IVIG, steroids, mycophenolate, anakinra, tofacitinib | Resolution of MAS, improvement in multi-organ involvement | Not mentioned |
| [12] | Strauss et al. | 2023 | 4-year-old patient with MDA5 antibody | Ruxolitinib | Fast and sustained remission | No major adverse events |
| [19] | Huang et al. | 2023 | 9 (previoulsy unreported) JDM patients | Ruxolitinib (n=6), Baricitinib (n=3) | Rash, muscle strength, and lab markers improved; 39.6% stopped steroids | Leukopenia, cough |
| [22] | Kaplan et al. | 2023 | Anti-MDA5+ JDM, ILD, cardiac involvement | Tofacitinib | Disease control with steroid tapering | Not specified |
| [24] | Mastrolia et al. | 2023 | Refractory JDM, calcinosis | Baricitinib | Disease and calcinosis improved | Not specified |
| [35] | Wang et al. | 2023 | 20 children with refractory/severe JDM | Baricitinib (n=20) + steroids + immunosuppressants | 95% improvement in skin rash, better muscle strength, reduced disease activity, 49% steroid reduction at 24 weeks | No serious side effects reported |
| [36] | Xue Y | 2023 | 9 anti-MDA5-positive children with JDM & ILD | Tofacitinib | 55.5% showed ILD improvement; 44.5% had worsened ILD; high IgG and T-cell levels correlated with poor response | Not mentioned |
| [37] | Zhang J | 2023 | A total of eighty-eight patients with JDM | Tofacitinib | Skin and muscle symptoms improved markedly. Nearly half achieved complete response, six remained on tofacitinib monotherapy. Lung disease improved in 60%, and calcinosis in 75% (complete resolution in 25%). | Only one patient had herpes zoster infection in 9 months after initiation. After drug withdrawal, herpes recovered and tofacitinib was given again. |
| [38] | Yu et al. | 2023 | 3 children with refractory JDM | Tofacitinib | Improved muscle strength, skin lesions, quality of life, steroid tapering | No severe adverse events |
| [39] | Xiangyuan C. et al. |
2024 | 12-year-old girl with JDM who developed multiple GI perforations | Tofacitinib | Leading to gradual improvement in muscle strength and reduction in inflammation | No severe adverse events |
| [40] | Kinkor M et al. | 2024 | 14-month-old female with anti-MDA5 | Tofacitinib | Remission of skin and muscle disease | Not mentioned |
| [41] | Huang B et al. | 2024 | 11-year-old girl with juvenile dermatomyositis (JDM), anti-MDA5 antibodies and multiple skin ulcers | Tofacitinib | At the 2-month follow-up visit, early healing of the ulcers Within 8 months, the skin ulcers hadhealed, and muscle enzyme markers and ESR returned to normal. |
Not mentioned |
| [42] | Bader-Meunier B et al. | 2025 | Thirty-nine patients with JDM | Various therapies | A significant decrease in the median Type 1 IFN score and serum IFN-α from the diagnosis of JDM to the 6-month follow-up | JAKi-related adverse events consisted of infections in nine patients (including five herpes zoster infections) and weight gain in three patients. |
| [43] | Arguelles Balas D et al. |
2025 |
9-year-old Spanish boy | Tofacitinib | Remission with tofacitinib monotherapy following the failure of previous therapies | No AEs related to tofacitinib have been observed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





