Submitted:
14 December 2025
Posted:
15 December 2025
You are already at the latest version
Abstract
Keywords:
Introduction
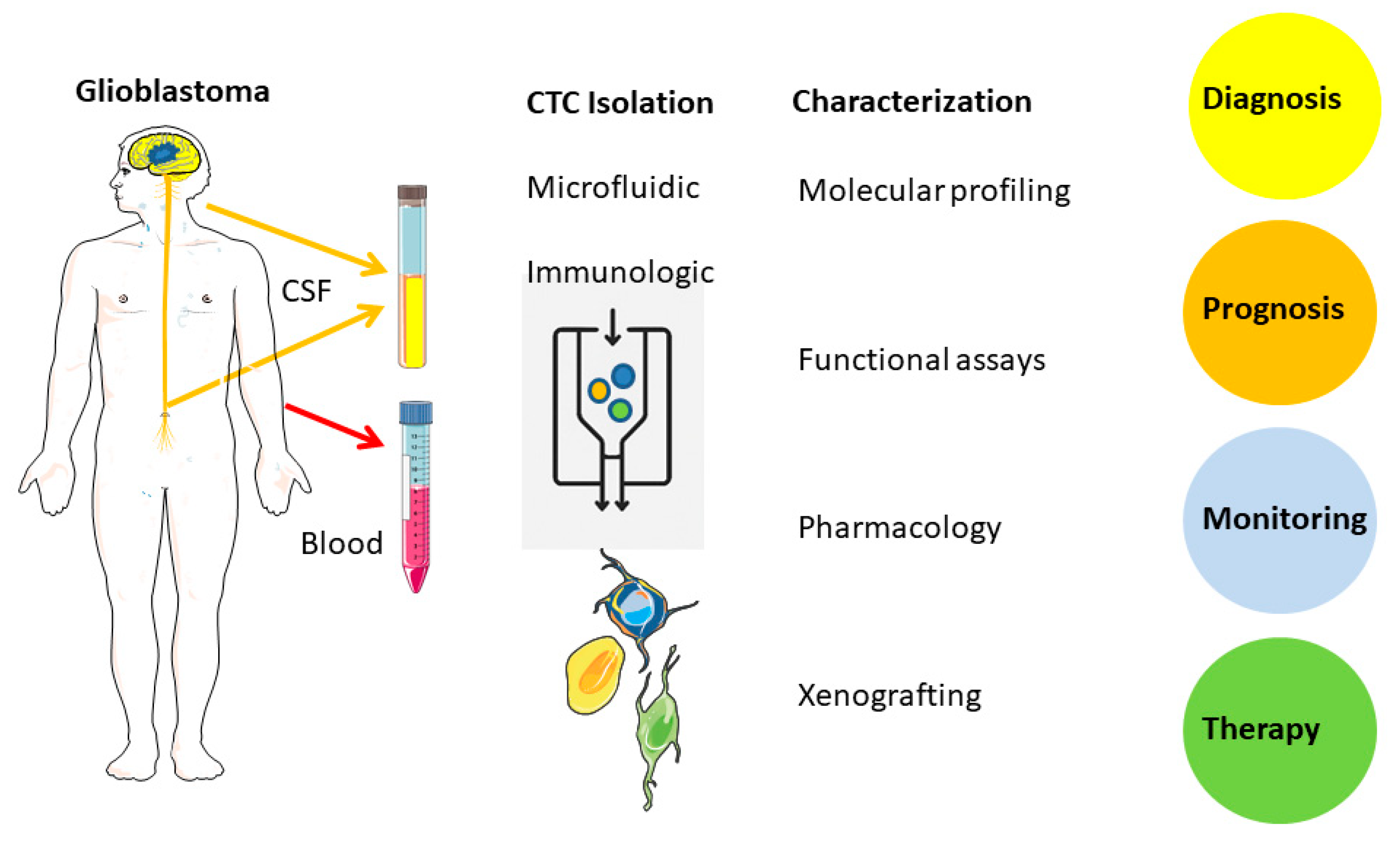
Circulating Tumor Cells (CTCs)
Comparative Perspective on Liquid Biopsy Modalities in Glioblastoma
Isolation, Enrichment, Characterization
Limitations and Current Barriers of CTC-Based Liquid Biopsy in Glioblastoma
Glioblastoma vs. Extracranial Tumors
Extracranial Tumors
Glioblastoma
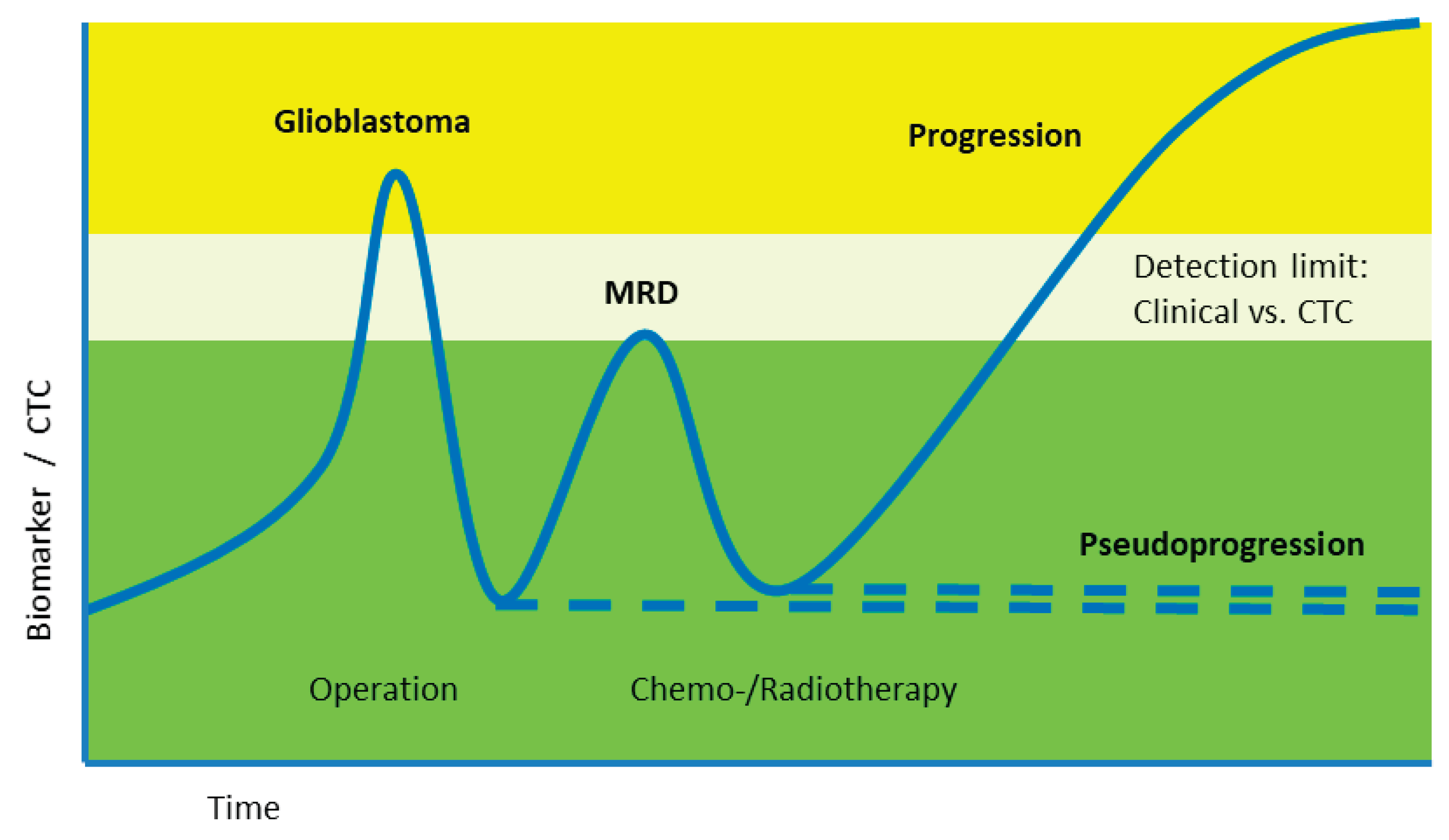
Clinical Studies
Challenges
Conclusions
Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Ohgaki, H.; Kleihues, P. Genetic Pathways to Primary and Secondary Glioblastoma. The American Journal of Pathology 2007, 170, 1445-1453. [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1-iii105. [CrossRef]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Rothenberg, S.M.; Sequist, L.V.; et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discovery 2014, 4, 1299-1309. [CrossRef]
- Bailey, P.; Cushing, H. A classification of the tumours of the glioma group on a histogenetic basis, with a correlated study of prognosis; J. B. Lippincott Company: Philadelphia, London and Montreal, 1926; p. 175.
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology 2021, 23, 1231-1251. [CrossRef]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 2015, 129, 829-848. [CrossRef]
- Müller, C.; Holtschmidt, J.; Auer, M.; Heitzer, E.; Lamszus, K.; Schulte, A.; Matschke, J.; Langer-Freitag, S.; Gasch, C.; Stoupiec, M.; et al. Hematogenous dissemination of glioblastoma multiforme. Science Translational Medicine 2014, 6, 247ra101-247ra101. [CrossRef]
- Perryman, L.; Erler, J.T. Brain cancer spreads. Science Translational Medicine 2014, 6, 247fs228. [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bauerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013, 31, 539-544. [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid biopsy for monitoring medulloblastoma. Extracell Vesicles Circ Nucleic Acids 2022, 3, 263-274. [CrossRef]
- Eibl, R.H.; Schneemann, M. Cell-free DNA as a biomarker in cancer. Extracell Vesicles Circ Nucleic Acids 2022, 3, 178-198. [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid biopsy and glioblastoma. Exploration of Targeted Anti-tumor Therapy 2023, 4, 28-41. [CrossRef]
- Eibl, R.H.; Schneemann, M. Medulloblastoma: From TP53 Mutations to Molecular Classification and Liquid Biopsy. Biology 2023, 12, 267. [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid Biopsy and Primary Brain Tumors. Cancers 2021, 13, 5429. [CrossRef]
- Martínez-Ricarte, F.; Mayor, R.; Martínez-Sáez, E.; Rubio-Pérez, C.; Pineda, E.; Cordero, E.; Cicuéndez, M.; Poca, M.A.; López-Bigas, N.; Cajal, S.R.y.; et al. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid. Clinical Cancer Research 2018, 24, 2812-2819. [CrossRef]
- Miller, A.M.; Shah, R.H.; Pentsova, E.I.; Pourmaleki, M.; Briggs, S.; Distefano, N.; Zheng, Y.; Skakodub, A.; Mehta, S.A.; Campos, C.; et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 2019, 565, 654-658. [CrossRef]
- Mouliere, F.; Mair, R.; Chandrananda, D.; Marass, F.; Smith, C.G.; Su, J.; Morris, J.; Watts, C.; Brindle, K.M.; Rosenfeld, N. Detection of cell-free DNA fragmentation and copy number alterations in cerebrospinal fluid from glioma patients. EMBO Mol Med 2018, 10, e9323. [CrossRef]
- Pan, Y.; Long, W.; Liu, Q. Current Advances and Future Perspectives of Cerebrospinal Fluid Biopsy in Midline Brain Malignancies. Current Treatment Options in Oncology 2019, 20, 88. [CrossRef]
- Wang, Y.; Springer, S.; Zhang, M.; McMahon, K.W.; Kinde, I.; Dobbyn, L.; Ptak, J.; Brem, H.; Chaichana, K.; Gallia, G.L.; et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A 2015, 112, 9704-9709. [CrossRef]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015, 6, 8839. [CrossRef]
- Majchrzak-Celińska, A.; Paluszczak, J.; Kleszcz, R.; Magiera, M.; Barciszewska, A.-M.; Nowak, S.; Baer-Dubowska, W. Detection of MGMT, RASSF1A, p15INK4B, and p14ARF promoter methylation in circulating tumor-derived DNA of central nervous system cancer patients. J Appl Genet 2013, 54, 335-344. [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid Biopsy and Cancer. In Cancer Treatment Modalities: An Interdisciplinary Approach, Rezaei, N., Ed.; Springer Nature Switzerland: Cham, 2025; pp. 367-391.
- Ashworth, A. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869, 14, 146.
- Paget, S. The distribution of secondary growths in cancer of the breast. The Lancet 1889, 133, 571-573. [CrossRef]
- Fidler, I.J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Research 1975, 35, 218-224.
- Eibl, R.H.; Pietsch, T.; Moll, J.; Skroch-Angel, P.; Heider, K.H.; von Ammon, K.; Wiestler, O.D.; Ponta, H.; Kleihues, P.; Herrlich, P. Expression of variant CD44 epitopes in human astrocytic brain tumors. Journal of Neuro-Oncology 1995, 26, 165-170. [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105-111. [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clinical Cancer Research 2004, 10, 6897-6904. [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. New England Journal of Medicine 2004, 351, 781-791. [CrossRef]
- Maheswaran, S.; Sequist, L.V.; Nagrath, S.; Ulkus, L.; Brannigan, B.; Collura, C.V.; Inserra, E.; Diederichs, S.; Iafrate, A.J.; Bell, D.W.; et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008, 359, 366-377. [CrossRef]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008, 26, 3213-3221. [CrossRef]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clinical Cancer Research 2008, 14, 6302-6309. [CrossRef]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010, 16, 398-406. [CrossRef]
- Dawson, S.-J.; Tsui, D.W.Y.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.-F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. New England Journal of Medicine 2013, 368, 1199-1209. [CrossRef]
- Neves, R.P.L.; Ammerlaan, W.; Andree, K.C.; Bender, S.; Cayrefourcq, L.; Driemel, C.; Koch, C.; Luetke-Eversloh, M.V.; Oulhen, M.; Rossi, E.; et al. Proficiency Testing to Assess Technical Performance for CTC-Processing and Detection Methods in CANCER-ID. Clinical Chemistry 2021, 67, 631-641. [CrossRef]
- Polzer, B.; Medoro, G.; Pasch, S.; Fontana, F.; Zorzino, L.; Pestka, A.; Andergassen, U.; Meier-Stiegen, F.; Czyz, Z.T.; Alberter, B.; et al. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol Med 2014, 6, 1371-1386. [CrossRef]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015, 9, 1773-1782. [CrossRef]
- Krol, I.; Castro-Giner, F.; Maurer, M.; Gkountela, S.; Szczerba, B.M.; Scherrer, R.; Coleman, N.; Carreira, S.; Bachmann, F.; Anderson, S.; et al. Detection of circulating tumour cell clusters in human glioblastoma. British Journal of Cancer 2018, 119, 487-491. [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553-557. [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98-112 e114. [CrossRef]
- Chowdhury, T.; Cressiot, B.; Parisi, C.; Smolyakov, G.; Thiébot, B.; Trichet, L.; Fernandes, F.M.; Pelta, J.; Manivet, P. Circulating Tumor Cells in Cancer Diagnostics and Prognostics by Single-Molecule and Single-Cell Characterization. ACS Sensors 2023. [CrossRef]
- Pantel, K.; Alix-Panabières, C. The clinical significance of circulating tumor cells. Nat Rev Clin Oncol 2007, 4, 62-63. [CrossRef]
- Dai, C.S.; Mishra, A.; Edd, J.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Blood-based detection, molecular biology, and clinical applications. Cancer Cell 2025, 43, 1399-1422. [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.I.; Morgan, B.; Trautwein, J.; et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013, 5, 179ra147. [CrossRef]
- Jung, C.S.; Foerch, C.; Schänzer, A.; Heck, A.; Plate, K.H.; Seifert, V.; Steinmetz, H.; Raabe, A.; Sitzer, M. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 2007, 130, 3336-3341. [CrossRef]
- Gao, F.; Cui, Y.; Jiang, H.; Sui, D.; Wang, Y.; Jiang, Z.; Zhao, J.; Lin, S. Circulating tumor cell is a common property of brain glioma and promotes the monitoring system. Oncotarget 2016, 7, 71330-71340. [CrossRef]
- Preuss, I.; Haas, S.; Eichhorn, U.; Eberhagen, I.; Kaufmann, M.; Beck, T.; Eibl, R.H.; Dall, P.; Bauknecht, T.; Hengstler, J.; et al. Activity of the DNA repair protein O6-methylguanine-DNA methyltransferase in human tumor and corresponding normal tissue. Cancer Detect Prev 1996, 20, 130-136.
- Preuss, I.; Eberhagen, I.; Haas, S.; Eibl, R.H.; Kaufmann, M.; von Minckwitz, G.; Kaina, B. O6-methylguanine-DNA methyltransferase activity in breast and brain tumors. International Journal of Cancer 1995, 61, 321-326. [CrossRef]
- Romero, D. Tracking cancer in liquid biopsies. Nature Research 2020.
- Ohgaki, H.; Eibl, R.H.; Wiestler, O.D.; Yasargil, M.G.; Newcomb, E.W.; Kleihues, P. p53 mutations in nonastrocytic human brain tumors. Cancer Research 1991, 51, 6202-6205.
- Kleihues, P.; Ohgaki, H.; Eibl, R.H.; Reichel, M.B.; Mariani, L.; Gehring, M.; Petersen, I.; Höll, T.; von Deimling, A.; Wiestler, O.D. Type and frequency of p53 mutations in tumors of the nervous system and its coverings. Recent Results Cancer Res 1994, 135, 25-31. [CrossRef]
- Eibl, R.H.; Kleihues, P.; Jat, P.S.; Wiestler, O.D. A model for primitive neuroectodermal tumors in transgenic neural transplants harboring the SV40 large T antigen. The American Journal of Pathology 1994, 144, 556-564.
- Louis, D.N.; von Deimling, A.; Chung, R.Y.; Rubio, M.P.; Whaley, J.M.; Eibl, R.H.; Ohgaki, H.; Wiestler, O.D.; Thor, A.D.; Seizinger, B.R. Comparative study of p53 gene and protein alterations in human astrocytic tumors. J Neuropathol Exp Neurol 1993, 52, 31-38. [CrossRef]
- Ohgaki, H.; Eibl, R.H.; Schwab, M.; Reichel, M.B.; Mariani, L.; Gehring, M.; Petersen, I.; Höll, T.; Wiestler, O.D.; Kleihues, P. Mutations of the p53 tumor suppressor gene in neoplasms of the human nervous system. Mol Carcinog 1993, 8, 74-80. [CrossRef]
- Radner, H.; el-Shabrawi, Y.; Eibl, R.H.; Brüstle, O.; Kenner, L.; Kleihues, P.; Wiestler, O.D. Tumor induction by ras and myc oncogenes in fetal and neonatal brain: modulating effects of developmental stage and retroviral dose. Acta Neuropathol 1993, 86, 456-465. [CrossRef]
- Wiestler, O.D.; Brüstle, O.; Eibl, R.H.; Radner, H.; Von Deimling, A.; Plate, K.; Aguzzi, A.; Kleihues, P. A new approach to the molecular basis of neoplastic transformation in the brain. Neuropathol Appl Neurobiol 1992, 18, 443-453. [CrossRef]
- Wiestler, O.D.; Brüstle, O.; Eibl, R.H.; Radner, H.; Aguzzi, A.; Kleihues, P. Retrovirus-mediated oncogene transfer into neural transplants. Brain Pathol 1992, 2, 47-59.
- von Deimling, A.; Eibl, R.H.; Ohgaki, H.; Louis, D.N.; von Ammon, K.; Petersen, I.; Kleihues, P.; Chung, R.Y.; Wiestler, O.D.; Seizinger, B.R. p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma. Cancer Research 1992, 52, 2987-2990.
- Wiestler, O.D.; Aguzzi, A.; Schneemann, M.; Eibl, R.; von Deimling, A.; Kleihues, P. Oncogene complementation in fetal brain transplants. Cancer Research 1992, 52, 3760-3767.
- Eibl, R.H.; Wiestler, O.D. Induction of primitive neuroectodermal tumors following retrovirus-mediated transfer of SV40 large T antigen into neural transplants. Clinical Neuropathology 1991, 10, 245.
- Wiestler, O.D.; Aguzzi, A.; Brüstle, O.; Eibl, R.; Radner, H.; Kleihues, P. Oncogene complementation in transgenic neural transplants. 1991, 4, 304-309.
- Muller Bark, J.; Kulasinghe, A.; Hartel, G.; Leo, P.; Warkiani, M.E.; Jeffree, R.L.; Chua, B.; Day, B.W.; Punyadeera, C. Isolation of Circulating Tumour Cells in Patients With Glioblastoma Using Spiral Microfluidic Technology - A Pilot Study. Front Oncol 2021, 11, 681130. [CrossRef]
- Lessi, F.; Morelli, M.; Franceschi, S.; Aretini, P.; Menicagli, M.; Marranci, A.; Pasqualetti, F.; Gambacciani, C.; Pieri, F.; Grimod, G.; et al. Innovative Approach to Isolate and Characterize Glioblastoma Circulating Tumor Cells and Correlation with Tumor Mutational Status. Int J Mol Sci 2023, 24. [CrossRef]
- Chih, Y.C.; Dietsch, A.C.; Koopmann, P.; Ma, X.; Agardy, D.A.; Zhao, B.; De Roia, A.; Kourtesakis, A.; Kilian, M.; Kramer, C.; et al. Vaccine-induced T cell receptor T cell therapy targeting a glioblastoma stemness antigen. Nat Commun 2025, 16, 1262. [CrossRef]
- Liu, T.; Xu, H.; Huang, M.; Ma, W.; Saxena, D.; Lustig, R.A.; Alonso-Basanta, M.; Zhang, Z.; O’Rourke, D.M.; Zhang, L.; et al. Circulating Glioma Cells Exhibit Stem Cell-like Properties. Cancer Res 2018, 78, 6632-6642. [CrossRef]
- Carvedilol With Chemotherapy in Second Line Glioblastoma and Response of Circulating Tumor Cells. Source: National Library of Medicine; [cited 2023 01 11].
- Liquid biopsy in low-grade glioma patients (GLIOLIPSY) [Internet]. Source: National Library of Medicine; [cited 2023 01 11].
- Aslan, K.; Turco, V.; Blobner, J.; Sonner, J.K.; Liuzzi, A.R.; Núñez, N.G.; De Feo, D.; Kickingereder, P.; Fischer, M.; Green, E.; et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat Commun 2020, 11, 931. [CrossRef]
- Wiestler, O.D.; Brüstle, O.; Eibl, R.H.; Radner, H.; Aguzzi, A.; Kleihues, P. Oncogene transfer into the brain. Recent Results Cancer Res 1994, 135, 55-66. [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.; et al. The nanomechanical signature of breast cancer. Nat Nanotechnol 2012, 7, 757-765. [CrossRef]
- Xiao, R.; Qi, X.; Li, Y.; Yang, Y.; Liu, L.; Li, M. High-throughput atomic force microscopy measurements reveal mechanical signatures of cell mixtures for liquid biopsy. Nanoscale 2025, 17, 28195-28208. [CrossRef]
- Eibl, R.H.; Benoit, M. Molecular resolution of cell adhesion forces. IEE Proc Nanobiotechnol 2004, 151, 128-132. [CrossRef]
- Eibl, R.H.; Moy, V.T. Atomic force microscopy measurements of protein-ligand interactions on living cells. Methods Mol Biol 2005, 305, 439-450. [CrossRef]
- Eibl, R.H.; Moy, V.T. AFM-based adhesion measurements of single receptor-ligand bonds on living cells. In Recent research developments in biophysics, Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, 2004; pp. 235-246.
- Eibl, R.H. Comment on “A method to measure cellular adhesion utilizing a polymer micro-cantilever” [Appl. Phys. Lett. 103, 123702 (2013)]. Appl. Phys. Lett. 2014, 104, 236103. [CrossRef]
- Eibl, R.H. Single-Molecule Studies of Integrins by AFM-Based Force Spectroscopy on Living Cells. In Scanning Probe Microscopy in Nanoscience and Nanotechnology 3, Bhushan, B., Ed.; NanoScience and Technology; Springer: Berlin, Heidelberg, 2013; pp. 137-169.
- Eibl, R.H. Cell Adhesion Receptors Studied by AFM-Based Single-Molecule Force Spectroscopy. In Scanning Probe Microscopy in Nanoscience and Nanotechnology 2, Bhushan, B., Ed.; NanoScience and Technology; Springer: Berlin, Heidelberg, 2011; pp. 197-215.
- Eibl, R.H. Direct Force Measurements of Receptor–Ligand Interactions on Living Cells. In Applied Scanning Probe Methods XII: Characterization, Bhushan, B., Fuchs, H., Eds.; NanoScience and Technology; Springer: Berlin, Heidelberg, 2009; pp. 1-31.
- Eibl, R.H. First measurement of physiologic VLA-4 activation by SDF-1 at the single-molecule level on a living cell. In Proceedings of the VIII. Linz Winter Workshop 2006: Advances in Single Molecule Research for Biology and Nanoscience, Hinterdorfer, P., Schütz, G., Pohl, P., Eds.; Trauner: Linz, 2007; pp. 40-43.
- Eibl, R.H. Atomic force microscopy measurement of SDF-1 mediated affinity modulation of single VLA-4 - VCAM-1 bonds. In Immunology 2004: Cytokine Network, Regulatory Cells, Signaling and Apoptosis.; Medimond: Bologna, 2004; pp. 115-120.
- Riva, M.; Sciortino, T.; Secoli, R.; D’Amico, E.; Moccia, S.; Fernandes, B.; Conti Nibali, M.; Gay, L.; Rossi, M.; De Momi, E.; et al. Glioma biopsies Classification Using Raman Spectroscopy and Machine Learning Models on Fresh Tissue Samples. Cancers (Basel) 2021, 13. [CrossRef]
- Ranasinghe, J.C.; Wang, Z.; Huang, S. Unveiling brain disorders using liquid biopsy and Raman spectroscopy. Nanoscale 2024, 16, 11879-11913. [CrossRef]
- Zhu, L.; Li, J.; Pan, J.; Wu, N.; Xu, Q.; Zhou, Q.Q.; Wang, Q.; Han, D.; Wang, Z.; Xu, Q.; et al. Precise Identification of Glioblastoma Micro-Infiltration at Cellular Resolution by Raman Spectroscopy. Adv Sci (Weinh) 2024, 11, e2401014. [CrossRef]
- Ranasinghe, J.C.; Wang, Z.; Huang, S. Raman Spectroscopy on Brain Disorders: Transition from Fundamental Research to Clinical Applications. Biosensors (Basel) 2022, 13. [CrossRef]
- Pax, M.; Rieger, J.; Eibl, R.H.; Thielemann, C.; Johannsmann, D. Measurements of fast fluctuations of viscoelastic properties with the quartz crystal microbalance. Analyst 2005, 130, 1474-1477. [CrossRef]
- Gonzalez-Beltran, A.N.; Masuzzo, P.; Ampe, C.; Bakker, G.-J.; Besson, S.; Eibl, R.H.; Friedl, P.; Gunzer, M.; Kittisopikul, M.; Dévédec, S.E.L.; et al. Community standards for open cell migration data. Gigascience 2020, 9. [CrossRef]
| Year | Author(s) | Method | Tumor Type | Milestone |
|---|---|---|---|---|
| 1869 | Ashworth T.R. [23] | Autopsy; microscopy; case report | Unknown primary tumor | First description of tumor cells in blood; morphologically identical to metastatic lesions |
| 1889 | Paget S. [24] | Autopsy | Breast cancer | Formulation of the ‘seed and soil’ hypothesis of metastasis |
| 1975 | Fidler I.J. [25] | Experimental metastasis assay | B16 melanoma | Only a small fraction of injected tumor cells forms metastases |
| 1995 | Eibl R.H. et al. [26] | Molecular and functional characterization | Glioblastoma and astrocytoma | First detection of CD44 splice variants; potential CTC markers |
| 2001 | Reya [27] T. et al. | Stem-cell biology applied to cancer heterogeneity | Solid tumors and leukemia; migratory CSCs | Development of the cancer stem cell concept |
| 2004 | Allard W.J. et al. [28] | CellSearch™ | Prostate, breast, ovarian, CRC, lung cancers | CTC detection in 7.5 mL blood samples |
| 2004 | Cristofanilli M. et al. [29] | CellSearch™ (CTC enumeration) | Metastatic breast cancer | CTCs as independent predictor of reduced PFS and OS |
| 2008 | Maheswaran S. et al. [30] | Molecular profiling; EGFR mutation detection | NSCLC | CTC-based therapy monitoring |
| 2008 | Cohen S.J. et al. [31] | CellSearch™; clinical study | Colorectal cancer | Clinical feasibility of CTC enumeration |
| 2008 | De Bono J.S. et al. [32] | Clinical study | Prostate cancer | CTC count as strongest independent predictor of OS |
| 2010 | Pantel K., Alix-Panabières C. [33] | Conceptual review | Metastatic cancers | Introduction of the term ‘liquid biopsy’ |
| 2013 | Dawson S.J. et al. [34] | Disease monitoring | Breast cancer | ctDNA more sensitive than CTCs for therapy monitoring |
| 2013 | Baccelli et al. [9] | Xenograft | Breast cancer | Identification of metastasis-initiating CTC subsets |
| 2014 | Sullivan J.P. et al. [3] | CTC-iChip (negative depletion) | GBM | Demonstration of CTCs in glioblastoma |
| 2014 | Neves R.P. et al. [35] | Microfluidic enrichment | NSCLC | EGFR variant detection via single-cell sequencing |
| 2014 | Polzer B. et al. [36] | CTC genome/transcriptome profiling | Breast cancer | Diagnostic potential; heterogeneity to primary tumors |
| 2015 | Mazel M. et al. [37] | CellSearch™ | Breast cancer | PD-L1 detection on CTCs |
| 2018 | Krol et al. [38] | — | GBM | Identification of CTC clusters in blood |
| 2019 | Szczerba P. et al. [39] | CTC analysis | GBM | Neutrophils escort CTCs; support proliferation and metastasis |
| 2019 | Gkountela S. et al. [40] | CTC analysis | GBM | CTC clusters show distinct methylation and higher metastatic potential |
| 2023 | Chowdhury et al. [41] | Advanced CTC detection; single-cell profiling | Various cancers | Technical advances in CTC analysis |
| Pro | Con | Clinical utility |
|---|---|---|
| Sufficient sensitivity in some advanced cancers | Limited sensitivity in screening, early-stage cancers, many advanced cancers | Prognostic markers in metastatic breast, prostate, colorectal cancers |
| FDA-approved enumeration for specific applications | Not standardized, experimental methods; centralized high-tech laboratories | Prediction of relapse, incl. treatment response using living CTCs (cell culture, xenograft) |
| High specificity (mutations) | Sophisticated technology, no easy/common standards; expensive; no remuneration; extra challenges for brain tumors (lacking epithelial markers) | Clinical potential; research use; high cost; limited availability |
| Liquid biopsy | Biological material | Strengths | Limitations | Clinical maturity |
|---|---|---|---|---|
| Circulating tumor cells (CTCs) | Intact, viable tumor cells | Preserve cellular phenotype; allow functional assays; enable single-cell multi-omics; potential insight into invasion and resistance | Ultra-rare; no standardized markers; EpCAM-negative phenotype in GBM; technical variability; limited validation | Exploratory / research use |
| Circulating tumor DNA (ctDNA) | Fragmented tumor-derived DNA | High specificity for mutations; increasingly standardized assays; suitable for longitudinal monitoring; CSF often informative | No cellular/functional information; limited sensitivity in plasma for CNS tumors; reflects mainly genomic alterations | Closest to clinical routine |
| Extracellular vesicles (EVs) | Vesicles carrying proteins, RNA, DNA | Relatively stable; reflect active secretion; multi-analyte potential | Heterogeneous populations; tumor attribution can be difficult; limited standardization | Experimental / early translational |
| CSF biomarkers (proteins, ctDNA) | Cell-free molecules in CSF | Higher proximity to CNS tumors; improved sensitivity vs blood in many settings | Invasive sampling; not suitable for frequent monitoring in all patients | Translational / selective clinical use |
| Method | Principle | Characteristics | Utility for GBM |
|---|---|---|---|
| CellSearch™ | EpCAM-based immunomagnetic selection | FDA-cleared; isolates EpCAM-positive CTCs; cytokeratin/CD45 staining; automated workflow | Not suitable (GBM typically EpCAM-negative) |
| iChip | Microfluidic inertial focusing + immunomagnetic depletion | High-throughput, marker-independent; preserves viability and heterogeneity | Research tool; potential with GBM-specific markers |
| ScreenCell™ | Microfiltration based on cell size (antigen-independent) | Fast, antigen-independent; efficient for heterogeneous viable CTCs | Suitable for EpCAM-negative GBM |
| pluriBead™ | Bead sieving with bound target cells | High purity, gentle isolation; minimal blood contamination | Potentially advantageous for rare GBM CTCs |
| Year | Study | Tumor | Outcome measure |
|---|---|---|---|
| 2016 | Gao et al.[46] | GBM, other gliomas | CTC incidence |
| 2018 | Liu et al.[65] | GBM | Similarity of GBM CTCs with CSC (in both mouse and human) |
| 2019-21 | NCT03861598[66] Early phase 1 study (Morgantown, West Virginia, USA) |
GBM | Carvedilol added to standard chemotherapy, correlating MRI controls with new RT-PCR test for CTC detection |
| 2021 | Müller-Bark et al.[62] | GBM | CTC number post-surgery correlated with survival |
| 2021-25 | GLIOLIPSY: LIQUID BIOPSY IN Low-grade Glioma Patients NCT05133154[67] Interventional study (University Hospital, Montpellier) |
Low-/High-grade glioma | Pre- and post-surgery detection and characterization of CTC and TEP |
| 2023-27 |
INCIPIENT: INtraventricular CARv3-TEAM-E T Cells for PatIENTs With GBM NCT05660369 Phase 1 study (MGH, Boston, USA) |
GBM | CAR-T cell study (dose/safety) in glioblastoma with EGFRvIII mutation, incl. CTC analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





