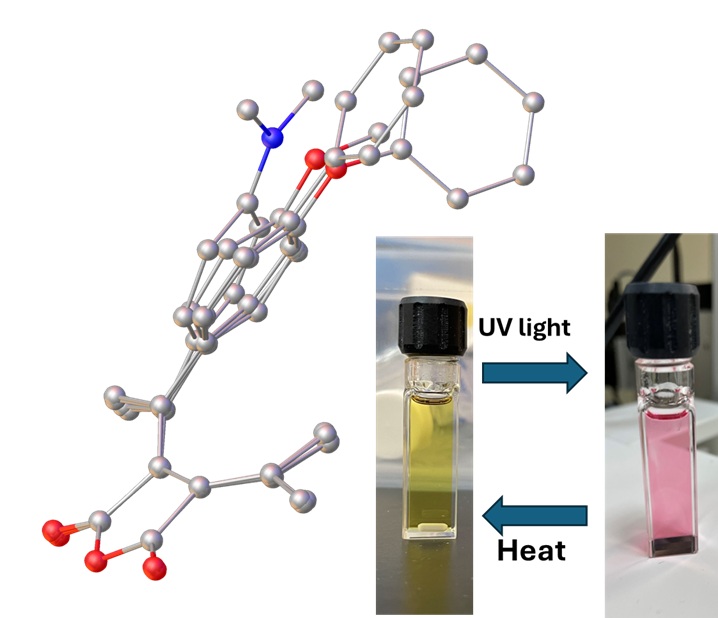
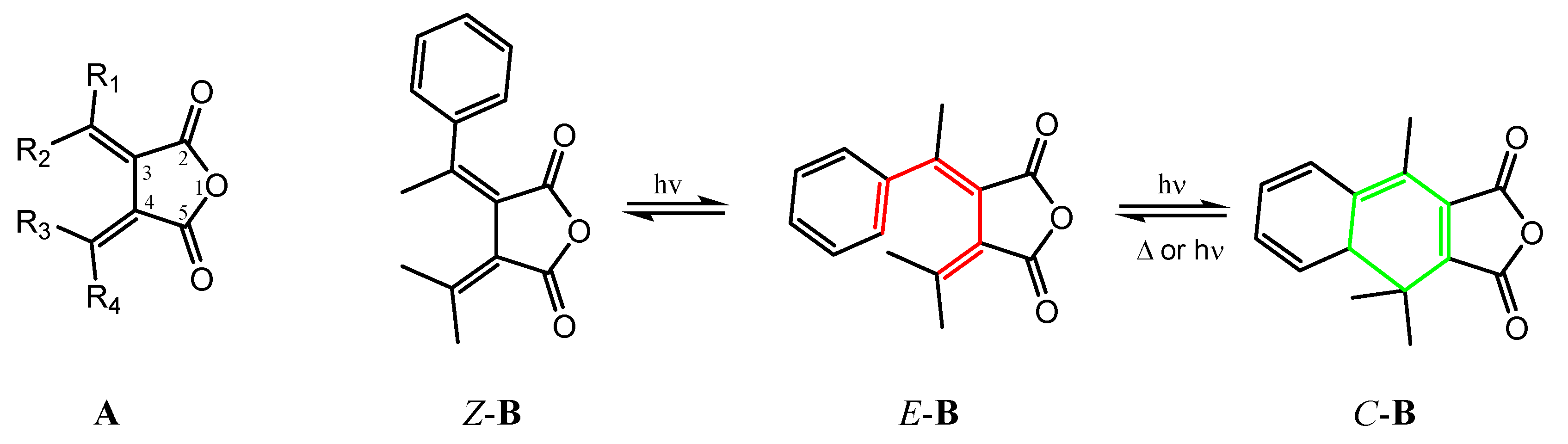
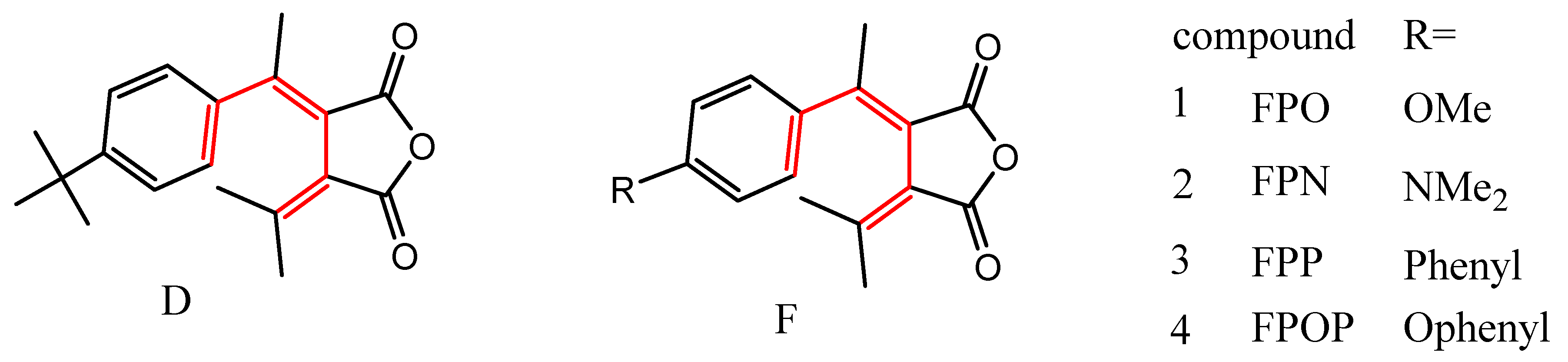
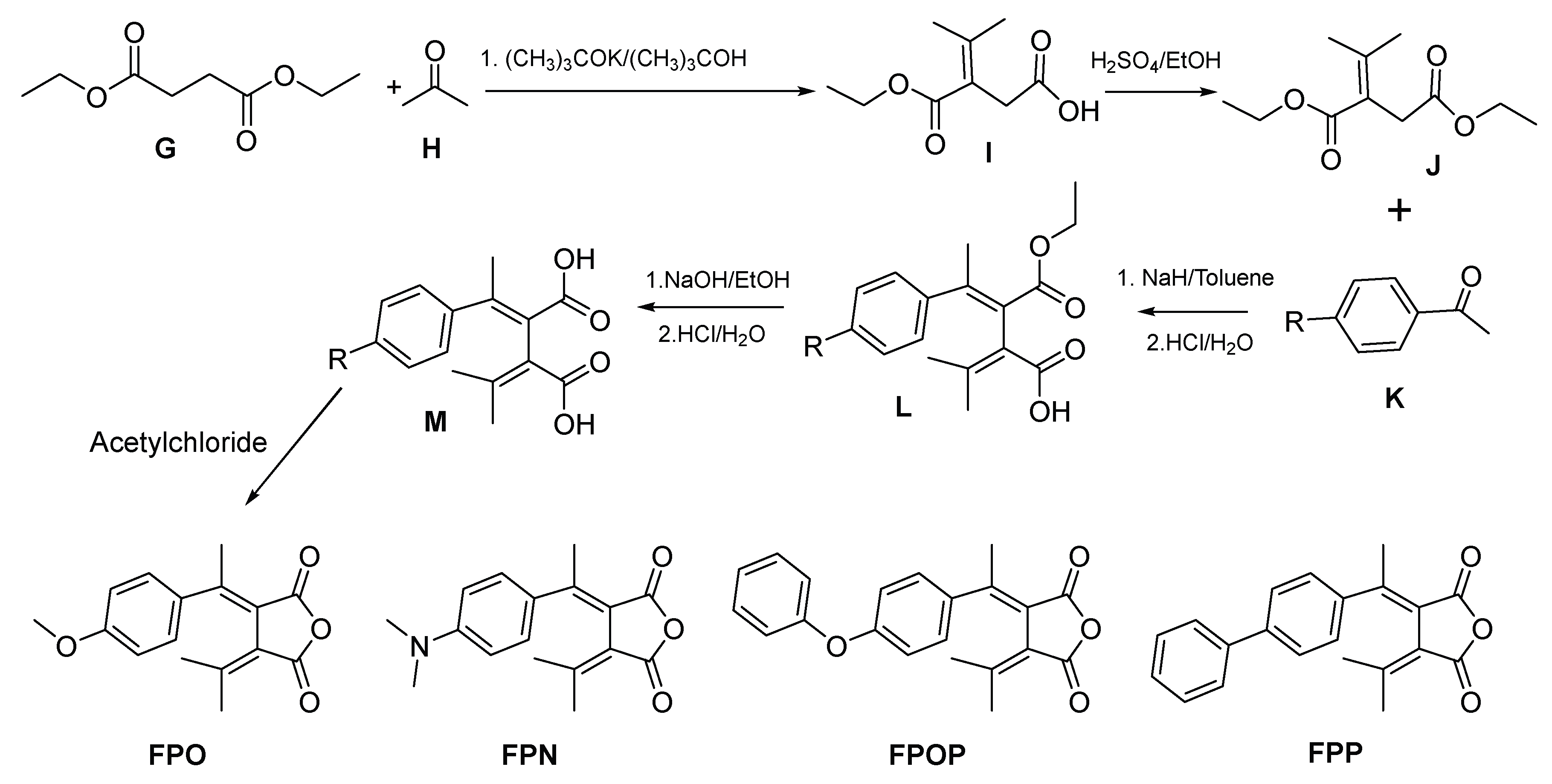
3.1. Synthesis and Crystallizations
The synthesis of the fulgides has been carried out following a slightly modified pathway illustrated in
Scheme 1, which was reported previously.
Diethyl succinate G was reacted with one equivalent acetone H and one equivalent potassium tert-butoxide in tert-butanol under reflux for 8 hours. After removal of solvent tert-butanol with a rotavapor under vacuum, the residue was poured into ice water. The water solution was washed with diethyl ether and was then acidified to pH around 2 and transferred into a separatory funnel, the top layer was collected, and the aqueous phase was washed with ethyl acetate three times. The resulting ethyl acetate solution is combined with the top layer and dried with granule anhydrous sodium sulfate. 3-(ethoxycarbonyl)-4-methylpent-3-enoic acid I yielded when the ethyl acetate solution is condensed to dryness by a rotavapor under vacuum. The carboxylic acid I without further purification was esterified in refluxing ethanol using sulfuric acid as catalyst for 10 hours to yield crude diethyl 2-(propan-2-ylidene) succinate J after removal of solvent from the reaction mixture. The crude product was transferred to a flask and applied to vacuum distillation to remove components of boiling point lower than 60 oC under vacuum of 80 millitorr. The material left in the flask was distillated to almost dryness to yield product J with a purity of around 85% as estimated from 1H NMR spectrum.
Each of the four para-substituted
K acetophenone reacted with compound
J to produce the corresponding
F as detailed in the following. Sodium hydride (1.2 equivalent) was added into the solution of K in toluene, and the suspension was stirred for 24 hours. The reaction mixture was poured into ice water to form a suspension which was transferred into a separatory funnel and the top layer was removed. The aqueous solution was washed with toluene 3 times and then acidified to pH around 2 to yield compound
L as precipitate. The precipitate was collected by filtration and dried by a rotavapor and then transferred into a flask. To the flask containing compound
L was added ethanol and sodium hydroxide (10 equivalent to
L). The reaction mixture was refluxed for 12 hours, cooled down to room temperature, and then the sodium salt of
M yielded as precipitate. The precipitate was collected by filtration, washed with chilled ethanol, dried by a rotavapor and transferred into a beaker. Water was added to the beaker until the precipitate was dissolved. The resulting solution was acidified with concentrated hydrochloric acid to pH around 2 to yield compound
M as precipitate. The precipitate
M was collected by filtration, washed with chilled water, transferred to a round bottom flask and dried with a rotavapor. Acetyl chloride was added to the flask containing
M (50 ml acetyl chloride per gram of
M), the resulting solution was stirred for 24 hours at room temperature, then the acetyl chloride was removed by a rotavapor. The resulting solid in the flask was applied to silica gel chromatography, fractions that exhibited red color on the wet TLC plate under UV detector were collected and condensed to yield the corresponding
F, which was proved to be of
E configuration for all the produce fulgides
FPO,
FPN,
FPOP and
FPP.
FPO was synthesized previously [
10], while its crystal structure has not been reported. The products of
Z configuration were not the major target of our focus; therefore, they were not isolated from the reaction mixture in this study.
3.1.1. (E)-3-(1-(4-methoxyphenyl) ethylidene)-4-(propan-2-ylidene) dihydrofuran-2,5-dione (FPO)
FPO yielded as yellow plate-shaped crystal;
1H NMR in CDCl3 (chemical shift in ppm): 1.07, singlet, 3 H; 2.11, singlet, 3H, 2.57, singlet, 3 H; 3.70, singlet 3 H; 6.78-6.80, doublet, 2 H; 7.16-7.18, doublet, 2H.
13C NMR in CDCl3 (chemical shift in ppm): 22.64, 22.17, 55.48, 114.55, 119.66, 120.10, 129.37, 134.80, 154.12, 154.57, 160.27, 154.57, 160.27, 163.59, 164.13.
ESI-MS, C16H16O4, [M+H+]: 273.1115, expected: 273.1121
3.1.2. (E)-3-(1-(4-(dimethylamino) phenyl) ethylidene)-4-(propan-2-ylidene) dihydrofuran-2,5-dione (FPN)
FPN yielded as orange plate-shaped crystal.
1H NMR in CDCl3 (chemical shift in ppm): 1.25, singlet, 3 H; 2.23, singlet, 3H, 2.67, singlet, 3 H; 3.01, singlet 3 H; 6.62-6.65, doublet, 2 H; 7.24-7.26, doublet, 2H.
13C NMR in CDCl3 (chemical shift in ppm): 22.42, 22.63, 26.27, 40.21, 111.87, 117.64, 120.83, 129.43, 150.86, 153.23, 155.35, 164.11, 164.45.
ESI-MS, C17H19NO3, [M+H+]: 286.1430, expected: 286.1438
3.1.3. (E)-3-(1-(4-phenoxyphenyl) ethylidene)-4-(propan-2-ylidene) dihydrofuran-2,5-dione (FPOP)
FPOP yielded as yellow block-shaped crystal;
1H NMR in CDCl3 (chemical shift in ppm): 1.14, singlet, 3 H; 2.13, singlet, 3H, 2.57, singlet, 3 H; 2.61, singlet 3 H; 6.89-7.30, multiple peaks, 9H.
13C NMR in CDCl3 (chemical shift in ppm):22.57, 22.60, 26.18, 118.59, 119.61, 119.88, 120.18, 124.27, 129.41, 130.02, 136.93, 153.41, 154.82, 156.00, 158.31, 16.31, 163.93.
ESI-MS, C21H18O4, [M+H+]: 335.1269, expected: 335.1278
3.1.4. (E)-3-(1-([1,1′-biphenyl]-4-yl) ethylidene)-4-(propan-2-ylidene) dihydrofuran-2,5-dione (FPP)
FPP yielded as yellow plate-shaped crystal;
1H NMR in CDCl3 (chemical shift in ppm): 1.08, singlet, 3 H; 2.12, singlet, 3H, 2.64, singlet, 3 H; 7.26-7.30 multiple peak 3 H; 7.34-7.38, multiple peaks, 2 H; 7.51-7.54. multiple peaks, 4 H.
13C NMR in CDCl3 (chemical shift in ppm): 22.64, 22.17, 55.48, 114.55, 119.66, 120.10, 129.37, 134.80, 154.12, 154.57, 160.27, 154.57, 160.27, 163.59, 164.13.
ESI-MS, C21H18O3, [M+H+]: 329.1322, expected: 329.1329
3.2. Crystal Structure and Discussion
A single crystal was picked from the crystals of each fulgide:
FPO,
FPN,
FPOP and
FPP and applied to X-ray crystallography analysis. The major parameter and results are summarized in
Table 1.
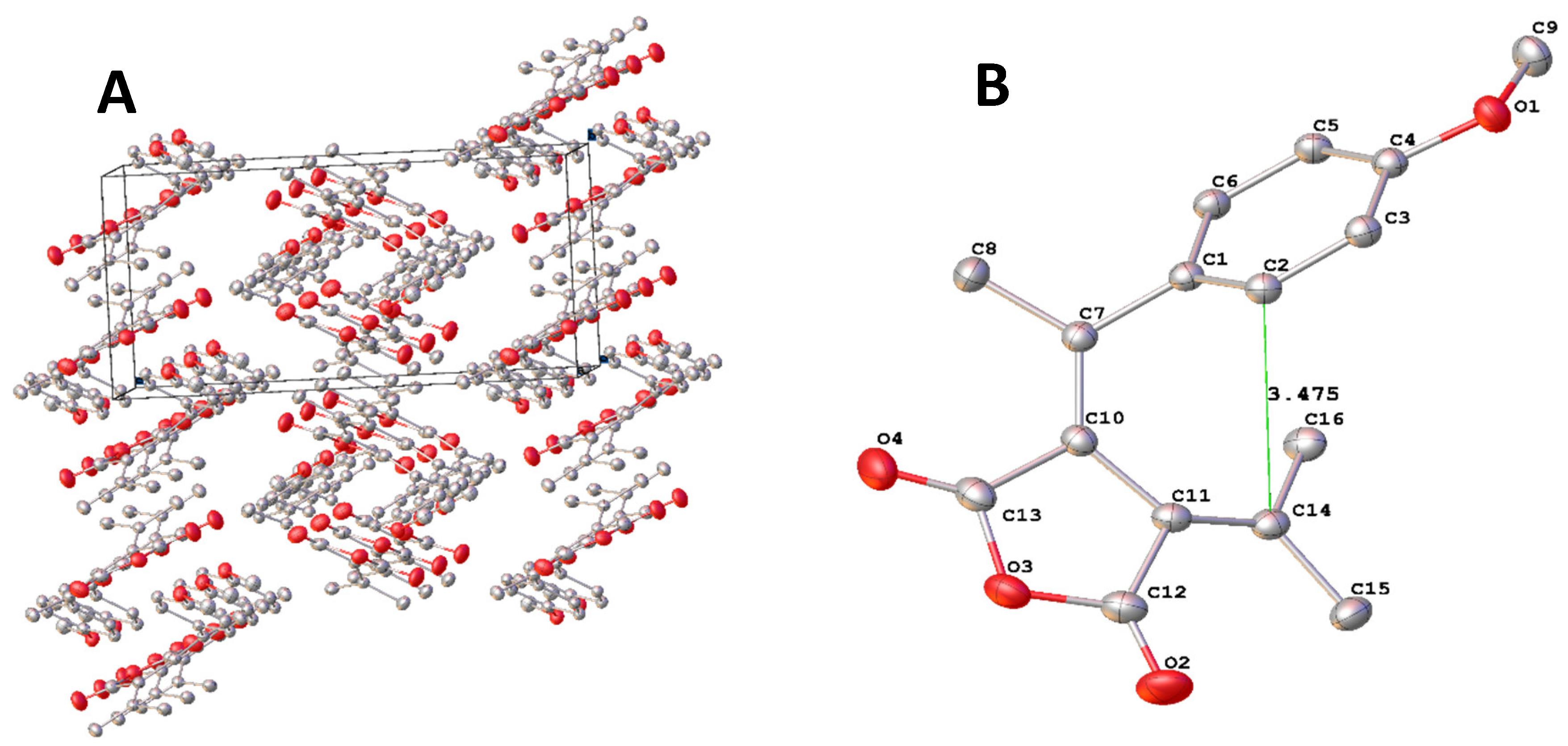
To determine the crystal structure of
FPO, a suitable single yellow plate-shaped crystal with dimensions 0.20 × 0.20 × 0.10 mm
3 was selected and mounted on a XtaLAB Synergy, Single source at home/near, Eiger2 1M diffractometer. Data was measured using
ω scans with Ag K
α radiation. The crystal was kept at a steady
T = 110.00 K during data collection. The structure was solved with the ShelXT 2018/2 (Sheldrick, 2018) solution program using dual methods and by using Olex2 1.5 [
11] as the graphical interface. The model was refined with ShelXL 2019/2 [
12,
13] using full matrix least squares minimization on
F2. The final
wR2 was 0.1426 (all data) and
R1 was 0.0473 (I≥2
s(I)). Major parameters and results are listed in
Table 1. The structure was determined to be monoclinic within space group of
P2
1/
n (No. 14). The molecular packing of
FPO in crystal was illustrated in
Figure 3. A. The structure of FPO was illustrated in
Figure 3. B.
The bond distance of nonhydrogen atoms, selected bond angles and torsions angles were measured and presented in
Table 2,
Table 3 and
Table 4.
The structure of fulgide can be considered of three moieties: the succinic anhydride, the phenyl group, and the conjugated system of C7=C10-C11=C14. The bond lengths among carbons C1-6 in the phenyl group fall into the range 1.385-1.402 Å which is consistent with those in a substituted benzene. The two C=C double bonds C7=C10 and C13=C14 are 1.3662 Å and 1.3578 Å respectively, which are slightly longer than the average C=C length of 1.34 Å due to distorted conjugation. Single C-C bonds as exemplified by C14-C15 and C14-C16 with lengths of 1.5022 Å and 1.4932 Å are slightly shorter than average 1.54 Å due to their adjacent to double bond. The torsion of C7-C10-C11-C14 is -35.8°, which shows the great distortion of the C7=C10-C11=C14 conjugated system. The torsion of C2-C1-C7-C10 is -50.76° shows the C11=C14 double bond is far from perfect conjugation of 0° torsion. The torsions C1-C7-C10-C11 and C13-C10-C11-C12 indicate that the double bond of C7=C10 and C11=C14 are distorted by similar extent of -11.19° and 10.42°. The significant distortion of the conjugated system and the double bonds are caused by the stereo repulsion between the methyl group of C14 and the phenyl group. The stereo strain energy drives the molecule to undergo intramolecular cyclization. The distance between the C14 and C2 was measured to be 3.475 Å.
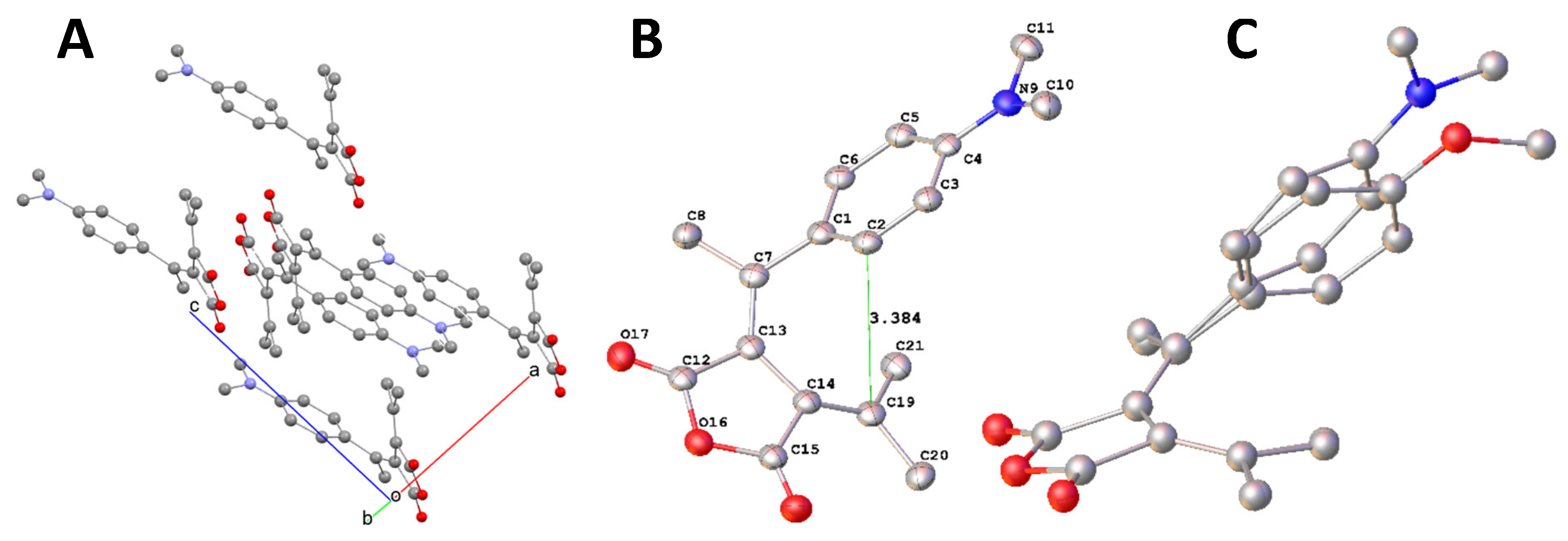
To see the effect of electron-donating group on the structure of the fulgide, we prepared the
FPO’s analogue
FPN which has a stronger electron-donating dimethyl amino group than methoxy group at the
para position of phenyl group. To determine the crystal structure of
FPN, a suitable single orange plate-shaped crystals of dimensions 0.20 × 0.10 × 0.02 mm
3 was selected from the crystalized product and mounted on a XtaLAB Synergy, Dualflex, HyPix diffractometer. Data was measured using
w scans with Cu K
a radiation. The crystal was kept at a steady
T = 100.00(10) K during data collection. The structure was solved and refined as that of
FPO. The parameters of determination and crystal structures are listed in
Table 1. The molecular packing of
FPN in crystal is illustrated in
Figure 4.A. The structure of FPN is illustrated in
Figure 4.B.
The results showed that the selected bond length in
FPN exhibited no change in comparison to those in
FPO. Regarding the bond angles, the angle of C12-C11-C10 changed by 16.9
°from 105.6
°in
FPO to 122.5
° in
FPN. The torsion angles of C2-C1-C7-C10, C1-C7-C10-C11, and C8-C7-C10-C13 changed significantly from -50.8
°, -11.2
° and -17.4
° in
FPO to -38.2
°, -17.6
° and -25.0
° in
FPN. Overlay of the structure of
FPO and
FPN by matching C12, C11, C10 and C13 showed clearly that the differences of torsion angles are caused by a rotation around the C1-C7 single bond as shown in
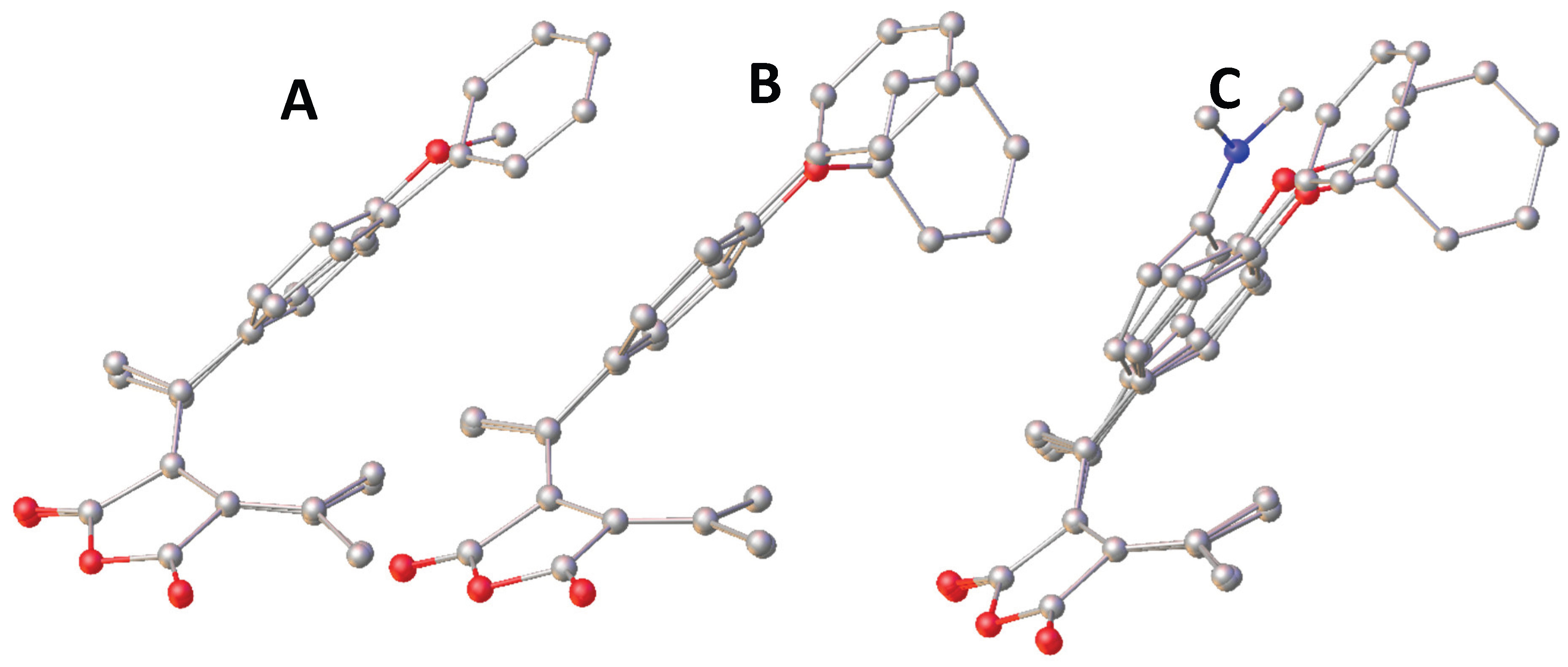
Figure 4. C. Such a rotation slightly decreased the distance between the C2 and C14 atoms, the ones forming C-C single bond in cyclization reaction, from 3.475 Å to 3.384 Å.
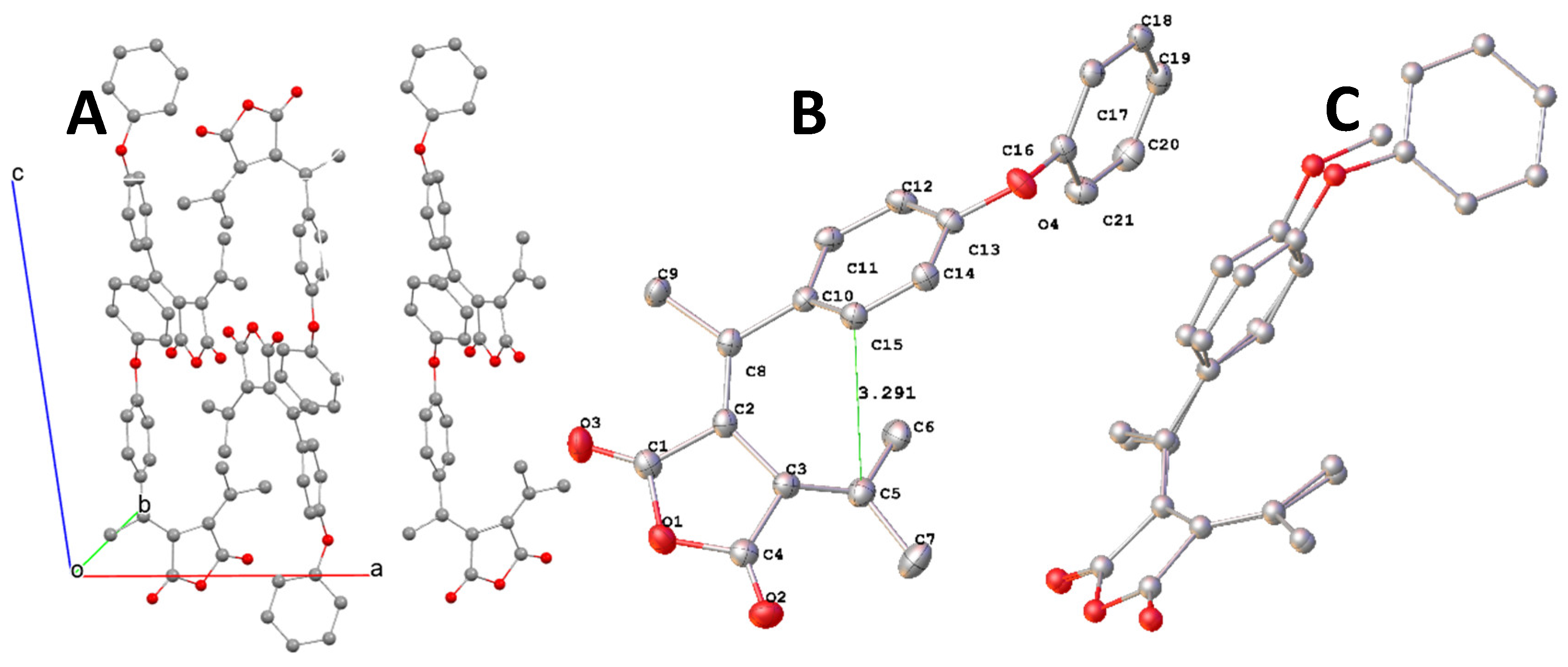
To study the size effect of the
para substitute group, a phenyl group with a larger size that methyl group in
FPO was introduced into fulgide
FPOP. A single yellow block-shaped crystal of
FPOP was subjected to X-ray crystallography. A suitable crystal with dimensions 0.34 × 0.23 × 0.18 mm
3 was selected and analyzed by using the same diffractometer, parameters and conditions. The structure was solved and refined as
FPO. The parameters of determination and crystal structures are listed in
Table 1. The molecular packing of
FPOP in crystal is illustrated in
Figure 5. A. The structure of
FPOP is illustrated in
Figure 5. B.
To show the difference in crystal structure caused by the size increasement, the selected bond lengths, bond angles and torsions in
FPOP are included in
Table 5,
Table 6, Table 7 and Table 8 for comparison. The length of bonds related to the conjugated system showed no difference between
FPO and
FPOP. Among the selected bond angles related to the conjugated system, the angle of C12-C11-C10 increased to 121.8
° in
FPOP from 105.6
° in
FPO, like the change observed in FPN. Among the torsion angles, the torsion of C1-C7-C10-C11 changed most to -4.3
° from -11.2
° in
FPO, while C8-C7-C10-C13 changed, with the least extent, to -14.3
°from 17.4
°in
FPO. Such changes are caused by distortion around double bonds C7=C10 and C11-C14 and by slight rotation around bond C1-C7. These changes have led to the decrease of in the distance from 3.475 Å
FPO to 3.302 Å
FPOP.
Table 5.
The selected bond angles of FPO, FPN, FPOP and FPP.
Table 5.
The selected bond angles of FPO, FPN, FPOP and FPP.
| Number |
Bond Angle |
Angle in Degree |
| FPO |
FPN |
FPOP |
FPP |
| 1 |
C1-C7-C10 |
122 |
122.1 |
120.7 |
122.1 |
| 2 |
C8-C7-C10 |
122.8 |
120.9 |
122.3 |
122.6 |
| 3 |
C13-C10-C7 |
120.9 |
120.8 |
121.6 |
122.3 |
| 4 |
C11-C10-C7 |
132.5 |
132.2 |
132.1 |
131.4 |
| 5 |
C16-C14-C11 |
122.2 |
121.9 |
122.8 |
122.6 |
| 6 |
C15-C14-C11 |
123.9 |
123.7 |
121.8 |
123.3 |
| 7 |
C12-C11-C10 |
105.6 |
122.5 |
121.8 |
122.9 |
| 8 |
C14-C11-C10 |
131.2 |
130.1 |
131.1 |
131.4 |
Table 6.
Selected torsion of FPO, FPN, FPOP and FPP.
Table 6.
Selected torsion of FPO, FPN, FPOP and FPP.
| Number |
Torsion |
Torsion Angles |
| FPO |
FPN |
FPOP |
FPP |
| 1 |
C2-C1-C7-C10 |
-50.8 |
-38.2 |
-46.5 |
-46 |
| 2 |
C1-C7-C10-C11 |
-11.2 |
-17.6 |
-4.3 |
-4.9 |
| 3 |
C7-C10-C11-C14 |
-35.8 |
-37 |
-39.3 |
-41.8 |
| 4 |
C10-C11-C14-C16 |
1.5 |
2.2 |
-3.6 |
-2.4 |
| 5 |
C8-C7-C10-C13 |
-17.4 |
-25 |
-14.3 |
-15 |
| 6 |
C15-C14-C11-C12 |
-12.3 |
-12.5 |
-18.1 |
-18.3 |
| 7 |
C13-C10-C11-C12 |
-10.4 |
-10.4 |
-14.1 |
-16 |
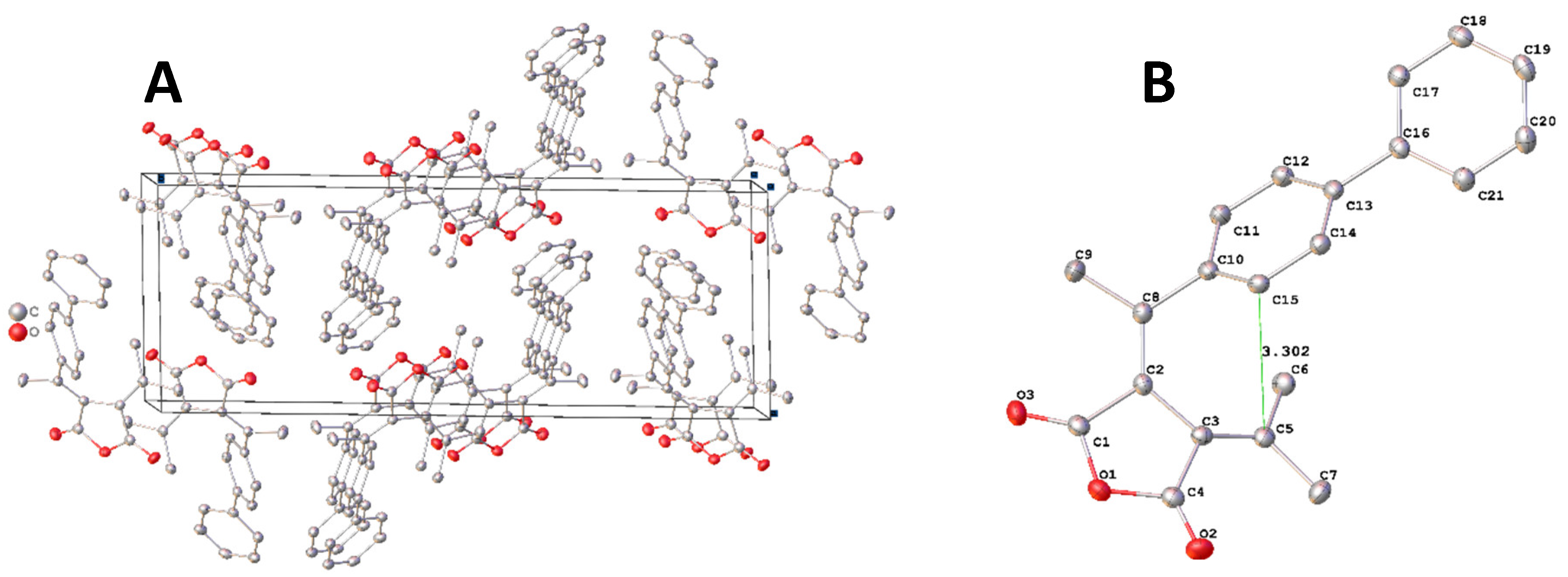
The fulgide
FPP with a phenyl substituent of no significant electron-donating properties an analogue of
FPO to evaluate both electronic and size effect of the substitution on the crystal structures. A yellow block-shaped single crystal with dimensions 0.62 × 0.50 × 0.32 mm
3 of
FPP was selected and subjected to single crystal using the same diffractometer. The structure was solved and refined as
FPOP. The parameters of determination and crystal structures are listed in
Table 1. The molecular packing of
FPP in crystal is illustrated in
Figure 6. A. The structure of
FPP is illustrated in
Figure 6. B.
For convenient comparison of the structures, the selected bond lengths, bond angles and torsions are summarized in
Table 5-8. The length of bonds related to the conjugated system showed no difference among all analyzed structures. C12-C11-C10 angle in
FPP changed to 122.9
°, like those in
FPN and
FPOP, from 105.6
°in
FPO. The selected torsion angles in
FPP are close to those in
FPOP, but significantly different from those in
FPO and
FPN. The distance between C15 and C5 was measured to be 3.302 Å in comparison to those of 3.475 Å and 3.384 Å in
FPO and
FPOP respectively. The changes can be easily observed in the overlay of structures of
FPO to
FPP and
FPOP to
FPP as shown in
Figure 7 A and B. The phenyl group in FBB tilted a little closer to the carbon involved in cyclization as shown in
Figure 7A. The phenyl and phenoxy of similar size as substituents led to almost the exact overlap of the phenyl group position, although they oriented quite different in the crystal.
All the four crystal structures have been overlayed by matching the position of the four carbons in the succinic moiety and displayed in
Figure 7. C. The succinic moiety showed well overlapping among all the structures. The bond lengths showed no changes. The bond angles related to the conjugate system showed no significant change either. The distortion around the double bond of C7=C10 caused the significant displacement of the carbon C8 and C7. The rotation around C7-C1 led to the tilt of the phenyl moiety. The phenyl moiety in
FPOP and
FPP overlaps well with each other, while this moieties in
FPO and FPN tilted sightly and significantly from those in
FPOP and
FPP away from the carbon C14. Such distortion contributes most to the distance between C2 and C14, which is most important in the photo cyclization involved in photochromism. All the four fulgides exhibited photochromic property as observed in the purification process of each compound. Although the fulgide
D with a tert-butyl group crystalizes into a non-centrosymmetric group of Pc and exhibit ferroelectric property, each of the fulgide:
FPO,
FPN,
FPOP and
FPP packed into centrosymmetric group of
P2
1/
n, P-1,
P2
1/
c and
P2
1/
c respectively. The results suggest the new crystalized compounds are not ferroelectric. Such a crystal packing difference among fulgide of similar molecular structures are to be understood.