1. Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality worldwide, accounting for approximately 18 million deaths annually and representing a substantial proportion of global disability-adjusted life years [1,2]. Despite significant advancements in identifying modifiable risk factors - such as hypertension, dyslipidaemia, diabetes, smoking, obesity and physical inactivity, and the widespread availability of effective interventions, the global burden of CVD continues to escalate.
The 2021 European Society of Cardiology (ESC) guidelines emphasize that most CVD events are preventable through lifestyle modifications and optimal management of risk factors [3]. Nevertheless, a substantial "implementation gap" remains between guideline recommendations and current clinical practice [4]. Recent multinational observational studies demonstrate that only a minority of high- and very-high-risk patients achieve the low-density lipoprotein cholesterol (LDL-C) goals recommended by guidelines, with some cohorts indicating that less than one-quarter of such patients meet targets, despite the availability of potent lipid-lowering therapies [5,6]. Even among patients with established CVD, large-scale registries have highlighted significant gaps in risk factor control, with over 40% experiencing uncontrolled blood pressure and 80% having suboptimal cholesterol levels despite being on medication. Furthermore, more than half remain physically inactive and continue smoking after an event, one-third are obese, while one-quarter have diabetes [7].
Preventive cardiology faces a confluence of critical implementation barriers. Recent evidence highlights that organizational constraints, specifically severe time pressure, workforce shortages, and inadequate clinical decision support, constitute primary bottlenecks in daily practice [8]. These systemic challenges, compounded by the competing priorities of managing multimorbidity, contribute directly to the widespread underuse of objective risk stratification [9]. International surveys indicate that less than half of physicians regularly employ validated cardiovascular (CV) risk calculators, relying instead on clinical judgment which underestimates risk in nearly two-thirds of high-risk patients [10,11]. This gap is largely driven by the perceived complexity of guidelines, with over 60% of physicians reporting that the volume and intricacy of recommendations discourage usage, particularly within time-constrained primary care settings [8].
Even when high risk is identified, therapeutic inertia often persists. Evidence from organisational interventions shows that overcoming hesitation to intensify treatment requires structured, team-based care and regular monitoring systems, rather than relying solely on individual clinician vigilance [6,8]. Crucially, this workload is exacerbated by fragmented data management: essential prognostic information remains dispersed across poorly integrated electronic health record (EHR) sections, forcing clinicians to manually extract critical insights during brief consultations [12,13].
Parallel challenges exist on the patient side, where limited health literacy and inadequate risk perception, often exacerbated by complex medical communication and variable-quality online information, severely undermine adherence [14]. Despite evidence establishing that simplified, plain-language strategies are a ‘clinical necessity’ for effective engagement, such tools remain critically underutilized in routine practice [15].
Large Language Models (LLMs) represent a technological paradigm shift with unique relevance to preventive cardiology. Unlike traditional and more focused artificial intelligence (AI) applications that dominate the field, such as electrocardiogram interpretation or imaging segmentation, LLMs are foundation models trained on vast text corpora [16,17]. This architecture enables them to generate coherent language, synthesise complex information, and perform reasoning tasks without the need for task-specific training [16,17]. These capabilities align remarkably with the core implementation barriers in preventive cardiology, and LLMs offer the potential to automate the extraction of risk factors from unstructured clinical notes, translate technical guideline recommendations into personalized advice, and facilitate the iterative, plain-language communication essential for improving patient health literacy [18–20].
However, early evaluations have demonstrated that this potential is accompanied by significant limitations. While LLMs have displayed the capacity to pass medical licensing examinations and provide generally appropriate prevention advice, critical safety concerns persist [21]. Studies evaluating generative artificial intelligence (AI) responses to CV prevention scenarios found that, while broadly accurate, the outputs often lacked the clinical nuance required for individualized treatment intensity decisions [22]. Furthermore, comparative analyses have revealed variability in accuracy across different models and languages, alongside the persistent risk of “hallucinations”, the generation of plausible but factually incorrect information [22,23]. These reliability issues constitute a major barrier to clinical deployment that must be rigorously addressed.
Although several recent reviews have examined the applications of LLMs in medicine broadly or cardiology in general, few have addressed the specific challenges of CV prevention [22,24,25]. Current literature predominantly focuses on diagnostics or imaging analysis, frequently overlooking the longitudinal and behavioural complexities inherent to risk factor management [26]. Furthermore, the transition from theoretical capability to clinical utility is hindered by a paucity of robust implementation frameworks, specifically those capable of reconciling generative AI with established risk scores and rigorous safety governance [27,28].
This review examines the practical use of LLMs in CV prevention to ensure they help reduce the global CVD burden instead of adding unnecessary complexity. We synthesise current evidence across three functional domains: (1) patient applications aimed at enhancing health literacy and supporting behaviour change; (2) clinician applications designed to expand risk stratification, early diagnosis of CVD, guideline adherence, and workflow efficiency; and (3) system applications that operate at the infrastructure level to enable automated data extraction, registry construction, and quality surveillance. Building on these insights and a critical analysis of safety risks, we propose a conceptual framework (C.A.R.D.I.O.) for the responsible integration of LLM-based tools into preventive cardiology workflows.
2. Methods and Literature Search
This work was conducted as a comprehensive narrative review, with data extraction and synthesis organised around the three core application domains (patient, clinician, and system) outlined in the introduction.
We searched electronic databases (PubMed/MEDLINE, Embase, Scopus) and preprint repositories (arXiv, medRxiv) for English-language articles published between 1 January 2015 and 30 November 2025. The search strategy combined terms related to LLMs and generative AI (e.g. “large language model*”, “LLM*”, “generative AI”, “ChatGPT”, “transformer*”) with cardiovascular and prevention-related terms (e.g. “cardiovascular”, “cardiology”, “primary prevention”, “secondary prevention”, “risk factor*”, “risk stratification”, “clinical decision support”).
The exact syntax was adapted for each database. Additional records were identified through manual cross-referencing and backward citation searching. We included original studies describing the development, validation, or implementation of LLM-based or closely related generative language tools in cardiology or cardiovascular prevention, as well as narrative reviews, position papers, and consensus documents from major professional societies addressing AI or LLMs with explicit implications for CV prevention. Foundational computer science papers on transformer architectures and general-purpose LLMs were included when directly relevant to clinical or health-system applications. We excluded studies focusing exclusively on computer vision applications without a substantive text-based or language-model component, and purely technical descriptions of non-medical AI models without clear relevance to healthcare or prevention.
After removal of duplicates, titles and abstracts were screened for relevance, followed by full-text assessment of potentially eligible articles. Given the rapid pace of AI development, particular emphasis was placed on work published after the introduction of the transformer architecture in 2017, although earlier contributions were retained when necessary to contextualize current LLM-based approaches [29]. Because of the heterogeneity of study designs (technical benchmarks, observational studies, pilot interventions, conceptual and policy papers), no formal risk-of-bias assessment or meta-analysis was undertaken. Instead, findings were synthesised narratively and organised according to the three levels of applications. These findings informed the development of the conceptual governance framework (C.A.R.D.I.O.) proposed in the final section.
3. Fundamentals of Large Language Models for Clinicians
From Calculators to Probabilistic Engines. The emergence of LLMs represents a transformative advancement in AI, dramatically demonstrated with ChatGPT’s release in late 2022 revealing unprecedented conversational capabilities [30]. To understand the utility of these tools in preventive cardiology, clinicians must clearly distinguish them from the tools currently employed in practice. Traditional risk tools, such as the SCORE2 or Pooled Cohort Equations, are deterministic: they apply fixed regression equations to a set of variables to produce a consistent numerical output [3,31]. Similarly, "narrow" AI applications are constrained to specific tasks like electrocardiogram interpretation [26,32]. In contrast, LLMs are probabilistic engines trained on petabytes of text [17,33]. Rather than "calculating" risk, they generate language by predicting the next statistically likely token (word fragment) based on context [16]. This architecture allows them to process unstructured clinical narratives that break traditional software, but it also introduces the risk of generating plausible yet factually incorrect statements, known as "hallucinations" [17,21,36,37]. To aid clinicians in navigating this new landscape, key technical concepts and their practical implications are defined in
Table 1.
Development and Training Stages. The creation of a clinically applicable LLM typically follows three progressive stages. First, during pre-training, the model is exposed to vast text corpora from the internet. It acquires a broad comprehension of medical language and concepts but lacks clinical specificity or safety constraints [16,33,36]. Second, in supervised fine-tuning, the model is adapted to the medical domain [38]. Developers train it on curated datasets of clinical guidelines, question–answer pairs, and de-identified notes, effectively transforming broad linguistic knowledge into focused medical expertise [16,33,36,38]. The final stage, alignment, often uses reinforcement learning from human feedback [36]. Human evaluators rate model outputs for safety, training the model to refuse harmful queries. However, alignment remains imperfect; models can still be manipulated ("jailbroken") and may generate incorrect medical information with high confidence [36,39].
Deployment Strategies. In practice, clinicians will encounter three primary deployment strategies. General-purpose models (e.g., GPT-4) offer flexible reasoning but may generate medically incorrect advice that diverges from guidelines [17,21]. Domain-adapted models (e.g., Med-PaLM 2) are fine-tuned on biomedical literature for higher accuracy but often remain "frozen" in time, unable to incorporate new evidence [38,40]. Composite systems represent the most reliable approach for prevention; they embed LLMs into broader workflows—using retrieval-augmented generation (RAG) to cite external guidelines or integrating with electronic health records (EHR)—to improve clinical utility and reduce errors [39,41,42].
Capabilities in Cardiovascular Prevention. Within this framework, LLMs offer four capabilities relevant to preventive cardiology:
Information Synthesis: Consolidating fragmented patient histories into concise summaries [18,38].
Risk Factor Extraction: Identifying unstructured variables (e.g., family history, symptoms) from free-text notes [25,28,42].
Guideline Translation: Converting complex technical recommendations into personalized care pathways [21,37].
Generative Communication: Creating plain-language explanations to support patient health literacy [14,43].
Evaluation and Risks. As LLMs enter clinical use, evaluation must move beyond standardized exams to focus on guideline concordance, calibration, and robustness [21,34,38]. True readiness requires prospective assessment in real-world workflows to monitor for automation bias, where clinicians uncritically accept AI outputs [20,27,36]. Despite their potential, significant risks remain. Models may hallucinate, fabricating trials or misquoting guidelines [21,23,36,37]. Limited context windows may cause models to "forget" relevant history in long records [35,39]. Finally, without retrieval mechanisms, models may provide outdated recommendations or amplify biases present in training data, potentially affecting care for under-represented groups [33,37,44].
4. Current Evidence on Large Language Models for Cardiovascular Prevention
Having defined the technical architecture and capabilities of LLMs, we now examine the emerging evidence supporting their translation into preventive cardiology. The transition from general-purpose chatbots to medical-grade tools requires rigorous evaluation against the specific challenges of risk factor management [17,20]. To structure the heterogeneous evidence base, we organize the current landscape of literature around the three key stakeholders in the prevention pathway: the patient, the clinician, and the health system (
Figure 1).
While the theoretical potential of these models is vast, current practice is defined by relatively narrow applications that target specific barriers within CV prevention workflows [20,22]. To structure this heterogeneous evidence base, we categorise existing applications into three functional domains: (1) patient applications aimed at enhancing health literacy and supporting behaviour change; (2) clinician applications designed to expand risk stratification, guideline adherence, and workflow efficiency; and (3) system applications that operate at the infrastructure level, including automated data extraction, registry construction, quality surveillance, and multimodal prognostic modelling. Together, these domains illustrate how LLM-based tools begin to address the “implementation gap” in CV prevention by informing patients, supporting clinicians, and enabling scalable, system-level optimisation of preventive care. A summary of key capabilities, benefits, and risks across these three domains is provided in
Table 2.
4.1. Patient Applications
The most immediate application of LLMs involves their direct use by patients for medical information retrieval and lifestyle support. Unlike traditional search engines or rigid rule-based chatbots, LLMs function as interactive agents capable of synthesising information rather than merely retrieving static links [18,20]. This capability has prompted extensive investigation into their utility for improving health literacy and facilitating shared decision-making regarding modifiable risk factors [17,23].
Information accuracy and safety. The primary determinant of clinical utility in this domain is the factual accuracy and safety of the generated advice. A recent systematic review synthesised evidence from 35 observational studies evaluating LLM applications in CVD, the majority of which focused on patient education and risk factor management [22]. The review found that current models, particularly versions of ChatGPT (OpenAI), demonstrate a high capacity for answering common patient inquiries. Across multiple studies, the aggregated evidence suggests that LLMs provide "accurate, comprehensive, and generally safe" responses to questions covering diverse domains, including heart failure, atrial fibrillation, traditional risk factors such as hypertension and diabetes, and lifestyle interventions [22].
This broad reliability is exemplified by early pivotal studies evaluating LLM responses to fundamental prevention questions. Cardiologists graded the model’s outputs as "appropriate" in approximately 84% of cases, noting that advice on diet, exercise, and lipid management was largely consistent with current guidelines [45]. However, this high baseline accuracy is accompanied by significant limitations. Models often suffer from "temporal obsolescence", lacking awareness of recently published guidelines, novel drug approvals, or new procedures [22,37,38]. Furthermore, performance variability has been noted across languages, with some studies showing accuracy drops when queries are posed in non-English languages [22]. While dangerous advice is rare, the persistent risk of hallucination and the confident fabrication of references remains a safety barrier for unsupervised use [21,22,36].
Crucially, there is a distinction between "broad accuracy" and "clinical precision". While models are generally correct on high-level concepts, they frequently lack the nuance required for personalized treatment decisions. For instance, an LLM may correctly identify the general benefit of statins, but fail to distinguish between the varying intensity targets for primary versus secondary prevention, or neglect to tailor advice for patients with specific comorbidities [22,45].
Communication quality and health literacy. Beyond factual content, the way in which information is conveyed is critical for patient engagement. In this regard, LLMs have demonstrated a notable capacity to outperform physicians in communication tone. In direct comparisons of responses to patient inquiries, evaluators have rated LLM-generated answers as significantly more empathetic and higher in quality than those drafted by clinicians [46]. By utilising validating language and avoiding the brevity that often characterizes time-pressured medical correspondence, these models offer a potential solution to the "empathy deficit" frequently cited in digital health interactions [46].
Nevertheless, this empathetic answering is often undermined by a paradox in technical accessibility. While the tone is supportive, the default linguistic complexity often exceeds the health literacy of the average patient [22]. CV prevention relies on the comprehension of nuanced concepts, yet evaluations of LLM-generated education materials consistently find them written at a high school or college reading level (11th–13th grade), far surpassing the recommended 6th-grade standard [47]. Although LLMs possess the capability to simplify text when explicitly prompted, this requires user initiative. Without specific prompt engineering (e.g., "explain this to a 12-year-old"), the inherent complexity of LLM outputs remains a significant barrier to equitable access, particularly for vulnerable populations [48].
Lifestyle behaviour and risk factor modification. The frontier of patient-facing LLM applications lies in their potential to promote active behaviour change. Theoretical frameworks suggest that the generative nature of these models enables the simulation of motivational interviewing, delivering unique, context-aware responses that address specific patient barriers [49,50]. Unlike static apps restricted to pre-scripted reminders, an LLM-based coach can theoretically engage in iterative dialogue, exploring the reasons for medication non-adherence or proposing personalized dietary and exercise adjustments. While pilot studies targeting general lifestyle risk factors have shown promise, robust evidence supporting the translation of these capabilities into effective CV interventions remains limited [49,50].
Taken together, current evidence indicates that patient-facing LLMs can provide generally accurate, guideline-concordant, and empathetic information across common CV topics [22,45,46]. However, their safe use remains constrained by static training data, variable performance across languages and literacy levels, the potential for hallucinated content, and the absence of prospective trials demonstrating improvements in risk factor control or clinical events [21,22,36–38,49–51]. At present, these systems are best regarded as adjuncts for patient education and engagement rather than validated standalone advisors for individualized CV prevention [22,45].
Risks, limitations, and evidence gaps in patient applications. Ultimately, while patient education represents the most extensively studied application, accounting for nearly three-quarters of the current literature, there is a paucity of prospective trials evaluating its impact on clinical endpoints [51]. The existing evidence base remains predominantly evaluative, focusing on the quality of textual outputs rather than interventional efficacy. Consequently, the specific capability of patient-facing LLMs to drive sustained improvements in risk factor control and prevent CV events remains to be established through rigorous clinical trials [22].
4.2. Clinicians Applications
The highest-yield applications of LLMs in preventive cardiology arguably lie in improving clinical workflows and clinician performance. In this domain, LLMs function not merely as encyclopaedic references but as "reasoning engines" capable of processing unstructured data to support diagnostic and therapeutic decision-making [17,18]. Unlike “narrow” AI tools designed for specific tasks, clinician-facing LLMs can operate as broad-spectrum support systems.
Guideline retrieval and reference consultation. The sheer volume of CV prevention guidelines, spanning hypertension, dyslipidaemia, diabetes, and lifestyle modification, alongside secondary prevention protocols, presents a cognitive challenge for time-pressured clinicians [3,31]. A primary utility of LLMs is the instant retrieval and synthesis of these complex recommendations. Initial evaluations of LLM performance on standardized medical examinations and cardiology-specific board questions have been promising, with general-purpose models often achieving passing scores without specialised training [16,52].
Recent studies evaluating LLMs against ESC guidelines demonstrate that these models can accurately interpret Class I and III recommendations in over 80% of selected clinical scenarios [21]. Similarly, when presented with vignette-based queries regarding CVD prevention, widely available LLMs have demonstrated the capacity to provide advice that is "broadly appropriate" and concordant with guidelines in most cases [53,54]. Recognizing this potential, professional societies are increasingly exploring RAG systems, tools that ground LLM answers specifically in verified guideline texts, to allow clinicians to query trusted repositories directly. This approach mitigates the risk of hallucination inherent in open-ended models, a strategy exemplified by the ESC Chat recently made available to medical professionals [55].
Decision support and risk stratification. A critical distinction must be made between guideline and literature recall and clinical decision making. In preventive cardiology, the ability to recite a guideline is less valuable than the ability to apply it to a specific patient. LLMs often struggle with the nuance required for complex decision-making [56–58]. Evidence suggests that when presented with multimorbid scenarios, LLMs may default to aggressive, standard-of-care recommendations that fail to account for competing risks, frailty, or pill burden [57,59,60]. Furthermore, model outputs often lack the specificity required for immediate action; for instance, a model might suggest "initiating high-intensity statin therapy" without specifying the agent or dosage appropriate for a patient’s specific renal function or drug-drug interaction profile [61].
A common misconception is that LLMs, being computational tools, are inherently capable of mathematical risk prediction. In reality, the token-prediction architecture of LLMs makes them unreliable arithmeticians, and studies benchmarking LLMs on medical calculations reveal high error rates [41]. Consequently, current stand-alone LLMs should not be used to replace deterministic risk calculators. Instead, the emerging approach involves a hybrid architecture known as "function calling." In this workflow, the LLM extracts the necessary variables (e.g., age, systolic blood pressure, smoking status, HDL levels) from unstructured clinical notes and transmits this data to an external interface to perform the calculation [24,42]. The LLM then receives the precise result and generates a plain-language interpretation for the clinician. While this architectural approach is currently being validated in broader medicine contexts, it represents a logical evolution for CV prevention, effectively bridging the gap between unstructured narrative data and rigid risk scoring systems [24,37,42].
Documentation, summarization, and administrative workflow. Perceived time pressure is a leading barrier to the implementation of preventive strategies [9,12]. Clinicians frequently cite the difficulty of synthesising a patient‘s CV history from the EHR as a deterrent to performing comprehensive risk assessments. LLMs integrated into the EHR infrastructure offer a solution to this “information overload.” Models trained on clinical notes have demonstrated the ability to summarise complex, longitudinal patient histories into concise “problem lists” with high accuracy in pilot studies [42].
In the context of prevention, such tools can automatically scan years of progress notes to flag relevant history that might be missed during a brief consultation. Furthermore, LLMs can act as a safety net for "diagnostic rescue" by identifying overlooked data that are not formally coded in the problem list (e.g., scanning historical imaging reports to identify incidental coronary calcification) [25,41]. By presenting this summary information directly to the clinician at the point of care and contextualizing a forgotten report against the patient’s current profile, these tools may reduce the cognitive load required to identify high-risk patients who are frequently under-identified and undertreated [8,25].
One of the most transformative clinician-facing applications is "ambient listening" technology, which is seeing rapid adoption in hospital settings [62,63]. These systems utilise automatic speech recognition paired with LLMs to transcribe clinician-patient encounters in real time and generate structured clinical documentation [63]. For preventive cardiology, the implications of ambient AI extend beyond mere convenience. By automating the creation of clinical notes, these tools theoretically liberate the clinician to maintain eye contact and engage in the nuanced dialogue required for motivational interviewing and shared decision-making. Early trials suggest that ambient AI tools can significantly reduce time spent on documentation and are associated with reduced measures of burnout [64–66]. Furthermore, these systems can be configured to automatically generate patient-friendly "After Visit Summaries" that translate the consultation’s medical jargon into clear, actionable steps for lifestyle change, thereby reinforcing the prevention plan discussed during the visit [12,65,67].
Risks, limitations, and evidence gaps in clinician applications. Despite these potential benefits, the deployment of clinical LLM applications introduces novel safety risks that require rigorous governance. The most prominent risk remains the generation of confident but false information ("hallucinations") [21,36]. In the context of guidelines, an LLM might invent a recommendation or fabricate a citation to support a treatment decision. Additionally, as already previously noted, standard LLMs suffer from "temporal obsolescence" and are frozen at the time of their training [37]. A model trained prior to recent updates will be unaware of new guidelines or novel therapies, necessitating that clinicians maintain independent, up-to-date knowledge to verify model outputs.
Finally, as models become more reliable, there is a paradoxical risk of "automation bias," where clinicians may become complacent, accepting AI-generated risk assessments or treatment plans without scrutiny [20,24]. This is particularly dangerous in preventive cardiology, where subtle patient preferences or social determinants of health, which the model may not appreciate, often dictate the success of a long-term treatment plan [23]. Furthermore, the use of ambient listening raises undefined legal questions regarding liability if an AI-generated note omits a critical symptom that leads to a missed diagnosis [68].
In summary, LLM applications in direct medical care hold the potential to act as powerful extensions of the clinician, handling the computational burden of data extraction, guideline retrieval, and documentation [20,41]. This technological support may allow the physician to focus on the uniquely human task of personalized risk negotiation [15,18]. However, these applications lack the reliability to function as autonomous decision-makers, necessitating a "human-in-the-loop" workflow where every AI-generated suggestion is rigorously verified by a supervising clinician [34,39].
4.3. System Applications
System-facing applications of LLMs operate at the level of health systems, payers, and research infrastructures rather than individual encounters. Outputs from these tools are typically not presented directly to patients or clinicians but are used to populate registries, drive performance dashboards, support population health programmes, and underpin real-world research. Within cardiovascular prevention, such “back-end” uses are central to addressing structural contributors to the implementation gap by enabling scalable phenotyping, continuous quality assurance, and integration of heterogeneous diagnostic and prognostic data [4,27,42].
Automated population phenotyping and data extraction. Effective system-level prevention depends on the accurate identification of high-risk cohorts across large populations. Traditional methods rely heavily on structured fields such as diagnosis codes and problem lists, which frequently fail to capture nuanced risk modifiers like family history, statin intolerance, functional limitations and psychosocial factors, that are often recorded only in free text [25,42]. System-facing transformer models leverage dense contextual representations to process large volumes of clinical narratives and can detect complex disease states and risk phenotypes with sensitivity that exceeds earlier rule-based extraction approaches [33,42].
Foundational work with transformer encoders pretrained on EHR data, such as Med-BERT, has demonstrated superior performance in phenotyping tasks compared with conventional models, learning clinically meaningful patterns from sequences of diagnostic and procedural codes [69]. When fine-tuned on cardiology narratives, such architectures achieve high fidelity in extracting core CV risk factors directly from unstructured notes, effectively creating “computable phenotypes” independent of billing codes [25,42]. Applications in heart failure, where models have been used to characterise New York Heart Association (NYHA) class and identify precipitants of decompensation from discharge summaries, provide proof of concept for this paradigm [70–72]. Translated to prevention, these approaches offer a means to capture uncodified prognostic variables, including frailty descriptors, barriers to medication adherence, and social determinants of health, at scale [73]. A key organisational opportunity is the repurposing of these pipelines from episodic analyses to automated, near-real-time registries. By continuously scanning clinical narratives for predefined risk patterns, health systems could systematically detect care gaps invisible to structured queries alone and maintain up-to-date lists of high-risk patients requiring intensified prevention [8,27].
Quality assurance and clinical registry automation. Automated phenotyping naturally feeds into quality assurance and registry maintenance. Historically, quality measurement in cardiovascular prevention has relied on manual chart review or analyses of structured administrative data, approaches that are labor-intensive, delayed, and prone to misclassification [21,70,74]. Early work with rule-based natural language processing (NLP) showed that key quality metrics, such as documentation of discharge instructions or left ventricular ejection fraction (LVEF), could be extracted from narrative text with performance comparable to human abstractors [75,76].
Modern NLP and LLM-based architectures extend this concept by refining diagnostic coding, correcting misclassified cases, and linking narrative descriptors to prescription and laboratory data [70,77]. This enables automated detection of complex “care gaps,” such as patients with established atherosclerotic cardiovascular disease who lack statin therapy or individuals with persistently elevated blood pressure despite frequent clinical encounters [74]. In principle, these systems allow a transition from periodic sample-based audits to continuous, system-wide surveillance of guideline adherence and attainment of prevention targets. High-resolution registries derived in this way would be valuable both for internal quality improvement and for benchmarking across institutions [78].
Resource optimization and cost efficiency. Health systems face a growing mismatch between the number of individuals requiring long-term CV risk management and the available specialist workforce [1,4]. In this context, system-level AI can function as an intelligent triage and planning engine [27,62]. By analysing longitudinal records, models can stratify populations not only by estimated risk but also by complexity profiles, distinguishing those needing referral to specialised preventive cardiology services from those who can be managed in primary care [25,77].
Beyond triage, LLM-based pipelines can reduce the marginal cost of high-resolution population surveillance by automating labor-intensive tasks such as chart abstraction for registries, manual coding of discharge summaries, and repeated screening of eligibility criteria for disease management programmes [70,75,76]. This automation could allow health systems to monitor large cohorts continuously, identify care gaps earlier, and reallocate human effort from data handling to clinical decision-making and patient engagement [27,62].
Big data, multimodal integration, and precision medicine. Conventional CV prevention strategies are inherently minimalist, relying on a limited set of variables to estimate risk. While such scores perform well at the population level, they omit most of the biological and environmental information that shapes individual trajectories [27,77]. The next frontier for system-facing AI is “deep phenotyping”, integrating diverse data streams into a high-dimensional representation of CV risk [25,27].
Achieving this requires expansion of the input layer beyond structured EHR data to encompass: (1) multi-omics information (e.g., genomics including polygenic risk scores, proteomics, microbiomics); (2) environmental and social exposures, quantified via geospatial data on air pollution, noise, and neighborhood deprivation; and (3) continuous physiological data from wearables, capturing heart rate variability, sleep patterns, and physical activity [27,77]. Additionally, narrow AI models play a crucial intermediate role by extracting high-value features from complex modalities, such as radiomic markers from imaging (e.g., perivascular fat attenuation on computed tomography angiography) or signal-based biomarkers from electrocardiograms (e.g., AI-estimated biological age or inferred atrial fibrillation risk) [26,32,74]. Within a system-level architecture, LLMs can serve as a semantic orchestration layer that links these heterogeneous outputs with clinical narratives, producing a unified, interpretable risk profile [20,42]. This multimodal integration underpins visions of precision prevention and “digital twin” models in cardiology, although significant challenges remain in terms of data interoperability, privacy, and regulatory oversight [27,39,51].
Novel prognostic systems. Transformer-based and LLM-related architectures also enable a transition from static, one-off risk scores to dynamic prognostic systems that model full patient trajectories. Rather than summarising history into a single baseline snapshot, these models encode longitudinal sequences of diagnoses, prescriptions, laboratory trends, and textual notes into high-dimensional embeddings. These representations can then be used to predict future cardiovascular events with greater temporal resolution and discriminatory performance than traditional regression approaches [25,27,67].
Work with Med-BERT has shown that transformers pretrained on structured EHR sequences outperform conventional deep-learning models on various disease prediction tasks by capturing contextual relationships between clinical events [69]. Building on this, the TRisk model has applied transformer-based learning to longitudinal datasets in primary prevention, achieving a C-index of 0.910 and substantially outperforming standard guideline-recommended risk scores for cardiovascular risk estimation [79]. Similarly, multimodal frameworks such as “PreCog” have begun to combine clinical transformers with high-fidelity diagnostic data, including cardiac magnetic resonance imaging features, polygenic risk, and metabolic profiles, identifying subgroups with markedly elevated relative risk of incident coronary artery disease compared with baseline estimates [77].
In parallel, investigators have evaluated whether general-purpose LLMs can perform risk stratification using structured inputs. When tested against conventional American College of Cardiology/American Heart Association (ACC/AHA) risk scores using cohorts such as the UK Biobank and KoGES (Korean Genome and Epidemiology Study), GPT-4 has demonstrated broadly comparable discrimination, suggesting that general LLMs can interpret risk variables and approximate standard calculators when appropriately prompted [80]. Nevertheless, even when discrimination is improved, calibration, clinical utility, and safety remain critical concerns, particularly when models are applied across populations and healthcare settings different from those used for development [37,39,57,59].
Bridge to research and clinical trials. System-level LLM applications also intersect with CV research, especially in real-world evidence generation and optimisation of clinical trial workflows. Trial recruitment is a persistent bottleneck that delays evaluation and dissemination of new preventive therapies. By parsing complex eligibility criteria and matching them against unstructured EHR data, LLM-based systems can identify potentially eligible participants at scale and across multiple sites, streamlining both feasibility assessments and active recruitment [78].
More broadly, LLM-enriched registries and phenotyping pipelines can support observational studies of preventive therapies in populations that more closely resemble routine practice than highly selected trial cohorts [27,42]. Such systems may help characterize patterns of uptake of novel interventions, quantify therapeutic inertia in high-risk primary prevention, and evaluate heterogeneous treatment effects across subgroups and health systems [25,27]. However, these applications are sensitive to the accuracy of labels, stability of underlying data distributions, and potential biases introduced by algorithmic processing [39,44,57]. Regulatory authorities are increasingly attentive to the provenance, transparency, and auditability of AI-derived real-world evidence, implying that LLM-based research workflows will require rigorous validation, version control, and explicit documentation before their outputs can inform guidelines or regulatory decisions [51,74].
Risks, limitations, and evidence gaps in system applications. Across system-facing use cases, several shared limitations constrain translation into routine cardiovascular prevention. Most reported implementations remain retrospective, single-center, or proof-of-concept analyses, with limited evidence on external validity across institutions, healthcare systems, and demographic subgroups [22,51,57]. Model performance is tightly coupled to documentation practices and data quality; settings with sparse or low-quality clinical notes may see degraded performance, raising concerns that automated phenotyping and quality metrics could systematically under-represent disadvantaged or under-resourced populations [39,44]. The opacity of LLM-derived representations complicates governance, as it may be difficult to distinguish genuine differences in care quality from algorithmic artefacts when interpreting performance dashboards or risk stratification outputs [20,74]. For advanced multimodal and prognostic systems, issues of calibration, drift over time, and robustness to shifts in practice patterns remain incompletely characterized, and prospective impact evaluations are missing [39,51,59]. Finally, large-scale linkage and processing of genomic, environmental, and behavioral data raise substantial concerns regarding privacy, consent, and acceptable secondary uses of data [17,68]. Collectively, these factors indicate that while system-facing LLM applications are central to the long-term vision of precision, population-level cardiovascular prevention, their safe deployment will require staged implementation, rigorous validation, and explicit fairness and governance frameworks [51,62,74].
5. Future Directions: From Hype to Clinical Utility
Despite rapid advances in model capability, current evaluations of LLMs in CV prevention remain dominated by retrospective benchmarks, vignette studies, and technical demonstrations rather than interventional trials embedded in care pathways [22,51,57]. The next phase of research must therefore shift from describing what models can do in principle to demonstrating what carefully governed systems achieve for patients, clinicians, and health systems in practice [26,62,74].
To bridge this gap, the field must undergo a fundamental translational shift from in silico performance to bedside utility [22,51]. The research agenda must move beyond evaluating text quality toward measuring clinical impact through prospective, pragmatic trials [51,62]. While the capacity to summarise patient history or explain guidelines is valuable, true utility must be demonstrated by tangible improvements in real-world care [27,42]. Future investigations should prioritize a spectrum of endpoints, ranging from intermediate biomarkers to hard clinical outcomes, including reductions in myocardial infarction and CV mortality [25,77]. Additionally, researchers must evaluate process metrics that reflect the quality of preventive care, such as medication adherence, the utilisation rates of validated risk scores, and the appropriateness of specialist referrals among others [9,27].
Equally critical is the rigorous evaluation of safety and trials should explicitly monitor for “automation bias”, the tendency for clinicians to uncritically accept AI-generated recommendations, particularly in complex, multimorbid cases [20,24,58]. Furthermore, for patient-facing applications, surveillance must extend to unintended behavioural harms, quantifying risks such as inappropriate reassurance, delayed help-seeking, and the propagation of subtle but clinically significant misinformation [21,23,34].
In parallel, technological development must evolve from passive information retrieval chatbots to more sophisticated “agentic workflows” and composite systems [81]. Building on the function-calling capabilities, in this emerging paradigm, the LLM functions not merely as a text generator but as an orchestrator authorized to perform a series of constrained, auditable actions via function calling [24,81]. Rather than simply suggesting that a risk assessment be performed, an agentic system could autonomously extract relevant variables from unstructured notes, execute a validated SCORE2 calculation through an API, query a drug–drug interaction database, and prepare a draft referral to a lipid clinic, all for clinician review and signature [41,81]. The clinician’s role shifts from manual data entry to executive verification of extracted data, calculations, and proposed orders [62,64].
To ensure safety and consistency, such systems must rely on RAG, thereby anchoring outputs in versioned, authoritative repositories, such as current ESC or AHA guidelines and institutional protocols, rather than the model’s opaque internal training data or uncurated web sources [37,55]. Future evaluation frameworks will need to consider the behaviour of the entire composite workflow, including robustness to atypical inputs and strict adherence to predefined safety boundaries [51,57].
Finally, the clinical translation of these tools must rigorously address equity, regulation, and global scalability [17,44]. There is a substantial risk that models trained on unrepresentative data will not only reproduce but amplify historical disparities in CV care [44,82]. Future validation studies must therefore necessitate the reporting of performance stratified by sex, ethnicity, and, where possible, socioeconomic status and language, scrutinizing calibration and error patterns in under-represented groups [39,82].
From a governance standpoint, regulatory frameworks must treat LLM-based tools for CV prevention as adaptive clinical decision support systems rather than static software [51,74]. This requires adopting a total product lifecycle perspective that includes pre-deployment validation, transparent documentation of intended use, structured processes for updating knowledge bases as guidelines evolve, and continuous post-deployment monitoring for model drift or unsafe performance [57,83]. If these conditions are met and systems are rigorously validated across diverse populations, LLMs could act as transformative force-multipliers in resource-limited environments [84]. By equipping primary care clinicians with accessible, guideline-based decision support and automated synthesis of complex preventive recommendations, they may help democratize access to specialist-level expertise and partially mitigate workforce shortages that currently hinder effective cardiovascular risk management outside tertiary centres [4,8,84].
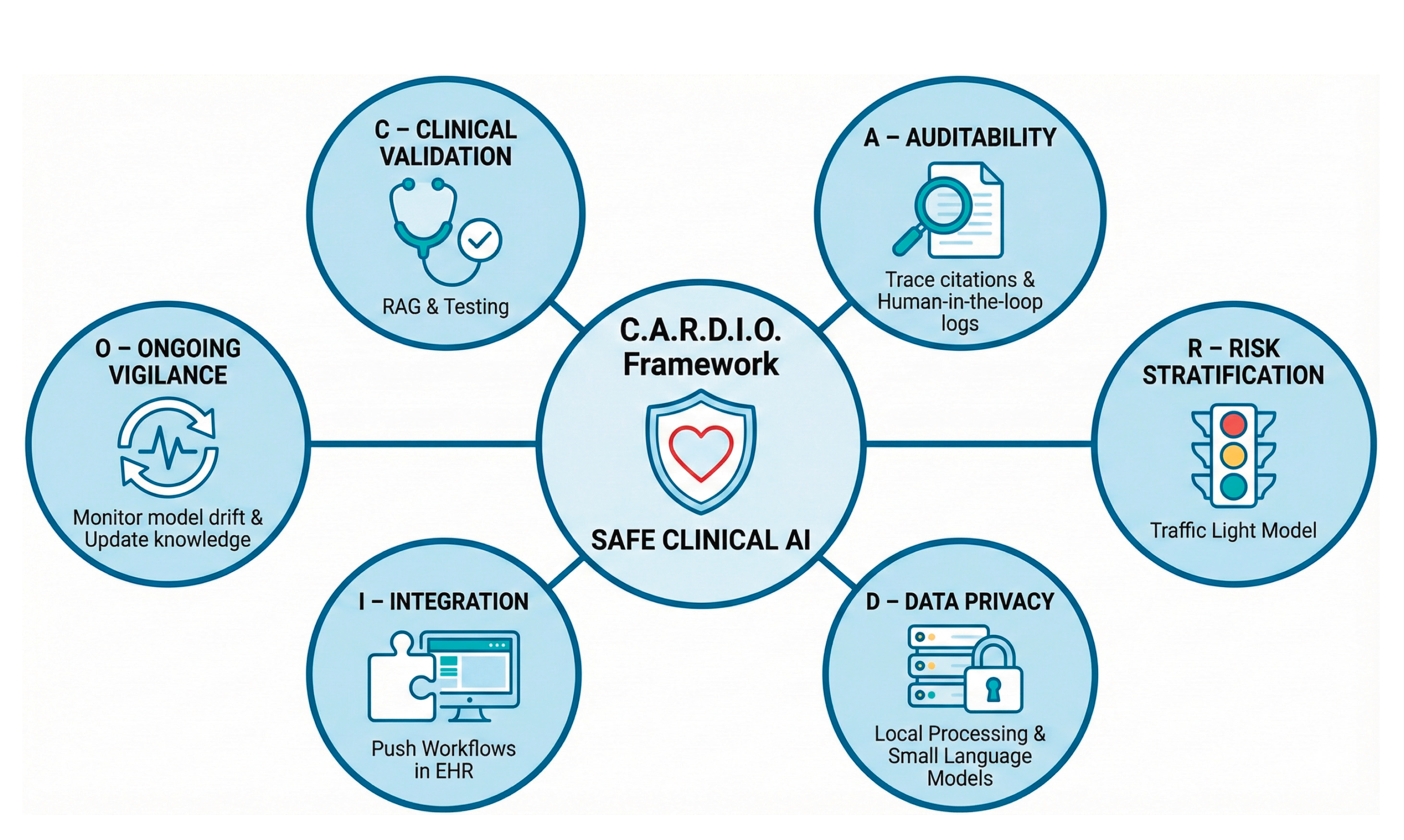
6. A Conceptual Framework for Safe Clinical Translation
To move LLMs from experimental tools to reliable clinical instruments, ad hoc adoption must be replaced with structured governance. The unique risks posed by generative AI, specifically hallucination, non-determinism, and temporal obsolescence, require a departure from traditional software regulation. Building on the evidence synthesised in this review, we propose the
C.A.R.D.I.O. Framework: a pragmatic roadmap designed to align generative AI with the rigor of preventive cardiology (
Figure 2). This framework prioritizes safety, transparency, and integration, ensuring that technological capability does not outpace ethical responsibility.
C - Clinical Validation. Safety in CV prevention dictates that LLMs must never rely solely on their internal parameters for clinical advice, as this renders them prone to hallucination [36]. Systems must employ RAG to anchor responses to versioned, authoritative sources [37,57]. Furthermore, validation metrics must evolve beyond multiple-choice accuracy. Models must be tested against "Gold Standard" clinical vignettes specifically designed to stress-test performance in multimorbid and complex scenarios, where standard algorithms frequently fail to account for competing risks [54,57].
A – Auditability. The "black box" nature of neural networks is incompatible with clinical accountability [20]. To ensure auditability, every clinical assertion generated by an LLM must include a direct citation to the source text [37]. Additionally, institutions must maintain "Human-in-the-Loop" logs of prompts, outputs, and subsequent clinician edits. These logs serve a dual purpose: they act as an audit trail in the event of adverse outcomes and provide a feedback dataset for continuous quality improvement [39,62].
R - Risk Stratification of Tasks. Not all AI tasks carry equal consequences. Deployment should follow a risk-tiered "traffic light" model [74,83]:
Low Risk (Green): Tasks such as drafting discharge summaries or patient education letters, where errors are easily detected by the patient or clinician.
Medium Risk (Orange): Clinical decision support, such as suggesting risk factors extracted from notes, which requires mandatory clinician verification.
High Risk (Red): Autonomous actions, such as initiating medication changes or auto-signing orders. Currently, these tasks remain prohibited for generative AI due to the risk of error. LLMs should act as "reasoning engines" that provide options and rationales, reserving the final decision for the human clinician.
D - Data Privacy. The widespread transmission of sensitive patient health information to public cloud-based models raises significant privacy and data sovereignty concerns [17,68]. To mitigate this, healthcare systems should prioritize the deployment of small language models and AI solutions that run locally within hospital firewalls [85,86]. This approach brings the model to the data, rather than sending data to the model, ensuring compliance with strict privacy regulations while reducing latency [40,85,86].
I - Integration into Workflow. To overcome the "implementation gap," LLM tools must reduce, not increase, the clinician’s cognitive load [4]. Integration must move beyond separate browser tabs to be fully embedded within the EHR infrastructure [42,62]. The interaction model should shift from a "Pull" system, where the user must actively query the AI, to a "push" system, where the LLM automatically flags care gaps during the routine chart review [8,27].
O - Ongoing Vigilance. Clinical validation is not a one-time event [51]. Systems require continuous post-deployment monitoring to detect "model drift" or performance degradation over time [39,83]. Furthermore, as medical knowledge evolves, models suffer from temporal obsolescence. Governance protocols must ensure that the underlying knowledge bases are updated dynamically as new guidelines or pivotal trial results are published, preventing the propagation of outdated medical advice [37,55].
7. Conclusions
LLMs offer a realistic opportunity to bridge the gap between guideline recommendations and clinical practice in preventive cardiology. By processing unstructured clinical data, they can identify high-risk patients, synthesise complex histories and personalise health communication, extending specialist-level expertise into routine care. Current models, limited by hallucinations, outdated knowledge, and non-deterministic behavior, are unsuitable as autonomous decision-makers and are best used as “reasoning engines” to support clinicians within supervised workflows. Realising their potential requires prospective, outcome-focused evaluation and structured governance frameworks, such as the C.A.R.D.I.O. model, to ensure safety, auditability, workflow integration, and equity. Ultimately, their value will depend on demonstrable improvements in risk factor control and CV outcomes across diverse populations.
Author Contributions
Both authors contributed equally to the preparation of the manuscript. Both authors have read and approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
During the preparation of this manuscript, the authors used the large language model Gemini 3 Pro to assist with figure generation and language editing. All AI-assisted content was reviewed and revised by the authors, who take full responsibility for the final content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC |
American College of Cardiology |
| AHA |
American Heart Association |
| AI |
Artificial intelligence |
| API |
Application programming interface |
| CV |
Cardiovascular |
| CVD |
Cardiovascular disease |
| EHR |
Electronic health record |
| ESC |
European Society of Cardiology |
| LLM |
Large language model |
| NLP |
Natural language processing |
| RAG |
Retrieval-augmented generation |
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. JACC 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 November 2025).
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Hoes, A.; Perk, J.; McKee, G.; Svavarsdóttir, M.H.; Czerwińska-Jelonkiewicz, K.; Janssen, A.; Oleksiak, A.; Dendale, P.; Graham, I.M. Optimising Implementation of European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: What Is Needed? Eur. J. Prev. Cardiol. 2021, 28, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Calabrò, P.; Solini, A.; Pirillo, A.; Gambacurta, R.; Ray, K.K.; Catapano, A.L.; Group*. The S.I. Lipid-Lowering Treatment and LDL-C Goal Attainment in High and Very High Cardiovascular Risk Patients: Evidence from the SANTORINI Study-The Italian Experience: The SANTORINI Italian Subgroup Study. Eur. Atheroscler. J. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; et al. Treatment Gaps in the Implementation of LDL Cholesterol Control among High- and Very High-Risk Patients in Europe between 2020 and 2021: The Multinational Observational SANTORINI Study. Lancet Reg. Health – Eur. 2023, 29. [Google Scholar] [CrossRef]
- Kotseva, K.; Wood, D.; De Bacquer, D.; De Backer, G.; Rydén, L.; Jennings, C.; Gyberg, V.; Amouyel, P.; Bruthans, J.; Castro Conde, A.; et al. EUROASPIRE IV: A European Society of Cardiology Survey on the Lifestyle, Risk Factor and Therapeutic Management of Coronary Patients from 24 European Countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar] [CrossRef]
- Uchmanowicz, I.; Czapla, M.; Lomper, K.; Iovino, P.; Rosiek-Biegus, M.; Surma, S.; Rahimi, K. Effective Implementation of Preventive Cardiology Guidelines: Pathways to Success. Eur. J. Prev. Cardiol. 2025, zwaf448. [Google Scholar] [CrossRef]
- Tokgozoglu, L.; Bruckert, E. Implementation, Target Population, Compliance and Barriers to Risk Guided Therapy. Eur. J. Prev. Cardiol. 2012, 19, 37–41. [Google Scholar] [CrossRef]
- Sposito, A.C.; Ramires, J.A.F.; Jukema, J.W.; Molina, J.C.; da Silva, P.M.; Ghadanfar, M.M.; Wilson, P.W.F. Physicians’ Attitudes and Adherence to Use of Risk Scores for Primary Prevention of Cardiovascular Disease: Cross-Sectional Survey in Three World Regions. Curr. Med. Res. Opin. 2009, 25, 1171–1178. [Google Scholar] [CrossRef]
- Liew, S.M.; Lee, W.K.; Khoo, E.M.; Ismail, I.Z.; Ambigapathy, S.; Omar, M.; Suleiman, S.Z.; Saaban, J.; Zaidi, N.F.M.; Yusoff, H. Can Doctors and Patients Correctly Estimate Cardiovascular Risk? A Cross-Sectional Study in Primary Care. BMJ Open 2018, 8, e017711. [Google Scholar] [CrossRef]
- Muthee, T.B.; Kimathi, D.; Richards, G.C.; Etyang, A.; Nunan, D.; Williams, V.; Heneghan, C. Factors Influencing the Implementation of Cardiovascular Risk Scoring in Primary Care: A Mixed-Method Systematic Review. Implement. Sci. 2020, 15, 57. [Google Scholar] [CrossRef]
- Smits, G.; Romeijnders, A.; Rozema, H.; Wijnands, C.; Hollander, M.; van Doorn, S.; Bots, M.; on behalf of the PoZoB primary care group. Stepwise Implementation of a Cardiovascular Risk Management Care Program in Primary Care. BMC Prim. Care 2022, 23, 1. [Google Scholar] [CrossRef]
- Bakhit, M.; Fien, S.; Abukmail, E.; Jones, M.; Clark, J.; Scott, A.M.; Glasziou, P.; Cardona, M. Cardiovascular Disease Risk Communication and Prevention: A Meta-Analysis. Eur. Heart J. 2024, 45, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Dennison Himmelfarb, C.R.; Beckie, T.M.; Allen, L.A.; Commodore-Mensah, Y.; Davidson, P.M.; Lin, G.; Lutz, B.; Spatz, E.S.; on behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing; on behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing; et al. Shared Decision-Making and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Singhal, K.; Azizi, S.; Tu, T.; Mahdavi, S.S.; Wei, J.; Chung, H.W.; Scales, N.; Tanwani, A.; Cole-Lewis, H.; Pfohl, S.; et al. Large Language Models Encode Clinical Knowledge. Nature 2023, 620, 172–180. [Google Scholar] [CrossRef]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large Language Models in Medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef]
- Quer, G.; Topol, E.J. The Potential for Large Language Models to Transform Cardiovascular Medicine. Lancet Digit. Health 2024. [Google Scholar] [CrossRef]
- Rao, S.J.; Iqbal, S.B.; Isath, A.; Virk, H.U.H.; Wang, Z.; Glicksberg, B.S.; Krittanawong, C. An Update on the Use of Artificial Intelligence in Cardiovascular Medicine. Hearts 2024, 5, 91–104. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Weissenbacher, D.; Moore, J.H.; Gonzalez-Hernandez, G.; Asselbergs, F.W. Artificial Intelligence: Revolutionizing Cardiology with Large Language Models. Eur. Heart J. 2024, 45, 332–345. [Google Scholar] [CrossRef]
- Olariu, M.-E.; Burlacu, A.; Brinza, C.; Iftene, A. Large Language Models for ESC Guideline Interpretation: A Targeted Review of Accuracy and Applicability. Future Cardiol. 2025, 21, 961–968. [Google Scholar] [CrossRef]
- Ferreira Santos, J.; Ladeiras-Lopes, R.; Leite, F.; Dores, H. Applications of Large Language Models in Cardiovascular Disease: A Systematic Review. Eur. Heart J. Digit. Health 2025, 6, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Hoffmann, L.; Rueger, C.; van Dijk, E.H.; Kader, R.; Ortiz-Prado, E.; Makowski, M.R.; Saba, L.; Hadamitzky, M.; Kather, J.N.; et al. Current Applications and Challenges in Large Language Models for Patient Care: A Systematic Review. Commun. Med. 2025, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Parsa, S.; Somani, S.; Dudum, R.; Jain, S.S.; Rodriguez, F. Artificial Intelligence in Cardiovascular Disease Prevention: Is It Ready for Prime Time? Curr. Atheroscler. Rep. 2024, 26, 263–272. [Google Scholar] [CrossRef] [PubMed]
- El Sherbini, A.; Rosenson, R.S.; Al Rifai, M.; Virk, H.U.H.; Wang, Z.; Virani, S.; Glicksberg, B.S.; Lavie, C.J.; Krittanawong, C. Artificial Intelligence in Preventive Cardiology. Prog. Cardiovasc. Dis. 2024, 84, 76–89. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef]
- Meder, B.; Asselbergs, F.W.; Ashley, E. Artificial Intelligence to Improve Cardiovascular Population Health. Eur. Heart J. 2025, 46, 1907–1916. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Ge, J. Artificial Intelligence Large Language Models in Cardiology. Rev. Cardiovasc. Med. 2025, 26, 39452. [Google Scholar] [CrossRef]
- Attention Is All You Need. Available online: https://arxiv.org/html/1706.03762v7 (accessed on 30 November 2025).
- Introducing ChatGPT. Available online: https://openai.com/index/chatgpt/ (accessed on 16 September 2024).
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An Artificial Intelligence-Enabled ECG Algorithm for the Identification of Patients with Atrial Fibrillation during Sinus Rhythm: A Retrospective Analysis of Outcome Prediction. Lancet Lond. Engl. 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Lei, J.; Shen, Y.; Wang, J.; Wei, P.; Chu, Z.; Qin, Z.; Ren, K. A Survey on Medical Large Language Models: Technology, Application, Trustworthiness, and Future Directions 2024.
- Lee, P.; Bubeck, S.; Petro, J. Benefits, Limits, and Risks of GPT-4 as an AI Chatbot for Medicine. N. Engl. J. Med. 2023, 388, 1233–1239. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, F.; Gu, B.; Zou, X.; Huang, J.; Wu, J.; Li, Y.; Chen, S.S.; Zhou, P.; Liu, J.; et al. A Survey of Large Language Models in Medicine: Progress, Application, and Challenge 2024.
- Jin, Q.; Wan, N.; Leaman, R.; Tian, S.; Wang, Z.; Yang, Y.; Wang, Z.; Xiong, G.; Lai, P.-T.; Zhu, Q.; et al. Demystifying Large Language Models for Medicine: A Primer 2024.
- Zakka, C.; Chaurasia, A.; Shad, R.; Dalal, A.R.; Kim, J.L.; Moor, M.; Alexander, K.; Ashley, E.; Boyd, J.; Boyd, K.; et al. Almanac: Retrieval-Augmented Language Models for Clinical Medicine 2023.
- Singhal, K.; Tu, T.; Gottweis, J.; Sayres, R.; Wulczyn, E.; Amin, M.; Hou, L.; Clark, K.; Pfohl, S.R.; Cole-Lewis, H.; et al. Toward Expert-Level Medical Question Answering with Large Language Models. Nat. Med. 2025, 31, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wornow, M.; Xu, Y.; Thapa, R.; Patel, B.; Steinberg, E.; Fleming, S.; Pfeffer, M.A.; Fries, J.; Shah, N.H. The Shaky Foundations of Large Language Models and Foundation Models for Electronic Health Records. Npj Digit. Med. 2023, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, Q.; Chen, A.; Peng, C.; Hu, Y.; Lin, F.; Peng, X.; Huang, J.; Zhang, J.; Keloth, V.; et al. Me-LLaMA: Foundation Large Language Models for Medical Applications. Res. Sq. 2024, rs.3.rs-4240043. [Google Scholar] [CrossRef]
- Khandekar, N.; Jin, Q.; Xiong, G.; Dunn, S.; Applebaum, S.S.; Anwar, Z.; Sarfo-Gyamfi, M.; Safranek, C.W.; Anwar, A.A.; Zhang, A.; et al. MedCalc-Bench: Evaluating Large Language Models for Medical Calculations 2024.
- Yang, X.; Chen, A.; PourNejatian, N.; Shin, H.C.; Smith, K.E.; Parisien, C.; Compas, C.; Martin, C.; Costa, A.B.; Flores, M.G.; et al. A Large Language Model for Electronic Health Records. Npj Digit. Med. 2022, 5, 194. [Google Scholar] [CrossRef]
- Chow, J.C.L.; Wong, V.; Li, K. Generative Pre-Trained Transformer-Empowered Healthcare Conversations: Current Trends, Challenges, and Future Directions in Large Language Model-Enabled Medical Chatbots. BioMedInformatics 2024, 4, 837–852. [Google Scholar] [CrossRef]
- Omiye, J.A.; Lester, J.C.; Spichak, S.; Rotemberg, V.; Daneshjou, R. Large Language Models Propagate Race-Based Medicine. Npj Digit. Med. 2023, 6, 195. [Google Scholar] [CrossRef]
- Sarraju, A.; Bruemmer, D.; Van Iterson, E.; Cho, L.; Rodriguez, F.; Laffin, L. Appropriateness of Cardiovascular Disease Prevention Recommendations Obtained From a Popular Online Chat-Based Artificial Intelligence Model. JAMA 2023, 329, 842–844. [Google Scholar] [CrossRef]
- Ayers, J.W.; Poliak, A.; Dredze, M.; Leas, E.C.; Zhu, Z.; Kelley, J.B.; Faix, D.J.; Goodman, A.M.; Longhurst, C.A.; Hogarth, M.; et al. Comparing Physician and Artificial Intelligence Chatbot Responses to Patient Questions Posted to a Public Social Media Forum. JAMA Intern. Med. 2023, 183, 589–596. [Google Scholar] [CrossRef]
- Behers, B.J.; Vargas, I.A.; Behers, B.M.; Rosario, M.A.; Wojtas, C.N.; Deevers, A.C.; Hamad, K.M. Assessing the Readability of Patient Education Materials on Cardiac Catheterization From Artificial Intelligence Chatbots: An Observational Cross-Sectional Study. Cureus 2024, 16, e63865. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Sarraju, A.; Kalwani, N.M.; Dexter, J.P. Evaluation of Prompts to Simplify Cardiovascular Disease Information Generated Using a Large Language Model: Cross-Sectional Study. J. Med. Internet Res. 2024, 26, e55388. [Google Scholar] [CrossRef]
- Bucher, A.; Blazek, E.S.; Symons, C.T. How Are Machine Learning and Artificial Intelligence Used in Digital Behavior Change Interventions? A Scoping Review. Mayo Clin. Proc. Digit. Health 2024, 2, 375–404. [Google Scholar] [CrossRef]
- Aggarwal, A.; Tam, C.C.; Wu, D.; Li, X.; Qiao, S. Artificial Intelligence–Based Chatbots for Promoting Health Behavioral Changes: Systematic Review. J. Med. Internet Res. 2023, 25, e40789. [Google Scholar] [CrossRef]
- Bedi, S.; Liu, Y.; Orr-Ewing, L.; Dash, D.; Koyejo, S.; Callahan, A.; Fries, J.A.; Wornow, M.; Swaminathan, A.; Lehmann, L.S.; et al. Testing and Evaluation of Health Care Applications of Large Language Models: A Systematic Review. JAMA 2025, 333, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, I.; Cagnina, A.; Luangphiphat, W.; Mahendiran, T.; Muller, O.; Abbe, E.; Fournier, S. ChatGPT Takes on the European Exam in Core Cardiology: An Artificial Intelligence Success Story? Eur. Heart J. - Digit. Health 2023, 4, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Zeljković, I.; Rode, F.; Lisičić, A.; Nola, I.A.; Pavlović, N.; Manola, Š. The Pulse of Artificial Intelligence in Cardiology: A Comprehensive Evaluation of State-of-the-Art Large Language Models for Potential Use in Clinical Cardiology 2024, 2023.08.08.23293689.
- Nastasi, A.J.; Courtright, K.R.; Halpern, S.D.; Weissman, G.E. A Vignette-Based Evaluation of ChatGPT’s Ability to Provide Appropriate and Equitable Medical Advice across Care Contexts. Sci. Rep. 2023, 13, 17885. [Google Scholar] [CrossRef]
- ESC Chat - Your AI-Powered Guidelines Companion. Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Guidelines-derivative-products/Chat (accessed on 24 November 2025).
- Sarraju, A.; Ouyang, D.; Itchhaporia, D. The Opportunities and Challenges of Large Language Models in Cardiology. JACC Adv. 2023, 2, 100438. [Google Scholar] [CrossRef]
- Hager, P.; Jungmann, F.; Holland, R.; Bhagat, K.; Hubrecht, I.; Knauer, M.; Vielhauer, J.; Makowski, M.; Braren, R.; Kaissis, G.; et al. Evaluation and Mitigation of the Limitations of Large Language Models in Clinical Decision-Making. Nat. Med. 2024, 30, 2613–2622. [Google Scholar] [CrossRef]
- Goh, E.; Gallo, R.; Hom, J.; Strong, E.; Weng, Y.; Kerman, H.; Cool, J.A.; Kanjee, Z.; Parsons, A.S.; Ahuja, N.; et al. Large Language Model Influence on Diagnostic Reasoning: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2440969. [Google Scholar] [CrossRef]
- Roeschl, T.; Hoffmann, M.; Hashemi, D.; Rarreck, F.; Hinrichs, N.; Trippel, T.D.; Gröschel, M.I.; Unbehaun, A.; Klein, C.; Kempfert, J.; et al. Assessing the Limitations of Large Language Models in Clinical Practice Guideline-Concordant Treatment Decision-Making on Real-World Data 2025, 2024.11.20.24313385.
- Griot, M.; Hemptinne, C.; Vanderdonckt, J.; Yuksel, D. Large Language Models Lack Essential Metacognition for Reliable Medical Reasoning. Nat. Commun. 2025, 16, 1–10. [Google Scholar] [CrossRef]
- Aydin, S.; Karabacak, M.; Vlachos, V.; Margetis, K. Navigating the Potential and Pitfalls of Large Language Models in Patient-Centered Medication Guidance and Self-Decision Support. Front. Med. 2025, 12. [Google Scholar] [CrossRef]
- Poon, E.G.; Lemak, C.H.; Rojas, J.C.; Guptill, J.; Classen, D. Adoption of Artificial Intelligence in Healthcare: Survey of Health System Priorities, Successes, and Challenges. J. Am. Med. Inform. Assoc. JAMIA 2025, 32, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.I.; Coristine, A.J.; Benis, A. AI Scribes in Health Care: Balancing Transformative Potential With Responsible Integration. JMIR Med. Inform. 2025, 13, e80898. [Google Scholar] [CrossRef]
- Kim, E.; Liu, V.X.; Singh, K. AI Scribes Are Not Productivity Tools (Yet). NEJM AI 2025, 2, AIe2501051. [Google Scholar] [CrossRef]
- Olson, K.D.; Meeker, D.; Troup, M.; Barker, T.D.; Nguyen, V.H.; Manders, J.B.; Stults, C.D.; Jones, V.G.; Shah, S.D.; Shah, T.; et al. Use of Ambient AI Scribes to Reduce Administrative Burden and Professional Burnout. JAMA Netw. Open 2025, 8, e2534976. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Devon-Sand, A.; Ma, S.P.; Jeong, Y.; Crowell, T.; Smith, M.; Liang, A.S.; Delahaie, C.; Hsia, C.; Shanafelt, T.; et al. Ambient Artificial Intelligence Scribes: Physician Burnout and Perspectives on Usability and Documentation Burden. J. Am. Med. Inform. Assoc. JAMIA 2025, 32, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.Y.K.; Subramanian, C.R.; Ali, S.S.; Apolinario, M.; Askin, E.; Barish, P.; Cheng, M.; Deardorff, W.J.; Donthi, N.; Ganeshan, S.; et al. Physician- and Large Language Model-Generated Hospital Discharge Summaries. JAMA Intern. Med. 2025, 185, 818–825. [Google Scholar] [CrossRef]
- Cohen, I.G.; Ritzman, J.; Cahill, R.F. Ambient Listening—Legal and Ethical Issues. JAMA Netw. Open 2025, 8, e2460642. [Google Scholar] [CrossRef]
- Rasmy, L.; Xiang, Y.; Xie, Z.; Tao, C.; Zhi, D. Med-BERT: Pretrained Contextualized Embeddings on Large-Scale Structured Electronic Health Records for Disease Prediction. NPJ Digit. Med. 2021, 4, 86. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Raman, V.K.; Patel, S.S.; Cheng, Y.; Parulkar, A.; Lam, P.H.; Moore, H.; Sheriff, H.M.; Fonarow, G.C.; et al. Artificial Intelligence Approaches for Phenotyping Heart Failure in U.S. Veterans Health Administration Electronic Health Record. ESC Heart Fail. 2024, 11, 3155–3166. [Google Scholar] [CrossRef]
- Topaz, M.; Radhakrishnan, K.; Blackley, S.; Lei, V.; Lai, K.; Zhou, L. Studying Associations Between Heart Failure Self-Management and Rehospitalizations Using Natural Language Processing. West. J. Nurs. Res. 2017, 39, 147–165. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Shanahan, L.; Munroe, J.; Horn, S.; Speedie, S. Discovering and Identifying New York Heart Association Classification from Electronic Health Records. BMC Med. Inform. Decis. Mak. 2018, 18, 48. [Google Scholar] [CrossRef]
- Guevara, M.; Chen, S.; Thomas, S.; Chaunzwa, T.L.; Franco, I.; Kann, B.H.; Moningi, S.; Qian, J.M.; Goldstein, M.; Harper, S.; et al. Large Language Models to Identify Social Determinants of Health in Electronic Health Records. Npj Digit. Med. 2024, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Wellnhofer, E. Real-World and Regulatory Perspectives of Artificial Intelligence in Cardiovascular Imaging. Front. Cardiovasc. Med. 2022, 9, 890809. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Garvin, J.H.; Goldstein, M.K.; Hwang, T.S.; Redd, A.; Bolton, D.; Heidenreich, P.A.; Meystre, S.M. Extraction of Left Ventricular Ejection Fraction Information from Various Types of Clinical Reports. J. Biomed. Inform. 2017, 67, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.H.; Elkin, P.L.; Shen, S.; Brown, S.; Trusko, B.; Wang, E.; Hoke, L.; Quiaoit, Y.; Lajoie, J.; Weiner, M.G.; et al. Automated Quality Measurement in Department of the Veterans Affairs Discharge Instructions for Patients with Congestive Heart Failure. J. Healthc. Qual. Off. Publ. Natl. Assoc. Healthc. Qual. 2013, 35, 16–24. [Google Scholar] [CrossRef]
- Pandey, D.; Xu, L.; Kun, E.; Li, C.; Wang, J.Y.; Melek, A.; DiCarlo, J.C.; Castillo, E.; Narula, J.; Taylor, C.A.; et al. Multimodal AI for Precision Preventive Cardiology 2025, 2025.10.09.25337677.
- Jin, Q.; Wang, Z.; Floudas, C.S.; Chen, F.; Gong, C.; Bracken-Clarke, D.; Xue, E.; Yang, Y.; Sun, J.; Lu, Z. Matching Patients to Clinical Trials with Large Language Models. Nat. Commun. 2024, 15, 9074. [Google Scholar] [CrossRef]
- Rao, S.; Li, Y.; Mamouei, M.; Salimi-Khorshidi, G.; Wamil, M.; Nazarzadeh, M.; Yau, C.; Collins, G.S.; Jackson, R.; Vickers, A.; et al. Refined Selection of Individuals for Preventive Cardiovascular Disease Treatment with a Transformer-Based Risk Model. Lancet Digit. Health 2025, 7. [Google Scholar] [CrossRef]
- Han, C.; Kim, D.W.; Kim, S.; Chan You, S.; Park, J.Y.; Bae, S.; Yoon, D. Evaluation of GPT-4 for 10-Year Cardiovascular Risk Prediction: Insights from the UK Biobank and KoGES Data. iScience 2024, 27, 109022. [Google Scholar] [CrossRef]
- Roeschl, T.; Hoffmann, M.; Unbehaun, A.; Dreger, H.; Hindricks, G.; Falk, V.; Balicer, R.; Tanacli, R.; Hohendanner, F.; Meyer, A. Development of an LLM Pipeline Surpassing Physicians in Cardiovascular Risk Score Calculation 2025, 2025.11.11.25340002.
- Mihan, A.; Pandey, A.; Spall, H.G.V. Mitigating the Risk of Artificial Intelligence Bias in Cardiovascular Care. Lancet Digit. Health 2024, 6, e749–e754. [Google Scholar] [CrossRef]
- Lekadir, K.; Frangi, A.F.; Porras, A.R.; Glocker, B.; Cintas, C.; Langlotz, C.P.; Weicken, E.; Asselbergs, F.W.; Prior, F.; Collins, G.S.; et al. FUTURE-AI: International Consensus Guideline for Trustworthy and Deployable Artificial Intelligence in Healthcare. BMJ 2025, 388, e081554. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, D.; Qin, Y.; Fan, Z.; Ci, F.N.Y.; Klonoff, D.C.; Ji, J.S.; Zhang, S.; Amissah-Arthur, K.N.; de Tavárez, M.M.J.; et al. Large Language Models and Global Health Equity: A Roadmap for Equitable Adoption in LMICs. Lancet Reg. Health – West. Pac. 2025, 63. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, H.; Lee, J.; Park, S.; Kim, D.; Lee, T.; Yoon, C.; Sohn, J.; Park, J.; Reykhart, O.; et al. Small Language Models Learn Enhanced Reasoning Skills from Medical Textbooks. Npj Digit. Med. 2025, 8, 240. [Google Scholar] [CrossRef]
- Garg, M.; Raza, S.; Rayana, S.; Liu, X.; Sohn, S. The Rise of Small Language Models in Healthcare: A Comprehensive Survey 2025.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).