1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that can lead to irreversible joint destruction and permanent disability if treatment is suboptimal [
1]. Contemporary treatment guidelines are encouraging to treat RA early and aggressively to reach treatment target - remission or at least low disease activity in order to avoid joint damage and preserve quality of life [
2,
3]. Advanced therapies have been in use in RA treatment for more than 25 years. In addition to first biologic disease modifying antirheumatic drugs (bDMARDs) - TNF inhibitors - later other cytokine or cell targeted monoclonal antibodies have become available, targeted synthetic DMARDs (tsDMARD) being the newest therapy line. Both biologic and targeted synthetic DMARDs have been revolutionary in achieving remission as treatment goal but still a significant proportion of RA patients fail to reach the target outcome with the first b/tsDMARD treatment courses
Although randomized double-blind, placebo-controlled trials (RCT) have been conducted for all biological drugs, their short duration and restrictive inclusion criteria limit generalizability to real- life settings. For this reason, biologics registries for RA have been established in Europe and elsewhere [
4]. From international registries (e.g., BSRBR-RA, DANBIO, RABBIT) it is known that each subsequent line of therapy may result in a lower treatment response, but remission can still be achieved even in later lines of therapy [
5,
6,
7], also a Finnish registry-based study showed that only up to ~40% of patients in their registry were eligible for inclusion in an RCT [
8].
A particular challenge in modern RA management is the subgroup of patients with
difficult-to-treat RA, recently defined by EULAR as patients who fail to reach treatment targets despite the use of multiple biologic or targeted synthetic DMARDs with different mechanisms of action [
9]. These patients often require several sequential lines of therapy and may still experience active disease, comorbidities, or treatment-limiting adverse events. Registry data show that although treatment response rates decrease with each additional line, meaningful improvements and even remission remain achievable in later lines of therapy [
10,
11]. The need for repeated switches of DMARDs reflects the real-world presence of difficult-to-treat RA and underscores the importance of registry studies in capturing their clinical course and outcomes.
In Estonia TNF inhibitors became available in late 2006, and access to advanced therapies improved over time: although EULAR removed the requirement for prior treatment with three synthetic DMARDs in 2016 [
12], Estonia — which had some of the strictest eligibility criteria in Europe — only aligned with this recommendation in 2020 when the national treatment requirements were finally updated [
13]. Biologic and target specific treatments are covered by the Estonian Health Insurance Fund (Tervisekassa) with a very high reimbursement rate—typically up to 100% of the treatment cost—once eligibility criteria are met.
This is the first study to provide an overview of Estonian RA patients focusing on patterns of sequential biologic therapy, treatment persistence, and the reasons for treatment discontinuation and switching, based on data from the Estonian Biologic Therapy Registry. By placing these findings in the context of international registry-based research, our study contributes to the broader understanding of how biologic and targeted therapies are used in real-world practice, and how treatment strategies evolve in patients with difficult-to-treat RA.
2. Materials and Methods
The Estonian Society for Rheumatology (ESR) established the Biologic Therapy Registry (BTR) in 2013 to collect information on the use of advanced therapies, their effectiveness and adverse events. Data from 2006-2013 were collected retrospectively and data collection has been prospective from 2013 onwards. It is a private registry belonging to ESR that collects digital data from all rheumatology units in Estonia on patients receiving biologic and targeted synthetic therapies. Data are provided by the local rheumatologist and entered into the registry by rheumatology nurses/specialized secretaries at the initiation and discontinuation of b/tsDMARD therapy, at 6 months, at 12 months and annually from there on. Adverse events are registered on an ongoing basis. Baseline data includes age at b/tsDMARD initiation, sex, BMI, education, comorbidities, ACPA/RF positivity, conventional DMARD therapy (including glucocorticosteroid use), disease activity - tender, swollen joint counts, ESR, CRP, DAS 28 score, health assessment questionnaire, tuberculosis and B/C hepatitis related information.
Analysis included data on all patients in ESR BTR with the diagnosis of RA (ICD-10 codes M05.8 and M06.0) who had received at least one dose of bDMARD or tsDMARD between 2006-2022. During this period drugs that have been available in Estonia are TNF inhibitors (infliximab, etanercept, adalimumab, golimumab), IL-6 inhibitors (tocilizumab, sarilumab), JAK inhibitors (tofacitinib, upadacitinib), abatacept (CTLA4 analogue) and rituximab (anti CD20).
Data were analysed according to sequential treatment lines, where each change of biologic or targeted therapy was considered a new line. Outcomes could therefore be evaluated across different stages of therapy, capturing both first-line initiation and subsequent switches. For the present study, analyses were restricted to the first five treatment lines, as these represented most patients, while later lines were too infrequent for meaningful comparison. This approach follows the framework applied in other registry studies, such as the British Society for Rheumatology Biologics Registry analysis by Zhao et al. [
10], which similarly reported outcomes up to the fifth line of therapy.
Effectiveness was evaluated at 6 months using three complementary approaches. First, we assessed the change in Disease Activity Score (DAS28-CRP) from baseline to 6 months as a continuous variable. Second, we categorized treatment responses according to remission (DAS28 ≤2.6), low disease activity (LDA, DAS28 ≤3.2), and the EULAR response criteria, distinguishing between good, moderate, and no response. Non-response was defined as a DAS28 reduction of less than 0.6 or a DAS28 score remaining above 5.1, while moderate response comprised all cases between non-response and good response. Finally, treatment survival was analysed as the time from therapy initiation to discontinuation.
In addition to line-specific outcomes, we examined overall patterns of sequential therapy by capturing each switch of b/tsDMARD. This allowed us to describe both within-class transitions (e.g., TNF inhibitor to another TNF inhibitor) and switches across classes such as IL-6 inhibitors, JAK inhibitors, CTLA4, or CD20 therapy. This approach follows the framework applied in other registry studies [
10,
14], which similarly mapped sequential treatment trajectories to highlight real-world therapeutic strategies in RA.
Reasons for discontinuation of biologic or targeted therapies were systematically categorized, allowing us to distinguish between lack or loss of efficacy, adverse events, remission, and other patient- or physician-related factors. By analysing these data, we aimed to better understand the clinical circumstances leading to treatment discontinuation and how these patterns may influence subsequent therapy choices.
To describe baseline characteristics, frequencies and proportions are presented for categorical variables. For continuous variables, means with standard deviations (SD) or medians with interquartile ranges (IQRs) were calculated, depending on the distribution of the variables. To compare differences between treatment categories for remission-related indicators (e.g., change in DAS28, low disease activity, EULAR response criteria, median drug survival time) 95% confidence intervals (CI) were calculated. Kaplan-Meier survival curves were performed to estimate the probability of treatment discontinuation according to the line of therapy. Statistical analyses were conducted using Stata, version 14.2. For visualizing treatment transitions, a Sankey diagram was used
Generative artificial intelligence (ChatGPT, OpenAI) was used to support the writing process of this manuscript. Specifically, it was applied to assist with drafting, restructuring, and improving the clarity and flow of text. The authors reviewed and edited all AI-assisted content to ensure accuracy and scientific integrity. No AI was used for data collection, analysis, or interpretation.
This study was approved by the Research Ethics Committee of the National Institute for Health Development, Estonia (Tervise Arengu Instituudi inimuuringute eetikakomitee (TAIEK); Study No. 2396, TAIEK meeting protocol No. 44. Decision No. 1122 issued on October 17, 2022.).
3. Results
Altogether 1074 RA patients were included in the study. The follow-up time in the registry ranged from 0 to 16 years. The median follow-up time was 3.72 (interquartile range 1,5- 8,1) years.
3.1. Baseline Characteristics
The baseline characteristics for lines of treatment are shown in
Table 1.
Of the total of 1074 patients initiating a first course of biologic therapy, 42% received a second line of treatment, 18% a third line, 8% a fourth, and 3% a fifth. The mean age at treatment initiation was around 53 years, with approximately 80% of patients being female, and the mean body mass index close to 26. These characteristics remained relatively stable across treatment lines. Seropositivity was considerably high in our patients being around 86% in the first line of therapy and decreasing to 71% in the 5th line.
We also observed that the mean age at diagnosis decreased, while the prevalence of at least one comorbidity increased, with each additional treatment line. Baseline disease activity was highest at first-line therapy, as reflected by a mean DAS28 of 5.3. In subsequent treatment lines, mean DAS28 scores as well as median ESR and CRP values were numerically lower, although disease activity remained clinically relevant.
3.2. Effectiveness Assessments
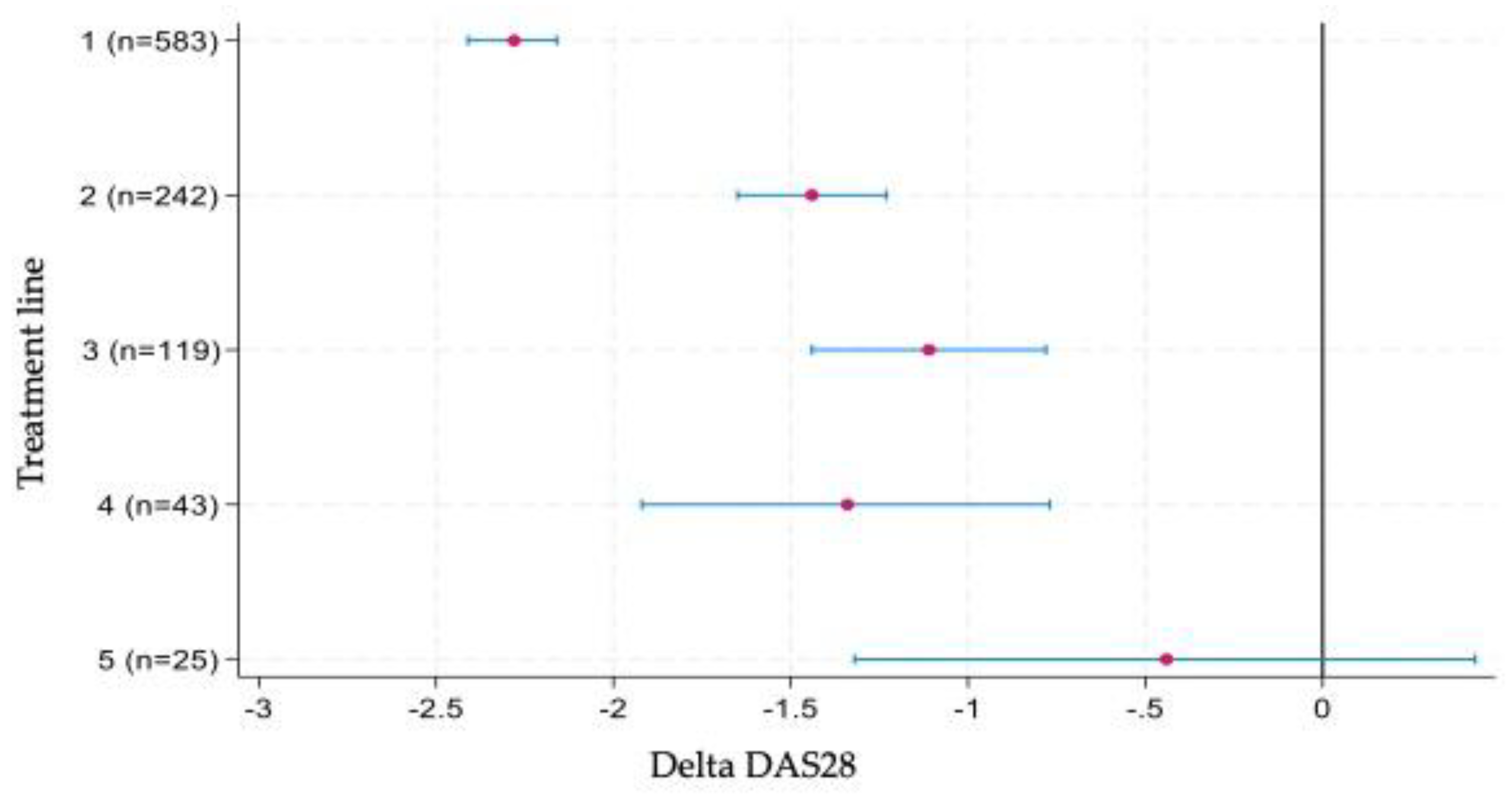
3.2.1. Delta DAS28
Outcomes for the continuous change in DAS28 at 6 months are presented in Figure 1. The greatest improvement was observed during first-line therapy, with a mean reduction of approximately 2.3 points. Substantial decreases were also seen in the second to fourth treatment lines, although the magnitude of change was smaller compared with first-line therapy. In contrast, by the fifth treatment line, the improvement in DAS28 had become marginal.
3.2.2. Categorical Response
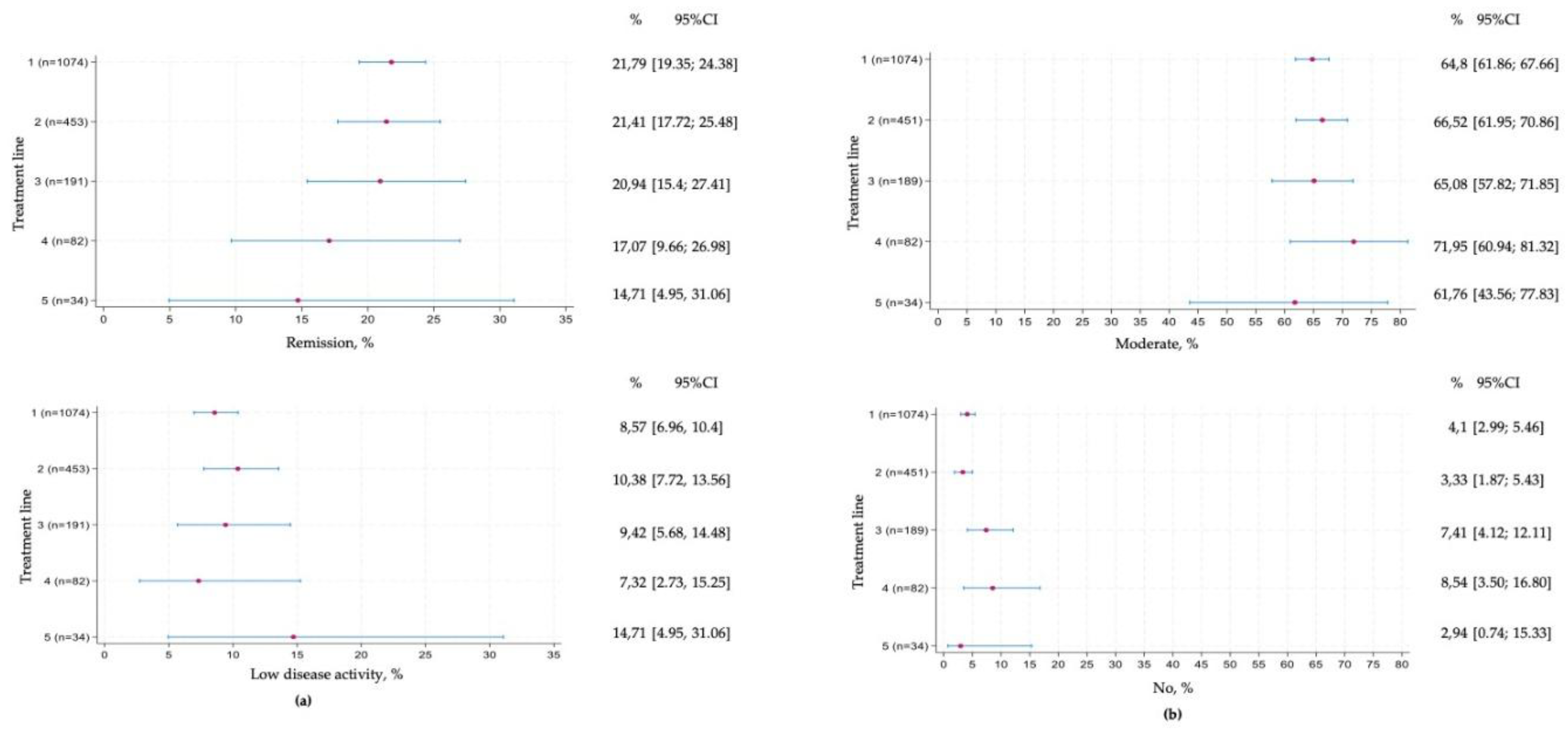
Remission according to DAS28 was achieved in approximately 20% of patients during treatment lines 1 to 3, declining to around 15% in later lines, with progressively wider confidence intervals reflecting the smaller patient numbers (section (a) of
Figure 2). Low disease activity was observed in a smaller proportion of patients, remaining relatively stable at 7–10% across lines 1 to 4.
Section (b) of
Figure 2 shows the distribution of EULAR responses. The proportion of patients achieving a moderate response was high, at around 60%, and remained constant across treatment lines, although with widening confidence intervals in later lines. The proportion of non-responders was consistently low, ranging between 2% and 10% across therapy lines.
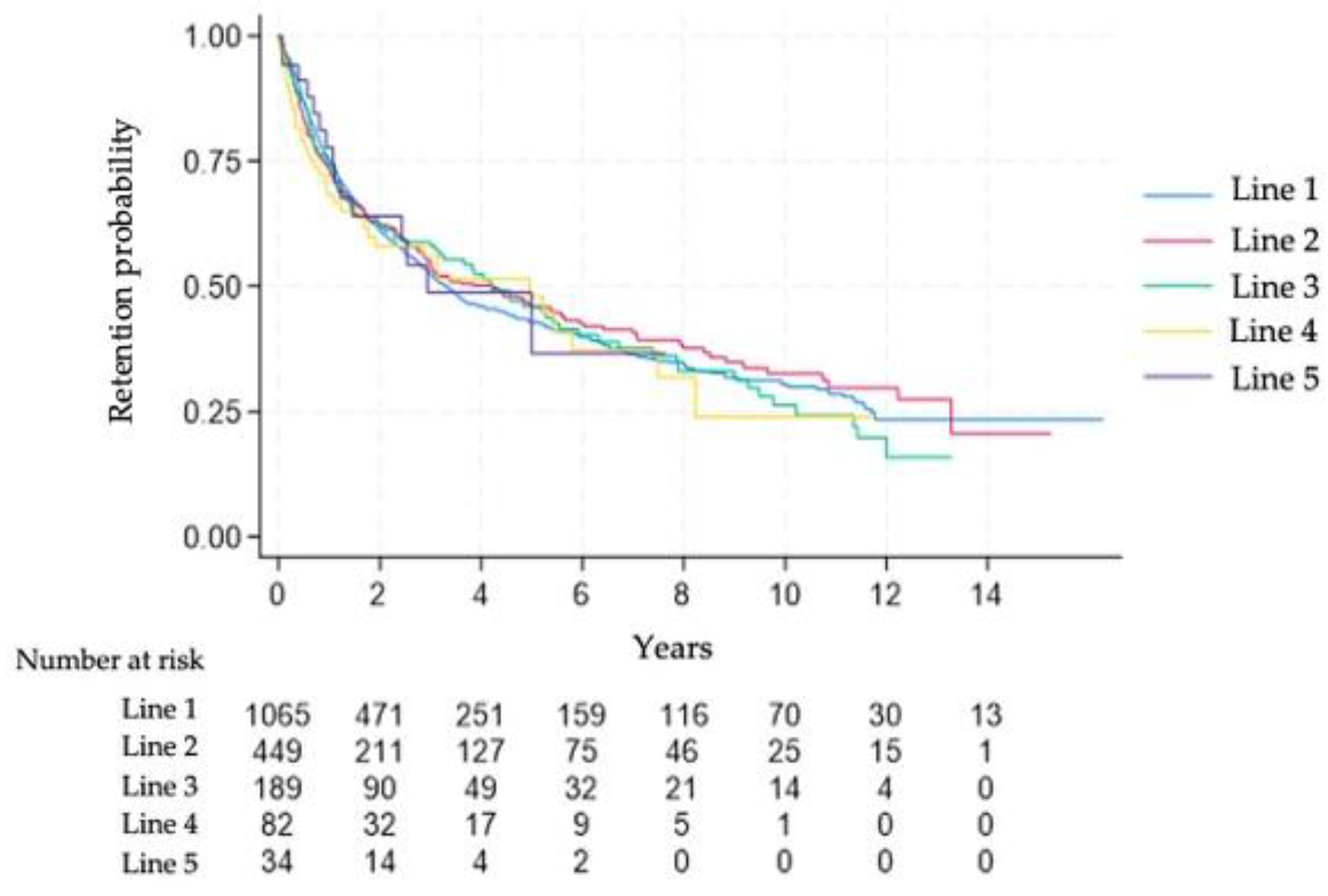
3.2.3. Drug Survival
Figure 3 shows the Kaplan–Meier curves for drug survival across treatment lines. The survival curves of the first and subsequent therapies largely overlapped, indicating similar persistence across treatment lines. By year 2, nearly 60% of patients remained on therapy.
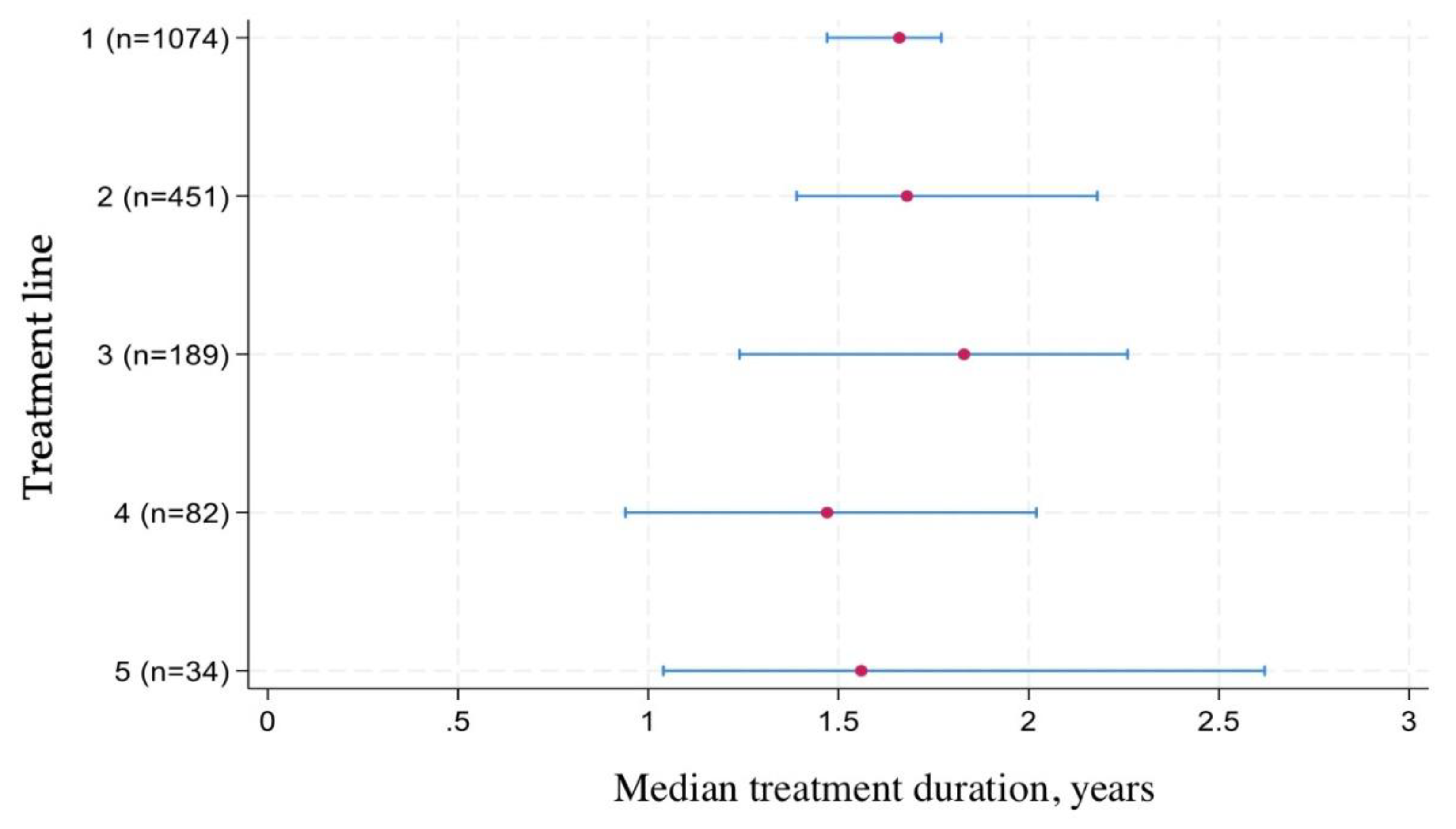
Figure 4 illustrates the median time to treatment discontinuation across different lines of therapy. For first-line treatment, the median drug survival was 1.66 years, meaning that half of the patients remained on their initial biologic or targeted therapy beyond this time point. Survival times for subsequent lines were slightly shorter, reflecting the challenges of maintaining long-term treatment effectiveness after switching.
3.3. Treatment Transitions
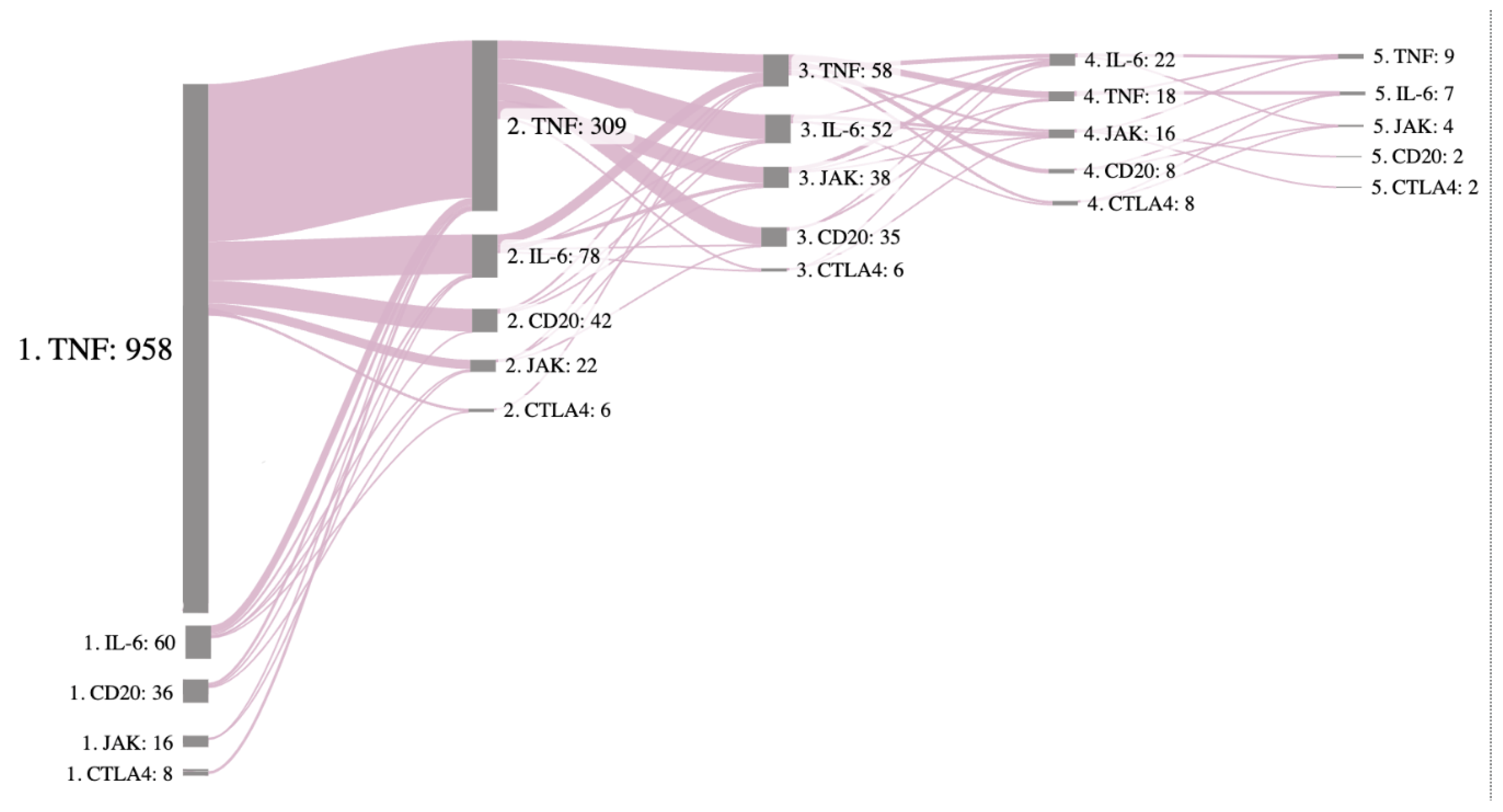
On
Figure 5 is a Sankey diagram visualizing the transitions between different lines of therapy starting with line 1 on the left and ending with line 5 on the right. The size of the gray node represents the number of patients on that therapy, and the pink curves represent transitions, with the thicker line having more patients who transitioned to next treatment. The majority of patients started with a TNF inhibitor and the first and even the third switch were still to a TNF inhibitor. However, a notable shift appears from second treatment line towards IL-6 inhibitors and Rituximab, the use of JAK inhibitors becomes more frequent from the third line.
3.4. Reasons for Treatment Discontinuation
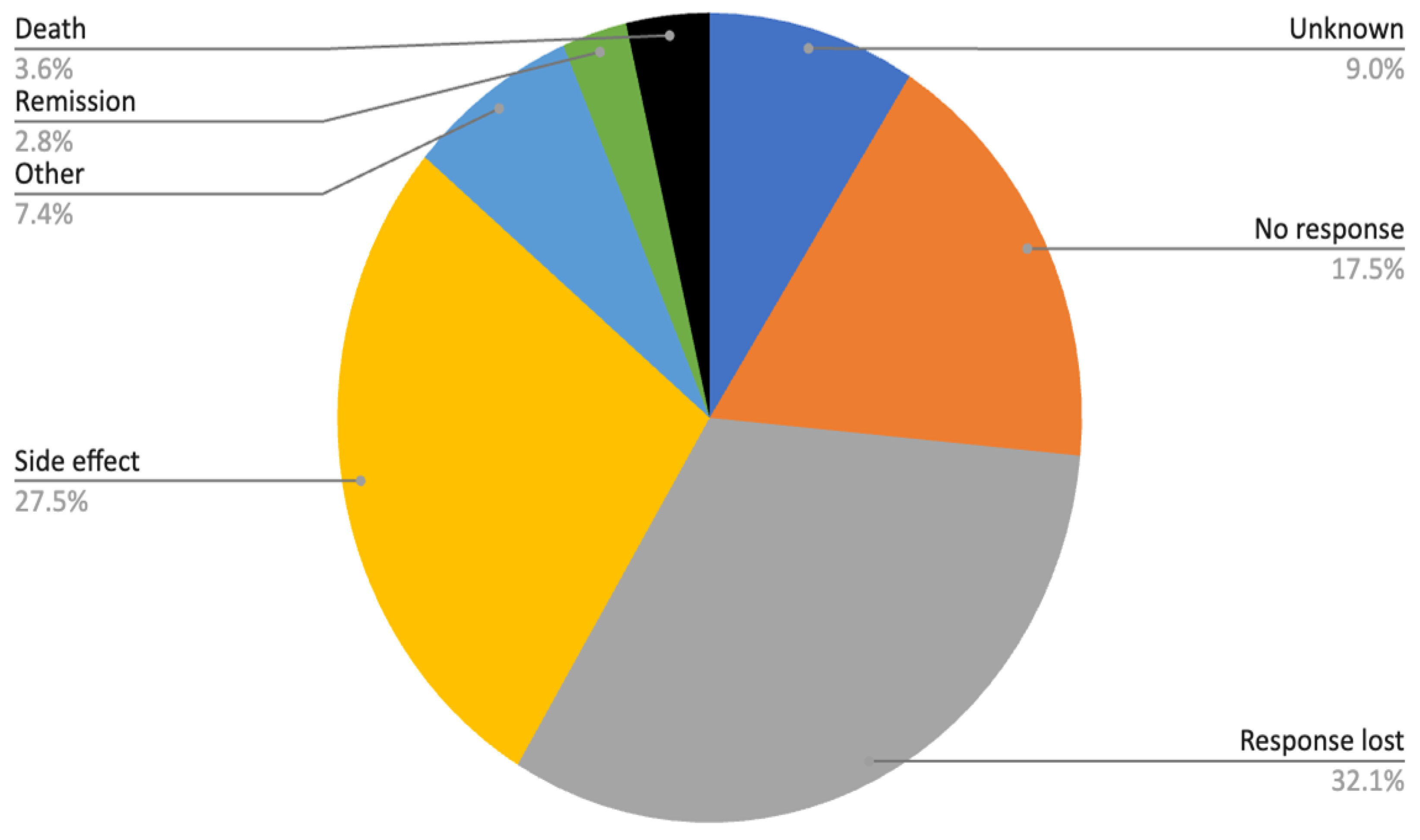
Approximately half of all treatment discontinuations were attributed to either primary ineffectiveness or secondary loss of response (reasons for discontinuation are presented in
Figure 6). Among the group categorized as “other reasons,” patient-related factors, such as personal preference, relocation to another region, or family planning considerations were most prevalent.
Adverse events constituted another major cause of treatment discontinuation (27.5%). The most documented side effects were allergic reactions and infections, each accounting for roughly one quarter of cases, while around one third of events were classified as unknown. Less frequent but clinically important adverse events included malignancies (3%) as well as various other complications.
Mortality data further underscore the burden of comorbidities and complications in this patient population. In total, 32 deaths were recorded, most frequently associated with infections (n = 11), of which six were attributable to COVID-19 and one to tuberculosis. Cancer (n = 7) and cardiovascular disease (n = 7) represented equally important contributors, while the remaining seven deaths were due to other or unknown causes.
4. Discussion
In this nationwide registry of 1,074 RA patients, the mean 6-month change in DAS28-CRP during first-line therapy was ~2.3 points, with remission achieved in ~20% across lines 1–3 (≈15% in later lines) and low disease activity in ~7–10% across lines 1–4. Treatment persistence was broadly similar across lines: ~60% of patients remained on therapy by year 2, and the median time to discontinuation on the first line was 1.66 years, with only a modest shortening on subsequent lines. This study provides the first nationwide description of biologic and targeted synthetic DMARD use in Estonian patients with rheumatoid arthritis, focusing on sequential treatment lines, persistence, and reasons for discontinuation. Compared with several other registry cohorts [
10,
14,
15], the proportion of seropositive patients in our study was notably high, indicating that biologic therapy in Estonia has predominantly been initiated in patients with more immunologically active disease. The gradual decline in seropositivity across treatment lines reflects a higher likelihood of difficult cases among seronegative patients, who often require multiple therapeutic switches to achieve adequate disease control.
As expected, the largest mean improvement in DAS28 was observed during first-line therapy, reflecting the initial strong response upon initiation of biologic or targeted treatment. The effect size decreased gradually across subsequent treatment lines, consistent with a more treatment-refractory patient population, a clinically relevant improvement was still achieved in most lines, suggesting preserved therapeutic responsiveness even after several switches. These findings align with results from large registry studies in the UK [
10], Australia [
14], and Japan [
15], all of which reported the greatest reductions in disease activity during the first treatment course. In contrast to these cohorts, our study showed a slightly higher proportion of remission compared with low disease activity, which is counterintuitive given their hierarchical relationship. This unexpected pattern is likely methodological rather than clinical, related to the narrow DAS28 range defining LDA (2.6–3.2), measurement variability near this threshold, or possible differences between DAS28-CRP and DAS28-ESR, the latter typically yielding higher values. Additionally, the inclusion of only patients with available 6-month data—without imputation—may have preferentially captured better responders, thereby inflating remission frequencies relative to LDA.
Drug survival in our cohort remained relatively stable across treatment lines, with only a modest decline from the first to the later biologic or targeted therapies. This contrasts with findings from the BSRBR-RA, where treatment persistence decreased more clearly after the first line [
10]. The overlapping Kaplan–Meier curves in the Estonian data may indicate consistent long-term tolerability and effectiveness despite multiple switches. A similar pattern of declining, yet sustained, retention across treatment lines has also been observed in other national registries [
14,
15,
16,
17]. These studies reported that while treatment duration tends to shorten with each subsequent line, many patients continue to derive benefit from additional biologic or targeted therapies, underscoring their long-term utility in real-world settings. The smaller differences observed in our cohort may relate to smaller patient numbers in later lines, differences in access, and national treatment-switching or reimbursement policies.
The observed treatment transitions reflect the typical evolution of biologic therapy use in rheumatoid arthritis. Most patients initiated therapy with a TNF inhibitor, and many remained within this class through the second or even third treatment line. This pattern of repeated TNF cycling has also been reported in other registries, where TNF inhibitors remain the most commonly used first- and second-line biologics despite increasing availability of alternative modes of action [
10,
11,
14]. The shift toward IL-6 inhibitors, rituximab, and later JAK inhibitors observed in our data mirrors therapeutic trends reported in other national registries [
10,
11,
14,
15] and reflects expanding treatment availability and a preference for switching to agents with alternative mechanisms of action after TNF. The relatively high proportion of continued TNF use across multiple lines in Estonia may be influenced by national reimbursement criteria and clinical experience accumulated with these agents. As seen in larger registries, repeated TNF cycling can still provide meaningful responses for a subset of patients, although overall effectiveness tends to decline with successive switches [
10].
Reasons for treatment discontinuation in our cohort were largely comparable to those reported in other European registries, where loss of response and adverse events are the predominant causes [
10,
16,
17]. In contrast to some larger cohorts, where secondary inefficacy tends to outweigh primary non-response [
10,
14], the proportions in our data were more balanced, suggesting that both early and late treatment failures contribute equally to discontinuation in routine practice. Adverse events, particularly infections and allergic reactions, followed by malignancies, were also in line with previously reported safety profiles of biologic and targeted therapies [
16,
17]. The mortality pattern dominated by infections and cardiovascular disease corresponds with other long-term registry findings [
15,
16,
18,
19], emphasizing the persistent comorbidity burden in real-world RA populations.
The strengths of this study include nationwide coverage, long follow-up, and the ability to analyse treatment outcomes across multiple lines of therapy. However, several limitations must be acknowledged. First, missing data were substantial for some variables, and no imputation methods were applied, which may have biased categorical response estimates. Second, the relatively small number of patients in later treatment lines resulted in wide confidence intervals and limited power to detect differences. Third, as with all observational registry data, treatment decisions may have been influenced by unmeasured confounders, such as socioeconomic status or patient preference, which cannot be fully accounted for.
5. Conclusions
In this nationwide registry-based study of 1,074 RA patients, biologic and targeted synthetic DMARDs demonstrated sustained clinical benefit across multiple lines of therapy. The mean 6-month improvement in DAS28-CRP during first-line treatment was approximately 2.3 points, with one in five patients achieving remission. Although treatment response and duration gradually declined with each additional line, most patients continued to derive meaningful benefit even in later therapies. Treatment persistence remained stable, and discontinuation patterns—dominated by loss of efficacy and adverse events—were consistent with international findings.
Overall, these results highlight that effective disease control remains achievable for many RA patients in Estonia even after several therapeutic switches, underscoring the importance of ongoing access to diverse biologic and targeted treatment options.
Author Contributions
Conceptualization, K.U., K.L. and R.M.; methodology, K.U. and S.V.; validation, K.U. and S.V.; formal analysis, K.U. and S.V.; investigation, K.U.; resources, K.L.; data curation, K.U.; writing—original draft preparation, K.U.; writing—review and editing, K.L., R.M. and S.V.; visualization, K.U. and S.V.; supervision, R.M. and K.L.; project administration, K.U.; funding acquisition, K.L and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by AbbVie Inc.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of the National Institute for Health Development, Estonia (Tervise Arengu Instituudi inimuuringute eetikakomitee (TAIEK); Study No. 2396, TAIEK meeting protocol No. 44. Decision No. 1122 issued on October 17, 2022.).
Informed Consent Statement
Informed consent was waivered due to the retrospective nature of the study (approved by the IRB)
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to patient confidentiality and the sensitive nature of the clinical data but are available from the corresponding author upon reasonable request. Requestors will be required to submit a methodologically sound proposal and sign a data access agreement.
Acknowledgments
The first author wishes to thank Mats Johanson for valuable advice on data cleaning and analysis. We also acknowledge all local rheumatologists, rheumatology nurses, and specialized secretaries who contributed and entered data to the Estonian Biologic Therapy Register. During the preparation of this manuscript, the authors used ChatGPT (GPT-5.1; OpenAI) for text revision, language polishing and structural suggestions. The authors reviewed and edited all outputs and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| RA |
Rheumatoid arthritis |
| b/tsDMARD |
Biologic or targeted synthetic disease-modifying antirheumatic drug |
| DAS28-CRP |
28-Joint Disease Activity Score |
| LDA |
Low disease activity |
| EULAR |
European Alliance of Associations for Rheumatology |
| TNF |
Tumor necrosis factor |
| IL-6 |
Interleukin-6 |
| JAK |
Janus kinase |
| CTLA4 |
Cytotoxic T-lymphocyte-associated protein 4 |
| CD20 |
Cluster of differentiation 20 |
| BMI |
Body mass index |
| RF |
Rheumatoid factor |
| ACPA |
Anti-citrullinated protein antibody |
| CRP |
C-reactive protein |
| ESR |
Erythrocyte sedimentation rate |
| TJC |
Tender joint count |
| SJC |
Swollen joint count |
| HAQ |
Health Assessment Questionnaire |
| IQR |
Interquartile range |
| SD |
Standard deviation |
| CI |
Confidence interval |
| RCT |
Randomized controlled trial |
| ICD-10 |
10th Revision of the International Classification of Diseases |
| BSRBR-RA |
British Society for Rheumatology Biologics Register for Rheumatoid Arthritis |
| DANBIO |
Danish nationwide DANBIO registry |
| RABBIT |
German biologics register RABBIT |
References
- Markusse, I.M.; Dirven, L.; Gerards, A.H.; van Groenendael, J.H.L.M.; Ronday, H.K.; Kerstens, P.J.S.M.; Lems, W.F.; Huizinga, T.W.J.; Allaart, C.F. Disease Flares in Rheumatoid Arthritis Are Associated with Joint Damage Progression and Disability: 10-Year Results from the BeSt Study. Arthritis Res. Ther. 2015, 17, 232. [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St.Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [CrossRef]
- Studenic, P.; Meissner, Y.; Kearsley-Fleet, L.; De Cock, D. Role of Rheumatoid Arthritis Registries Worldwide: What Have They Taught Us? Best Pract. Res. Clin. Rheumatol. 2025, 39, 102017. [CrossRef]
- Ibfelt, E.H.; Jensen, D.V.; Hetland, M.L. The Danish Nationwide Clinical Register for Patients with Rheumatoid Arthritis: DANBIO. Clin. Epidemiol. 2016, 8, 737–742. [CrossRef]
- Hamann, P.D.H.; Pauling, J.D.; McHugh, N.; Shaddick, G.; Hyrich, K.; the BSRBR-RA Contributors Group Predictors, Demographics and Frequency of Sustained Remission and Low Disease Activity in Anti-Tumour Necrosis Factor–Treated Rheumatoid Arthritis Patients. Rheumatology 2019, 58, 2162–2169. [CrossRef]
- Listing, J.; Strangfeld, A.; Rau, R.; Kekow, J.; Gromnica-Ihle, E.; Klopsch, T.; Demary, W.; Burmester, G.-R.; Zink, A. Clinical and Functional Remission: Even Though Biologics Are Superior to Conventional DMARDs Overall Success Rates Remain Low – Results from RABBIT, the German Biologics Register. Arthritis Res. Ther. 2006, 8, R66. [CrossRef]
- Aaltonen, K.J.; Ylikylä, S.; Tuulikki Joensuu, J.; Isomäki, P.; Pirilä, L.; Kauppi, M.; Rannio, T.; Eklund, K.; Blom, M.; Nordström, D. Efficacy and Effectiveness of Tumour Necrosis Factor Inhibitors in the Treatment of Rheumatoid Arthritis in Randomized Controlled Trials and Routine Clinical Practice. Rheumatology 2017, 56, 725–735. [CrossRef]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.J.; Kedves, M.; Hamar, A.; Goes, M.C. van der; Kent, A.; Bakkers, M.; Pchelnikova, P.; Blaas, E.; et al. EULAR Points to Consider for the Management of Difficult-to-Treat Rheumatoid Arthritis. Ann. Rheum. Dis. 2022, 81, 20–33. [CrossRef]
- Zhao, S.S.; Kearsley-Fleet, L.; Bosworth, A.; Watson, K.; Hyrich, K.L. Effectiveness of Sequential Biologic and Targeted Disease Modifying Anti-Rheumatic Drugs for Rheumatoid Arthritis. Rheumatol. Oxf. Engl. 2022, 61, 4678–4686. [CrossRef]
- Pappas, D.A.; O’Brien, J.; Guo, L.; Shan, Y.; Baker, J.F.; Kricorian, G.; Stryker, S.; Collier, D.H. Treatment Patterns and Clinical Outcomes in Patients with Rheumatoid Arthritis Initiating Etanercept, Adalimumab, or Janus Kinase Inhibitor as First-Line Therapy: Results from the Real-World CorEvitas RA Registry. Arthritis Res. Ther. 2023, 25, 166. [CrossRef]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; Vollenhoven, R. van; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2016 Update. Ann. Rheum. Dis. 2017, 76, 960–977. [CrossRef]
- Eesti Haigekassa Tervishoiuteenuste Loetelu–Riigi Teataja Available online: https://www.riigiteataja.ee/akt/128062019011 (accessed on 22 November 2025).
- Fletcher, A.; Lassere, M.; March, L.; Hill, C.; Barrett, C.; Carroll, G.; Buchbinder, R. Patterns of Biologic and Targeted-Synthetic Disease-Modifying Antirheumatic Drug Use in Rheumatoid Arthritis in Australia. Rheumatol. Oxf. Engl. 2022, 61, 3939–3951. [CrossRef]
- Fujii, T.; Murata, K.; Onizawa, H.; Onishi, A.; Tanaka, M.; Murakami, K.; Nishitani, K.; Furu, M.; Watanabe, R.; Hashimoto, M.; et al. Management and Treatment Outcomes of Rheumatoid Arthritis in the Era of Biologic and Targeted Synthetic Therapies: Evaluation of 10-Year Data from the KURAMA Cohort. Arthritis Res. Ther. 2024, 26, 16. [CrossRef]
- Flouri, I.; Markatseli, T.E.; Voulgari, P.V.; Boki, K.A.; Papadopoulos, I.; Settas, L.; Zisopoulos, D.; Skopouli, F.N.; Iliopoulos, A.; Bertsias, G.K.; et al. Comparative Effectiveness and Survival of Infliximab, Adalimumab, and Etanercept for Rheumatoid Arthritis Patients in the Hellenic Registry of Biologics: Low Rates of Remission and 5-Year Drug Survival. Semin. Arthritis Rheum. 2014, 43, 447–457. [CrossRef]
- Favalli, E.G.; Pregnolato, F.; Biggioggero, M.; Becciolini, A.; Penatti, A.E.; Marchesoni, A.; Meroni, P.L. Twelve-Year Retention Rate of First-Line Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: Real-Life Data From a Local Registry. Arthritis Care Res. 2016, 68, 432–439. [CrossRef]
- Aboulenain, S.; Li, X.; Movahedi, M.; Bombardier, C.; Kuriya, B. Cardiovascular Risk Factors and the Risk of Discontinuation of Advanced Therapies Due to Treatment Failure in Rheumatoid Arthritis: Results From the Ontario Best Practices Research Initiative. ACR Open Rheumatol. 2023, 5, 712–717. [CrossRef]
- Argnani, L.; Zanetti, A.; Carrara, G.; Silvagni, E.; Guerrini, G.; Zambon, A.; Scirè, C.A. Rheumatoid Arthritis and Cardiovascular Risk: Retrospective Matched-Cohort Analysis Based on the RECORD Study of the Italian Society for Rheumatology. Front. Med. 2021, 8, 745601. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).