1. Case Presentation
1.1. General Information
A 57-year-old female patient, Ms. Zou, was admitted to our hospital on July 24, 2025, with the chief complaint of “diagnosed lung adenocarcinoma for 6 years and discovered ovarian mass for 1 year”. She had no smoking history, was postmenopausal for 5 years, and had no family history of malignant tumors. This study was approved by the Ethics Committee of The First People’s Hospital of Neijiang (Approval No. 2025-072), and the patient signed the informed consent form.
1.2. Disease Progression and Treatment Course
1.2.1. Diagnosis and Treatment of Lung Adenocarcinoma
The patient presented with cough and expectoration. Chest computed tomography (CT) revealed multiple bilateral pulmonary nodules (maximum diameter: 3.5 cm) with moderate right pleural effusion (
Figure 1). Pathological examination of the pleural effusion confirmed adenocarcinoma (
Figure 2), and next-generation sequencing (NGS) detected an EGFR exon 19 deletion mutation. She was diagnosed with advanced lung adenocarcinoma (cT4N2M1 Stage Ⅳ). Treatment included the first-generation TKI gefitinib (250 mg orally once daily) and cisplatin intrapleural perfusion. Two months later, the pleural effusion completely resolved, and the pulmonary nodules reduced by 50%, achieving a partial response (PR) (
Figure 3).
Sixteen months after gefitinib treatment, the pulmonary nodules enlarged (maximum diameter: 4.2 cm) with new lesions (
Figure 4), indicating TKI resistance. Repeated biopsy and NGS detected a RET fusion mutation. Due to financial constraints, she refused cabozantinib (a RET inhibitor) and received 6 cycles of pemetrexed + carboplatin chemotherapy combined with anlotinib (anti-angiogenic therapy). The disease remained stable (SD), followed by anlotinib maintenance therapy for 19 months.
Cough worsened after chemotherapy, and chest CT showed a 5.0 cm nodule in the right upper lobe. She received 4 cycles of camrelizumab immunotherapy, with stable disease but no further improvement.
Cough and expectoration aggravated, with increased pleural effusion and enlarged abdominal lymph nodes (
Figure 5). She initially refused treatment, leading to disease progression. In April 2023, she underwent right pleural effusion drainage + bevacizumab intrapleural perfusion, combined with 1 cycle of paclitaxel (240 mg ivgtt d1) + nedaplatin (50 mg ivgtt d1-2) chemotherapy. Third NGS detected an EGFR T790M mutation, and she was switched to the third-generation TKI osimertinib (80 mg orally once daily). As of July 2025, the pulmonary lesions remained stable (maximum diameter: 2.1 cm) (
Figure 6).
1.2.2. Diagnosis and Treatment of Primary Ovarian Cancer
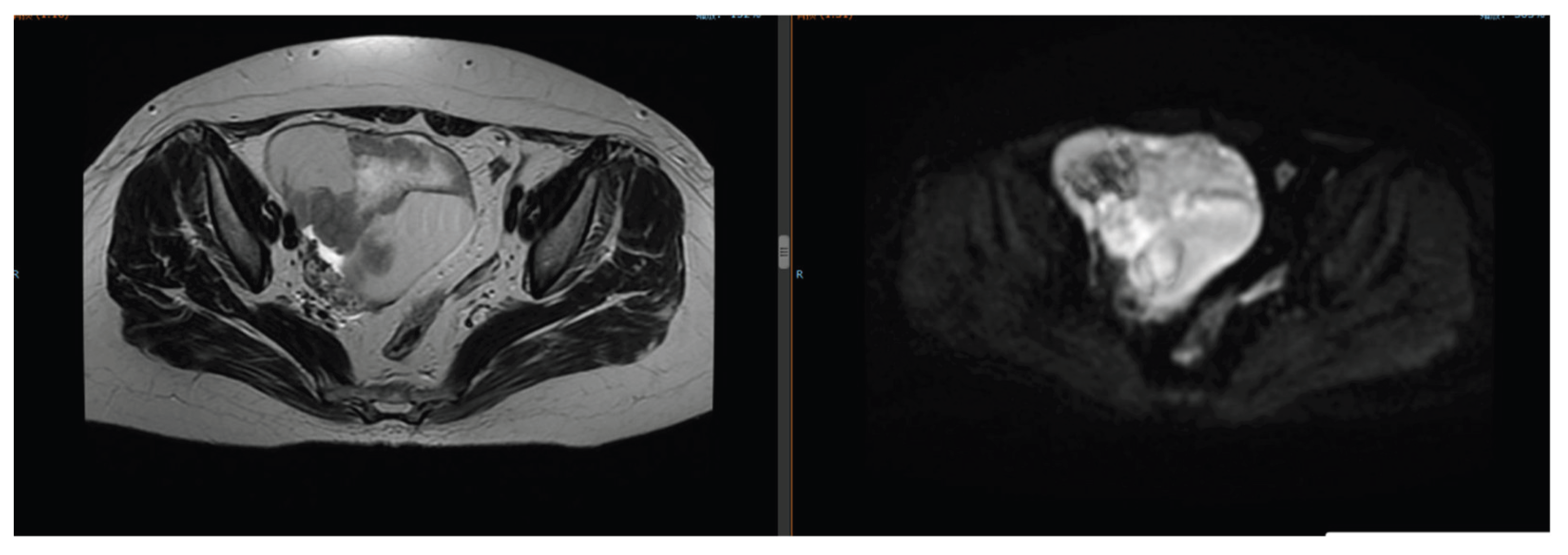
The patient developed lower abdominal distension and pain. Pelvic magnetic resonance imaging (MRI) revealed a left ovarian mass (6.8 cm × 5.2 cm) (
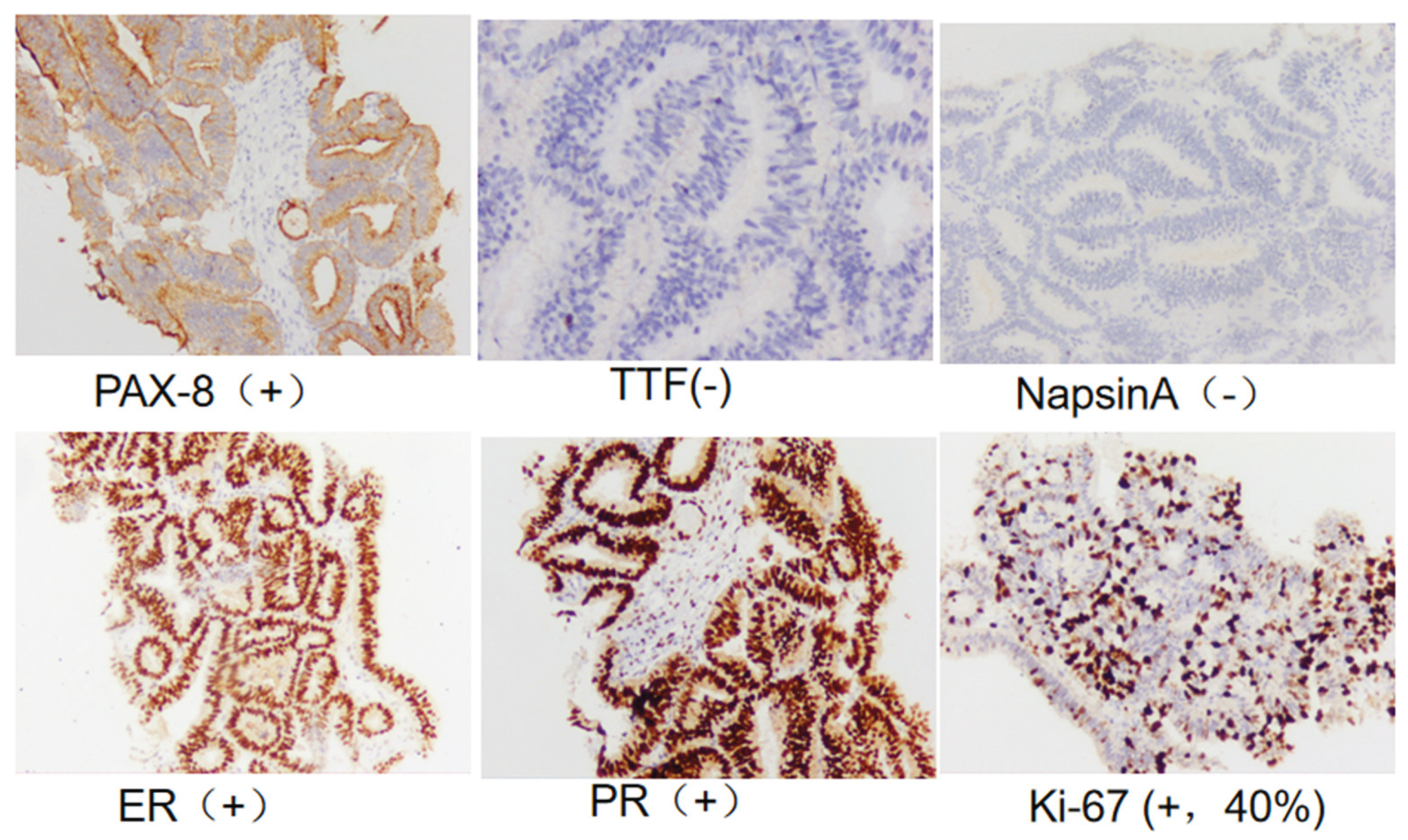
Figure 7). Needle biopsy confirmed an ovarian epithelial-derived tumor, morphologically consistent with endometrioid carcinoma. Immunohistochemistry results: PAX-8 (+), ER (strong +, 90%), PR (strong +, 80%), TTF-1 (-), Napsin A (-), Ki-67 (+, ~40%) (
Figure 8). She was diagnosed with primary ovarian cancer (FIGO Stage Ⅲ), excluding lung metastasis due to significant differences in pathological type and immunophenotype from lung adenocarcinoma.
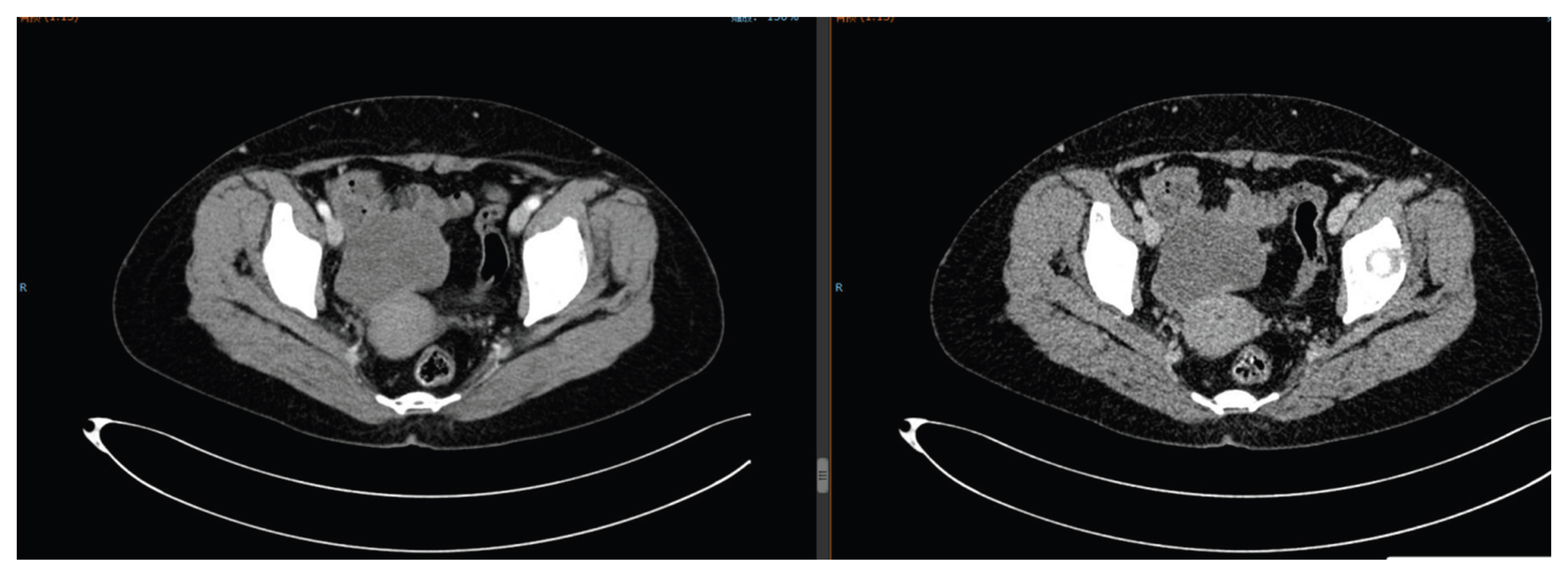
After a multidisciplinary team (MDT) discussion, she underwent HIFU treatment on September 9, 2024 (single dose: 3000 J, focusing on the ovarian lesion), followed by 4 cycles of docetaxel (80 mg ivgtt d1) + carboplatin (300 mg ivgtt d1) chemotherapy. The disease achieved PR (
Figure 9), but she developed grade Ⅲ leukopenia (nadir white blood cell count: 1.9 × 10
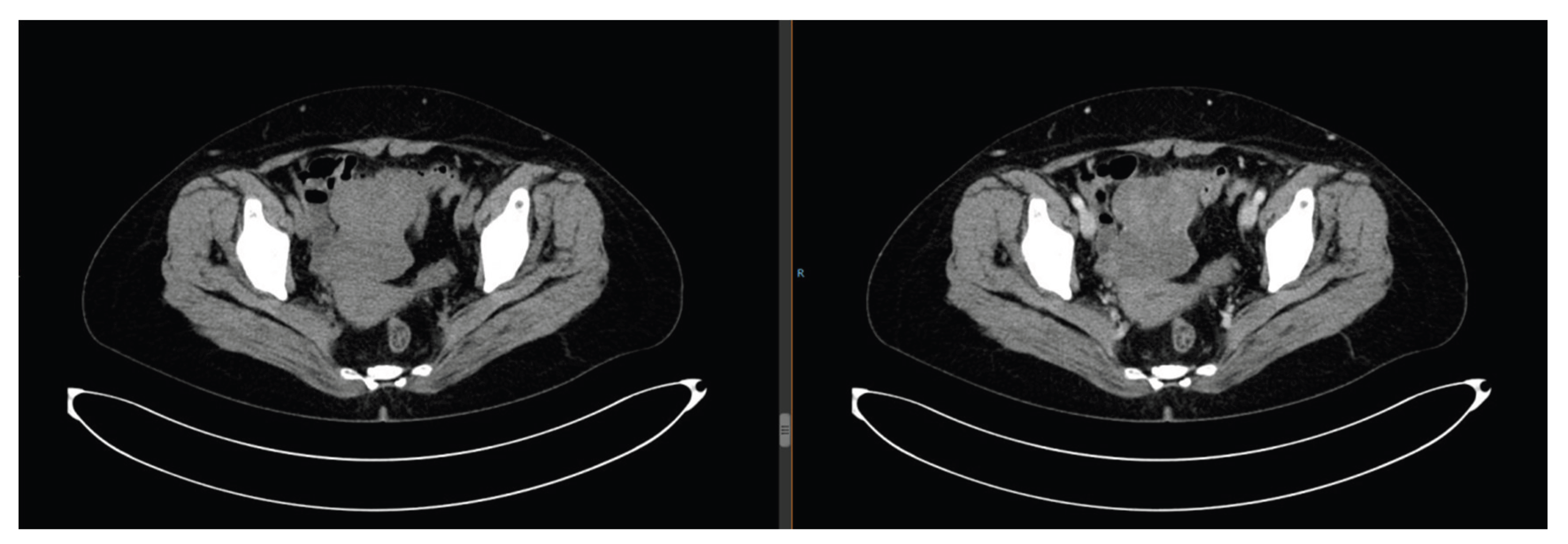
9/L) and grade Ⅲ nausea/vomiting. The regimen was adjusted to single-agent docetaxel for 2 cycles, which she refused due to persistent gastrointestinal discomfort. Megestrol acetate dispersible tablets (160 mg orally once daily) were added. In May 2025, pelvic CT showed the tumor enlarged to 5.7 cm. Two cycles of pemetrexed disodium chemotherapy were administered, but the tumor further increased to 7.2 cm (
Figure 10). The patient refused further chemotherapy and immunotherapy, continuing osimertinib for lung adenocarcinoma control.
2. Key Therapeutic Analysis
2.1. Individualized Treatment for Lung Adenocarcinoma
The patient’s lung adenocarcinoma management followed the standardized pathway for advanced EGFR-mutated lung cancer: the first-generation TKI achieved 16 months of benefit, followed by chemotherapy combined with anlotinib. The 19-month stable disease with anlotinib maintenance aligns with a pooled analysis of 2 single-arm trials, which confirmed that oral anlotinib could have promising efficacy and manageable safety, and could be an option as maintenance treatment for patients with locally advanced or metastatic NSCLC without known sensitive driver gene mutations after standard first-line platinum-based doublet chemotherapy [
1]. The detection of the EGFR T790M mutation and subsequent osimertinib use reflects current precision medicine principles—recent FLAURA2 trial data further validate osimertinib’s superiority, with combination therapy extending the median PFS to 25.5 months [
2]. Notably, the SAVANNAH trial (2025) provides new insights for osimertinib resistance management via MET inhibition, which could guide future treatment if the patient progresses [
3].
2.2. Application of HIFU in Ovarian Cancer Treatment
HIFU achieved local tumor control in this inoperable patient, consistent with emerging evidence that local ablative therapy improves outcomes in advanced ovarian cancer. A feasibility study (HIFU-related thermal therapy) demonstrated that HIFU is feasible for the treatment of metastatic pelvic tumors or recurrent ovarian cancer without serious complications [
4]. The patient’s chemotherapy resistance may relate to cancer stem cell (CSC) activation—a 2023 study identified ALDH1A1 as a prognostic marker for ovarian cancer progression, suggesting targeted CSC therapy could prevent relapse [
5]. For subsequent treatment, senaparib (a novel PARP inhibitor) showed promising efficacy in the 2024 FLAMES phase 3 trial; the trial enrolled 128 patients with platinum-resistant disease, with an objective response rate of 28.9%, making it a suitable option given its approval status [
6].
3. Discussion
Double primary malignancies (DPMs)—defined as two histologically distinct malignant tumors occurring in the same patient without evidence of metastasis between them—are clinically rare, with an overall incidence of 0.60–2.67% among all cancer patients in China [
7]. DPMs require rigorous differential diagnosis, where immunohistochemistry plays a key role. Traditional markers like PAX-8 and TTF-1 remain reliable, while recent studies (e.g., IMP2/IMP3 analysis, 2023) explore novel targets for ovarian tumor subtyping, though their clinical utility is still limited [
8] The patient’s long-term lung cancer control underscores the value of dynamic genomic testing, as supported by 2024–2025 EGFR-TKI trials [
2]. For ovarian cancer, combining HIFU with PARP inhibitors (e.g., senaparib) could balance efficacy and tolerance, addressing the limitations observed in this case (e.g., chemotherapy-related adverse reactions).
For patients with financial constraints, priority can be given to medical insurance-covered chemotherapy drugs (e.g., docetaxel), and minimally invasive treatments such as HIFU can be explored to reduce tumor burden, thereby prolonging survival and improving quality of life.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
References
- Liu Y, Miao L, Chen X, et al. Maintenance therapy with anlotinib after induction therapy with platinum-based chemotherapy for advanced non-small-cell lung cancer: A pooled analysis of 2 single-arm trials. Medicine. 2024;103(27):e38459. [CrossRef]
- Roy PG, Reingold D, Pathak N, et al. Recent advances in the management of EGFR-mutated advanced non-small cell lung cancer—a narrative review. Curr Oncol. 2025;32:448. [CrossRef]
- Ahn M, Kim TM, Bonanno L, et al. Savannah: Savolitinib + osimertinib in patients with EGFRm advanced NSCLC and MET overexpression/amplification following progression on osimertinib. J Thorac Oncol. 2025;20(Suppl 3):S4–S5. [CrossRef]
- Lei T, Guo X, Gong C, et al. High-intensity focused ultrasound ablation in the treatment of recurrent ovarian cancer and metastatic pelvic tumors: a feasibility study. Int J Hyperthermia. 2021;38(1):282–287. [CrossRef]
- Sterzyńska K, Nowak M, Wysocka J, et al. The prognostic value of cancer stem cell markers (CSCs) expression—ALDH1A1, CD133, CD44—for survival and long-term follow-up of ovarian cancer patients. Int J Mol Sci. 2023;24(3):2400. [CrossRef]
- Wu XH, Liu JH, Zhang M, et al. Senaparib as first-line maintenance therapy in advanced ovarian cancer: A randomized phase 3 trial. Nat Med. 2024;30(5):1021–1030. [CrossRef]
- Chinese Anti-Cancer Association Integrative Rehabilitation Committee for Multiple Primary and Unknown Primary Tumors, Shaanxi Anti-Cancer Association Rare Tumor Committee. Chinese Expert Consensus on Diagnosis and Treatment of Multiple Primary Malignancies (2024 Edition). 360doc. 2025. https://www.360doc.cn/article/31446682_1158869112.html.
- Němejcová K, Müller M, Vávra J, et al. A comprehensive immunohistochemical analysis of IMP2 and IMP3 in 542 cases of ovarian tumors. Diagn Pathol. 2023;18(1):32. [CrossRef]
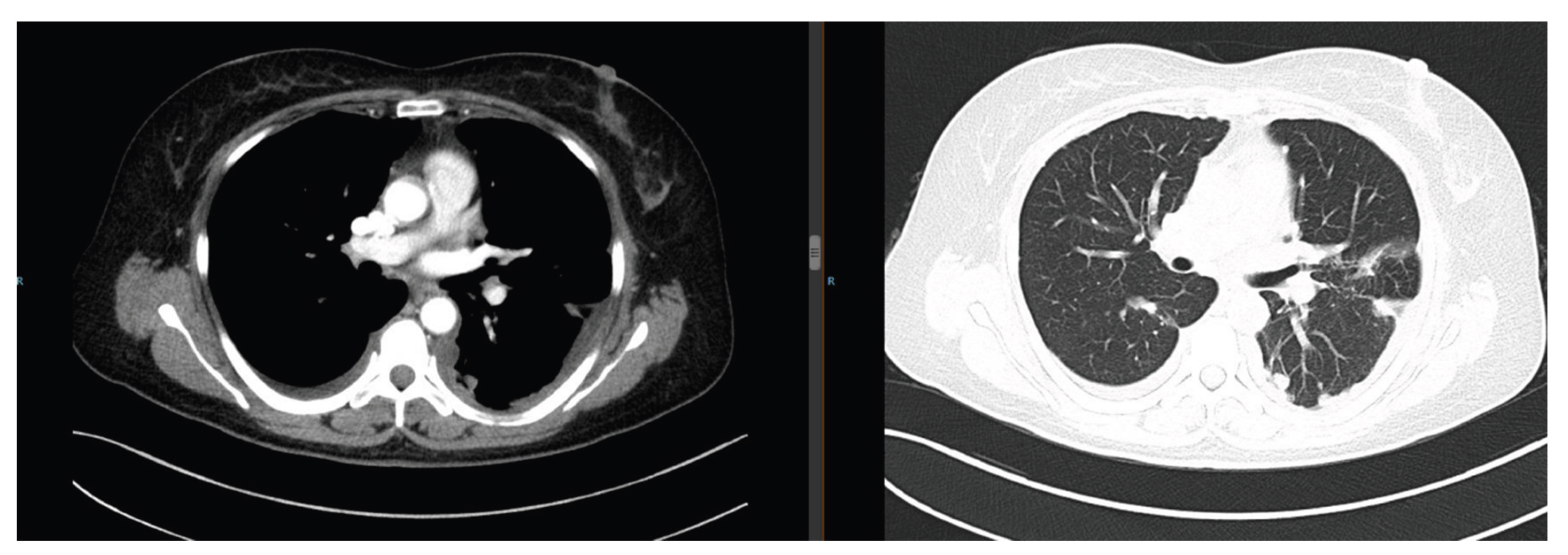
Figure 1.
Chest CT on September 11, 2019: Multiple bilateral pulmonary nodules (maximum diameter 3.5 cm) with right pleural effusion.
Figure 1.
Chest CT on September 11, 2019: Multiple bilateral pulmonary nodules (maximum diameter 3.5 cm) with right pleural effusion.
Figure 2.
Pleural effusion pathology on September 9, 2019: Adenocarcinoma cells (AE1/AE3 (+), CK7 (+), Napsin A (+), TTF-1 (+), Ki-67 (+, ~10%)).
Figure 2.
Pleural effusion pathology on September 9, 2019: Adenocarcinoma cells (AE1/AE3 (+), CK7 (+), Napsin A (+), TTF-1 (+), Ki-67 (+, ~10%)).
Figure 3.
Chest CT on February 13, 2020: Complete resolution of pleural effusion, 50% reduction in pulmonary nodules.
Figure 3.
Chest CT on February 13, 2020: Complete resolution of pleural effusion, 50% reduction in pulmonary nodules.
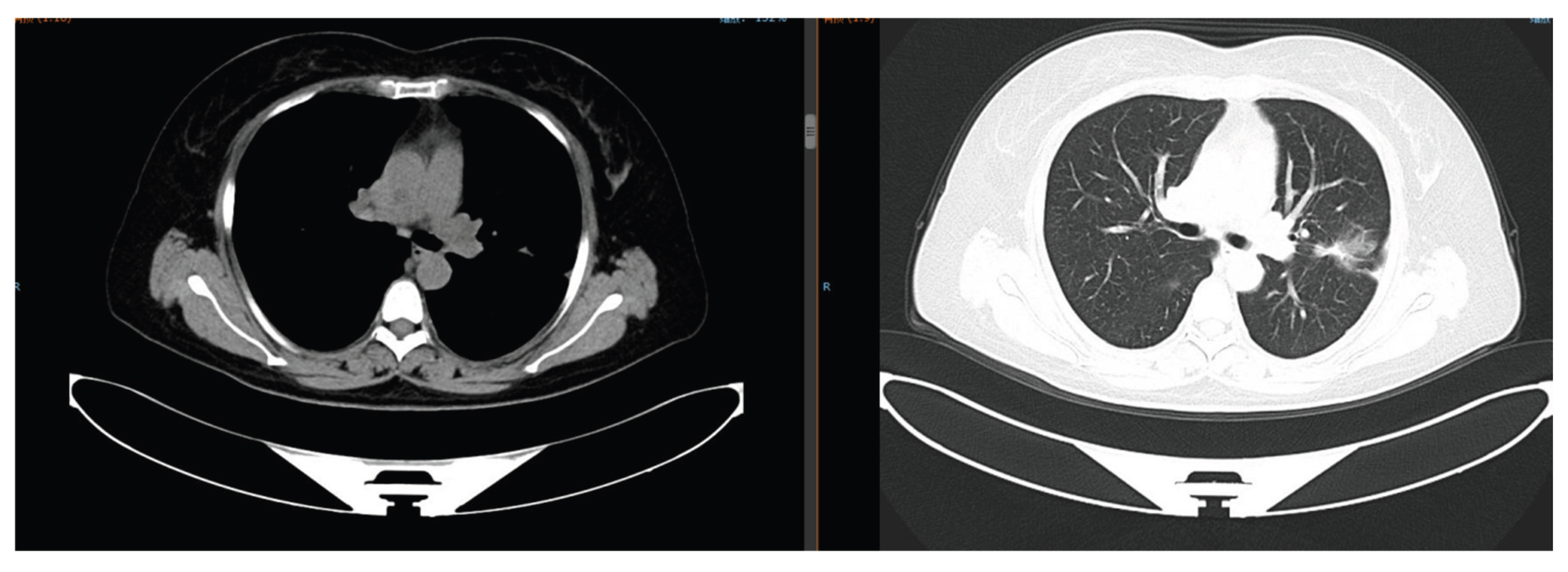
Figure 4.
Chest CT on January 16, 2021: Enlargement of pulmonary nodules (maximum diameter 4.2 cm) with new lesions.
Figure 4.
Chest CT on January 16, 2021: Enlargement of pulmonary nodules (maximum diameter 4.2 cm) with new lesions.
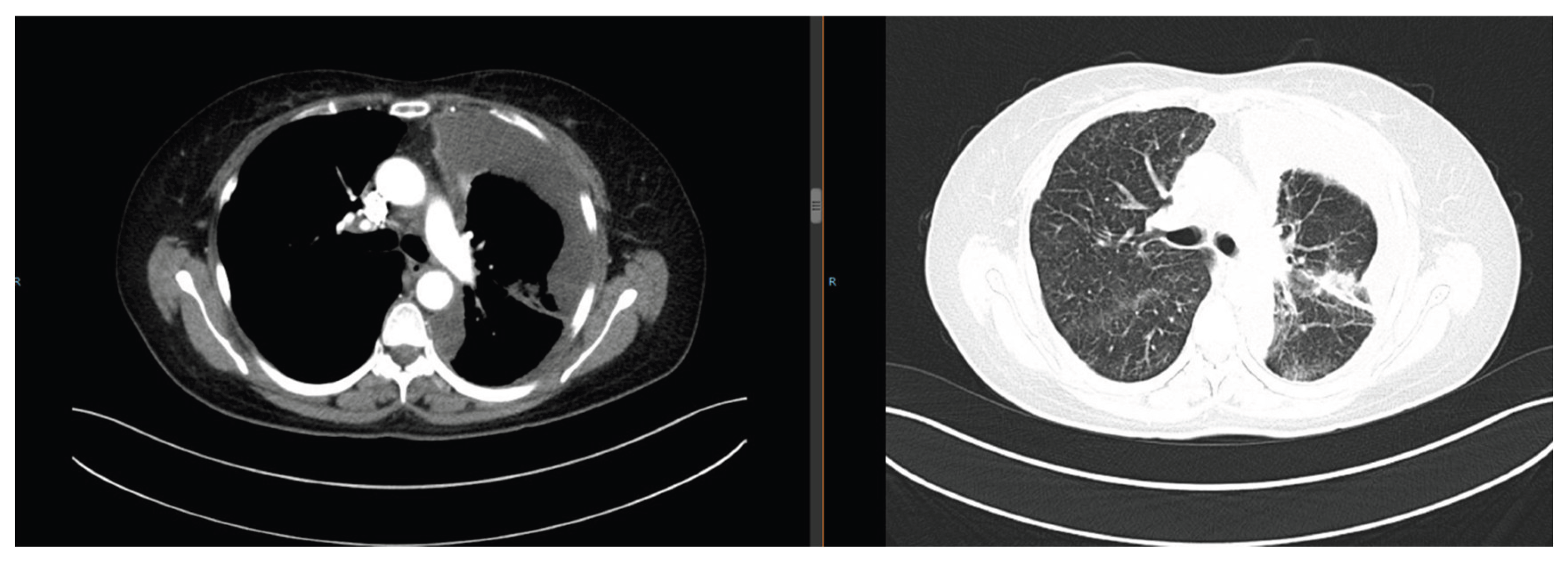
Figure 5.
Chest CT in January 2023: Increased pleural effusion and enlarged abdominal lymph nodes.
Figure 5.
Chest CT in January 2023: Increased pleural effusion and enlarged abdominal lymph nodes.
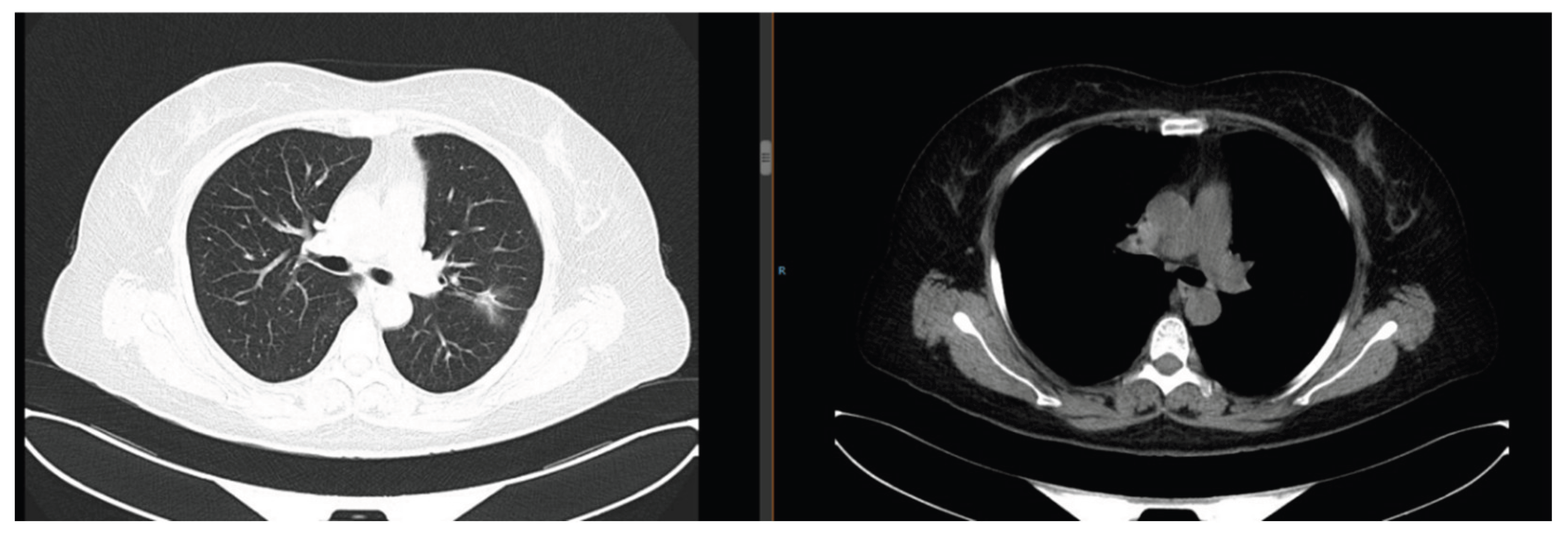
Figure 6.
Chest CT in July 2025: Stable pulmonary lesions (maximum diameter 2.1 cm).
Figure 6.
Chest CT in July 2025: Stable pulmonary lesions (maximum diameter 2.1 cm).
Figure 7.
Pelvic MRI on August 29, 2024: Left ovarian mass (6.8 cm × 5.2 cm).
Figure 7.
Pelvic MRI on August 29, 2024: Left ovarian mass (6.8 cm × 5.2 cm).
Figure 8.
Pelvic mass pathology on September 2, 2024: Ovarian endometrioid carcinoma (PAX-8 (+), ER (strong +, 90%), PR (strong +, 80%), TTF-1 (-), Napsin A (-), Ki-67 (+, ~40%)).
Figure 8.
Pelvic mass pathology on September 2, 2024: Ovarian endometrioid carcinoma (PAX-8 (+), ER (strong +, 90%), PR (strong +, 80%), TTF-1 (-), Napsin A (-), Ki-67 (+, ~40%)).
Figure 9.
Pelvic CT on December 19, 2024: Partial response of ovarian tumor after HIFU and chemotherapy.
Figure 9.
Pelvic CT on December 19, 2024: Partial response of ovarian tumor after HIFU and chemotherapy.
Figure 10.
Pelvic CT on July 24, 2025: Further enlargement of ovarian tumor after chemotherapy failure.
Figure 10.
Pelvic CT on July 24, 2025: Further enlargement of ovarian tumor after chemotherapy failure.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).