1. Introduction
In contemporary healthcare, improving care quality and ensuring patient safety have emerged as primary goals, yet the risk of clinical errors and adverse effects remains unresolved despite advancements in treatment technologies [
1]. Recently, the demand for the field of aesthetics has significantly increased, driven by the improvement of living standards and global beauty trends, accompanied by a parallel increase in post-procedure complications, long-term side effects and related legal disputes [
2].
The physiological risks of aesthetic plastic procedures are relatively lower in terms of directly threatening life compared to those in other fields of medicine [
3]. However, it has been reported that 86 percent of patients who experience physical complications such as infection, hematoma, nerve injury and pigmentation change develop psychological sequelae of disfigurement leading to a diagnosis of post-traumatic stress disorder [
4].
Facial injectable applications are one of the procedures that are continuously growing in the global aesthetic and plastic surgery market, primarily used to improve wrinkles, restore volume in depressed facial areas, and enhance the three-dimensional (3D) contour of specific regions such as the nose and chin [
5,
6]. Recently, with the growing public understanding of age-related changes in facial appearance and increasing social interest in rejuvenation and aesthetic enhancement, minimally invasive injections into the face that provide immediate results without surgery have gained significant attention [
7].
However, insufficient anatomical knowledge or a lack of technical proficiency of the clinicians can lead to serious complications after injection into the face, such as skin necrosis or blindness [
8]. Due to concerns and anxiety about procedural failure, clinicians engaged in the field of aesthetic and plastic medicine experience occupational stress [
9]. Although beginners require repetitive training to improve the success rate of procedures, adequate training facilities and practical environments remain lacking in the medical fields [
10]. Currently, education on injection procedures is provided through various academic conferences and training workshops; however, such programs have limitations in improving technical proficiency for the clinicians [
11]. Facial injection requires a personalized approach that considers the facial morphology, skin condition, age and sex for each patient, making it difficult to improve skills through theoretical education or short-term practice [
12].
Recently, clinical simulators have been adopted in various educational tools and are recognized as effective instruments for integrating basic and clinical sciences [
13]. Rather than replacing learning in real clinical environments, simulators enhance procedural competence by providing learners with opportunities to practice repeatedly beyond time and space, thereby helping them deliver high-quality medical care to patients in actual clinical settings [
14]. They also enable multiple users to experience similar situations and exchange immediate feedback, and allow instructors to plan clinical cases independently of patient availability [
15]. Increasing attention has been directed toward developing clinical simulators to supplement the limited opportunities for clinical education and training, resulting in expanded research in simulation-based education [
16]. The need for simulator application in facial injection training has also been emphasized [
17].
In this study, to address complications and medical accidents caused by unskilled techniques during facial procedures and the lack of opportunities for adequate procedural training, a clinical simulator for injection training was developed and implemented based on anatomical data as an alternative to improve procedural proficiency without temporal or spatial constraints.
2. Materials and Methods
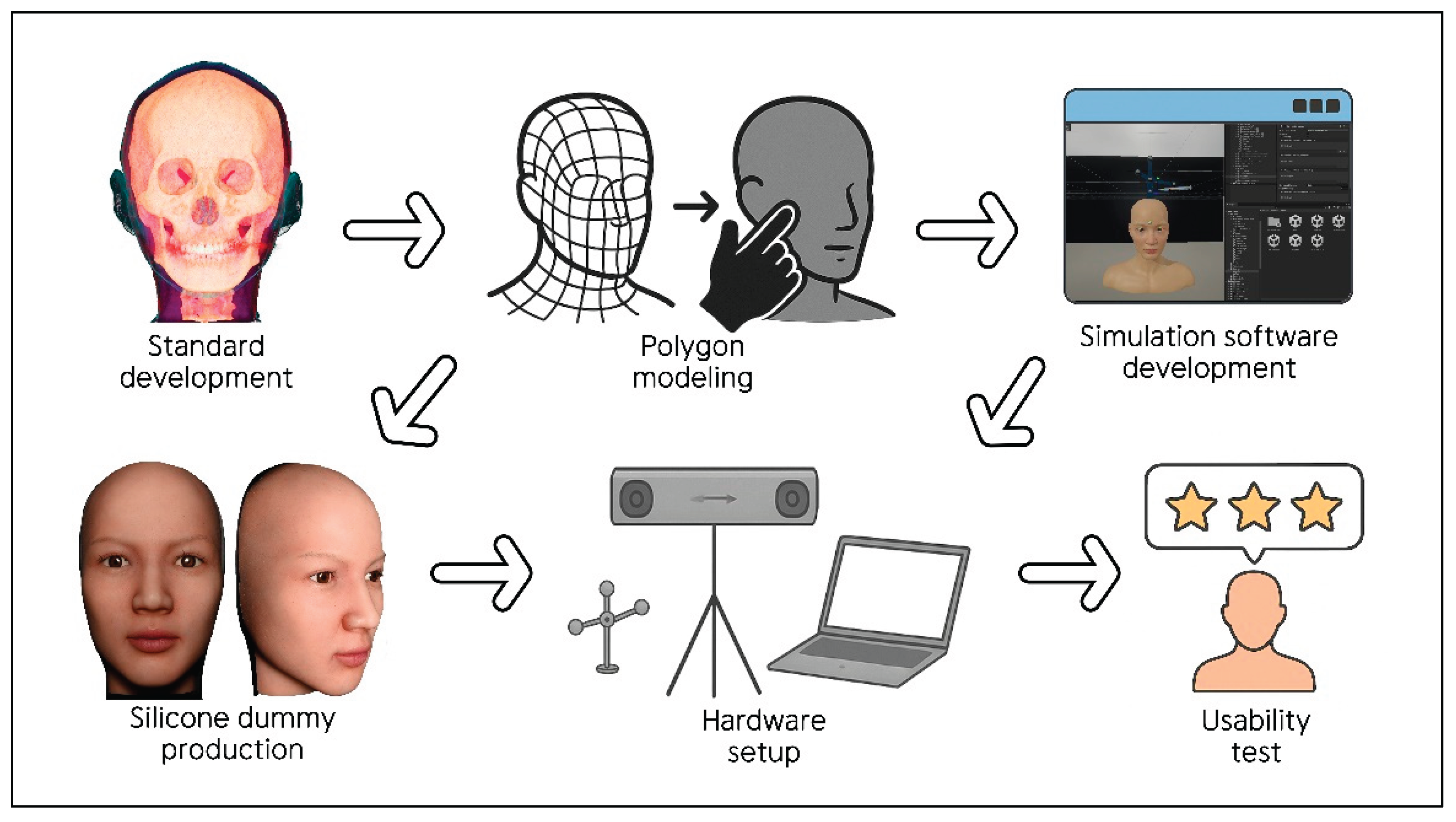
The development and implementation of the interactive clinical simulator for facial injection training comprised six steps: 2.1. Standard development with facial anatomy data; 2.2. Polygon modeling; 2.3. Development of simulation software; 2.4. Production of a facial silicone dummy; 2.5. Hardware setup; and 2.6. Pilot usability test. Details of each stage are provided below (
Figure 1).
2.1. Standard Development with Facial Anatomy Data
The data used for the facial model of the clinical simulator were constructed to accurately represent the anatomical structures of the face from the most superficial to the deepest layers. These data were based on detailed human morphology information, including the gross features such as the skeletal and soft tissue structures and the courses of the neurovascular structures.
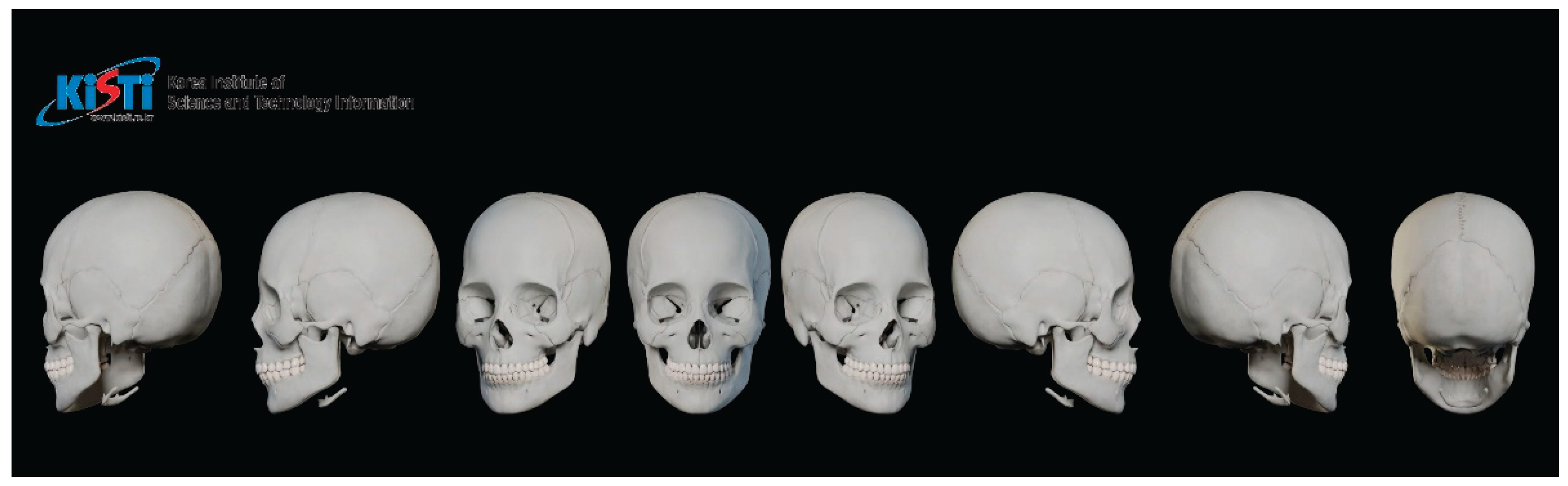
The skeletal framework and morphometric data forming the basic shape and contour of the face were obtained from the Digital Korean Human Model Database of the Korea Institute of Science and Technology Information [
18] (
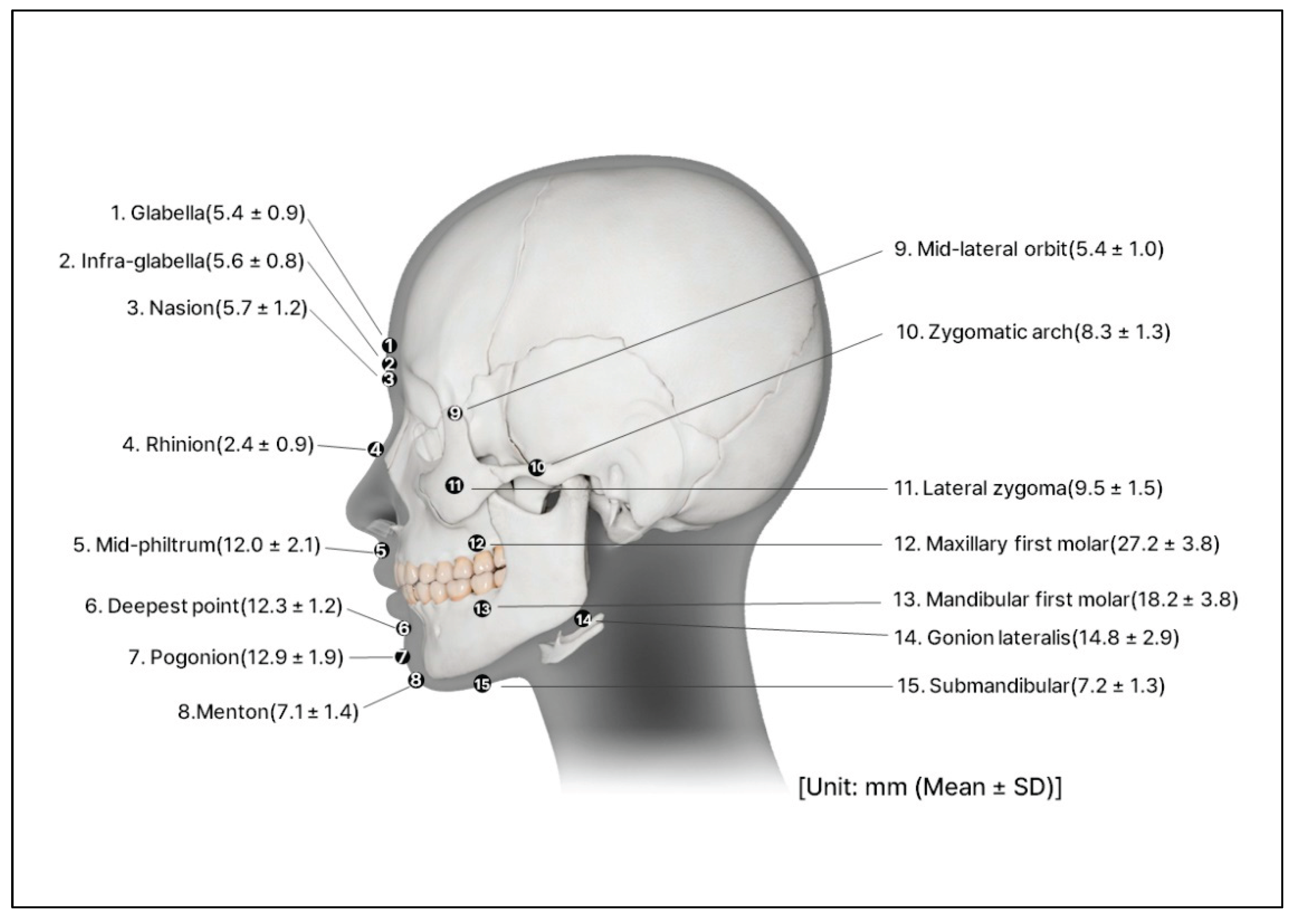
Figure 2). The thickness of the soft tissue was determined from cone-beam computed tomography by measuring the vertical depth at the various facial landmarks (
Figure 3) [
19].
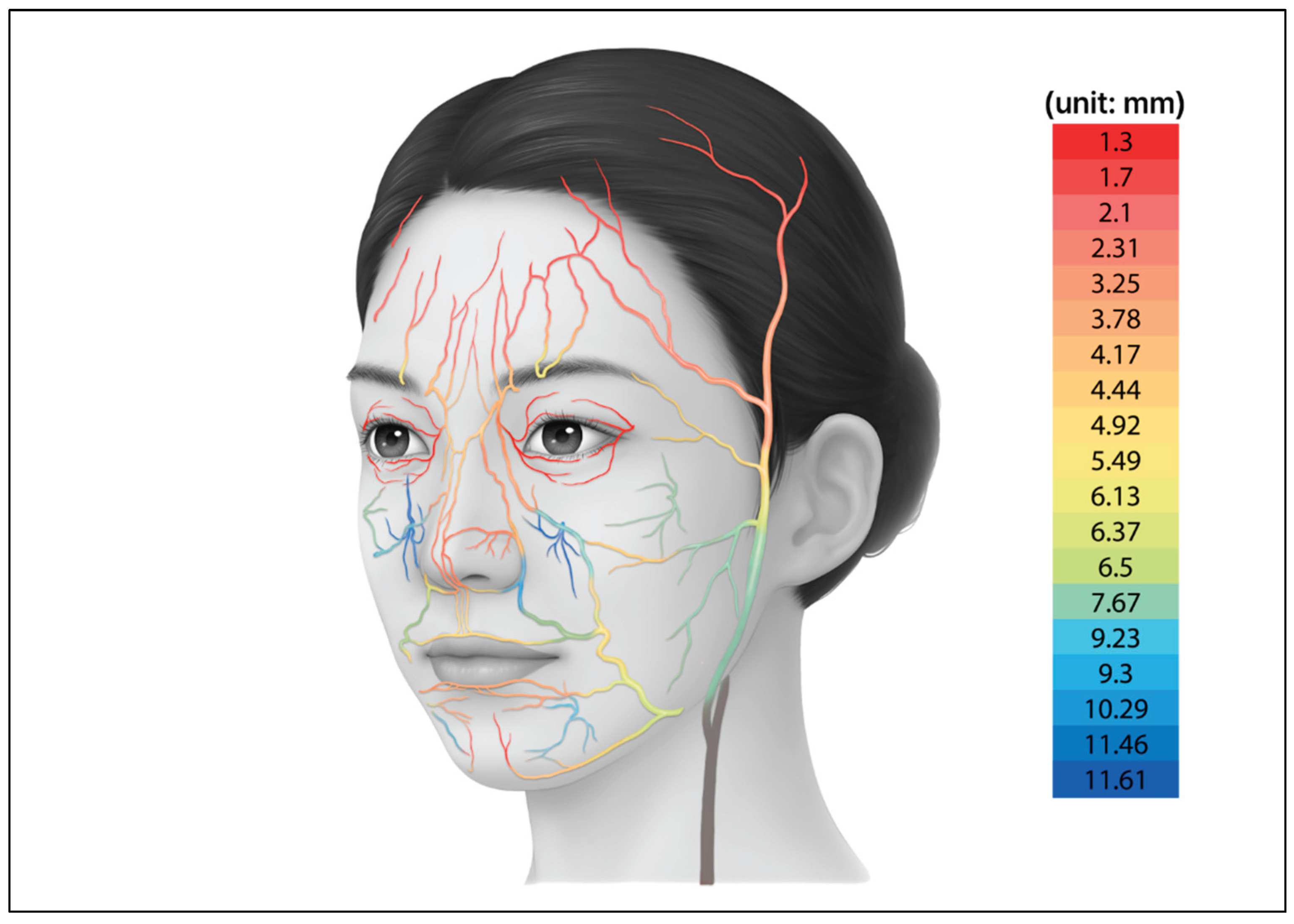
Information on major vascular structures with high risk of complications during the injection of materials, particularly the depth and course of the arteries in the face, was collected from previous anatomical studies [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. Based on these data, two-dimensional illustration was created to visualize the arterial distribution (
Figure 4). The arterial dataset was composed by integrating quantitative values reported in multiple population studies, allowing the vascular patterns of the facial model to reproduce anatomy observed in real human specimens.
2.2. Polygon Modeling
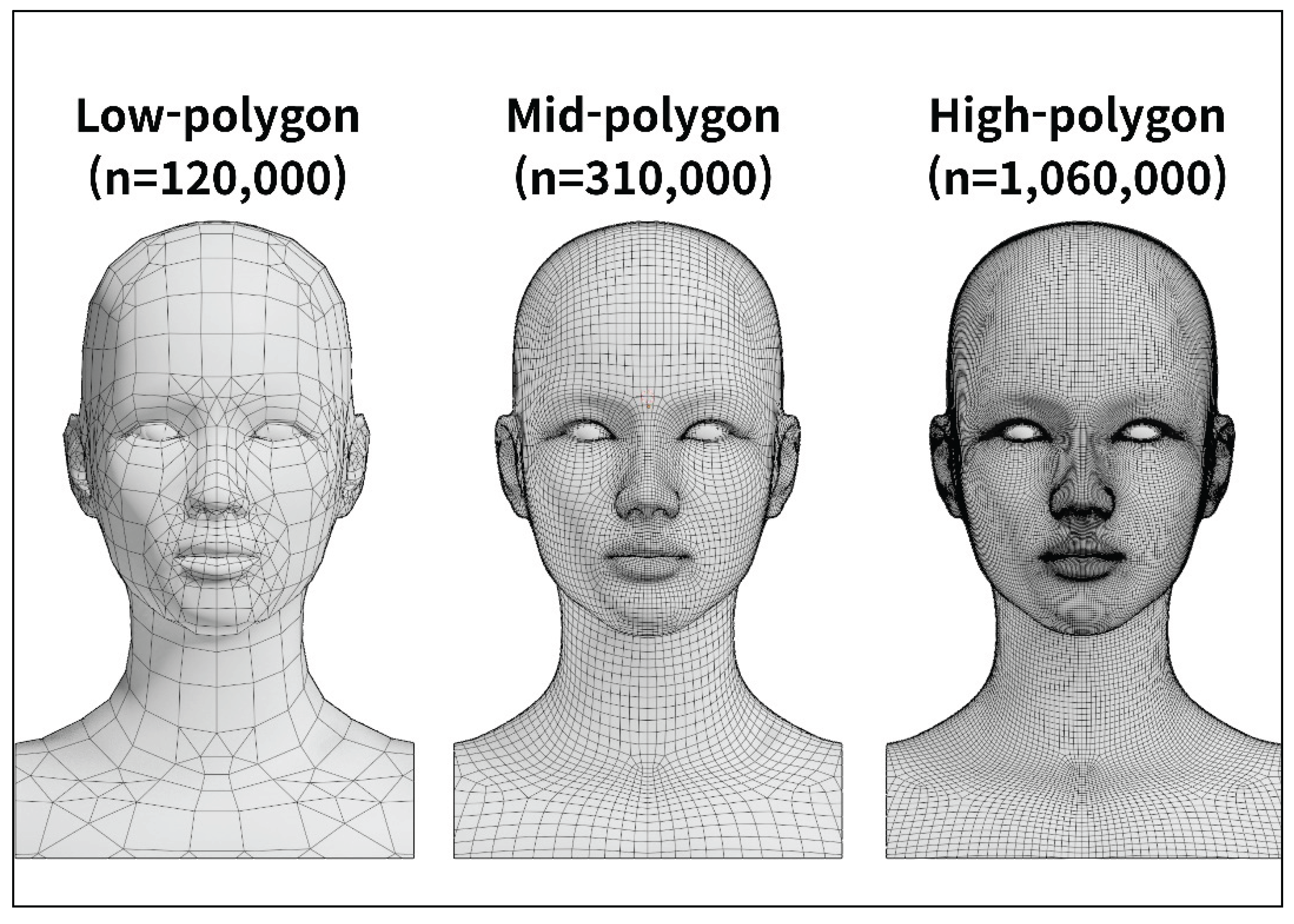
Based on the anatomical data of the standardized East Asian face, a 3D polygonal model was designed to reflect the morphology of the real human face. A human subject was selected and facial photographs were taken from multiple directions to capture the contour of the face. These photographs were processed using the 3D reconstruction software (Meshroom, AliceVision, Paris, France) to generate an initial low-polygon model.
The automatically generated polygonal mesh reflected the general facial contour but remained a simple form. To improve anatomical precision, the mesh density was adjusted using the modeling software (Blender, Blender Foundation, Amsterdam, Netherlands) while continuously comparing the model with the standardized East Asian facial dataset (
Figure 5).
In the refinement stage, the high-precision modeling software (ZBrush, Pixologic, Los Angeles, CA, USA) was used to align the overall facial proportions with those of the standardized East Asian facial ratio, focusing particularly on the position and size of the eyes and nose as well as the contour of the lips. During the texturing and rendering process, skin details such as pores, wrinkles and fine surface irregularities were added to reproduce the natural texture of human skin. Color tone and surface gloss were also applied, resulting in a visually realistic high-polygon facial model.
2.3. Development of Simulation Software
The software program for a clinical simulator (AXNavDermaInjection, Surgical Mind Inc., Seoul, Republic of Korea) was developed for the procedural guidance of injection. This software development was conducted over a period of 18 months, from July 2023 to December 2024, and was built on the development platform (version 2022.3.39f1; Unity, Unity Technologies, San Francisco, CA, USA). The 3D modeling of the syringe and other instruments used in the procedure was implemented using the modeling software (version 3.5; Blender, Blender Foundation, Amsterdam, Netherlands).
The previously constructed high-polygon facial model was optimized to improve computational efficiency by applying a normal vector–based compression technique. This process preserved fine surface shading details while reducing data size, enabling real-time rendering performance. The facial model consisting of approximately 10 million polygons was reduced to about 1 million polygons. Potential losses of micro-surface details due to simple polygon reduction were minimized through the same correction procedure. Curved anatomical structures such as blood vessels were simplified from circular cross-sections to hexagonal or octagonal forms, while curvature and texture were maintained using the same refinement method.
The finalized software was designed to synchronize simultaneously with the virtual model by detecting the spatial positions of the physical syringe and silicone dummy through an external optical tracking system.
The implemented main functions are summarized in
Table 1.
2.4. Production of a Facial Silicone Dummy
In this study, a facial silicone dummy was fabricated to serve as a physical model that receives actual needle insertions during facial injection training and operates in synchronization with the simulation software. A master pattern, representing the original form of the silicone dummy, was produced by 3D printing based on the standardized 3D facial polygonal model. The surface details of the pattern, including skin texture, wrinkles and pores, were refined using the modeling program (ZBrush, Pixologic Inc., Los Angeles, USA).
A negative mold was then created by applying a mixture of plaster and synthetic resin over the completed pattern using a reverse casting technique. An external supporting frame was fabricated to prevent deformation during the curing process. The mold was cured for 90 min with a final hardness of Shore 70D. After curing, the surface was evenly coated with a release agent (ER-200 Spray, Mann Release Technologies Inc., Macungie PA, USA) to prevent surface damage and the mold was manually demolded.
The inner cavity of the completed mold was filled with addition-cure silicone (SJS-1330, Sejin Silicone Inc., Goyang, Republic of Korea) to form the outer structure. The soft tissue was created using platinum-cured silicones (Ecoflex 00-10; and Ecoflex 00-20, Smooth-On Inc., Macungie, USA). Both materials were processed at a 1:1 mixing ratio and cured for approximately 6 h.
To reproduce a natural skin tone, the pigment (Slic Pig, Smooth-On Inc., Macungie PA, USA) was blended in proportions not exceeding 3% of the total silicone weight. For surface finishing, a mixture of pigment (Psycho Paint; and Novocs, Smooth-On Inc., Macungie PA, USA) was applied at a 1:1 mixing ratio to achieve a realistic texture and a typical East Asian skin tone. This coating layer had a working time of 45 min and was fully cured within 24 h.
To ensure the structural stability of the silicone dummy during repeated injection training, an internal supporting framework made of a lightweight, high-strength thermoplastic polymer was embedded within the silicone structure. This framework was 3D-printed via additive manufacturing, providing rigidity while maintaining material compatibility with the silicone soft tissue layers.
2.5. Hardware Setup
The hardware system for operating the clinical simulator and providing simultaneous feedback was installed in Ewha Medical Academy at Ewha Womans University Medical Center. This facility represents one of the first integrated medical simulation laboratories in Korea, supporting an interactive environment for simulator operation and user feedback.
The system consisted of a computer for running the simulation software, a large monitor for real-time visual feedback, an optical tracking camera for detecting the motion of the user and the position of the silicone dummy and tracking markers attached to the target objects for positional recognition. The recommended specifications for the operating computer included an Intel Core i5-12600K CPU, NVIDIA GeForce RTX 4060 GPU, 16 GB RAM and an NVMe SSD (500 GB with at least 10 GB of free space).
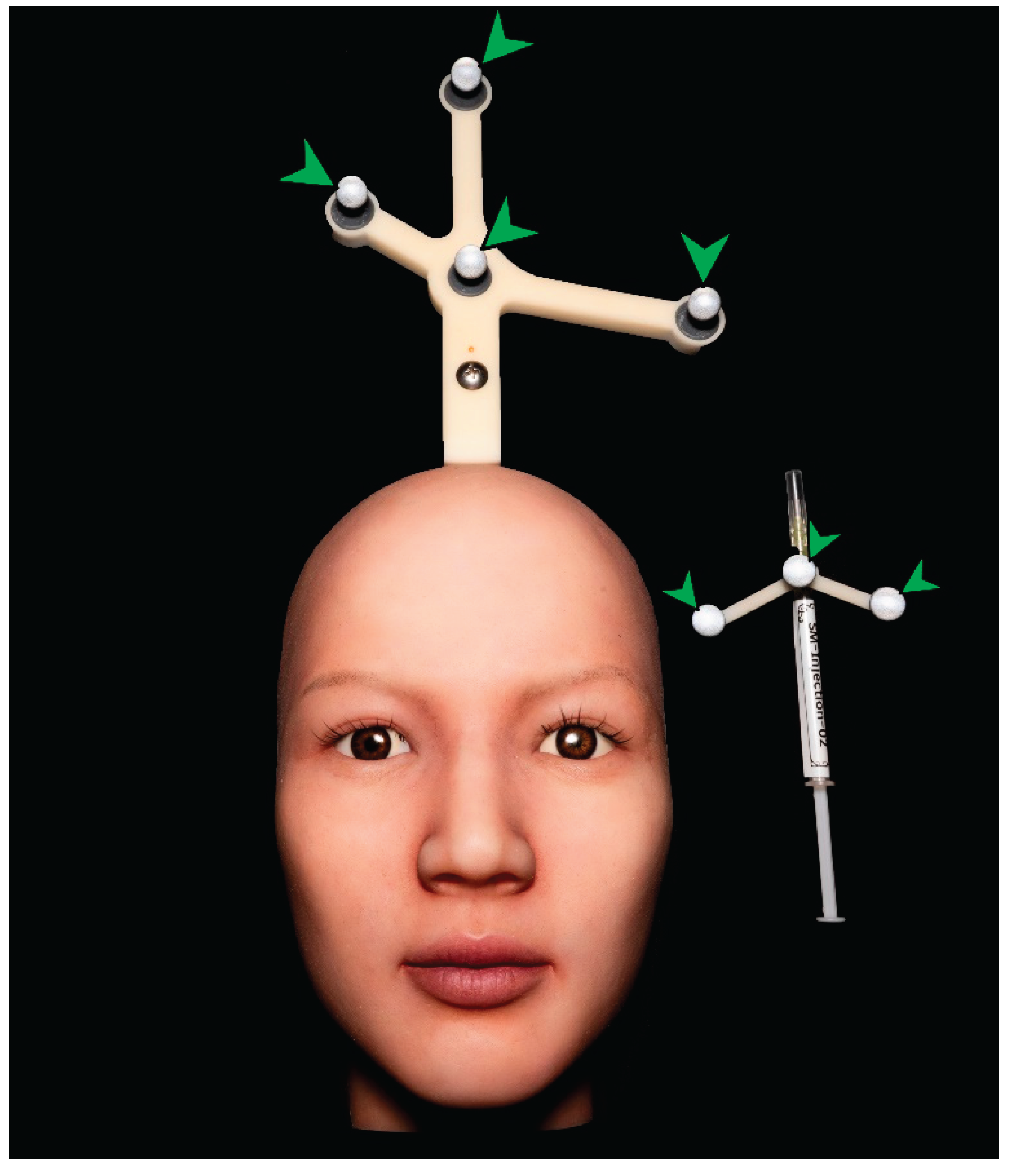
For real-time visualization, a large portable monitor (86TN3FLG, LG Electronics, Seoul, Republic of Korea) was connected to the system. An optical tracking camera (NDI Vega VICRA, Northern Digital Incorporated, Canada) was installed to detect the movement of the syringe and the positional changes of the silicone dummy. To enhance motion-tracking accuracy, passive reflective markers were attached near the vertex region of the silicone dummy and along the body of the syringe, enabling the optical camera to detect reflected signals (
Figure 6).
The complete hardware setup of the clinical simulator is shown in
Figure 7.
2.6. Pilot Usability Test
An internal verification was conducted to assess the technical usability of the simulator after system integration. The evaluation involved a surgeon, two anatomists and two biomedical engineers from the development team.
Each member independently interacted with the simulator and provided structured feedback on five aspects: manipulability, visual completeness, system stability, intuitiveness and expected learning effect.
3. Results
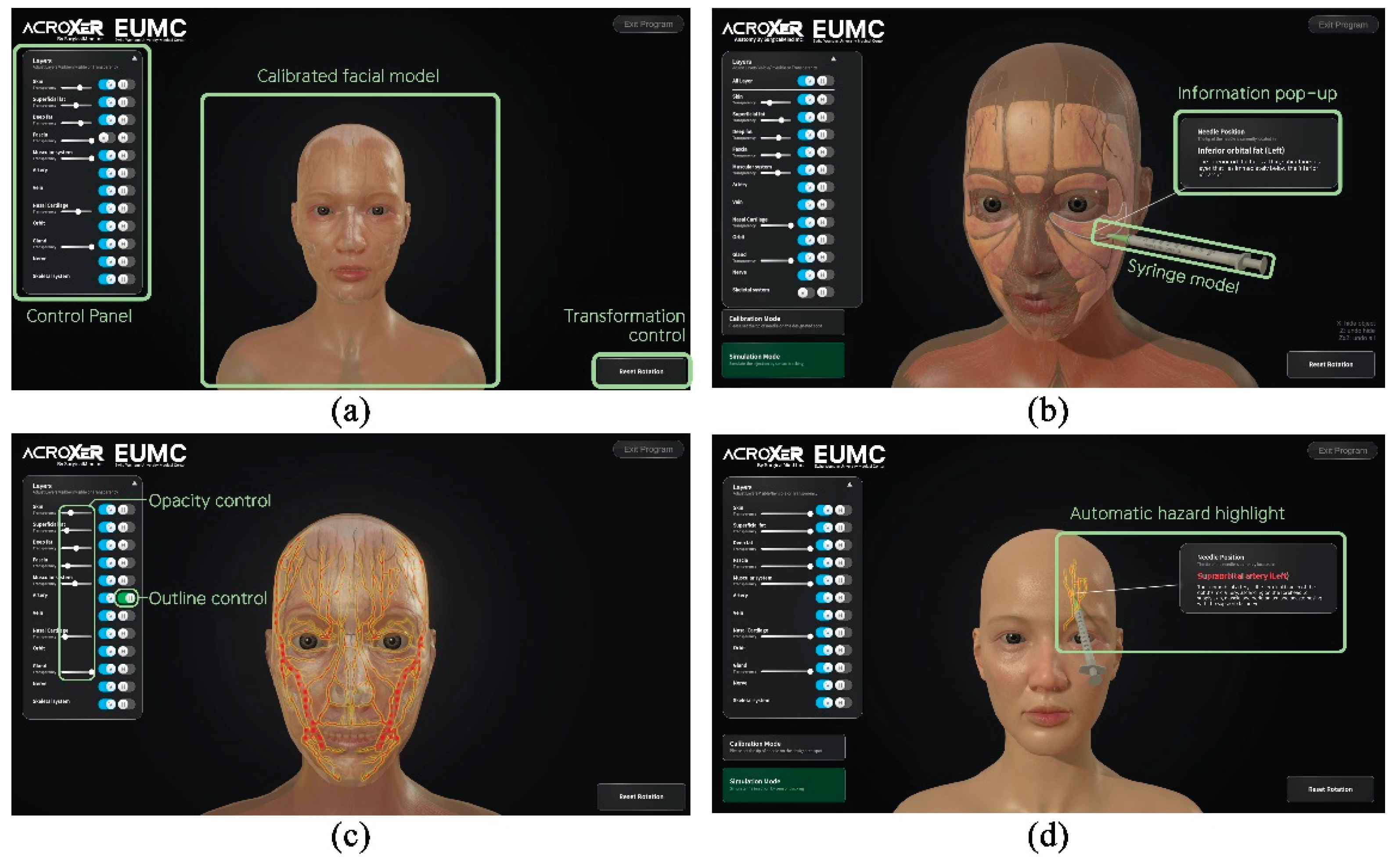
During clinical simulator operation, the volumetric root mean square accuracy was 0.25 mm and the 95 percent confidence interval for volumetric accuracy was 0.50 mm. The maximum frame rate, which represents the measurement update rate, reached 20 Hz. The optical tracking camera, positioned approximately 90 to 100 cm from the silicone dummy, demonstrated a spatial measurement reproducibility of 0.029 to 0.045 mm and a dynamic tracking error of less than one millimeter. When the optical camera detected the passive reflective markers, the system continuously calculated the spatial coordinates of the dummy and syringe in real time and projected their movements onto the monitor screen. The basic user interface design is presented in
Figure 8a. The trajectory of the syringe was simultaneously visualized on the screen, and when the needle tip contacted with a virtual anatomical structure, the system calculated the coordinate data and displayed the structure name and anatomical description in a pop-up window within approximately 0.05 s (
Figure 8b). Through this process, users were able to recognize the anatomical context of each injection site immediately.
The simulator incorporated visibility control and transparency adjustment functions, allowing users to explore the relative positions and depth distribution of anatomical structures step by step (
Figure 8c). When the needle approached critical vascular structures such as the dorsal nasal artery, supraorbital artery or supratrochlear artery, the system issued a visual warning by activating a red and yellow outline glow shader, clearly indicating regions that required anatomical caution (
Figure 8d). When a target structure had been highlighted in advance, the highlight disappeared once the needle tip reached the corresponding layer, providing an immediate visual indication of correct placement. The facial model could be rotated and tilted freely, enabling users to observe anatomical relationships from multiple perspectives and to perform repeated procedural training with improved spatial awareness.
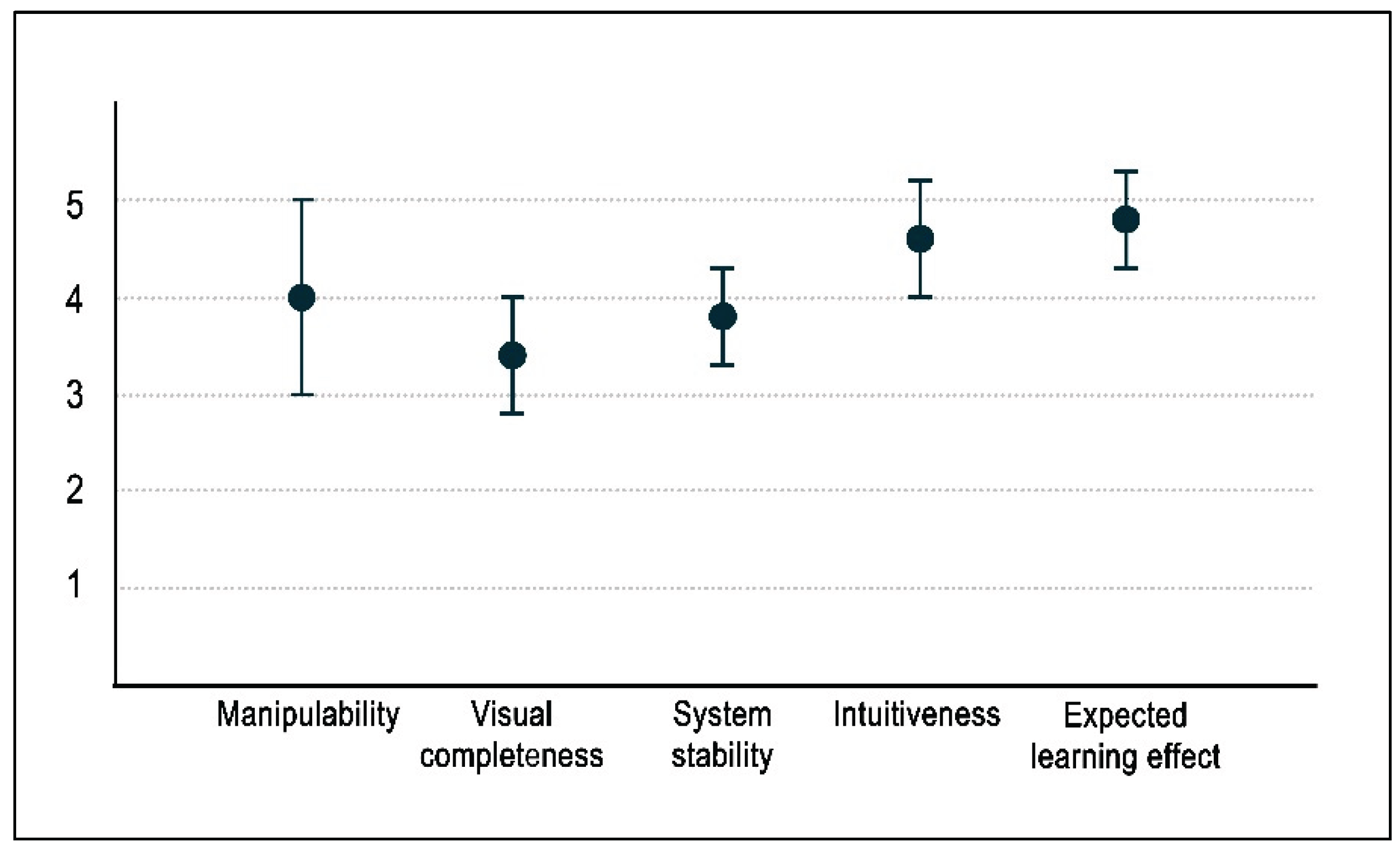
In the preliminary usability test, all items were evaluated on a five-point scale. The mean scores with standard deviations for manipulability, visual completeness, system stability, intuitiveness and expected learning effect were 4.0 ± 1.0, 3.4 ± 0.6, 3.8 ± 0.5, 4.6 ± 0.6 and 4.8 ± 0.5, respectively, as summarized in
Figure 9.
4. Discussion
The face is closely related to social identity of an individual beyond the concept of external image and plays a central role in human social interaction [
33]. Facial expression, the eyes and the skin function as primary mediators that convey emotional states to others and serve important roles in social communication [
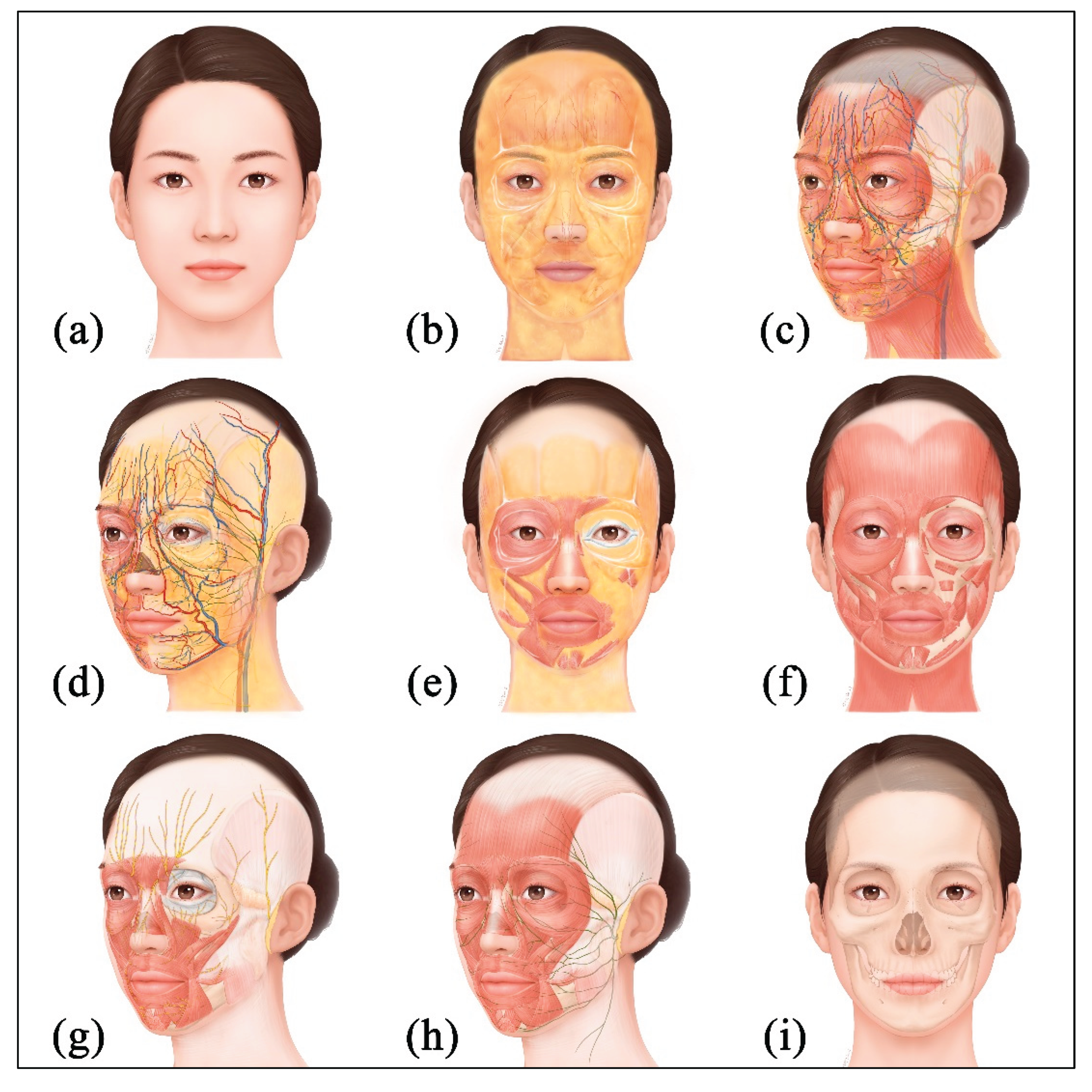
34]. Aesthetic investment is concentrated on the face because it is one of the most visually exposed body regions and the self-image expressed through the face leaves strong impressions and memories in others. For these reasons, the face has become a central area of aesthetic medicine, and the complex anatomy of the face is an important consideration for clinicians. The face has a multilayered structure and vessels and nerves are intricately arranged (
Figure 10); therefore, high anatomical precision and fine technical skill for medical procedures are required.
Demand for education and procedural training in facial anatomy continues to increase in clinical settings, but most training still remains limited to formats such as seminars and symposiums. Education in clinical environments is restricted for patient safety, and although training using donated cadavers is highly effective for understanding human anatomy, widespread adoption is difficult due to a shortage of cadavers, ethical constraints, limitations of educational facilities and a lack of specialized personnel [
35,
36]. Accordingly, procedural training using simulators has attracted attention as an alternative that can prevent medical accidents and disputes caused by insufficient anatomical knowledge and limited procedural experience [
37]. Clinical simulators were originally introduced in anesthesiology and have advanced through the convergence of computer engineering, biomedical engineering and behavioral science [
13]. These systems combine life-size dummies with interactive software to reproduce diverse clinical scenarios and enable repetitive, standardized, anatomy-based training [
38]. Simulation-based education is evaluated as an efficient method that complements the ethical and practical limitations of cadaver-based education by allowing repeated practice in a safe and controlled environment.
Previous studies have reported that simulator-based training improves theoretical and practical knowledge, procedural confidence, teamwork and patient outcomes [
39,
40]. Not only beginners but also experts with experience in clinical medicine can seek skill enhancement through such training; therefore, clinical simulator learning has a wide scope of application. Above all, the greatest advantage of simulators is the possibility of repetitive training. When vessels are damaged during use or when physical contact is made with neural tissues that should be avoided, users can recognize and verify this through immediate visual feedback. In addition, self-directed learning can be conducted without an instructor and substantial progress in procedural competence can be achieved through the acquisition of greater anatomical knowledge and training. In the clinical simulator developed in this study, a larger set of standard data for East Asian faces was incorporated with the previously developed VR-based injection simulator [
17]. The expanded dataset enabled the anatomical structures such as muscles, vessels and nerves, to be modeled with greater 3D fidelity and quantitative precision. The use of optical tracking camera with higher resolution and higher capability allowed syringe motion to be monitored in real time without delay, which resulted in improved procedural training compared with the earlier system. A dummy model with an external appearance and skin texture similar to those of a real human face was developed and linked with the software, thereby further enhancing the realism and educational effect of the simulator. The polygonal model developed in this study was efficiently compressed to a small size without image quality degradation based on vector-quantization compression during application-software implementation, providing the technical advantage that implementation is possible without dependence on high-performance hardware. The differences between the existing and the presently developed simulators are summarized in
Table 2.
Meanwhile, some users of clinical simulators may experience cognitive fatigue due to excessive focus on digital learning, and there is a risk that the simulation experience may blur the distinction from reality [
41]. In addition, low-quality simulation content and a shortage of qualified simulator faculty can degrade the quality of learning and hinder educational effectiveness [
42]. Initial equipment-setup costs such as software licenses and hardware procurement, costs for technical maintenance and management, and requirements for personnel training and programming remain major challenges in implementing simulators [
43]. Although the clinical simulator for facial injection training developed in this study reflects improved facial anatomical data compared with existing systems, an ongoing task is to update skeletal and soft tissues with sample datasets from more population groups studied to date [
44,
45,
46], not only East Asian data (
Table 3).
In this study, the silicone dummy and syringe were produced to be more realistic than those previously developed to improve the user’s training experience, but technical limitations still exist in perfectly reproducing the actual clinical environment. For example, whereas tissues move or change during actual procedures depending on the amount and material properties of the injectables, the simulator has the limitation that it cannot reflect movement or deformation of anatomical structures in real time. Therefore, users should fully recognize the differences between simulator training and actual procedures and concurrently adopt training approaches that can compensate for them. In addition, although the simulator can analyze user manipulations and provide feedback, its responses are relatively limited. Injection procedures are highly delicate tasks, and during actual procedures the method must be adjusted according to changes in the face, but current simulators find it difficult to respond perfectly to this. For instance, the training content should differ depending on whether a hypodermic needle or a cannula with a blunted needle tip is used as the procedural instrument, but there is the inconvenience that a single system supports only one type of instrument. Future clinical simulators to be developed need to add systems that are compatible with multiple procedural instruments.
For continuous improvement, it is essential to gather and analyze feedback from diverse users and to regularly inspect the performance of the simulator. However, the analytical methodologies proposed in other studies have primarily relied on subjective evaluations such as user satisfaction and confidence, which limits the assessment of the objective reliability and validity of simulators [
47]. Future research needs to employ validated assessment tools to measure users’ knowledge, technical competence and performance, and to quantitatively demonstrate the educational effectiveness of simulators.
An ideal future simulator should include diverse simulation scenarios such as wrinkle improvement, volume correction and line refinement in addition to technical enhancements. Although the simulator developed in this study is currently specialized for facial injection training, it has the potential to be extended to other clinical procedural domains such as lumbar puncture, intravenous injection and various ostomy management and care training on the basis of the same technical architecture. Such expansion of the system could be advanced into a more comprehensive clinical simulation platform by integrating anatomical datasets for different body regions.
5. Conclusions
This study presented the detailed process for developing and implementing a clinical simulator for facial injection training and proposed an alternative learning tool that can address the limitations of traditional anatomy education.
This simulator, based on precise anatomical knowledge, provides an accurate and realistic learning environment and is anticipated to enhance the anatomical understanding and procedural skills of clinicians in aesthetic medicine.
Author Contributions
Conceptualization, J.S., S.C. and S.H.; methodology, J.S., S.H.; software, H.C., I.K. and D.Y.; validation, J.S., I.K. and S.H.; formal analysis, J.S., D.Y.; investigation, J.S., H.C., B.K.; resources, S.C., B.K.; data curation, J.S., D.Y.; writing—original draft preparation, J.S., S.C.; writing—review and editing, J.S., S.H.; visualization, J.S., H.C. and I.K.; supervision, S.H.; project administration, J.S., S.C. and B.K.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korea Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by Ministry of Health & Welfare, Republic of Korea (RS-2023-KH134708), and by Korea Institute of Science and Technology Information (KISTI) (No. K25L3M1C2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Access will be considered for academic or research purposes following review and approval.
Acknowledgments
The authors gratefully acknowledge the support of Ewha Medical Academy at Ewha Womans University Medical Center for providing research facilities and technical assistance during the development of the clinical simulator. The authors also express their appreciation to Suhyun Chae for creating the two-dimensional illustration of the facial anatomy used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garrouste-Orgeas M, Philippart F, Bruel C, Max A, Lau N, Misset B. Overview of medical errors and adverse events. Ann Intensive Care. 2012;2(1):2. Published 2012 Feb 16. [CrossRef]
- Park BY, Kim MJ, Kang SR, Hong SE. A Legal Analysis of the Precedents of Medical Disputes in the Cosmetic Surgery Field. Arch Plast Surg. 2016;43(3):278-283. [CrossRef]
- Montrief T, Bornstein K, Ramzy M, Koyfman A, Long BJ. Plastic Surgery Complications: A Review for Emergency Clinicians. West J Emerg Med. 2020;21(6):179-189. Published 2020 Sep 25. [CrossRef]
- Borah G, Rankin M, Wey P. Psychological complications in 281 plastic surgery practices. Plast Reconstr Surg. 1999;104(5):1241-1246. [CrossRef]
- Fagien S, Klein AW. A brief overview and history of temporary fillers: evolution, advantages, and limitations. Plast Reconstr Surg. 2007;120(6 Suppl):8S-16S. [CrossRef]
- Signorini M, Liew S, Sundaram H, et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence- and Opinion-Based Review and Consensus Recommendations. Plast Reconstr Surg. 2016;137(6):961e-971e. [CrossRef]
- Greco TM, Antunes MB, Yellin SA. Injectable fillers for volume replacement in the aging face. Facial Plast Surg. 2012;28(1):8-20. [CrossRef]
- Sito G, Manzoni V, Sommariva R. Vascular Complications after Facial Filler Injection: A Literature Review and Meta-analysis. J Clin Aesthet Dermatol. 2019;12(6):E65-E72.
- Vitous CA, Byrnes ME, De Roo A, Jafri SM, Suwanabol PA. Exploring Emotional Responses After Postoperative Complications: A Qualitative Study of Practicing Surgeons. Ann Surg. 2022;275(1):e124-e131. [CrossRef]
- Childs S, Blenkinsopp E, Hall A, Walton G. Effective e-learning for health professionals and students--barriers and their solutions. A systematic review of the literature--findings from the HeXL project. Health Info Libr J. 2005;22 Suppl 2:20-32. [CrossRef]
- Morris MP, Toyoda Y, Christopher AN, Broach RB, Percec I. A Systematic Review of Aesthetic Surgery Training Within Plastic Surgery Training Programs in the USA: An In-Depth Analysis and Practical Reference. Aesthetic Plast Surg. 2022;46(1):513-523. [CrossRef]
- Kumar N, Rahman E, Adds PJ. An effective and novel method for teaching applied facial anatomy and related procedural skills to esthetic physicians. Adv Med Educ Pract. 2018;9:905-913. Published 2018 Dec 6. [CrossRef]
- Ypinazar VA, Margolis SA. Clinical simulators: applications and implications for rural medical education. Rural Remote Health. 2006;6(2):527.
- Maran NJ, Glavin RJ. Low- to high-fidelity simulation—a continuum of medical education?. Med Educ. 2003;37 Suppl 1:22-28. [CrossRef]
- Hammoud MM, Nuthalapaty FS, Goepfert AR, et al. To the point: medical education review of the role of simulators in surgical training. Am J Obstet Gynecol. 2008;199(4):338-343. [CrossRef]
- Lamé G, Dixon-Woods M. Using clinical simulation to study how to improve quality and safety in healthcare. BMJ Simul Technol Enhanc Learn. 2020;6(2):87-94. [CrossRef]
- Oh SM, Kim JY, Han S, et al. Development and Usability of a Virtual Reality-Based Filler Injection Training System. Aesthetic Plast Surg. 2020;44(5):1833-1842. [CrossRef]
- Digital Korean Human Information System for better project. Available online: https://dk.kisti.re.kr/(accessed on 20 April 2023).
- Hwang HS, Choe SY, Hwang JS, et al. Reproducibility of Facial Soft Tissue Thickness Measurements Using Cone-Beam CT Images According to the Measurement Methods. J Forensic Sci. 2015;60(4):957-965. [CrossRef]
- Alfertshofer MG, Frank K, Moellhoff N, et al. Ultrasound Anatomy of the Dorsal Nasal Artery as it Relates to Liquid Rhinoplasty Procedures. Facial Plast Surg Clin North Am. 2022;30(2):135-141. [CrossRef]
- Chen CL, Cong LY, Kong XX, et al. Three-Dimensional Computed Tomography Scanning of Temporal Vessels to Assess the Safety of Filler Injections. Aesthet Surg J. 2021;41(11):1306-1313. [CrossRef]
- Choi DH, Eom JR, Lee JW, et al. Zygomatico-orbital artery: The largest artery in the temporal area. J Plast Reconstr Aesthet Surg. 2018;71(4):484-489. [CrossRef]
- Cong LY, Liao ZF, Zhang YS, Li DN, Luo SK. Three-Dimensional Arterial Distribution Over the Midline of the Nasal Bone. Aesthet Surg J. 2022;42(7):784-790. [CrossRef]
- Dagistan S, Miloǧlu Ö, Altun O, Umar EK. Retrospective morphometric analysis of the infraorbital foramen with cone beam computed tomography. Niger J Clin Pract. 2017;20(9):1053-1064. [CrossRef]
- Hwang SH, Kim SW, Park CS, Kim SW, Cho JH, Kang JM. Morphometric analysis of the infraorbital groove, canal, and foramen on three-dimensional reconstruction of computed tomography scans. Surg Radiol Anat. 2013;35(7):565-571. [CrossRef]
- Khorasanizadeh F, Delazar S, Gheidari O, et al. Anatomic evaluation of the normal variants of the arteries of face using color Doppler ultrasonography: Implications for facial aesthetic procedures. J Cosmet Dermatol. 2023;22(6):1844-1851. [CrossRef]
- Lee KL, Lee HJ, Youn KH, Kim HJ. Positional relationship of superior and inferior labial artery by ultrasonography image analysis for safe lip augmentation procedures. Clin Anat. 2020;33(2):158-164. [CrossRef]
- Money SM, Wall WB, Davis LS, Edmondson AC. Lumen Diameter and Associated Anatomy of the Superior Labial Artery With a Clinical Application to Dermal Filler Injection. Dermatol Surg. 2020;46(5):678-684. [CrossRef]
- Phumyoo T, Jiirasutat N, Jitaree B, Rungsawang C, Uruwan S, Tansatit T. Anatomical and Ultrasonography-Based Investigation to Localize the Arteries on the Central Forehead Region During the Glabellar Augmentation Procedure. Clin Anat. 2020;33(3):370-382. [CrossRef]
- Tansatit T, Phumyoo T, Sawatwong W, McCabe H, Jitaree B. Implication of Location of the Ascending Mental Artery at the Chin Injection Point. Plast Reconstr Surg. 2020;145(1):51e-57e. [CrossRef]
- Ten B, Kara T, Kaya Tİ, et al. Evaluation of facial artery course variations and depth by Doppler ultrasonography. J Cosmet Dermatol. 2021;20(7):2247-2258. [CrossRef]
- Yang W, He R, Chen T, Haining W, Goossens R, Huysmans T. Soft tissue thickness estimation for head, face, and neck from CT data for product design purposes. Ergonomics in Design. 2022;47:558-565. [CrossRef]
- Bargiela-Chiappini F; Haugh M. Face communication and social interaction; University of Toronto Press: Toronto, Canada, 2009; pp. 1-344.
- Kret ME. Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Front Psychol. 2015;6:711. Published 2015 May 27. [CrossRef]
- Azer SA, Eizenberg N. Do we need dissection in an integrated problem-based learning medical course? Perceptions of first- and second-year students. Surg Radiol Anat. 2007;29(2):173-180. [CrossRef]
- Raja DS, Sultana B. Potential health hazards for students exposed to formaldehyde in the gross anatomy laboratory. J Environ Health. 2012;74(6):36-40.
- Hunt EA, Nelson KL, Shilkofski NA. Simulation in medicine: addressing patient safety and improving the interface between healthcare providers and medical technology. Biomed Instrum Technol. 2006;40(5):399-404. [CrossRef]
- Akaike M, Fukutomi M, Nagamune M, et al. Simulation-based medical education in clinical skills laboratory. J Med Invest. 2012;59(1-2):28-35. [CrossRef]
- Green R, Curry N. Simulation training improves clinical knowledge of major haemorrhage management in foundation year doctors. Transfus Med. 2014;24(6):379-384. [CrossRef]
- Okuda Y, Bryson EO, DeMaria S Jr, et al. The utility of simulation in medical education: what is the evidence?. Mt Sinai J Med. 2009;76(4):330-343. [CrossRef]
- Larue C, Pepin J, Allard E. Simulation in preparation or substitution for clinical placement: a systematic review of the literature. J Nurs Educ Pract. 2015;5(9):132–140. [CrossRef]
- Sørensen JL, Østergaard D, LeBlanc V, et al. Design of simulation-based medical education and advantages and disadvantages of in situ simulation versus off-site simulation. BMC Med Educ. 2017;17(1):20. Published 2017 Jan 21. [CrossRef]
- Mussi E, Furferi R, Volpe Y, Facchini F, McGreevy KS, Uccheddu F. Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering (Basel). 2019;6(1):14. Published 2019 Feb 5. [CrossRef]
- Krogman WM. The human skeleton in forensic medicine, 2nd ed.; Charles C Thomas: Springfield, IL, USA, 1962; pp. 268–280.
- Matsumura H, Tanijiri T, Kouchi M, et al. Global patterns of the cranial form of modern human populations described by analysis of a 3D surface homologous model. Sci Rep. 2022;12(1):13826. Published 2022 Aug 15. [CrossRef]
- Stephan CN, Simpson EK. Facial soft tissue depths in craniofacial identification (part I): An analytical review of the published adult data. J Forensic Sci. 2008;53(6):1257-1272. [CrossRef]
- Krishnan DG, Keloth AV, Ubedulla S. Pros and cons of simulation in medical education:A review. Education. 2017;3(6):84–87.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).