Submitted:
17 November 2025
Posted:
19 November 2025
You are already at the latest version
Abstract
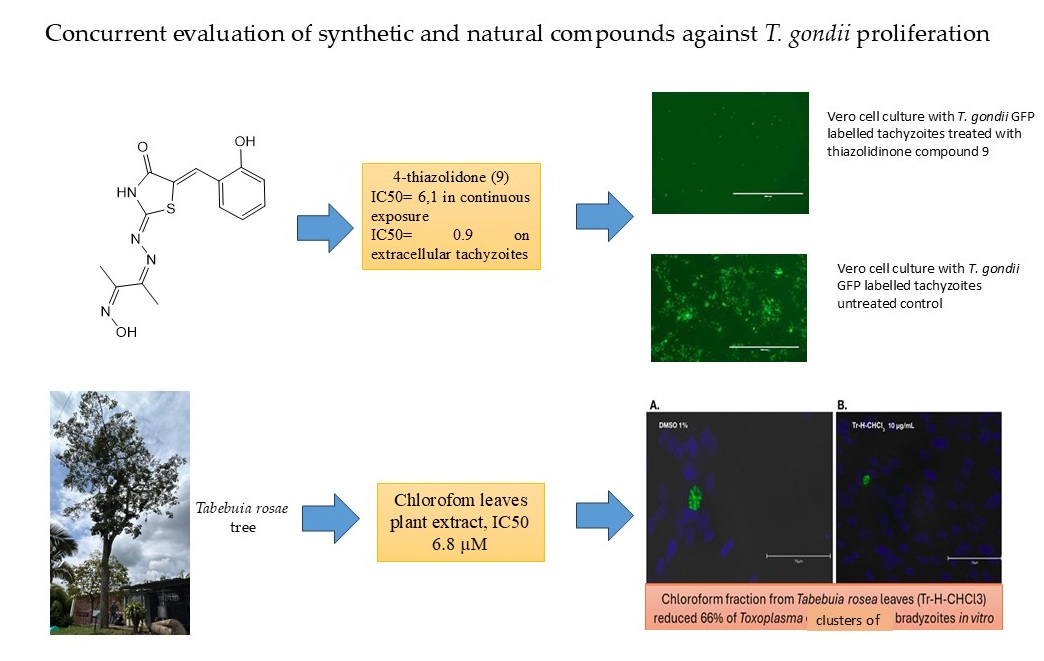
Concurrent evaluation of the antiparasitic efficacy of synthetic and natural compounds can provide novel insights into the development of anti-Toxoplasma drugs. We assessed 16 synthetic compounds and fractions derived from the leaves of two Tabebuia tree species for their in vitro activity against live parasites, employing strains that express green fluorescent protein and specific identification of bradyzoites with an anti-BAG1 monoclonal antibody. This study successfully identified several promising synthetic compounds with potent anti-Toxoplasma activity and favorable in vitro selectivity profiles, notably pyrazoline 2 and thiazolidinone 9. One thiazolidinone compound exhibited significant activity against extracellular tachyzoites, whereas one tree fraction demonstrated excellent activity against both tachyzoites and bradyzoites. Additionally, their in silico ADMET properties suggest their potential for good in vivo performance and CNS penetration. Although the natural extracts showed less potency in their crude form, they provide a basis for future purification efforts. The simultaneous evaluation of compounds sourced from diverse discovery pipelines can offer valuable insights into the development of drugs that target various biological pathways.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of Pyrazole, Pyrazoline and Thiazolidinone Derivatives Compounds
2.2. Fraction from Tabebuia Rosae and T. chrysantha Tree
2.2. Host Cell Lines and Cultivation of T. gondii Parasites
2.3. Cytotoxicity Evaluation of the Selected Compounds in Vero Cells by Alamar Blue Assay
2.4. T. gondii RH GFP Tachyzoite Viability Assay
2.5. Assessment of T. gondii RH GFP Tachyzoite Viability Following a 24-Hour Pretreatment of Host Cells
2.6. Assessment of the Viability of T. gondii RH GFP Tachyzoite Following a 3-Hour Pretreatment of Parasites
2.7. Half Maximal Cytotoxic Concentration (CC50), Half Maximal Inhibitory Concentration (IC50) and Selectivity Index (SI) Calculation
2.8. In Silico Pharmacokinetics, Drug-Likeliness, and ADMET Depiction Exploration
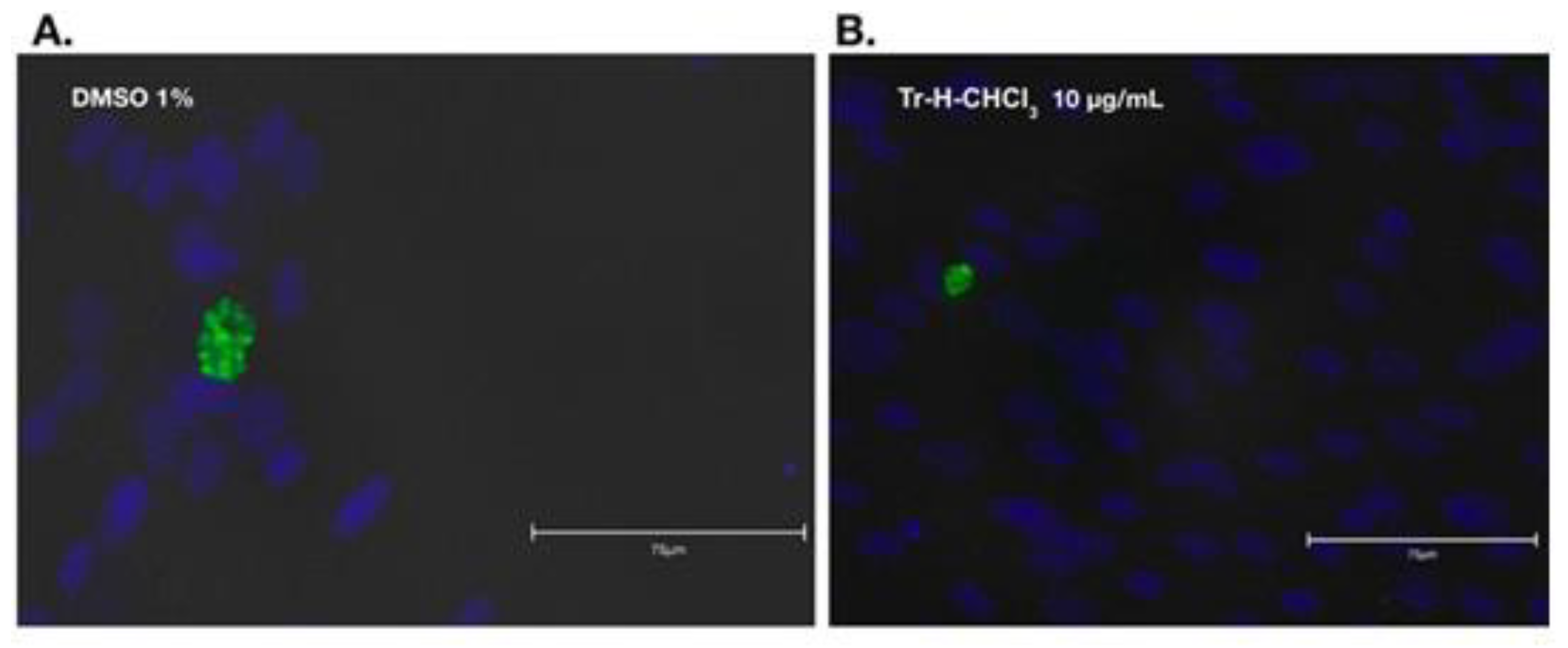
2.9. Anti-Bradyzoite Activity of T. rosae Fractions
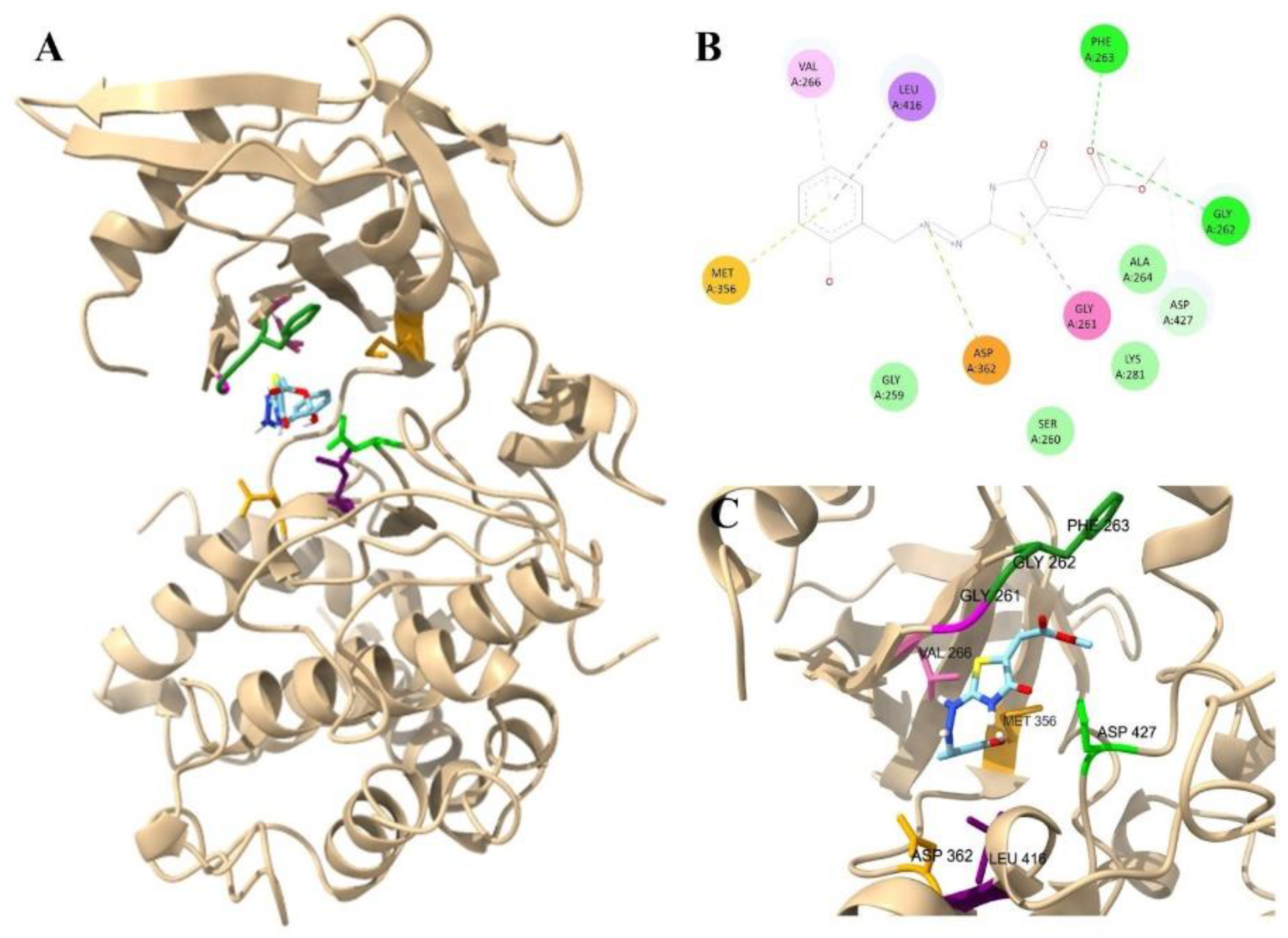
2.10. Molecular Docking
3. Results
3.1. Cytotoxicity, Anti-Toxoplasma Inhibitory Effect and Selectivity Index of the Synthetic and Natural Compounds
3.2. In Silico Prediction of Pharmacokinetics, Drug-Likeliness, and ADMET Depiction Exploration
3.3. Result of Anti-Bradyzoite Evaluation of T. rosae Fraction
3.4. Molecular Docking of Thiazolidinone Compound 9 with Target Proteins Candidates of T. gondii
4. Discussion
- -
- Benchmarking and controls: Synthetic compounds serve as reliable internal standards, helping to measure assay drift and enabling cross-comparisons among experiments.
- -
- Discovery funneling: Plant extracts with promising activity may feed into synthetic-lead optimization pipelines, combining the novelty of natural chemistry with the reproducibility of synthetic optimization.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandelbrot, L.; Gomez-Marin, J.E. Protozoan Diseases: Toxoplasmosis. In International Encyclopedia of Public Health; Elsevier: Amsterdam, 2025; Vol. 3, pp. 830–854 ISBN 978-0-323-97280-2.
- Rahmanian, V.; Rahmanian, K.; Jahromi, A.; Bokaie, S. Seroprevalence of Toxoplasma Gondii Infection: An Umbrella Review of Updated Systematic Reviews and Meta-Analyses. J Family Med Prim Care 2020, 9, 3848. [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin Microbiol Rev 2018, 31, e00057-17.
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin Microbiol Rev 2018, 31. [CrossRef]
- Teil, J.; Dupont, D.; Charpiat, B.; Corvaisier, S.; Vial, T.; Leboucher, G.; Wallon, M.; Peyron, F. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr Infect Dis J 2016, 35, 634–638. [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin Microbiol Rev 2018, 31. [CrossRef]
- McPhillie, M.; Zhou, Y.; El Bissati, K.; Dubey, J.; Lorenzi, H.; Capper, M.; Lukens, A.K.; Hickman, M.; Muench, S.; Verma, S.K.; et al. New Paradigms for Understanding and Step Changes in Treating Active and Chronic, Persistent Apicomplexan Infections. Sci Rep 2016, 6. [CrossRef]
- McFarland, M.M.; Zach, S.J.; Wang, X.; Potluri, L.P.; Neville, A.J.; Vennerstrom, J.L.; Davis, P.H. Review of Experimental Compounds Demonstrating Anti-Toxoplasma Activity. Antimicrob Agents Chemother 2016, 60, 7017. [CrossRef]
- Montazeri, M.; Sharif, M.; Sarvi, S.; Mehrzadi, S.; Ahmadpour, E.; Daryani, A. A Systematic Review of in Vitro and in Vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006-2016). Front Microbiol 2017, 8, 228484. [CrossRef]
- Molina, D.A.; Ramos, G.A.; Zamora-Vélez, A.; Gallego-López, G.M.; Rocha-Roa, C.; Gómez-Marin, J.E.; Cortes, E. In Vitro Evaluation of New 4-Thiazolidinones on Invasion and Growth of Toxoplasma Gondii. Int J Parasitol Drugs Drug Resist 2021, 16, 129–139. [CrossRef]
- Cardona-Trujillo, M.C.; Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. In Vitro Anti-Toxoplasma Activity of Extracts Obtained from Tabebuia Rosea and Tabebuia Chrysantha: The Role of β-Amyrin. Molecules 2024, 29, 920. [CrossRef]
- Bekhit, A.A.; Ashour, H.M.A.; Abdel Ghany, Y.S.; Bekhit, A.E.D.A.; Baraka, A. Synthesis and Biological Evaluation of Some Thiazolyl and Thiadiazolyl Derivatives of 1H-Pyrazole as Anti-Inflammatory Antimicrobial Agents. Eur J Med Chem 2008, 43, 456–463. [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently Reported Biological Activities of Pyrazole Compounds. Bioorg Med Chem 2017, 25, 5891–5903. [CrossRef]
- Huang, W.; Ojo, K.K.; Zhang, Z.; Rivas, K.; Vidadala, R.S.R.; Scheele, S.; Derocher, A.E.; Choi, R.; Hulverson, M.A.; Barrett, L.K.; et al. SAR Studies of 5-Aminopyrazole-4-Carboxamide Analogues as Potent and Selective Inhibitors of Toxoplasma Gondii CDPK1. ACS Med Chem Lett 2015, 6, 1184–1189. [CrossRef]
- Montoya, A.; Quiroga, J.; Abonia, R.; Derita, M.; Sortino, M.; Ornelas, A.; Zacchino, S.; Insuasty, B. Hybrid Molecules Containing a 7-Chloro-4-Aminoquinoline Nucleus and a Substituted 2-Pyrazoline with Antiproliferative and Antifungal Activity. Molecules 2016, Vol. 21, Page 969 2016, 21, 969. [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Vélez, I.D.; Upegui, Y.; Muñoz, J.A.; et al. An Efficient Synthesis of New Caffeine-Based Chalcones, Pyrazolines and Pyrazolo[3,4- b ][1,4]Diazepines as Potential Antimalarial, Antitrypanosomal and Antileishmanial Agents. Eur J Med Chem 2015, 93, 401–413. [CrossRef]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.D.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of Novel Analogs of 2-Pyrazoline Obtained from [(7-Chloroquinolin-4-Yl)Amino]Chalcones and Hydrazine as Potential Antitumor and Antimalarial Agents. Eur J Med Chem 2013, 67, 252–262. [CrossRef]
- Rocha-Roa, C.; Molina, D.; Cardona, N. A Perspective on Thiazolidinone Scaffold Development as a New Therapeutic Strategy for Toxoplasmosis. Front Cell Infect Microbiol 2018, 8.
- Dzitko, K.; Kaproń, B.; Paneth, A.; Bekier, A.; Plech, T.; Paneth, P.; Trotsko, N. TZD-Based Hybrid Molecules Act as Dual Anti- Mycobacterium Tuberculosis and Anti- Toxoplasma Gondii Agents. Int J Mol Sci 2023, 24. [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J Med Chem 2015, 58, 4066–4072. [CrossRef]
- Mayoral, J.; Di Cristina, M.; Carruthers, V.B.; Weiss, L.M. Toxoplasma Gondii: Bradyzoite Differentiation In Vitro and In Vivo. Methods in Molecular Biology 2020, 2071, 269–282. [CrossRef]
- McPhillie, M.J.; Zhou, Y.; Hickman, M.R.; Gordon, J.A.; Weber, C.R.; Li, Q.; Lee, P.J.; Amporndanai, K.; Johnson, R.M.; Darby, H.; et al. Potent Tetrahydroquinolone Eliminates Apicomplexan Parasites. Front Cell Infect Microbiol 2020, 10. [CrossRef]
- Tomita, T.; Bzik, D.J.; Ma, Y.F.; Fox, B.A.; Markillie, L.M.; Taylor, R.C.; Kim, K.; Weiss, L.M. The Toxoplasma Gondii Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence. PLoS Pathog 2013, 9, 1–15. [CrossRef]
- D’Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordón, C.; Jones-Brando, L. Design, Synthesis and Biological Characterization of Thiazolidin-4-One Derivatives as Promising Inhibitors of Toxoplasma Gondii. Eur J Med Chem 2014, 86, 17–30. [CrossRef]
- Molina, D.; Cossio-Pérez, R.; Rocha-Roa, C.; Pedraza, L.; Cortes, E.; Hernández, A.; Gómez-Marín, J.E. Protein Targets of Thiazolidinone Derivatives in Toxoplasma Gondii and Insights into Their Binding to ROP18. BMC Genomics 2018, 19, 856. [CrossRef]
- Carvalho, C.S.; de Melo, E.J.T. Anti-Parasitic Action and Elimination of Intracellular Toxoplasma Gondii in the Presence of Novel Thiosemicarbazone and Its 4-Thiazolidinone Derivatives. Brazilian Journal of Medical and Biological Research 2009, 43, 139–149. [CrossRef]
- Sepulveda-Arias, J.; Veloza, L.; Mantilla-Muriel, L. Anti-Toxoplasma Activity of Natural Products: A Review. Recent Pat Antiinfect Drug Discov 2015, 9, 186–194. [CrossRef]
- El-Hawary, S.S.; Taher, M.A.; Amin, E.; AbouZid, S.F.; Mohammed, R. Genus Tabebuia: A Comprehensive Review Journey from Past Achievements to Future Perspectives. Arabian Journal of Chemistry 2021, 14, 103046. [CrossRef]
- Darme, P.; Escotte-Binet, S.; Cordonnier, J.; Remy, S.; Hubert, J.; Sayagh, C.; Borie, N.; Villena, I.; Voutquenne-Nazabadioko, L.; Dauchez, M.; et al. Anti- Toxoplasma Gondii Effect of Lupane-Type Triterpenes from the Bark of Black Alder ( Alnus Glutinosa ) and Identification of a Potential Target by Reverse Docking. Parasite 2022, 29, 7. [CrossRef]
- Pfaff, A.W.; de-la-Torre, A.; Rochet, E.; Brunet, J.; Sabou, M.; Sauer, A.; Bourcier, T.; Gomez-Marin, J.E.; Candolfi, E. New Clinical and Experimental Insights into Old World and Neotropical Ocular Toxoplasmosis. Int J Parasitol 2014, 44, 99–107. [CrossRef]

| Host cell pre-treatment | Tachyzoite pre-treatment | |||||||
| Number of the compound | Group |
CC50 (µM) |
IC50 (µM) | SI | IC50 (µM) | SI | IC50 (µM) | SI |
| 1 | Pyrazoline | 131.9 | 6.7 | 19.6 | 7.5 | 17.5 | 23.4 | 5.6 |
| 2 | 123.3 | 1.6 | 73.3 | 7.5 | 16.4 | 6.6 | 18.6 | |
| 3 | >160 | 18.1 | >8.3 | - | - | - | - | |
| 4 | 154.2 | 3.6 | 42.6 | 5.3 | 28.9 | 31.6 | 4.8 | |
| 5 | Pyrazole | >160 | 14.0 | >11.4 | - | - | - | - |
| 6 | >160 | 21.3 | >7.5 | - | - | - | - | |
| 7 | >160 | 15.9 | >10.0 | - | - | - | - | |
| 8 | >160 | 21.0 | >7.6 | - | - | - | - | |
| 9 | Thiazolidinone | >160 | 6.1 | >25.9 | 10.7 | >14.9 | 0.9 | >164.9 |
| 10 | 61.2 | 5.7 | 10.6 | 15.4 | 3.9 | 4.9 | 12.4 | |
| 11 | 53.2 | 3.6 | 14.5 | 22.5 | 2.3 | 29.5 | 1.8 | |
| 12 | 40.3 | 3.9 | 10.2 | 10.5 | 3.8 | 5.8 | 6.9 | |
| 13 | >160 | 8.8 | >18.1 | 254.6 | >0.6 | 19.8 | >8.0 | |
| 14 | >160 | 141.6 | >1.1 | - | - | - | - | |
| 15 | >160 | 9.5 | >16.8 | 54.4 | >2.9 | 4.0 | >39.7 | |
| 16 | >160 | 16.7 | >9.5 | - | - | - | - | |
| Tr-HCHCl3 | Plant extract | 50.1 | 6.8 | 7.3 | - | - | - | - |
| Tc-HCHCL3 | Plant extract | 24 | 5.6 | 4.4 | ||||
| Pyrimethamine | >200 | 0.3 | >666 | 1.0 | >190 | 6.1 | >32.4 | |
| Ligand | Group | Physical and chemical properties | |||||
|---|---|---|---|---|---|---|---|
|
Molecular weight (g/mol) |
Rotatable bonds Number |
Log P (Octanol/ Water) |
H-bond acceptors Number |
H-bond donors Number |
Lipinski
violations |
||
| 1 | Pyrazoline |
286.30 | 3 | 2.97 | 4 | 2 | 0/5 |
| 2 | 302.76 | 3 | 3.49 | 4 | 2 | 0/5 | |
| 3 | 298.34 | 4 | 2.84 | 5 | 2 | 0/5 | |
| 4 | 312.32 | 3 | 2.56 | 6 | 2 | 0/5 | |
| 5 | Pyrazole |
409.32 | 3 | 6.63 | 4 | 1 | 1/5 |
| 6 | 374.87 | 3 | 5.97 | 4 | 1 | 1/5 | |
| 7 | 358.42 | 3 | 5.46 | 4 | 1 | 1/5 | |
| 8 | 419.32 | 3 | 6.08 | 4 | 1 | 1/5 | |
| 9 | Thiazolidinone | 305.31 | 4 | 1.00 | 7 | 2 | 0/5 |
| 10 | 318.35 | 3 | 2.17 | 7 | 3 | 0/5 | |
| 11 | 327.36 | 3 | 2.34 | 7 | 2 | 0/5 | |
| 12 | 332.38 | 4 | 2.48 | 7 | 2 | 0/5 | |
| 13 | 334.35 | 3 | 1.88 | 8 | 4 | 0/5 | |
| 14 | 336.80 | 3 | 3.12 | 6 | 2 | 0/5 | |
| 15 | 369.40 | 3 | 2.31 | 8 | 3 | 0/5 | |
| 16 | 284.297 | 4 | 0.488 | 8 | 2 | 0/5 | |
| β-amyrin | 396.69 | 0 | 8.66 | 0 | 0 | 1/5 | |
| Absorption | Distribution | Metabolism | Excretion | Toxicity | |||||||||||||||||||
| Ligand | Group | Intestinal absorption (human) |
VDss (human) | BBB permeability | CNS permeability | Substrate | Inhibitor | Total Clearance |
AMES toxicity | ||||||||||||||
| CYP | |||||||||||||||||||||||
| 2D6 | 3A4 | 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | |||||||||||||||||
| Numeric (% Absorbed) |
Numeric (Log L/ kg) |
Numeric (Log BBB) |
Numeric (Log PS) |
Categorical (Yes/No) | Numeric (Log ml/min/ kg) |
Categorical (Yes/ No) | |||||||||||||||||
| 1 | Pyrazoline | 92.349 | 0.390 | 0.265 | -2.131 | No | Yes | Yes | Yes | Yes | No | No | 0.183 | No | |||||||||
| 2 | 91.392 | 0.478 | 0.272 | -1.968 | No | Yes | Yes | Yes | Yes | No | No | 0.133 | Yes | ||||||||||
| 3 | 93.901 | 0.260 | 0.022 | -2.245 | No | Yes | Yes | Yes | No | No | No | 0.32 | Yes | ||||||||||
| 4 | 93.218 | 0.369 | -0.137 | -2.322 | No | Yes | Yes | Yes | No | No | Yes | 0.17 | Yes | ||||||||||
| 5 | Pyrazole | 90.699 | 0.078 | 0.22 | -1.087 | No | Yes | Yes | Yes | Yes | No | Yes | 0.34 | Yes | |||||||||
| 6 | 92.074 | 0.013 | 0.193 | -1.202 | No | Yes | Yes | Yes | Yes | No | Yes | 0.221 | Yes | ||||||||||
| 7 | 93.142 | -0.099 | 0.066 | -1.365 | No | Yes | Yes | Yes | Yes | No | Yes | 0.452 | Yes | ||||||||||
| 8 | 92.007 | 0.03 | 0.191 | -1.18 | No | Yes | Yes | Yes | Yes | No | Yes | 0.199 | Yes | ||||||||||
| 9 | Thiazolidinone | 71.211 | -0.449 | -0.587 | -3.006 | No | No | No | No | No | No | No | 0.134 | No | |||||||||
| 10 | 80.849 | -0.306 | -0.945 | -2.494 | No | No | No | No | No | No | No | -0.052 | No | ||||||||||
| 11 | 81.681 | -0.358 | -0.537 | -2.365 | No | No | No | No | No | No | No | 0.03 | Yes | ||||||||||
| 12 | 82.429 | -0.304 | -0.774 | -2.506 | No | Yes | No | No | No | No | No | 0.097 | No | ||||||||||
| 13 | 70.116 | -0.166 | -1.181 | -3.48 | No | No | No | No | No | No | No | 0.054 | Yes | ||||||||||
| 14 | 90.208 | -0.26 | -0.609 | -2.189 | No | No | Yes | No | No | No | No | -0.067 | No | ||||||||||
| 15 | 78.711 | -0.132 | -1.019 | -3.038 | No | Yes | No | No | No | No | No | -0.152 | No | ||||||||||
| 16 | 70.155 | -0.601 | -1.008 | -3.181 | No | No | No | No | No | No | No | 0.276 | No | ||||||||||
| β Amyrin | 100.000 | - | 0.97 | -1.277 | Yes | Yes | No | No | Yes | Yes | Yes | - | No |
| Condition |
DMEM alone |
DMSO 1% | JAG21 (2µM) |
Tr-H-CHCl3 1 µg/mL |
Tr-H-CHCl3 5 µg/mL |
Tr-H-CHCl3 10 µg/mL |
| Mean number of cysts in 10 fields | 52±5.4 | 38±2.8 | 26.67±5.8 | 21.1±2.2 | 19.3±3.0 | 12.8±3.3 |
| % reduction vs DMEM | - | 27.2 | 48.9 | 59.4 | 62.9 | 75.4 |
| p vs DMEM | - | 0.00065 | 0.000015 | 0.0000066 | 0.0000017 | 0.0000003 |
| % reduction vs DMSO | - | - | 29.8 | 44.3 | 49.1 | 66.2 |
| p vs DMSO | - | - | 0.01 | 0.00039 | 0.00013677 | 1.2005E-05 |
| % reduction vs JAG21 | - | - | - | 20.6 | 34.6 | 71.6 |
| p vs JAG21 | - | - | - | 0.073 | 0.028 | 0.001 |
| Ligand | Group | Affinity TgCDPK1 (kcal/mol) | Affinity TgROP18 (kcal/mol) |
| 9 | Thiazolidinone | -6.6 | -6.5 |
| ATP | Control | -7.3 | -7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





