1. Introduction
The progressive aging of the global population has established age-related pathologies as a paramount medical challenge. Osteoarthritis (OA) is a prevalent and debilitating condition among the elderly, and its etiology extends beyond simple mechanical “wear and tear.” It is now understood as a “whole-joint disease” affecting the entire articular structure, with a complex pathological process involving progressive cartilage degradation, subchondral bone remodeling, synovial inflammation, and osteophyte formation. The central pathology is the degradation of articular cartilage, a highly specialized tissue responsible for frictionless joint movement. As an avascular and aneural tissue, its capacity for self-repair is exceedingly limited following injury. This irreversible damage, characterized by the loss of proteoglycans and the disruption of the collagen network, ultimately leads to direct bone-on-bone friction, causing severe pain, joint stiffness, and a significant decline in patient quality of life. Current non-surgical treatments primarily offer symptomatic relief through analgesics or viscosupplementation, whereas the definitive surgical solution, total knee arthroplasty, while effective, does not represent an ideal long-term solution for younger, more active patients due to its finite lifespan and the potential need for revision surgery. Consequently, the development of therapies capable of regenerating durable, biological hyaline cartilage constitutes a significant and urgent unmet clinical need [
1,
2,
3].
To address this challenge, surgical techniques have continuously evolved. Among them, microfracture, a first-line therapy, aims to initiate repair by creating perforations in the subchondral bone to induce bone marrow-derived stem cells to form a fibrin clot. However, this technique is beset by a fundamental limitation: the resultant repair tissue is predominantly fibrocartilage, which possesses inferior biomechanical properties—such as lower compressive stiffness and poor durability—compared to native hyaline cartilage. This fibrocartilaginous tissue often demonstrates clinical deterioration within several years post-surgery and may cause permanent alterations to the subchondral bone, thereby complicating subsequent advanced treatments[
4].
To regenerate higher-quality cartilage, cell-based therapies have emerged, represented by Autologous Chondrocyte Implantation (ACI) and its third-generation variant, matrix-induced ACI (MACI). These techniques involve the in vitro expansion of a patient’s own chondrocytes, which are subsequently transplanted into the defect site. MACI further simplifies the surgical procedure and enhances cell retention by pre-seeding the cells onto a biological scaffold, such as porcine collagen, and has received FDA approval. Despite these significant advancements, ACI and MACI are commonly confronted with the “scaffold dilemma.” While an exogenous scaffold provides a three-dimensional environment for cell growth, it may also elicit adverse inflammatory responses, act as a barrier to nutrient diffusion, and physically impede the seamless biochemical and mechanical integration between the graft and the surrounding native tissue[
5,
6,
7,
8].
To completely bypass the reliance on exogenous scaffolds, a revolutionary scaffold-free approach, Cell Sheet Engineering (CSE), was developed. This technology, pioneered by Professor Teruo Okano at Tokyo Women’s Medical University, utilizes specialized culture dishes grafted with the thermoresponsive polymer pNIPAAm. Cells adhere and proliferate normally at 37 °C[
9,
10]. When the temperature is lowered below 32 °C, the polymer surface undergoes a hydrophilic-hydrophobic transition, allowing an entire confluent cell layer to detach spontaneously and intact. This gentle harvesting method completely obviates the need for conventional enzymatic digestion, thus preserving crucial biological structures essential for tissue function. It maintains cell-cell junctions (e.g., gap junctions and desmosomes), which are vital for intercellular communication and coordinated tissue function. More critically, it retains the basal extracellular matrix (ECM) secreted by the cells during cultivation. This endogenous ECM, rich in adhesive proteins like fibronectin and laminin, functions as a biological adhesive, facilitating the rapid and secure attachment of the cell sheet to the defect surface without the need for sutures or fibrin glue. This exceptional adhesiveness ensures high rates of cell engraftment and survival, fundamentally overcoming the substantial cell loss associated with conventional cell suspension injections[
11,
12].
The efficacy of CSE is well-substantiated by extensive evidence. In various preclinical animal models (including rats, rabbits, and minipigs), studies have consistently demonstrated that transplanted cell sheets can induce the regeneration of high-quality tissue that closely resembles native hyaline cartilage in structure and biochemical composition. Histological analysis reveals that the neo-tissue is rich in proteoglycans (Safranin-O positive) and type II collagen, while being deficient in type I collagen, a marker for fibrocartilage[
13]. Furthermore, results from human clinical trials in Japan and Taiwan are encouraging [
14,
15,
16]. Patients receiving autologous or allogeneic chondrocyte sheet transplants have shown statistically significant and lasting improvements in knee function scores (KOOS and LKS). Post-operative arthroscopy and MRI have also confirmed that the defects were well-covered with smooth neo-tissue, with biopsies further verifying the formation of high-quality hyaline-like cartilage.
Future advancements in CSE are contingent upon the transition from complex and costly “autologous” therapies to standardized, readily available “allogeneic” off-the-shelf products. A highly promising source for allogeneic cells is juvenile chondrocytes (JCCs) isolated from surgically discarded polydactyly tissues. These cells not only possess a much higher proliferative capacity than adult chondrocytes but also exhibit low immunogenicity due to the immune-privileged nature of cartilage, minimizing the risk of rejection. The establishment of a master cell bank of JCCs would enable the production of standardized, quality-controlled cell sheet products, simplifying the treatment to a single surgical procedure and significantly reducing costs, thereby benefiting a broader patient population[
17].
In summary, CSE represents a tissue reconstruction strategy that more closely aligns with principles of developmental biology. It successfully circumvents the “scaffold dilemma” of traditional tissue engineering, providing a reliable pathway for regenerating high-quality hyaline cartilage. Globally, Japan and Taiwan are at the forefront of the clinical application of this technology, facilitated by their more flexible regulatory frameworks (e.g., Japan’s conditional approval system and Taiwan’s “Regulations for Special Medical Techniques”). In contrast, the US FDA and European EMA have adopted more stringent approval standards, often classifying such products as advanced therapy medicinal products (ATMPs), which require more extensive clinical data. Despite these challenges, as allogeneic cell sheet technology matures, it holds the potential to become a paradigm-shifting therapy for OA[
18,
19,
20].
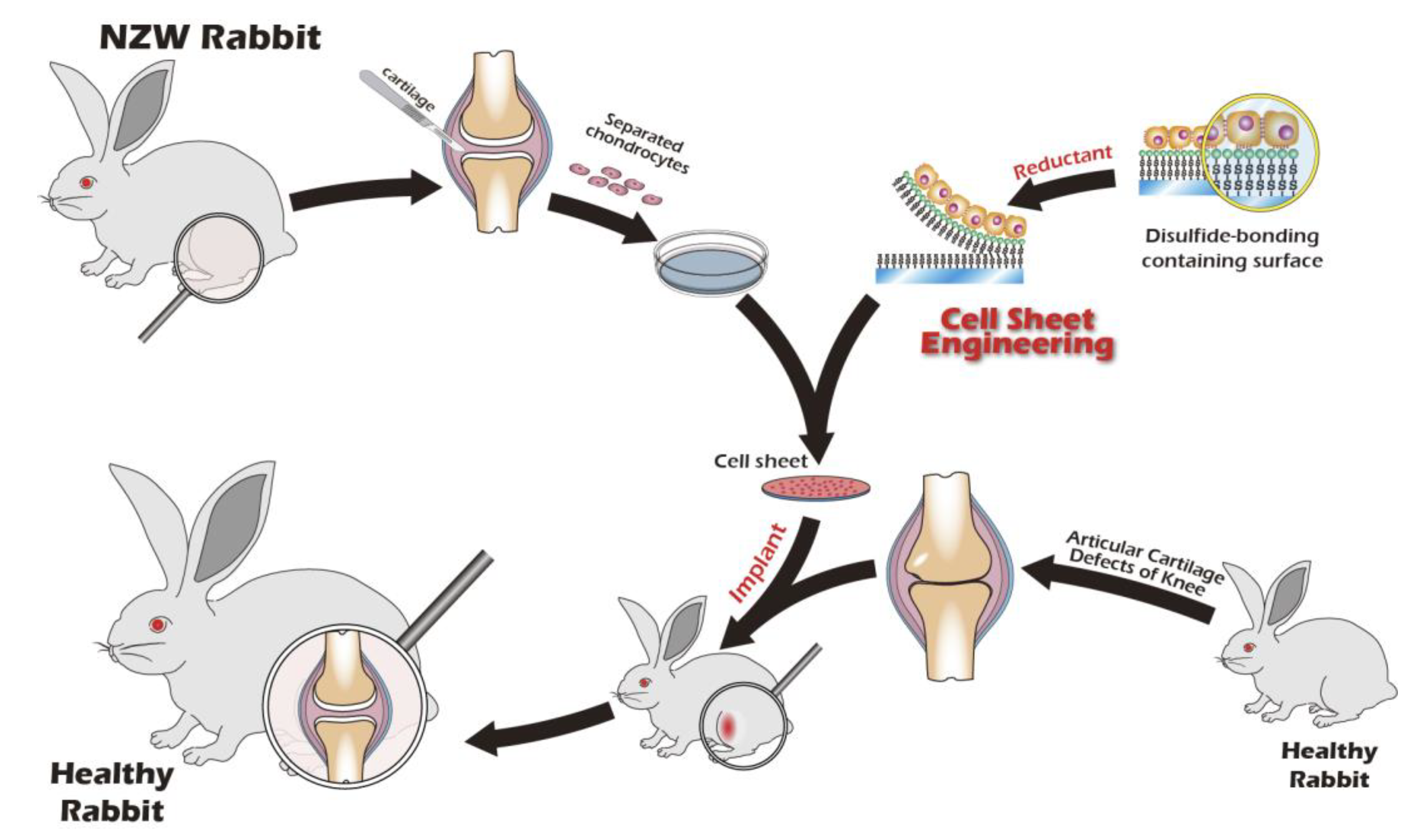
Recognizing the remarkable efficacy of Cell Sheet Engineering (CSE), our research group has dedicated years to developing an innovative cell sheet fabrication system that does not require poly(N-isopropylacrylamide) (pNIPAAm). This system involves a specialized surface treatment of culture substrates, allowing a confluent cell sheet, complete with its ECM, to be harvested intact through a gentle treatment with a reducing amino acid. Given the urgent clinical need for articular cartilage reconstruction, the present study was designed to apply this non-pNIPAAm-based CSE technology to the field of articular cartilage regeneration. We have designed specialized chondrocyte culture inserts and established a New Zealand white rabbit knee defect model to validate the preliminary therapeutic efficacy of these cell sheets. The ultimate objective of this research is to provide an effective regenerative strategy for patients with knee osteoarthritis and to lay a solid foundation for future human clinical trials. The objective we seek to accomplish is depicted in
Figure 1.
2. Materials and Methods
2.1. Preparation of Cell Culture Inserts
As described in a previous publication by our group [
21,
22], a surface chemical modification of porous polyethylene terephthalate (PET) membranes was performed. Initially, 0.25 M gamma-polyglutamic acid (γ-PGA) (Vedan, Taiwan) was dissolved in a 0.25 M boric acid buffer (pH 6.0) and mixed overnight. Subsequently, the solution was combined with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and
N-hydroxysuccinimide (NHS) powder (ACROS Organics, Geel, Belgium) to create solution A, thereby activating the carboxyl groups of γ-PGA. Concurrently, cystamine was dissolved in a 0.25 M boric acid buffer (pH 6.0) to form solution B, which was preactivated for 20 minutes. Solution A was then added to solution B, and the mixture was allowed to react for 2.5 hours at pH 6.0, resulting in solution C.
In the second stage, a 6-well-sized porous PET membrane was pre-activated using a low-pressure plasma apparatus (PDC-002-HP, Harrick Plasma, New York, NY, USA) to introduce reactive functional groups on its inert surface. The pressure was maintained at 400 mTorr for 20 minutes in an oxygen atmosphere and at 500 mTorr for 45 minutes in a carbon dioxide atmosphere, with the plasma power set to 45 W. The activated PET membrane was then immersed in a 0.25 M EDC/NHS solution in 0.25 M boric acid buffer at 4 °C for 20 minutes, followed by the addition of an equal volume of solution C. The reaction was maintained under continuous agitation for 4 hours to facilitate covalent bond formation.
Finally, following the surface chemical modification, the disulfide bond-containing γ-PGA-modified porous PET membrane was rinsed gently with deionized water to remove unreacted residues and was subsequently air-dried overnight. The membrane was then affixed to the insert via ultrasonic welding. The assembled culture inserts were sterilized with ethylene oxide gas for subsequent use in cell culture.
2.2. Characterization of Surface-Modified PET Membrane
Water contact angle measurements were performed to assess surface hydrophilicity. Prepared films were cut into 20 mm diameter discs and positioned on the instrument stage. An aliquot of 0.2 ml of distilled water was dispensed onto the specimen, and the contact angle was measured using a surface tension meter equipped with CCD dynamic image capture analysis (SEO Phoenix MT, Gyeonggi-do, Korea).
The atomic composition and chemical states of the functionalized membrane surface were quantified using Electron Spectroscopy for Chemical Analysis (ESCA/XPS, ULVAC-PHI QuantERA II, Chanhassen, MN, USA) with a 200 µm spot size. Grafted film samples (5 x 5 mm) were positioned on a carrier, and high-resolution spectra of C1s, O1s, N1s, and S2p were acquired to confirm the presence of the grafted molecules and the disulfide linker.
The nanomechanical properties, specifically the elastic modulus (stiffness), of the surface-modified membranes were measured using Atomic Force Microscopy (AFM, Bruker Dimension Icon, Billerica, MA, USA) in PeakForce QNM mode. Measurements were conducted on both dry and fully hydrated samples to assess the effect of hydration on surface stiffness.
2.3. Juvenile Rabbit Articular Chondrocyte Isolation and Cultivation
All procedures were executed in accordance with institutional guidelines and received approval from the Institutional Animal Care and Use Committee at Taipei Medical University (No. LAC2023-0039). Knee joints were excised from juvenile rabbits, sectioned into small fragments, and homogenized. The particles were treated with Pronase (30 minutes, 37 °C) and subsequently with Collagenase type II (16 hours, 37 °C). After enzymatic digestion, the isolated cells were resuspended in growth medium, filtered through a 70 µm strainer, and cultured on type II collagen-coated dishes at 37 °C in 5% CO
2. Upon reaching 90% confluency, chondrocytes were passaged, with the medium being replaced every two days. All procedures were recorded as
Figure 2.
2.4. Rabbit Articular Chondrocyte Isolation and Cultivation
Following surgical exposure of adult rabbit knee joints, cartilage was meticulously scraped using a scalpel to obtain homogenized fragments. A full-thickness cartilage defect measuring 1.5 cm in diameter, a critically-sized defect for this model, was then created using a dental drill. The harvested cartilage fragments were treated with pronase for 30 minutes at 37 °C. After removal of pronase by centrifugation (800 rpm, 5 minutes), the tissue was digested with collagenase type II at 37 °C for 16 hours. The collagenase was subsequently removed by centrifugation (800 rpm, 5 minutes). The isolated cells were resuspended in growth medium (DMEM/F12 with 5% FBS, 30 µg/mL vitamin C, 30 µg/mL insulin, and 1% antibiotic-antimycotic), seeded onto type II collagen-coated dishes, and cultured at 37 °C in a 5% CO
2 atmosphere. Upon reaching 90% confluency, the primary (P0) chondrocytes were passaged to generate P1 chondrocytes, with medium changes performed every two days. All procedures were recorded as
Figure 3.
2.5. Preparation and Transplantation of Chondrocyte Sheets
P1 chondrocytes were used for the fabrication of cell sheets. The inserts were coated with type II collagen, and 1 x 10^6 cells were seeded into each insert and cultured for 14 days at 37 °C in a 5% CO
2 atmosphere. The cell sheets were then detached by the addition of a cystamine solution. The first harvested cell sheet was transferred onto a type II collagen-coated tissue culture dish and incubated at 37 °C to allow for attachment. This procedure was repeated twice more to generate triple-layered chondrocyte sheets. A second surgical procedure was performed to expose the previously created defect. The triple-layered cell sheet was trimmed to an appropriate size and transplanted into the target wound. All procedures for preparing the chondrocyte sheet are documented in
Figure 4, and the comprehensive diagram outlining the transplantation concept is presented in
Figure 5.
2.6. Histological and Immunocytochemistry (ICC) Staining
Chondrocytes were fixed with a 4% formaldehyde solution. Standard hematoxylin and eosin (H&E) staining was performed. For ICC, fixed cells on slides were permeabilized with Triton X-100 for 20 minutes and blocked with 1% bovine serum albumin (BSA) for two hours. Specimens were incubated overnight with primary antibodies against collagen I (1:100, Arigo, Hsinchu, Taiwan), collagen II (1:100, Arigo), and aggrecan (1:100, Thermo Fisher, Waltham, MA, USA). An HRP-conjugated goat anti-mouse IgG (1:100, Arigo) was used as the secondary antibody, followed by hematoxylin counterstaining. For immunofluorescent staining, a FITC-conjugated goat anti-mouse IgG (H+L) (1:100, Arigo) was used as the secondary antibody, with DAPI for nuclear counterstaining.
2.7. Chondrocyte Sheet Histological and Immunohistochemical Staining
Chondrocyte sheets were fixed with a 4% formaldehyde solution. For cross-sectional analysis, fixed sheets were processed into paraffin-embedded sections. H&E staining was performed according to conventional methodologies. The same protocols and antibodies described for ICC were used for IHC and immunofluorescence staining of the sheets. For ECM analysis, sheets were fixed in 4% paraformaldehyde. Staining was performed with 1% alcian blue for 30 minutes, 0.1% safranin O for 5 minutes (following a 10-minute hematoxylin incubation), or 0.04% toluidine blue for 10 minutes to visualize GAG distribution. Following staining, sections were dehydrated through an ethanol series and cleared with xylene. Staining results were examined using a bright-field microscope.
2.8. Magnetic Resonance Imaging (MRI) and Micro-CT Protocol for Ex vivo Rabbit Knee Specimens
2.8.1. Ethical Approval and Specimen Preparation
All animal procedures were conducted in accordance with institutional guidelines and regulations for the care and use of laboratory animals. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University (Approval No. TMU LAC2023-0039). Following humane euthanasia of New Zealand White rabbits, the hind limbs containing the operated knee joints were immediately disarticulated. To prevent tissue dehydration, each specimen was wrapped in saline-soaked gauze, sealed in a container, and stored at 4 °C. All imaging was completed within 24 hours of harvest.
2.8.2. Micro-Computed Tomography (Micro-CT) Imaging
Ex vivo high-resolution imaging was performed using a Skyscan 1176 in vivo micro-CT scanner (Bruker, Kontich, Belgium). Each specimen was immobilized in a non-metallic holder. Scans were acquired with the following parameters: Source Voltage: 80 kVp; Source Current: 280 µA; Resolution: 12.2 µm; Rotation Step: 0.3 degrees; Frame Averaging: 2; Filter: 1-mm aluminum. Raw projection images were reconstructed into 3D datasets using NRecon software (Bruker) for analysis of subchondral bone morphology, osteophyte formation, and bone microarchitecture.
2.8.3. Magnetic Resonance Imaging (MRI)
Following micro-CT, specimens were imaged using a 7T PharmaScan 70/16 USR MRI system (Bruker, Ettlingen, Germany) with a dedicated rodent knee coil. The multi-sequence protocol included: Sagittal T2-Weighted Fast Spin-Echo (FSE) for evaluating cartilage defects, meniscal integrity, and joint effusion (TR/TE: 2500/33 ms; FOV: 40×40×20 mm; Matrix: 256×256×128; Slice Thickness: 0.16 mm; NEX: 4) and Coronal Proton Density-Weighted Fat-Suppressed (PD-FS) FSE for detailed assessment of cartilage morphology (TR/TE: 2500/33 ms). This protocol was selected as T2-weighted imaging is particularly sensitive to changes in tissue water content and the integrity of the collagen fibril network, which are key indicators of cartilage health. DICOM images were analyzed for qualitative and semi-quantitative assessment of cartilage damage using scoring systems such as the International Cartilage Repair Society (ICRS) scale.
3. Results
3.1. ESCA (XPS) Analysis Confirms Successful Surface Modification and Reversible Detachment Chemistry
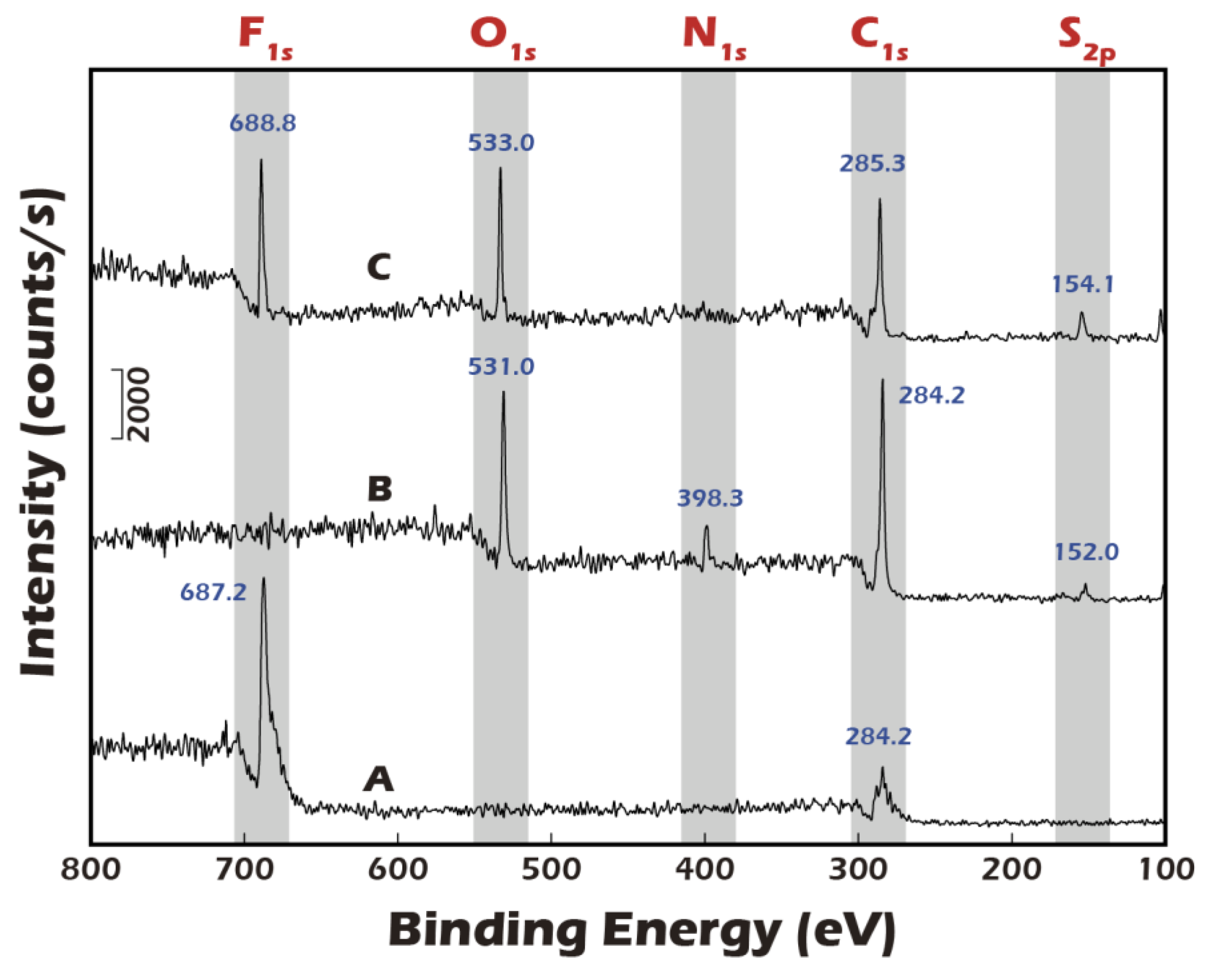
A foundational requirement of this technology is the precise, multi-step chemical modification of the substrate surface. To validate this process, Electron Spectroscopy for Chemical Analysis (ESCA), a highly surface-sensitive technique, was employed.
Figure 6 presents the high-resolution XPS spectra for a polyvinylidene fluoride (PVDF) substrate at the key stages of modification. The pristine PVDF, a fluoropolymer, exhibited a characteristic elemental signature dominated by strong chemical shifts at 687.2 eV, corresponding to the F1s orbital, and at 284.2 eV, corresponding to the C1s orbital of the polymer backbone. Following the plasma treatment and subsequent covalent grafting of the disulfide linker and γ-PGA biopolymer, a dramatic change in the surface chemistry was observed. The prominent F1s peak was almost completely attenuated, indicating that the underlying PVDF substrate was thoroughly covered by a uniform layer of the grafted material. Concurrently, new peaks emerged corresponding to the elemental composition of the grafted layer: a significant O1s peak from the carboxyl and amide groups of γ-PGA, a distinct N1s peak from the amide bonds and amine groups, and a small but crucial S2p peak, which is the definitive signature of the sulfur atoms within the disulfide bond of the linker. This new elemental profile unequivocally confirms the successful grafting of the complete molecular construct. The final step was to validate the detachment mechanism. Upon treatment with a Cysteine solution, which specifically reduces and cleaves disulfide bonds, the surface chemistry was reversed. The XPS analysis showed a strong resurgence of the F1s peak, indicating the re-exposure of the underlying PVDF substrate. Simultaneously, the N1s peak, representative of the γ-PGA layer, disappeared. This dynamic change provides direct and compelling evidence that the disulfide linker was successfully cleaved and the entire grafted polymer-cell complex was lifted off, validating the viability of our chemically-induced detachment strategy.
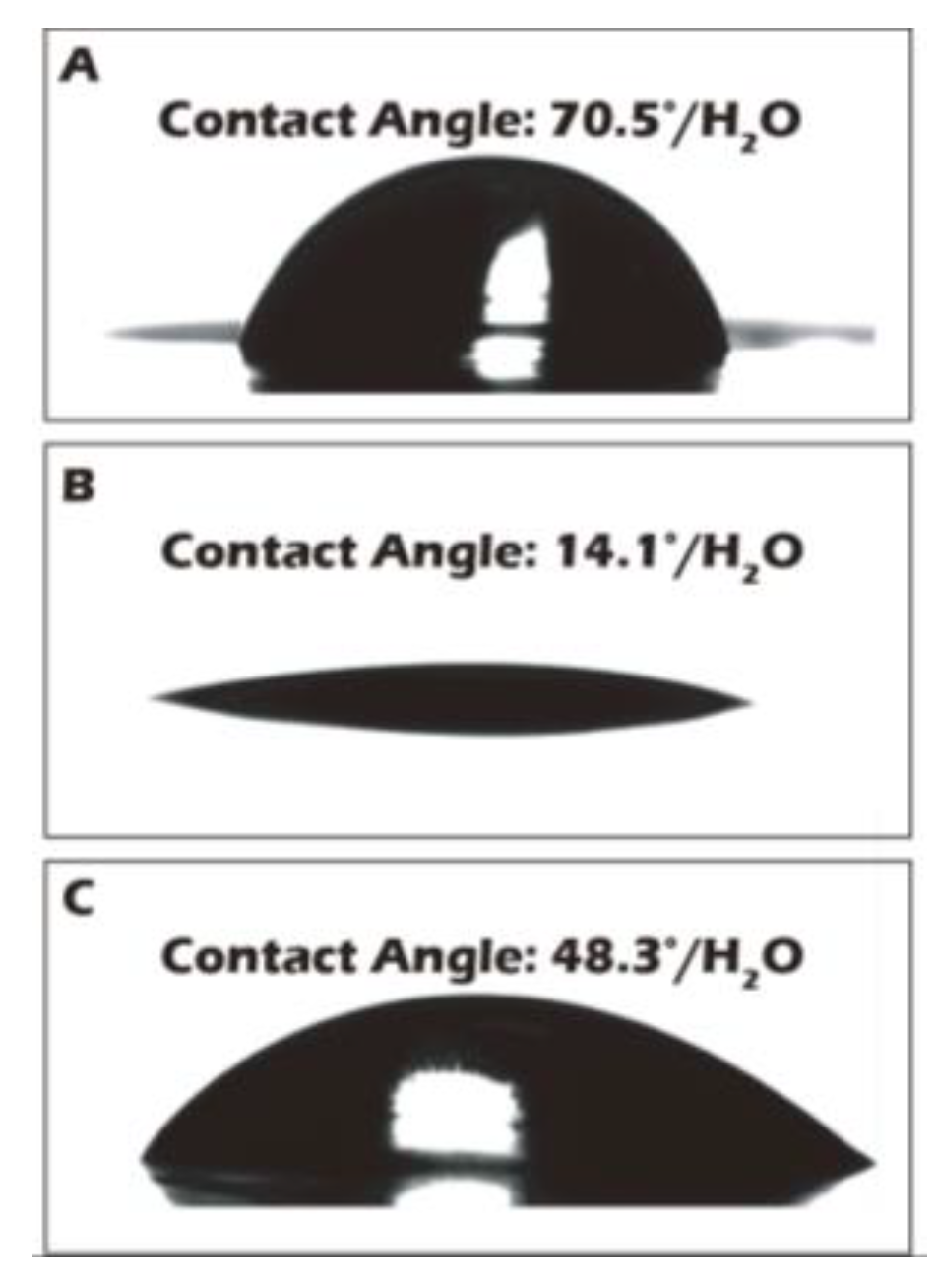
3.2. Water Contact Angle Measurements Corroborate Macroscopic Surface Property Changes
To complement the molecular-level validation from XPS, macroscopic changes in surface properties were assessed using water contact angle measurements, which directly reflect surface wettability and energy. As illustrated in
Figure 7, the native polyethylene terephthalate (PET) substrate exhibited a water contact angle of approximately 70.5°, indicating a moderately hydrophobic surface that is generally suboptimal for promoting uniform cell adhesion and spreading. After the chemical grafting of the highly hydrophilic biopolymer γ-PGA, rich in polar carboxyl and amide functional groups, the surface properties were radically altered. The contact angle decreased dramatically to an average of 14.1°, signifying a transition to a highly hydrophilic, high-energy surface. This state is known to be highly conducive to the adsorption of vitronectin and fibronectin from the culture medium, thereby facilitating robust cell attachment and proliferation. Subsequently, to trigger cell sheet detachment, the surface was treated with Cysteine. This cleavage of the disulfide bond and release of the γ-PGA layer resulted in the contact angle increasing to 48.3°. This intermediate value suggests the exposure of a surface less hydrophilic than the γ-PGA layer but more hydrophilic than the original PET, a condition that reduces cell-substrate affinity and aids in the gentle, spontaneous lifting of the intact cell sheet. To ensure the potential for clinical-scale production, where reproducibility is paramount, a multi-point water contact angle analysis was performed on a large-area reacted membrane (
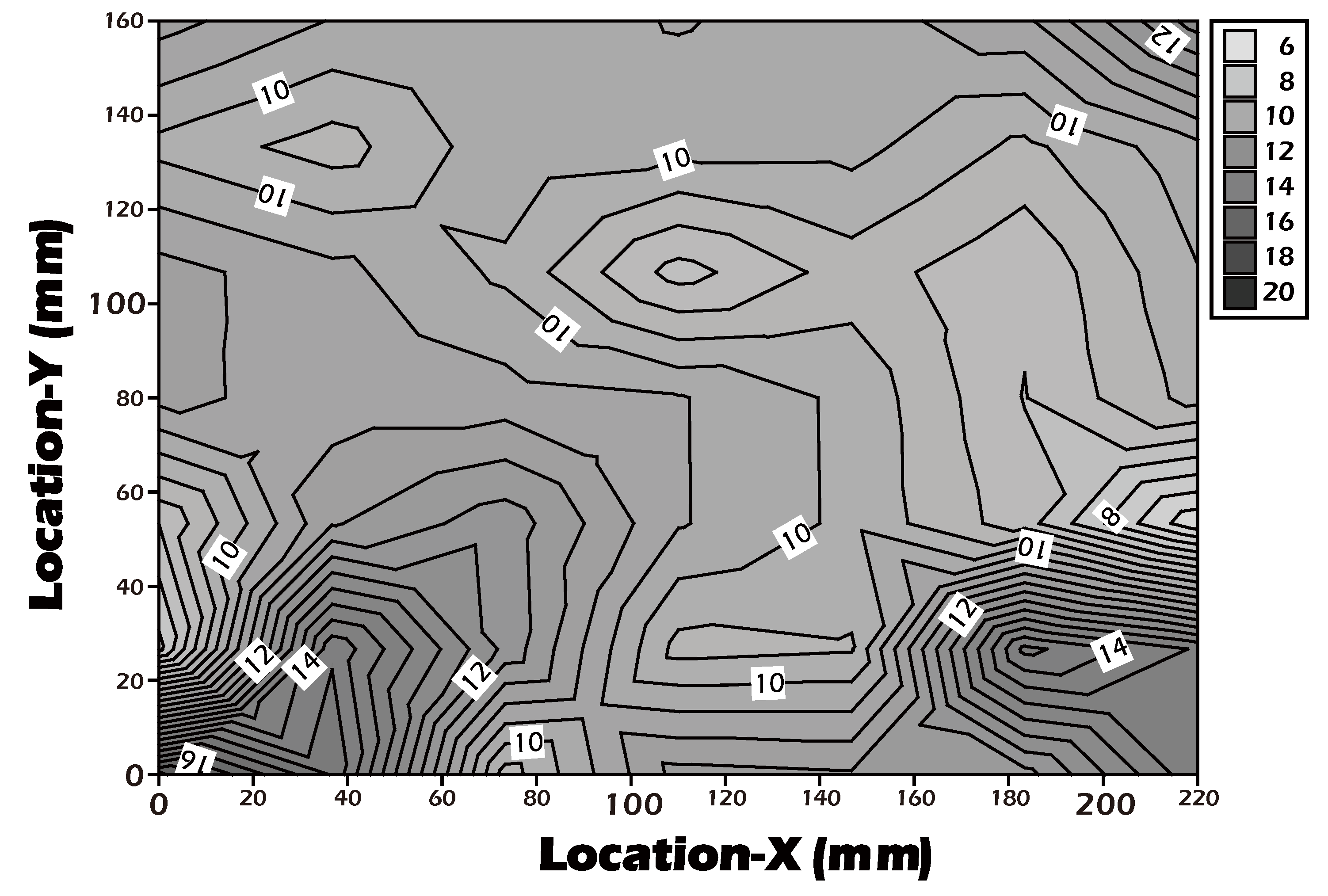
Figure 8). The contour plot analysis revealed that the contact angle was uniformly controlled below the target threshold of 15° across the vast majority of the surface area, confirming the high consistency and reliability of our grafting process. This result is a critical quality control metric, indicating that the fabricated culture inserts possess a consistently uniform surface for predictable cell growth.
3.3. AFM Reveals Biomimetic Stiffness of the Hydrated Culture Substrate
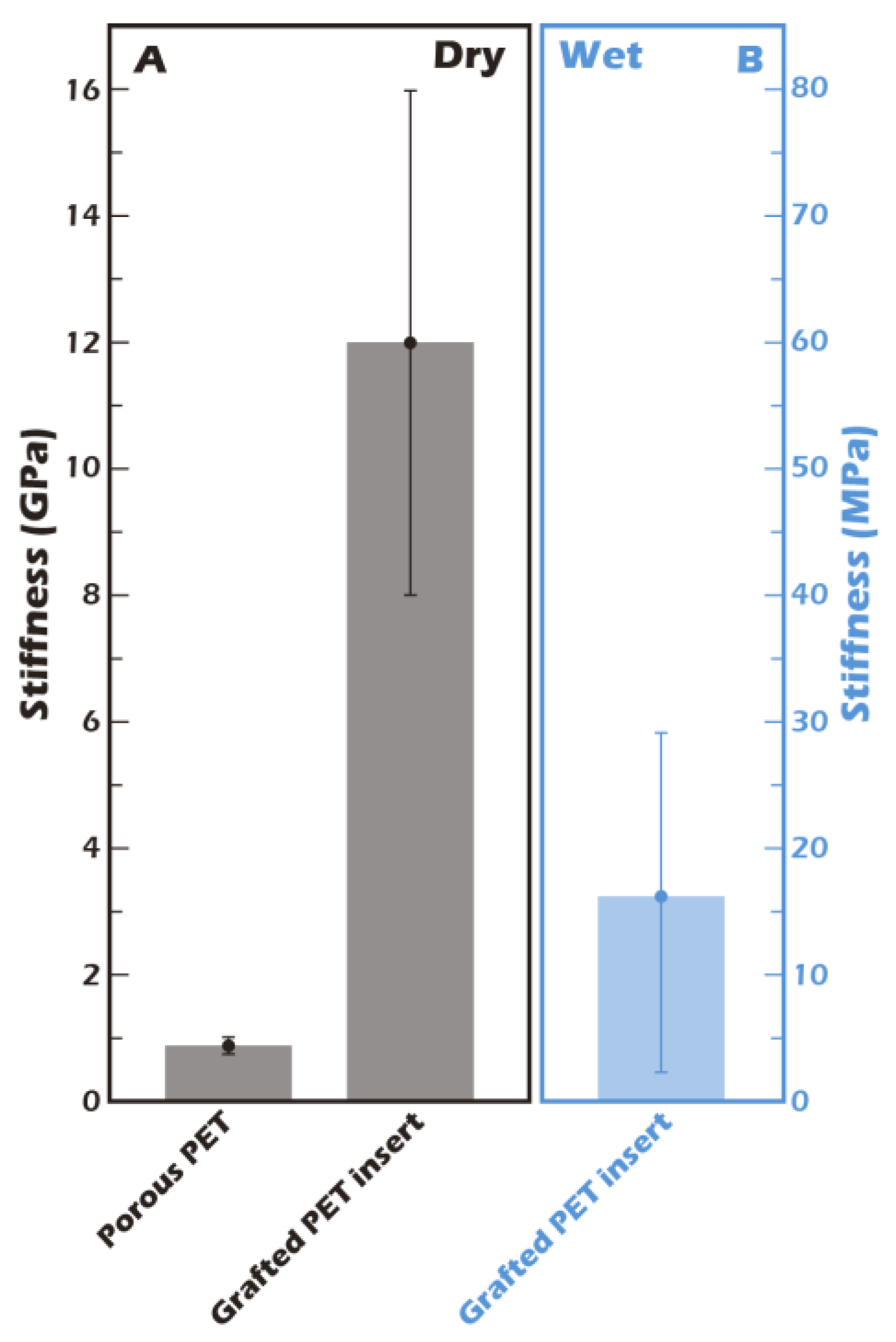
The mechanical properties of the cellular microenvironment are increasingly recognized as critical regulators of cell behavior, a concept known as mechanotransduction. For chondrocytes, substrate stiffness is a key determinant of phenotype maintenance. The stiffness of our culture substrate was therefore quantified by AFM. As shown in
Figure 9, the stiffness of the ungrafted porous PET membrane in its dry state was approximately 0.8 GPa. This value increased substantially to approximately 12 GPa after the grafting of the γ-PGA layer, reflecting the formation of a dense, rigid polymer coating. However, the physiologically relevant value is that of the hydrated state. Crucially, when the grafted membrane was fully hydrated, its stiffness decreased precipitously by three orders of magnitude to approximately 16.2 MPa. This dramatic softening is attributed to the plasticizing effect of water on the hydrophilic γ-PGA polymer network. This value of 16.2 MPa closely approximates the compressive modulus of normal human knee cartilage (typically reported in the range of 15-25 MPa) [
23]. This finding is of profound biological significance, as it suggests that our hydrated culture surface provides a physiologically relevant mechanical microenvironment for chondrocytes, unlike excessively rigid glass or polystyrene culture dishes. It is hypothesized that this biomimetic stiffness is a key factor in preventing the chondrocyte dedifferentiation into fibroblast-like cells that is often observed on conventional culture substrates.
3.4. Grafted Membrane Exhibits Excellent Biocompatibility and Promotes Cell Proliferation
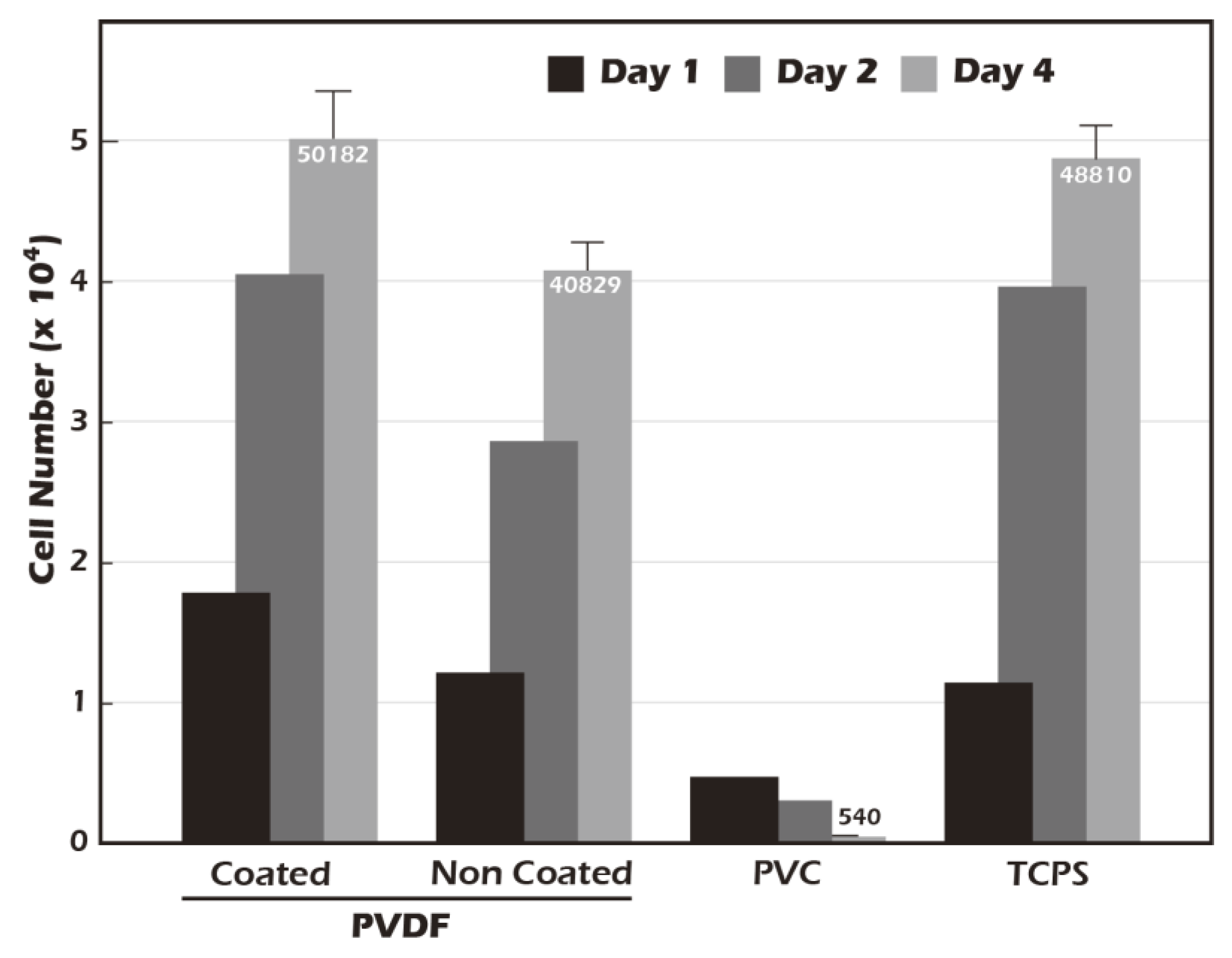
A fundamental prerequisite for any material intended for tissue engineering applications is biocompatibility. In accordance with the ISO 10993-5 standard [
24], a cytotoxicity test was conducted using NIH3T3 fibroblasts (
Figure 10) to assess the biological safety of our modified surface. The cell count for the negative control, polyvinyl chloride (PVC), decreased significantly over the four-day period, confirming its cytotoxic nature. In contrast, both the positive control, standard tissue culture polystyrene (TCPS), and the ungrafted PET membrane supported robust cell proliferation, indicating they are non-toxic. Notably, the surface-grafted PET membrane demonstrated a capacity for cell proliferation that surpassed that of the TCPS control. Cell numbers on our modified surface increased from an initial seeding of 17,911 on day 1 to a final count of 50,182 on day 4, representing a 2.8-fold increase. This was superior to the proliferation observed on TCPS. These results not only confirm that our surface modification process introduces no cytotoxic elements but also suggest that the resulting surface, characterized by its hydrophilicity, biomimetic stiffness, and the presence of γ-PGA (which may act as a nutrient source), actively creates a highly favorable and pro-proliferative environment for cell growth. This enhanced biocompatibility is a significant advantage for producing high-density cell sheets for therapeutic applications.
3.5. Cultured Rabbit Chondrocytes Maintain a Stable, Healthy Phenotype
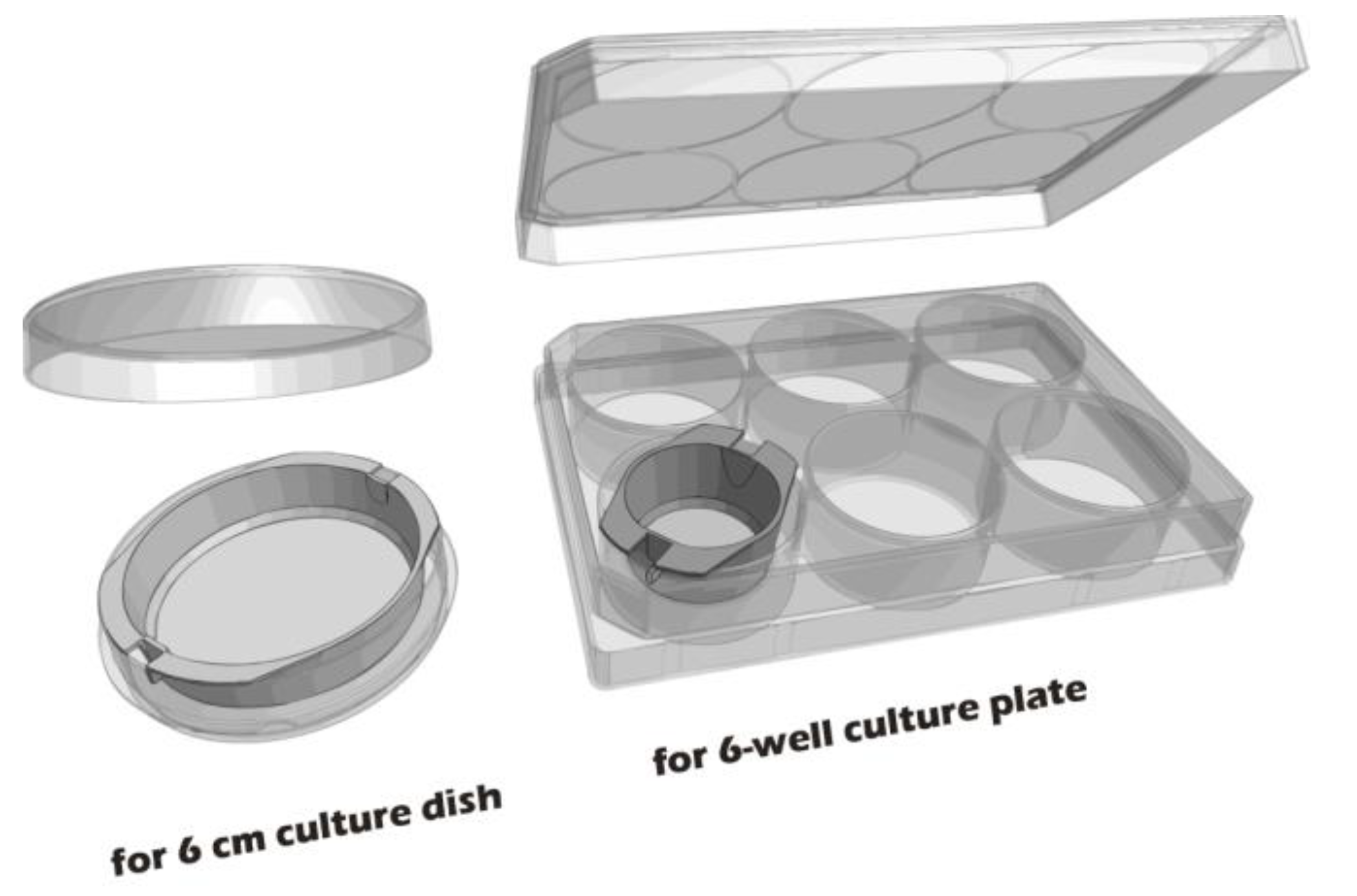
To develop culture inserts for future clinical applications, in addition to the 6-well culture plate, a 6-cm culture dish culture insert capable of cultivating large chondrocyte sheets has also been developed. The appearance of these two culture inserts is depicted in
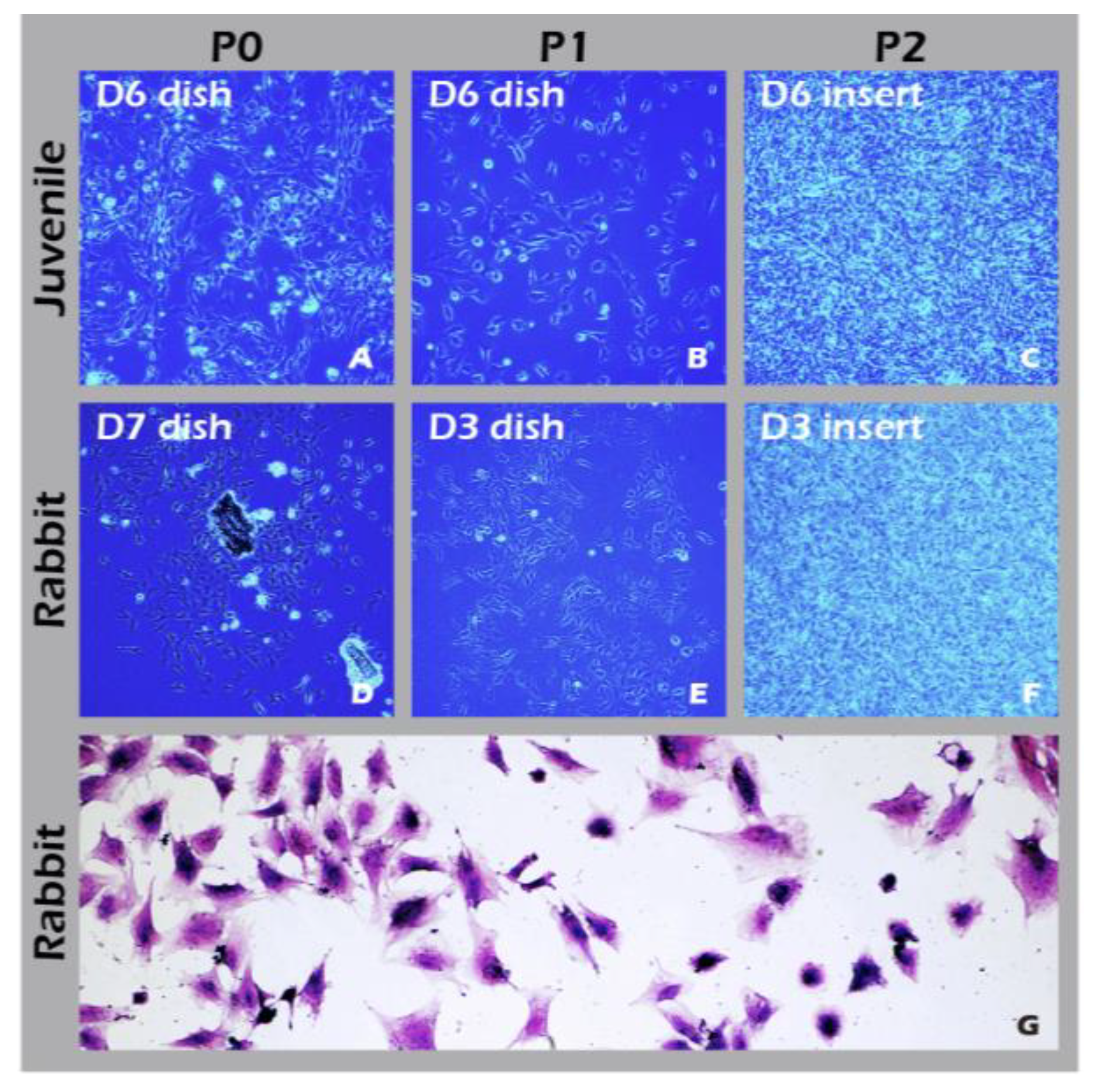
Figure 11. To establish a clinically relevant protocol, cartilage was first harvested from juvenile rabbits (1-week-old) as a robust, highly proliferative cell source to validate the system. Subsequently, to better model the clinical scenario of treating age-related or traumatic cartilage damage, cells were also sourced from adult rabbits (>3.5 kg), which typically exhibit a lower proliferative capacity. Chondrocytes from both sources were successfully isolated and expanded on our system. Microscopic evaluation confirmed that cells from both juvenile (
Figure 12A-C) and adult (
Figure 12D-F) rabbits could adhere and proliferate normally on the grafted PET membrane, displaying the characteristic polygonal, cobblestone-like morphology of healthy, differentiated chondrocytes. H&E staining further confirmed this typical morphology and the formation of a confluent monolayer (
Figure 12G). To confirm their phenotype at a molecular level, Immunocytochemistry (ICC) analysis was performed (
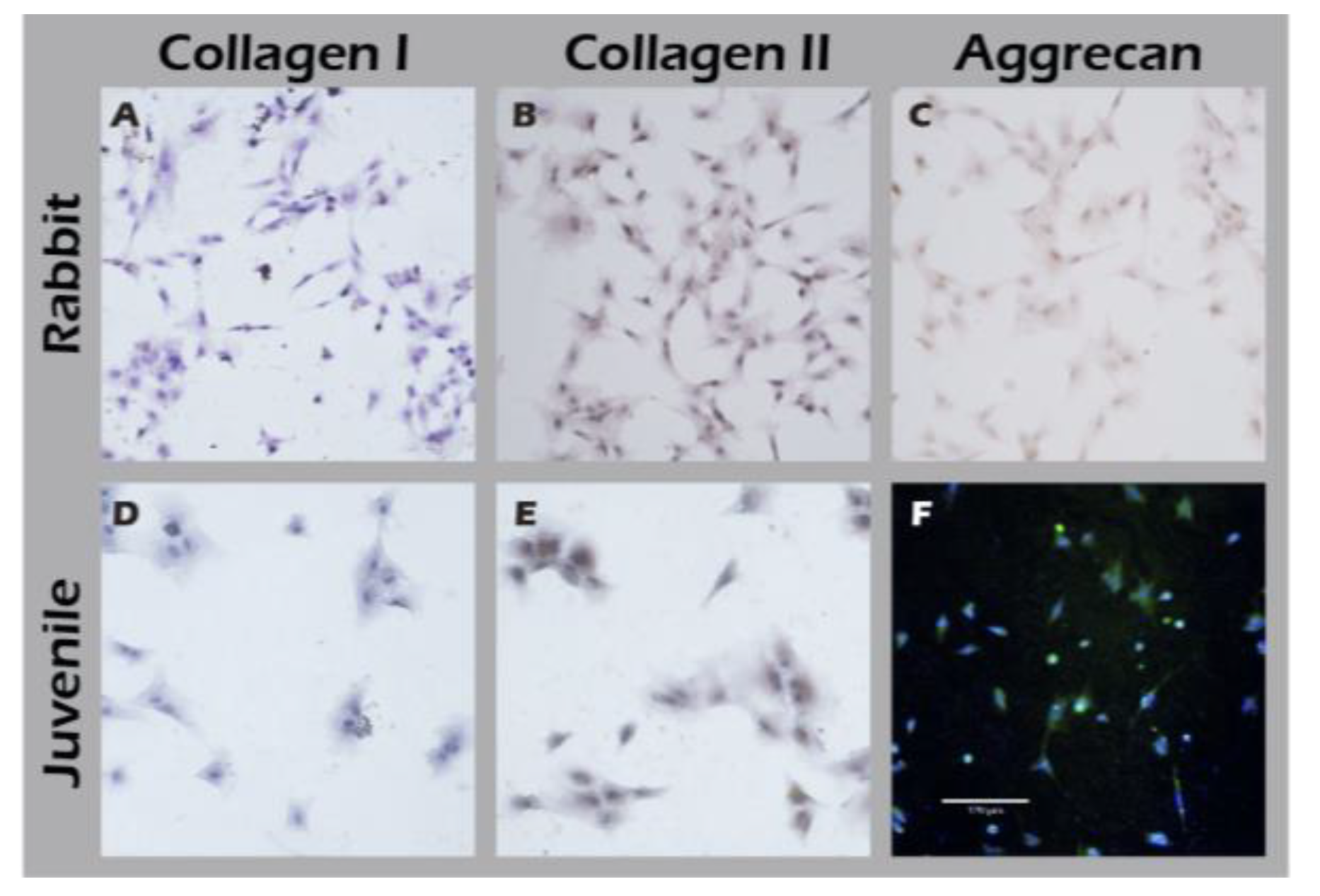
Figure 13). This analysis demonstrated that chondrocytes from both age groups maintained the correct and desired phenotype: there was strong, uniform expression of Collagen II, the primary structural protein of hyaline cartilage, and Aggrecan, the major proteoglycan responsible for its compressive resistance. Critically, there was minimal to no expression of Collagen I, which is a marker for fibrocartilage and cellular dedifferentiation. These findings are consistent with the definitive molecular signature of healthy, functional articular chondrocytes and confirm that our culture system successfully supports the maintenance of the desired cell type.
3.6. Robust Fabrication and Characterization of Multilayered Chondrocyte Sheets with Developed ECM
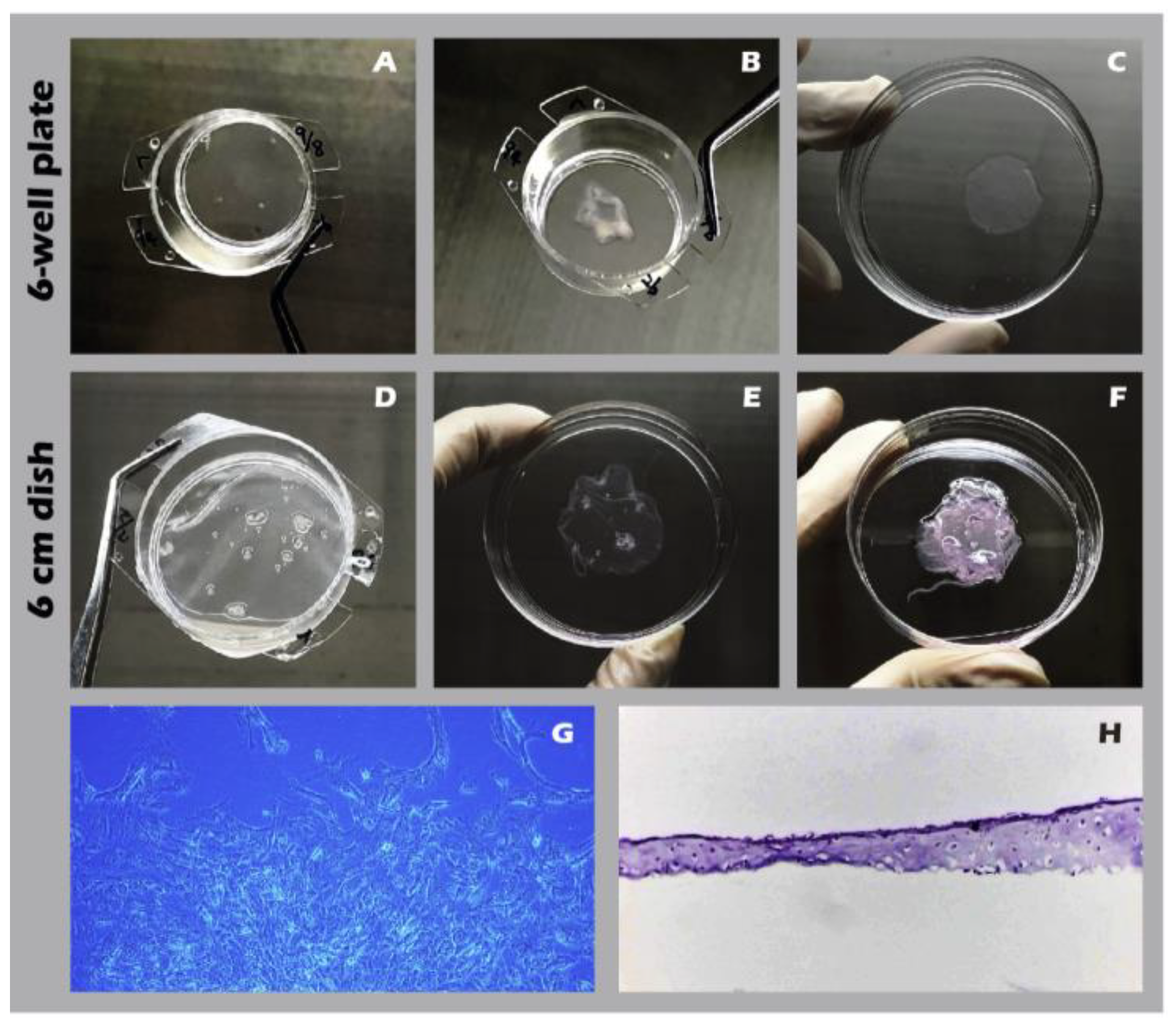
Using the developed inserts, viable, cohesive cell sheets were successfully fabricated from adult rabbit chondrocytes. A noticeable, uniform contraction of the cell sheet was observed upon detachment. This phenomenon was attributed to the release of cytoskeletal tension from cell-substrate junctions and the intrinsic contractile properties of the intact, cell-secreted ECM (
Figure 14A-F). This contraction is indicative of a well-formed, interconnected tissue construct. The viability and regenerative potential of these detached sheets were confirmed by their ability to give rise to migrating and proliferating cells when transferred to a new culture dish as an explant (
Figure 14G), demonstrating their capacity to actively participate in a healing process. Recognizing that many clinical defects require a substantial volume of tissue for repair, we demonstrated that our technique allows for the straightforward stacking of multiple sheets. A construct of three stacked sheets resulted in a tissue of two to three cell layers in thickness, confirming that a sufficient cell mass for transplantation can be achieved (
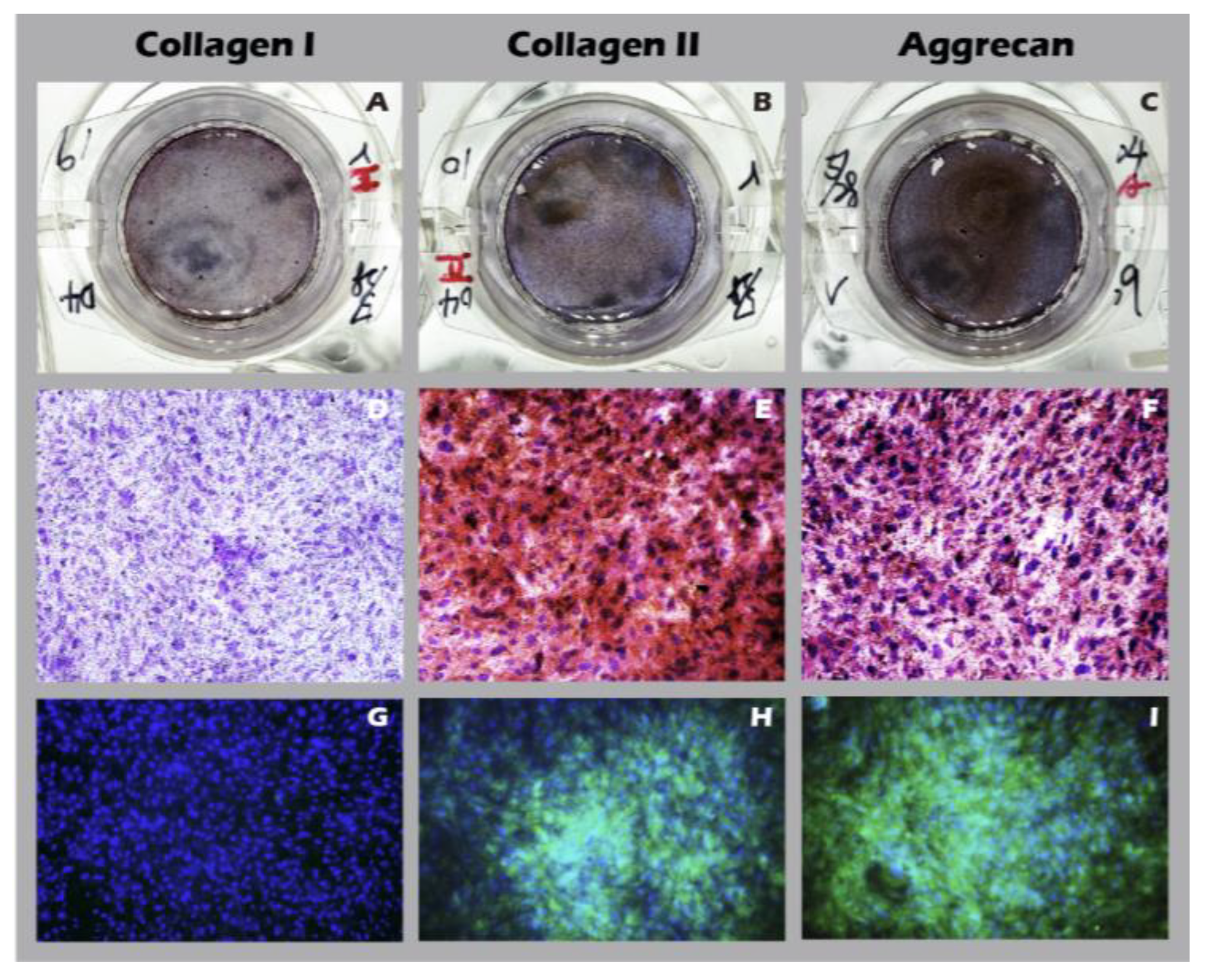
Figure 14H). Comprehensive IHC and IF analyses of these engineered sheets (
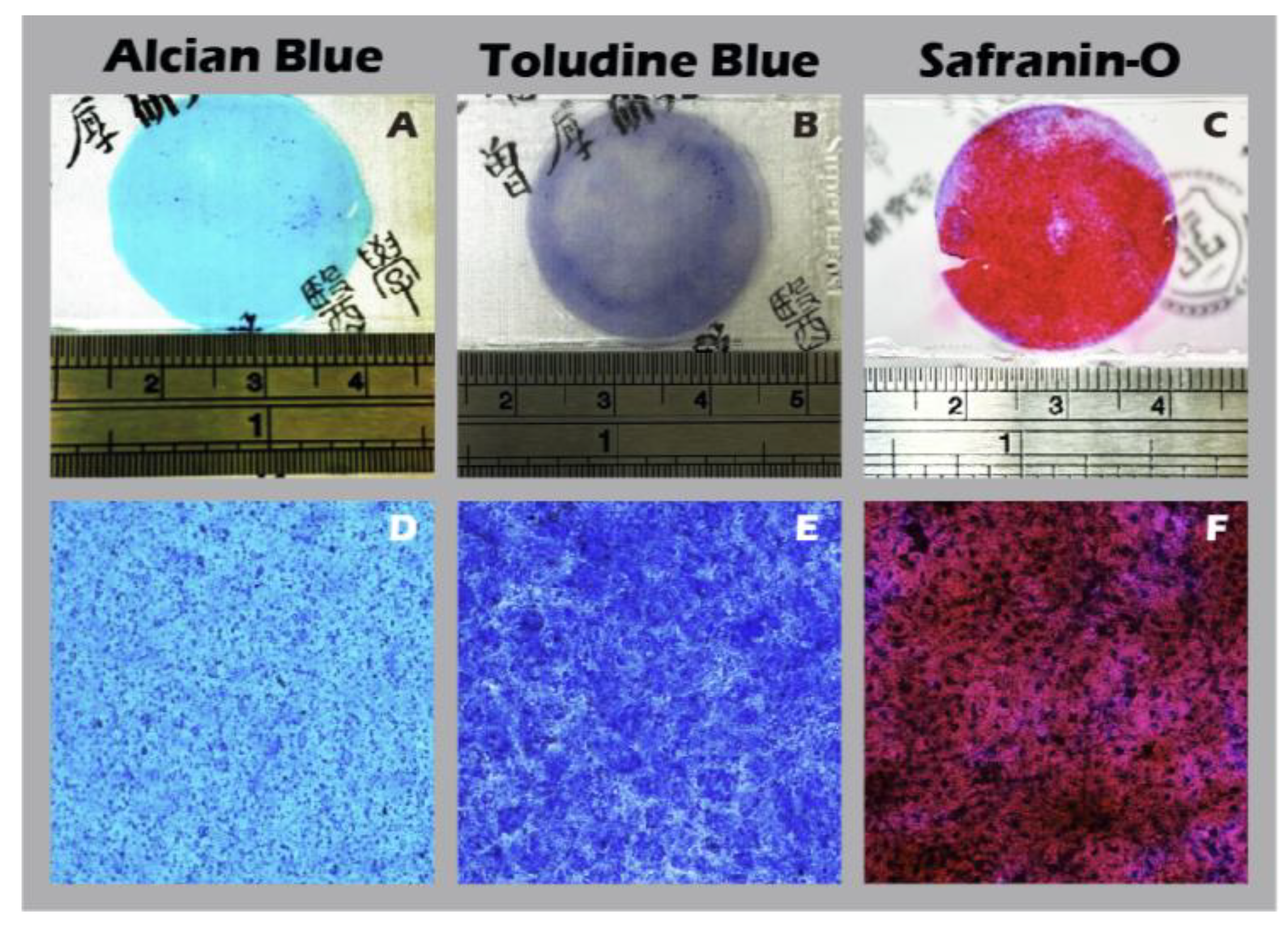
Figure 15) further confirmed a hyaline cartilage phenotype, with strong, matrix-associated expression of Collagen II and Aggrecan and negligible Collagen I. Furthermore, histochemical staining for GAGs using Alcian blue, Toluidine blue, and Safranin-O (
Figure 16) all yielded strong positive results, indicating the presence of a well-developed, proteoglycan-rich ECM distributed throughout the full thickness of the sheet structure. This mature matrix is essential for providing the immediate biomechanical functionality required upon transplantation.
3.7. In Vivo Transplantation Leads to Successful Regeneration of Hyaline-Like Cartilage
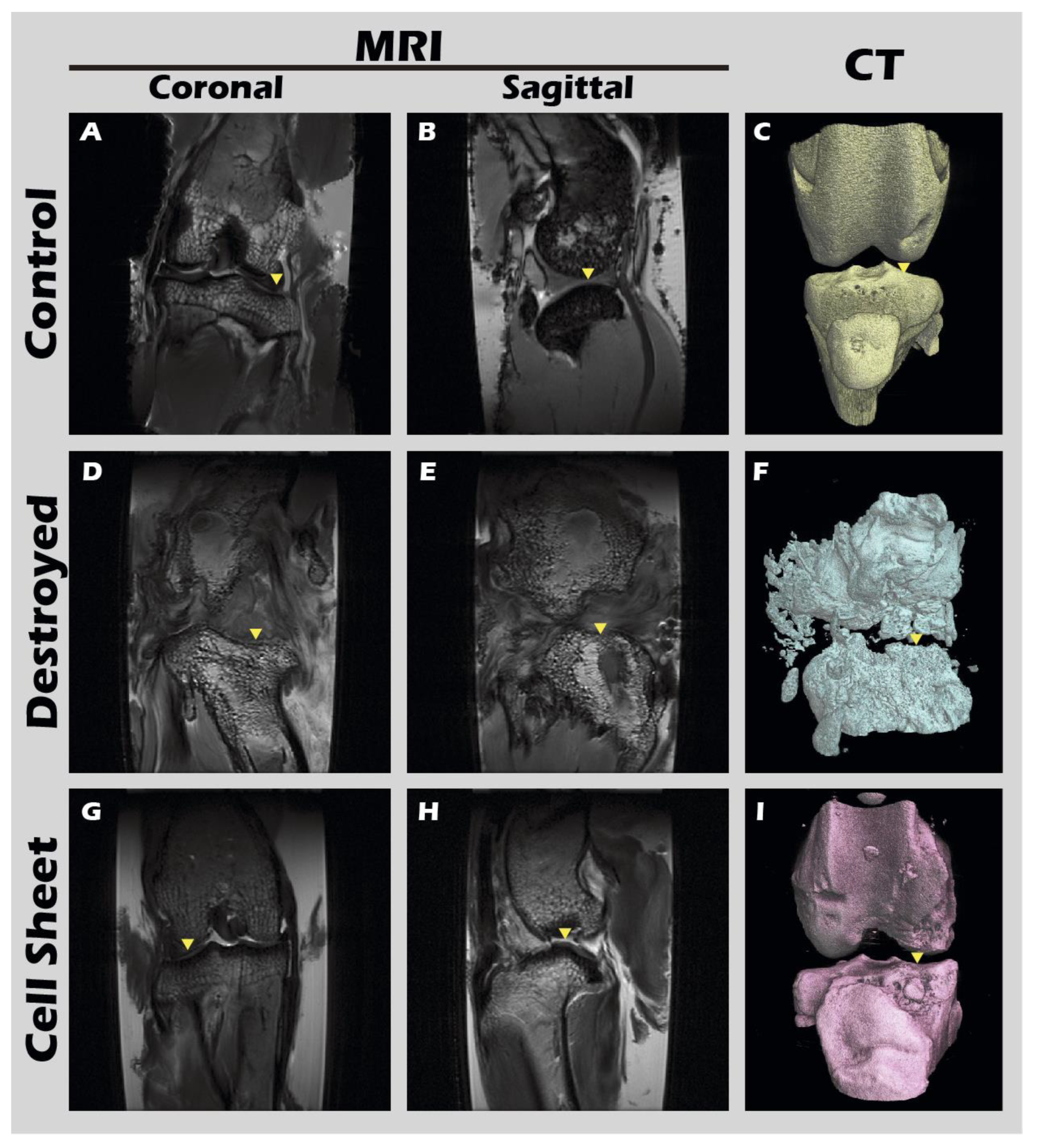
The ultimate test of our engineered tissue was its ability to regenerate cartilage in vivo. Autologous chondrocyte sheets were transplanted into full-thickness cartilage defects in adult rabbit knees. After a 12-week healing period, comprehensive imaging and histological analyses were performed. High-resolution MRI and Micro-CT scans (
Figure 16) provided a clear comparison between the outcomes. The untreated defect in the left knee resulted in extensive pathology, including persistent inflammation, significant tissue degradation, loss of joint space, and the formation of substantial subchondral bone irregularities and osteophytes. In stark contrast, the right knee treated with the chondrocyte sheet exhibited a remarkable degree of healing. The MRI scans showed significant cartilage regeneration, with the repaired tissue appearing nearly restored to the level of the uninjured control, displaying a smooth, continuous articular surface and a normal signal intensity indicative of healthy tissue hydration and composition. H&E staining of the harvested joints (
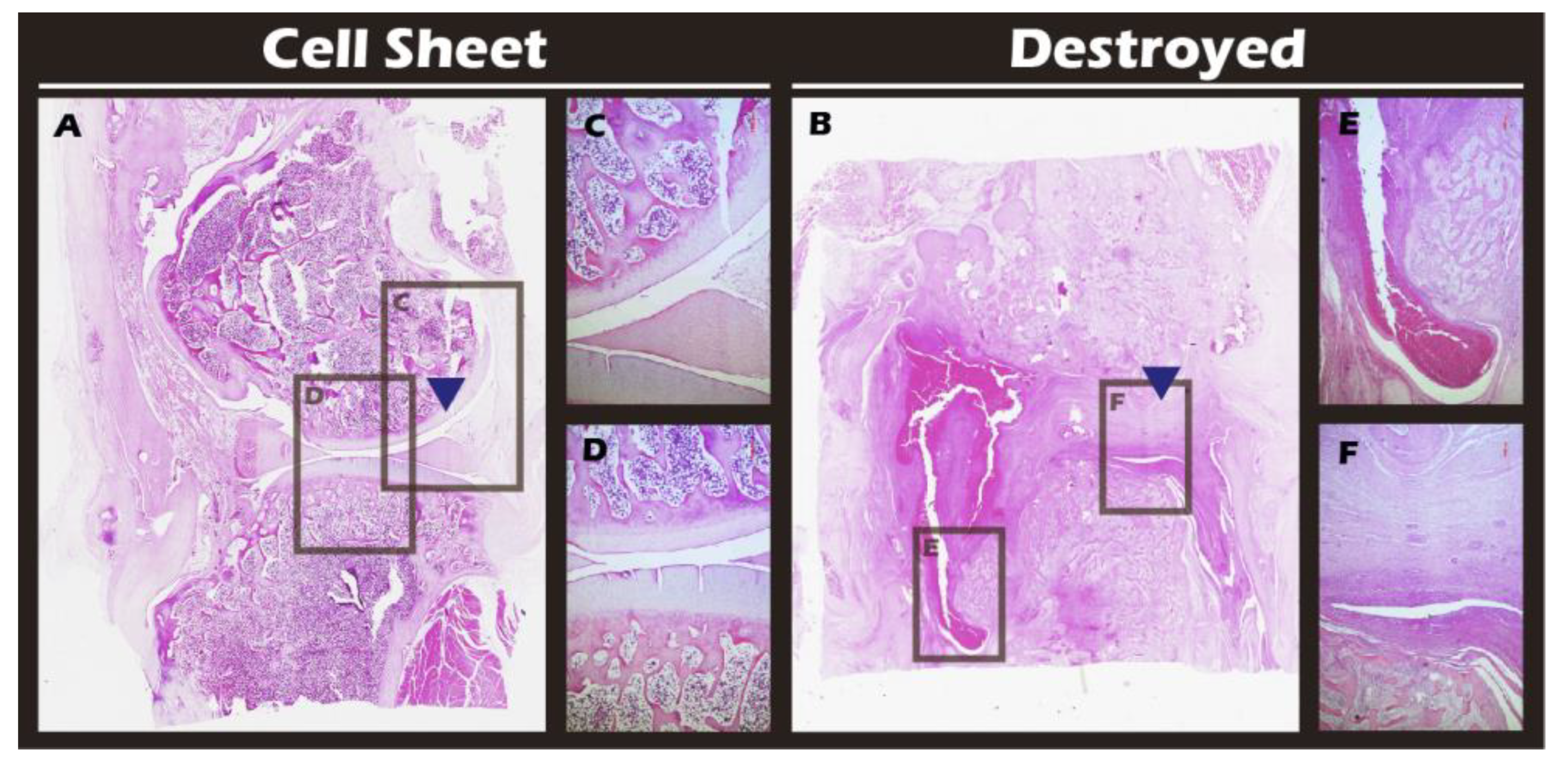
Figure 17) corroborated these imaging findings at the microscopic level, revealing substantial tissue recovery, good surface congruity, and excellent integration with the adjacent native cartilage and subchondral bone in the treated group. Finally, IHC analyses for Collagen II (
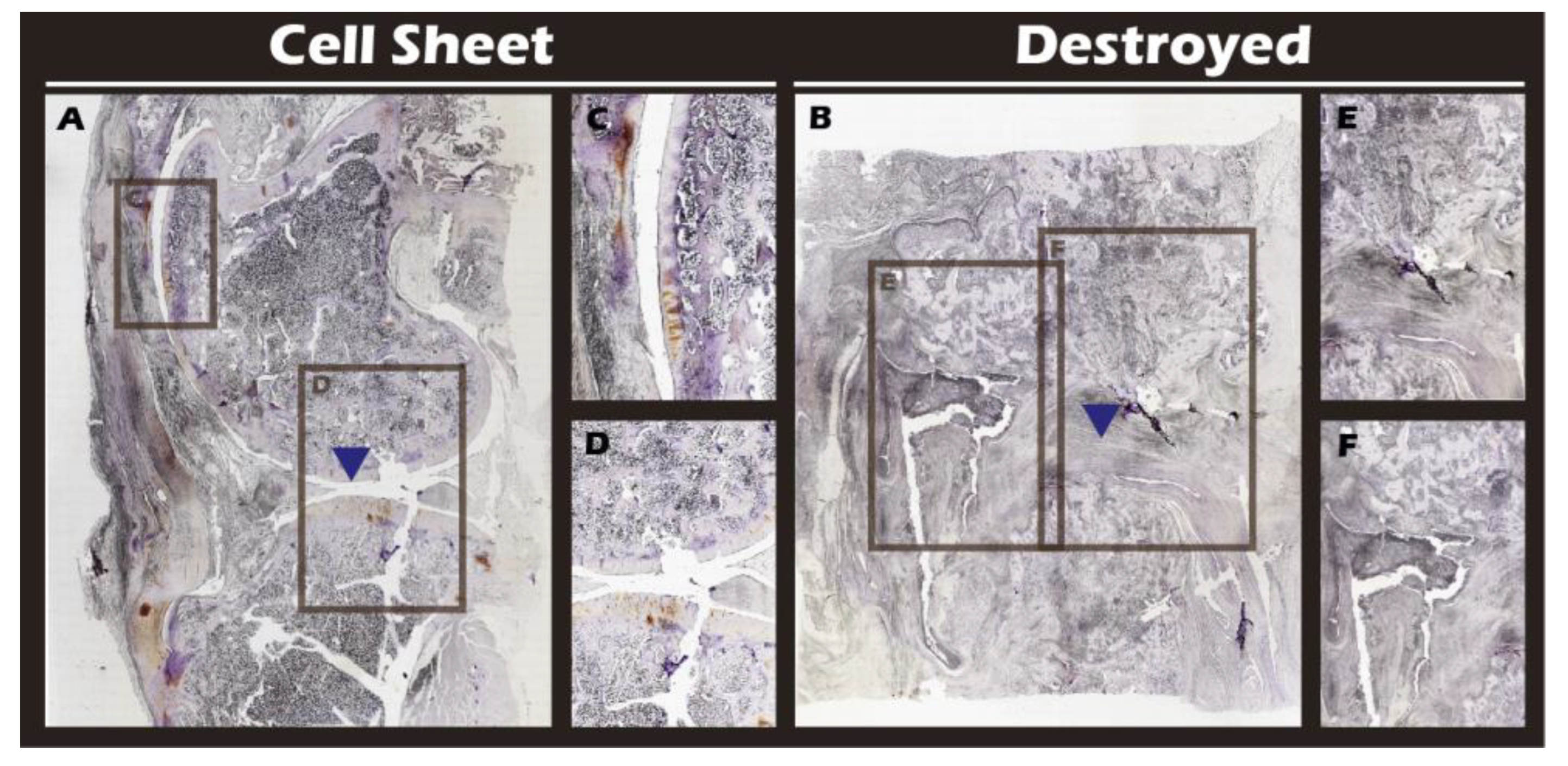
Figure 18) and Collagen I (
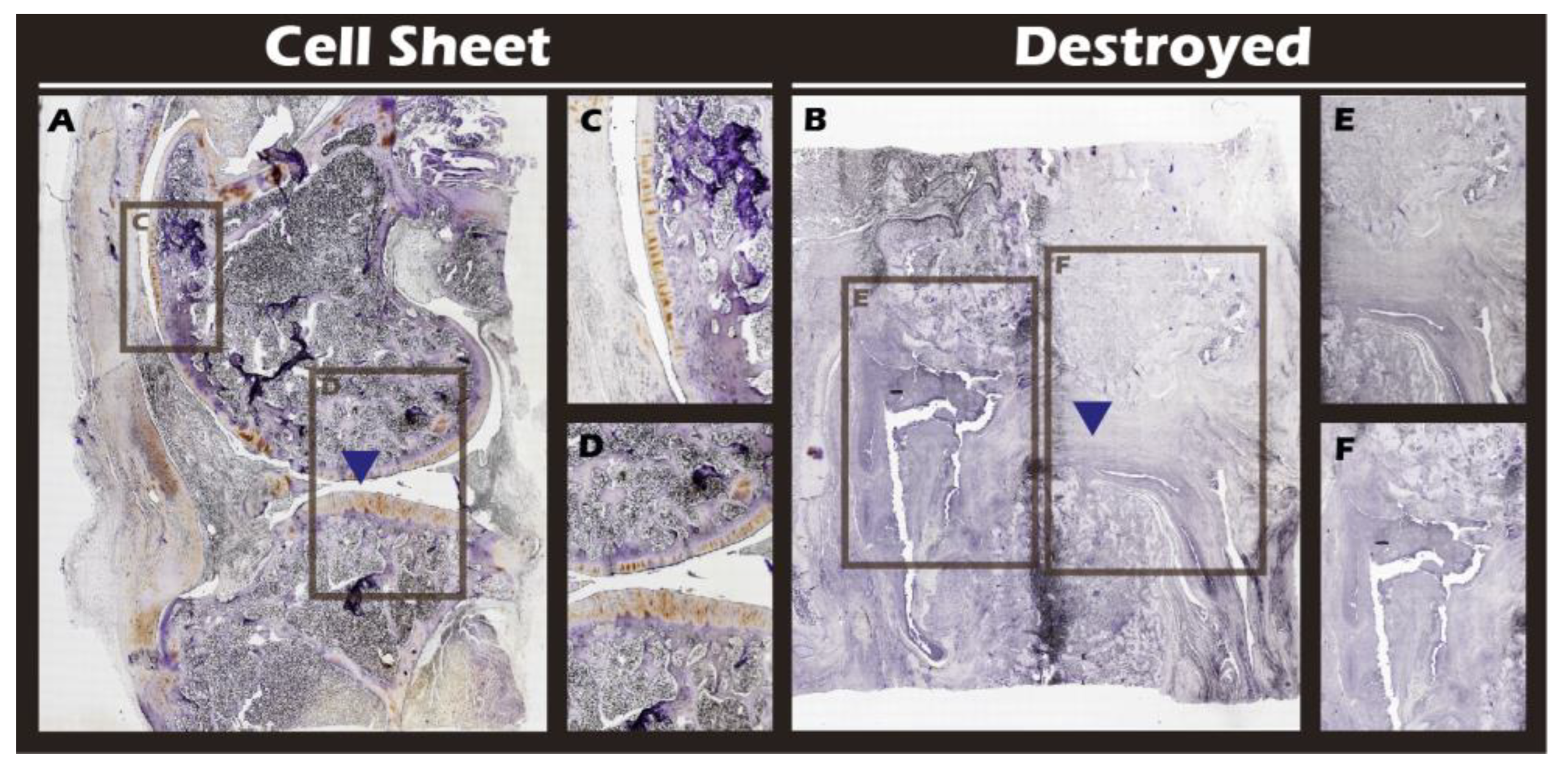
Figure 19) were performed to confirm the biochemical nature of the regenerated tissue. The repaired tissue in the treated knee showed strong and widespread expression of Collagen II, characteristic of hyaline cartilage, with a distribution pattern that mirrored the native tissue. Conversely, the untreated defect showed extensive expression of Collagen I, indicative of the formation of non-functional fibrous scar tissue. Collectively, these multi-modal results demonstrate the clear therapeutic efficacy of our autologous chondrocyte sheet in regenerating structurally and biochemically appropriate, hyaline-like cartilage in a challenging large-animal model.
Figure 17.
MRI and CT imaging of rabbit knee joints. Representative images from the (A-C) untreated Control group, (D-F) defect-only Destroyed group, and (G-I) Cell Sheet-treated group. First row (A, D, G): coronal MRI scans. Second row (B, E, H): sagittal MRI scans. Third row (C, F, I): 3D-reconstructed CT images. Yellow triangles indicate the defect site.
Figure 17.
MRI and CT imaging of rabbit knee joints. Representative images from the (A-C) untreated Control group, (D-F) defect-only Destroyed group, and (G-I) Cell Sheet-treated group. First row (A, D, G): coronal MRI scans. Second row (B, E, H): sagittal MRI scans. Third row (C, F, I): 3D-reconstructed CT images. Yellow triangles indicate the defect site.
Figure 18.
Histological analysis of sagittal sections of knee joints with H&E staining. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of the repaired tissue in (A). (E, F) Magnified views of the defect area in (B). Blue triangles indicate the defect site.
Figure 18.
Histological analysis of sagittal sections of knee joints with H&E staining. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of the repaired tissue in (A). (E, F) Magnified views of the defect area in (B). Blue triangles indicate the defect site.
Figure 19.
Immunohistochemical analysis for Collagen I in sagittal sections of knee joints. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of (A). (E, F) Magnified views of (B). Blue triangles indicate the defect site.
Figure 19.
Immunohistochemical analysis for Collagen I in sagittal sections of knee joints. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of (A). (E, F) Magnified views of (B). Blue triangles indicate the defect site.
Figure 20.
Immunohistochemical analysis for Collagen II in sagittal sections of knee joints. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of the hyaline-like repaired tissue in (A). (E, F) Magnified views of the defect area in (B). Blue triangles indicate the defect site.
Figure 20.
Immunohistochemical analysis for Collagen II in sagittal sections of knee joints. (A) Cell Sheet-treated right knee. (B) Defect-only Destroyed left knee. (C, D) Magnified views of the hyaline-like repaired tissue in (A). (E, F) Magnified views of the defect area in (B). Blue triangles indicate the defect site.
4. Discussion
The present study describes the successful development and validation of a novel, patented cell sheet engineering technology. This technology utilizes a disulfide-containing amino acid as a cleavable linker combined with the biocompatible polymer γ-PGA to produce functional, autologous chondrocyte sheets possessing a complete and well-developed extracellular matrix (ECM) [
25]. Crucially, it was demonstrated in a New Zealand white rabbit model that these cell sheets can effectively repair damaged knee cartilage, regenerating tissue that is structurally and functionally similar to native hyaline cartilage, thereby offering a highly promising solution for cartilage regenerative medicine.
The prevailing CSE technology relies on the phase transition of the thermoresponsive polymer, pNIPAAm, for cell sheet detachment. However, this mechanism, being a physical phenomenon, is dependent on a specific molecular weight range of pNIPAAm, which consequently constrains the stiffness of the culture surface to a narrow range (approx. 10-500 kPa)[
23]. The system described herein employs a distinct chemical mechanism, utilizing the mild reducing agent Cysteine to specifically cleave disulfide bonds for gentle cell sheet release. The core advantage of this design is its high degree of customizability and chemical specificity. By altering the surface biopolymer (in this study, γ-PGA), it is possible to modulate the physicochemical properties of the culture substrate, such as stiffness and charge [
26], to more accurately mimic the microenvironment of a specific target tissue[
27]. In the past, some studies have indicated that the cells themselves lack strength, different tissues have varying stiffnesses, so the stiffness of the extracellular matrix in different tissues significantly influences cell behavior [
28,
29,
30]. This unique design concept imparts the platform with the potential for broader applications in tissue engineering beyond cartilage [
31].
The biomimicry of physical properties is critical for guiding appropriate cell differentiation and proliferation, a process governed by mechanotransduction. The health of cartilage tissue is intrinsically linked to its well-developed ECM, which can constitute up to 80% of the tissue. Our AFM measurements confirmed that the stiffness of our hydrated γ-PGA-grafted membrane is approximately 16.2 MPa, a value remarkably close to that of native human knee cartilage (approx. 20 MPa). In contrast to the hydrophobic state of pNIPAAm systems at a 37 °C culture temperature, our platform provides a more physiologically relevant mechanical environment. It is postulated that this high degree of biomechanical fidelity is a key determinant in the successful cultivation of phenotypically stable hyaline chondrocyte sheets, as appropriate mechanical cues are known to be essential for maintaining chondrocyte phenotype and preventing dedifferentiation via pathways such as YAP/TAZ signaling. Moreover, γ-PGA was selected not only for its excellent hydrophilicity but also based on literature indicating its potential utilization by cells as a nutrient source. This hypothesis was indirectly supported by our cytotoxicity assays, which showed that the grafted PET membrane promoted cell proliferation more effectively than traditional TCPS. The resultant cell sheets stained strongly positive for GAGs, confirming the successful construction of cartilage-like tissue with a developed ECM [
32,
33,
34].
To ensure robustness and potential for future scale-up, a multi-level validation system was established. At the molecular level, high-surface-sensitivity ESCA (XPS) analysis clearly demonstrated the expected changes in elemental composition and chemical states pre- and post-grafting and after Cysteine treatment, confirming the feasibility of the underlying chemical reactions. At the macroscopic level, the significant change in water contact angle (from 70.5° down to 14.1° and back up to 48.3°) provided physical evidence for the mechanism. Importantly, our multi-point analysis of large-area reacted membranes showed uniform contact angles, demonstrating the high consistency and reproducibility of the manufacturing process. This uniformity is a critical prerequisite for Good Manufacturing Practice (GMP) and for providing stable, standardized clinical-grade products in the future [
35].
To ensure the potential for clinical translation, the study design closely mirrored clinical scenarios. We first utilized chondrocytes from highly proliferative juvenile rabbits to confirm platform stability, then transitioned to cells from older, adult rabbits, as the target patient population for cartilage reconstruction is typically older or has sustained traumatic injury. Our results showed that chondrocytes from both sources proliferated normally and maintained their correct phenotype. The high transparency of the detached sheets is a hallmark of hyaline cartilage. These sheets not only exhibited excellent viability but also demonstrated the ability to migrate and re-attach in a simulated transplant setting, indicating a strong potential for tissue integration in vivo. Furthermore, our technique allows for the straightforward stacking of multiple sheets, creating a graft 2-3 layers thick, a crucial feature for repairing the deep, often irregular defects found in osteoarthritic joints [
36].
The most significant outcome of this research was observed in the autologous transplantation animal study. Through a combination of MRI, CT, and histopathological analyses, it was observed that while the untreated knee joint exhibited severe inflammation and tissue destruction after 16 weeks, the knee treated with autologous chondrocyte sheets for 12 weeks demonstrated almost complete restoration of the continuity, thickness, and smoothness of the cartilage tissue. Strong IHC staining for Collagen II confirmed the regenerated tissue was functional hyaline cartilage, while the minimal expression of Collagen I ruled out the formation of fibrous scar tissue. These results support the conclusion that the engineered autologous chondrocyte sheets can guide true, functional cartilage regeneration in vivo [
37].
Despite these encouraging results, the study has certain limitations. Due to the large size of the adult rabbits, longitudinal in vivo imaging with standard small-animal MRI or CT scanners was not feasible. Therefore, our analysis was limited to the endpoint after euthanasia, precluding continuous observation of the repair process. Future investigations should consider the use of larger animal models, such as pigs or sheep, which not only better approximate the dimensions and mechanical loading of human joints but also facilitate long-term in vivo imaging. Additionally, while histological outcomes were excellent, future work should include biomechanical testing of the repaired cartilage to quantitatively assess its functional properties and long-term durability [
38,
39].
5. Conclusions
In summary, the present study successfully developed and validated a novel cell sheet engineering platform based on a disulfide cleavage mechanism. This platform offers multiple advantages, including a highly biomimetic physical stiffness, tunable surface properties, a stable manufacturing process, and a mild, chemically specific detachment condition. Using this platform, autologous hyaline chondrocyte sheets with a well-developed extracellular matrix were successfully cultured from adult rabbit chondrocytes. In a rabbit knee cartilage defect model, we demonstrated that transplantation of these cell sheets could guide the regeneration of structurally and functionally complete hyaline cartilage tissue, achieving excellent repair outcomes. This technology addresses many of the limitations associated with traditional therapies and provides a highly promising clinical solution for treating osteoarthritis and other cartilage injuries, laying a solid foundation for the advancement of regenerative medicine.
6. Patents
A relevant patent (US9,546,349 B2) has been issued to H. Tseng and is licensed to Taipei Medical University.
Author Contributions
T.-L.T. and H.T. designed the study and drafted this manuscript. Y.T., J.-W.L. and C.-T.Y. prepared cell culture insert. Y.T., H.-I L. supported the materials analyses. T.-L.T., H.-Y.C., J.-W.L. and H.-I L. carried out animal study. C.-T.Y. and J.-W.L. analyzed the quality of H&E, ICC and IHC. J.-J.L. and Y.-Y.Y. took part in the discussion and edited the English manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Point Robotics MedTech Inc. (grant no. A-110-091) and the National Science and Technology Council, Taiwan (grant no. 112-2622-E-038-002).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University (Approval No. LAC2023-0039; date of approval: 28 March 2023) for a project entitled “Preparation of chondrocytes cell sheet to reconstruct cartilage tissue by a novel cell sheet engineering.”
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in the study are presented in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CSE |
Cell Sheet Engineering |
| OA |
Osteoarthritis |
| ACI |
Autologous Chondrocyte Implantation |
| MACI |
matrix-induced ACI |
| ECM |
extracellular matrix |
| KOOS |
Knee Injury and Osteoarthritis Outcome Score |
| LKS |
Lysholm Knee Score |
| JCCs |
juvenile chondrocytes |
| ATMPs |
advanced therapy medicinal products |
| pNIPAAm |
poly(N-isopropylacrylamide) |
| γ-PGA |
gamma-polyglutamic acid |
| EDC |
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS |
N-hydroxysuccinimide |
| PVDF |
Poly vinyl difluoride |
| PET |
polyethylene terephthalate |
| CCD |
Charge-coupled Device |
| ESCA |
Electron Spectroscopy for Chemical Analysis |
| XPS |
X-ray photoelectron spectroscopy |
| H&E |
hematoxylin and eosin |
| ICC |
Immunocytochemistry |
| IHC |
Immunohistochemistry |
| IF |
Immuno-fluorescence |
| IACUC |
Institutional Animal Care and Use Committee |
| Micro-CT |
Micro-Computed Tomography |
| MRI |
Magnetic Resonance Imaging |
| PVC |
Poly vinyl chloride |
| TCPS |
Tissue Culture Polystyrene |
| AFM |
Atomic Force Microscope |
| GMP |
Good Manufacturing Practice |
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. The Lancet 2019, 393, 1745–1759, doi:. [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis & Rheumatism 2012, 64, 1697–1707, doi:10.1002/art.34453.
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health: A Multidisciplinary Approach 2009, 1, 461–468, doi:10.1177/1941738109350438.
- Drummer, D.; McAdam, J.; Seay, R.; Ferrando, A.; Bridges, S.L.; Singh, J.A.; Bamman, M. Osteoarthritis Progression: Mitigation and Rehabilitation Strategies. Frontiers in Rehabilitation Sciences 2021, 2, doi:10.3389/fresc.2021.724052.
- Seed, S.M.; Dunican, K.C.; Lynch, A.M. Osteoarthritis: a review of treatment options. Geriatrics 2009, 64, 20–29.
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. New England Journal of Medicine 1994, 331, 889–895, doi:10.1056/nejm199410063311401.
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous Chondrocyte Implantation: A Systematic Review. JBJS 2010, 92, 2220–2233, doi:10.2106/jbjs.J.00049.
- Olson, A.; Graver, A.; Grande, D.A. Scaffolds for articular cartilage repair. Journal of Long-Term Effects of Medical Implants 2012, 22.
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. Journal of Biomedical Materials Research 1999, 45, 355–362, doi:10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7.
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromolekulare Chemie, Rapid Communications 1990, 11, 571–576, doi:. [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Regenerative Therapies Using Cell Sheet-Based Tissue Engineering for Cardiac Disease. Cardiology Research and Practice 2011, 2011, 1–8, doi:10.4061/2011/845170.
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. Journal of Internal Medicine 2010, 267, 54–70, doi:10.1111/j.1365-2796.2009.02185.x.
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Articular Cartilage Repair with Mesenchymal Stem Cells After Chondrogenic Priming: A Pilot Study. Tissue Engineering Part A 2018, 24, 761–774, doi:10.1089/ten.tea.2017.0235.
- Sato, M.; Yamato, M.; Mitani, G.; Takagaki, T.; Hamahashi, K.; Nakamura, Y.; Ishihara, M.; Matoba, R.; Kobayashi, H.; Okano, T.; et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. npj Regenerative Medicine 2019, 4, doi:10.1038/s41536-019-0069-4.
- Sato, M.; Toyoda, E.; Hamahashi, K. Regenerative Medicine for Osteoarthritis of the Knee Using Cell Sheets. In Regenerative Medicine in Sports and Orthopaedics: A New Approach, Gobbi, A., Nakamura, N., Lane, J.G., Dallo, I., Eds.; Springer Nature Switzerland: Cham, 2025; pp. 363–373.
- Yu, J.; Tu, Y.-K.; Tang, Y.-B.; Cheng, N.-C. Stemness and transdifferentiation of adipose-derived stem cells using l-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials 2014, 35, 3516–3526, doi:. [CrossRef]
- Kondo, M.; Kameishi, S.; Grainger, D.W.; Okano, T. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerging Topics in Life Sciences 2020, 4, 677–689, doi:10.1042/etls20200151.
- Abolarinwa, B.A.; Shaw, M.K.; Lee, C.-H. Perspectives on Challenges to Cell Therapy Development in Taiwan: Strengthening Evidential Standards and Ways Forward. Frontiers in Bioengineering and Biotechnology 2021, 9, doi:10.3389/fbioe.2021.789043.
- MacPherson, J. From the Editors — Cell Therapy Regulation in 2025: A Turning Point for Global Innovation and Access. Available online: https://www.isctglobal.org/telegrafthub/blogs/isct-head-office1/2025/10/07/from-the-editors-cell-therapy-regulation-in-2025-a (accessed on 2025/10/20).
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnology Journal 2014, 9, 904–914, doi:10.1002/biot.201300432.
- Dang, L.H.; Hung, S.-H.; Tseng, Y.; Quang, L.X.; Le, N.T.N.; Fang, C.-L.; Tseng, H. Partial Decellularized Scaffold Combined with Autologous Nasal Epithelial Cell Sheet for Tracheal Tissue Engineering. International Journal of Molecular Sciences 2021, 22, 10322, doi:10.3390/ijms221910322.
- Viet-Nhi, N.-K.; Chen, Y.-C.; Dang, L.H.; Tseng, H.; Hung, S.-H. Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. Journal of Functional Biomaterials 2023, 14, 147, doi:10.3390/jfb14030147.
- Kim, H.; Witt, H.; Oswald, T.A.; Tarantola, M. Adhesion of Epithelial Cells to PNIPAm Treated Surfaces for Temperature-Controlled Cell-Sheet Harvesting. ACS Applied Materials & Interfaces 2020, 12, 33516–33529, doi:10.1021/acsami.0c09166.
- Biological evaluation of medical devices—Part 5: Tests for <i>in vitro</i> cytotoxicity. In ANSI/AAMI/ISO 10993-5:2009/(R)2022; Biological evaluation of medical devices—Part 5: Tests for <i>in vitro</i> cytotoxicity.
- Yoshida, H.; Matsusaki, M.; Akashi, M. Development of Thick and Highly Cell-Incorporated Engineered Tissues by Hydrogel Template Approach with Basic Fibroblast Growth Factor or Ascorbic Acid. Journal of Biomaterials Science, Polymer Edition 2010, 21, 415–428, doi:10.1163/156856209x423135.
- Khalil, I.; Irorere, V.; Radecka, I.; Burns, A.; Kowalczuk, M.; Mason, J.; Khechara, M. Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses. Materials 2016, 9, 28, doi:10.3390/ma9010028.
- Dadsetan, M.; Pumberger, M.; Casper, M.E.; Shogren, K.; Giuliani, M.; Ruesink, T.; Hefferan, T.E.; Currier, B.L.; Yaszemski, M.J. The effects of fixed electrical charge on chondrocyte behavior. Acta Biomaterialia 2011, 7, 2080–2090, doi:10.1016/j.actbio.2011.01.012.
- Tsimbouri, P. Adult Stem Cell Responses to Nanostimuli. Journal of Functional Biomaterials 2015, 6, 598–622, doi:10.3390/jfb6030598.
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease Models & Mechanisms 2011, 4, 165–178, doi:10.1242/dmm.004077.
- Chen, C.-H.; Shih, T.-C.; Liu, Y.-L.; Peng, Y.-J.; Huang, Y.-L.; Chen, B.S.; Tseng, H. Engineered Immature Testicular Tissue by Electrospun Mats for Prepubertal Fertility Preservation in a Bioluminescence Imaging Transgenic Mouse Model. International Journal of Molecular Sciences 2022, 23, 12145, doi:10.3390/ijms232012145.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689, doi:10.1016/j.cell.2006.06.044.
- Zhang, K.; Wang, L.; Liu, Z.; Geng, B.; Teng, Y.; Liu, X.; Yi, Q.; Yu, D.; Chen, X.; Zhao, D.; et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels 2021, 15, 339–359, doi:10.1080/19336950.2021.1903184.
- Lu, M.; Zhu, M.; Wu, Z.; Liu, W.; Cao, C.; Shi, J. The role of <scp>YAP/TAZ</scp> on joint and arthritis. The FASEB Journal 2024, 38, doi:10.1096/fj.202302273rr.
- Liu, S.; Li, P.; Liu, X.; Wang, P.; Xue, W.; Ren, Y.; Yang, R.; Chi, B.; Ye, Z. Bioinspired mineral-polymeric hybrid hyaluronic acid/poly (γ-glutamic acid) hydrogels as tunable scaffolds for stem cells differentiation. Carbohydrate Polymers 2021, 264, 118048, doi:. [CrossRef]
- Melocchi, A.; Schmittlein, B.; Sadhu, S.; Nayak, S.; Lares, A.; Uboldi, M.; Zema, L.; di Robilant, B.N.; Feldman, S.A.; Esensten, J.H. Automated manufacturing of cell therapies. Journal of Controlled Release 2025, 381, 113561, doi:. [CrossRef]
- Takahashi, T.; Sato, M.; Toyoda, E.; Maehara, M.; Takizawa, D.; Maruki, H.; Tominaga, A.; Okada, E.; Okazaki, K.; Watanabe, M. Rabbit xenogeneic transplantation model for evaluating human chondrocyte sheets used in articular cartilage repair. Journal of Tissue Engineering and Regenerative Medicine 2018, doi:10.1002/term.2741.
- Guilak, F.; Estes, B.T.; Moutos, F.T. Functional tissue engineering of articular cartilage for biological joint resurfacing—The 2021 Elizabeth Winston Lanier Kappa Delta Award. Journal of Orthopaedic Research 2022, 40, 1721–1734, doi:10.1002/jor.25223.
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. Journal of Experimental Orthopaedics 2016, 3, doi:10.1186/s40634-015-0037-x.
- Schmidt, T.; Xiang, Y.; Bao, X.; Sun, T. A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy. Processes 2021, 9, 935, doi:10.3390/pr9060935.
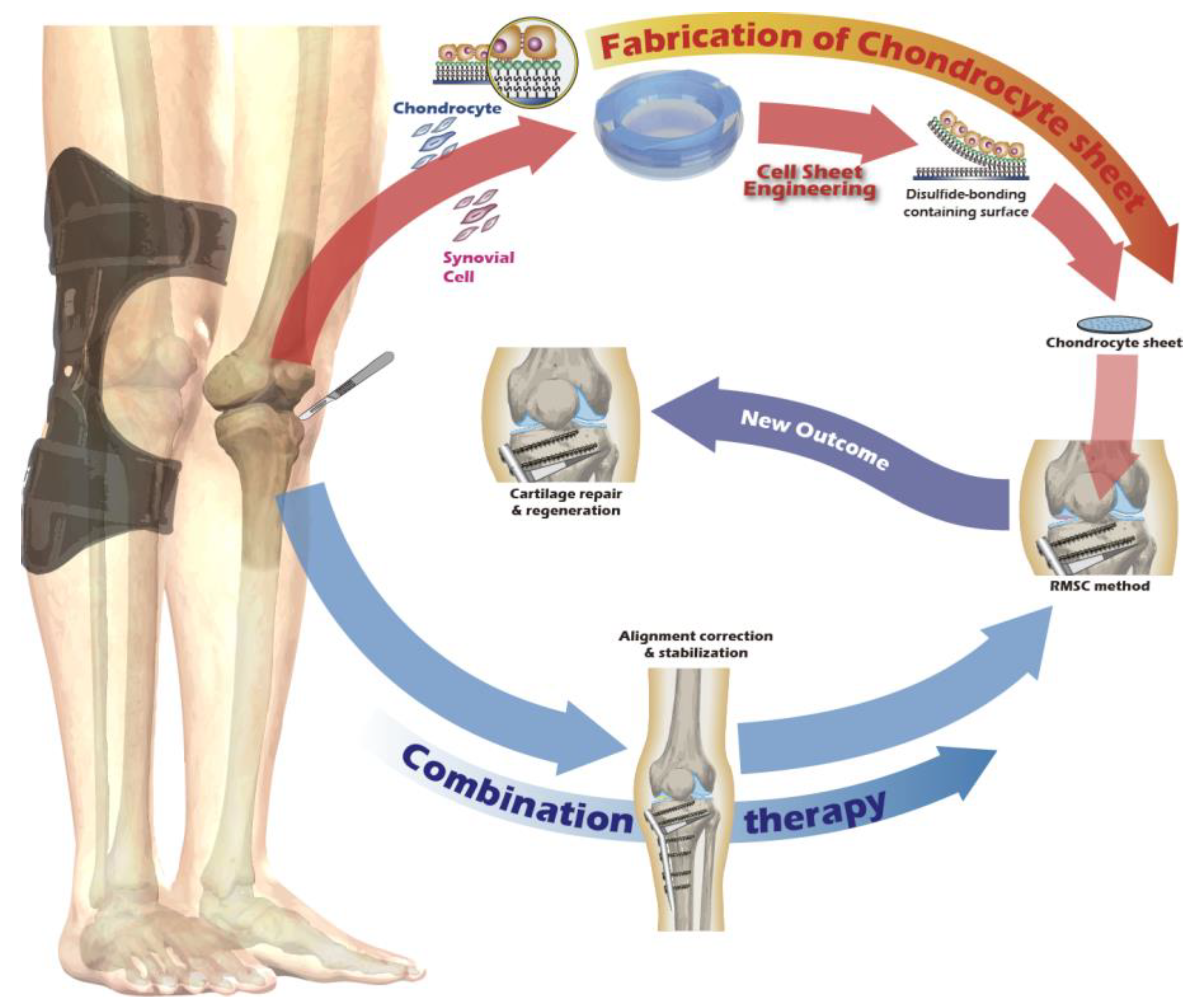
Figure 1.
Schematic of the proposed combination therapy for comprehensive knee joint regeneration, integrating cell sheet transplantation with ACI/MACI and an assistive exoskeleton.
Figure 1.
Schematic of the proposed combination therapy for comprehensive knee joint regeneration, integrating cell sheet transplantation with ACI/MACI and an assistive exoskeleton.
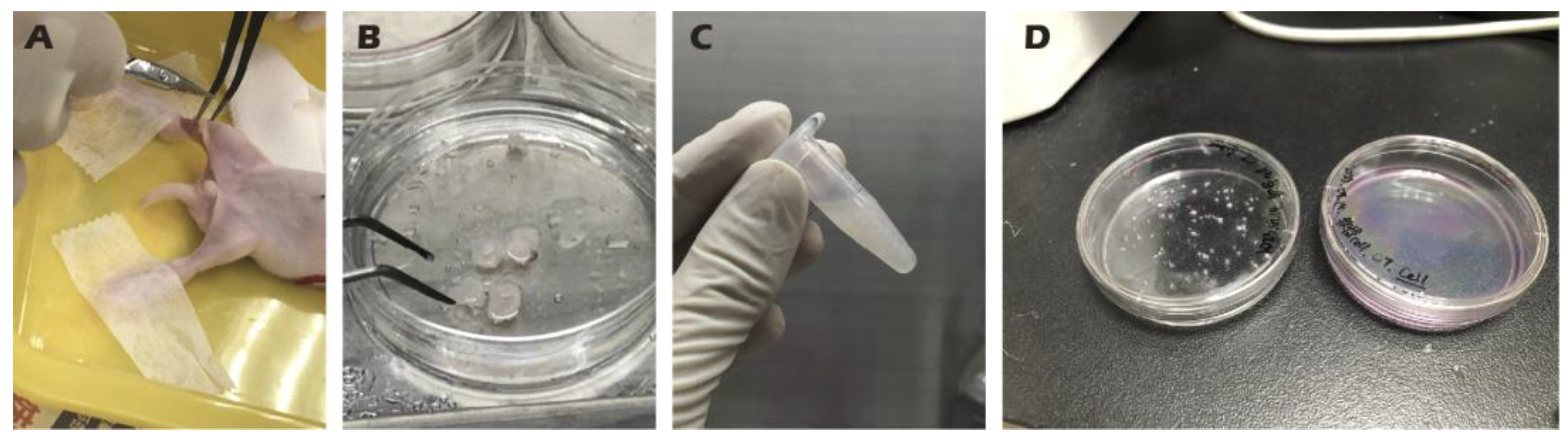
Figure 2.
Procedure for harvesting (A, B), isolating (C), and culturing (D) chondrocytes from juvenile rabbit knee cartilage.
Figure 2.
Procedure for harvesting (A, B), isolating (C), and culturing (D) chondrocytes from juvenile rabbit knee cartilage.
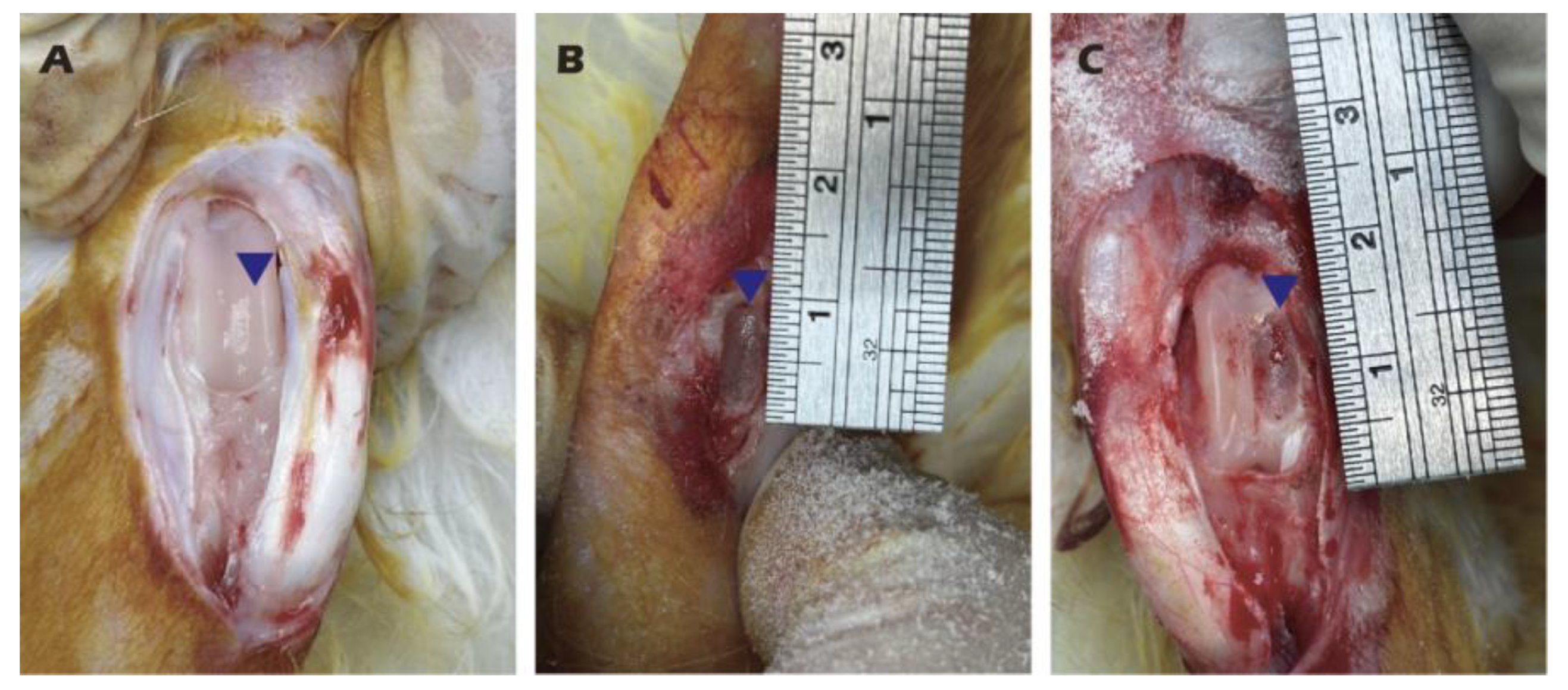
Figure 3.
Harvesting of knee cartilage from adult rabbits (A, B) and creation of a critical-sized articular cartilage defect (C).
Figure 3.
Harvesting of knee cartilage from adult rabbits (A, B) and creation of a critical-sized articular cartilage defect (C).
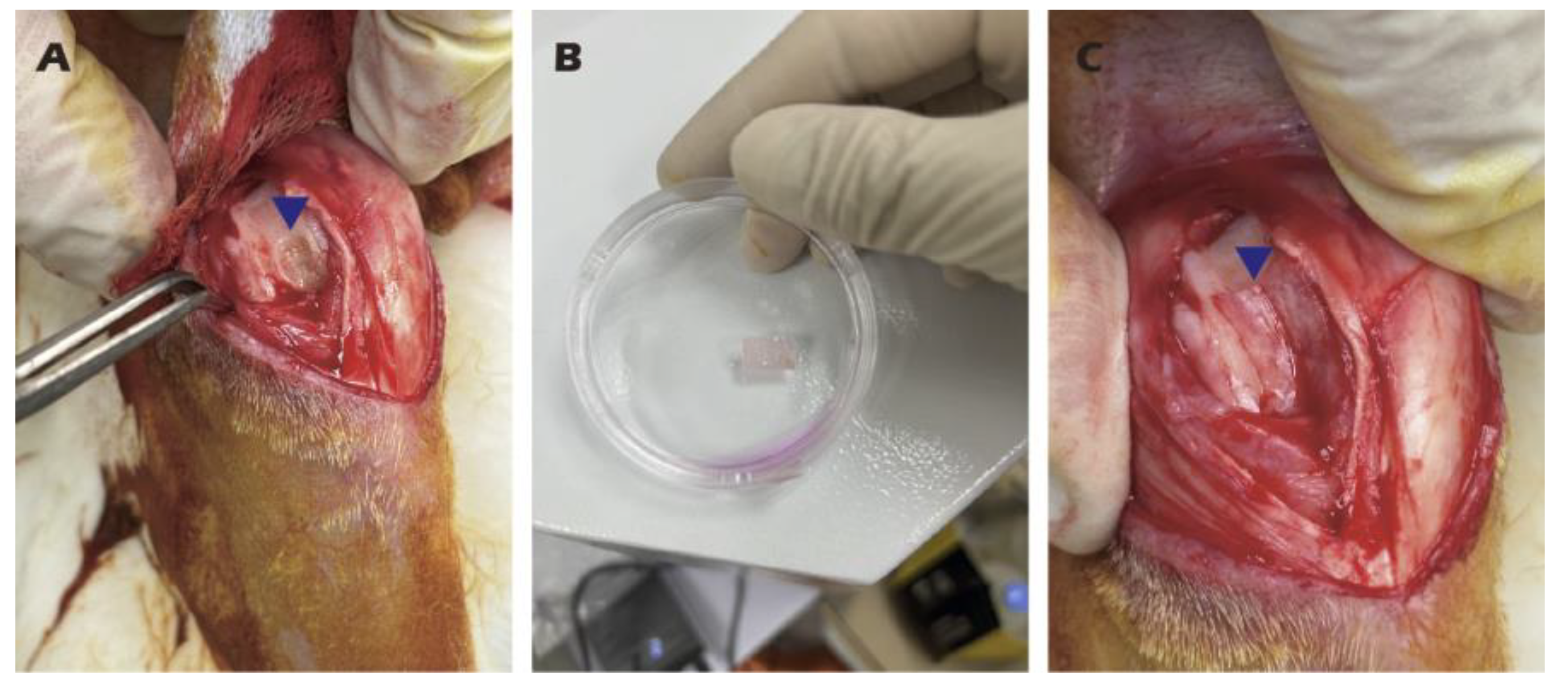
Figure 4.
Surgical procedure for autologous chondrocyte sheet transplantation in adult rabbits. (A) Creation of the cartilage defect and harvesting of cartilage tissue. (B) The harvested cartilage tissue. (C) Transplantation of the chondrocyte sheet onto the defect, covered with an occlusive membrane. Blue triangles indicate the surgical site.
Figure 4.
Surgical procedure for autologous chondrocyte sheet transplantation in adult rabbits. (A) Creation of the cartilage defect and harvesting of cartilage tissue. (B) The harvested cartilage tissue. (C) Transplantation of the chondrocyte sheet onto the defect, covered with an occlusive membrane. Blue triangles indicate the surgical site.
Figure 5.
Schematic overview of the autologous chondrocyte transplantation procedure in adult rabbits, from cartilage harvest to cell sheet reimplantation.
Figure 5.
Schematic overview of the autologous chondrocyte transplantation procedure in adult rabbits, from cartilage harvest to cell sheet reimplantation.
Figure 6.
ESCA (XPS) analysis of PVDF membrane surfaces. (A) Pristine PVDF membrane. (B) PVDF membrane after grafting with disulfide-containing and biopolymer. (C) The grafted membrane after treatment with a reducing agent.
Figure 6.
ESCA (XPS) analysis of PVDF membrane surfaces. (A) Pristine PVDF membrane. (B) PVDF membrane after grafting with disulfide-containing and biopolymer. (C) The grafted membrane after treatment with a reducing agent.
Figure 7.
Water contact angle measurements of the membrane surfaces: (A) before grafting, (B) after grafting, and (C) after reduction.
Figure 7.
Water contact angle measurements of the membrane surfaces: (A) before grafting, (B) after grafting, and (C) after reduction.
Figure 8.
Contour plot of water contact angles measured across a large-area (22 × 16 cm²) surface-grafted PET porous membrane, demonstrating grafting uniformity.
Figure 8.
Contour plot of water contact angles measured across a large-area (22 × 16 cm²) surface-grafted PET porous membrane, demonstrating grafting uniformity.
Figure 9.
Stiffness measurement of the grafted membrane by Atomic Force Microscopy (AFM) (A) in the dry state and (B) in a hydrated state.
Figure 9.
Stiffness measurement of the grafted membrane by Atomic Force Microscopy (AFM) (A) in the dry state and (B) in a hydrated state.
Figure 10.
Biocompatibility assessment of the grafted membrane according to ISO 10993-5 guidelines.
Figure 10.
Biocompatibility assessment of the grafted membrane according to ISO 10993-5 guidelines.
Figure 11.
Custom-made culture inserts designed for 6-cm culture dishes and 6-well culture plates.
Figure 11.
Custom-made culture inserts designed for 6-cm culture dishes and 6-well culture plates.
Figure 12.
Characterization of cultured chondrocytes. (A-F) Phase-contrast micrographs (40×) of chondrocytes at (A, D) passage 0 (P0) and (B, E) passage 1 (P1) on culture dishes, and (C, F) passage 2 (P2) on culture inserts. (G) H&E staining of fixed P1 chondrocytes (200×).
Figure 12.
Characterization of cultured chondrocytes. (A-F) Phase-contrast micrographs (40×) of chondrocytes at (A, D) passage 0 (P0) and (B, E) passage 1 (P1) on culture dishes, and (C, F) passage 2 (P2) on culture inserts. (G) H&E staining of fixed P1 chondrocytes (200×).
Figure 13.
Immunocytochemical analysis of cultured chondrocytes from adult (top row) and juvenile (bottom row) rabbits for (A, D) Collagen I, (B, E) Collagen II, and (C, F) Aggrecan.
Figure 13.
Immunocytochemical analysis of cultured chondrocytes from adult (top row) and juvenile (bottom row) rabbits for (A, D) Collagen I, (B, E) Collagen II, and (C, F) Aggrecan.
Figure 14.
Macroscopic and microscopic appearance of engineered chondrocyte sheets. (A) Sheet on a 6-well plate insert before detachment. (B) The detached sheet from (A). (C) The sheet from (A) unfolded in a 6-cm dish. (D) Sheet on a 6-cm dish insert. (E) The detached sheet from (D). (F) A three-layer stacked construct. (G) Micrograph (40×) showing cell outgrowth from a sheet explant. (H) H&E staining of a vertical cross-section of a single-layer sheet.
Figure 14.
Macroscopic and microscopic appearance of engineered chondrocyte sheets. (A) Sheet on a 6-well plate insert before detachment. (B) The detached sheet from (A). (C) The sheet from (A) unfolded in a 6-cm dish. (D) Sheet on a 6-cm dish insert. (E) The detached sheet from (D). (F) A three-layer stacked construct. (G) Micrograph (40×) showing cell outgrowth from a sheet explant. (H) H&E staining of a vertical cross-section of a single-layer sheet.
Figure 15.
Immunohistochemical (IHC) and immunofluorescence (IF) analysis of chondrocyte sheets. Staining for (A, D, G) Collagen I, (B, E, H) Collagen II, and (C, F, I) Aggrecan. The first column (A-C) shows macroscopic images, the second column (D-F) shows IHC micrographs (40×), and the third column (G-I) shows IF images.
Figure 15.
Immunohistochemical (IHC) and immunofluorescence (IF) analysis of chondrocyte sheets. Staining for (A, D, G) Collagen I, (B, E, H) Collagen II, and (C, F, I) Aggrecan. The first column (A-C) shows macroscopic images, the second column (D-F) shows IHC micrographs (40×), and the third column (G-I) shows IF images.
Figure 16.
Histochemical staining for extracellular matrix components in chondrocyte sheets. Staining for glycosaminoglycans (GAGs) using (A, D) Alcian Blue, (B, E) Toluidine Blue, and (C, F) Safranin-O. The top row (A-C) shows macroscopic images, and the bottom row (D-F) shows corresponding micrographs (40×).
Figure 16.
Histochemical staining for extracellular matrix components in chondrocyte sheets. Staining for glycosaminoglycans (GAGs) using (A, D) Alcian Blue, (B, E) Toluidine Blue, and (C, F) Safranin-O. The top row (A-C) shows macroscopic images, and the bottom row (D-F) shows corresponding micrographs (40×).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).