Introduction
The evolution of surgery has been anchored in the progressive refinement of anatomical knowledge. Concepts like "membrane anatomy" and "stratigraphic surgery" have contributed significantly to precision by detailing fascial planes. However, these approaches are inherently reductive, simplifying the complex anatomical environment into a series of static separators. They successfully answer "where to dissect" but fall short of explaining dynamic pathophysiologies, such as the sustained hydrodynamics of a pancreatic fistula or the mechanisms of selective cancer cell dissemination beyond lymphatic routes. These persistent challenges necessitate a leap from a static description of structure to a dynamic understanding of system function. This leap is enabled by reconceptualizing the interstitium as an integrated physiological network, the Interstitial-Cleft Network (ICN), a concept grounded in the
Interstitial Integration Hypothesis (IIH)—a unified physical framework that identifies the structured spaces between components as the fundamental substrate for emergence and self-organization across scales [
1].
A New Physiological Framework: From Static Structure to Dynamic System
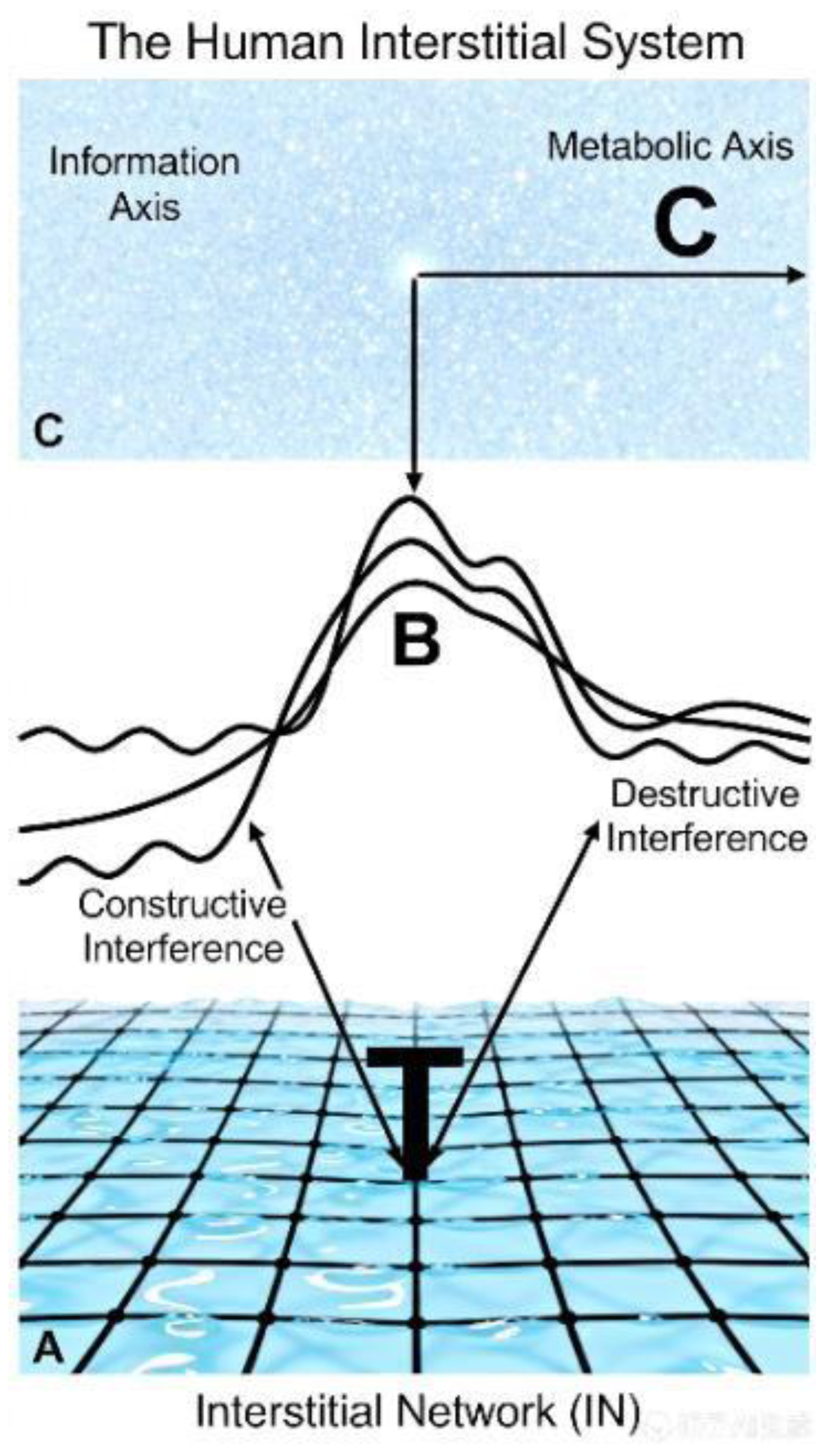
The cornerstone of this paradigm is the ICN, a pervasive, fluid-suffused continuum that integrates microscopic pericellular clefts with macroscopic fascial planes into a cohesive body-wide network [
2]. This is not a potential space but the anatomic substrate of a dynamic system. The ICN's architecture enables three critical, interdependent functions, which are direct instantiations of the core principles of the IIH [
1]:
A Mechanical and Integrative System: It distributes physiological stress, maintaining structural integrity and organ position. This exemplifies the IIH principle of Constrained Connectivity, where physical geometry defines mechanical interaction pathways.
A Fluid and Solute Transport System: It governs interstitial fluid flow and pressure, providing the pathophysiological basis for edema and fistula formation [
3]. This demonstrates
State- Dependent Permeability, where material properties dynamically modulate flow.
An Information Conduction and Cell Migration System: It serves as a conduit for signaling molecules, immune cells, and cancer cells [
4], elucidating patterns of recurrence along anatomical pathways. This embodies
Information Encoding, where the physical state of the interstice directs cellular behavior.
This systemic view recontextualizes complications. A pancreatic fistula represents a failure of the interstitial containment system, and local tumor recurrence often reflects residual disease within an underappreciated interstitial compartment.
Paradigmatic Integration and Transcendence
Surgical Interstitialogy does not negate prior anatomical concepts but subsumes them into a more powerful, overarching system model. The previously described "membranes" or "strata" are understood as the dense, organized collagenous boundaries of the ICN's fluid-filled channels. This is akin to meticulously mapping the banks of a river while overlooking the fluid dynamics, composition, and functional role of the water itself—the very medium that defines the river as a transport system and ecosystem. The IIH clarifies that traditional anatomy has been describing the
structure of the riverbanks, while Surgical Interstitialogy focuses on the
flow within the river, which is the actual determinant of system function [
1]. Our theory thus integrates prior knowledge by explaining why dissection along these planes is effective (they are the ICN's natural interfaces) and transcends it by answering deeper questions about fluid pressure, signal propagation, and postoperative modulation. Thus, a "membrane" is redefined from a passive wall to an intelligent guardrail on a highway, shifting the surgical focus from topographical cartography to dynamic traffic engineering of the human body.
Principles of Surgical Interstitialogy
This paradigm is operationalized through three core principles:
System-Guided Navigation: Following Nature's Pathways. Surgical access is optimized by following the natural low-resistance pathways within the ICN, utilizing technologies like balloon dissection to expand these pre-formed planes, thereby minimizing trauma.
Oncological Compartmentalization: Resecting the System, Not Just the Organ. Malignancies spread within defined compartments delineated by the ICN [
5]. "Interstitialectomy"—the en-bloc resection of the tumor within its functional ICN compartment—extends the principle of total mesorectal excision, aiming to eradicate microscopic systemic invasion.
Postoperative System Rehabilitation: Engineering the Microenvironment. Recovery is actively managed by modulating the interstitial microenvironment—normalizing pressure, employing biomimetic scaffolds for functional regeneration, or leveraging the network for targeted therapy [
6].
A Call to Action: Leading the Shift to Systems Surgery
Adopting this paradigm requires a concerted global effort:
Education: Revise anatomy curricula to teach the interstitium as a dynamic functional system (the ICN).
Technology: Develop novel imaging for real-time ICN mapping and specialized instruments for system-specific dissection.
Evidence Generation: Prioritize randomized trials comparing system-based interstitial approaches against conventional techniques.
Accessibility and Standardization: Simplify and standardize system-based techniques to ensure enhanced safety and efficacy across diverse healthcare resource settings.
6. Conclusion
The advancement of surgery demands a paradigm shift. Membrane anatomy and stratigraphic surgery represent the pinnacle of refinement within the classical anatomical paradigm. Surgical Interstitialogy, with its systems perspective centered on the ICN and grounded in the physical principles of the IIH [
1], elevates the surgeon from a "navigator of planes" to an "engineer of the human body's internal microenvironment." We urge the global surgical community to shift its focus from static structures to dynamic systems and collectively pioneer the era of systems surgery.
Figure 1.
The paradigm shift in surgical anatomy. (Left) The conventional view of organs separated by static anatomical planes. (Right) The new paradigm of a dynamic interstitial system (the Interstitial-Cleft Network, ICN), a pervasive network that governs fluid flow, information conduction, and cell migration, redefining surgical anatomy and physiology.
Figure 1.
The paradigm shift in surgical anatomy. (Left) The conventional view of organs separated by static anatomical planes. (Right) The new paradigm of a dynamic interstitial system (the Interstitial-Cleft Network, ICN), a pervasive network that governs fluid flow, information conduction, and cell migration, redefining surgical anatomy and physiology.
Author Contributions
Qingbao Wang: Conceptualization, Methodology, Writing – Original Draft, Supervision, Funding Acquisition. Yi Wang: Validation, Formal Analysis, Investigation, Data Curation, Writing – Review & Editing.
Funding
This research received no external funding.
Declaration of Interests
The author declares no competing interests.
References
- Wang, Q.; Wang, Y. The Interstitial Integration Hypothesis: A Unified Physical Framework for Emergence and Self-Organization. Preprints 2025, 2025100923. [Google Scholar] [CrossRef]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci Rep 2018, 8, 4947. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Swartz, M.A. Interstitial Fluid and Lymph Formation and Transport: Physiological Regulation and Roles in Inflammation and Cancer. Physiol Rev 2012, 92, 1005–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y. A New Physiological Framework: The Human Interstitial System as the Body's Relational Matrix. Preprints 2025, 2025101531. [Google Scholar] [CrossRef]
- Heald, R.J.; Husband, E.M.; Ryall, R.D. The Mesorectum in Rectal Cancer Surgery—the Clue to Pelvic Recurrence? Br J Surg 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Tatrái, P.; Eppert, M.; Bojarski, L.; et al. The Extracellular Matrix as a Key Regulator of Intracellular Communication in the Tumor Microenvironment: Implications for Cancer Therapy. Biochim Biophys Acta Rev Cancer 2023, 1878, 188885. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).