Submitted:
16 October 2025
Posted:
17 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introductions
2. Molecular Markers of OSA and T2DM
2.1. Hypoxia-Inducible Factor-1α (HIF-1α)
2.2. Sirtuin 1 (SIRT1)
2.3. MicroRNAs
2.3.1. MicroRNA-181a
2.3.2. MicroRNA-199a
2.4. Inflammatory Markers
2.4.1. Tumor Necrosis Factor-α
2.4.2. Interleukin-6
2.4.3. C-Reactive Protein
2.5. Adipokine Markers
2.5.1. Adiponectin
2.5.2. Leptin
2.5.3. Resistin
2.5.4. Chemerin
2.5.5. Omentin-1
2.6. Other Biomarkers
2.6.1. Melatonin
2.6.2. Orexin
2.6.3. Ghrelin
3. Management of OSA in T2DM Patients
3.1. Gold Standard Treatment
3.2. Current and Emerging Therapeutic Interventions
4. Anti-Diabetic Drugs Used in OSA: Molecular Mechanisms and Clinical Evidence
4.1. GLP-1 Receptor Agonists
4.1.1. Molecular Mechanisms of Action
- o Core Molecular Signaling Pathways
- o Weight Loss-Mediated Mechanisms
- o Central Nervous System (CNS) and Respiratory Control
- o Upper Airway Muscle Tone Enhancement
- o Anti-inflammatory and Cytoprotective Effects
- o Pulmonary-Specific Mechanisms
4.1.2. Clinical Evidence:
4.2. SGLT2 Inhibitors
4.2.1. Molecular Mechanisms of Action
- o Core Molecular Signaling Pathways
- o Weight loss mechanisms
- o Cardiovascular risk reduction mechanisms
4.2.2. Clinical Evidence
4.3. Metformin
4.3.1. Molecular Mechanisms of Action
4.3.2. Clinical Evidence
5. Future Therapeutic Research Directions
6. Materials and Methods
- o Molecular mechanisms: Grouped by biomarker categories (transcription factors, inflammatory markers, adipokines, hormonal factors)
- o Therapeutic interventions: Organized by drug class with emphasis on mechanism of action and clinical evidence
- o Clinical implications: Integration of molecular insights with therapeutic potential
7. Essential Considerations
Limitations:
- o This narrative review is subject to several limitations:
- o Possible selection bias in literature identification despite a comprehensive search strategy
- o Lack of formal statistical analysis due to study heterogeneity
- o Potential publication bias favoring positive results
- o Heterogeneity in OSA diagnostic criteria and severity classification across studies
- o Limited long-term follow-up data for many interventions
Author Contributions
Funding
Conflicts of Interest
References
- Gomase, V.G.; Deshmukh, P.; Lekurwale, V.Y. Obstructive Sleep Apnea and Its Management: A Narrative Review. Cureus 2023. [Google Scholar] [CrossRef]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Akset, M.; Poppe, K.G.; Kleynen, P.; Bold, I.; Bruyneel, M. Endocrine Disorders in Obstructive Sleep Apnoea Syndrome: A Bidirectional Relationship. Clin Endocrinol (Oxf) 2023, 98, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Naughton, M.T.; Tamisier, R.; Cowie, M.R.; Bradley, T.D. Sleep Apnoea and Heart Failure. European Respiratory Journal 2022, 59. [Google Scholar] [CrossRef]
- Kalaydzhiev, P.; Poroyliev, N.; Somleva, D.; Ilieva, R.; Markov, D.; Kinova, E.; Goudev, A. Sleep Apnea in Patients with Exacerbated Heart Failure and Overweight. Sleep Med X 2023, 5. [Google Scholar] [CrossRef]
- Song, S.O.; He, K.; Narla, R.R.; Kang, H.G.; Ryu, H.U.; Boyko, E.J. Metabolic Consequences of Obstructive Sleep Apnea Especially Pertaining to Diabetes Mellitus and Insulin Sensitivity. Diabetes Metab J 2019, 43. [Google Scholar] [CrossRef]
- Shih, N.C.; Wei, J.C.C. Comparative Analysis of Diabetes Risk in Patients with Obstructive Sleep Apnea Undergoing Different Treatment Approaches. Journal of Otolaryngology - Head and Neck Surgery 2023, 52. [Google Scholar] [CrossRef]
- Afzal, H.; Butt, N.I.; Ashfaq, F.; Habib, O.; Anser, A.; Aftab, S. Obstructive Sleep Apnea in Type 2 Diabetes Mellitus. Rawal Medical Journal 2023, 48. [Google Scholar] [CrossRef]
- Andayeshgar, B.; Janatolmakan, M.; Soroush, A.; Azizi, S.M.; Khatony, A. The Prevalence of Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sleep Sci Pract 2022, 6. [Google Scholar] [CrossRef]
- Van Dijk, M.; Donga, E.; Van Dijk, J.G.; Lammers, G.J.; Van Kralingen, K.W.; Dekkers, O.M.; Corssmit, E.P.M.; Romijn, J.A. Disturbed Subjective Sleep Characteristics in Adult Patients with Long-Standing Type 1 Diabetes Mellitus. Diabetologia 2011, 54. [Google Scholar] [CrossRef]
- de Mattos, A.C.M.T.; Campos, Y.S.; Fiorini, V.O.; Sab, Y.; Tavares, B.L.; Velarde, L.G.C.; Lima, G.A.B.; Filho, R.A. da C. Relationship between Sleep Disturbances, Lipid Profile and Insulin Sensitivity in Type 1 Diabetic Patients: A Cross-Sectional Study. Arch Endocrinol Metab 2020, 64. [Google Scholar] [CrossRef]
- Tan, H.L.; Babwah, F.; Waheed, N.; Butt, M.I. Obstructive Sleep Apnoea and Type 1 Diabetes Mellitus. British Journal of Diabetes and Vascular Disease 2015, 15. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Rolim, L.C. de S.P.; Sá, J.R. de; Poyares, D.; Tufik, S.; Silva, A.B.; Dib, S.A. Cardiovascular Autonomic Neuropathy Contributes to Sleep Apnea in Young and Lean Type 1 Diabetes Mellitus Patients. Front Endocrinol (Lausanne) 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.L.; Perfect, M.M.; Janovsky, C.C.P.S.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep Characteristics in Type 1 Diabetes and Associations with Glycemic Control: Systematic Review and Meta-Analysis. Sleep Med 2016, 23. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, P.; Wang, L.; Wang, D.; Sun, K.; Ma, Y.; Wang, H.; Xu, C.; Zhang, R.; Zhang, X.; et al. Prevalence of Obstructive Sleep Apnea Syndrome in Hospitalized Patients with Type 2 Diabetes in Beijing, China. J Diabetes Investig 2022, 13. [Google Scholar] [CrossRef]
- Jeon, B.; Luyster, F.S.; Sereika, S.M.; DiNardo, M.M.; Callan, J.A.; Chasens, E.R. Comorbid Obstructive Sleep Apnea and Insomnia and Its Associations with Mood and Diabetes-Related Distress in Type 2 Diabetes Mellitus. Journal of Clinical Sleep Medicine 2022, 18. [Google Scholar] [CrossRef]
- Nagayoshi, M.; Punjabi, N.M.; Selvin, E.; Pankow, J.S.; Shahar, E.; Iso, H.; Folsom, A.R.; Lutsey, P.L. Obstructive Sleep Apnea and Incident Type 2 Diabetes. Sleep Med 2016, 25. [Google Scholar] [CrossRef]
- Gabryelska, A.; Chrzanowski, J.; Sochal, M.; Kaczmarski, P.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Czupryniak, L.; Białasiewicz, P. Nocturnal Oxygen Saturation Parameters as Independent Risk Factors for Type 2 Diabetes Mellitus among Obstructive Sleep Apnea Patients. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Fallahi, A.; Jamil, D.I.; Karimi, E.B.; Baghi, V.; Gheshlagh, R.G. Prevalence of Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes and Metabolic Syndrome: Clinical Research and Reviews 2019, 13. [Google Scholar] [CrossRef]
- Worku, A.; Ayele, E.; Alemu, S.; Legese, G.L.; Yimam, S.M.; Kassaw, G.; Diress, M.; Asres, M.S. Obstructive Sleep Apnea Risk and Determinant Factors among Type 2 Diabetes Mellitus Patients at the Chronic Illness Clinic of the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Siddiquee, A.T.; Kim, S.; Thomas, R.J.; Lee, M.H.; Lee, S.K.; Shin, C. Obstructive Sleep Apnoea and Long-Term Risk of Incident Diabetes in the Middle-Aged and Older General Population. ERJ Open Res 2023, 9. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.; Miao, Y.; Zhang, Q. Obstructive Sleep Apnea, Prediabetes and Progression of Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Diabetes Investig 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Sweed, R.A.; Wahab, N.H.A. El; El Hooshy, M.S.; Morsy, E.Y.; Shetta, D.M. Obstructive Sleep Apnea in Patients with Type 2 Diabetes Mellitus in Egyptian Population. The Egyptian Journal of Bronchology 2023, 17. [Google Scholar] [CrossRef]
- Balkau, B.; Vol, S.; Loko, S.; Andriamboavonjy, T.; Lantieri, O.; Gusto, G.; Meslier, N.; Racineux, J.-L.; Tichet, J. High Baseline Insulin Levels Associated With 6-Year Incident Observed Sleep Apnea. Diabetes Care 2010, 33, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Bahnasy, W.S.; El-Heneedy, Y.A.E.; El-Seidy, E.A.S.; Labib, N.A.A.; Ibrahim, I.S.E. Sleep Disturbances in Diabetic Peripheral Neuropathy Patients: A Clinical and Polysomnographic Study. Egypt J Neurol Psychiatr Neurosurg 2018, 54, 23. [Google Scholar] [CrossRef]
- Huang, L.E.; Bunn, H.F. Hypoxia-Inducible Factor and Its Biomedical Relevance. Journal of Biological Chemistry 2003, 278, 19575–19578. [Google Scholar] [CrossRef]
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia Regulates Hypoxia-Inducible Factor-1α Protein Stability and Function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef]
- Shao, Y.; Lv, C.; Yuan, Q.; Wang, Q. Levels of Serum 25(OH)VD3, HIF-1 α, VEGF, VWF, and IGF-1 and Their Correlation in Type 2 Diabetes Patients with Different Urine Albumin Creatinine Ratio. J Diabetes Res 2016, 2016. [Google Scholar] [CrossRef]
- Discher, D.J.; Bishopric, N.H.; Wu, X.; Peterson, C.A.; Webster, K.A. Hypoxia Regulates β-Enolase and Pyruvate Kinase-M Promoters by Modulating Sp1/Sp3 Binding to a Conserved GC Element. Journal of Biological Chemistry 1998, 273, 26087–26093. [Google Scholar] [CrossRef]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-Inducible Factor 1. Journal of Biological Chemistry 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc Natl Acad Sci U S A 1995, 92. [Google Scholar] [CrossRef]
- Badawi, Y.; Shi, H. Relative Contribution of Prolyl Hydroxylase-Dependent and -Independent Degradation of HIF-1alpha by Proteasomal Pathways in Cerebral Ischemia. Front Neurosci 2017, 11. [Google Scholar] [CrossRef]
- Gabryelska, A.; Białasiewicz, P. Association between Excessive Daytime Sleepiness, REM Phenotype and Severity of Obstructive Sleep Apnea. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Kai, J.; Wang, F.; Wang, Z.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Wang, S.; et al. HIF-1α-Upregulated LncRNA-H19 Regulates Lipid Droplet Metabolism through the AMPKα Pathway in Hepatic Stellate Cells. Life Sci 2020, 255. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Finger, E.C.; Krieg, A.J.; Wu, C.; Diep, A.N.; Lagory, E.L.; Wei, K.; McGinnis, L.M.; Yuan, J.; Kuo, C.J.; et al. Cross-Talk between Hypoxia and Insulin Signaling through Phd3 Regulates Hepatic Glucose and Lipid Metabolism and Ameliorates Diabetes. Nat Med 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Rha, J.; Selak, M.A.; Unger, T.L.; Keith, B.; Liu, Q.; Haase, V.H. Hypoxia-Inducible Factor 2 Regulates Hepatic Lipid Metabolism. Mol Cell Biol 2009, 29. [Google Scholar] [CrossRef]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional Regulation of Genes Encoding Glycolytic Enzymes by Hypoxia-Inducible Factor 1. Journal of Biological Chemistry 1994, 269, 23757–23763. [Google Scholar] [CrossRef]
- Minchenko, A.; Leshchinsky, I.; Opentanova, I.; Sang, N.; Srinivas, V.; Armstead, V.; Caro, J. Hypoxia-Inducible Factor-1-Mediated Expression of the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase-3 (PFKFB3) Gene: Its Possible Role in the Warburg Effect. Journal of Biological Chemistry 2002, 277. [Google Scholar] [CrossRef]
- Hackenbeck, T.; Huber, R.; Schietke, R.; Knaup, K.X.; Monti, J.; Wu, X.; Klanke, B.; Frey, B.; Gaipl, U.; Wullich, B.; et al. The GTPase RAB20 Is a HIF Target with Mitochondrial Localization Mediating Apoptosis in Hypoxia. Biochim Biophys Acta Mol Cell Res 2011, 1813. [Google Scholar] [CrossRef]
- Li, Y.; Miao, L.Y.; Xiao, Y.L.; Huang, M.; Yu, M.; Meng, K.; Cai, H.R. Hypoxia Induced High Expression of Thioredoxin Interacting Protein (TXNIP) in Non-Small Cell Lung Cancer and Its Prognostic Effect. Asian Pacific Journal of Cancer Prevention 2015, 16. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C. V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Padwad, Y.S. HIF-1 in Cancer Therapy: Two Decade Long Story of a Transcription Factor. Acta Oncol (Madr) 2017, 56. [Google Scholar] [CrossRef] [PubMed]
- Graven, K.K.; Yu, Q.; Pan, D.; Roncarati, J.S.; Farber, H.W. Identification of an Oxygen Responsive Enhancer Element in the Glyceraldehyde-3-Phosphate Dehydrogenase Gene. Biochimica et Biophysica Acta - Gene Structure and Expression 1999, 1447. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, W.S.; Ratcliffe, P.; Harris, A.L. Expression of the Hypoxia-Inducible and Tumor-Associated Carbonic Anhydrases in Ductal Carcinoma in Situ of the Breast. American Journal of Pathology 2001, 158. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front Physiol 2020, 11. [Google Scholar] [CrossRef]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Haneda, M. Loss of HIF-1α Impairs GLUT4 Translocation and Glucose Uptake by the Skeletal Muscle Cells. Am J Physiol Endocrinol Metab 2014, 306. [Google Scholar] [CrossRef]
- Görgens, S.W.; Benninghoff, T.; Eckardt, K.; Springer, C.; Chadt, A.; Melior, A.; Wefers, J.; Cramer, A.; Jensen, J.; Birkeland, K.I.; et al. Hypoxia in Combination With Muscle Contraction Improves Insulin Action and Glucose Metabolism in Human Skeletal Muscle via the HIF-1a Pathway. Diabetes 2017, 66. [Google Scholar] [CrossRef]
- Thomas, A.; Belaidi, E.; Moulin, S.; Horman, S.; van der Zon, G.C.; Viollet, B.; Levy, P.; Bertrand, L.; Pepin, J.-L.; Godin-Ribuot, D.; et al. Chronic Intermittent Hypoxia Impairs Insulin Sensitivity but Improves Whole-Body Glucose Tolerance by Activating Skeletal Muscle AMPK. Diabetes 2017, 66, 2942–2951. [Google Scholar] [CrossRef]
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr Drug Targets 2017, 18. [Google Scholar] [CrossRef]
- Karuga, F.F.; Kaczmarski, P.; Sochal, M.; Szmyd, B.; Białasiewicz, P.; Gabryelska, A. Cross-Sectional Analysis of Hypoxia-Regulated MiRNA-181a, MiRNA-199a, HIF-1α, and SIRT1 in the Development of Type 2 Diabetes in Patients with Obstructive Sleep Apnea—Preliminary Study. J Clin Med 2024, 13, 7644. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating MicroRNA Profile as a Potential Biomarker for Obstructive Sleep Apnea Diagnosis. Sci Rep 2019, 9, 13456. [Google Scholar] [CrossRef]
- Karuga, F.F.; Jaromirska, J.; Malicki, M.; Sochal, M.; Szmyd, B.; Białasiewicz, P.; Strzelecki, D.; Gabryelska, A. The Role of MicroRNAs in Pathophysiology and Diagnostics of Metabolic Complications in Obstructive Sleep Apnea Patients. Front Mol Neurosci 2023, 16. [Google Scholar] [CrossRef]
- Gu, N.; You, L.; Shi, C.; Yang, L.; Pang, L.; Cui, X.; Ji, C.; Zheng, W.; Guo, X. Expression of MiR-199a-3p in Human Adipocytes Is Regulated by Free Fatty Acids and Adipokines. Mol Med Rep 2016, 14, 1180–1186. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem 2018, 119. [Google Scholar] [CrossRef] [PubMed]
- Alzamil, H. Elevated Serum TNF- α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J Obes 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.; Jayaraman, A.; Ahmad, J.; Joshi, S.; Yerra, S.K. TNF-Alpha −308G/A and −238G/A Polymorphisms and Its Protein Network Associated with Type 2 Diabetes Mellitus. Saudi J Biol Sci 2017, 24, 1195–1203. [Google Scholar] [CrossRef]
- Patel, R.; Palit, S.P.; Rathwa, N.; Ramachandran, A.V.; Begum, R. Genetic Variants of Tumor Necrosis Factor-α and Its Levels: A Correlation with Dyslipidemia and Type 2 Diabetes Susceptibility. Clinical Nutrition 2019, 38, 1414–1422. [Google Scholar] [CrossRef]
- Bowker, N.; Shah, R.L.; Sharp, S.J.; Luan, J.; Stewart, I.D.; Wheeler, E.; Ferreira, M.A.R.; Baras, A.; Wareham, N.J.; Langenberg, C.; et al. Meta-Analysis Investigating the Role of Interleukin-6 Mediated Inflammation in Type 2 Diabetes. EBioMedicine 2020, 61, 103062. [Google Scholar] [CrossRef]
- Kreiner, F.F.; Kraaijenhof, J.M.; von Herrath, M.; Hovingh, G.K.K.; von Scholten, B.J. Interleukin 6 in Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: Mechanisms and Therapeutic Perspectives. Expert Rev Clin Immunol 2022, 18, 377–389. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Abd-Elrahman, M.Z.; Bahriz, R.; Albehairy, A. Inflammatory Cytokine and Plasma C-Reactive Protein Response to Ketoacidosis in Adults with Type 1 Diabetes: Egyptian Multicenter Study. Egypt J Intern Med 2020, 32. [Google Scholar] [CrossRef]
- Lam, D.C.L.; Lam, K.S.L.; Ip, M.S.M. Obstructive Sleep Apnoea, Insulin Resistance and Adipocytokines. Clin Endocrinol (Oxf) 2015, 82, 165–177. [Google Scholar] [CrossRef]
- Chen, B.; Lam, K.S.L.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.C.; Zhang, J.; Xu, A. Hypoxia Dysregulates the Production of Adiponectin and Plasminogen Activator Inhibitor-1 Independent of Reactive Oxygen Species in Adipocytes. Biochem Biophys Res Commun 2006, 341. [Google Scholar] [CrossRef]
- Lindberg, S.; Jensen, J.S.; Bjerre, M.; Pedersen, S.H.; Frystyk, J.; Flyvbjerg, A.; Galatius, S.; Jeppesen, J.; Mogelvang, R. Adiponectin, Type 2 Diabetes and Cardiovascular Risk. Eur J Prev Cardiol 2015, 22, 276–283. [Google Scholar] [CrossRef]
- Berger, S.; Polotsky, V.Y. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxid Med Cell Longev 2018, 2018. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, Cardiovascular Diseases and Type 2 Diabetes Mellitus. Acta Pharmacol Sin 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Abed, B.A.; Farhan, L.O.; Dawood, A.S. Relationship between Serum Nesfatin-1, Adiponectin, Resistin Concentration, and Obesity with Type 2 Diabetes Mellitus. Baghdad Science Journal 2023, 21. [Google Scholar] [CrossRef]
- Cherneva, R.V.; Georgiev, O.B.; Petrova, D.S.; Mondeshki, T.L.; Ruseva, S.R.; Cakova, A.D.; Mitev, V.I. Resistin - the Link between Adipose Tissue Dysfunction and Insulin Resistance in Patients with Obstructive Sleep Apnea. J Diabetes Metab Disord 2013, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W. Trash Talk by Fat. Hypertension 2015, 66, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum Omentin Levels in Patients with Obstructive Sleep Apnea. Sleep and Breathing 2014, 18, 391–395. [Google Scholar] [CrossRef]
- Zhang, D.; Pang, X.; Huang, R.; Gong, F.; Zhong, X.; Xiao, Y. Adiponectin, Omentin, Ghrelin, and Visfatin Levels in Obese Patients with Severe Obstructive Sleep Apnea. Biomed Res Int 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- She, N.; Liu, N.; Ren, X.; Liu, H. Association between Omentin and Obstructive Sleep Apnea: A Meta-analysis. Clin Respir J 2023, 17, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Von Frankenberg, A.D.; Reis, A.F.; Gerchman, F. Relationships between Adiponectin Levels, the Metabolic Syndrome, and Type 2 Diabetes: A Literature Review. Arch Endocrinol Metab 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yan, B.; Yang, D.; Guo, J.; Wang, C.; Zhang, Q.; Shi, Y.; Shi, X.; Tian, G.; Liang, X. Serum Adiponectin Levels Are Positively Associated With Diabetic Peripheral Neuropathy in Chinese Patients With Type 2 Diabetes. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.; Jain, S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci 2017, 18, 1321. [Google Scholar] [CrossRef]
- Huang, K.; Liang, Y.; Ma, Y.; Wu, J.; Luo, H.; Yi, B. The Variation and Correlation of Serum Adiponectin, Nesfatin-1, IL-6, and TNF-α Levels in Prediabetes. Front Endocrinol (Lausanne) 2022, 13. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Horm Mol Biol Clin Investig 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Ciriello, J.; Moreau, J.M.; Caverson, M.M.; Moranis, R. Leptin: A Potential Link Between Obstructive Sleep Apnea and Obesity. Front Physiol 2022, 12. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J. Effects of Different Obesity-Related Adipokines on the Occurrence of Obstructive Sleep Apnea. Endocr J 2020, 67, 485–500. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum Leptin, Resistin, and Adiponectin Levels in Obese and Non-Obese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Polonio-González, M.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.; Gimeno-Orna, J.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int J Mol Sci 2024, 25, 2338. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-Diabetes Is Associated with Elevated Levels of TNF-Alpha, IL-6 and Adiponectin and Low Levels of Leptin in a Population of Mexican Americans: A Cross-Sectional Study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Gavrilova, O.; Yakar, S.; Jou, W.; Pack, S.; Asghar, Z.; Wheeler, M.B.; LeRoith, D. Leptin Improves Insulin Resistance and Hyperglycemia in a Mouse Model of Type 2 Diabetes. Endocrinology 2005, 146, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Bidulescu, A.; Dinh, P.C.; Sarwary, S.; Forsyth, E.; Luetke, M.C.; King, D.B.; Liu, J.; Davis, S.K.; Correa, A. Associations of Leptin and Adiponectin with Incident Type 2 Diabetes and Interactions among African Americans: The Jackson Heart Study. BMC Endocr Disord 2020, 20. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the Crossroads of Inflammation and Obesity. Trends in Endocrinology & Metabolism 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Zhou, C.; Jia, X.; Kang, J. Elevated Levels of Serum Chemerin in Patients with Obstructive Sleep Apnea Syndrome. Biomarkers 2012, 17, 248–253. [Google Scholar] [CrossRef]
- Senthilkumar, G.P.; Anithalekshmi, M.S.; Yasir, Md.; Parameswaran, S.; Packirisamy, R. muthu; Bobby, Z. Role of Omentin 1 and IL-6 in Type 2 Diabetes Mellitus Patients with Diabetic Nephropathy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2018, 12, 23–26. [Google Scholar] [CrossRef]
- Karel, P.; Schilperoord, M.; Reichman, L.J.A.; Krabbe, J.G. The Dark Side of Apnea: Altered 24-Hour Melatonin Secretion in Obstructive Sleep Apnea (OSAS) Is Disease Severity Dependent. Sleep and Breathing 2024, 28, 1751–1759. [Google Scholar] [CrossRef]
- Ramracheya, R.D.; Muller, D.S.; Squires, P.E.; Brereton, H.; Sugden, D.; Huang, G.C.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Function and Expression of Melatonin Receptors on Human Pancreatic Islets. J Pineal Res 2008, 44. [Google Scholar] [CrossRef]
- Patel, R.; Parmar, N.; Pramanik Palit, S.; Rathwa, N.; Ramachandran, A.V.; Begum, R. Diabetes Mellitus and Melatonin: Where Are We? Biochimie 2022, 202, 2–14. [Google Scholar] [CrossRef]
- Gestreau, C.; Bévengut, M.; Dutschmann, M. The Dual Role of the Orexin/Hypocretin System in Modulating Wakefulness and Respiratory Drive. Curr Opin Pulm Med 2008, 14. [Google Scholar] [CrossRef]
- Polito, R.; Francavilla, V.C.; Ambrosi, A.; Tartaglia, N.; Tafuri, D.; Monda, M.; Messina, A.; Sessa, F.; Di Maio, G.; Ametta, A.; et al. The Orexin-A Serum Levels Are Strongly Modulated by Physical Activity Intervention in Diabetes Mellitus Patients. In Proceedings of the Journal of Human Sport and Exercise - 2020 - Winter Conferences of Sports Science; Universidad de Alicante, 2020; Vol. 15.
- Zarifkar, M.; Noshad, S.; Shahriari, M.; Afarideh, M.; Khajeh, E.; Karimi, Z.; Ghajar, A.; Esteghamati, A. Inverse Association of Peripheral Orexin-A with Insulin Resistance in Type 2 Diabetes Mellitus: A Randomized Clinical Trial. The Review of Diabetic Studies 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Lotfy, M.; D’Souza, C.; Alseiari, S.M.; Alsaadi, A.A.; Qahtan, S.A. Hypocretin/Orexin Modulates Body Weight and the Metabolism of Glucose and Insulin. Diabetes Metab Res Rev 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, I.; Sadeghi, M.; Tajmiri, G.; Brühl, A.B.; Sadeghi Bahmani, L.; Brand, S. Evaluation of Blood Levels of Omentin-1 and Orexin-A in Adults with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Life 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Pradhan, G.; Zhou, Y.; Xue, B.; Sun, Y. Diverse and Complementary Effects of Ghrelin and Obestatin. Biomolecules 2022, 12, 517. [Google Scholar] [CrossRef]
- Dezaki, K.; Sone, H.; Yada, T. Ghrelin Is a Physiological Regulator of Insulin Release in Pancreatic Islets and Glucose Homeostasis. Pharmacol Ther 2008, 118, 239–249. [Google Scholar] [CrossRef]
- Pardak, P.; Filip, R.; Woliński, J. The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight. J Clin Med 2022, 11, 2032. [Google Scholar] [CrossRef]
- Lindqvist, A.; Shcherbina, L.; Prasad, R.B.; Miskelly, M.G.; Abels, M.; Martínez-Lopéz, J.A.; Fred, R.G.; Nergård, B.J.; Hedenbro, J.; Groop, L.; et al. Ghrelin Suppresses Insulin Secretion in Human Islets and Type 2 Diabetes Patients Have Diminished Islet Ghrelin Cell Number and Lower Plasma Ghrelin Levels. Mol Cell Endocrinol 2020, 511, 110835. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine 2017, 13. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. Journal of Clinical Sleep Medicine 2019, 15, 301–334. [Google Scholar] [CrossRef]
- Liu, T.; Li, W.; Zhou, H.; Wang, Z. Verifying the Relative Efficacy between Continuous Positive Airway Pressure Therapy and Its Alternatives for Obstructive Sleep Apnea: A Network Meta-Analysis. Front Neurol 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Uniken Venema, J.A.M.; Doff, M.H.J.; Joffe-Sokolova, D.; Wijkstra, P.J.; van der Hoeven, J.H.; Stegenga, B.; Hoekema, A. Long-Term Obstructive Sleep Apnea Therapy: A 10-Year Follow-up of Mandibular Advancement Device and Continuous Positive Airway Pressure. Journal of Clinical Sleep Medicine 2020, 16, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, S.; Wang, J.; Chen, R. Predictors of the Efficacy for Daytime Sleepiness in Patients With Obstructive Sleep Apnea With Continual Positive Airway Pressure Therapy: A Meta-Analysis of Randomized Controlled Trials. Front Neurol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Amendolara, M.; Di Lecce, V.; Santomasi, C.; Quaranta, V.N.; Portacci, A.; Lazzaretti, I.D.; Cuccaro, L.A.S.; Casparrini, M.; Spierto, S.; Picerno, V.; et al. The Impact of PAP Therapy First Impression on Short-Term Treatment Adherence. Sleep and Breathing 2025, 29, 152. [Google Scholar] [CrossRef]
- Missey, F.; Ejneby, M.S.; Ngom, I.; Donahue, M.J.; Trajlinek, J.; Acerbo, E.; Botzanowski, B.; Cassarà, A.M.; Neufeld, E.; Glowacki, E.D.; et al. Obstructive Sleep Apnea Improves with Non-Invasive Hypoglossal Nerve Stimulation Using Temporal Interference. Bioelectron Med 2023, 9, 18. [Google Scholar] [CrossRef]
- Thuler, E.R.; Rabelo, F.A.W.; Santos Junior, V.; Kayamori, F.; Bianchini, E.M.G. Hypoglossal Nerve Trunk Stimulation: Electromyography Findings during Drug-Induced Sleep Endoscopy: A Case Report. J Med Case Rep 2023, 17, 187. [Google Scholar] [CrossRef]
- Olson, M.D.; Junna, M.R. Hypoglossal Nerve Stimulation Therapy for the Treatment of Obstructive Sleep Apnea. Neurotherapeutics 2021, 18, 91–99. [Google Scholar] [CrossRef]
- Lorenzi-Filho, G.; Almeida, F.R.; Strollo, P.J. Treating <scp>OSA</Scp> : <scp>C</Scp> Urrent and Emerging Therapies beyond <scp>CPAP</Scp>. Respirology 2017, 22, 1500–1507. [Google Scholar] [CrossRef]
- Sultana, R.; Sissoho, F.; Kaushik, V.P.; Raji, M.A. The Case for Early Use of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Patients with Comorbid Diabetes and Metabolic Syndrome. Life 2022, 12, 1222. [Google Scholar] [CrossRef]
- Malhotra, A.; Grunstein, R.R.; Fietze, I.; Weaver, T.E.; Redline, S.; Azarbarzin, A.; Sands, S.A.; Schwab, R.J.; Dunn, J.P.; Chakladar, S.; et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. New England Journal of Medicine 2024, 391, 1193–1205. [Google Scholar] [CrossRef]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of Liraglutide 3.0 Mg in Individuals with Obesity and Moderate or Severe Obstructive Sleep Apnea: The SCALE Sleep Apnea Randomized Clinical Trial. Int J Obes 2016, 40, 1310–1319. [Google Scholar] [CrossRef]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, S.; Yu, X.; Wei, Q.; Yu, F.; Tong, J. DAHOS Study: Efficacy of Dapagliflozin in Treating Heart Failure with Reduced Ejection Fraction and Obstructive Sleep Apnea Syndrome — A 3-Month, Multicenter, Randomized Controlled Clinical Trial. Eur J Clin Pharmacol 2024, 80, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; van Egmond, L.; Chapman, C.D.; Cedernaes, J.; Benedict, C. Aiding Sleep in Type 2 Diabetes: Therapeutic Considerations. Lancet Diabetes Endocrinol 2018, 6, 60–68. [Google Scholar] [CrossRef]
- Soreca, I.; Arnold, N.; Dombrovski, A.Y. Bright Light Therapy for CPAP-Resistant OSA Symptoms. Journal of Clinical Sleep Medicine 2024, 20, 211–219. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Gajewski, A.; Jaromirska, J.; Strzelecki, D.; Białasiewicz, P.; Chałubiński, M.; Sochal, M. Assessment of Continuous Positive Airway Pressure Effect on the Circadian Clock Signaling Pathway in Obstructive Sleep Apnea Patients. Sci Rep 2025, 15, 11273. [Google Scholar] [CrossRef]
- Papaetis, G. GLP-1 Receptor Agonists, SGLT-2 Inhibitors, and Obstructive Sleep Apnoea: Can New Allies Face an Old Enemy? Archives of Medical Science – Atherosclerotic Diseases 2023, 8, 19–34. [Google Scholar] [CrossRef]
- Kusunoki, M.; Hisano, F.; Wakazono, N.; Tsutsumi, K.; Oshida, Y.; Miyata, T. Effect of Treatment With Sodium-Glucose Cotransporter 2 Inhibitor on the Initiation of Continuous Positive Airway Pressure Therapy in Type 2 Diabetic Patients With Obstructive Sleep Apnea Syndrome. J Clin Med Res 2021, 13, 497–501. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Patel, S.R.; Strollo, P.J.; Cronin, J.; Yee, J.; Pho, H.; Werner, A.; Farkas, R. Aroxybutynin and Atomoxetine (AD109) for the Treatment of Obstructive Sleep Apnea: Rationale, Design and Baseline Characteristics of the Phase 3 Clinical Trials. Contemp Clin Trials Commun 2025, 47. [Google Scholar] [CrossRef]
- Mason, M.; Welsh, E.J.; Smith, I. Drug Therapy for Obstructive Sleep Apnoea in Adults. Cochrane Database of Systematic Reviews 2013, 2013. [Google Scholar] [CrossRef]
- Dragonieri, S.; Portacci, A.; Quaranta, V.N.; Carratu, P.; Lazar, Z.; Carpagnano, G.E.; Bikov, A. Therapeutic Potential of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Syndrome Management: A Narrative Review. Diseases 2024, 12, 224. [Google Scholar] [CrossRef]
- Janić, M.; Škrgat, S.; Harlander, M.; Lunder, M.; Janež, A.; Pantea Stoian, A.; El-Tanani, M.; Maggio, V.; Rizzo, M. Potential Use of GLP-1 and GIP/GLP-1 Receptor Agonists for Respiratory Disorders: Where Are We At? Medicina (B Aires) 2024, 60, 2030. [Google Scholar] [CrossRef]
- Yu, M.; Wang, R.; Pei, L.; Zhang, X.; Wei, J.; Wen, Y.; Liu, H.; Ye, H.; Wang, J.; Wang, L. The Relationship between the Use of GLP-1 Receptor Agonists and the Incidence of Respiratory Illness: A Meta-Analysis of Randomized Controlled Trials. Diabetol Metab Syndr 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Tirzepatide Reduced Sleep Apnea Severity by up to Nearly Two-Thirds in Adults with Obstructive Sleep Apnea (OSA) and Obesity Secondary Endpoint-Percent Change in AHI from Baseline Tirzepatide*-55.0 %-50.7 % Placebo-5.0 %-3.0 % Secondary Endpoint-Percent Change in Body Weight from Baseline Tirzepatide*-18.1 %-17.7 % Placebo-1.3 %-1.6 % SURMOUNT-OSA Study 2 Participants Used PAP Therapy Efficacy Estimand Results at 52 Weeks Treatment-Regimen Estimand Results at 52 Weeks; 2024.
- Jiang, W.; Li, W.; Cheng, J.; Li, W.; Cheng, F. Efficacy and Safety of Liraglutide in Patients with Type 2 Diabetes Mellitus and Severe Obstructive Sleep Apnea. Sleep and Breathing 2023, 27, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Crilly, S.; O’Mahony, A.; O’Riordan, B.; Traynor, M.; Gitau, R.; McDonald, K.; Ledwidge, M.; O’Shea, D.; Murphy, D.J.; et al. Continuous Positive Airway Pressure but Not GLP1-Mediated Weight Loss Improves Early Cardiovascular Disease in Obstructive Sleep Apnea: A Randomized Proof-of-Concept Study. Ann Am Thorac Soc 2024, 21, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Sprung, V.S.; Kemp, G.J.; Wilding, J.P.; Adams, V.; Murphy, K.; Burgess, M.; Emegbo, S.; Thomas, M.; Needham, A.J.; Weimken, A.; et al. Randomised, COntrolled Multicentre Trial of 26 Weeks Subcutaneous Liraglutide (a Glucagon-like Peptide-1 Receptor Agonist), with or without ContiNuous Positive Airway Pressure (CPAP), in Patients with Type 2 Diabetes Mellitus (T2DM) and Obstructive Sleep ApnoEa (OSA) (ROMANCE): Study Protocol Assessing the Effects of Weight Loss on the Apnea–Hypnoea Index (AHI). BMJ Open 2020, 10, e038856. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Abreu, C.; Castro, J.C.; Alcarria, E.; Cruz-Bravo, M.; Garcia-Llorente, M.J.; Albornos, C.; Moreno, C.; Cepeda, M.; Almodóvar, F. An Association between Liraglutide Treatment and Reduction in Excessive Daytime Sleepiness in Obese Subjects with Type 2 Diabetes. BMC Endocr Disord 2015, 15, 78. [Google Scholar] [CrossRef]
- Baser, O.; Lu, Y.; Chen, S.; Chen, S.; Baser, E. Tirzepatide and Semaglutide for the Treatment of Obstructive Sleep Apnea and Obesity: A Retrospective Analysis. Med Res Arch 2024, 13. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef]
- Peng, Y.; Qin, D.; Wang, Y.; Xue, L.; Qin, Y.; Xu, X. The Effect of SGLT-2 Inhibitors on Cardiorespiratory Fitness Capacity: A Systematic Review and Meta-Analysis. Front Physiol 2023, 13. [Google Scholar] [CrossRef]
- Abdelmasih, R.; Abdelmaseih, R.; Thakker, R.; Faluk, M.; Ali, A.; Alsamman, M.M.; Hasan, S.M. Update on the Cardiovascular Benefits of Sodium-Glucose Co-Transporter-2 Inhibitors: Mechanism of Action, Available Agents and Comprehensive Review of Literature. Cardiol Res 2021, 12, 210–218. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl Sci 2020, 5, 632–644. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Hajdys, J.; Radzioch, E.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Cardiovascular Diseases: Therapeutic Potential of SGLT-2 Inhibitors. Biomedicines 2023, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. Molecular Determinants of Renal Glucose Reabsorption. Focus on “Glucose Transport by Human Renal Na + / <scp>d</Scp> -Glucose Cotransporters SGLT1 and SGLT2.”. American Journal of Physiology-Cell Physiology 2011, 300, C6–C8. [Google Scholar] [CrossRef]
- Chavda, V.; Vashi, R.; Patel, S. Cerebrovascular Complications of Diabetes: SGLT-2 Inhibitors as a Promising Future Therapeutics. Curr Drug Targets 2021, 22, 1629–1636. [Google Scholar] [CrossRef]
- Vrhovac, I.; Eror, D.B.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na+-D-Glucose Cotransporters SGLT1 and SGLT2 in Human Kidney and of SGLT1 in Human Small Intestine, Liver, Lung, and Heart. Pflugers Arch 2015, 467. [Google Scholar] [CrossRef]
- Miller, E.M. Elements for Success in Managing Type 2 Diabetes With SGLT-2 Inhibitors: Role of the Kidney in Glucose Homeostasis: Implications for SGLT-2 Inhibition in the Treatment of Type 2 Diabetes Mellitus. J Fam Pract 2017, 66. [Google Scholar]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Kolesnik, E.; Scherr, D.; Rohrer, U.; Benedikt, M.; Manninger, M.; Sourij, H.; von Lewinski, D. SGLT2 Inhibitors and Their Antiarrhythmic Properties. Int J Mol Sci 2022, 23, 1678. [Google Scholar] [CrossRef]
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z.I. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu Rev Med 2023, 74, 369–384. [Google Scholar] [CrossRef]
- Green, J.B.; McCullough, P.A. Roles for SGLT2 Inhibitors in Cardiorenal Disease. Cardiorenal Med 2022, 12, 81–93. [Google Scholar] [CrossRef]
- Sy, G.L.L.; Te, M.T.; Payawal, D.A. Effect of SGLT 2 Inhibitors on Reducing Liver Enzymes: A Meta-Analysis. J Gastroenterol Hepatol 2021, 36. [Google Scholar]
- Krishnan, R.; Subramanian, R.; Selvarajan, R. SGLT2 Inhibitor: A Cardio-Renal Metabolic Pill. Int J Health Sci Res 2023, 13, 133–141. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-M.; Feng, S.-T.; Wen, Y.; Tang, T.-T.; Wang, B.; Liu, B.-C. Cardiorenal Protection of SGLT2 Inhibitors—Perspectives from Metabolic Reprogramming. EBioMedicine 2022, 83, 104215. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 143. [Google Scholar] [CrossRef]
- Qiu, M.; Ding, L.-L.; Zhan, Z.-L.; Liu, S.-Y. Use of SGLT2 Inhibitors and Occurrence of Noninfectious Respiratory Disorders: A Meta-Analysis of Large Randomized Trials of SGLT2 Inhibitors. Endocrine 2021, 73, 31–36. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu Rev Physiol 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Feder, D.; de Fatima Veiga Gouveia, M.R.; Govato, T.C.P.; Nassis, C.D.Z. SGLT2 Inhibitors and the Mechanisms Involved in Weight Loss. Curr Pharmacol Rep 2020, 6, 346–353. [Google Scholar] [CrossRef]
- Elian, V.I.; Dragomirescu, L.; Cheta, D.M.; Stoian, A.P.; Serafinceanu, C. Weight Loss Improves Metabolic Status in Overweight and Obese Subjects. Diabetes 2014, 63. [Google Scholar]
- Serafinceanu, C.; Crăciun, A.M.; Dobjanschi, C.; Elian, V. Sglt2 Inhibitors – Is the Paradigm in Type 2 Diabetes Mellitus Management Changing ? Rom J Diabetes Nutr Metab Dis 2014, 21, 261–265. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Karampelas, O.; Pircalabioru, G.G.; Radulian, G.; Musat, M. Risks and Benefits of SGLT-2 Inhibitors for Type 1 Diabetes Patients Using Automated Insulin Delivery Systems—A Literature Review. Int J Mol Sci 2024, 25, 1972. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, Q.; Bai, X.-Y.; Zhou, Y.-F.; Zhou, Q.-L.; Zhang, M. Effect of Dapagliflozin on Obstructive Sleep Apnea in Patients with Type 2 Diabetes: A Preliminary Study. Nutr Diabetes 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Armentaro, G.; Pelaia, C.; Condoleo, V.; Severini, G.; Crudo, G.; De Marco, M.; Pastura, C.A.; Tallarico, V.; Pezzella, R.; Aiello, D.; et al. Effect of SGLT2-Inhibitors on Polygraphic Parameters in Elderly Patients Affected by Heart Failure, Type 2 Diabetes Mellitus, and Sleep Apnea. Biomedicines 2024, 12, 937. [Google Scholar] [CrossRef]
- Mir, T.; Bin Atique, H.; Regmi, N.; Sattar, Y.; Sundus, S.; Ambreen, S.; Lohia, P.; Qureshi, W.T.; Soubani, A. SGLT2 Inhibitors and Sleep Apnea; How Helpful Are the Medications: A Meta-Analysis. Endocrine and Metabolic Science 2021, 2, 100084. [Google Scholar] [CrossRef]
- Sawada, K.; Karashima, S.; Kometani, M.; Oka, R.; Takeda, Y.; Sawamura, T.; Fujimoto, A.; Demura, M.; Wakayama, A.; Usukura, M.; et al. Effect of Sodium Glucose Cotransporter 2 Inhibitors on Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Endocr J 2018, 65, 461–467. [Google Scholar] [CrossRef]
- Furukawa, S.; Miyake, T.; Senba, H.; Sakai, T.; Furukawa, E.; Yamamoto, S.; Niiya, T.; Matsuura, B.; Hiasa, Y. The Effectiveness of Dapagliflozin for Sleep-Disordered Breathing among Japanese Patients with Obesity and Type 2 Diabetes Mellitus. Endocr J 2018, 65, 953–961. [Google Scholar] [CrossRef]
- BUTT, J.H.; JERING, K.; DE BOER, R.A.; CLAGGETT, B.L.; DESAI, A.S.; HERNANDEZ, A.F.; INZUCCHI, S.E.; JHUND, P.S.; KØBER, L.; KOSIBOROD, M.N.; et al. Heart Failure, Investigator-Reported Sleep Apnea and Dapagliflozin: A Patient-Level Pooled Meta-Analysis of DAPA-HF and DELIVER. J Card Fail 2024, 30, 436–448. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.; Higuchi, K.; Ristic, B.; Sato, T.; Ramachandran, S.; Ganapathy, V. The Hepatic Plasma Membrane Citrate Transporter NaCT (SLC13A5) as a Molecular Target for Metformin. Sci Rep 2020, 10, 8536. [Google Scholar] [CrossRef] [PubMed]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int J Mol Sci 2020, 21, 3240. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. Journal of Clinical Investigation 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of Action of Metformin in Type 2 Diabetes: Effects on Mitochondria and Leukocyte-Endothelium Interactions. Redox Biol 2020, 34, 101517. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat Rev Endocrinol 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Johanns, M.; Hue, L.; Rider, M.H. AMPK Inhibits Liver Gluconeogenesis: Fact or Fiction? Biochemical Journal 2023, 480, 105–125. [Google Scholar] [CrossRef]
- Cho, K.; Chung, J.Y.; Cho, S.K.; Shin, H.-W.; Jang, I.-J.; Park, J.-W.; Yu, K.-S.; Cho, J.-Y. Antihyperglycemic Mechanism of Metformin Occurs via the AMPK/LXRα/POMC Pathway. Sci Rep 2015, 5, 8145. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat Rev Endocrinol 2019, 15, 569–589. [Google Scholar] [CrossRef]
- An, H.; He, L. Current Understanding of Metformin Effect on the Control of Hyperglycemia in Diabetes. Journal of Endocrinology 2016, 228, R97–R106. [Google Scholar] [CrossRef]
- Shohrati, M.; Karbasi-Afshar, R.; Saburi, A. Remarks in Metformin and Sleep Disorders in Diabetic Patients. Indian J Endocrinol Metab 2012, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Zunica, E.R.M.; Heintz, E.C.; Dantas, W.S.; Hebert, R.C.; Tanksley, M.; Beyl, R.A.; Mader, E.C.; Kirwan, J.P.; Axelrod, C.L.; Singh, P. Effects of Metformin on Glucose Metabolism and Mitochondrial Function in Patients with Obstructive Sleep Apnea: A Pilot Randomized Trial. Physiol Rep 2024, 12. [Google Scholar] [CrossRef]
- Lin D, R.L.T.S.W.B.M.JR. The Relationship between Metformin and Obstructive Sleep Apnea. J Sleep Med Disord. 2015, 2, 1027. [Google Scholar] [PubMed]
- Kajbaf, F.; Fendri, S.; Basille-Fantinato, A.; Diouf, M.; Rose, D.; Jounieaux, V.; Lalau, J. -D. The Relationship between Metformin Therapy and Sleep Quantity and Quality in Patients with Type 2 Diabetes Referred for Potential Sleep Disorders. Diabetic Medicine 2014, 31, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, E.S.; Mackiewicz, M.; Gislason, T.; Teff, K.L.; Pack, A.I. Molecular Signatures of Obstructive Sleep Apnea in Adults: A Review and Perspective. Sleep 2009, 32, 447–470. [Google Scholar] [CrossRef]
- Fernandes, M.; Spanetta, M.; Vetrugno, G.; Nuccetelli, M.; Placidi, F.; Castelli, A.; Manfredi, N.; Izzi, F.; Laganà, G.; Bernardini, S.; et al. The Potential Role of Interleukin-6 in the Association between Inflammation and Cognitive Performance in Obstructive Sleep Apnea. Brain Behav Immun Health 2024, 42. [Google Scholar] [CrossRef]
- Verma, S.; Bhatta, M.; Davies, M.; Deanfield, J.E.; Garvey, W.T.; Jensen, C.; Kandler, K.; Kushner, R.F.; Rubino, D.M.; Kosiborod, M.N. Effects of Once-Weekly Semaglutide 2.4 Mg on C-Reactive Protein in Adults with Overweight or Obesity (STEP 1, 2, and 3): Exploratory Analyses of Three Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trials. EClinicalMedicine 2023, 55, 101737. [Google Scholar] [CrossRef]
- Kang, N.; Oh, S.; Kim, S.-Y.; Ahn, H.; Son, M.; Heo, S.-J.; Byun, K.; Jeon, Y.-J. Anti-Obesity Effects of Ishophloroglucin A from the Brown Seaweed Ishige Okamurae (Yendo) via Regulation of Leptin Signal in Ob/Ob Mice. Algal Res 2022, 61, 102533. [Google Scholar] [CrossRef]
- Amorim, M.R.; Aung, O.; Mokhlesi, B.; Polotsky, V.Y. Leptin-Mediated Neural Targets in Obesity Hypoventilation Syndrome. Sleep 2022, 45. [Google Scholar] [CrossRef]
- Suriyagandhi, V.; Nachiappan, V. Therapeutic Target Analysis and Molecular Mechanism of Melatonin - Treated Leptin Resistance Induced Obesity: A Systematic Study of Network Pharmacology. Front Endocrinol (Lausanne) 2022, 13. [Google Scholar] [CrossRef]
- Pau, M.C.; Mangoni, A.A.; Zinellu, E.; Pintus, G.; Carru, C.; Fois, A.G.; Pirina, P.; Zinellu, A. Circulating Superoxide Dismutase Concentrations in Obstructive Sleep Apnoea (OSA): A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1764. [Google Scholar] [CrossRef]
- Hosseini, H.; Homayouni-Tabrizi, M.; Amiri, H.; Safari-Faramani, R.; Moradi, M.-T.; Fadaei, R.; Khazaie, H. The Effect of Continuous Positive Airway Pressure on Total Antioxidant Capacity in Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep and Breathing 2023, 27, 1237–1245. [Google Scholar] [CrossRef]
- Saruhan, E.; Sertoglu, E.; Unal, Y.; Bek, S.; Kutlu, G. The Role of Antioxidant Vitamins and Selenium in Patients with Obstructive Sleep Apnea. Sleep and Breathing 2021, 25. [Google Scholar] [CrossRef]
- Bakshi, G.K.; Khurana, S.; Srivastav, S.; Kumar, R.; Chourasia, M.; Bose, S. Integrative Analysis of Candidate MicroRNAs and Gene Targets for OSA Management Using in Silico and In-Vitro Approach. Biotechnology Notes 2025, 6, 79–88. [Google Scholar] [CrossRef]
- Moriondo, G.; Soccio, P.; Tondo, P.; Scioscia, G.; Sabato, R.; Foschino Barbaro, M.P.; Lacedonia, D. Obstructive Sleep Apnea: A Look towards Micro-RNAs as Biomarkers of the Future. Biology (Basel) 2023, 12. [Google Scholar] [CrossRef]
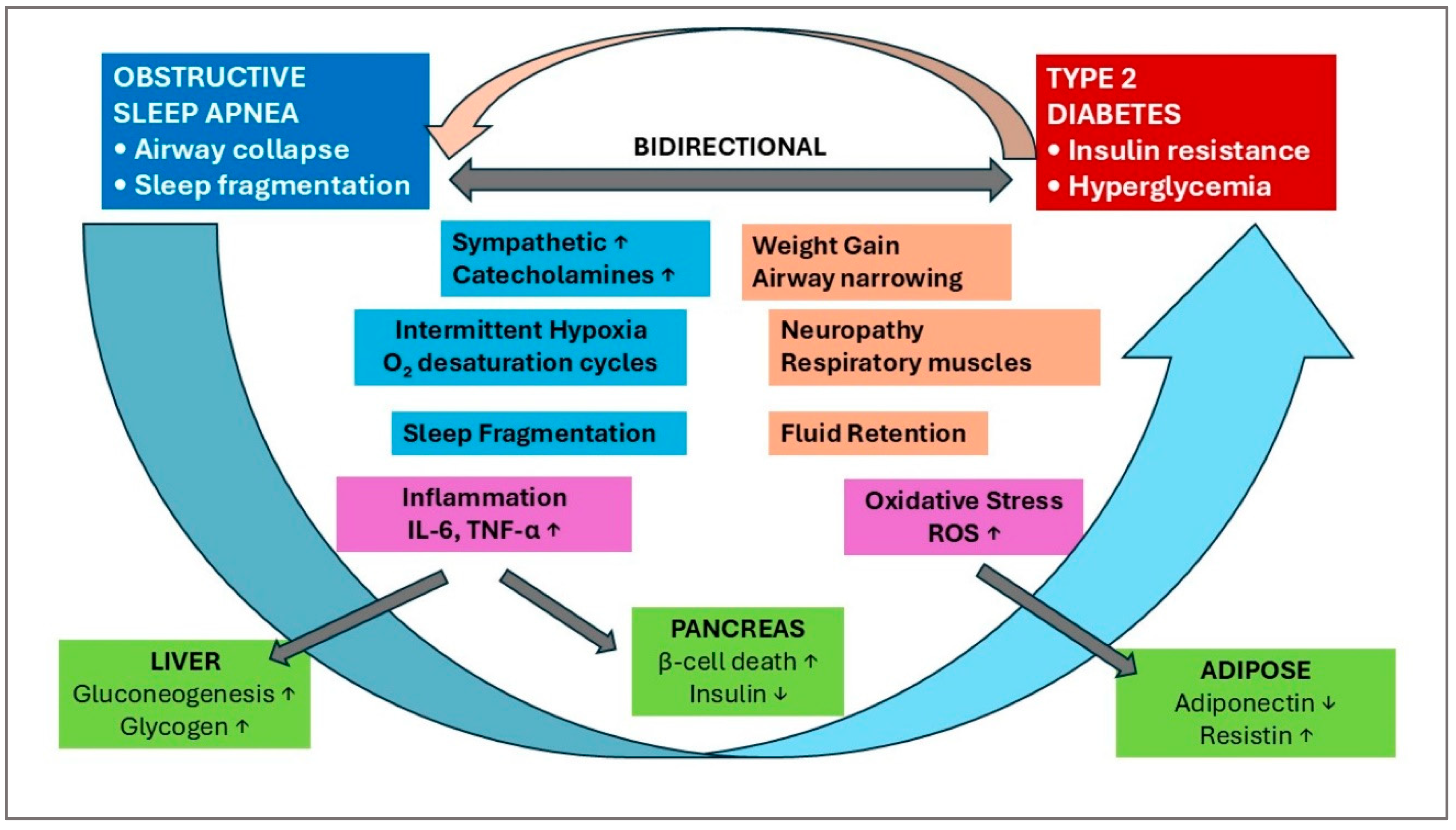
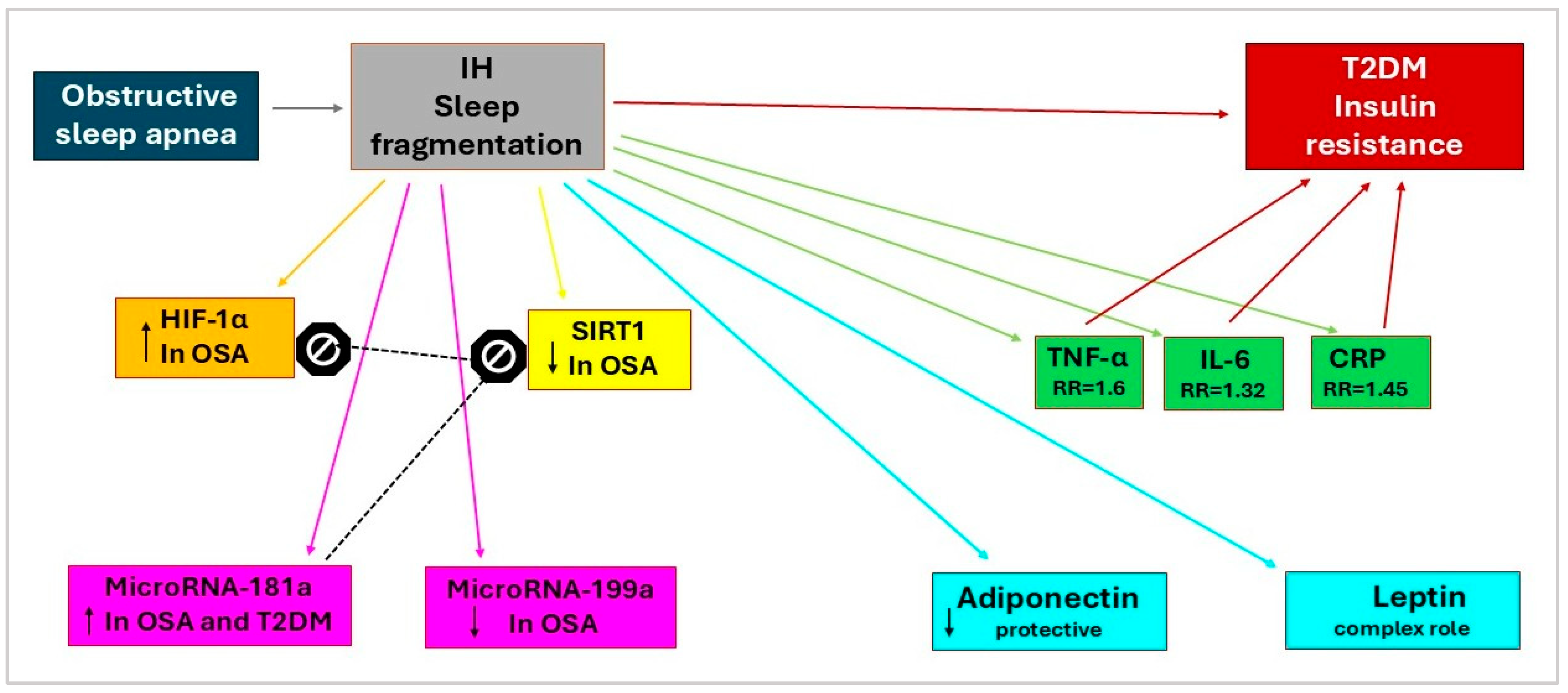
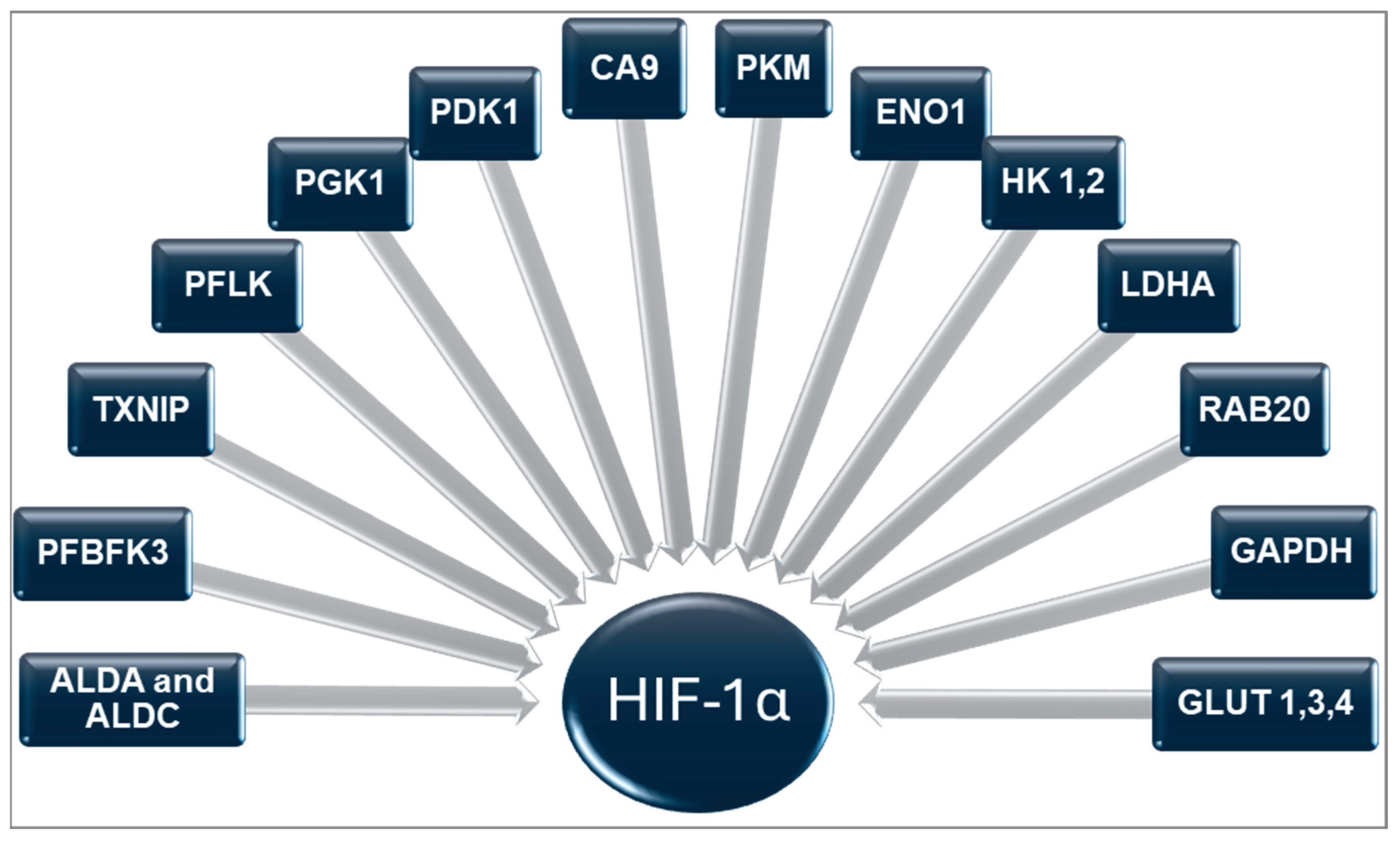
| Adipokine | Sources | Receptor | Actions | Reference |
| Adiponectin | Adipocyte | AdipoR1 and AdipoR2; Cadherin-T |
Increases insulin sensitivity; Anti-inflammatory |
[62,63] |
| Leptin | White adipose tissue (Obesity gene encoding) | Leptin receptor, OB-R |
Increases energy consumption; Inhibits fat synthesis; Induces fat decomposition; Inhibits insulin synthesis and secretion. |
[64,65] |
| Resistin | Adipose tissue; Immune and epithelial cells | No mention | Inhibits insulin’s ability to stimulate glucose cellular uptake; Pro-inflammatory |
[66,67] |
| Chemerin | Adipose tissue | Specific receptor proteins: ChemR23 (CMKLR1), and RARRES2 | Acts in an immune response; Anti-inflammatory; Regulates glucose metabolism. |
[68] |
| Omentin-1 | Omental adipose tissue | No mention | Anti-inflammatory; Regulates fat metabolism; Improves insulin sensitivity. |
[69,70,71] |
| Therapy Class | Specific Agent | Mechanism | OSA Benefits | T2DM Benefits | Clinical Evidence | FDA Status | Key Studies |
| GLP-1 Agonists |
Tirzepatide | Dual GIP/GLP-1 agonist |
52-week study: significant AHI reduction | Superior glycemic control and weight loss vs GLP-1 alone | Phase III positive results | Approved for obesity, T2DM, and OSA | [110] |
| Semaglutide | GLP-1 receptor agonist; |
AHI reduction, improved sleep quality, and weight loss | Established weight and glycemic; cardiovascular protection | Phase III trials completed | Approved for obesity, T2DM | [111] | |
| Liraglutide | GLP-1 receptor agonist |
AHI improvement | Established weight and glycemic benefit | RCT evidence | Approved for obesity, T2DM | [112] | |
| SGLT2 Inhibitors |
Empagliflozin | SGLT2 inhibition; natriuretic effects | 65% OSA occurrence reduction; improved SpO2 | Cardiovascular protection; renal benefits, Glycemic control | Meta-analysis evidence | Approved for T2DM, HF, CKD | [113] |
| Dapagliflozin | SGLT2 inhibition; natriuretic effects | Reduced fluid retention; improved AHI | Cardiovascular protection; renal benefits, Glycemic control | Observational studies | Approved for T2DM, HF, CKD | [114] | |
| Chronotherapy | Melatonin | Circadian synchronization; antioxidant effects | Sleep architecture improvement | Insulin sensitivity enhancement |
Preclinical evidence | OTC supplement | [115] |
| Light Therapy | Circadian entrainment; PER2 enhancement | Potential sleep quality improvement | Metabolic rhythm restoration | Early studies | Nonpharmacological | [116] | |
| CRY Stabilizers | Clock gene stabilization (TW68) | Potential circadian restoration | Hepatic glucose suppression | Preclinical only | Investigational | [117] | |
| Combinations | GLP-1 + SGLT2 | Synergistic metabolic effects | Additive OSA benefits potential | Enhanced glycemic/CV outcomes | Ongoing trials | Individual approvals | [118] |
| CPAP + GLP-1 /SGLT2 | Mechanical + metabolic intervention | Optimal AHI reduction + weight loss | Comprehensive metabolic control | Limited studies | Standard + approved | [119] | |
| Aroxybutynin +atomoxetine | A selective norepinephrine reuptake inhibitor and a selective antimuscarinic | Activation of the upper airway dilator muscles | No mention | Phase III trials are ongoing | Submitted for approval in OSA | [120] |
| Reference |
Primary and Secondary Objectives |
Population / Participants | Sample Size | Intervention / Exposure | Outcome Measures | Major Findings |
| Malhotra A et al., 2024 [111] |
Primary: To evaluate the change in AHI from baseline. Secondary: To assess percent change in AHI, body weight, hypoxic burden, patient-reported sleep impairment and disturbance (PROMIS scales), hsCRP concentration, and SBP. | Adults with moderate-to-severe OSA (AHI ≥15 events/hour) and obesity (BMI ≥30) | 469 (Trial 1: 234 [no PAP], Trial 2: 235 [with PAP]) |
Tirzepatide (maximum tolerated dose of 10 mg or 15 mg subcutaneously once weekly) vs. placebo for 52 weeks | Change in AHI, percent change in AHI, percent change in body weight, hypoxic burden, PROMIS-SRI and PROMIS-SD scores, hsCRP concentration, and SBP. | In Trial 1, tirzepatide reduced AHI by -25.3 events/hour vs. -5.3 with placebo (difference -20.0, P<0.001); body weight by -17.7% vs. -1.6%. In Trial 2, AHI was reduced by -29.3 vs. -5.5 (difference -23.8, P<0.001); body weight by -19.6% vs. -2.3%. Significant improvements in hypoxic burden, PROMIS scores, hsCRP, and SBP |
| Jiang W et al., 2023 [126] | Primary: To assess liraglutide’s effect on OSA severity in patients with T2DM. Secondary: To evaluate glycemic control, body weight, and safety. |
Patients with T2DM and severe OSA | 60 | Liraglutide (1.8 mg/day) vs. control | AHI, HbA1c, body weight, adverse events | Liraglutide reduced AHI by 12.2 events/hour (p < 0.001), improved HbA1c, and reduced body weight, with a tolerable safety profile. |
| Blackman A et al., 2016 [112] |
Primary: To evaluate liraglutide’s effect on OSA severity in obese individuals. Secondary: To assess changes in body weight and cardio-metabolic outcomes. |
Individuals with obesity and with moderate-to-severe OSA | 359 | Liraglutide (3.0 mg/day) vs. placebo | AHI, body weight, HbA1c, blood pressure | Liraglutide reduced AHI by 12.2 events/hour (p = 0.015), body weight by 5.7%, and improved cardiometabolic markers compared to the placebo. |
| O’Donnell C et. al, 2024 [127] |
Primary: To compare CPAP and liraglutide on early CV disease markers in OSA. Secondary: To assess changes in AHI and metabolic parameters. |
Adults with OSA and obesity | 30 | CPAP vs. liraglutide (3.0 mg/day) | Carotid intima-media thickness, AHI, HbA1c, body weight | CPAP improved cardiovascular markers (p = 0.02) more than liraglutide; however, liraglutide reduced AHI and body weight, with no significant cardiovascular benefit. |
| Sprung et al., 2020 [128] |
Primary: To assess liraglutide with or without CPAP on OSA in patients with T2DM. Secondary: To evaluate glycemic control, body weight, and CV risk markers. |
Type 2 diabetes patients with OSA | 72 | Liraglutide, CPAP, or both vs. placebo | AHI, HbA1c, body weight, cardiovascular risk markers | Study protocol: designed to assess the combined effects of liraglutide and CPAP; results not reported in this paper. |
| Gomez-Peralta F et. al, 2015 [129] |
Primary: To investigate liraglutide’s effect on excessive daytime sleepiness in obese type 2 diabetes patients. Secondary: To assess glycemic control and body weight changes. |
Obese patients with type 2 diabetes | 158 | Liraglutide (1.2–1.8 mg/day) | Epworth Sleepiness Scale (ESS), HbA1c, body weight | Liraglutide reduced ESS scores by 2.9 points (p < 0.001), improved HbA1c, and decreased body weight, suggesting benefits for daytime sleepiness. |
| Baser O et al., 2024 [130] |
Primary: To assess the association between AOMs and the incidence of OSA. Secondary: To compare OSA risk between tirzepatide and semaglutide users. | Patients with obesity (AOM cohort: tirzepatide or semaglutide users; non-AOM cohort: no AOM use) | 105,402 (AOM: 20,384; non-AOM: 85,018) | Tirzepatide or semaglutide vs. no AOM | Incidence of OSA, hazard ratio of OSA |
The AOM cohort had a lower incidence of OSA (3.12%) compared to the non-AOM cohort (12.56%, p < 0.001); AOM use reduced the likelihood of OSA by 40% (HR = 0.60, p < 0.0001). Additionally, tirzepatide (2.65%) and semaglutide (3.18%) showed no significant difference (p = 0.1664). |
| Characteristic | GLP-1RAs | SGLT2 Inhibitors |
| Weight Loss Mechanism | Central appetite suppression | Peripheral caloric loss |
| Primary Site | CNS/GI tract | Kidney |
| Fluid Effects | Minimal | Diuretic |
| Respiratory Control | Direct CNS effects and indirect | Indirect via metabolic changes |
| Onset of Action | Rapid (days-weeks) | Gradual (weeks-months) |
| Dependency | Receptor-mediated | Non-receptor-mediated |
| Reference | Primary and Secondary Objectives | Population / Participants | Sample Size | Intervention / Exposure | Outcome Measures | Major Findings |
| Qiu M et al., 2021 [150] |
Primary: To assess the association between SGLT2i and noninfectious respiratory disorders. Secondary: To evaluate specific respiratory outcomes. |
Patients with T2DM from randomized trials | 42,151 | SGLT2 inhibitors vs. placebo or other therapies | Incidence of noninfectious respiratory disorders | SGLT2i were not associated with an increased risk of noninfectious respiratory disorders (RR, 0.95; 95% CI, 0.84-1.07), suggesting safety in this context. |
| Tang Y et al., 2019 [156] |
Primary: To evaluate dapagliflozin’s effect on OSA in T2DM. Secondary: To assess changes in glycemic control and body weight. |
Patients with T2DM and OSA | 24 | Dapagliflozin (10 mg/day) | AHI, HbA1c, body weight | Dapagliflozin reduced AHI (p = 0.03), improved glycemic control, and decreased body weight, suggesting potential benefits for OSA in individuals with type 2 diabetes. |
| Armentaro G et al., 2024 [157] |
Primary: To assess SGLT2 inhibitors’ effect on OSA parameters in elderly patients. Secondary: To evaluate CV and metabolic outcomes. |
Elderly patients with heart failure, T2DM, and OSA | 60 | SGLT2i | AHI, oxygen saturation, CV events | SGLT2i improved AHI and oxygen saturation (p < 0.05), with benefits in cardiovascular and metabolic parameters in elderly patients. |
| Mir T et al., 2021 [158] |
Primary: To investigate the effect of SGLT2i on sleep apnea in T2DM. Secondary: To assess safety and metabolic outcomes. |
Patients with T2DM and OSA from randomized trials | NA | SGLT2 inhibitors vs. control | AHI, AE, glycemic control | SGLT2i significantly reduced AHI (p < 0.05) and improved glycemic control, indicating a beneficial role in managing sleep apnea. |
| Kusunoki M et al., 2021 [119] |
Primary: To assess SGLT2 inhibitors’ effect on CPAP initiation in patients with T2DM and OSA. Secondary: To evaluate glycemic control and body weight. |
Patients with T2DM and OSA | 30 | SGLT2i | CPAP initiation rate, HbA1c, body weight | SGLT2i reduced the need for CPAP initiation (p < 0.05), with improvements in HbA1c and body weight, suggesting benefits in the management of OSA. |
| Neeland IJ et al., 2020 [113] |
Primary: To evaluate empagliflozin’s effect on OSA in T2DM. Secondary: To assess CV and renal outcomes. |
Patients with T2DM and CV disease | 7,020 | Empagliflozin vs. placebo | OSA events, CV death, renal outcomes | Empagliflozin reduced OSA events (HR 0.76, 95% CI 0.59-0.98) and improved cardiovascular and renal outcomes, suggesting broader benefits. |
| Sawada K et al., 2018 [159] |
Primary: To investigate the SGLT2i effect on OSA severity in T2DM. Secondary: To assess metabolic and anthropometric changes. |
Type 2 diabetes patients with OSA | 24 | SGLT2 inhibitors | Apnea-hypopnea index (AHI), body mass index, HbA1c | SGLT2 inhibitors significantly reduced AHI (p = 0.02) and improved BMI and HbA1c, indicating potential therapeutic benefits for OSA. |
| Furukawa S et al., 2018 [160] |
Primary: To assess dapagliflozin’s effect on sleep-disordered breathing in obese T2DM. Secondary: To evaluate body weight and glycemic control. |
Japanese patients with obesity and T2DM | 30 | Dapagliflozin (5 mg/day) | Apnea-hypopnea index (AHI), body weight, HbA1c | Dapagliflozin reduced AHI (p < 0.05), body weight, and HbA1c, demonstrating its efficacy in improving sleep-disordered breathing. |
| Butt JH et al., 2024 [161] |
Primary: To evaluate dapagliflozin’s effect on sleep apnea in heart failure and type 2 diabetes patients. Secondary: To assess CV outcomes. |
Heart failure patients with or without T2DM | 11,007 | Dapagliflozin vs. placebo | Sleep apnea events, heart failure hospitalization, CV death | Dapagliflozin reduced sleep apnea events (HR 0.79, 95% CI 0.64-0.97) and improved heart failure and cardiovascular outcomes. |
| Molecular Marker | Clinical Relevance |
Therapeutic Target |
References |
| HIF-1α | Increase in OSA patients; correlates with insulin resistance; promotes inflammation. |
HIF-1α stabilizers; circadian modulators | [45] |
| TNF-α | Elevated in OSA; correlates with CIH severity |
Anti-TNF therapies; adipokine modulators | [177] |
| IL-6 | Acute phase reactant; hepatic glucose production |
JAK inhibitors; IL-6 blockers | [178] |
| CRP | Correlates with OSA severity and diabetes risk | Anti-inflammatory agents | [179] |
| Leptin | Resistance in obesity; maintains inflammation despite metabolic dysfunction. |
Leptin sensitizers; circadian modulators | [180,181,182] |
| Adiponectin | Reduced in both OSA and T2DM; protective against metabolic dysfunction |
Adiponectin receptor agonists | [78] |
| Resistin | Elevated in metabolic dysfunction | Adipokine modulators | [67] |
| ROS/Antioxidants | Activates NF-κB; impairs insulin signaling |
Antioxidant supplementation; SOD mimetics | [183,184,185] |
| miRNA-181a | Altered in OSA; links to insulin resistance |
miRNA modulators | [186,187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





