Submitted:
13 October 2025
Posted:
14 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Novel Therapeutics
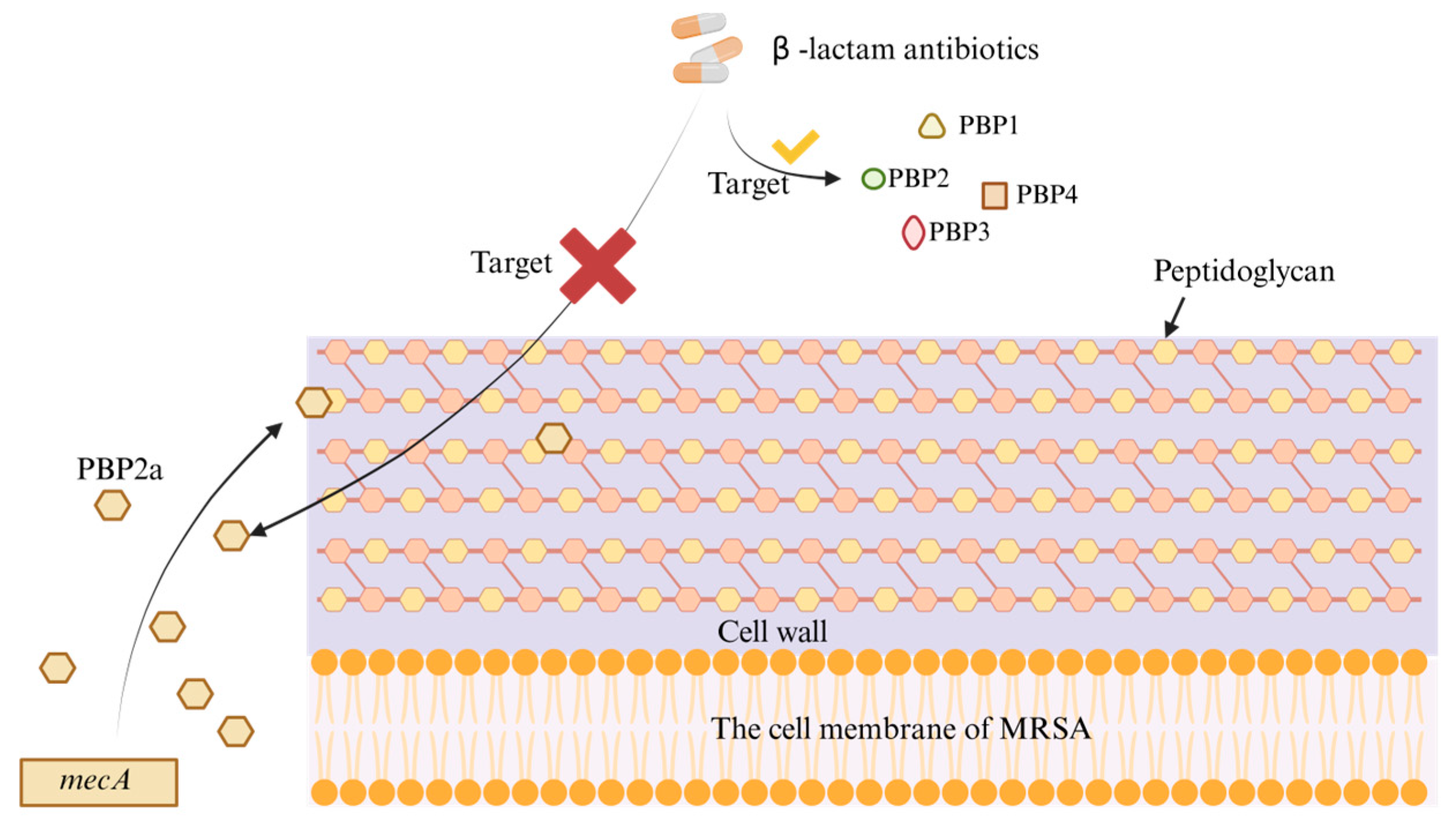
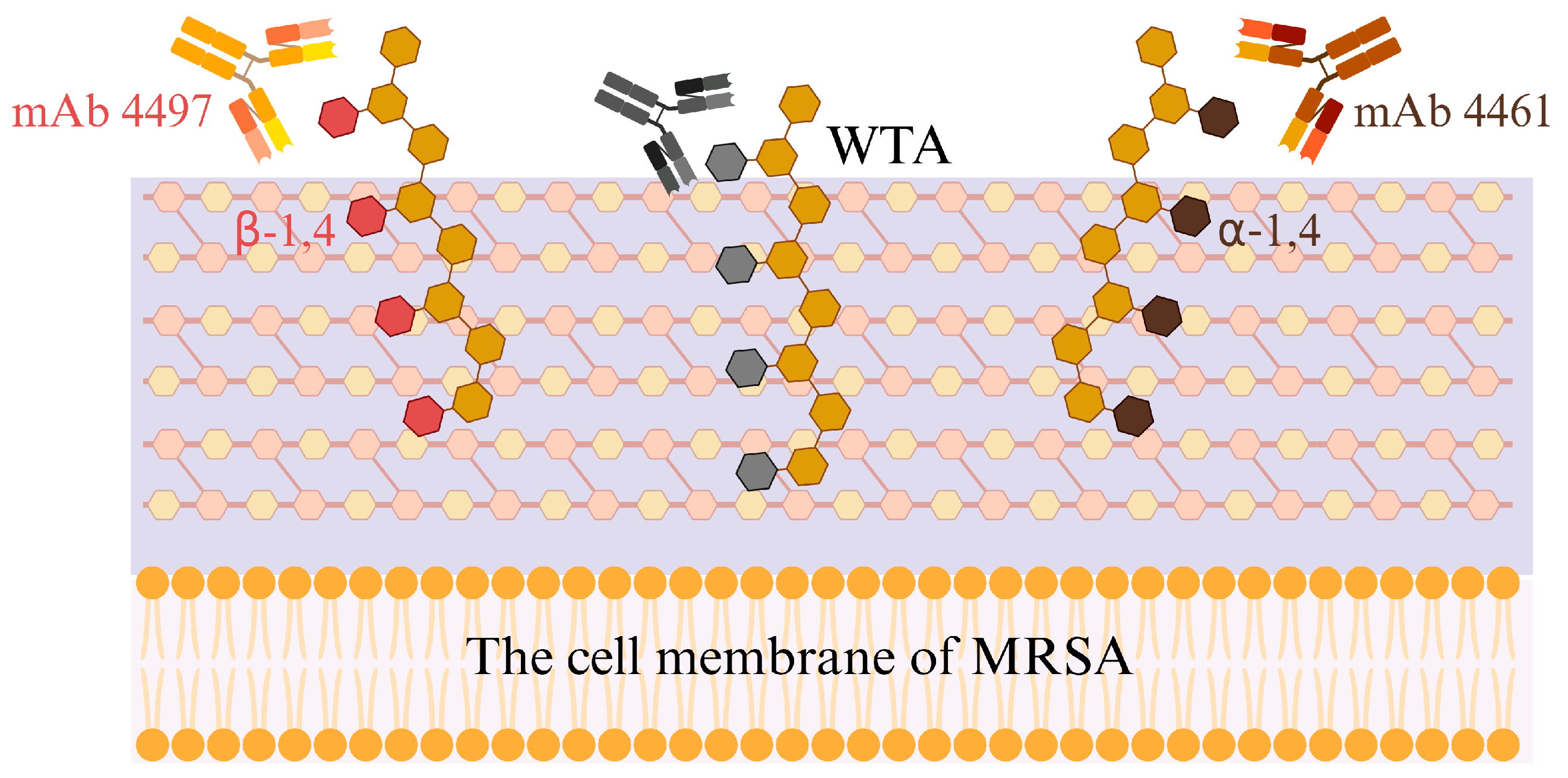
2.1. Monoclonal Antibody Therapy
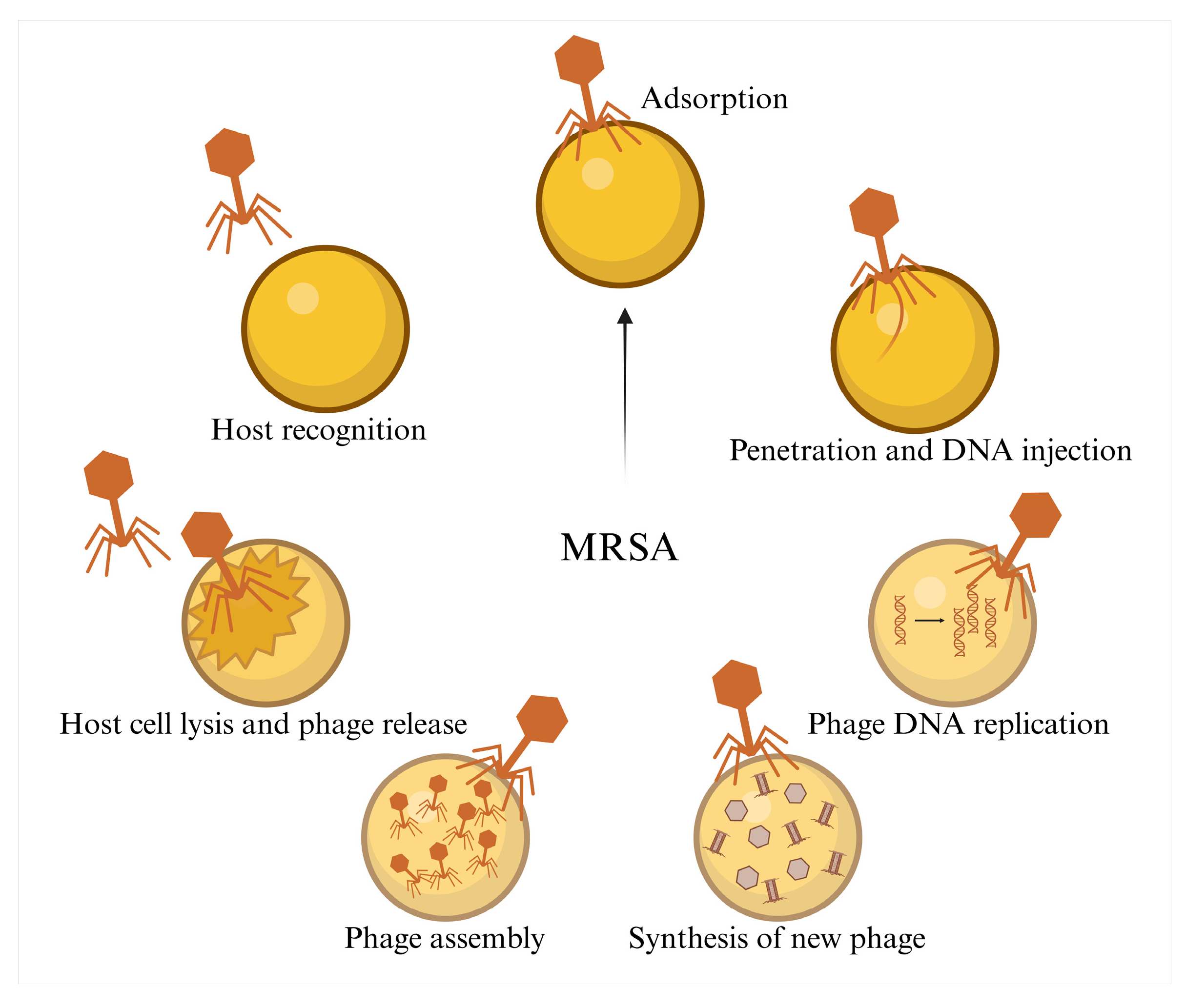
2.2. Phage Therapy
2.3. Antimicrobial Peptide Therapy
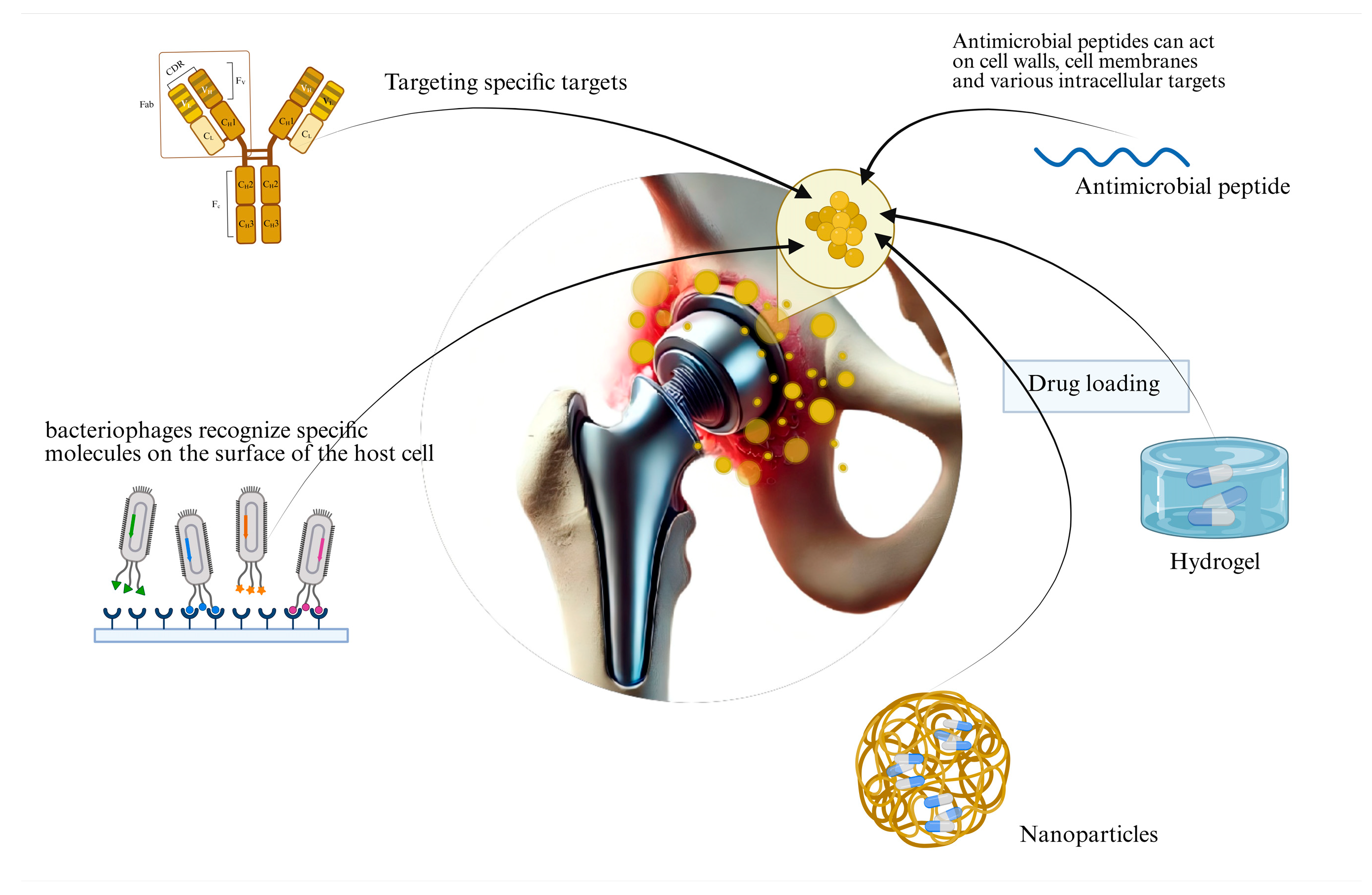
3. Emerging Therapeutic Approaches
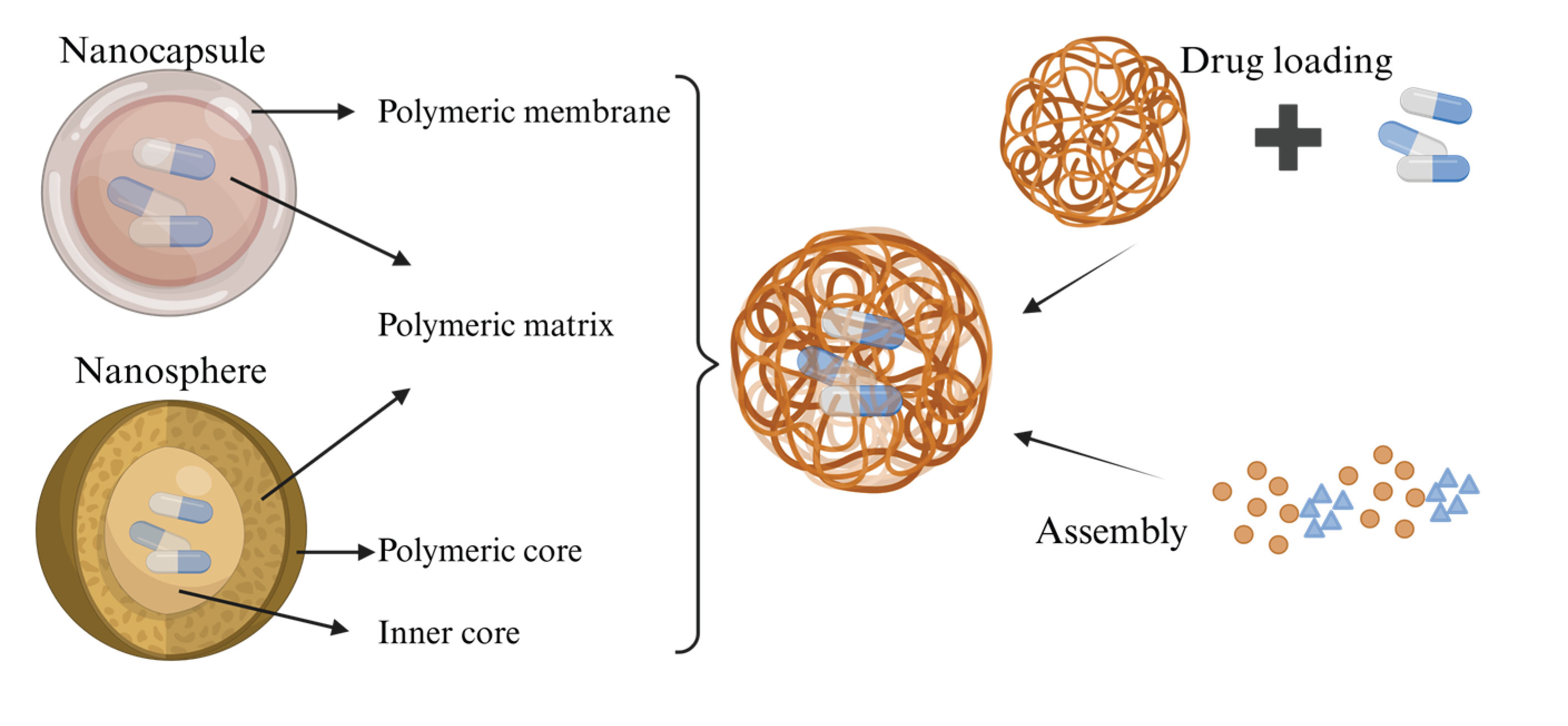
3.1. Nanoparticles
3.2. Hydrogels
4. Discussion
| Treatment Strategy | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Monoclonal Antibodies | Specific antibodies targeting resistant bacteria | Strong specificity, low side effects | High production cost, low bioavailability |
| Phage Therapy | Utilizing viruses to directly kill resistant bacteria | Strong targeting, effective against resistant bacteria | Narrow host range, potential for immune rejection |
| Antimicrobial Peptides | Disrupting cell membranes or cell walls leading to cell lysis | Low toxicity, strong thermal stability | Long-term use may induce bacterial resistance, Poor bioavailability, short half-life |
Author Contributions
Funding (Acknowledgments)
Conflicts of Interest
Abbreviations
| TJR | Total joint replacement |
| PJI | Periprosthetic Joint Infection |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| PBP2a | The mec gene on the Staphylococcus chromosome |
| MRSA-PJI | Periprosthetic Joint Infection caused by Methicillin-Resistant Staphylococcus Aureus |
| mAbs | Monoclonal Antibodies |
| IgG | Gamma Immunoglobulin |
| Fab | Antigen-Binding Fragment |
| Fc | Crystallizable Fragment |
| AMP | Antimicrobial Peptides |
| MPS | mononuclear phagocyte system |
| PSA | Poly-sialic acid |
| AI | artificial intelligence |
References
- Baratta, J. L., Deiling, B., Hassan, Y. R., et al. (2023). Total joint replacement in ambulatory surgery. Best practice & research. Clinical anaesthesiology, 37(3), 269-284. [CrossRef]
- Vrancianu, C. O., Serban, B., Gheorghe-Barbu, I., et al. (2023). The Challenge of Periprosthetic Joint Infection Diagnosis: From Curren t Methods to Emerging Biomarkers. International journal of molecular sciences, 24(5), 4320. [CrossRef]
- Magruder, M. L., Heckmann, N. D., Lieberman, J. R., et al. Novel Technologies in Periprosthetic Joint Infections: Emerging Therap eutics. The Journal of arthroplasty, S0883-5403(0825)00837-X. [CrossRef]
- Tornero, E., García-Ramiro, S., Martínez-Pastor, J. C., et al. Prophylaxis with teicoplanin and cefuroxime reduces the rate of prosth etic joint infection after primary arthroplasty. Antimicrobial agents and chemotherapy, 59(2), 831-837. [CrossRef]
- Tillander, J. A. N., Rilby, K., Svensson Malchau, K., et al. Treatment of periprosthetic joint infections guided by minimum biofilm eradication concentration (MBEC) in addition to minimum inhibitory co ncentration (MIC): protocol for a prospective randomised clinical tria l. BMJ open, 12(9), e058168. [CrossRef]
- Hieda, Y., Choe, H., Maruo, A., et al. Clinical outcomes of continuous local antibiotic perfusion in combinat ion with debridement antibiotics and implant retention for periprosthe tic hip joint infection. Scientific reports, 15(1), 26017. [CrossRef]
- Hu, L., Fu, J., Zhou, Y., et al. (2023). Microbiological profiles and antibiotic resistance of periprosthetic j oint infection after hip replacement in patients with fracture or non- fracture: A comparative study. Journal of back and musculoskeletal rehabilitation, 36(1), 147-154. [CrossRef]
- Li, Y., Quan, X., Zhou, C., et al. Risk factors for metachronous periprosthetic joint infection in patien ts with multiple prosthetic joints: a systematic review and meta-analy sis. Journal of orthopaedic surgery and research, 20(1), 293. [CrossRef]
- Xu, Z. 1,2,3-Triazole-containing hybrids with potential antibacterial activit y against methicillin-resistant Staphylococcus aureus (MRSA). European journal of medicinal chemistry, 206, 112686. [CrossRef]
- Lee, A. S., de Lencastre, H., Garau, J., et al. Methicillin-resistant Staphylococcus aureus. Nature reviews. Disease primers, 4, 18033. [CrossRef]
- Peacock, S. J., Paterson, G. K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annual review of biochemistry, 84, 577-601. [CrossRef]
- Sun, Z., Hu, L., Sankaran, B., et al. (2018). Differential active site requirements for NDM-1 β-lactamase hydrolysis of carbapenem versus penicillin and cephalosporin antibiotics. Nature Communications, 9(1), 4524. [CrossRef]
- Lai, L.-Y., Satishkumar, N., Cardozo, S., et al. Altered PBP4 and GdpP functions synergistically mediate MRSA-like high -level, broad-spectrum β-lactam resistance in Staphylococcus aureus. mBio, 15(5), e0288923. [CrossRef]
- Ju, Y., An, Q., Zhang, Y., et al. Recent advances in Clp protease modulation to address virulence, resis tance and persistence of MRSA infection. Drug discovery today, 26(9), 2190-2197. [CrossRef]
- Piuzzi, N. S., Klika, A. K., Lu, Q., et al. Periprosthetic joint infection and immunity: Current understanding of host-microbe interplay. Journal of orthopaedic research : official publication of the Orthopae dic Research Society, 42(1), 7-20. [CrossRef]
- Liang, S., Pan, Y., Wang, J., et al. Bone-targeting ZIF-8 based nanoparticles loaded with vancomycin for th e treatment of MRSA-induced periprosthetic joint infection. Journal of controlled release : official journal of the Controlled Rel ease Society, 385, 113965. [CrossRef]
- Joel, J., Graham, S. M., Peckham-Cooper, A., et al. Clinical results of linezolid in arthroplasty and trauma MRSA related infections. World journal of orthopedics, 5(2), 151-157. [CrossRef]
- Jiang, G., Wang, W., Yang, Y., et al. Organism profiles and empirical treatments for periprosthetic joint in fections. Journal of orthopaedic surgery and research, 20(1), 698. [CrossRef]
- Chang, Y.-J., Lee, M. S., Lee, C.-H., et al. Daptomycin treatment in patients with resistant staphylococcal peripro sthetic joint infection. BMC infectious diseases, 17(1), 736. [CrossRef]
- Kuo, F.-C., Yen, S.-H., Peng, K.-T., et al. Methicillin-resistant Staphylococcal periprosthetic joint infections c an be effectively controlled by systemic and local daptomycin. BMC infectious diseases, 16, 48. [CrossRef]
- Buss, N. A. P. S., Henderson, S. J., McFarlane, M., et al. Monoclonal antibody therapeutics: history and future. Current opinion in pharmacology, 12(5), 615-622. [CrossRef]
- Tkaczyk, C., Hua, L., Varkey, R., et al. Identification of anti-alpha toxin monoclonal antibodies that reduce t he severity of Staphylococcus aureus dermonecrosis and exhibit a corre lation between affinity and potency. Clinical and vaccine immunology : CVI, 19(3), 377-385. [CrossRef]
- Robinson, K. M., Ramanan, K., Tobin, J. M., et al. Survival during influenza-associated bacterial superinfection improves following viral- and bacterial-specific monoclonal antibody treatment. JCI insight, 4(14), e125554. [CrossRef]
- Di Carluccio, C., Soriano-Maldonado, P., Berni, F., et al. Antibody Recognition of Different Staphylococcus aureus Wall Te ichoic Acid Glycoforms. ACS central science, 8(10), 1383-1392. [CrossRef]
- Zhang, W., Liu, L., Zhang, Q., et al. Inducing bacterial calcification for systematic treatment and immunomo dulation against methicillin-resistant Staphylococcus aureus. Nature biotechnology, 10.1038/s41587-41025-02736-41583. [CrossRef]
- van Dijk, B., Hooning van Duyvenbode, J. F. F., de Vor, L., et al. Evaluating the Targeting of a Staphylococcus-aureus-Infected Im plant with a Radiolabeled Antibody In Vivo. International journal of molecular sciences, 24(5), 4374. [CrossRef]
- Qin, L., Hu, N., Zhang, Y., et al. Antibody-antibiotic conjugate targeted therapy for orthopedic implant- associated intracellular S. aureus infections. Journal of advanced research, 65, 239-255. [CrossRef]
- Hansel, T. T., Kropshofer, H., Singer, T., et al. The safety and side effects of monoclonal antibodies. Nature reviews. Drug discovery, 9(4), 325-338. [CrossRef]
- Daugherty, A. L., Mrsny, R. J. Formulation and delivery issues for monoclonal antibody therapeutics. Advanced drug delivery reviews, 58(5-6), 686-706. [CrossRef]
- Karimi, M., Aslanabadi, A., Atkinson, B., et al. Subcutaneous liposomal delivery improves monoclonal antibody pharmacok inetics in vivo. Acta biomaterialia, 195, 522-535. [CrossRef]
- Kakasis, A., Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections . A comprehensive review. International journal of antimicrobial agents, 53(1), 16-21. [CrossRef]
- Strathdee, S. A., Hatfull, G. F., Mutalik, V. K., et al. Phage therapy: From biological mechanisms to future directions. Cell, 186(1), 17-31. [CrossRef]
- Onsea, J., Wagemans, J., Pirnay, J. P., et al. Bacteriophage therapy as a treatment strategy for orthopaedic-device-r elated infections: where do we stand? European cells & materials, 39, 193-210. [CrossRef]
- Young, J., Lee, S. W., Shariyate, M. J., et al. Bacteriophage therapy and current delivery strategies for orthopedic i nfections: A SCOPING review. The Journal of infection, 88(3), 106125. [CrossRef]
- Pires, D. P., Oliveira, H., Melo, L. D. R., et al. Bacteriophage-encoded depolymerases: their diversity and biotechnologi cal applications. Applied microbiology and biotechnology, 100(5), 2141-2151. [CrossRef]
- Kim, S. G., Giri, S. S., Yun, S., et al. (2020). Synergistic phage–surfactant combination clears IgE-promoted Staphylococcus aureus aggregation in vitro and enhances the effect in vivo. International journal of antimicrobial agents, 56(1). [CrossRef]
- Lu, Y., Lu, Y., Li, B., et al. StAP1 phage: an effective tool for treating methicillin-resistant Staphylococcus aureus infections. Frontiers in microbiology, 14, 1267786. [CrossRef]
- Arens, D. K., Rodriguez, A. R., Huh, E. Y., et al. Enhancing orthopedic infection control: carbon scaffold-mediated phage therapy for methicillin-resistant staphylococcus aureus in fracture-r elated infections. Biomedical physics & engineering express, 11(1), 10.1088/2057-1976/ad1089c1087b. [CrossRef]
- Liu, M.-Y., Liu, X., Wang, C.-Y., et al. Inhalable Polymeric Microparticles for Phage and Photothermal Synergis tic Therapy of Methicillin-Resistant Staphylococcus aureus Pneu monia. Nano letters, 24(28), 8752-8762. [CrossRef]
- Dedrick, R. M., Smith, B. E., Cristinziano, M., et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clinical infectious diseases : an official publication of the Infectio us Diseases Society of America, 76(1), 103-112. [CrossRef]
- Ooi, M. L., Drilling, A. J., Morales, S., et al. Safety and Tolerability of Bacteriophage Therapy for Chronic Rhinosinu sitis Due to Staphylococcus aureus. JAMA otolaryngology-- head & neck surgery, 145(8), 723-729. [CrossRef]
- Gu Liu, C., Green, S. I., Min, L., et al. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibact erial Mechanism of Action and Stoichiometry. mBio, 11(4), e01462-01420. [CrossRef]
- Li, X., He, Y., Wang, Z., et al. (2021). A combination therapy of Phages and Antibiotics: Two is better than one. International Journal of Biological Sciences, 17(13), 3573-3582. [CrossRef]
- Tagliaferri, T. L., Jansen, M., Horz, H.-P. (2019). Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Frontiers in Cellular and Infection Microbiology, 9. [CrossRef]
- Kunz Coyne, A. J., Stamper, K., Bleick, C., et al. Synergistic bactericidal effects of phage-enhanced antibiotic therapy against MRSA biofilms. Microbiology spectrum, 12(4), e0321223. 0321. [CrossRef]
- Young, J., Lee, S. W., Shariyate, M. J., et al. (2024). Bacteriophage therapy and current delivery strategies for orthopedic infections: A SCOPING review. Journal of Infection, 88(3). [CrossRef]
- Kaur, S., Harjai, K., Chhibber, S. In Vivo Assessment of Phage and Linezolid Based Implant Coatings for T reatment of Methicillin Resistant S. aureus (MRSA) Mediated Orthopaedi c Device Related Infections. PloS one, 11(6), e0157626. [CrossRef]
- Antonelli, B., Chen, A. F. Reducing the risk of infection after total joint arthroplasty: preoper ative optimization. Arthroplasty (London, England), 1(1), 4. [CrossRef]
- Luo, Y., Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. International journal of molecular sciences, 22(21), 11401. [CrossRef]
- Deo, S., Turton, K. L., Kainth, T., et al. Strategies for improving antimicrobial peptide production. Biotechnology advances, 59, 107968. [CrossRef]
- Bin Hafeez, A., Jiang, X., Bergen, P. J., et al. Antimicrobial Peptides: An Update on Classifications and Databases. International journal of molecular sciences, 22(21), 11691. [CrossRef]
- Luo, Y., Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. International journal of molecular sciences, 22(21), 11401. [CrossRef]
- Li, X., Zuo, S., Wang, B., et al. Antimicrobial Mechanisms and Clinical Application Prospects of Antimic robial Peptides. Molecules (Basel, Switzerland), 27(9), 2675. [CrossRef]
- Shi, J., Chen, C., Kong, P., et al. Non-Membrane Active Peptide Resensitizes MRSA to β-Lactam Antibiotics and Inhibits S. aureus Virulence. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 12(15), e2416260. [CrossRef]
- Songnaka, N., Lertcanawanichakul, M., Hutapea, A. M., et al. Purification and Characterization of Novel Anti-MRSA Peptides Produced by Brevibacillus sp. SPR-20. Molecules (Basel, Switzerland), 27(23), 8452. [CrossRef]
- Melicherčík, P., Kotaška, K., Jahoda, D., et al. Antimicrobial peptide in polymethylmethacrylate bone cement as a proph ylaxis of infectious complications in orthopedics-an experiment in a m urine model. Folia microbiologica, 67(5), 785-791. [CrossRef]
- Zhu, Y., Weng, X., Zhang, J., et al. Protective effect of additional cathelicidin antimicrobial peptide PR- 39 on prosthetic-joint infections. Journal of orthopaedic surgery (Hong Kong), 31(2), 10225536231175237. [CrossRef]
- Baetke, S. C., Lammers, T., Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. The British journal of radiology, 88(1054), 20150207. [CrossRef]
- Chen, C.-W., Hsu, C.-Y., Lai, S.-M., et al. Metal nanobullets for multidrug resistant bacteria and biofilms. Advanced drug delivery reviews, 78, 88-104. [CrossRef]
- Salatin, S., Bazmani, A., Shahi, S., et al. Antimicrobial Benefits of Flavonoids and their Nanoformulations. Current pharmaceutical design, 28(17), 1419-1432. [CrossRef]
- Zielińska, A., Carreiró, F., Oliveira, A. M., et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules (Basel, Switzerland), 25(16), 3731. [CrossRef]
- Liu, Y., Yang, G., Jin, S., et al. Development of High-Drug-Loading Nanoparticles. ChemPlusChem, 85(9), 2143-2157. [CrossRef]
- Fu, S., Yi, X., Li, Y., et al. Berberine and chlorogenic acid-assembled nanoparticles for highly effi cient inhibition of multidrug-resistant Staphylococcus aureus. Journal of hazardous materials, 473, 134680. [CrossRef]
- Hong, Q., Zhang, W., Liu, Z., et al. (2024). Infection microenvironment-triggered nanoparticles eradicate MRSA by thermally amplified chemodynamic therapy and M1 macrophage. Journal of Nanobiotechnology, 22(1), 448. [CrossRef]
- Foster, A. L., Boot, W., Stenger, V., et al. Single-stage revision of MRSA orthopedic device-related infection in s heep with an antibiotic-loaded hydrogel. Journal of orthopaedic research : official publication of the Orthopae dic Research Society, 39(2), 438-448. [CrossRef]
- Bédouet, L., Beilvert, A., Servais, E., et al. Degradable Hydrophilic Poly(ethylene glycol) Microspheres for the Sust ained Delivery of Peptide-Based Antibiotics for Local Anti-infective T herapies. ACS infectious diseases, 11(6), 1673-1685. [CrossRef]
- Yang, Q., Xiang, X., Wang, H., et al. Oral natural material hydrogels: a new strategy for enhancing oral dru g delivery efficiency. Journal of biomaterials science. Polymer edition, 1-28. [CrossRef]
- Zhang, J., Ye, X., Li, W., et al. Copper-containing chitosan-based hydrogels enabled 3D-printed scaffold s to accelerate bone repair and eliminate MRSA-related infection. International journal of biological macromolecules, 240, 124463. [CrossRef]
- Liu, W., Gao, R., Yang, C., et al. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus-infected chronic skin wound healing. Science advances, 8(27), eabn7006. [CrossRef]
- Tu, C., Lu, H., Zhou, T., et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS -scavenging, oxygen and nitric oxide-generating properties. Biomaterials, 286, 121597. [CrossRef]
- Zhang, L., Niu, W., Lin, Y., et al. Multifunctional antibacterial bioactive nanoglass hydrogel for normal and MRSA infected wound repair. Journal of Nanobiotechnology, 21(1), 162. [CrossRef]
- Cheng, J., Wang, H., Gao, J., et al. First-Aid Hydrogel Wound Dressing with Reliable Hemostatic and Antibac terial Capability for Traumatic Injuries. Advanced healthcare materials, 12(25), e2300312. [CrossRef]
- Indelli, P. F., Iannotti, F., Ferretti, A., et al. "Recommendations for periprosthetic joint infections (PJI) prevention: the European Knee Associates (EKA)-International Committee American A ssociation of Hip and Knee Surgeons (AAHKS)-Arthroplasty Society in As ia (ASIA) survey of members". Knee surgery, sports traumatology, arthroscopy : official journal of t he ESSKA, 30(12), 3932-3943. [CrossRef]
- Brooks, J. R., Dusane, D. H., Moore, K., et al. Pseudomonas aeruginosa biofilm killing beyond the spacer by ant ibiotic-loaded calcium sulfate beads: an in vitro study. Journal of bone and joint infection, 6(5), 119-129. [CrossRef]
- Indelli, P. F., Iannotti, F., Ferretti, A., et al. "Recommendations for periprosthetic joint infections (PJI) prevention: the European Knee Associates (EKA)-International Committee American A ssociation of Hip and Knee Surgeons (AAHKS)-Arthroplasty Society in As ia (ASIA) survey of members". Knee surgery, sports traumatology, arthroscopy : official journal of t he ESSKA, 30(12), 3932-3943. [CrossRef]
- Brooks, J. R., Dusane, D. H., Moore, K., et al. <i>Pseudomonas aeruginosa</i> biofilm killing beyond the spacer by ant ibiotic-loaded calcium sulfate beads: an in vitro study. Journal of bone and joint infection, 6(5), 119-129. [CrossRef]
- Antonelli, B., Chen, A. F. Reducing the risk of infection after total joint arthroplasty: preoper ative optimization. Arthroplasty (London, England), 1(1), 4. [CrossRef]
- han, A. C., Martyn, G. D., Carter, P. J. Fifty years of monoclonals: the past, present and future of antibody t herapeutics. Nature reviews. Immunology, 10.1038/s41577-41025-01207-41579. [CrossRef]
- Zhang, Y. Evolution of phage display libraries for therapeutic antibody discover y. mAbs, 15(1), 2213793. [CrossRef]
- Kim, J. W., Min, S. W., Lee, J., et al. Development and Characterization of Phage-Display-Derived Novel Human Monoclonal Antibodies against the Receptor Binding Domain of SARS-CoV- 2. Biomedicines, 10(12), 3274. [CrossRef]
- Ferguson, M. R., Delgado, K. N., McBride, S., et al. Use of Epivolve phage display to generate a monoclonal antibody with o psonic activity directed against a subdominant epitope on extracellula r loop 4 of Treponema pallidum BamA (TP0326). Frontiers in immunology, 14, 1222267. [CrossRef]
- Keizer, R. J., Huitema, A. D. R., Schellens, J. H. M., et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clinical pharmacokinetics, 49(8), 493-507. [CrossRef]
- Kortright, K. E., Chan, B. K., Koff, J. L., et al. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacte ria. Cell host & microbe, 25(2), 219-232. [CrossRef]
- Ramirez-Sanchez, C., Gonzales, F., Buckley, M., et al. Successful Treatment of <i>Staphylococcus aureus</i> Prosthetic Joint Infection with Bacteriophage Therapy. Viruses, 13(6), 1182. [CrossRef]
- Lenneman, B. R., Fernbach, J., Loessner, M. J., et al. Enhancing phage therapy through synthetic biology and genome engineeri ng. Current opinion in biotechnology, 68, 151-159. [CrossRef]
- Erdem Büyükkiraz, M., Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial comp ounds. Journal of applied microbiology, 132(3), 1573-1596. [CrossRef]
- Xuan, J., Feng, W., Wang, J., et al. Antimicrobial peptides for combating drug-resistant bacterial infectio ns. Drug resistance updates : reviews and commentaries in antimicrobial an d anticancer chemotherapy, 68, 100954. [CrossRef]
- Gao, N., Sun, J., Li, X., et al. Overcoming delivery challenges of antimicrobial peptides for clinical translation: From nanocarriers to molecular modifications. Drug resistance updates : reviews and commentaries in antimicrobial an d anticancer chemotherapy, 83, 101289. [CrossRef]
- Sharma, L., Bisht, G. S. Short Antimicrobial Peptides: Therapeutic Potential and Recent Advance ments. Current pharmaceutical design, 29(38), 3005-3017. [CrossRef]
- Gutierrez, A. M., Frazar, E. M., X Klaus, M. V., et al. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Hu man and Environmental Treatment. Advanced healthcare materials, 11(7), e2101820. [CrossRef]
- Liang, S., Pan, Y., Wang, J., et al. (2025). Bone-targeting ZIF-8 based nanoparticles loaded with vancomycin for the treatment of MRSA-induced periprosthetic joint infection. Journal of Controlled Release, 385, 113965. [CrossRef]
- Jacqueline, C., Caillon, J., Meyer, O., et al. (2021). Efficacy of Nanoencapsulated Daptomycin in an Experimental Methicillin-Resistant Staphylococcus aureus Bone and Joint Infection Model. Antimicrobial agents and chemotherapy, 65(12), 10.1128/aac.00768-00721. [CrossRef]
- Boot, W., Schmid, T., D'Este, M., et al. (2021). A Hyaluronic Acid Hydrogel Loaded with Gentamicin and Vancomycin Successfully Eradicates Chronic Methicillin-Resistant Staphylococcus aureus Orthopedic Infection in a Sheep Model. Antimicrobial agents and chemotherapy, 65(4). [CrossRef]
- Isler, B., Welyczko, Z., Jorgensen, N., et al. Advancing the management of prosthetic joint infections: a review of r andomized controlled trials and emerging evidence. Antimicrobial agents and chemotherapy, 69(10), e0033825. [CrossRef]
- Santos-Júnior, C. D., Torres, M. D. T., Duan, Y., et al. Discovery of antimicrobial peptides in the global microbiome with mach ine learning. Cell, 187(14), 3761-3778.e3716. [CrossRef]
- Li, F., Gan, L., Yang, X., et al. Progress of AI assisted synthesis of polysaccharides-based hydrogel an d their applications in biomedical field. International journal of biological macromolecules, 287, 138643. [CrossRef]
- Jian, T., Wang, M., Hettige, J., et al. Self-Assembling and Pore-Forming Peptoids as Antimicrobial Biomaterial s. ACS nano, 18(34), 23077-23089. [CrossRef]
- Shariati, A., Moradabadi, A., Azimi, T., et al. Wound healing properties and antimicrobial activity of platelet-derive d biomaterials. Scientific reports, 10(1), 1032. [CrossRef]
- Zamora-Mendoza, L., Guamba, E., Miño, K., et al. Antimicrobial Properties of Plant Fibers. Molecules (Basel, Switzerland), 27(22), 7999. [CrossRef]
- Kelley, B., De Moor, P., Douglas, K., et al. Monoclonal antibody therapies for COVID-19: lessons learned and implic ations for the development of future products. Current opinion in biotechnology, 78, 102798. [CrossRef]
- Garfall, A. L. New Biological Therapies for Multiple Myeloma. Annual review of medicine, 75, 13-29. [CrossRef]
- Hatfull, G. F., Dedrick, R. M., Schooley, R. T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annual review of medicine, 73, 197-211. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





