Submitted:
06 October 2025
Posted:
06 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biothreats and Their History
2.1. Biowarfare and Bioterrorism
3. Particle Capture
3.1. Microfluidic-Based Capture
3.2. Condensation-Based Growth-Tube for Submicron Particles
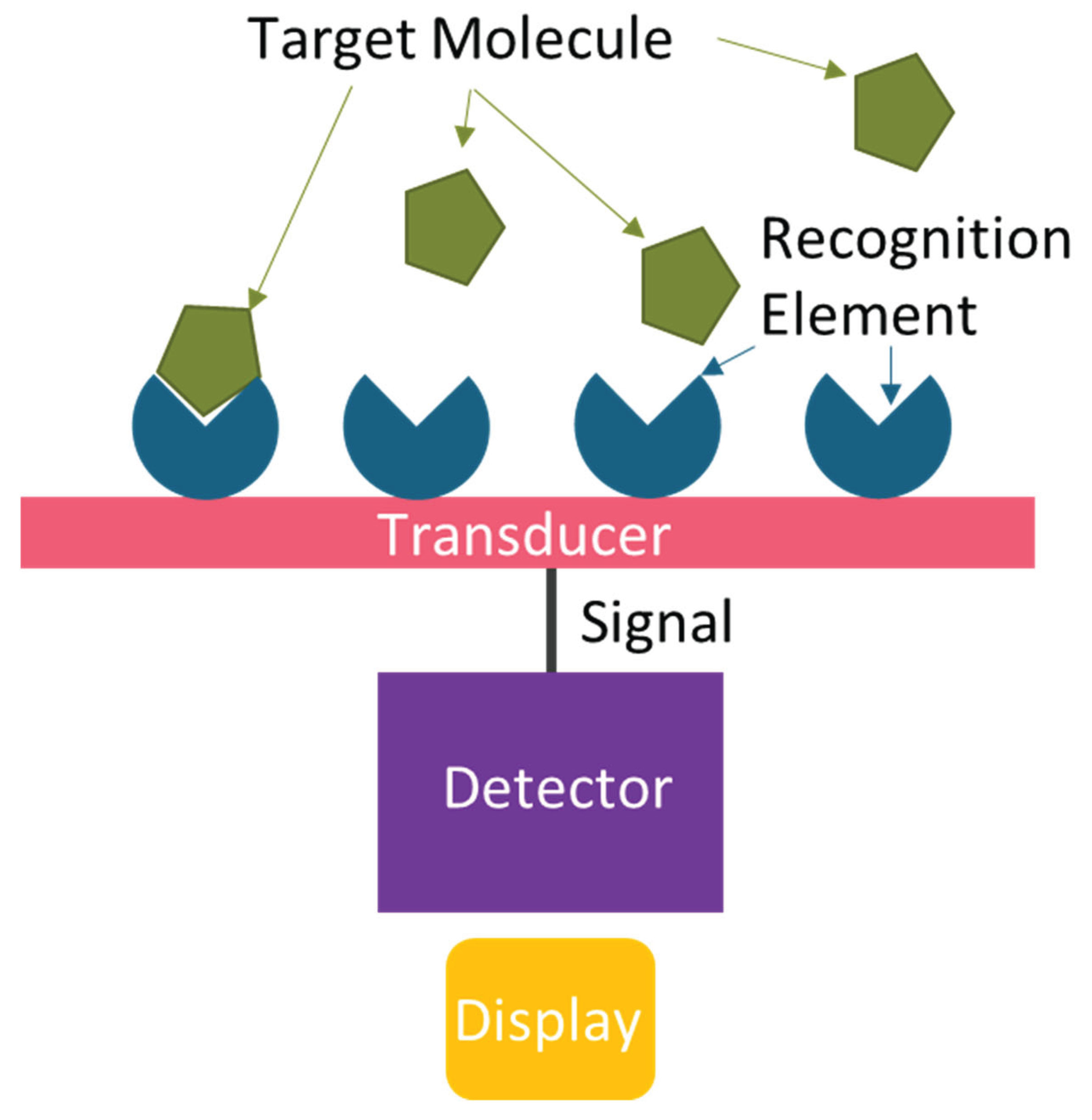
4. Biosensors
4.1. Recognition Elements
4.2. Transduction Mechanisms
4.2.1. Optical Biosensors
4.2.2. Electrochemical Biosensors
4.3. Enhancement Using Nanomaterials
5. Challenges and Future Directions
5.1. Capture Efficiency
5.2. Detection Sensitivity and Specificity
5.3. Real-Time and Continuous Monitoring
5.4. Multiplexing and Broader Detection Capabilities
5.5. Scalability and Field Deployment
5.6. Future Research Directions
6. Conclusions
Acknowledgments
References
- National Research Council Globalization, Biosecurity, and the Future of the Life Sciences; National Academies Press: Washington, DC, 2006; ISBN 9780309653885.
- Mousavian, Z.; Fahimi-Kashani, E.; Nafisi, V.; Fahimi-Kashani, N. Recent Advances in Development of Biosensors for Monitoring of Airborne Microorganisms. Iran J Biotechnol 2024, 22, e3722. [CrossRef]
- Ma, J.; Du, M.; Wang, C.; Xie, X.; Wang, H.; Zhang, Q. Advances in Airborne Microorganisms Detection Using Biosensors: A Critical Review. Front Environ Sci Eng 2021, 15, 47. [CrossRef]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for Chemists of Terms Used in Biotechnology (IUPAC Recommendations 1992). Pure and Applied Chemistry 1992, 64, 143–168. [CrossRef]
- Leffel, E.K.; Reed, D.S. Marburg and Ebola Viruses as Aerosol Threats. Biosecur Bioterror 2004, 2, 186–191. [CrossRef]
- Jernigan, D.B.; Raghunathan, P.L.; Bell, B.P.; Brechner, R.; Bresnitz, E.A.; Butler, J.C.; Cetron, M.; Cohen, M.; Doyle, T.; Fischer, M.; et al. Investigation of Bioterrorism-Related Anthrax, United States, 2001: Epidemiologic Findings. Emerging Infectious Diseases 2002, 8, 1019–1028. [CrossRef]
- Borio, L.; Inglesby, T.; Peters, C.J.; Schmaljohn, A.L.; Hughes, J.M.; Jahrling, P.B.; Ksiazek, T.; Johnson, K.M.; Meyerhoff, A.; O’Toole, T.; et al. Hemorrhagic Fever Viruses as Biological WeaponsMedical and Public Health Management. JAMA 2002, 287, 2391–2405. [CrossRef]
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin Infect Dis 2020, 71, 2311–2313. [CrossRef]
- Pan, M.; Lednicky, J.A.; Wu, C. -Y. Collection, Particle Sizing and Detection of Airborne Viruses. J. Appl. Microbiol. 2019, 127, 1596–1611. [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Warkiani, M.E.; Li, W. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab Chip 2015, 16, 10–34. [CrossRef]
- Ahasan, K.; Schnoebelen, N.J.; Shrotriya, P.; Kingston, T.A. Continuous Sampling of Aerosolized Particles Using Stratified Two-Phase Microfluidics. ACS Sens. 2024. [CrossRef]
- Bian, J.; Gui, H.; Xie, Z.; Yu, T.; Wei, X.; Wang, W.; Liu, J. Simulation of Three-Stage Operating Temperature for Supersaturation Water-Based Condensational Growth Tube. Journal of Environmental Sciences 2020, 90, 275–285. [CrossRef]
- Banerjee, S.; Hemmat, M.A.; Shubham, S.; Gosai, A.; Devarakonda, S.; Jiang, N.; Geekiyanage, C.; Dillard, J.A.; Maury, W.; Shrotriya, P.; et al. Structurally Different Yet Functionally Similar: Aptamers Specific for the Ebola Virus Soluble Glycoprotein and GP1,2 and Their Application in Electrochemical Sensing. International Journal of Molecular Sciences 2023, 24, 4627. [CrossRef]
- Walper, S.A.; Lasarte Aragonés, G.; Sapsford, K.E.; Brown, C.W.I.; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting Biothreat Agents: From Current Diagnostics to Developing Sensor Technologies. ACS Sensors 2018, 3, 1894–2024. [CrossRef]
- Piret, J.; Boivin, G. Pandemics Throughout History. Frontiers in Microbiology 2021, 11. [CrossRef]
- Alchon, S.A. A Pest in the Land: New World Epidemics in a Global Perspective; UNM Press, 2003.
- Number of Deaths Due to HIV/AIDS Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-deaths-due-to-hiv-aids.
- WHO Coronavirus (COVID-19) Dashboard Available online: https://covid19.who.int.
- Shipman, P.L. The Bright Side of the Black Death Available online: https://www.americanscientist.org/article/the-bright-side-of-the-black-death (accessed on 27 November 2023).
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. Proc (Bayl Univ Med Cent) 2005, 18, 21–25. [CrossRef]
- Dye, C. After 2015: Infectious Diseases in a New Era of Health and Development. Philos Trans R Soc Lond B Biol Sci 2014, 369, 20130426. [CrossRef]
- Christian, M.D. Biowarfare and Bioterrorism. Critical Care Clinics 2013, 29, 717–756. [CrossRef]
- Hilleman, M.R. Overview: Cause and Prevention in Biowarfare and Bioterrorism. Vaccine 2002, 20, 3055–3067. [CrossRef]
- Frischknecht, F. The History of Biological Warfare. EMBO Rep 2003, 4, S47–S52. [CrossRef]
- Jansen, H.J.; Breeveld, F.J.; Stijnis, C.; Grobusch, M.P. Biological Warfare, Bioterrorism, and Biocrime. Clinical Microbiology and Infection 2014, 20, 488–496. [CrossRef]
- Rathjen, N.A.; Shahbodaghi, S.D. Bioterrorism. American Family Physician 2021, 104, 376–385.
- Borio, L.L.; Henderson, D.A.; Hynes, N.A. Bioterrorism. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; 2015; pp. 178-190.e2.
- Barras, V.; Greub, G. History of Biological Warfare and Bioterrorism. Clinical Microbiology and Infection 2014, 20, 497–502. [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [CrossRef]
- Islam, M.S. Hybrid Separation of Circulating Tumor Cells in Zigzag and Contraction-Expansion Microfluidic Channels. M.S., Washington State University: United States -- Washington, 2022.
- Islam, M.S.; Uddin, M.R.; Chen, X. Circulating Tumor Cell Separation in a Zigzag Channel Using Dielectrophoresis Based Inertial Microfluidics.; American Society of Mechanical Engineers Digital Collection, February 8 2023.
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; Stetten, F. von; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms: Requirements, Characteristics and Applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [CrossRef]
- Ahasan, K.; Landry, C.M.; Chen, X.; Kim, J.-H. Effect of Angle-of-Attacks on Deterministic Lateral Displacement (DLD) with Symmetric Airfoil Pillars. Biomed Microdevices 2020, 22, 42. [CrossRef]
- Bhagat, A.A.S.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for Cell Separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [CrossRef]
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Global Challenges 2019, 3, 1800112. [CrossRef]
- Hamacher, T.; Berendsen, W.J.T.; Dongen, J.E. van; Hee, R.M. van der; Cornelissen, M.J.J.L.; Broekhuijse, J.M.L.W.; Segerink, L.I. Virus Removal from Semen with a Pinched Flow Fractionation Microfluidic Chip. Lab. Chip 2021, 21, 4477–4486. [CrossRef]
- Narayana Iyengar, S.; Kumar, T.; Mårtensson, G.; Russom, A. High Resolution and Rapid Separation of Bacteria from Blood Using Elasto-Inertial Microfluidics. ELECTROPHORESIS 2021, 42, 2538–2551. [CrossRef]
- Wu, Z.; Willing, B.; Bjerketorp, J.; Jansson, K.J.; Hjort, K. Soft Inertial Microfluidics for High Throughput Separation of Bacteria from Human Blood Cells. Lab. Chip 2009, 9, 1193–1199. [CrossRef]
- Islam, M.S.; Chen, X. Continuous CTC Separation through a DEP-Based Contraction–Expansion Inertial Microfluidic Channel. Biotechnology Progress 2023, 39, e3341. [CrossRef]
- Sarowar, M.T.; Islam, M.S.; Chen, X. Separation of CTCs From Blood Cells Using Curved Contraction-Expansion Microchannel Equipped With DEP Force.; American Society of Mechanical Engineers Digital Collection, February 5 2024.
- Ahasan, K.; Kim, J.-H. Study of Angle-of-Attack (AoA) for Airfoil in Deterministic Lateral Displacement (DLD). In Proceedings of the Volume 10: Micro- and Nano-Systems Engineering and Packaging; American Society of Mechanical Engineers: Salt Lake City, Utah, USA, November 11 2019; p. V010T12A020.
- Ahasan, K.; Senf, B.L.; Kim, J.-H. Controls on the Transport of Particles/Cells in Deterministic Lateral Displacement via Symmetric Airfoil with Angle of Attacks. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC); IEEE: Orlando, FL, USA, June 2020; pp. 2185–2190.
- Ahasan, K.; Islam, M.S.; Shrotriya, P.; Kingston, T.A. Stratified Two-Phase Microfluidic Device for Continuous Sampling of Sub-Micron Aerosolized Particles. Journal of Aerosol Science 2025, 106697. [CrossRef]
- Jiang, X.; Jing, W.; Sun, X.; Liu, Q.; Yang, C.; Liu, S.; Qin, K.; Sui, G. High-Throughput Microfluidic Device for LAMP Analysis of Airborne Bacteria. ACS Sensors 2016, 1, 958–962. [CrossRef]
- Jing, W.; Jiang, X.; Zhao, W.; Liu, S.; Cheng, X.; Sui, G. Microfluidic Platform for Direct Capture and Analysis of Airborne Mycobacterium Tuberculosis. Analytical Chemistry 2014, 86, 5815–5821. [CrossRef]
- Jing, W.; Zhao, W.; Liu, S.; Li, L.; Tsai, C.-T.; Fan, X.; Wu, W.; Li, J.; Yang, X.; Sui, G. Microfluidic Device for Efficient Airborne Bacteria Capture and Enrichment. Analytical Chemistry 2013, 85, 5255–5262. [CrossRef]
- Liu, Q.; Zhang, X.; Yao, Y.; Jing, W.; Liu, S.; Sui, G. A Novel Microfluidic Module for Rapid Detection of Airborne and Waterborne Pathogens. Sensors and Actuators B: Chemical 2018, 258, 1138–1145. [CrossRef]
- Liu, Q.; Zhang, Y.; Jing, W.; Liu, S.; Zhang, D.; Sui, G. First Airborne Pathogen Direct Analysis System. Analyst 2016, 141, 1637–1640. [CrossRef]
- Li, X.; Zhang, X.; Liu, Q.; Zhao, W.; Liu, S.; Sui, G. Microfluidic System for Rapid Detection of Airborne Pathogenic Fungal Spores. ACS Sensors 2018, 3, 2095–2103. [CrossRef]
- Bian, X.; Lan, Y.; Wang, B.; Zhang, Y.S.; Liu, B.; Yang, P.; Zhang, W.; Qiao, L. Microfluidic Air Sampler for Highly Efficient Bacterial Aerosol Collection and Identification. Analytical Chemistry 2016, 88, 11504–11512. [CrossRef]
- Inami, H.; Tsuge, K.; Matsuzawa, M.; Sasaki, Y.; Togashi, S.; Komano, A.; Seto, Y. Semi-Automated Bacterial Spore Detection System with Micro-Fluidic Chips for Aerosol Collection, Spore Treatment and ICAN DNA Detection. Biosensors and Bioelectronics 2009, 24, 3299–3305. [CrossRef]
- Shen, F.; Tan, M.; Wang, Z.; Yao, M.; Xu, Z.; Wu, Y.; Wang, J.; Guo, X.; Zhu, T. Integrating Silicon Nanowire Field Effect Transistor, Microfluidics and Air Sampling Techniques For Real-Time Monitoring Biological Aerosols. Environ. Sci. Technol. 2011, 45, 7473–7480. [CrossRef]
- Mirzaee, I.; Song, M.; Charmchi, M.; Sun, H. A Microfluidics-Based on-Chip Impinger for Airborne Particle Collection. Lab on a Chip 2016, 16, 2254–2264. [CrossRef]
- Kang, J.S.; Lee, K.S.; Kim, S.S.; Bae, G.-N.; Jung, J.H. Real-Time Detection of an Airborne Microorganism Using Inertial Impaction and Mini-Fluorescent Microscopy. Lab on a Chip 2014, 14, 244–251. [CrossRef]
- Damit, B. Droplet-Based Microfluidics Detector for Bioaerosol Detection. Aerosol Science and Technology 2017, 51, 488–500. [CrossRef]
- Hong, S.C.; Kang, J.S.; Lee, J.E.; Kim, S.S.; Jung, J.H. Continuous Aerosol Size Separator Using Inertial Microfluidics and Its Application to Airborne Bacteria and Viruses. Lab. Chip 2015, 15, 1889–1897. [CrossRef]
- Ma, Z.; Zheng, Y.; Cheng, Y.; Xie, S.; Ye, X.; Yao, M. Development of an Integrated Microfluidic Electrostatic Sampler for Bioaerosol. Journal of Aerosol Science 2016, 95, 84–94. [CrossRef]
- Resina-Pelfort, O.; Comas-Riu, J.; Vives-Rego, J. Effects of Deflected Droplet Electrostatic Cell Sorting on the Viability and Exoproteolytic Activity of Bacterial Cultures and Marine Bacterioplankton. Systematic and Applied Microbiology 2001, 24, 31–36. [CrossRef]
- Liu, Q.; Zhang, X.; Yao, Y.; Jing, W.; Liu, S.; Sui, G. A Novel Microfluidic Module for Rapid Detection of Airborne and Waterborne Pathogens. Sensors and Actuators B: Chemical 2018, 258, 1138–1145. [CrossRef]
- Choi, J.; Hong, S.C.; Kim, W.; Jung, J.H. Highly Enriched, Controllable, Continuous Aerosol Sampling Using Inertial Microfluidics and Its Application to Real-Time Detection of Airborne Bacteria. ACS Sensors 2017, 2, 513–521. [CrossRef]
- Ahasan, K.; Islam, M.S.; Shrotriya, P.; Kingston, T.A. Stratified Two-Phase Microfluidic Device for Continuous Sampling of Sub-Micron Aerosolized Particles. Journal of Aerosol Science 2026, 191, 106697. [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020. [CrossRef]
- Englert, N. Fine Particles and Human Health—a Review of Epidemiological Studies. Toxicol. Lett. 2004, 149, 235–242. [CrossRef]
- Kowalski, W.J.; Bahnfleth, W. Airborne Respiratory Diseases and Mechanical Systems for Control of Microbes. HPAC Heating, Piping, Air Conditioning 1998, 70.
- Chatterjee, S. Understanding the Nature of Variations in Structural Sequences Coding for Coronavirus Spike, Envelope, Membrane and Nucleocapsid Proteins of SARS-CoV-2 2020.
- Influenza Virus Pleiomorphy Characterized by Cryoelectron Tomography | PNAS Available online: https://www.pnas.org/doi/full/10.1073/pnas.0607614103 (accessed on 10 July 2025).
- Battles, M.B.; McLellan, J.S. Respiratory Syncytial Virus Entry and How to Block It. Nat Rev Microbiol 2019, 17, 233–245. [CrossRef]
- Colf, L.A.; Juo, Z.S.; Garcia, K.C. Structure of the Measles Virus Hemagglutinin. Nat Struct Mol Biol 2007, 14, 1227–1228. [CrossRef]
- Frost, J.R.; Shaikh, S.; Severini, A. Exploring the Mumps Virus Glycoproteins: A Review. Viruses 2022, 14, 1335. [CrossRef]
- Tang, J.W. The Effect of Environmental Parameters on the Survival of Airborne Infectious Agents. J. R. Soc. Interface 2009, 6, S737–S746. [CrossRef]
- Haddrell, A.E.; Lewis, D.; Church, T.; Vehring, R.; Murnane, D.; Reid, J.P. Pulmonary Aerosol Delivery and the Importance of Growth Dynamics. Therapeutic Delivery 2017, 8, 1051–1061. [CrossRef]
- Yurt, A.; Daaboul, G.H.; Connor, J.H.; Goldberg, B.B.; Ünlü, M.S. Single Nanoparticle Detectors for Biological Applications. Nanoscale 2012, 4, 715–726. [CrossRef]
- Podzimek, J. John Aitken’s Contribution to Atmospheric and Aerosol Sciences—One Hundred Years of Condensation Nuclei Counting. Bull. Am. Meteorol. Soc. 1989, 70, 1538–1545. [CrossRef]
- Esq, J.A. On Improvements in the Apparatus for Counting the Dust Particles in the Atmosphere. Proc. R. Soc. Edinb. 1890, 16, 135–172. [CrossRef]
- Ahasan, K.; Hu, H.; Shrotriya, P.; Kingston, T.A. Heterogeneous Condensation on Simplified Viral Envelope Protein Structures. ACS Appl. Mater. Interfaces 2025, 17, 27829–27838. [CrossRef]
- Tammaro, M.; Di Natale, F.; Salluzzo, A.; Lancia, A. Heterogeneous Condensation of Submicron Particles in a Growth Tube. Chem. Eng. Sci. 2012, 74, 124–134. [CrossRef]
- Thomson, W. On the Equilibrium of Vapour at a Curved Surface of Liquid. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 42, 448–452. [CrossRef]
- Holländer, W.; Dunkhorst, W.; Lödding, H.; Windt, H. Theoretical Simulation and Experimental Characterization of an Expansion-Type Kelvin Spectrometer with Intrinsic Calibration. 2002.
- Smorodin, V.Y.; Hopke, P.K. Condensation Activation and Nucleation on Heterogeneous Aerosol Nanoparticles. J. Phys. Chem. B 2004, 108, 9147–9157. [CrossRef]
- Fan, Y.; Qin, F.; Luo, X.; Lin, L.; Gui, H.; Liu, J. Heterogeneous Condensation on Insoluble Spherical Particles: Modeling and Parametric Study. Chemical Engineering Science 2013, 102, 387–396. [CrossRef]
- Agarwal, J.K.; Sem, G.J. Continuous Flow, Single-Particle-Counting Condensation Nucleus Counter. J. Aerosol Sci. 1980, 11, 343–357. [CrossRef]
- McMurry, P.H. The History of Condensation Nucleus Counters. Aerosol Science and Technology 2000, 33, 297–322. [CrossRef]
- Sinclair, D.; Hoopes, G.S. A Continuous Flow Condensation Nucleus Counter. J. Aerosol Sci. 1975, 6, 1–7. [CrossRef]
- Hering, S.V.; Stolzenburg, M.R. A Method for Particle Size Amplification by Water Condensation in a Laminar, Thermally Diffusive Flow. Aerosol Science and Technology 2005, 39, 428–436. [CrossRef]
- Hering, S.V.; Stolzenburg, M.R.; Quant, F.R.; Oberreit, D.R.; Keady, P.B. A Laminar-Flow, Water-Based Condensation Particle Counter (WCPC). Aerosol Sci. Technol. 2005, 39, 659–672. [CrossRef]
- Lewis, G.S.; Hering, S.V. Minimizing Concentration Effects in Water-Based, Laminar-Flow Condensation Particle Counters. Aerosol Sci. Technol. 2013, 47, 645–654. [CrossRef]
- Hering, S.V.; Spielman, S.R.; Lewis, G.S. Moderated, Water-Based, Condensational Particle Growth in a Laminar Flow. Aerosol Sci. Technol. 2014, 48, 401–408. [CrossRef]
- Hering, S.V.; Lewis, G.S.; Spielman, S.R.; Eiguren-Fernandez, A.; Kreisberg, N.M.; Kuang, C.; Attoui, M. Detection near 1-Nm with a Laminar-Flow, Water-Based Condensation Particle Counter. Aerosol Sci. Technol. 2017, 51, 354–362. [CrossRef]
- Hering, S.V.; Lewis, G.S.; Spielman, S.R.; Eiguren-Fernandez, A. A MAGIC Concept for Self-Sustained, Water-Based, Ultrafine Particle Counting. Aerosol Sci. Technol. 2019, 53, 63–72. [CrossRef]
- Oh, S.; Anwar, D.; Theodore, A.; Lee, J.-H.; Wu, C.-Y.; Wander, J. Development and Evaluation of a Novel Bioaerosol Amplification Unit (BAU) for Improved Viral Aerosol Collection. Journal of Aerosol Science 2010, 41, 889–894. [CrossRef]
- Pan, M.; Eiguren-Fernandez, A.; Hsieh, H.; Afshar-Mohajer, N.; Hering, S.V.; Lednicky, J.; Fan, Z.H.; Wu, C.-Y. Efficient Collection of Viable Virus Aerosol through Laminar-Flow, Water-Based Condensational Particle Growth. Journal of Applied Microbiology 2016, 120, 805–815. [CrossRef]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.-Y. Determination of the Distribution of Infectious Viruses in Aerosol Particles Using Water-Based Condensational Growth Technology and a Bacteriophage MS2 Model. Aerosol Science and Technology 2019, 53, 583–593. [CrossRef]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.-Y. Collection of Airborne Bacteria and Yeast through Water-Based Condensational Growth. Aerobiologia 2018, 34, 337–348. [CrossRef]
- Pan, M.; Bonny, T.S.; Loeb, J.; Jiang, X.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.-Y. Collection of Viable Aerosolized Influenza Virus and Other Respiratory Viruses in a Student Health Care Center through Water-Based Condensation Growth. mSphere 2017, 2, e00251-17. [CrossRef]
- Lee, S.; Park, J.; Im, H.; Jung, H. A Microfluidic ATP-Bioluminescence Sensor for the Detection of Airborne Microbes. Sensors and Actuators B: Chemical 2008, 132, 443–448. [CrossRef]
- Yu, Y.; Zhang, J.; Zhong, H. Heterogeneous Condensation of Water Vapor on Fine SiO 2 Particles in Two-Section Growth Tube. Energy Fuels 2018, 32, 12750–12757. [CrossRef]
- Tammaro, M. Heterogeneous Condensation for Submicronic Particles Abatement, 2010.
- Xu, J.; Yu, Y.; Zhang, J.; Meng, Q.; Zhong, H. Heterogeneous Condensation of Water Vapor on Particles at High Concentration. Powder Technology 2017, 305, 71–77. [CrossRef]
- Yu, Y.; Lu, Y.; Sang, C.; Xu, C.; Nie, T.; Xing, S.; Fu, C. Growth Characteristics of Submicron Particles by Water Vapor Condensation in the Multi-Section Growth Tube. Powder Technology 2024, 440, 119797. [CrossRef]
- Yu, Y.; Zhang, J.; Xu, C. Numerical Simulation on the Growth of Polydisperse Fine SiO2 Particles by Water Vapor Condensation. Powder Technology 2021, 385, 537–545. [CrossRef]
- Kwon, H.-B.; Yoo, S.-J.; Kim, Y.-J. Microfluidic Condensation Nanoparticle Counter Using Water as the Condensing Liquid for Assessing Individual Exposure to Airborne Nanoparticles. Lab on a Chip 2020, 20, 1092–1102. [CrossRef]
- Kwon, H.-B.; Yoo, S.-J.; Hong, U.-S.; Kim, K.; Han, J.; Kim, M.-K.; Kang, D.-H.; Hwang, J.; Kim, Y.-J. MEMS-Based Condensation Particle Growth Chip for Optically Measuring the Airborne Nanoparticle Concentration. Lab on a Chip 2019, 19, 1471–1483. [CrossRef]
- Balendra, S.; Kale, A.; Pongetti, J.; Kazemimanesh, M.; Haugen, M.; Weller, L.; Boies, A. Condensation Particle Counters: Exploring the Limits of Miniaturisation. Journal of Aerosol Science 2024, 175, 106266. [CrossRef]
- Yoo, S.-J.; Oh, J.; Hong, S.-J.; Kim, M.; Hwang, J.; Kim, Y.-J. Microfluidics-Based Condensation Bioaerosol Sampler for Multipoint Airborne Virus Monitoring. Biosensors and Bioelectronics 2024, 264, 116658. [CrossRef]
- Chen, L.; Zhou, Q.; Li, G.; Chang, L.; Chen, L.; Li, Y. Investigation into Detection Efficiency Deviations in Aviation Soot and Calibration Particles Based on Condensation Particle Counting. Symmetry 2024, 16, 244. [CrossRef]
- Li, Y.; Chen, X.; Wu, J.; Zhang, Q.; Zhang, Z.; Hao, J.; Jiang, J. A Convertible Condensation Particle Counter Using Alcohol or Water as the Working Fluid. Aerosol Science and Technology 2025, 59, 185–194. [CrossRef]
- Chen, K.; Xu, W.; Wang, J.; Li, Q.; Lin, Y. Optimization of Guide Blades Structure for Swirl Growth Tube Based on Revised Steam Phase Change Particle Agglomeration Model. Chemical Engineering Research and Design 2025, 213, 66–77. [CrossRef]
- Dai, A.; Zhang, J.; Li, A. Growth Characteristics of Atmospheric Fine Particles in Turbulent Water Vapor Environment. Journal of Environmental Engineering 2024, 150, 04024033. [CrossRef]
- Wang, Q.; Wang, L.; Wu, H.; Yang, H. Promoting Fine Particle Removal in Double-Tower Cascade Wet Flue Gas Desulfurization System by Flue Gas Temperature Reduction. Powder Technology 2020, 373, 581–589. [CrossRef]
- Yang, L.; Bao, J.; Yan, J.; Liu, J.; Song, S.; Fan, F. Removal of Fine Particles in Wet Flue Gas Desulfurization System by Heterogeneous Condensation. Chemical Engineering Journal 2010, 156, 25–32. [CrossRef]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A Review of Methods for the Detection of Pathogenic Microorganisms. 2019. [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; S. Kaushik, K.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [CrossRef]
- Parida, M.M.; Dash, P.K.; Shukla, J. Advance Detection Technologies for Select Biothreat Agents. Handbook on Biological Warfare Preparedness 2020, 83–102. [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosensors and Bioelectronics 2020, 159, 112214. [CrossRef]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023.
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J Nat Med 2018, 72, 32–42. [CrossRef]
- Garibyan, L.; Avashia, N. Research Techniques Made Simple: Polymerase Chain Reaction (PCR). J Invest Dermatol 2013, 133, e6. [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem 2016, 60, 1–8. [CrossRef]
- Mehrotra, P. Biosensors and Their Applications – A Review. Journal of Oral Biology and Craniofacial Research 2016, 6, 153. [CrossRef]
- Naresh, Varnakavi.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors (Basel) 2021, 21, 1109. [CrossRef]
- Samuel, V.R.; Rao, K.J. A Review on Label Free Biosensors. Biosensors and Bioelectronics: X 2022, 11, 100216. [CrossRef]
- Morales, M.A.; Mark Halpern, J. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug Chem 2018, 29, 3231–3239. [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and Antibody-Derived Analytical Biosensors. Essays Biochem 2016, 60, 9–18. [CrossRef]
- Clark Jr., L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Annals of the New York Academy of Sciences 1962, 102, 29–45. [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors (Basel) 2016, 16, 780. [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [CrossRef]
- Xu, Y.; Jiang, X.; Zhou, Y.; Ma, M.; Wang, M.; Ying, B. Systematic Evolution of Ligands by Exponential Enrichment Technologies and Aptamer-Based Applications: Recent Progress and Challenges in Precision Medicine of Infectious Diseases. Front Bioeng Biotechnol 2021, 9, 704077. [CrossRef]
- Ning, Y.; Hu, J.; Lu, F. Aptamers Used for Biosensors and Targeted Therapy. Biomed Pharmacother 2020, 132, 110902. [CrossRef]
- Sett, A.; Das, S.; Sharma, P.; Bora, U. Aptasensors in Health, Environment and Food Safety Monitoring. Open Journal of Applied Biosensor 2012, 1, 9–19. [CrossRef]
- De Penning, S.; Murphy, M.P.; Kingston, T.A.; Nilsen-Hamilton, M.; Shrotriya, P. Influence of Crosslinker on Aptamer Immobilization and Aptasensor Sensing Response for Non-Metallic Surfaces. Biosensors and Bioelectronics 2025, 270, 116933. [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [CrossRef]
- Lv, L.; Xu, Z.; Zhou, Y.; Wu, J.; Zhang, X.; Qi, H. A Biosensor Based on Commercial R-SAW for Rapid and Sensitive Detection of E. Coli. Chemosensors 2025, 13, 308. [CrossRef]
- Alanazi, N.; Almutairi, M.; Alodhayb, A.N. A Review of Quartz Crystal Microbalance for Chemical and Biological Sensing Applications. Sens Imaging 2023, 24, 10. [CrossRef]
- Liu, Y.; Tuleouva, N.; Ramanculov, E.; Revzin, A. Aptamer-Based Electrochemical Biosensor for Interferon Gamma Detection. Anal. Chem. 2010, 82, 8131–8136. [CrossRef]
- Sassolas, A.; Leca-Bouvier, B.D.; Blum, L.J. DNA Biosensors and Microarrays. Chem. Rev. 2008, 108, 109–139. [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive Optical Biosensors for Unlabeled Targets: A Review. Analytica Chimica Acta 2008, 620, 8–26. [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem 2016, 60, 91–100. [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.; Kajikawa, K.; Mihara, H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays (Basel) 2015, 4, 228–244. [CrossRef]
- Sikora, T.; Osuchowski, Ł.; Maziejuk, M.; Lisowski, W. Mobile Device for Detection of Biological Threat. In Proceedings of the 2017 International Carnahan Conference on Security Technology (ICCST); October 2017; pp. 1–4.
- Petrovszki, D.; Valkai, S.; Gora, E.; Tanner, M.; Bányai, A.; Fürjes, P.; Dér, A. An Integrated Electro-Optical Biosensor System for Rapid, Low-Cost Detection of Bacteria. Microelectronic Engineering 2021, 239–240, 111523. [CrossRef]
- Janik, M.; Brzozowska, E.; Czyszczoń, P.; Celebańska, A.; Koba, M.; Gamian, A.; Bock, W.J.; Śmietana, M. Optical Fiber Aptasensor for Label-Free Bacteria Detection in Small Volumes. Sensors and Actuators B: Chemical 2021, 330, 129316. [CrossRef]
- Fernández Blanco, A.; Hernández Pérez, M.; Moreno Trigos, Y.; García-Hernández, J. Development of Optical Label-Free Biosensor Method in Detection of Listeria Monocytogenes from Food. Sensors 2023, 23, 5570. [CrossRef]
- Shen, R.; Hui, W.; Wu, W.; Yang, N.; Lin, X.; Mak, P.-I.; Martins, R.P.; Liu, A.; Jia, Y. A Cost-Effective and Field-Deployable Sensing System for Chip-Integrated Detection of Bacteria with the Naked Eye. Sensors and Actuators B: Chemical 2024, 410, 135668. [CrossRef]
- Jiao, C.; Li, X.; Zhang, Z.; Wu, Y.; Ren, X.; Pawliszyn, J.; Zeng, J. Field-Deployable Immuno-Solid-Phase Microextraction Coupled with Photothermal Imaging for Rapid Pathogen Surveillance in Environmental and Clinical Matrices. Anal. Chem. 2025, 97, 20602–20610. [CrossRef]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-Based Biosensors: A Critical Review and Perspectives. TrAC Trends in Analytical Chemistry 2016, 79, 317–325. [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials (Basel) 2018, 11, 448. [CrossRef]
- Lazcka, O.; Campo, F.J.D.; Muñoz, F.X. Pathogen Detection: A Perspective of Traditional Methods and Biosensors. Biosensors and Bioelectronics 2007, 22, 1205–1217. [CrossRef]
- Turner, A.P.F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors - Sensor Principles and Architectures. Sensors (Basel) 2008, 8, 1400–1458. [CrossRef]
- Setterington, E.B.; Alocilja, E.C. Electrochemical Biosensor for Rapid and Sensitive Detection of Magnetically Extracted Bacterial Pathogens. Biosensors 2012, 2, 15–31. [CrossRef]
- Pintavirooj, C.; Vongmanee, N.; Sukjee, W.; Sangma, C.; Visitsattapongse, S. Biosensors for Klebsiella Pneumoniae with Molecularly Imprinted Polymer (MIP) Technique. Sensors (Basel) 2022, 22, 4638. [CrossRef]
- Gao, J.; Jeffries, L.; Mach, K.E.; Craft, D.W.; Thomas, N.J.; Gau, V.; Liao, J.C.; Wong, P.K. A Multiplex Electrochemical Biosensor for Bloodstream Infection Diagnosis. SLAS Technol 2017, 22, 466–474. [CrossRef]
- Wei, H.; Bu, S.; Zhang, W.; Ma, L.; Liu, X.; Wang, Z.; Li, Z.; Hao, Z.; He, X.; Wan, J. An Electrochemical Biosensor for the Detection of Pathogenic Bacteria Based on Dual Signal Amplification of Cu3(PO4)2-Mediated Click Chemistry and DNAzymes. Analyst 2021, 146, 4841–4847. [CrossRef]
- Hannah, A.J.; Ward, A.C.; Connolly, P. Rapidly Detected Common Wound Pathogens via Easy-to-Use Electrochemical Sensors. Journal of Biomedical Engineering and Biosciences (JBEB) 2021, 8, 11–20. [CrossRef]
- Wu, Q.; Ya, Y.; Jin, C.; Zhao, Y.; Yan, F.; Feng, D.; Huang, K.-J.; Xie, S.; Tan, X. From Leaf to Lab-on-Cloth: Spatial DNA Nanorobotics and 2D Graphyne Synergy Enable Ultra-Precise Electrochemical Tracking of Sugarcane Pokkah Boeng Disease. Biosensors and Bioelectronics 2025, 283, 117548. [CrossRef]
- Qiu, W.; Xu, H.; Takalkar, S.; Gurung, A.S.; Liu, B.; Zheng, Y.; Guo, Z.; Baloda, M.; Baryeh, K.; Liu, G. Carbon Nanotube-Based Lateral Flow Biosensor for Sensitive and Rapid Detection of DNA Sequence. Biosensors and Bioelectronics 2015, 64, 367–372. [CrossRef]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.-C.; Cheng, Y.-W.; Sun, H.-Y.; et al. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. Nano Lett 2021, 21, 2272–2280. [CrossRef]
- Jiang, N.; Shrotriya, P.; Dassanayake, R.P. NK-Lysin Antimicrobial Peptide-Functionalized Nanoporous Alumina Membranes as Biosensors for Label-Free Bacterial Endotoxin Detection. Biochemical and Biophysical Research Communications 2022, 636, 18–23. [CrossRef]
- Anisuzzaman, S.; Alimoradi, N.; Singappuli-Arachchige, D.; Banerjee, S.; Pogorelko, G.V.; Kaiyum, Y.A.; Johnson, P.E.; Shrotriya, P.; Nilsen-Hamilton, M. Pyoverdine Binding Aptamers and Label-Free Electrochemical Detection of Pseudomonads. Front. Chem. 2024, 12. [CrossRef]
- Gosai, A.; Hau Yeah, B.S.; Nilsen-Hamilton, M.; Shrotriya, P. Label Free Thrombin Detection in Presence of High Concentration of Albumin Using an Aptamer-Functionalized Nanoporous Membrane. Biosensors and Bioelectronics 2019, 126, 88–95. [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous Anodic Aluminum Oxide for Chemical Sensing and Biosensors. TrAC Trends in Analytical Chemistry 2013, 44, 25–38. [CrossRef]
- Zelada-Guillén, G.A.; Blondeau, P.; Rius, F.X.; Riu, J. Carbon Nanotube-Based Aptasensors for the Rapid and Ultrasensitive Detection of Bacteria. Methods 2013, 63, 233–238. [CrossRef]
| Date (Year) | Epidemics / Pandemics | Pathogen | Death Toll (Millions) |
|---|---|---|---|
| 165–180 | Antonine Plague | Variola virusa | 5–10 |
| 541–549 | Plague of Justinian | Yersinia pestis | 15–100 |
| 735–737 | Japanese smallpox | Variola virus | 2 |
| 1346–1353 | Black Death | Yersinia pestis | 75–200 |
| 1519–1520 | Mexico smallpox epidemic | Variola virus | 5–8 |
| 1545–1548 | Cocoliztli epidemic | Salmonella entericaa | 5–15 |
| 1576–1580 | Cocoliztli epidemic | Salmonella entericaa | 2–2.5 |
| 1629–1631 | Italian plague | Yersinia pestis | 1 |
| 1656–1658 | Naples Plague | Yersinia pestis | 1.25 |
| 1772–1773 | Persian Plague | Yersinia pestis | 2 |
| 1846–1860 | Cholera pandemic | Vibrio cholerae | 1+ |
| 1855–1960 | Third Plague Pandemic | Yersinia pestis | 12–15 |
| 1889–1890 | Flu pandemic | Influenza A/H3N8a | 1 |
| 1918–1920 | Spanish flu | Influenza A/H1N1 | 17–100 |
| 1918–1922 | Russia typhus epidemic | Rickettsia prowazekii | 2–3 |
| 1957–1958 | Influenza pandemic | Influenza A/H2N2 | 1–4 |
| 1968–1969 | Hong Kong flu | Influenza A/H3N2 | 1–4 |
| 1981–present | HIV/AIDS pandemic | HIV-1 | 44+ (as of July 2025) |
| 2019–present | COVID-19 pandemic | SARS-CoV-2 | 7 (as of June 2025) |
| Category | Disease | Pathogen | Historical Abuse |
|---|---|---|---|
| A | Anthrax | Bacillus anthracis | World War I, World War II, Soviet Union (1979), Japan (1995), USA (2001) |
| Botulism | Clostridium botulinum | - | |
| Hemorrhagic Fever | Marburg virus | Soviet bioweapons program | |
| - | Ebola virus | - | |
| - | Arenaviruses | - | |
| Plague | Yersinia pestis | Fourteenth-century Europe, World War II | |
| Smallpox | Variola major | Eighteenth-century North America | |
| Tularemia | Francisella tularensis | World War II | |
| B | Brucellosis | Brucella | - |
| Cholera | Vibrio cholera | World War II | |
| Encephalitis | Alphaviruses | World War II | |
| Food Poisoning | Salmonella, Shigella | World War II, USA (1990s) | |
| Glanders | Burkholderia mallei | World War I, World War II | |
| Psittacosis | Chlamydia psittaci | - | |
| Q Fever | Coxiella burnetii | - | |
| Typhus | Rickettsia prowazekii | World War II | |
| Various Toxic Syndromes | Various bacteria | World War II | |
| C | Emerging pathogens | ||
| Operating Mechanism | Microfluidic Design | Target Particle / Organism | Advantages / Limitations |
|---|---|---|---|
| Inertia-based / Passive mixing | Staggered herringbone microchannels [45,46,47,48,49] | E. coli, M. smegmatis, M. tuberculosis | Simple design, efficient for bacteria; longer capture times (1–3 h) |
| Herringbone microchannels [50] | Aspergillus niger spores | Good for spores; limited real-time sensitivity | |
| Three-loop spiral with herringbone & sawtooth [51] | General aerosol particles | Improved mixing; manual steps required | |
| On-chip impinger [54] | Microorganisms | Moderate throughput; simple operation | |
| Inertial forces + mini fluorescent microscopy [55] | Microorganisms | Real-time detection; limited scale | |
| Droplet-based + wet-cyclone sampler [56] | Particles 2–5 µm | Captures medium particles; poor submicron efficiency | |
| Size-based separation microfluidic system [57] | Submicron particles | Size-selective; complex operation, ~70% efficiency | |
| U-shaped stratified liquid stream [61] | Particles 0.6–2.1 µm | High submicron efficiency; more complex channel design | |
| U-shaped stratified flow microchannel [11,62] | Aerosol particles | Validated numerically & experimentally; improved capture | |
| Electrostatic | Electrostatic microfluidic sampler [58] | Particles <5 µm | Can target small particles; may alter biological properties, ~40% efficiency |
| Filtration-based | PDMS microfilter-based membrane [60] | General bioaerosols | Simple and passive; low throughput, time-intensive |
| Sensor-based / Electrical | Silicon nanowire FET + microfluidic [53] | Influenza virus | Enables electrical detection; limited sensitivity (20–30% signal increase) |
| Integrated / Multi-step | Semi-automated microfluidic chip [52] | Spores | Combines collection & amplification; multi-step, moderate throughput |
| Recognition Element | Typical Targets | Advantages | Limitations | Field-Deployability |
| Antibodies | Proteins, toxins, pathogens | High specificity and affinity; well-established | Expensive; sensitive to temperature, pH, humidity | Low–Moderate |
| Enzymes | Toxins, metabolites, small molecules | Rapid signal; high catalytic specificity | Short shelf life; denatures easily | Low |
| DNA/RNA Probes | Nucleic Acid Sequences | Sequence-specific; strong specificity | Limited to known nucleic acid targets; cannot detect whole pathogens | Moderate |
| Aptamers | Proteins, toxins, pathogens, small molecules | Chemically stable; easily modified; high affinity; suitable for portable devices | SELEX can be slow; some non-specific adsorption | High |
| Molecularly Imprinted Polymers (MIPs) | Small molecules, proteins, toxins | Synthetic and robust; long shelf life; inexpensive | Lower selectivity; less sensitive in complex samples | High |
| Author | Sensor Type | Target(s) | Performance (LoD / Time) | Specificity | Nanomaterial |
|---|---|---|---|---|---|
| Sikora et al. [141] | Optical | Multiple bacteria | 10²–10⁶ CFU/mL / Real-time | High | None |
| Petrovszki et al. [142] | Optical | E. coli | ~10² CFU/mL / N/A | High | None |
| Janik et al. [143] | Optical | E. coli O157:H7 | 10 CFU/mL / N/A | High | None |
| Fernández Blanco et al. [144] | Optical | Listeria monocytogenes | 10² CFU/mL / 4 h | High | None |
| Shen et al. [145] | Optical | E. coli, S. enterica | 3 copies / Few hours | High | None |
| Jiao et al. [146] | Optical | SARS-CoV-2 N, Flu A NP | 68–75 pg/mL / 15 min | High | None |
| Qiu et al. [158] | Optical | Specific DNA sequences | 40 pM / Minutes | High | Multi-walled carbon nanotubes (MWCNTs) |
| Pinals et al. [159] | Optical | SARS-CoV-2 spike protein | 12.6 nM / Minutes | High | Single-walled carbon nanotubes (SWCNTs) |
| Setterington & Alocilja [152] | Electrochemical | Bacillus cereus, E. coli O157:H7 | 40 CFU/mL / 65 min (B. cereus); 6 CFU/mL / 65 min (E. coli) | Moderate | None |
| Pintavirooj et al. [153] | Electrochemical | Klebsiella pneumoniae | 0.012 CFU/mL / N/A | High | None |
| Gao et al. [154] | Electrochemical | Bloodstream bacteria (16S rRNA) | 290 CFU/mL / 1 h | High | None |
| Wei et al. [155] | Electrochemical | Salmonella typhimurium | 10 CFU/mL / N/A | High | None |
| Hannah et al. [156] | Electrochemical | Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus | 7.4 × 10⁶ CFU/mL / 1–2.5 h | Moderate | None |
| Wu et al. [157] | Electrochemical | Pokkah boeng pathogen (sugarcane) | 16.6 aM / N/A | High | None |
| Jiang et al. [160] | Electrochemical | LPS (Gram-negative), LTA (Gram-positive) | 10 ng/mL / N/A | High | NAAO |
| Anisuzzaman et al. [161] | Electrochemical | Pyoverdine Pf5 (Pseudomonas) | 1.3 nM / N/A | High | NAAO |
| Banerjee et al. [13] | Electrochemical | Ebola virus sGP, GP1,2 | 150 pM / N/A | High | NAAO |
| Gosai et al. [162] | Electrochemical | α-Thrombin | 10 pM / N/A | High | NAAO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





