1. Introduction
Allergic rhinitis (AR) is a chronic, IgE-mediated type 2 inflammatory disease, commonly associated with OME [
1]. Inflammatory cytokines such as IL-4 and IL-13 may impair Eustachian tube function, leading to middle ear effusion, ear fullness, and conductive hearing loss. Current treatments for AR include allergen avoidance, H1-antihistamines, intranasal corticosteroids, allergen-specific immunotherapy (AIT), and patient education. However, despite standard-of-care (SoC) therapy, 62% of patients report dissatisfaction due to limited long-term efficacy and persistent residual symptoms [
2]. Stapokibart (CM310) is a humanized antibody that targets IL-4Rα and effectively inhibits its interaction with both IL-4 and IL-13(key cytokines involved in type 2 inflammation) [
3,
4]. It has been approved in China for Atopic Dermatitis (AD), Chronic Rhinosinusitis with Nasal Polyps (CRSwNP), and Seasonal AR(SAR). However, its effect on OME-related symptoms has not been reported [
3,
4]. This case explores the possible therapeutic value of Stapokibart in alleviating ear fullness secondary to type 2 inflammation. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
2. Case Report Description
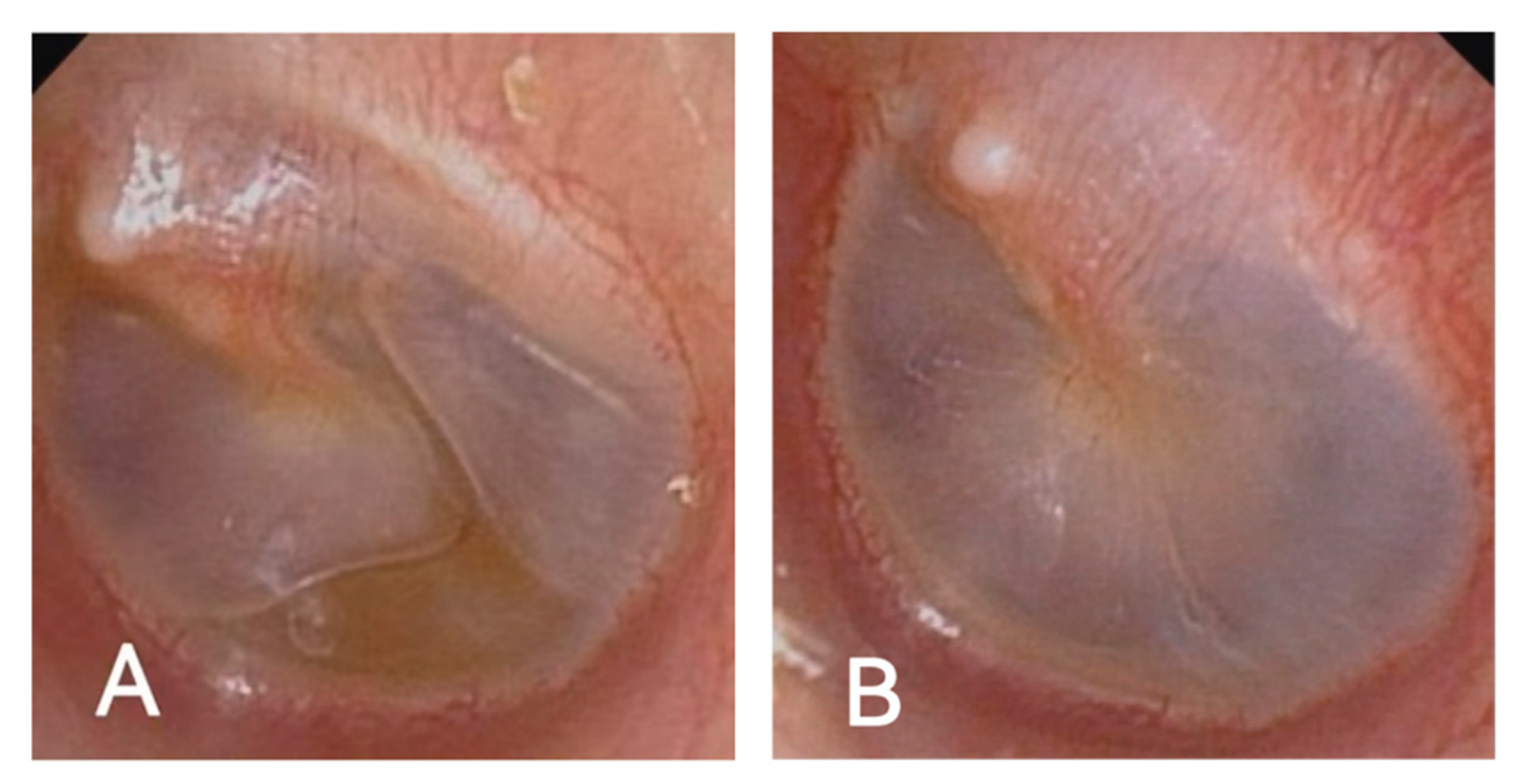
A 60-year-old man presented with recurrent bilateral aural fullness, hearing loss, and tinnitus. He had a 15-year history of allergic rhinitis and CRSwNP, and multiple asthma exacerbations. His symptoms had been recurrent and refractory to conventional therapies, including tympanic puncture, insufflation, and corticosteroid treatment. On otoscopic examination, both tympanic membranes appeared dull with visible effusion (
Figure 1A). Audiometry revealed conductive hearing loss with a B-type tympanogram in the left ear and A-type on the right. His peripheral eosinophil count was mildly elevated (0.13×10⁹/L), and Phadiatop screening was negative. He was diagnosed with OME, likely secondary to type 2 inflammation.
The patient was initiated on Stapokibart (CM310), a humanized anti-IL-4Rα monoclonal antibody, on 13 March 2025 (600 mg loading dose, followed by 300 mg biweekly).By June, his ear congestion symptoms had improved significantly. Otoscopic examination showed that both eardrums were intact with no obvious fluid accumulation(
Figure 1B).Tympanometry showed A-type curves bilaterally. Audiometric testing indicated an improvement in hearing thresholds, particularly in the left ear. At the most recent follow-up on 8 July 2025, he had received nine injections, with no recurrence of ear symptoms. CT imaging still showed mild sinusitis, and eosinophils remained mildly elevated.
Figure 2.
(A)Otoscopic image taken in February, demonstrating bilateral middle ear effusion. (B) Otoscopic images showing resolution of middle ear effusion in both ears, June 2025.
Figure 2.
(A)Otoscopic image taken in February, demonstrating bilateral middle ear effusion. (B) Otoscopic images showing resolution of middle ear effusion in both ears, June 2025.
3. Discussion
Based on the patient’s history and imaging, his ear symptoms were closely linked to Eustachian tube dysfunction, a well-recognized complication in upper airway inflammatory disorders such as CRSwNP and allergic rhinitis. The underlying mechanism is often attributable to edema and obstruction of the nasal and nasopharyngeal mucosa, which disrupts pressure regulation in the middle ear [
5]. The sensation of blockage in patient’s ear may be attributable to Eustachian tube dysfunction, a condition often associated with conductive hearing loss. Eustachian tube dysfunction should be considered a diagnosis of exclusion. Sinonasal inflammation—often transient and reversible—can precipitate dysfunction of the Eustachian tube, particularly when triggered by allergic responses [
6,
7].
Importantly, this patient had multiple type 2 inflammation–related conditions, including CRSwNP, allergic rhinitis, and asthma. Previous studies have shown that IL-4 and IL-13 are key cytokines driving type 2 inflammation, leading to chronic mucosal inflammation in the nasal cavity, paranasal sinuses, and middle ear [
2]. This inflammatory process can impair Eustachian tube patency and promote the development and persistence of OME. Conventional management—including polypectomy, anti-inflammatory medications, and physical ventilation therapies—may provide only short-term relief, as they do not address the underlying, sustained inflammatory drive, resulting in a high recurrence rate.
In this case, following failure of standard therapy, the patient was started on Stapokibart. Although Stapokibart is currently approved in China for atopic dermatitis, CRSwNP, and seasonal allergic rhinitis, its efficacy in OME has not been reported [
4]. In this patient, treatment was followed by marked resolution of bilateral aural fullness, normalization of tympanograms to A-type bilaterally, improvement in hearing thresholds, and sustained symptom control throughout follow-up without relapse.
The clinical course in this patient suggests that IL-4Rα inhibition may not only control sinonasal inflammation but also improve middle ear conditions secondary to Eustachian tube dysfunction, offering a novel therapeutic avenue for recurrent, treatment-refractory OME. Although this indication is not yet approved, these findings provide preliminary support for its potential role and highlight the need for prospective clinical trials to confirm its efficacy and safety. This case also reinforces the concept of unified airway disease and the importance of comprehensive management across the upper and lower airway in type 2 inflammatory disorders.
5. Conclusions
Otitis media is a common comorbidity in patients with CRSwNP [
8]. Type 2 inflammation, driven by IL-4 and IL-13, plays a key role in chronic nasal and middle ear pathology [
2]. This patient’s symptoms correlated with rhinitis exacerbations, indicating a unified airway involvement. Traditional therapies failed to provide long-term relief. Following Stapokibart treatment, symptoms markedly improved, suggesting potential benefits of IL-4Rα inhibition in restoring Eustachian tube function and resolving middle ear effusion. Although not currently an approved indication, this case provides preliminary evidence supporting Stapokibart's potential utility in managing OME symptoms in type 2 inflammatory conditions. Further clinical trials are needed to confirm its efficacy and safety in this context.
Author Contributions
Yiyun Zhang, Mengwen Shi and Yan Zhou: preparation, creation, and/or presentation of the published work, specifically writing the initial draft. Janjun Chen : ideas; formulation or evolution of overarching research goals and aims. Huabin Li and Yu Sun : ideas; formulation or evolution of overarching research goals and aims. Oversight and leadership responsibility for the research activity planning, including mentorship external to the core team.
Funding
This research was funded by the Key Program of the National Natural Science Foundation of China (No. 82430035), the Foundation for Innovative Research Groups of Hubei Province (No. 2023AFA038), the National Key Research and Development Program of China (Nos. 2021YFF0702303, 2021YFF0702301, 2024YFC2511100 /2024YFC2511101), and the Fundamental Research Funds for the Central Universities (No.2024BRA019).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Our institution does not require ethics approval for reporting individual cases or case series.
Informed Consent Statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OME |
Otitis Media with Effusion |
| CRSwNP |
Chronic Rhinosinusitis with Nasal Polyps |
| AD |
Atopic Dermatitis |
| SAR |
Seasonal Allergic Rhinitis |
References
- A Greiner, A.N.; Hellings, P.W.; Rotiroti, G.; Scadding, G.K. Allergic rhinitis. Lancet 2011, 378, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Li, H.; et al. Stapokibart for moderate-to-severe seasonal allergic rhinitis: a randomized phase 3 trial. Nat. Med. 2025, 31, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Stapokibart: first approval. Drugs 2025, 85, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.; Ahmad, I. Updates in eustachian tube dysfunction. Otolaryngol. Clin. N. Am. 2022, 55, 1151–1164. [Google Scholar]
- Sproat, R.; Burgess, C.; Lancaster, T.; Martinez-Devesa, P. Eustachian tube dysfunction in adults. BMJ 2014, 348, g1647. [Google Scholar] [CrossRef]
- Wang, E.; Liu, B.; Wang, Y.; Yao, W.; Sun, Y. Occlusion of the lateral semicircular canal through the external ear canal: a case report. Ear Nose Throat J. 2022, 101, NP447–NP450. [Google Scholar] [CrossRef] [PubMed]
- Rovers, M.M.; Schilder, A.G.; Zielhuis, G.A.; Rosenfeld, R.M. Otitis media. Lancet 2004, 363, 465–473. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).