Submitted:
22 September 2025
Posted:
24 September 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Anti-HER2 Therapies
Monoclonal Antibodies (MAB)
New Anti-HER2 mAB (Monoclonal Antibody)
Antibody-Drug Conjugate (ADCs)
- -
- Old Generation ADCs
- -
- New Generation ADCs
Tyrosine Kinase Inhibitor (TKI)
- -
- Old Generation TKI
- -
- New Generation TKI
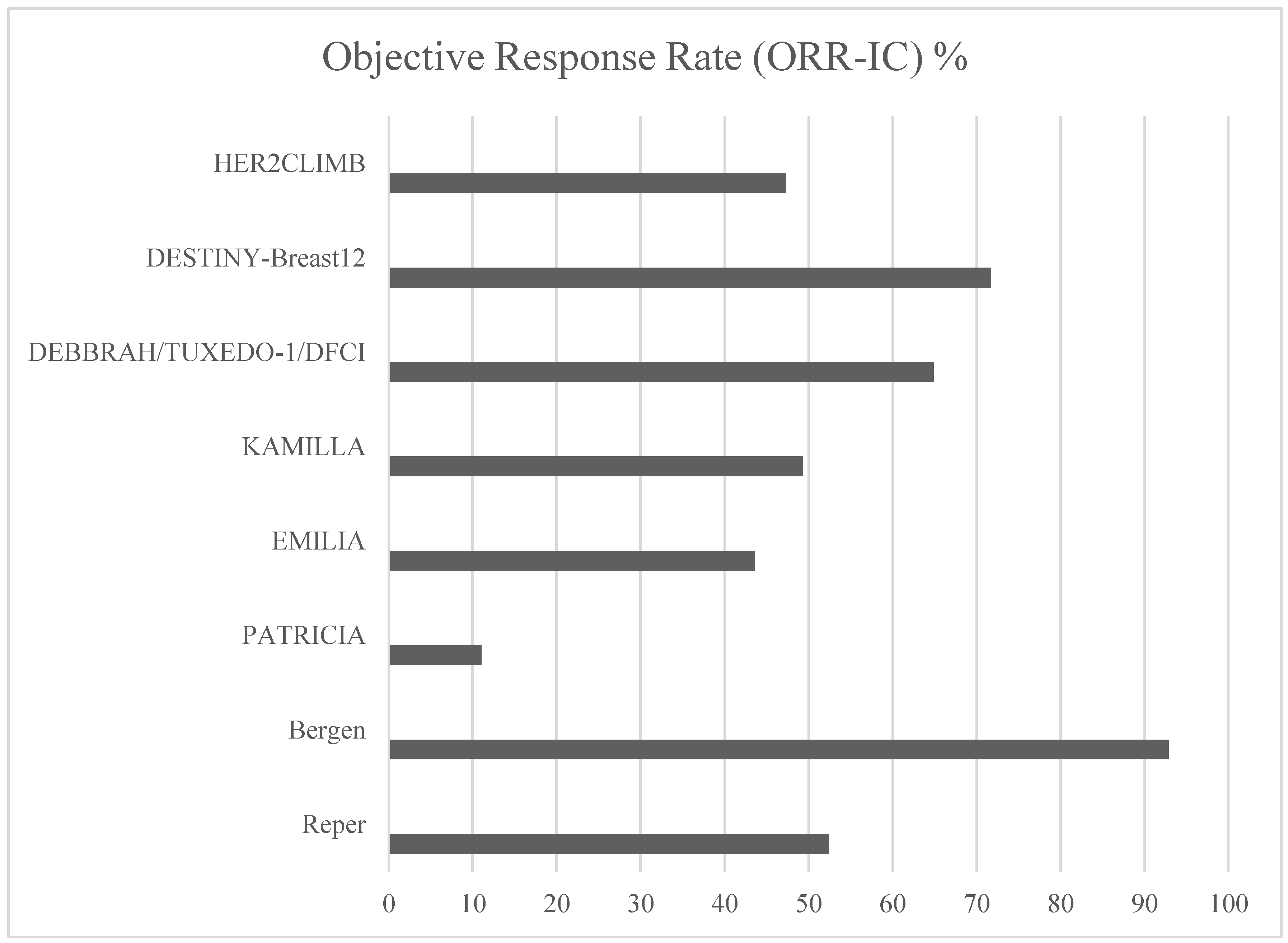
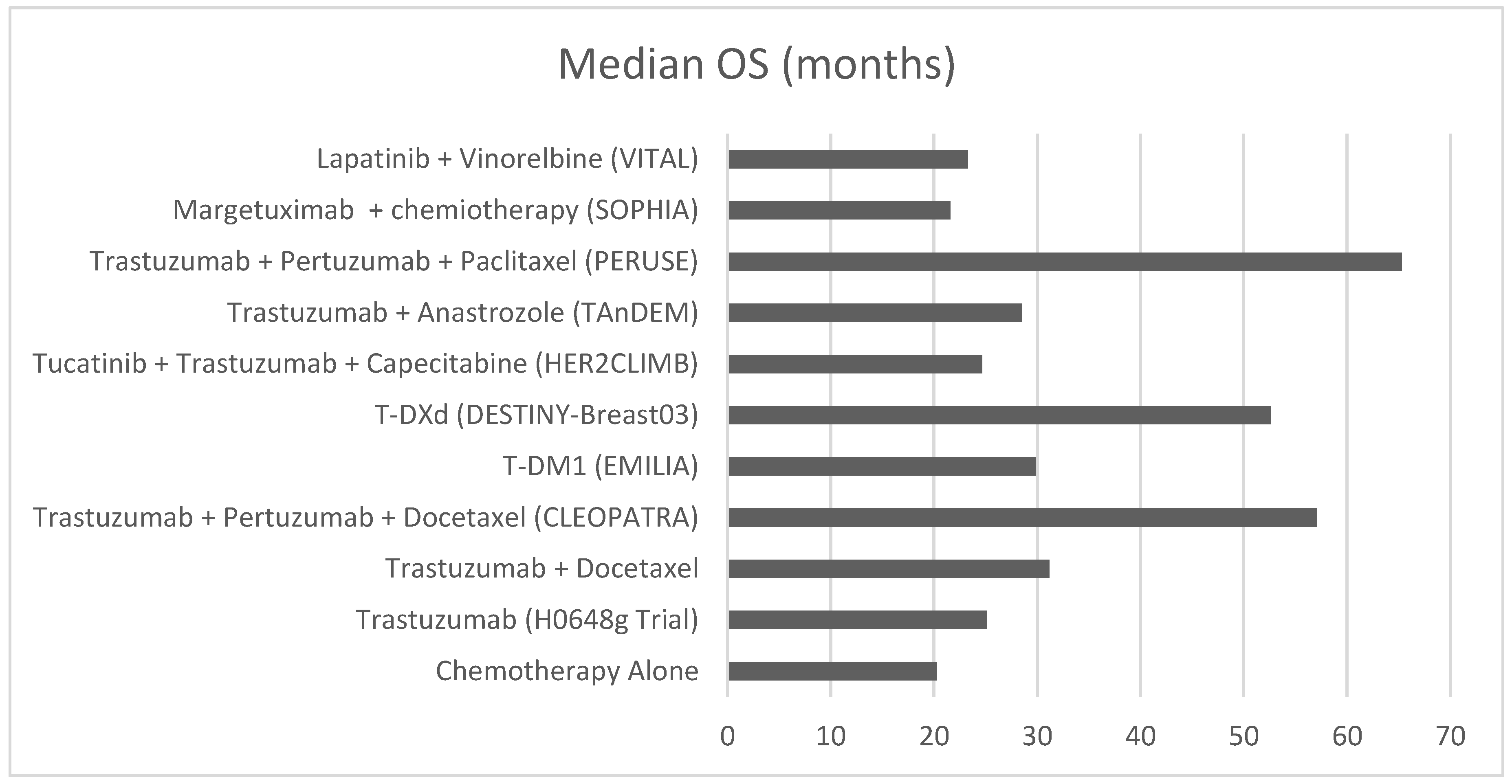
Intracranial Efficacy of Anti-HER2 Therapies
Discussion
Conclusions
References
- Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J, Wei W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 2021 Nov;41(11):1183-1194. Epub 2021 Aug 16. [CrossRef] [PubMed] [PubMed Central]
- Mercogliano MF, Bruni S, Mauro FL, Schillaci R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers (Basel). 2023 Mar 26;15(7):1987. [CrossRef] [PubMed] [PubMed Central]
- Li J, Chen Z, Su K, Zeng J. Clinicopathological classification and traditional prognostic indicators of breast cancer. Int J Clin Exp Pathol. 2015 Jul 1;8(7):8500-5. [PubMed] [PubMed Central]
- Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023 Feb;22(2):101-126. Epub 2022 Nov 7. [CrossRef] [PubMed] [PubMed Central]
- Jørgensen JT. Twenty-five years with HER2 targeted therapy. Ann Transl Med. 2024 Jun 10;12(3):53. Epub 2023 Jun 25. [CrossRef] [PubMed] [PubMed Central]
- Malenfant SJ, Eckmann KR, Barnett CM. Pertuzumab: a new targeted therapy for HER2-positive metastatic breast cancer. Pharmacotherapy. 2014 Jan;34(1):60-71. Epub 2013 Aug 5. [CrossRef] [PubMed]
- Bansal I, Pandey AK, Ruwali M. Small-molecule inhibitors of kinases in breast cancer therapy: recent advances, opportunities, and challenges. Front Pharmacol. 2023 Aug 30;14:1244597. [CrossRef] [PubMed] [PubMed Central]
- Mark C, Lee JS, Cui X, Yuan Y. Antibody-Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int J Mol Sci. 2023 Sep 6;24(18):13726. [CrossRef] [PubMed] [PubMed Central]
- De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: what is their clinical relevance? Cancer Treat Rev. 2013;39:925–34.
- Stanowicka-Grada M, Senkus E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Curr Treat Options Oncol. 2023 Nov;24(11):1633-1650. Epub 2023 Oct 25. [CrossRef] [PubMed] [PubMed Central]
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005 Jul 1;23(19):4265-74. Epub 2005 May 23. [CrossRef] [PubMed]
- Jackisch C, Welslau M, Schoenegg W, et al. Impact of trastuzumab treatment beyond disease progression for advanced/metastatic breast cancer on survival - results from a prospective, observational study in Germany. Breast. 2014;23:603–8. [CrossRef]
- Rocca A, Andreis D, Fedeli A, Maltoni R, Sarti S, Cecconetto L, Pietri E, Schirone A, Bravaccini S, Serra P, Farolfi A, Amadori D. Pharmacokinetics, pharmacodynamics and clinical efficacy of pertuzumab in breast cancer therapy. Expert Opin Drug Metab Toxicol. 2015;11(10):1647-63. Epub 2015 Aug 26. [CrossRef] [PubMed]
- Jagosky M, Tan AR. Combination of Pertuzumab and Trastuzumab in the Treatment of HER2-Positive Early Breast Cancer: A Review of the Emerging Clinical Data. Breast Cancer (Dove Med Press). 2021 Jun 14;13:393-407. [CrossRef] [PubMed] [PubMed Central]
- Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, Clark E, Knott A, Restuccia E, Benyunes MC, Cortés J; CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020 Apr;21(4):519-530. Epub 2020 Mar 12. [CrossRef] [PubMed]
- Bachelot T, Ciruelos E, Schneeweiss A, et al. on behalf of the PERUSE investigators. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol 2019; 30: 766-73. [CrossRef]
- Miles D, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Campone M, Bondarenko I, Nowecki Z, Errihani H, Paluch-Shimon S, Wardley A, Merot JL, Trask P, du Toit Y, Pena-Murillo C, Revelant V, Klingbiel D, Bachelot T; PERUSE investigators. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021 Oct;32(10):1245-1255. Epub 2021 Jul 2. [CrossRef] [PubMed]
- del Mastro L, De Laurentiis M. Applicazioni cliniche del trastuzumab nel trattamento del carcinoma mammario HER2+ Recenti Prog Med 2019;110(12):594-603. [CrossRef]
- Urruticoechea A, Rizwanullah M, Im SA et al. Final overall survival (OS) analysis of PHEREXA: A randomized phase III trial of trastuzumab (H) + capecitabine (X) ± pertuzumab (P)in patients with HER2-positive metastatic breast cancer (MBC)who experienced disease progression during or after H-based therapy. J Clin Oncol. 2018;36(Suppl.15):Abst. 1013.
- Bartsch R, Bergen E. ASCO 2018: highlights in HER2-positive metastatic breast cancer. Memo. 2018;11(4):280-283. Epub 2018 Oct 11. [CrossRef] [PubMed] [PubMed Central]
- Arpino G, de la Haba Rodríguez J, Ferrero JM, De Placido S, Osborne CK, Klingbiel D, Revelant V, Wohlfarth C, Poppe R, Rimawi MF; PERTAIN Study Group. Pertuzumab, Trastuzumab, and an Aromatase Inhibitor for HER2-Positive and Hormone Receptor-Positive Metastatic or Locally Advanced Breast Cancer: PERTAIN Final Analysis. Clin Cancer Res. 2023 Apr 14;29(8):1468-1476. [CrossRef] [PubMed] [PubMed Central]
- Nordstrom JL, Gorlatov S, Zhang W, et al.: Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res 13:R123, 2011. [CrossRef]
- Liu L, Yang Y, Burns R, et al.: Margetuximab mediates greater Fc-dependent anti-tumor activities than trastuzumab or pertuzumab in vitro. Cancer Res 79:1538, 2019 (abstr 1538). [CrossRef]
- Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Musolino A, Pegram MD, Bachelot T, Wright GS, Saura C, Escrivá-de-Romaní S, De Laurentiis M, Schwartz GN, Pluard TJ, Ricci F, Gwin WR 3rd, Levy C, Brown-Glaberman U, Ferrero JM, de Boer M, Kim SB, Petráková K, Yardley DA, Freedman O, Jakobsen EH, Gal-Yam EN, Yerushalmi R, Fasching PA, Kaufman PA, Ashley EJ, Perez-Olle R, Hong S, Rosales MK, Gradishar WJ; SOPHIA Study Group. Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial. J Clin Oncol. 2023 Jan 10;41(2):198-205. Epub 2022 Nov 4. [CrossRef] [PubMed] [PubMed Central]
- Gradishar WJ, O’Regan R, Rimawi MF, Nordstrom JL, Rosales MK, Rugo HS. Margetuximab in HER2-positive metastatic breast cancer. Future Oncol. 2023 May;19(16):1099-1112. Epub 2023 May 12. [CrossRef] [PubMed]
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014 Mar 5;16(2):209. [CrossRef] [PubMed] [PubMed Central]
- Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, Green M, Gianni L. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017 Jun;18(6):732-742. Epub 2017 May 16. Erratum in: Lancet Oncol. 2017 Aug;18(8):e433. doi: 10.1016/S1470-2045(17)30527-2. Erratum in: Lancet Oncol. 2018 Dec;19(12):e667. doi: 10.1016/S1470-2045(18)30848-9. PMID: 28526536; PMCID: PMC5531181. [CrossRef]
- Krop IE, Kim SB, Martin AG, LoRusso PM, Ferrero JM, Badovinac-Crnjevic T, Hoersch S, Smitt M, Wildiers H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017 Jun;18(6):743-754. Epub 2017 May 16. [CrossRef] [PubMed]
- Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L, Manzano A, Melichar B, Siena S, Stroyakovskiy D, Fielding A, Ma Y, Puvvada S, Shire N, Lee JY. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024 Jan 1;42(1):47-58. Epub 2023 Oct 23. [CrossRef] [PubMed] [PubMed Central]
- Daiichi Sankyo Co. Ltd. ENHERTU® Approved in the U.S. for Patients with HER2 Positive Metastatic Breast Cancer Treated with a Prior Anti-HER2-Based Regimen daiichisankyo.us/press-releases/-/article/enhertu-approved-in-the-u-s-for-patients-with-her2-positive-metastatic-breast-cancer-treated-with-a-prior-anti-he r2-based-regimen (2022).
- Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I; DESTINY-Breast01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020 Feb 13;382(7):610-621. Epub 2019 Dec 11. [CrossRef] [PubMed] [PubMed Central]
- André F, Hee Park Y, Kim SB, Takano T, Im SA, Borges G, Lima JP, Aksoy S, Gavila Gregori J, De Laurentiis M, Bianchini G, Roylance R, Miyoshi Y, Armstrong A, Sinha R, Ruiz Borrego M, Lim E, Ettl J, Yerushalmi R, Zagouri F, Duhoux FP, Fehm T, Gambhire D, Cathcart J, Wu C, Chu C, Egorov A, Krop I. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 May 27;401(10390):1773-1785. Epub 2023 Apr 20. Erratum in: Lancet. 2023 Dec 9;402(10418):2196. doi: 10.1016/S0140-6736(23)02709-5. Erratum in: Lancet. 2024 Mar 9;403(10430):912. doi: 10.1016/S0140-6736(24)00420-3. PMID: 37086745. [CrossRef]
- Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, Chiu JWY, Xu B, Hamilton E, Madhusudan S, Iwata H, Altintas S, Henning JW, Curigliano G, Perez-Garcia JM, Kim SB, Petry V, Huang CS, Li W, Frenel JS, Antolin S, Yeo W, Bianchini G, Loi S, Tsurutani J, Egorov A, Liu Y, Cathcart J, Ashfaque S, Cortés J. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023 Jan 14;401(10371):105-117. Epub 2022 Dec 7. Erratum in: Lancet. 2023 Feb 18;401(10376):556. doi: 10.1016/S0140-6736(22)00045-9. PMID: 36495879. [CrossRef]
- Cortés J, Hurvitz SA, Im SA, Iwata H, Curigliano G, Kim SB, Chiu JWY, Pedrini JL, Li W, Yonemori K, Bianchini G, Loi S, Borges GS, Wang X, Bachelot T, Nakatani S, Ashfaque S, Liang Z, Egorov A, Hamilton E. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-Breast03 trial. Nat Med. 2024 Aug;30(8):2208-2215. Epub 2024 Jun 2. [CrossRef] [PubMed] [PubMed Central]
- Guan M, Tong Y, Guan M, Liu X, Wang M, Niu R, Zhang F, Dong D, Shao J, Zhou Y. Lapatinib Inhibits Breast Cancer Cell Proliferation by Influencing PKM2 Expression. Technol Cancer Res Treat. 2018 Jan 1;17:1533034617749418. [CrossRef] [PubMed] [PubMed Central]
- Chintalaramulu N, Vadivelu R, Nguyen NT, Cock IE. Lapatinib inhibits doxorubicin induced migration of HER2-positive breast cancer cells. Inflammopharmacology. 2020 Oct;28(5):1375-1386. Epub 2020 May 6. [CrossRef] [PubMed]
- Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, Cabiddu M, Barni S. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer. 2017 Oct;84:141-148. Epub 2017 Aug 12. [CrossRef] [PubMed]
- Janni W, Sarosiek T, Karaszewska B, Pikiel J, Staroslawska E, Potemski P, Salat C, Brain E, Caglevic C, Briggs K, Mahood K, DeSilvio M, Marini L, Papadimitriou C. Final overall survival analysis of a phase II trial evaluating vinorelbine and lapatinib in women with ErbB2 overexpressing metastatic breast cancer. Breast. 2015 Dec;24(6):769-73. Epub 2015 Sep 16. [CrossRef] [PubMed]
- Xuhong JC, Qi XW, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019 Oct 1;9(10):2103-2119. [PubMed] [PubMed Central]
- Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, Kim SB, Moy B, Delaloge S, Gradishar W, Masuda N, Palacova M, Trudeau ME, Mattson J, Yap YS, Hou MF, De Laurentiis M, Yeh YM, Chang HT, Yau T, Wildiers H, Haley B, Fagnani D, 101 Lu YS, Crown J, Lin J, Takahashi M, Takano T, Yamaguchi M, Fujii T, Yao B, Bebchuk J, Keyvanjah K, Bryce R, Brufsky A; NALA Investigators. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol. 2020 Sep 20;38(27):3138-3149. Epub 2020 Jul 17. [CrossRef] [PubMed] [PubMed Central]
- Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res. 2022 Jul 13;9(1):39. [CrossRef] [PubMed] [PubMed Central]
- Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, Murthy R, Okines A, Paplomata E, Cameron D, Carey LA, Gelmon K, Hortobagyi GN, Krop I, Loibl S, Pegram M, Slamon D, Ramos J, Feng W, Winer E. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022 Mar;33(3):321-329. Epub 2021 Dec 23. Erratum in: Ann Oncol. 2023 Jul;34(7):630. doi: 10.1016/j.annonc.2022.12.005. PMID: 34954044. [CrossRef]
- Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020 Feb 13;382(7):597-609. Epub 2019 Dec 11. Erratum in: N Engl J Med. 2020 Feb 6;382(6):586. doi: 10.1056/NEJMx190039. PMID: 31825569.]. [CrossRef]
- Criscitiello C, Corti C, De Laurentiis M, Bianchini G, Pistilli B, Cinieri S, Castellan L, Arpino G, Conte P, Di Meco F, Gennari A, Guarneri V, Visani L, Livi L, Marchetti P, Puglisi F, Viale G, Del Mastro L, De Placido S, Curigliano G. Tucatinib’s journey from clinical development to clinical practice: New horizons for HER2-positive metastatic disease and promising prospects for brain metastatic spread. Cancer Treat Rev. 2023 Nov;120:102618. Epub 2023 Aug 22. [CrossRef] [PubMed]
- Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019 Nov 12;19(1):1091. [CrossRef] [PubMed] [PubMed Central]
- Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, Dhaliwal AJ, Sahgal A, Das S, Chan KK, Jerzak KJ. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021 Jun 1;23(6):894-904. [CrossRef] [PubMed] [PubMed Central]
- Lopes S, Vieira I, Abreu M, Pousa I, Ferreira A, Sousa S, Pereira D. Prognostic Factors and Survival According to Tumor Subtype in Women With Breast Cancer Brain Metastases. Curr Probl Cancer. 2022 Dec;46(6):100866. Epub 2022 Jun 11. [CrossRef] [PubMed]
- Raghavendra AS, Ibrahim NK. Breast Cancer Brain Metastasis: A Comprehensive Review. JCO Oncol Pract. 2024 Oct;20(10):1348-1359. Epub 2024 May 15. [CrossRef] [PubMed] [PubMed Central]
- E. Le Rhun, M. Guckenberger, M. Smits et al., on behalf of the EANO Guidelines Committee and the ESMO Guidelines Committee.
- Fontanella C, De Carlo E, Cinausero M, Pelizzari G, Venuti I, Puglisi F. Central nervous system involvement in breast cancer patients: Is the therapeutic landscape changing too slowly? Cancer Treat Rev. 2016 May;46:80-8. Epub 2016 Apr 1. [CrossRef] [PubMed]
- Gamucci, T.; Pizzuti, L.; Natoli, C.; Mentuccia, L.; Sperduti, I.; Barba, M.; Sergi, D.; Iezzi, L.; Maugeri-Saccà, M.; Vaccaro, A.; et al. A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol. Ther. 2019, 20, 192–200. [CrossRef]
- Bergen ES, Binter A, Starzer AM, Heller G, Kiesel B, Tendl-Schulz K, Bago-Horvath Z, Furtner J, Leitner J, Exner R, Fitzal F, Dieckmann K, Widhalm G, Preusser M, Berghoff AS, Bartsch R. Favourable outcome of patients with breast cancer brain metastases treated with dual HER2 blockade of trastuzumab and pertuzumab. Ther Adv Med Oncol. 2021 Apr 22;13:17588359211009002. [CrossRef] [PubMed] [PubMed Central]
- Lin NU, Pegram M, Sahebjam S, Ibrahim N, Fung A, Cheng A, Nicholas A, Kirschbrown W, Kumthekar P. Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study. J Clin Oncol. 2021 Aug 20;39(24):2667-2675. Epub 2021 May 4. [CrossRef] [PubMed] [PubMed Central]
- Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, Miles D, Samant M, Welslau M, Diéras V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015 Jan;26(1):113-119. Epub 2014 Oct 29. [CrossRef] [PubMed] [PubMed Central]
- Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, Hatschek T, Kelly CM, Peña-Murillo C, Yilmaz M, Donica M, Ellis P. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann Oncol. 2020 Oct;31(10):1350-1358. Epub 2020 Jul 5. [CrossRef] [PubMed]
- Bartsch R, Pérez-García JM, Furtner J, Berghoff AS, Marhold M, Starzer AM, Hughes M, Kabraji S, Sammons S, Anders C, Murthy RK, Van Swearingen AED, Pereslete A, Gion M, Vaz Batista M, Braga S, Pinto PBC, Sampayo-Cordero M, Llombart-Cussac A, Preusser M, Cortés J, Lin NU. Results of a patient-level pooled analysis of three studies of trastuzumab deruxtecan in HER2-positive breast cancer with active brain metastasis. ESMO Open. 2025 Jan;10(1):104092. Epub 2025 Jan 3. [CrossRef] [PubMed] [PubMed Central]
- Das G, Wong STC, Zhao H. Beyond Primary HER2 Expression: Trastuzumab Deruxtecan’s Efficacy in Brain Metastasis. Cancers (Basel). 2024 Oct 18;16(20):3525. [CrossRef] [PubMed] [PubMed Central]
- Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, Hurvitz S, Loi S, Okines A, Abramson V, Bedard PL, Oliveira M, Mueller V, Zelnak A, DiGiovanna MP, Bachelot T, Chien AJ, O’Regan R, Wardley A, Conlin A, Cameron D, Carey L, Curigliano G, Gelmon K, Loibl S, Mayor J, McGoldrick S, An X, Winer EP. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol. 2020 Aug 10;38(23):2610-2619. Epub 2020 May 29. [CrossRef] [PubMed] [PubMed Central]
- Cucciniello L, Blondeaux E, Bighin C, Gasparro S, Russo S, Dri A, Pugliese P, Fontana A, Cortesi E, Ferzi A, Riccardi F, Sini V, Boni L, Fabi A, Montemurro F, De Laurentiis M, Arpino G, Del Mastro L, Gerratana L, Puglisi F. Clinico-pathological predictors of radiologic complete response to first-line anti-HER2 therapy in metastatic breast cancer. NPJ Breast Cancer. 2024 Dec 18;10(1):105. [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



