1. Introduction
Gloves are articles of materials designed to resist hazardous physical, chemical, biological and mechanical stressors primarily potentially exposing the skin of the hands [
1]. In 2019 before the COVID-19 lockdown, there were 18,200 illnesses recorded across all industries in the United States from skin exposure, about 1.7 times those for inhalation [
2].
Many skin exposures occur through handling of chemicals for which Chemically Protective Clothing (CPC) gloves shall be worn as Personal Protective Equipment (PPE) [
3,
4]. To assess how well gloves perform as barriers, permeation (mass transfer through the material at the molecular level) and penetration (mass transfer through nano- or micro- holes and seams) tests are performed [
3,
4]. Most researchers and glove producers in the United States have used the gas-collection mode of ASTM F739 in its open- and closed-loop versions to generate permeation data from circular glove pieces [
5]. Methods that use liquid collection for nonvolatile chemicals are valid alternatives provided the permeant is adequately soluble, the solvent does not degrade or back-permeate the glove material, and the collection side is mixed. Water is usually the preferred collection solvent. The major permeation parameters measured at 27 ± 1
oC over eight or fewer hours sampled at 5-min intervals are the steady state permeation rate (SSPR) where the permeation rate is constant and the standardized breakthrough time (SBT) defined at 100 ng/cm
2/min for open- and closed- loop collection systems.
However, disposable gloves that permit manipulation of small items are extensively used in healthcare and clinical chemistry laboratories [
3,
4]. The major permeation protocol there, ASTM D6978-19 [
6], tests pieces of disposable medical gloves in the large ASTM-F739 permeation cell where circular glove pieces exposed to aqueous solutions of specific chemotherapy agents at their highest use concentrations over four hours are sampled at 30-min intervals from a mixed aqueous closed-loop collection system without recirculation. The ASTM D6978-19 permeation method differs from ASTM F739 in that permeations are at 35 ± 1
oC; a permeation rate of 10 ng/cm
2/min defines the breakthrough detection time; and the thinnest portion of the glove from either the cuff or the palm is evaluated rather than the palm or top of the palm for ASTM F739.
Similar types of permeation cells and procedures are mandated for glove permeation testing for occupational environments within the European Community through the EN 374-3 and ISO 6529 methods (now combined) with their past respective normalized breakthrough times at 1,000 and 100 ng/cm
2/min being analogous to the ASTM F739 SBT. Recent reviews [
1,
3,
4] have discussed how the results from the ASTM F739 and other permeation cell methods relate to actual glove protection in the workplace.
Double gloving with disposable gloves as a method to protect workers has been practiced in healthcare industries. Such research has usually involved aqueous challenges, double layering of circular glove pieces in permeation cells to simulate field double gloving, and closed-loop aqueous collection systems without recirculation [
3,
4]. The first peer-reviewed journal report of double layering of disposable glove material against a pure organic solvent, diethylene glycol mono-n-butyl ether, came in 2020, also featuring a review of the double gloving literature in its introduction [
7]. Double layers for four different disposable nitrile gloves from one company caused average normalized breakthrough times at 250 ng/cm
2 to be lengthened about fourfold and average SSPR to be lowered by about the same factor with coefficients of variation for each of about 20%. Since then, more reports have appeared on double gloving against toxic drugs like antineoplastics [
8,
9]. Its use in pandemics [
10] and surgeries [
11,
12,
13] has also been reviewed.
Triple gloving of disposable gloves is a much rarer technique employed by surgeons and dentists mostly for puncture protection to resist potent body fluid pathogens [
14,
15,
16,
17,
18] like the Ebola virus [
17] and COVID-19 [
18], and to protect against dental sensitizers such as acrylates [
19]. Firefighters at HazMat incidents have also triple gloved [
20]. There were no literature reports of comparisons of permeation of pure organic solvents through single, double and triple layers of disposable gloves of the same material.
Suitable compounds for testing must not degrade the glove [
3,
4]. Disposable gloves are designed to resist water H-O-H. The closest organic molecules to water are alcohols R-O-H where R is alkyl, alicyclyl, aryl or other non-polar multifunctional group entity. However, methanol, ethanol and n-butanol permeate the Kimberly-Clark Professional disposable Stirling Nitrile glove in 10 min or less at ASTM F739 conditions [
21]. This implies that higher carbon content alcohols would be better choices. 2-Butoxyethanol (2-BE; n-butyl-O-CH
2CH
2-OH; CAS RN 111-76-2; ethylene glycol mono-n-butyl ether, butyl cellosolve and butyl oxitol are among the many synonyms) is a water-miscible non-volatile solvent of 168
oC boiling point [
22]. It is used in paints and varnishes, household and healthcare facility soaps, cleaning liquids and detergents, pesticide formulations (as active ingredient and adjuvant), floor wax stripping agents, fracking, textile processing and dry-cleaning, as well as in adhesives, cosmetics, and oil slick dispersants [
22]. It has a 8-h Threshold Limit Value-Time Weighted Average (TLV-TWA) of 20 ppm (v/v) associated with eye and upper respiratory tract effects, and a urinary Biological Exposure Index (BEI) of after-shift total 200 mg 2-butoxyacetic acid/g creatinine [
23]. The Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) is 50 ppm (skin), and the National Institute for Occupational Safety and Health (NIOSH) recommended exposure limit (REL) is 5 ppm (skin), and its immediately dangerous to life or health level is 700 ppm [
24].
Ansell documented for 2-BE the following “Excellent” degradation and “Good Protection” ASTM F739 SBTs of its thinnest chemical protective clothing (CPC) gloves [
25]: >480 min for Butyl ChemTek 38-514 (14 mil or 360 µm), LLDPE Barrier 02-100 (2.5 mil or 64 µm); Neoprene 39-865 (18 mil or 460 µm), and Viton Butyl ChemTek 38-612 (12 mil or 310 µm); 240-480 min for nitrile Solvex 37-145 (11 mil or 280 µm) and nitrile AlphaTek 58-435 (18 mil or 460 µm). Butyl, neoprene and nitrile had the longest SBTs of the non-laminates/blends. Nitrile was chosen to be the disposable glove material investigated in terms of multilayers since it is the most used and available disposable glove material [
26] and the thickest powderless unsupported and unlined disposable nitrile glove of Kimtech Science was Blue (130 µm thickness or about 5.1 mil from previous research [
7]). Ansell in the latter half of the 2010s introduced its “thinnest chemical-resistant disposable glove”, the Microflex 93-260, 7.8 mil (200 µm) thick, comprised of three layers of nitrile and neoprene (polychloroprene) [
27,
28]. The Microflex 93-260 glove is also ASTM D6978 compliant for chemotherapy drugs at their highest use concentration in isotonic aqueous solution. Since butyl and chloroprene CPC gloves were more resistant to 2-BE than Solvex CPC nitrile relative to SBT [
25], the trilayered Microflex glove of nitrile/neoprene was thought worthy of testing relative to the nitrile layers since the resistance to 2-BE for both types of gloves was unknown.
Thus, 2-BE was to be used in a ASTM F739 permeation cell to investigate permeation of single, double and triple layers of the thickest Kimberly-Clark disposable nitrile glove, Kimtech Science Blue, and to compare with the results from Ansell’s Microflex 93-260. The hypothesis was that 3 layers of disposable nitrile would produce ASTM F739 SBTs not statistically different from one layer of Microflex 93-260.
2. Materials and Methods
2.1. Glove Selection
The gloves used were unsupported, unlined and powderless Kimberly-Clark Professional’s Kimtech Science Blue nitrile disposable gloves from Fisher Scientific, Pittsburgh, PA and Microflex 93-260 CPC nitrile/neoprene gloves from Ansell, Iselin, N.J.
2.2. Chemicals
2-BE was procured from Eastman Chemical Company, Miami FL. 4-Bromophenol internal standard (IS) was obtained from Aldrich, St Louis, MO. Sodium dichromate (99%) from Fisher Scientific Pittsburgh PA was used for a saturated salt aqueous solution to generate a (55 ± 4)% relative humidity atmosphere inside of Pyrex glass vacuum desiccators from Fisher Scientific.
All water for aqueous solutions was obtained from a Millipore Milli-Q Water System (Temecula, CA) and Millipore Simplicity Water Purification final polishing system (Temecula, CA). Helium (99.9999%) and nitrogen (99.9999%) were purchased from Air Liquide (El Segundo, CA).
2.3. Equipment
A Marathon digital micrometer from Fisher Scientific (Pittsburgh, PA) measured glove palm-piece thickness at three random locations. Vernier calipers facilitated the measurement of glove piece diameters. Two sampling-side ASTM-type-I-PTC-600 permeation cells from Pesce Lab Sales (Kennett Square, PA) were used in permeation testing. A Fisher moving tray Model 127 Shaking Water Bath from Fisher Scientific (Pittsburgh, PA), allowed temperature control and mixing in the four permeation cells that were immersed except for the top halves of their stems. The shaking water bath was modified with copper tubing bars to support clamps to hold the nearly immersed permeation cells in position. A torque wrench was used to tighten permeation cell nuts uniformly at 5 ft-pounds. A calibrated Fisher Scientific traceable printing hygrometer/thermometer allowed measurement of relative humidity and temperature.
The gas chromatograph-mass spectrometer (GC-MS) system for analyses was an Agilent (Santa Clara, CA) 6890N Network GC with a 30 m x 0.25 mm HP-5ms chemically bonded (0.25 μm thick film) fused silica capillary column in tandem with a quadrupole mass spectrometer, the Agilent 5973 Network Mass Selective Detector, operated at 70 eV electron impact energy at an ion source temperature of 230oC. The GC-MS transfer line and GC injector temperatures were at 280oC. Helium was the carrier gas at 3.0 ± 0.1 mL/min.
Microscopic magnification of the glove surface was used to determine whether micro holes or tears were present.
2.4. Permeation Testing
The ASTM test protocol with 2.54 cm Pyrex permeation cells in the closed loop without recirculation mode [
5] was modified: the temperature of permeation was 35.0 ± 0.4
oC from ASTM D6978-19 [
6]; two challenge half-cells were utilized instead of the challenge/collection half-cells; and sampling was done without collection solvent replenishment.
Test specimens were cut with scissors from the palm areas of glove previously conditioned at 54 ± 3% relative humidity at 25 ± 1oC for 24 hr. The test pieces were checked for microholes (microscopic examinations), and then their thicknesses measured (micrometer).
The test piece(s) (outer surface(s) facing the challenge chamber) were then mounted between the PTFE gaskets, sealed by the flanges, and the nuts tightened to a uniform torque that prevented leaks. For gloving simulations, two pieces were used instead of one for double gloving, and three for triple gloving. The assembled cells were held by clamps on the copper bars that allowed insertion of four cells into the water bath. The water bath was maintained at a shaking speed of 8.52 ± 0.05 cm/s to eliminate concentration gradients in the collection solvent. At the start of the 30-min equilibration period at 35oC, 10.0 mL of triply de-ionized water was added to the four collection sides. The test chemical (10.0 mL) was added at time zero to the three challenge cells and the fourth cell acted as the air method blank. The mixing was restarted. Collection side sampling occurred at 0.0, 5, 10, 20, 30, 40, 50, 60, 120 and 135 min. The samples were taken with a 100 µL Eppendorf pipet with long tips (moving tray stopped), placed into pre-chilled 1-mL Pyrex vials, and then stored at -20oC.
The glove samples were reconditioned at the original conditions before remeasuring all of the initial parameters.
2.5. Analyte Analysis
The samples were thawed and aliquots injected for GC-MS quantitation were 2.0 μL in volume containing 0.10 µg/µL of IS. The MS ions were mass-to charge ratio (m/z) of 57 and m/z of 87 for 2-BE, and m/z 172 for the IS. There was a solvent delay of four min at 120oC, held for two min, ramped at 40oC/min to 280oC, and kept there for four min.
GC-MS quantitations were performed by the internal standard method whereby the area response of analyte injected divided by the area of the IS was interpolated on a linear plot of (area of analyte standard divided by area of the IS) versus mass of analyte injected. The standards concentrations were 0, 0.1, 0.5, 1, 5, 10, 50, and 100 ng/µL for the low range and 100, 200, 300, and 400 ng/µL for the high range. The linear portions were characterized by their slopes, intercepts, their associated standard deviations, the correlation coefficients r, and p-values. For the permeation samples, dilution into a working linear range with water solvent occurred when necessary.
The analyte mass in the collection side was calculated by multiplying the injected sample mass by the permeation cell collection solvent volume in μL at the sampling time divided by two. The total mass collected in the collection side (corrected for mass removed by previous collection) divided by the exposed surface area was then plotted versus sampling time from zero time in minutes to generate the permeation curves for each individual glove tested. The sampling time interval where the permeation reached 100 ng/cm
2/min was determined to be the SBT. The time period of steepest slope was identified as the steady state permeation period and its slope (the SSPR) and standard deviation obtained through linear regression. The lag time t
l was calculated from this linear regression equation for the time when the cumulated mass divided by exposed area was zero. The diffusion coefficient D was then calculated from equation 1 [
3]:
where l is the initial thickness in cm, tl is the lag time in min and D is in cm2/min.
The mean and standard deviation data for each triplicate set for each glove type were then calculated.
2.6. Statistical Analyses
Linear regression was used to characterize linear relationships, including standard deviations of the slopes and intercepts as well as defining the correlation coefficients r and p-value for GC-MS internal standard analysis data that involved area ratios versus injected mass. The Student t-test was used to test the statistical significance of differences in means for all triplicate permeation experimental data, for example, thickness for swelling for each glove before and after permeation, and average SBT, cumulated mass/area, SSPR, and D for each glove type. The threshold p-value was p ≤0.05. These were obtained with Microsoft Excel 2007 software.
3. Results
3.1. Analyte Analyses
The retention times for the 2-BE and the 4-BP were 4.8 min and 7.9 min, respectively. The total run time for each injection was 10 min. The regression equation for the low concentration standards (0, 0.1, 0.5, 1, 5, 10, 50 and 100 ng/µL) was y= 0.0553x + 0.0126 (r2= 0.9904, p≤ 0.05). The lower quantifiable limit was 0.10 ng/µL. The regression equation for the high concentration standards range (100, 200, 300, and 400 ng/µL) was y= 0.7058x - 40.9 (r2= 0.9985, p< 0.05).
3.2. Glove Thickness
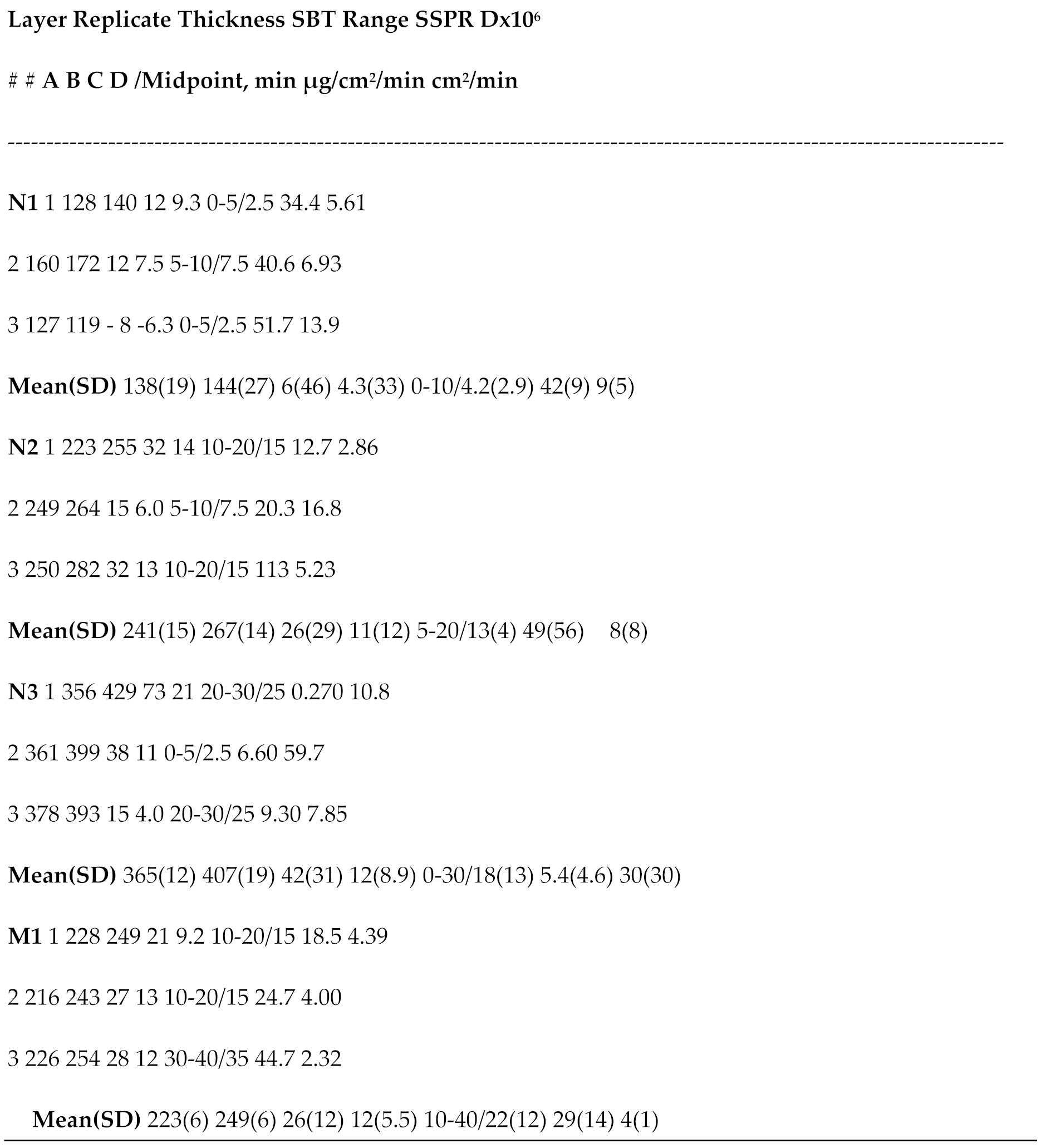
Table 1 shows all the intrarun and interrun data for glove thickness and swelling, SBT, SSPR and calculated D.
Table 1.
Thickness (A= initial µm; B= after permeation µm; C= swelling µm; D= change %), standardized breakthrough time (SBT), steady state permeation rate (SSPR), and calculated diffusion coefficient (D) of 2-butoxyethanol through layers of a Kimtech Science Blue disposable nitrile glove (N) and a single layer of Microflex 93-260 (M) in a ASTM F739 2.54 cm permeation cell with water collection without recirculation at 35oC. Standard deviations (SD) in parentheses are for triplicate data.
Table 1.
Thickness (A= initial µm; B= after permeation µm; C= swelling µm; D= change %), standardized breakthrough time (SBT), steady state permeation rate (SSPR), and calculated diffusion coefficient (D) of 2-butoxyethanol through layers of a Kimtech Science Blue disposable nitrile glove (N) and a single layer of Microflex 93-260 (M) in a ASTM F739 2.54 cm permeation cell with water collection without recirculation at 35oC. Standard deviations (SD) in parentheses are for triplicate data.
Microscopic examination revealed no obvious holes in the materials before and after permeation so that penetration did not occur. Relative to interrun initial glove thickness, the imprecision data in terms of % relative standard deviation (RSD) of layers were as follows: 14, single nitrile layer; 6.2, double nitrile layer; 3.3, triple nitrile layer; and 2.7, Microflex single layer. The after-permeation respective data were: 19, 5.2, 4.7 and 2.4. The respective percent difference between before and after challenge thickness RSDs were: 4.3, 11, 12 and 12. In terms of initial thickness, the Microflex glove was approximately equivalent to a double layer of the disposable nitrile glove, that not being statistically different at p ≤.0.05.
The intrarun thickness change was swelling in all but the one instance for a single nitrile layer. The resolution of the Marathon digital micrometer was 1 µm and hence that of thickness differences was 2 µm. The percent swelling ranges and their span are instructive: single nitrile, -6.3 to 9.3, 15.6; double nitrile, 6 to 14, 8; triple nitrile, 4 to 21, 17 and Microflex, 9.2 to 13, 3.8. The Microflex layer is clearly more uniformly affected by 2-BE than all the nitrile layers probably because of better quality assurance and control in the production process than for the disposable nitrile materials.
3.3. Standardized Breakthrough Time
When the midpoints of the triplicate sampling intervals that contained the SBT were averaged for each layering condition (Table 1), the average SBTs for the nitrile glove were: 4.2 ± 2.9 min (RSD, 69%) for the single layer; 13 ± 4 min (RSD, 31%) for the double layer; and 18 ± 13 min (RSD, 72%) for the triple layer. The comparison of the triple layer with the double resulted in no statistical significance at p ≤ 0.05 because of large standard deviations but comparison of single-/double- layer and single-/triple- layer nitrile data were significant at p ≤0.05.
The Microflex layer had an average SBT of 22 ± 12 min (RSD, 55%). In terms of average SBT, the Microflex layer behaved like the double and triple nitrile layers since they were not statistically different at p ≤0.05 though the average SBT for double nitrile layers was 3.1 times longer than that for a single nitrile layer, 4.3 times longer for triple nitrile layers and 5.2 times longer than the single Microflex layer. Based just on average SBT and ignoring standard deviations, the Microflex layer was most resistant to 2-BE. All the RSD were high.
3.4. Cumulated Permeated Mass/Area
Another permeation resistance parameter related to risk assessment is cumulated permeated mass per unit area CPM/A (µg/cm2) at critical times. By 30 min, this parameter average was: single layer nitrile, 1,040 ± 280 (RSD, 27%); double, 520 ± 560 (RSD, 108%); triple, 93 ± 93 (RSD, 100%) ; and Microflex, 210 ± 40 (RSD, 19%). While the only statistically significant pair comparisons at p ≤0.05 were between the triple layer and the two single layers and also between the two single layers, the clear implication is that the triple layer is about 2.3 times more resistant to 2-BE at 30 min than Microflex, and both are much more resistant (about 11 times for 3 nitrile layers and 5 times for Microflex) than a single nitrile layer. The Microflex layer provided resistance intermediate between 2 and 3 nitrile layers.
3.5. Steady State Permeation Rate
The average SSPR for the nitrile layers in μg/cm2/min were (Table 1): 42 ± 9 (RSD, 21%) for a single layer; 50 ± 60 (RSD, 120%) for the double layer; and 5 ± 5 (RSD, 100%) for the triple layer, the respective SSPR relative to the single layer being not significant for the double layer and about 8 times lower for the triple layer, the latter significantly different at p ≤0.05. The Microflex layer had an average SSPR of 29 ± 14 μg/cm2/min (RSD, 48%), about 1.4 times lower than for a single nitrile layer but 6 times higher than the triple layer. All other comparisons were not statistically significant at p ≤0.05 because of the large imprecisions.
3.6. Diffusion Coefficient
The calculated D values from equation 1 are presented in Table 1 but are placed there for reference because of the high degree of intrarun swelling reported in
Section 3.2. A swelling classification for individual gloves is available for reference [
29]: none (not statistically significant), moderate (about 4%) and complete (10-20%). When there is no swelling, Fick’s first law of diffusion can be used to interpret the permeation data and to calculate diffusion coefficients from equation 1. None of the average diffusion coefficients for nitrile layers differed from one another because of high imprecision.
4. Discussion
The data are consistent with a strong interaction between the glove test materials and 2-BE resulting in high RSDs for all the interrun data for swelling, SBTs, cumulated permeated mass/area at 30 min, and SSPRs. In a previous study with 2-BE permeating through a single layer of Kimtech Science Purple nitrile disposable glove under the same experimental conditions as the present, the SSPR had a RSD of 21% and the mass permeated at 20 min was calculated to be 32% [
30], comparable with the analogous data in the present study. SBTs for that Purple layer were between 0-20 min but are not comparable with the present single nitrile layer SBT results because a 20-min testing interval was used. While the Purple layer RSD for swelling was 100%, the average swelling was only 1.9%.
Though the physical thickness of the Microflex layer most resembled 2 nitrile layers, the permeation behavior was closer to 3 nitrile layers, and it generally exhibited the lowest RSD. In terms of the cumulated mass/area results for the Microflex and triple nitrile layers, the greater resistance of the nitrile triple layer can be explained by the Microflex layer having the longer SBT but also a higher SSPR than the triple nitrile layer so that how long a glove is worn becomes important. Disposable gloves are not meant to be worn for long periods unlike CPC gloves where SSPR assumes greater importance but still largely ignored as evident in the Kimberly-Clark Worldwide/Kimtech Science/Ansell classification [
21]. Its permeation ASTM F739 SBTs in min for their gloves are: Not Recommended, < 10; Splash Protection, 10-60; Medium Protection, 60-240; High Protection, >240, and is also subdivided into levels in min: Unclassified <10; 1, 10-30; 2, 30-60; 3, 60-120; 4, 120-240; 5, 240-480; 6, >480. Thus, the average SBTs of Table 1 would be classified as Not Recommended for one nitrile layer and Splash Protection for the other situations. This classification does not discriminate the SBTs of the multiple nitrile layers and the Microflex layer. The level classification allows better resolution and the best splash protection is shown by the Microflex layer but even at 30 min SSPR or the permeation before the steady state period may be important relative to the potential dose to the hands and therefore health effects.
These are the first permeation cell data published on triple layers of disposable glove materials for a pure organic solvent challenge simulating triple gloving. A similar study for the simulation of double gloving for a pure organic solvent challenge has been previously reported [
7].
The use of double or triple gloving with disposable gloves may be the only at-hand PPE recourse in emergencies when CPC gloves are unavailable. When donning multiple gloves, the most protective glove should be donned first [
31] and the smallest size that fits last to optimize manipulations with small implements or items to be handled [
32]. If only one type of glove is available in different sizes, this means the thickest should be donned first and the smallest size last.
It is hoped that more layer research with different and differing disposable gloves will occur to find more practical means to ensure safety during emergencies when CPC gloves may not be available.