1. Introduction
Pemphigus comprises a group of life-threatening autoimmune blistering diseases in which autoantibodies induce acantholysis, leading to cutaneous and mucosal erosions [
1]. Pathogenetically, the process is driven by autoantibodies binding to desmosomal proteins, specifically desmoglein-1 (Dsg1) and desmoglein-3 (Dsg3) [
2,
3]. The most prevalent forms of this group of diseases are pemphigus vulgaris (PV), which accounts for up to 65% of all diagnosed cases, and pemphigus foliaceous (PF), which accounts for up to 27% [
4,
5,
6]. Extensive erosion of the skin and mucous membranes in pemphigus profoundly impacts patients’ quality of life. The disruption of the skin-mucosal barrier has been shown to elevate the risk of infection. This disruption can lead to a worsening of systemic conditions, an exacerbation of cutaneous involvement, and potentially fatal outcomes.[
4] However, early diagnosis and severity assessment can be challenging due to heterogeneous clinical manifestations, particularly in patients with isolated mucosal involvement [
7,
8,
9,
10,
11].
At present, laboratory diagnosis of pemphigus principally relies on a thorough evaluation of histological findings, enzyme-linked immunosorbent assay (ELISA) results, and both direct and indirect immunofluorescence (IIF) [
12]. However, the clinical implementation of these tests is hindered by several factors, including cost, equipment limitations, and interpretation challenges, such as ELISA/IIF discrepancies. Despite histopathological confirmation, serological methods have been observed to demonstrate false-negative results [
13]. Conversely, in the absence of supporting pemphigus evidence, false-positives have been reported [
14]. The presence of such diagnostic contradictions has the potential to present a significant challenge to the diagnostic process. Despite the efficacy of current diagnostic methods, misdiagnosis rates persist, underscoring the need for more specific and minimally invasive pemphigus diagnostics [
12,
15].
In recent years, microRNAs have been identified as potential biomarkers, indicating their promise in various medical applications. These endogenous short (18–22 nucleotide) non-coding RNAs regulate post-transcriptional gene expression by blocking protein translation or inducing mRNA degradation. Their exceptional stability in various biological substrates further enhances their potential as diagnostic tools [
16,
17,
18,
19]. Significant advancements in the field have been made in recent years, primarily through active research, which has led to the identification of disease-specific microRNAs. These findings have offered a more profound comprehension of the mechanisms through which microRNAs function, both in physiological and pathological contexts, including pemphigus.
To date, more than 120 microRNAs have been analyzed in various substrates in the published literature (
Table 1), with particular attention directed toward miR-338-3p and miR-424-5p. Abnormalities in the expression of these microRNAs may be critical to the pathogenesis of pemphigus. Their consistent dysregulation across studies influencing key disease pathways supports the pathogenic role of miR-338-3p and miR-424-5p in pemphigus [
12,
20,
21]. Thus, multiple studies have established that miR-338-3p plays a key pathogenic role in pemphigus. It has been suggested that it induces Th1/Th2 imbalance by downregulating the expression of
RNF114 mRNA via transfection, thereby participating in the regulation of apoptosis and T-cell activation [
20,
22,
23]. Another hypothesis identifies
TRADD as a target gene of miR-338-3p in apoptosis regulation. This hypothesis is supported by in vitro and in vivo evidence showing reduced
TRADD mRNA levels following miR-338-3p overexpression [
24]. Together, these findings suggest that overexpression of miR-338-3p may contribute to the pathogenesis of pemphigus by disrupting the Th1/Th2 balance, which is consistent with current disease models [
25,
26,
27]. Multiple studies have demonstrated that patients with active pemphigus exhibit significantly higher levels of miR-338-3p expression than controls. These levels directly correlate with disease severity, as measured by the Pemphigus Disease Area Index (PDAI) [
20,
28]. These results suggest that miR-338-3p could serve as a biomarker for diagnosing and monitoring the activity of pemphigus and predicting its prognosis.
While the role of miR-338-3p has been examined in vitro, the role of miR-424-5p in pemphigus pathogenesis has only been examined through bioinformatics and gene prediction models [
21]. The study identified 52 potential target genes of miR-424-5p, all of which were found to be involved in p38 MAPK activation and the MAPK signaling pathway. This pathway plays a crucial role in regulating autoimmune responses, cell proliferation, differentiation, and apoptosis, while also contributing to cytoskeletal reorganization, desmosome disruption, and keratinocyte apoptosis [
29,
30,
31]. A notable finding was the observation that the levels of microRNA-424-5p expression were significantly higher in patients with PV compared to healthy controls (p < 0.05) [
21,
32]. These findings suggest that microRNA-338-3p and microRNA-424-5p may serve as promising diagnostic biomarkers for pemphigus.
The objective of this study was to investigate the microRNA profiles of peripheral blood mononuclear cells in patients with pemphigus (in both active and remission periods) in comparison to those of healthy subjects, with the aim of assessing their potential as diagnostic markers for pemphigus.
Table 1.
Comparison of publications devoted to the study of microRNA expression in pemphigus.
Table 1.
Comparison of publications devoted to the study of microRNA expression in pemphigus.
| Article |
Pemphigus subtype 1
|
Number of MicroRNAs studied |
Internal control |
Key
microRNAs |
Material and
internal control |
| Valentino A. et al. [43] |
PV |
86 |
U6 |
hsa-miR-148a-3p
hsa-miR-146b-5p
hsa-miR-126
hsa-miR-139 |
Blood plasma
derived
exosomes |
| Khabou B. et al. [44] |
PF |
6 |
n/a 2
|
hsa-miR-17-5p
hsa-miR-21-5
hsa-miR-146a-5p
hsa-miR-155-5p
hsa-miR-338-3p
hsa-miR-21 |
Peripheral blood
mononuclear cells/
skin biopsy samples |
He W. et al.
[32] |
PV |
12 |
5S рРНК |
hsa-miR-125b-5p
hsa-miR-146a-5p
hsa-miR-148a-3p
hsa-miR-150-5p
hsa-miR-155-5p
hsa-miR-181a-5p
hsa-miR181b-5p
hsa-miR-326
hsa-miR-338-3p
hsa-miR-423-5p
hsa-miR-424-5p
hsa-miR-584-5p |
Blood plasma |
Xu M. et al.
[45] |
PV |
1 |
U6 |
hsa-miR-338-3p |
Peripheral blood
mononuclear cells |
Lin N. et al.
[20] |
PV |
1 |
U6 |
hsa-miR-338-3p |
Peripheral blood
mononuclear cells |
Liu Q. et al.
[24] |
PV |
1 |
18srRNA |
hsa-miR-338-3p |
Peripheral blood
mononuclear cells |
Wang M. et al.
[21] |
PV |
124 |
U6 |
hsa-miR-424-5p |
Peripheral blood
mononuclear cells |
2. Results
2.1. Patient Selection
Four groups were included in the study.
Group 1 consisted of 37 patients with newly diagnosed or previously established pemphigus in the active stage. Among them were 9 males (24.3%) and 28 females (75.7%), with a mean age of 54 ± 13.93 years. The group included 28 patients (75.7%) with pemphigus vulgaris and 9 (24.3%) with pemphigus foliaceus. Nineteen patients (51.4%) had a relapse of previously diagnosed pemphigus, while 18 (48.6%) had disease onset. Lesion distribution was as follows: 12 patients (32.4%) had skin-only lesions, 18 (48.6%) had both skin and mucous membrane involvement, and 7 (18.9%) had lesions limited to mucous membranes. Disease severity, assessed by the Pemphigus Disease Area Index (PDAI), was mild in 12 patients (32.4%), moderate in 22 (59.5%), and severe in 3 (8.1%).
Samples for analysis in Group 1 were collected twice. The first sampling was performed at hospital admission: 18 patients (48.6%) had not yet received systemic glucocorticoids (0 mg), while 19 (51.4%) were already on therapy at a mean dose of 24.6 ± 16.7 mg. The second sampling occurred during the third week of pathogenetic therapy, at a mean dose of 81.5 ± 8.16 mg. Eleven patients withdrew from the study after three weeks of inpatient treatment. No repeated biomaterial sampling was obtained from them.
Diagnosis of pemphigus was based on clinical history, typical manifestations, detection of serum antibodies to desmoglein 1 and/or 3, histopathology showing intraepidermal suprabasal acantholysis with cleft formation, and direct immunofluorescence demonstrating IgG and/or C3 deposits in an intercellular pattern. After confirmation, all patients received inpatient treatment at the Department of Dermatovenereology of Sechenov University.
Group 2 included 20 patients in clinical remission lasting more than one year. All continued maintenance therapy with systemic glucocorticoids. The group comprised 7 males (35%) and 13 females (65%), with a mean age of 56 ± 12.81 years. Fourteen patients (70%) had pemphigus vulgaris and 6 (30%) had pemphigus foliaceus. The mean maintenance glucocorticoid dose was 12.53 ± 5.09 mg. The average remission duration was 1.7 ± 1.01 years. During prior exacerbations, 6 patients (30%) had skin-only lesions, 12 (60%) had both skin and mucosal involvement, and 2 (10%) had lesions limited to mucous membranes.
Group 3 consisted of 20 healthy controls: 15 females (75%) and 5 males (25%), with a mean age of 34 ± 11.5 years.
Group 4 included 10 patients with other bullous dermatoses in the active stage. Diagnoses were as follows: 4 with bullous pemphigoid (40%), 2 with erosive-ulcerative lichen planus (20%), 2 with dermatitis herpetiformis (20%), and 1 with cicatricial pemphigoid. The group comprised 7 females (70%) and 3 males (30%), with a mean age of 65 ± 18.58 years. Samples were collected before the initiation of oral glucocorticoid therapy.
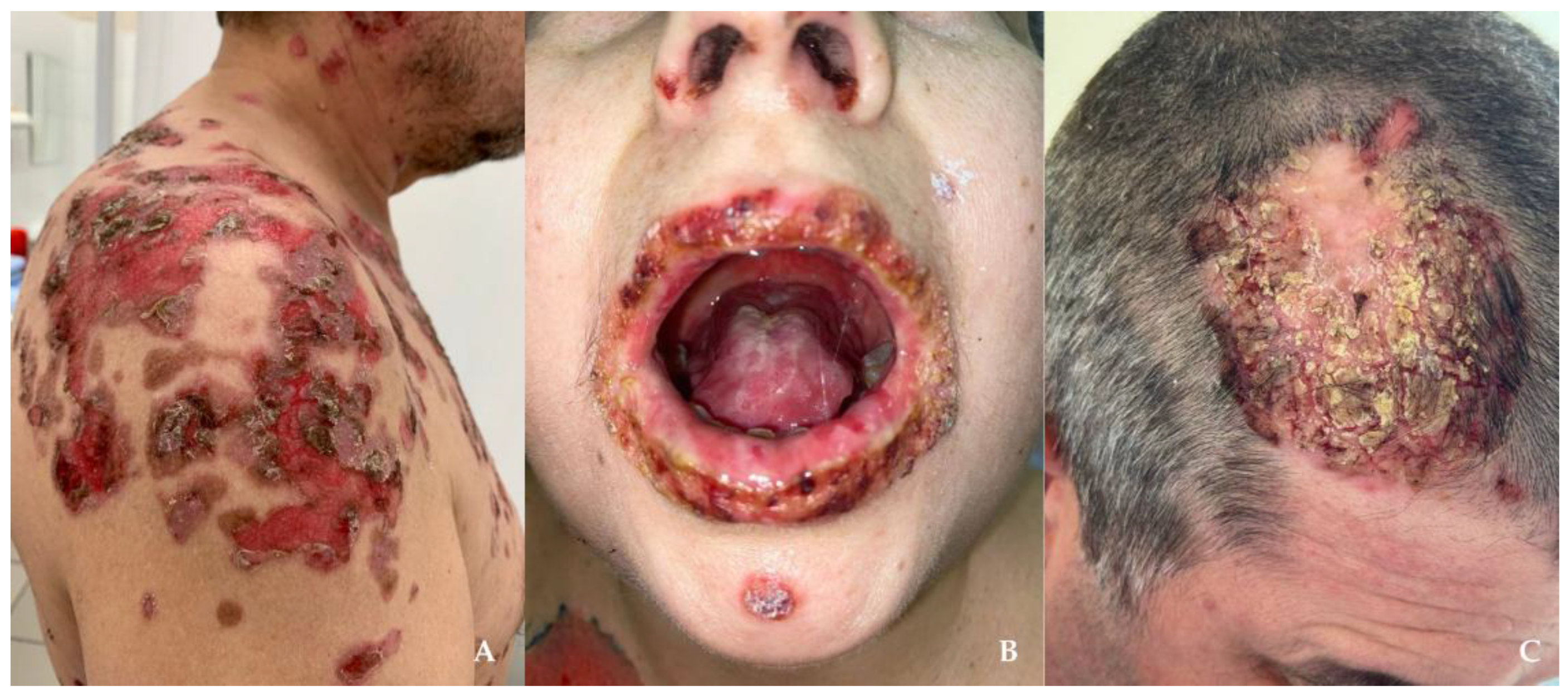
Figure 1.
Clinical presentation of pemphigus vulgaris on the skin (A) and mucous membranes (B), and pemphigus foliaceus (C).
Figure 1.
Clinical presentation of pemphigus vulgaris on the skin (A) and mucous membranes (B), and pemphigus foliaceus (C).
2.2. Analysis of Relative microRNA Expression Levels in Patients with Pemphigus and Control Groups
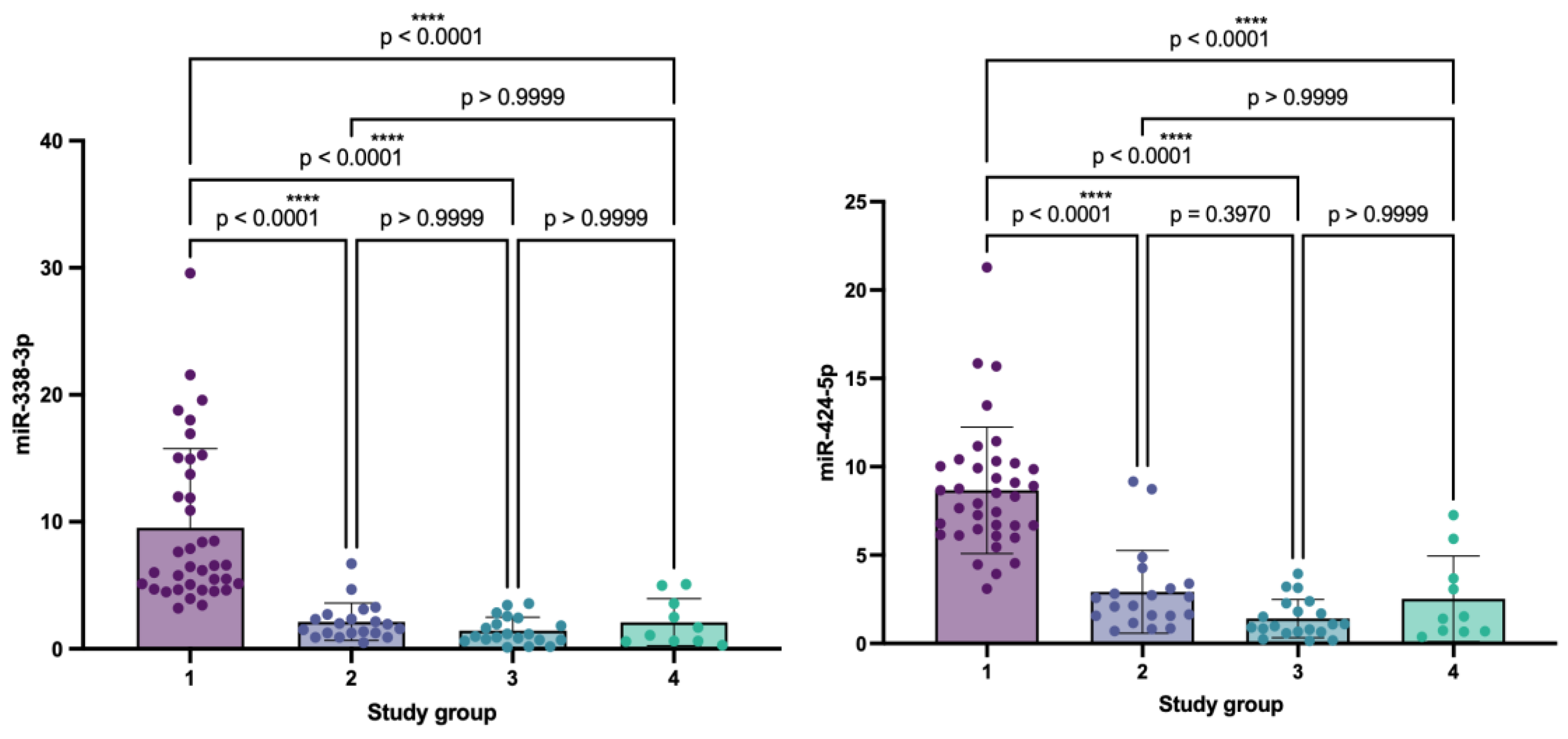
The mean expression level of miR-338-3p exhibited marked variation across the study groups. In patients with active pemphigus (group 1), the mean value was 9.53 (SD ± 6.24), while in patients in remission (group 2), it averaged 2.13 (SD ± 1.47). In healthy controls (group 3), microRNA expression was minimal — 1.42 (SD ± 1.06), and in patients with other bullous dermatoses (group 4), it was 2.10 (SD ± 1.85) (
Table 3).
Statistical analysis revealed significant differences among the groups (H = 57.40; p < 0.0001), indicating considerable variability in miR-338-3p expression. Post-hoc analysis demonstrated that expression levels in group 1 were significantly higher than in groups 2, 3, and 4 (p < 0.0001). Specifically, miR-338-3p expression in group 1 was 2.18-fold higher (118%) compared to group 2, 3.13-fold higher (213%) compared to group 3, and 2.44-fold higher (144%) compared to group 4. No statistically significant differences were observed between groups 2 and 3, 2 and 4, or 3 and 4 (p > 0.9999) (
Figure 2).
A similar trend was observed for miR-424-5p: in patients with active pemphigus, the mean expression level was 8.67 (SD ± 3.58), while in remission it decreased to 2.92 (SD ± 2.34). In conditionally healthy participants, miR-424-5p expression was 1.41 (SD ± 1.08), and in patients with other bullous dermatoses, it was 2.53 (SD ± 2.42). Significant intergroup differences were identified (H = 56.32; p < 0.0001). Multiple comparison testing revealed that expression levels in group 1 were significantly higher than in groups 2, 3, and 4 (p < 0.0001). In particular, miR-424-5p expression in group 1 was 1.95-fold higher (95%) compared to group 2, 3.40-fold higher (240%) compared to group 3, and 2.46-fold higher (146%) compared to group 4.
No significant differences were found between groups 2, 3, and 4 (p > 0.05) (
Figure 2). Overall, the analysis demonstrated a pronounced upregulation of both miR-338-3p and miR-424-5p in patients with autoimmune pemphigus compared to the control groups.
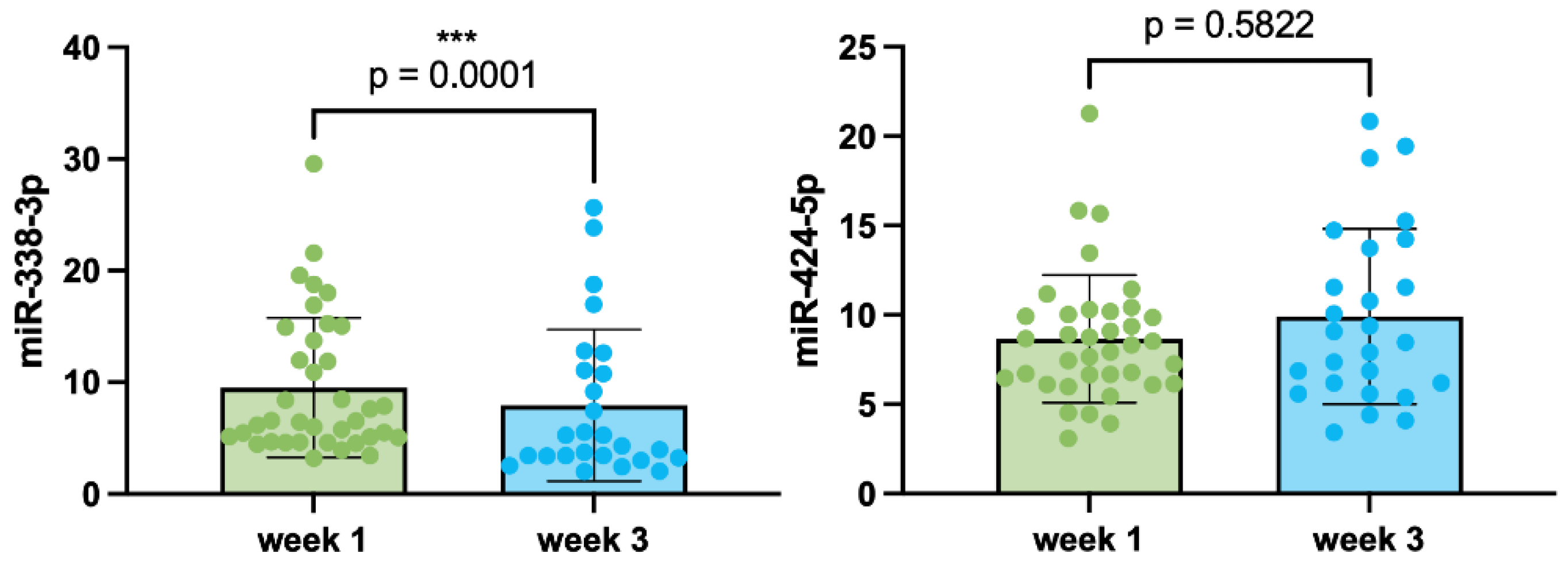
2.3. Relative Expression Levels of miR-338-3p and miR-424-5p in Patients with Pemphigus During Oral Systemic Glucocorticosteroid Therapy
To assess changes in the expression levels of miR-338-3p and miR-424-5p during oral systemic glucocorticosteroid (GCS) therapy, blood samples obtained from patients during the first and third weeks of inpatient treatment were analyzed. Sampling at week one was performed upon hospital admission: in 18 patients, blood samples were collected before the initiation of pathogenetic GCS therapy (0 mg), while 19 patients had already been receiving oral GCS at a mean prednisone-equivalent dose of 24.6 ± 16.7 mg at the time of admission; in these cases, sampling was carried out prior to dose adjustment and escalation of systemic GCS (
Table 2).
At week one, the mean expression levels of miR-338-3p and miR-424-5p were 9.53 (SD ± 6.24) and 8.67 (SD ± 3.58), respectively. By week three, the mean expression levels were 7.94 (SD ± 6.80) for miR-338-3p and 9.92 (SD ± 4.92) for miR-424-5p.
A comparison of miR-338-3p expression between the first and third weeks of GCS therapy revealed a statistically significant decrease (W = –283.0, p = 0.0001). The median expression level at week one was 6.55 (n = 37), compared with 4.80 at week three (n = 26). Thus, miR-338-3p expression decreased 1.36-fold (by 26.7%). The median difference was –2.974, indicating a consistent decline in expression by week three. Spearman’s correlation analysis confirmed that within-pair changes were statistically significant (rs = 0.8697, p < 0.0001) (
Figure 3).
In contrast, no statistically significant differences were observed for miR-424-5p between the first and third weeks of therapy (W = 45.0, p = 0.5822). The median expression level at week one was 8.318 (n = 37), and 8.767 at week three (n = 26). The median difference was 0.1230, corresponding to a 0.95-fold decrease (–5.4%), which was not statistically significant. Nevertheless, Spearman’s correlation analysis demonstrated a high degree of data consistency (rs = 0.7181, p < 0.0001) (
Figure 3).
Taken together, these findings indicate that the expression level of miR-338-3p significantly decreases by the third week of oral systemic GCS therapy (1.36-fold, or 26.7%), whereas the expression level of miR-424-5p remains stable.
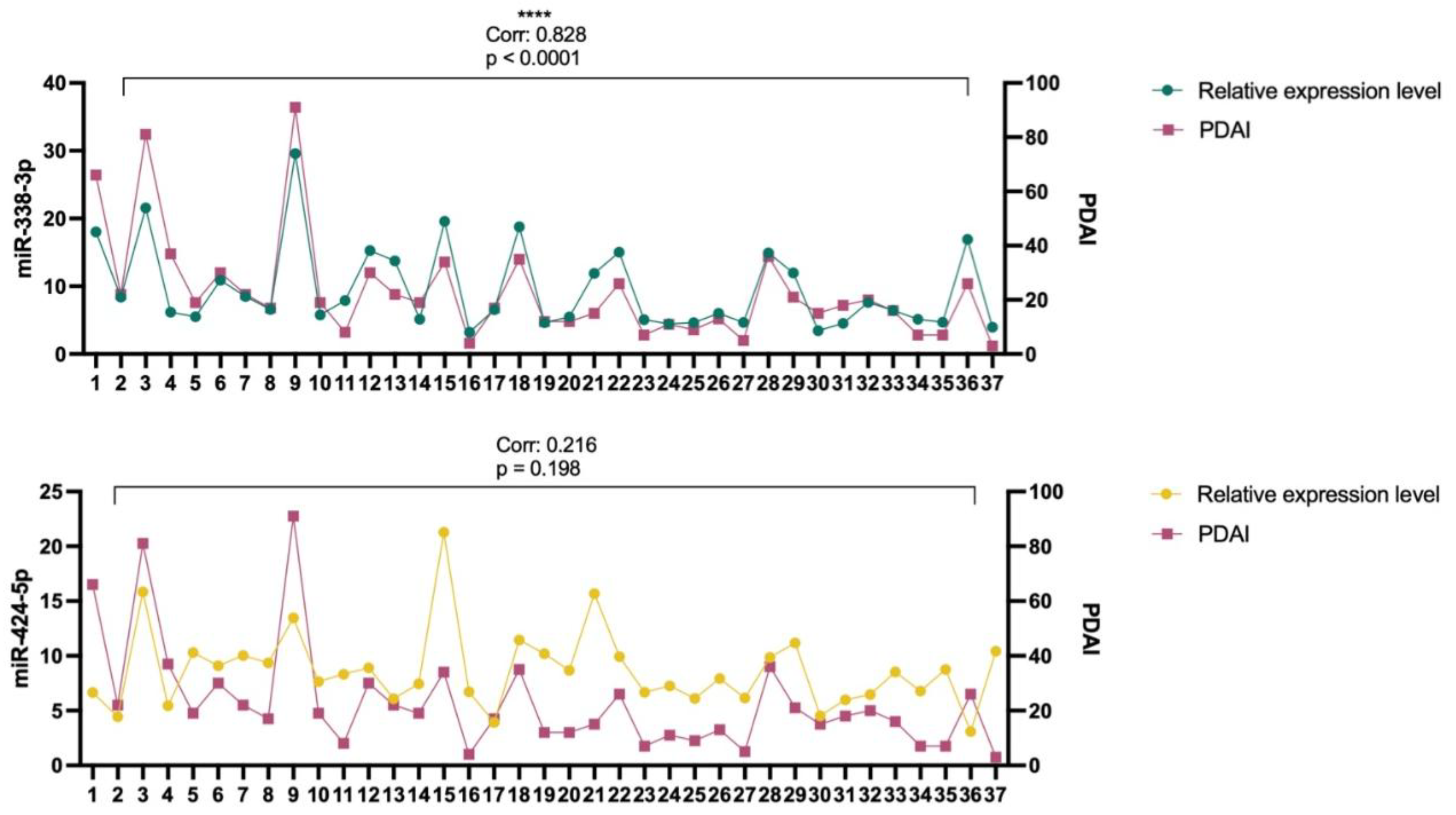
2.4. Correlation Analysis of miR-338-3p and miR-424-5p Expression Levels with Disease Severity Assessed by the Pemphigus Disease Area Index (PDAI)
To assess the association between miR-338-3p and miR-424-5p expression levels and disease severity, as quantified by the Pemphigus Disease Area Index (PDAI), a correlation analysis was conducted. The distributions of both microRNA expression levels and PDAI scores significantly deviated from normality (Shapiro–Wilk test: W = 0.8331, p < 0.0001; W = 0.7588, p < 0.0001); therefore, Spearman’s rank correlation coefficient was employed for statistical evaluation.
The analysis demonstrated a statistically significant positive correlation between miR-338-3p expression and PDAI scores (r = 0.8280, 95% CI: 0.6835–0.9101, p < 0.0001), indicating a robust direct association between elevated miR-338-3p levels and increased disease severity in pemphigus (
Figure 4). The high correlation coefficient underscores the strength of this association and suggests a potential role of miR-338-3p in the pathogenesis of the disease.
In contrast, miR-424-5p expression did not exhibit a statistically significant correlation with PDAI scores (r = 0.2166, 95% CI: –0.1254 to 0.5125, p = 0.1980). Although the correlation coefficient was positive, its magnitude was low and the confidence interval included zero, indicating the absence of a statistically robust association between miR-424-5p expression and disease severity (
Figure 4).
Taken together, these findings demonstrate that miR-338-3p expression is significantly correlated with PDAI scores, whereas miR-424-5p does not show a statistically significant relationship with this clinical parameter.
2.5. Diagnostic Value of Relative miR-338-3p and miR-424-5p Expression Levels in Pemphigus
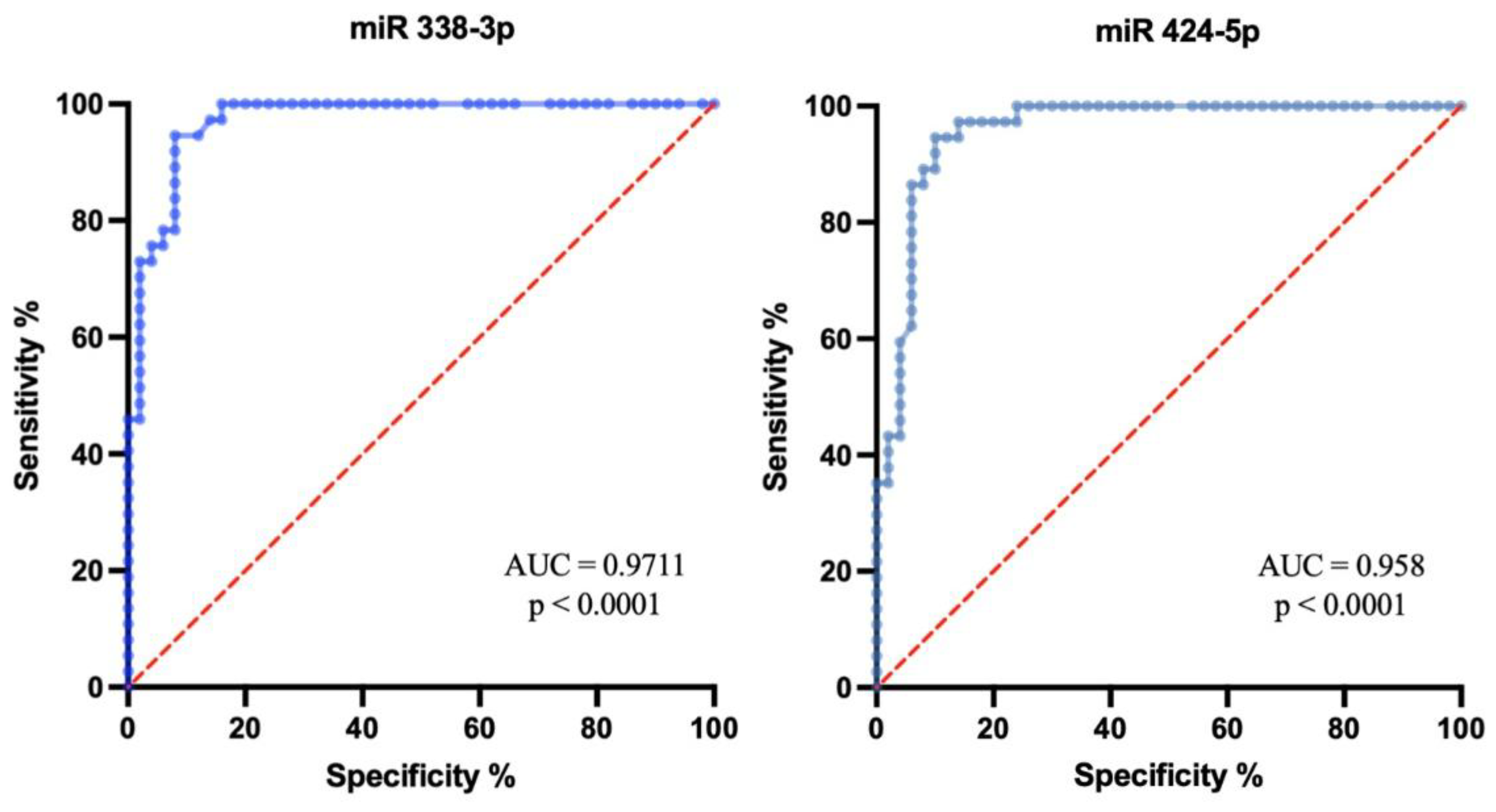
To evaluate the diagnostic significance of relative miR-338-3p and miR-424-5p expression levels in pemphigus, receiver operating characteristic (ROC) curve analysis was performed. The dataset included 50 control subjects from Groups 2, 3, and 4, as well as 37 patients with active pemphigus from Group 1. Since no statistically significant differences in microRNA expression were observed among control Groups 2, 3, and 4, they were pooled into a single control cohort for ROC analysis.
For miR-338-3p, the area under the ROC curve (AUC) was 0.9711 (95% CI: 0.9415–1.000; p < 0.0001), indicating high diagnostic accuracy for distinguishing patients with active pemphigus. The Youden index was applied to determine the optimal cutoff threshold. A cutoff value > 3.753 provided the best balance between sensitivity and specificity, with sensitivity of 94.59% (95% CI: 82.30–99.04%) and specificity of 92.00% (95% CI: 81.16–96.85%). The corresponding Youden index was 0.865. At this threshold, the likelihood ratio was 11.82, further confirming the strong diagnostic utility of miR-338-3p (
Figure 5).
For miR-424-5p, the AUC was 0.9581 (95% CI: 0.9183–0.9979; p < 0.0001). The optimal cutoff identified by the Youden index was > 4.367, yielding a sensitivity of 94.59% (95% CI: 82.30–99.04%) and specificity of 90.00% (95% CI: 78.64–95.65%), with a Youden index of 0.845. At this threshold, the likelihood ratio reached 9.459, suggesting that miR-424-5p levels above 4.367 are strongly associated with the presence of pemphigus (
Figure 5).
Taken together, these findings indicate that cutoff values ≥ 3.753 for miR-338-3p and ≥ 4.367 for miR-424-5p provide optimal diagnostic discrimination. Both microRNAs demonstrated high sensitivity, specificity, and likelihood ratios, underscoring their potential as reliable biomarkers for differentiating patients with pemphigus from healthy individuals and supporting their clinical applicability in diagnostic practice.
3. Discussion
MicroRNAs are a class of small (~22 nucleotides), endogenous non-coding RNAs that regulate gene expression at the post-transcriptional level. They are estimated to influence more than 60% of coding genes, primarily through binding to complementary mRNA sequences and inhibiting translation, promoting mRNA degradation, or inducing epigenetic modifications via chromatin remodeling complexes [
34,
35,
36]. Through these mechanisms, microRNAs play essential roles in cell differentiation, proliferation, and apoptosis [
16]. Despite intensive research, their precise contribution to disease pathogenesis remains incompletely understood, largely due to the fact that a single microRNA may regulate numerous genes across multiple signaling pathways. Identifying disease-specific microRNAs therefore remains a major challenge in translational medicine [
36].
To date, more than 120 microRNAs have been investigated in pemphigus across various biological substrates. Among these, miR-338-3p and miR-424-5p have emerged as the most promising candidates [
28,
37,
38]. Expression profiling in peripheral blood mononuclear cells (PBMCs), including lymphocytes, monocytes, and dendritic cells, is particularly valuable given the direct involvement of these immune cells in disease pathogenesis. An imbalance of Th1/Th2 cells in pemphigus may be associated with the overexpression of miR-338-3p and miR-424-5p. Lin et al. (2018) demonstrated that miR-338-3p-mediated
RNF114 suppression contributes to apoptotic control and T-cell activation [
20], while other studies suggested regulation of
TRADD expression, a key mediator of apoptosis [
24]. Collectively, miR-338-3p appears to be one of the most relevant microRNAs in pemphigus, with several studies supporting its utility for assessing disease severity and monitoring therapeutic response [
20,
28].
By contrast, studies of miR-424-5p remain limited. Existing reports have been restricted to baseline comparisons, demonstrating increased expression in active pemphigus patients relative to controls [
21]. However, longitudinal analyses, correlations with disease severity, and diagnostic threshold values have not been assessed. Bioinformatics analyses suggest that miR-424-5p targets genes involved in MAPK signaling, particularly p38 MAPK activation, a pathway central to autoimmune regulation, keratinocyte apoptosis, cytoskeletal reorganization, and desmosomal disruption [
21,
31]. These observations highlight miR-424-5p as a promising research target in pemphigus, warranting further functional validation.
In this study, we analyzed the relative expression levels of miR-338-3p and miR-424-5p in patients with PV and PF. Both microRNAs showed significant differential expression in active pemphigus compared with healthy controls, remission patients, and other active bullous dermatoses. Notably, miR-338-3p expression decreased at the third week of systemic oral GCS therapy, suggesting a treatment-responsive dynamic. Furthermore, expression levels of miR-338-3p and miR-424-5p correlated with disease severity, as assessed by PDAI scores. ROC analysis was conducted, and diagnostic thresholds for both miR-338-3p and miR-424-5p were established. Our findings are in agreement with Lin et al. (2018), who reported treatment-responsive decreases of miR-338-3p in active pemphigus patients with correlation to PDAI scores. However, their study was limited by small sample sizes and heterogeneous follow-up periods (2–6 weeks post-therapy). In contrast, only two studies to date have examined miR-424-5p expression, both demonstrating elevated levels in active pemphigus compared with controls [
21,
32]. Yet, neither addressed longitudinal dynamics, correlations with PDAI, nor diagnostic threshold values. To our knowledge, our study is the first to provide such data for miR-424-5p.
Our findings highlight the potential of miR-338-3p and miR-424-5p to serve not only as diagnostic biomarkers of pemphigus but also as tools for disease monitoring and prognostic assessment. Both microRNAs demonstrated significant associations with PDAI scores, suggesting their added value in developing more objective scoring systems for disease severity. Importantly, miR-338-3p expression decreased after three weeks of systemic GCS therapy, supporting its role as a treatment-responsive marker. These results complement previous studies and, for the first time, provide expression thresholds for miR-338-3p and miR-424-5p that may be applied in clinical practice.
In addition, the correlation of miR-338-3p and miR-424-5p with PDAI underscores their potential utility in stratifying patients by disease severity and predicting treatment efficacy, particularly in cases with isolated mucosal involvement where diagnostic challenges remain. Recent evidence further suggests that Th1/Th2 imbalance in pemphigus may be linked to the overexpression of miR-338-3p and miR-424-5p, influencing MAPK signaling pathways and T-cell activation via RNF114 and TRADD regulation. Taken together, these findings indicate that further investigation of these microRNAs and their molecular targets could open new avenues not only for biomarker validation but also for the development of personalized therapeutic strategies in pemphigus.
4. Materials and Methods
4.1. Patients, Study Design, and Approvals
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional ethics committee I.M. Sechenov First Moscow State Medical University (Sechenov University) (protocol N°02-23, January 26, 2023). Written informed consent was obtained from all participants prior to inclusion.
Patients with active pemphigus were sampled twice: before the initiation of systemic glucocorticoid therapy and during the third week of treatment. Patients in long-term remission, healthy controls, and individuals with other active bullous dermatoses were enrolled and sampled on an outpatient basis.
Eligibility criteria included age ≥18 years, confirmed diagnosis according to clinical, histological, immunopathological, and serological findings, and signed informed consent. Exclusion criteria comprised pregnancy or lactation, major comorbidities, other autoimmune diseases, or withdrawal of consent.
4.2. Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) is considered the most accurate and sensitive approach for determining relative and absolute microRNA levels in biological samples [
39]. Nevertheless, significant methodological heterogeneity persists across studies regarding the number of microRNAs analyzed, technical modifications of PCR, detection strategies, and, importantly, the choice of biological substrate and internal controls (
Table 1). These inconsistencies highlight the absence of a standardized protocol for assessing microRNA expression in pemphigus.
In the present study, peripheral blood mononuclear cells (PBMCs; lymphocytes, monocytes, dendritic cells) were selected as the biological substrate, as these cells are central to the regulation of immune responses and inflammatory processes. Peripheral venous blood was collected into EDTA K₂-coated tubes, and PBMCs were isolated within 5 h using Ficoll density-gradient centrifugation (density 1.077 g/cm³; PanEco, Russia). The interphase layer was harvested, washed, transferred into cryovials, and stored at −80 °C until further analysis. Samples were obtained from patients at baseline (prior to systemic therapy initiation) and at week 3 following hospitalization and corticosteroid treatment initiation.
Total RNA, including microRNAs, was extracted using the Lyra Total RNA and microRNA Isolation Kit (Biolabmix, Russia) in accordance with the manufacturer’s protocol. Complementary DNA (cDNA) synthesis was performed with Stem-Loop reverse transcription primers using the OT-1 kit (Syntol, Russia). The stem-loop approach was selected for its high specificity and ability to discriminate mature microRNAs from precursor sequences, thereby ensuring reliable quantification even at low expression levels [
40,
41].
Reverse transcription reactions were set up separately for miR-338-3p, miR-424-5p, and the endogenous control U6 snRNA (
Table 4). Each 25 µL RT reaction contained 10 µL of 2.5× Reaction Mix, 1 µL of a specific stem-loop primer, 1 µL of MMLV reverse transcriptase, 3 µL of nuclease-free water, and 10 µL of RNA template. The thermal profile was as follows: 37 °C for 30 min, 95 °C for 5 min, followed by cooling to 4 °C.
Quantitative Real-Time PCR was carried out in triplicate using a 2.5× SYBR Green I Reaction Mix (Syntol, Russia) on a Bio-Rad iCycler iQ5 detection system (Bio-Rad Laboratories, USA). Each 25 µL PCR reaction contained 10 µL of SYBR Green I Mix, 1 µL of primer mixture (10 pmol/µL, diluted 1:5), 0.5 µL of 25 mM MgCl₂, 1 µL of 1:5 diluted cDNA, and 12.5 µL of nuclease-free ddH₂O. The amplification protocol was: initial denaturation at 95 °C for 10 min; 40 cycles of 95 °C for 30 s, 60 °C for 20 s, and 72 °C for 1 min; final extension at 95 °C for 5 min.
Melting curve analysis (60–95 °C) was performed after each run to confirm amplification specificity and absence of primer-dimer formation. No-template controls (NTC) and no-reverse transcription controls (No-RT) were included to monitor for contamination and genomic DNA carryover.
Amplification output was expressed as threshold cycle (Ct) values, defined as the cycle number at which fluorescence intensity exceeded background levels. The Ct values were inversely proportional to target nucleic acid abundance. Relative quantification of miRNA expression was performed using the 2
−ΔΔCt method [
42] with U6 as the internal control.
4.3. Statistical Analysis
Data analysis was performed using Microsoft Excel, GraphPad Prism 10.1.1, and Jamovi 2.3.28. Relative microRNA expression levels were calculated using the standard 2
−ΔΔCt method [
42]. The normality of data distribution was assessed with the Shapiro–Wilk test. Statistical significance of differences between independent groups was evaluated using the Mann–Whitney U test and the Kruskal–Wallis test, while paired comparisons were performed using the Wilcoxon signed-rank test. Correlation analysis was conducted with Spearman’s rank correlation coefficient. To assess the diagnostic value of relative expression levels, receiver operating characteristic (ROC) curve analysis was applied, and optimal cut-off points were determined using the Youden index.
5. Conclusions
Pemphigus is a group of chronic, potentially life-threatening autoimmune blistering diseases that necessitate reliable diagnostic tools and continuous monitoring of disease activity and therapeutic response. In this study, we demonstrated that expression levels of miR-338-3p and miR-424-5p were significantly elevated in patients with active pemphigus compared with all control groups. Notably, miR-338-3p expression showed a strong positive correlation with disease severity, as assessed by PDAI scores. ROC analysis revealed robust discriminatory ability for active disease for both microRNAs (AUC = 0.9711 for miR-338-3p and AUC = 0.9581 for miR-424-5p). To the best of our knowledge, this is the first study in Russia to evaluate the diagnostic significance of miR-338-3p and miR-424-5p in pemphigus, and it is also the first study on this topic conducted with a cohort of such size. Collectively, these findings highlight the strong potential of miR-338-3p and miR-424-5p as diagnostic and monitoring biomarkers in pemphigus. Further studies with larger cohorts and longitudinal assessments of microRNA expression in relation to disease severity and systemic glucocorticoid therapy are warranted to validate these results and may ultimately enable more accurate disease assessment, improved monitoring, and better prediction of clinical outcomes.
Author Contributions
Conceptualization, N.P.T. and D.V.M.; Methodology, D.V.M. and Yu.V.K.; Investigation, D.V.M. and Yu.V.K.; Formal analysis, D.V.M. and Yu.V.K.; Resources, D.V.M.; Data curation, D.V.M.; Writing—original draft preparation, D.V.M. and Yu.V.K.; Writing—review and editing, N.P.T., N.L.S., A.A.L., T.A.F. and D.N.U.; Visualization, D.V.M.; Supervision, N.P.T.; Project administration, N.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Sechenov University (protocol code N◦ 02-23, January 26, 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olisova, O.Y.; Teplyuk, N.P. An Illustrated Guide to Dermatology. For Preparing Practitioners for Accreditation; GE-OTAR-Media: Moscow, Russia, 2023; p. 376. [Google Scholar]
- Malik, A.M.; Tupchong, S.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. An Updated Review of Pemphigus Diseases. Medicina 2021, 57, 1080. [Google Scholar] [CrossRef]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Amagai, M.; Tanikawa, A.; Committee for Guidelines for the Management of Pemphigus Disease. Japanese Guidelines for the Management of Pemphigus. J. Dermatol. 2014, 41, 471–486. [Google Scholar] [CrossRef]
- Kridin, K. Pemphigus Group: Overview, Epidemiology, Mortality, and Comorbidities. Immunol. Res. 2018, 66, 255–270. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Brito, M.M.; Salathiel, A.M.; Ferraz, T.S.; Alves, D.; Roselino, A.M. Incidence of Pemphigus Vulgaris Exceeds That of Pemphigus Foliaceus in a Region Where Pemphigus Foliaceus Is Endemic: Analysis of a 21-Year Historical Series. An. Bras. Dermatol. 2011, 86, 1109–1112. [Google Scholar] [CrossRef]
- Costan, V.V.; Popa, C.; Hâncu, M.F.; Porumb-Andrese, E.; Toader, M.P. Comprehensive Review on the Pathophysiology, Clinical Variants and Management of Pemphigus. Exp. Ther. Med. 2021, 22, 1335. [Google Scholar] [CrossRef] [PubMed]
- Harel-Raviv, M.; Srolovitz, H.; Gornitsky, M. Pemphigus Vulgaris: The Potential for Error. A Case Report. Spec. Care Dentist. 1995, 15, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Morishima-Koyano, M.; Nobeyama, Y.; Fukasawa-Momose, M.; et al. Case of Pemphigus Foliaceus Misdiagnosed as a Single Condition of Erythrodermic Psoriasis and Modified by Brodalumab. J. Dermatol. 2020, 47, e201–e202. [Google Scholar] [CrossRef] [PubMed]
- Daltaban, Ö.; Özçentik, A.; Karakaş, A.; et al. Clinical Presentation and Diagnostic Delay in Pemphigus Vulgaris: A Prospective Study from Turkey. J. Oral Pathol. Med. 2020, 49, 681–686. [Google Scholar] [CrossRef]
- Khamaganova, I.V.; Malyarenko, E.N.; Denisova, E.V.; Vorontsova, I.V.; Plieva, K.T. Mistakes of Diagnostics in Pemphigus Vulgaris: Case Report. Russ. J. Skin Venereal Dis. 2017, 20, 30–33. [Google Scholar] [CrossRef]
- Teplyuk, N.P.; Kolesova, Y.V.; Mak, D.V.; Lepekhova, A.A.; Toshchakov, S.V.; Fedotcheva, T.A. Pemphigus: New Approaches to Diagnosis and Disease Severity Assessment. Russ. J. Skin Venereal Dis. 2023, 26, 515–526. [Google Scholar] [CrossRef]
- Kridin, K.; Bergman, R. The Usefulness of Indirect Immunofluorescence in Pemphigus and the Natural History of Patients with Initial False-Positive Results: A Retrospective Cohort Study. Front. Med. 2018, 5, 266. [Google Scholar] [CrossRef] [PubMed]
- Giurdanella, F.; Nijenhuis, A.M.; Diercks, G.F.H.; Jonkman, M.F.; Pas, H.H. Keratinocyte Binding Assay Identifies Anti-Desmosomal Pemphigus Antibodies Where Other Tests Are Negative. Front. Immunol. 2018, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Dähnrich, C.; Rosemann, A.; et al. Novel ELISA Systems for Antibodies to Desmoglein 1 and 3: Correlation of Disease Activity with Serum Autoantibody Levels in Individual Pemphigus Patients. Exp. Dermatol. 2010, 19, 458–463. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Martin-Burriel, I.; Gil, A.; Cubero, P.; Forner, M.; Khalyfa, A.; Marin, J.M. Stability of Circulating Exosomal miRNAs in Healthy Subjects. Sci. Rep. 2018, 8, 10306. [Google Scholar] [CrossRef]
- Matias-Garcia, P.R.; Wilson, R.; Mussack, V.; Reischl, E.; Waldenberger, M.; Gieger, C.; Anton, G.; Peters, A.; Kuehn-Steven, A. Impact of Long-Term Storage and Freeze-Thawing on Eight Circulating microRNAs in Plasma Samples. PLoS ONE 2020, 15, e0227648. [Google Scholar] [CrossRef]
- Ward Gahlawat, A.; Lenhardt, J.; Witte, T.; Keitel, D.; Kaufhold, A.; Maass, K.K.; Pajtler, K.W.; Sohn, C.; Schott, S. Evaluation of Storage Tubes for Combined Analysis of Circulating Nucleic Acids in Liquid Biopsies. Int. J. Mol. Sci. 2019, 20, 704. [Google Scholar] [CrossRef]
- Lin, N.; Liu, Q.; Wang, M.; Wang, Q.; Zeng, K. Usefulness of miRNA-338-3p in the Diagnosis of Pemphigus and Its Correlation with Disease Severity. PeerJ 2018, 6, e5388. [Google Scholar] [CrossRef]
- Wang, M.; Liang, L.; Li, L.; et al. Increased miR-424-5p Expression in Peripheral Blood Mononuclear Cells from Patients with Pemphigus. Mol. Med. Rep. 2017, 15, 3479–3484. [Google Scholar] [CrossRef]
- Rodriguez, M.S.; Egaña, I.; Lopitz-Otsoa, F.; Aillet, F.; Lopez-Mato, M.P.; Dorronsoro, A.; Lobato-Gil, S.; Sutherland, J.D.; Barrio, R.; Trigueros, C.; Lang, V. The RING Ubiquitin E3 RNF114 Interacts with A20 and Modulates NF-κB Activity and T-Cell Activation. Cell Death Dis. 2014, 5, e1399. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Li, M.; Zhang, K.; Li, C.; Chen, H.; Tao, D.; Zhang, S.; Ma, Y. Identification of RNF114 as a Novel Positive Regulatory Protein for T Cell Activation. Immunobiology 2014, 219, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, F.; Wang, M.; Xiong, H.; Peng, X.; Liang, L.; Li, L.; Zhang, J.; Peng, X.; Zeng, K. Increased Expression of microRNA-338-3p Contributes to Production of Dsg3 Antibody in Pemphigus Vulgaris Patients. Mol. Med. Rep. 2018, 18, 550–556. [Google Scholar] [CrossRef]
- Satyam, A.; Khandpur, S.; Sharma, V.K.; Sharma, A. Involvement of TH1/TH2 Cytokines in the Pathogenesis of Autoimmune Skin Disease—Pemphigus Vulgaris. Immunol. Investig. 2009, 38, 498–509. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, W.J.; Kim, S.C. Analysis of Serum Cytokine Profile in Pemphigus. Ann. Dermatol. 2017, 29, 438–445. [Google Scholar] [CrossRef]
- Rizzo, C.; Fotino, M.; Zhang, Y.; et al. Direct Characterization of Human T Cells in Pemphigus Vulgaris Reveals Elevated Autoantigen-Specific Th2 Activity in Association with Active Disease. Clin. Exp. Dermatol. 2005, 30, 535–540. [Google Scholar] [CrossRef]
- Teplyuk, N.P.; Mak, D.V.; Kolesova, Y.V.; Lepekhova, A.A.; Fedotcheva, T.A.; Ulchenko, D.N. The miR-338-3p Expression Level in Pemphigus Diagnosis. Russ. J. Skin Venereal Dis. 2024, 27, 448–462. [Google Scholar] [CrossRef]
- Li, X.; Ishii, N.; Ohata, C.; Furumura, M.; Hashimoto, T. Signalling Pathways in Pemphigus Vulgaris. Exp. Dermatol. 2014, 23, 155–156. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Kitajima, Y.; Sato-Nagai, M.; Grando, S.A. Desmoglein versus Non-Desmoglein Signaling in Pemphigus Acantholysis: Characterization of Novel Signaling Pathways Downstream of Pemphigus Vulgaris Antigens. J. Biol. Chem. 2007, 282, 13804–13812. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, P.; Hu, P.; Liu, Z.; Diaz, L.A.; Enghild, J.J.; Chua, M.P.; Rubenstein, D.S. Desmosome Signaling. Inhibition of p38MAPK Prevents Pemphigus Vulgaris IgG-Induced Cytoskeleton Reorganization. J. Biol. Chem. 2005, 280, 23778–23784. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xing, Y.; Li, C.; et al. Identification of Six microRNAs as Potential Biomarkers for Pemphigus Vulgaris: From Diagnosis to Pathogenesis. Diagnostics 2022, 12, 3058. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA Delivery. J. Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating microRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cannavicci, A.; Dai, S.-C.; Wang, C.; Kutryk, M.J.B. MicroRNA Signature of Human Blood Mononuclear Cells. Mol. Cell. Biochem. [CrossRef]
- Papara, C.; Zillikens, D.; Sadik, C.D.; Baican, A. MicroRNAs in Pemphigus and Pemphigoid Diseases. Autoimmun. Rev. 2021, 20, 102852. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xu, M.; Tian, X.; et al. Research Advances in the Detection of miRNA. J. Pharm. Anal. 2019, 9, 217–226. [Google Scholar] [CrossRef]
- Kramer, M.F. Stem-Loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 2011. Chapter 15, Unit15.10. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; et al. Real-Time Quantification of MicroRNAs by Stem–Loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(−ΔΔCT) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Valentino, A.; Leuci, S.; Galderisi, U.; et al. Plasma Exosomal microRNA Profile Reveals miRNA 148a-3p Downregulation in the Mucosal-Dominant Variant of Pemphigus Vulgaris. Int. J. Mol. Sci. 2023, 24, 11493. [Google Scholar] [CrossRef] [PubMed]
- Khabou, B.; Fakhfakh, R.; Tahri, S.; et al. miRNA Implication in the Pathogenesis and the Outcome of Tunisian Endemic Pemphigus Foliaceus. Exp. Dermatol. 2023, 32, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, Q.; Li, S.; et al. Increased Expression of miR-338-3p Impairs Treg-Mediated Immunosuppression in Pemphigus Vulgaris by Targeting RUNX1. Exp. Dermatol. 2020, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).