1. Introduction
Fine powders with a given chemical and phase composition and high uniformity of component distribution are required for creation of inorganic materials or composite materials with unique functional properties [
1,
2,
3]. The simplest and most obvious is the method of mechanical homogenization of powder mixtures, which is carried out using special equipment, such as a planetary mill [
4]. High-temperature solid-phase synthesis from a homogeneous mixture of salts obtained by drying a solution of these salts at subzero temperatures is more difficult for implementation [
5]. The maximum level of homogenization is achieved in mixed-anionic compounds, which are produced using high-temperature (synthesis in the solid phase, synthesis by interaction of gas and solid phases, synthesis using pressure) and low-temperature reactions (topochemical, solvothermal synthesis, synthesis in thin films) [
6]. Precipitation of hydroxides [
7], carbonates or oxalates are also used to obtain homogeneous precursors of oxide powders [
8]. Synthesis of inorganic powders consisting of small particles with a high specific surface area and activity via precipitation from solution is the most convenient for implementation [
9].
High uniformity of the distribution of components in a powder intended for the production of biocompatible materials can be achieved using synthesis from both mixed-cationic [
10] and mixed-anionic solutions, for example, containing HPO
42-/P
2O
74- [
11], HPO
42-/CO
32- [
12,
13,
14], P
2O
74−/CO
32− [
15], HPO
42-/SiO
32- [
16].
The mineral component of bone tissue is mainly represented by carbonate-substituted hydroxyapatite [
17]. Calcium hydroxyapatite Ca
10(PO
4)
6(OH)
2 is a well-known and unique ion exchanger [
18,
19,
20], therefore, various cations such as Na
+, K
+, Mg
2+, Zn
2+, Ba
2+, or Sr
2+ [
21] and anions such as CO
32− or SiO
44−, F
− or Cl
− can be included in the composition of bone tissue [
22,
23].
Various powders obtained by one of the methods of chemical synthesis are used for the manufacture of bone implants [
24]. Precipitation from solutions including a hydrophosphate ion, a carbonate ion, and a calcium ion is preferable due to the possibility of obtaining powders similar in chemical and phase composition to natural bone tissue [
12,
13,
14,
25]. Powders of calcium phosphates with molar ratio Ca/P<1.67 and calcium carbonates are used to creating materials for temporary bone implants. At the same time, hydroxyapatite-based materials (molar ratio Ca/P=1.67) are resistant to dissolution and can be used as implants for long time substituting [
26].
Materials based on calcium carbonates [
27,
28,
29] and calcium phosphates with a molar ratio of Ca/P<1.67 are able to dissolve gradually during implantation and therefore they are used in regenerative methods of bone defects treating [
30]. Synthetic powders of calcium carbonate CaCO
3 and calcium phosphates with a molar ratio of Ca/P<1.67 can be used as fillers in biocompatible and biodegradable composites with polymer [
31] or mineral (obtained as a result of chemical bonding reactions) matrices [
32]. In addition, these powders can also be used to produce biocompatible ceramic materials with the phase composition belonging to oxide systems, including calcium oxide and phosphorus oxide [
33].
The aim of the present work consisted in preparing and investigating powders synthesized from an aqueous solution of calcium chloride CaCl2 and a mixed anionic solution containing orthophosphate and carbonate ions. Potassium hydroorthophosphate K2HPO4 was used as the source of orthophosphate ions, and potassium carbonate K2CO3 was used as the source of carbonate ions. For comparison, as references, powders were also synthesized from an aqueous solution of calcium chloride CaCl2 and aqueous solutions including either potassium hydrophosphate K2HPO4 or potassium carbonate K2CO3.
2. Materials and Methods
For the synthesis of powders, calcium chloride CaCl2 (CAS No. 10043-52-4, Rushim, Moscow, Russia), potassium hydrophosphate trihydrate K2HPO4 3H2O (CAS No. 16788-57-1, Rushim, Moscow, Russia) and potassium carbonate K2CO3 (CAS No. 584-08-7, Rushim, Moscow, Russia) were used.
The following reactions were used to calculate the amounts of starting salts and expected products:
The labeling and synthesis conditions of the powders from CaCl
2 and K
2HPO
4 and/or K
2CO
3 are shown in
Table 1.
500 ml of 0.5M aqueous solution of potassium hydrophosphate K
2HPO
4 (
PO4 powder); 500 ml of 0.5M aqueous solution of potassium carbonate K
2CO
3 (
CO3 powder); 500 ml aqueous solution containing 0.125M potassium hydrophosphate K
2HPO
4 and 0.125M potassium carbonate K
2CO
3 (
PO4_CO3 powder) were added to the 500 ml of 0.5M aqueous CaCl
2 solutions. The resulting suspensions were kept on a stirrer for 30 minutes. The resulting precipitates were separated from the mother liquor using vacuum filtration. The synthesized powders were dried in a thin layer at room temperature for a week. After drying, the powders were crushed using an agate mortar and pestle, and then passed through a polyester sieve with a mesh size of 200 microns. For the isolation of salts dissolved in the mother liquors they were dried at 40 °C for a month. The synthesized powders and isolated reaction by-products were weighed to determine their mass and to estimate the yield of synthesized powders and reaction by-products relatively to the theoretically possible amounts.
Table 2 shows the quantities of the initial, expected target products and reaction by-product calculated in accordance with reactions (1-3).
To study the thermal evolution of the phase composition of the synthesized powders, they were placed in porcelain boats and heated at a heating rate of 5 °C/min, followed by exposure for 2 hours at various temperatures in the range of 200-1000 °C. The labeling of the powders after heat treatment used in the figures is shown in
Table 3.
The phase composition of powders after synthesis and after heat treatment was performed by X-ray powder diffraction (XRD) analysis using CuKa radiation (λ = 1.5418 Å, step 2θ – 0.02°) using Rigaku D/Max-2500 diffractometers (Rigaku Corporation, Tokyo, Japan) in the angle range 2θ = 2…70° or Tongda TD-3700 (Dandong Tongda Science & Technology Co., Ltd., Dandong, China) in the angle range 2θ = 3…70°. The X-ray images were analyzed using the WinXPOW program using the ICDD PDF-2 (
http://www.icdd.com/products/pdf2.htm, accessed on 18 August 2025) [
34] databases and the Match! program. (
https://www.crystalimpact.com/, accessed on 18 August 2025). The quantitative ratio of the target and related products in the obtained powders was determined using the Match!3 program,
https://www.crystalimpact.com/. The infrared (IR) spectra were collected in the wavelength range 500-4000 cm
−1 using the Spectrum Three IR spectrometer (Perkin Elmer, Waltham, Massachusetts, USA) in the mode of disturbed total internal reflection using the Universal ATR accessory (crystal diamond/KRS-5). The bands in the spectra were assigned based on the literature data [
35]. Synchronous thermal analysis (TA), including thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), was performed on a NETZSCH STA 449 F3 Jupiter thermal analyzer (NETZSCH, Selb, Germany) in air in the temperature range of 40-1000 °C at a heating rate of 10 °C/min, using pre-thermostating at 40 °C for 30 minutes. The mass of the sample was at least 10 mg. The composition of the gas phase was monitored using a Netzsch QMS 403 Quadro quadrupole mass spectrometer (NETZSCH, Germany) combined with a NETZSCH STA 449 F3 Jupiter thermal analyzer (NETZSCH, Selb, Germany). Mass spectra (MS) were recorded for m/z=44 (CO
2). The microstructure of the powders was studied by scanning electron microscopy (SEM) using a scanning electron microscope with an auto emission source JEOL JSM-6000PLUS Neoscope II (JEOL Ltd., Japan). For the study, the samples were glued onto a copper substrate using carbon tape, and a layer of gold ~ 15nm was sprayed. The survey was carried out in vacuum mode. The accelerating voltage of the electron gun was up to 5 kV. The images were obtained in secondary electrons at magnifications up to 1000× and recorded in digitized form on a computer.
3. Results and Discussion
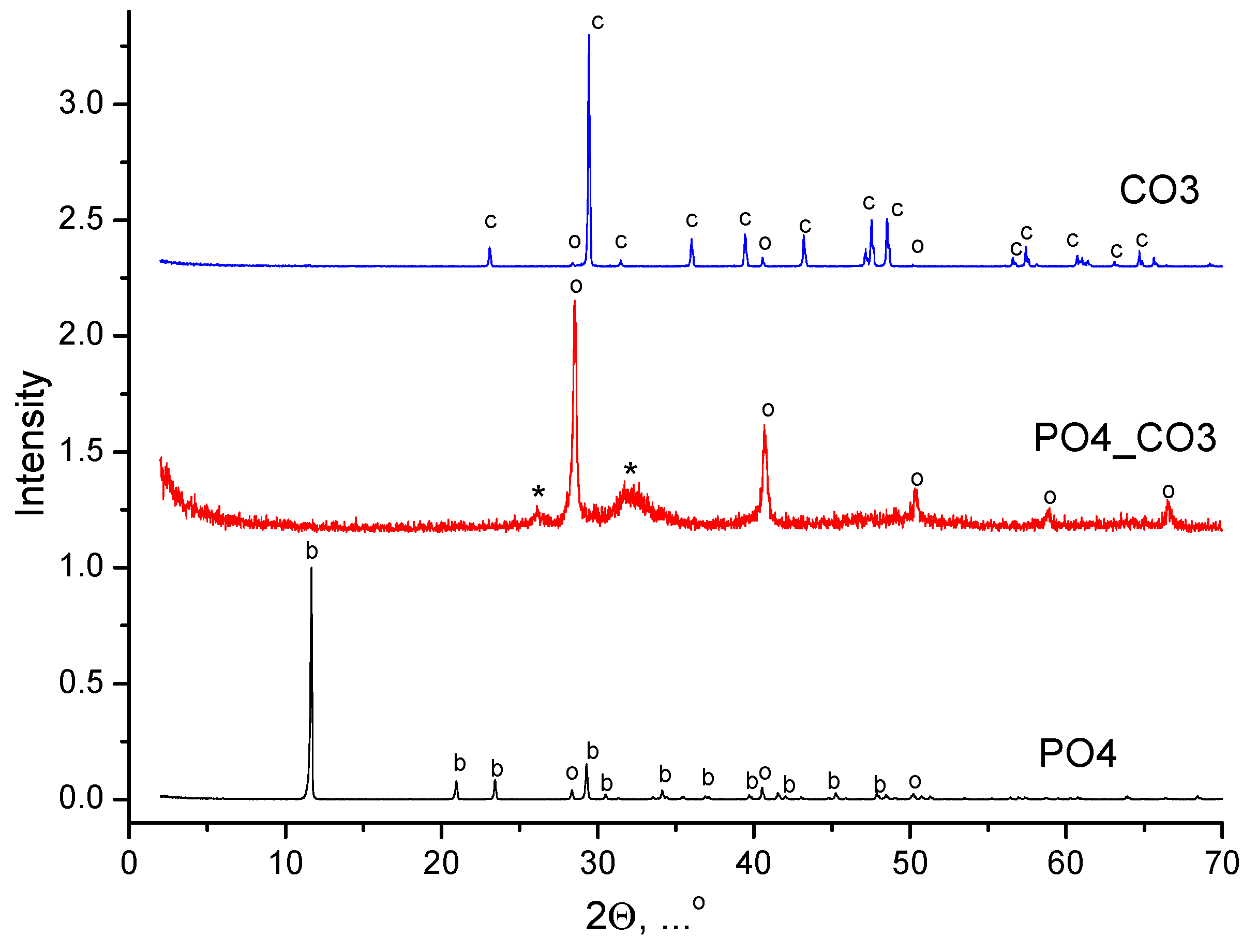
The XRD data of synthesized powders are shown in
Figure 1.
Phase composition of powder, synthesized from water solutions CaCl
2 and K
2CO
3, included calcite CaCO
3. Phase composition of powder, synthesized from water solutions CaCl
2 and K
2HPO
4, included brushite CaHPO
4·2H
2O. Phase composition of powder, synthesized from water solutions CaCl
2 and mixed-anionic solution containing K
2CO
3 и K
2HPO
4, included a low crystalline product with the main peaks corresponding to hydroxyapatite Ca
10(PO
4)
6(OH)
2. All synthesized powders included potassium chloride (sylvine) KCl as a reaction by-product. XRD data of reaction by-products isolated from mother liquors is presented in
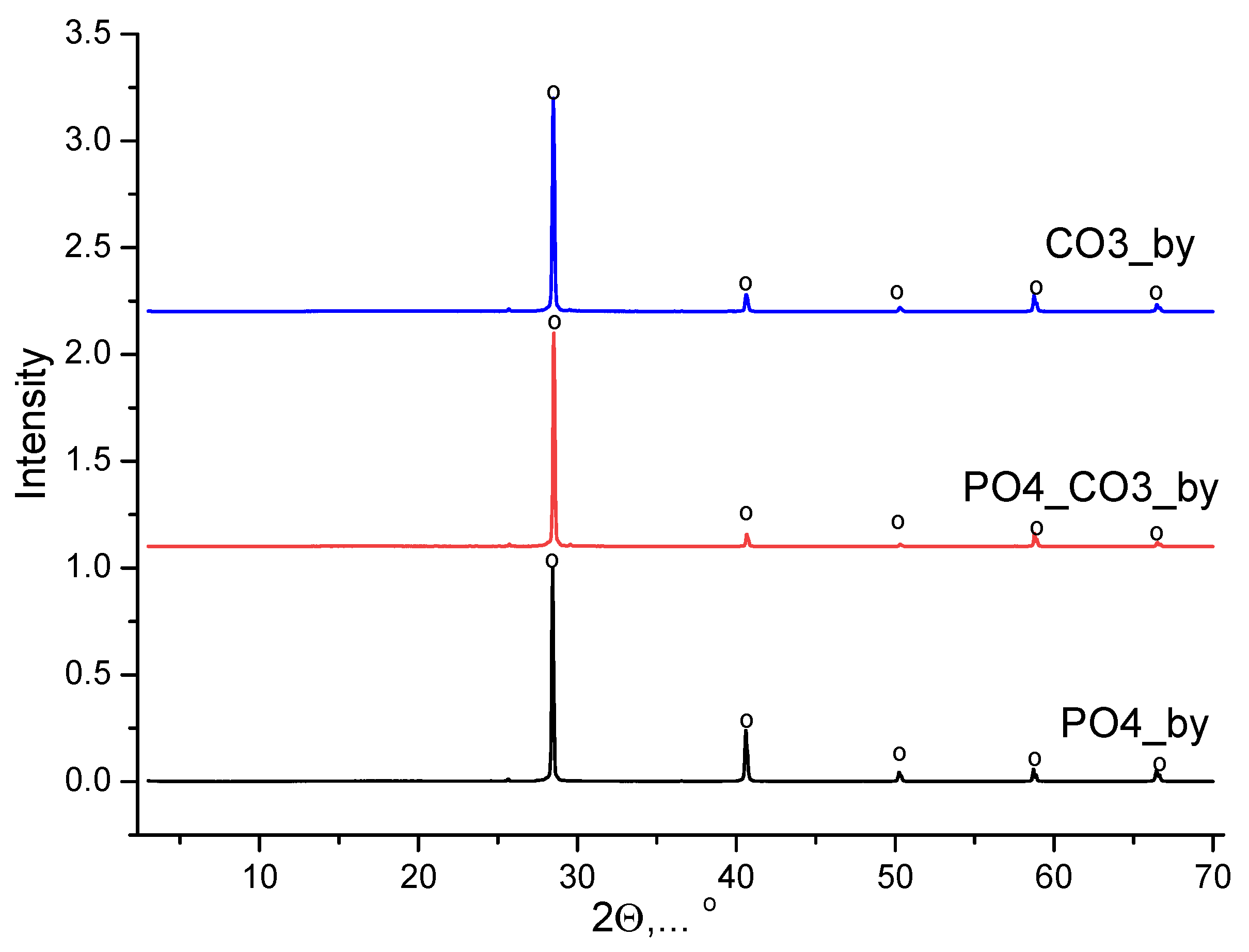
Figure 2. The phase composition of the by-products isolated from the mother liquors was presented by potassium chloride (sylvine) KCl.
The formation of brushite CaHPO
4·2H
2O and calcite CaCO
3 can be caused by reactions (1) and (3), respectively. During the preparation of the mixed-anionic aqua solution from potassium hydrophosphate K
2HPO
4 and potassium carbonate K
2CO
3 taken in an equimolar ratio, not only the release of CO
2 was observed, but also the formation of potassium orthophosphate K
3PO
4, occurred. Reaction (4) indicates that when mixing solutions of these two salts the presence of potassium carbonate K
2CO
3 also persists.
So, due to hydrolysis (reactions 5 and 6) the mixed-anionic solution had an alkaline pH, characteristic of aqua solution of salts formed by a strong base and a weak acid:
The synthesis of hydroxyapatite can be represented formally as reaction (7) taking into account the molar ratio of the salts used for synthesis as it was described in Materials and methods (reaction 2):
In this case, the theoretically possible amount of hydroxyapatite Ca
10(PO
4)
6(OH)
2 (0.0208 mol=20.9 g) can be calculated as 1/6 of the amount of potassium hydrophosphate K
2HPO
4 (0.125 mol) used for preparation of a mixed-anionic solution (
Table 1). Taking into account the possibility of formation of calcium carbonate CaCO
3, the mass of the precipitate can be calculated as the sum of the possible amounts of hydroxyapatite and calcium carbonate. The amount of calcium carbonate CaCO
3 (0.0417 mol=4.17 g) can be calculated as 1/6 of the amount of CaCl
2 (0.25 mol). Thus, the mass of the precipitate in the synthesis of
PO4_CO3 can be calculated as 25.07 g. Theoretically calculated (expected) and experimentally obtained masses of target products and by-products, as well as their comparison, are presented in
Table 4.
The data presented in the
Table 4 indicate that the total yield of synthesis products was close to ~90% in all cases. According to the estimation obtained using the Match! program, the content of the reaction by-product (potassium chloride, sylvine KCl) was maximal (30.9%) in the powder synthesized from a mixed-anionic solution. The amount of potassium chloride (sylvine) KCl in the
PO4 powder was determined as 2.8%, and in the
CO3 powder it was determined as 1.8%. The estimation of the amount of by-product in synthesized powders obtained using the Match! program is consistent with the estimation of the amount of by-product retained in
PO4_CO3 and
CO3 powders (
Table 4). A small particle size and a significant specific surface area may determine the ability of the synthesized
PO4_CO3 powder to retain (occlude) mother liquors and the by-product dissolved in it, as it has been shown in other studies [
36].
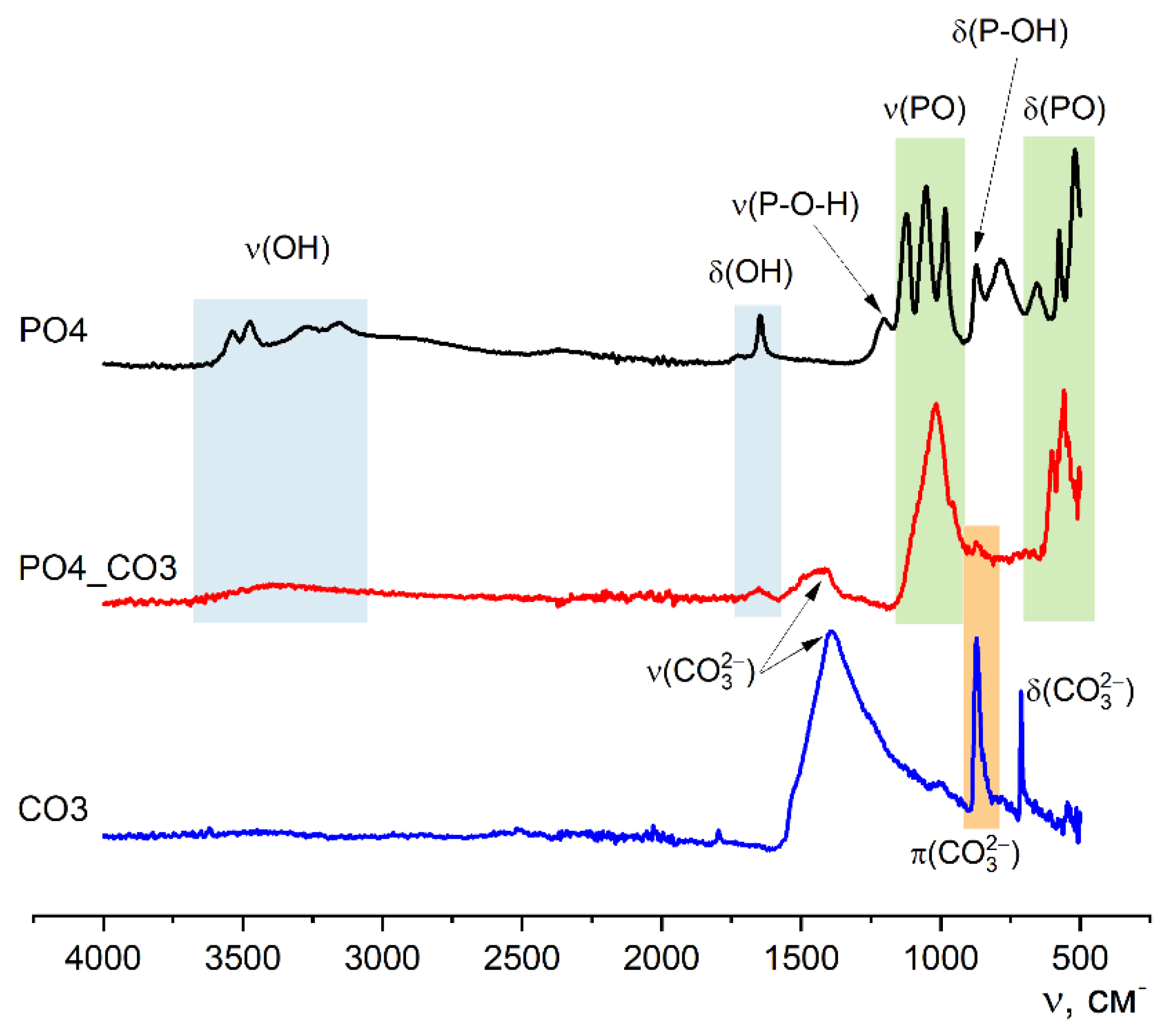
Figure 3 shows the FTIR spectroscopy data of synthesized powders.
The FTIR spectroscopy data (
Figure 3) is consistent with the X-ray diffraction data, since the appearance of the curves corresponds to the reference and literature data for brushite (SpectraBase Spectrum ID 9u0yn3G2J6i
https://spectrabase.com/spectrum/9u0yn3G2J6i [
37,
38,
39]), calcite (SpectraBase Compound ID YYVCfbcpX1
https://spectrabase.com/spectrum/YYVCfbcpX1 [
40,
41,
42]), hydroxyapatite (SpectraBase Spectrum ID BxeLPnr9PTc
https://spectrabase.com/spectrum/BxeLPnr9PTc [
43], and carbonate-hydroxyapatite [
14,
44,
45]). On the spectra of
PO4 and
CO3 powders, there are valence (ν) and deformation (δ) vibrations which are characteristic of brushite CaHPO
4·2H
2O: ν(OH) – 3539, 3473, 3270, 3153
cm-1; ν(P-O-H) – 1204
cm-1; ν(PO) – 1120, 1052, 980
cm-1 δ(OH) – 1645
cm-1; 980
cm-1; δ(P-OH) – 871
cm-1; δ(PO) – 656, 575, 519
cm-1) and calcite CaCO
3: ν(CO
32-) – 1390
cm-1; π(CO
32-) – 874
cm-1; δ(CO
32-) – 710
cm-1) respectively.
Characteristic vibrations of the phosphate group PO
43-, which confirm the formation of weakly crystallized hydroxyapatite, can also be seen on the spectrum of the powder
PO4_CO3. Vibrations of ν(CO
32-) – 1410
cm-1; and π(CO
32-) – 874
cm-1 in the spectrum of
PO4_CO3 powder suggest the presence of carbonate groups in the structure of the synthesized low crystalline hydroxyapatite. The presence of potassium chloride KCl, regardless of its content in the synthesized powders, is not determined due to the absence of absorption bands in the studied region [
46,
47].
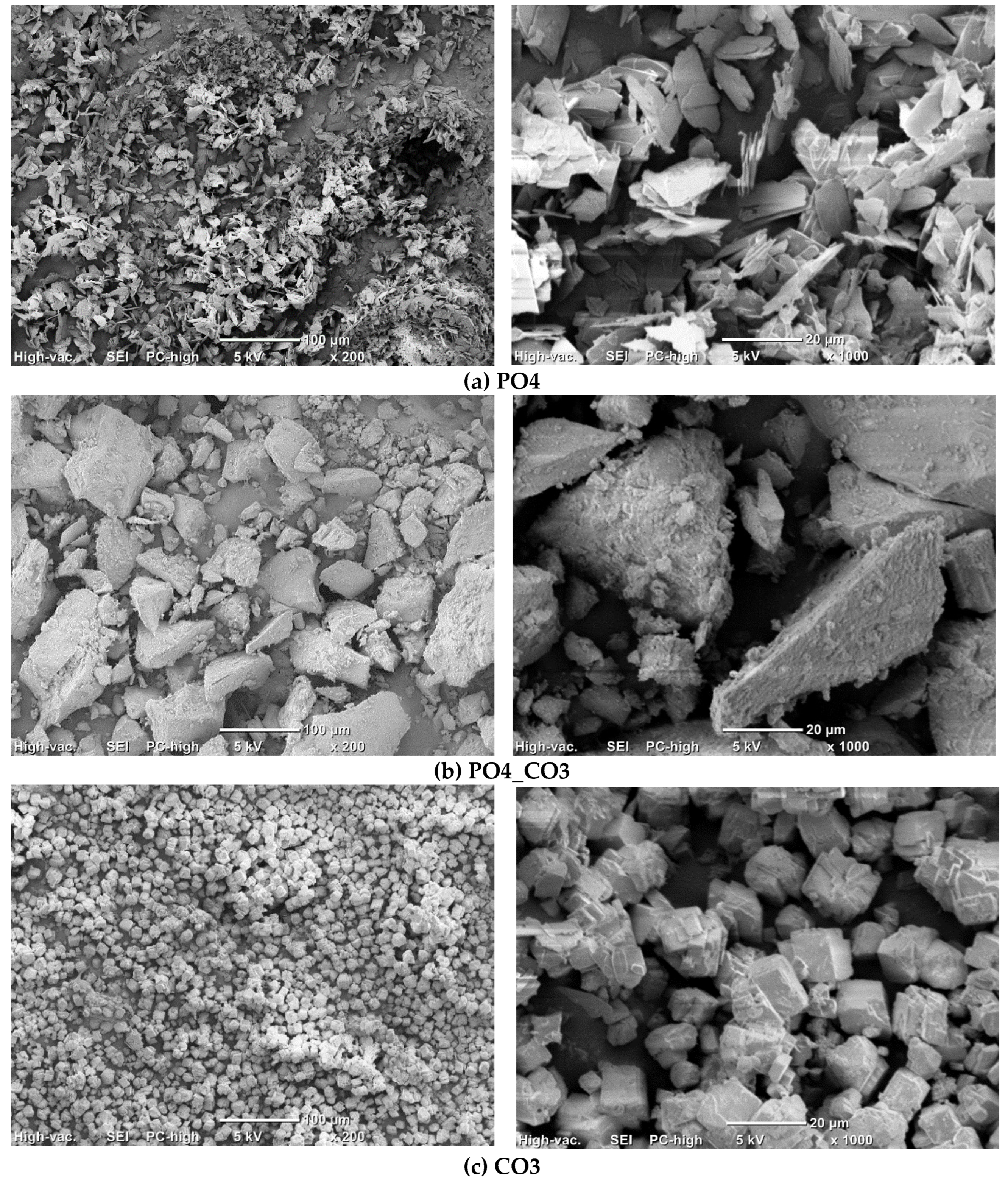
Figure 4 shows micrographs of synthesized powders.
The particles of
PO4 powder (
Figure 4, a) with a size of 10-20 microns and a thickness of 1-2 μm have a lamellar morphology characteristic for brushite CaHPO
4·2H
2O [
48]. The particles of
CO3 powder (
Figure 4, c) with a size of 10-20 μm have the cubic shapes which is one of typical for calcite CaCO
3 [
49]. The powder
PO4_CO3 (
Figure 4, b) is composed of large aggregates of 5-60 μm in size, consisting of particles less than 1 μm in size. Hydroxyapatite powders synthesized by precipitation from solutions, as a rule, consist of particles having a size of less than a micron [
44]. It was estimated that the mass (33.5 g) of the synthesized
PO4_CO3 powder after drying was 8.4 g higher than the expected (25.1 g) mass of the powder (
Table 4). The mass of reaction by-product (
PO4_CO3_by) isolated from the mother liquor was 11.8 g less than the expected (37.3 g) mass. Thus, after drying and grinding in a mortar, KCl (reaction by-product) retaining in synthesized powder acted as a binder, binding hydroxyapatite particles in large aggregates visible in the micrographs (
Figure 4, b).
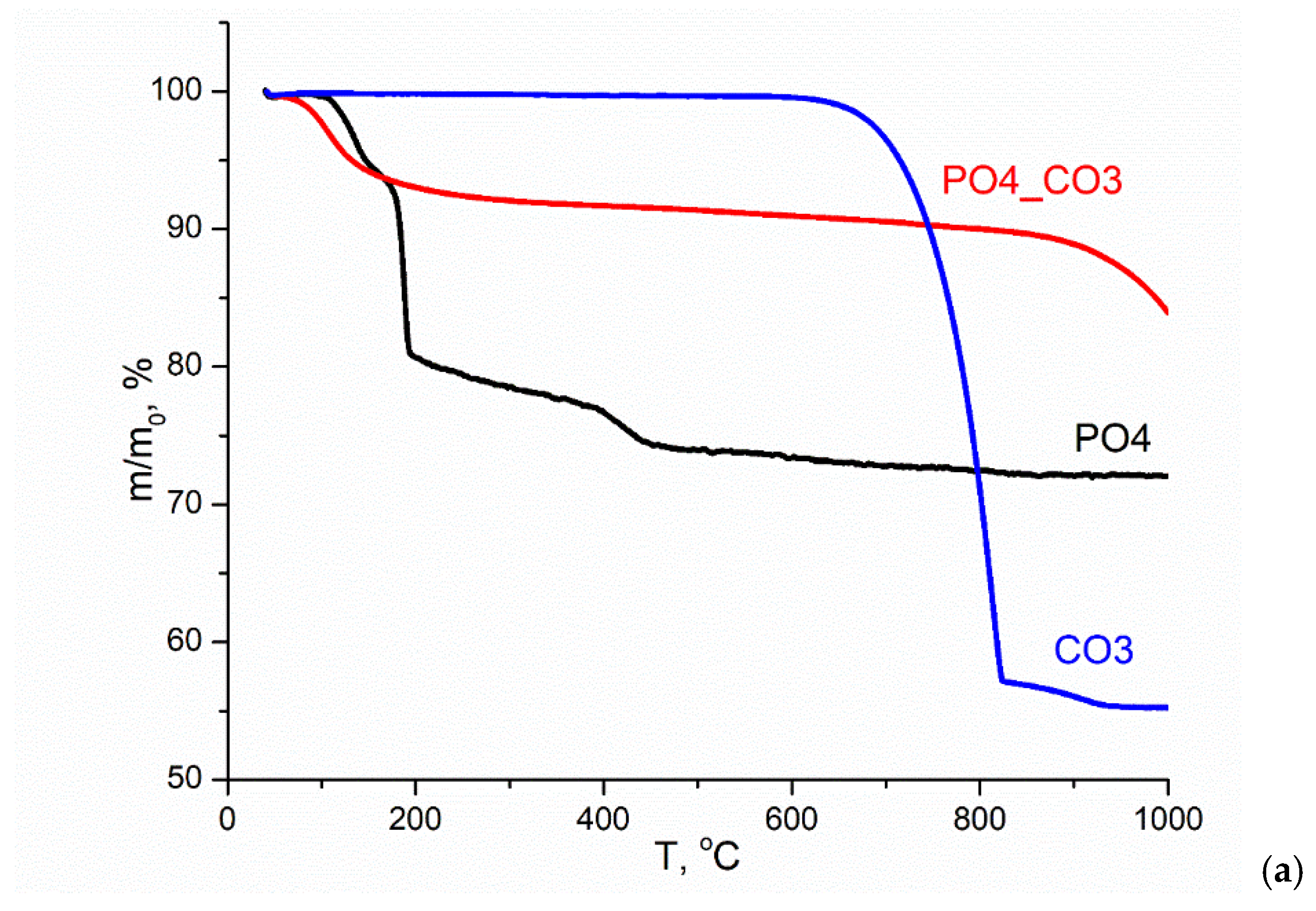
Figure 5 shows the TA data of the synthesized powders. According to the TGA data (
Figure 5, a), the total weight loss at 1000 °C for
PO4 powder was 27.9%. The change in the weight of the PO4 powder is possible due to the removal of physically bounded water, dehydration of brushite CaHPO
4·2H
2O and the formation of monetite CaHPO
4 (reaction 8), and the conversion of monetite CaHPO
4 into Ca
2P
2O
7 pyrophosphate (reaction 9).
The theoretically possible mass loss during reactions 8 and 9 is 26.16%, which is close to the value of the total mass loss for
PO4 powder determined by the TGA method. All the processes indicated for the
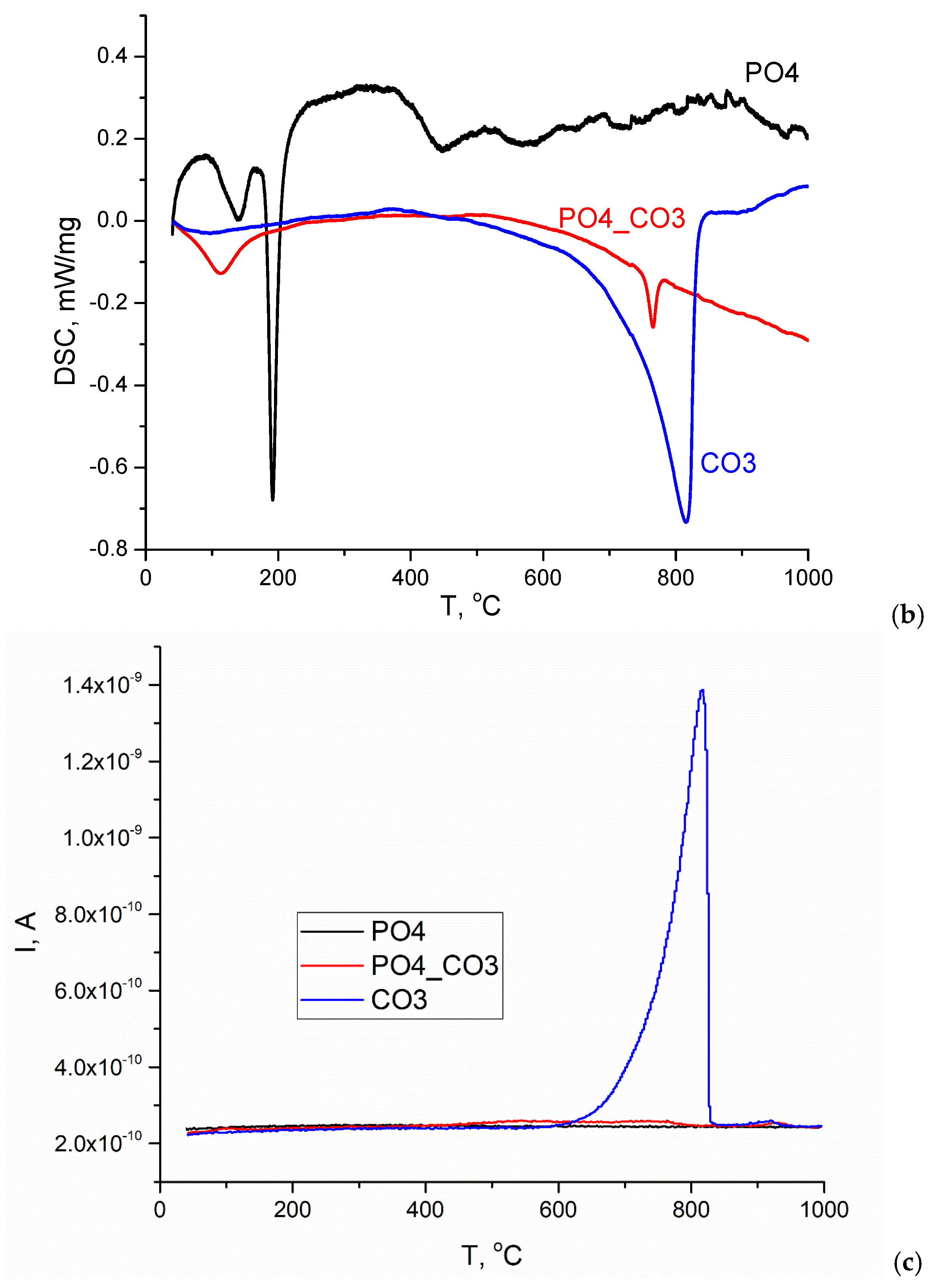
PO4 powder, leading to weight loss, occur with heat absorption in the ranges 87-168 °C, 168-243 °C, 375-477 °C (
Figure 5, b). It can be assumed that the presence of KCl in the
PO4 powder, as well as the stage of thermostating the sample at 40 °C for 30 minutes before heating at a set rate (10 °C/min), causes decomposition of the metastable brushite at lower temperatures in the range of 87-168 °C (reaction 8). In article [
50] the authors conclude that two-stage conversion of brushite into monetite was possible with formation of an amorphous phase at the first stage. The range 168-243 °C should be considered as characteristic of the monetite CaHPO
4 formation from brushite CaHPO
4·2H
2O; and the range 375-477 °C should be considered as characteristic of the calcium pyrophosphate Ca
2P
2O
7 from CaHPO
4 monetite formation [
51]. In the range of 477-840 °C, the smooth change in mass was 3%, while it is difficult to identify the intervals for processes occur with heat absorption or release.
According to the TGA data (
Figure 5, a), the total weight loss at 1000 °C for the
CO3 powder was 44.9%. The theoretically possible mass change for calcite during thermal decomposition in accordance with reaction (10) is 44%. The change in the mass of the
CO3 powder occurred with heat absorption in the range 622-844 °C, amounted to 42.9% due to the decomposition of calcite and was accompanied by the release of CO
2 (reaction 10).
The graph of the mass change of the
CO3 powder (
Figure 5, a) and the dependence of the ion current on temperature (
Figure 5, c) have the forms characteristic for calcite CaCO
3 [
52]. In
Figure 5, c, for the
CO3 powder, one can see a graph of the dependence of the ion current on temperature for m/z=44 (CO
2), typical for CaCO
3. It should be noted that in the range 844-949 °C, a loss (2%) of mass is observed, which is most likely associated with both the removal of CO
2 and the removal of that insignificant amount of KCl (
Table 4), which could be captured by the synthesized
CO3 powder above the melting point of KCl (776 °C [
53] or 769 ± 2 °C (1042 ± 2 K) [
54]).
According to the TGA data (
Figure 5, a), the total weight loss at 1000 °C for
PO4_CO3 powder was 16.2%. Three sections can be distinguished on the curve of mass versus temperature for
PO4_CO3 powder: removal of physically bound water (7.5% mass loss), which proceeds with heat absorption in the range of 43-240 °C; a section of smooth weight reduction (2.6%) in the range of 240-829 °C; and a section of mass loss (6.1%), apparently related to the removal of KCl, in the range of 829-1000 °C. The endothermic peak at 765 °C clearly seen on the DSC curve (
Figure 5, b) can be attributed to the melting of KCl, since according to the estimate (
Table 4), the mass of the retained reaction by-product (KCl) in the
PO4_CO3 powder was the maximum. No endothermic peaks that could be attributed to the KCl melting process were found in the DSC graphs for
PO4 and
CO3 powders synthesized for comparison. Significantly lower values of the ion current can be seen on the curve m/z =44 (CO2) for
PO4-CO3 powder (
Figure 5, c) in wide ranges of 460-800 °C and 900-940 °C than for
CO3 powder.
Figure 6 shows the XRD of the
CO3 powder synthesized from aqueous solutions of CaCl
2 and K
2CO
3 after heat treatment at various temperatures.
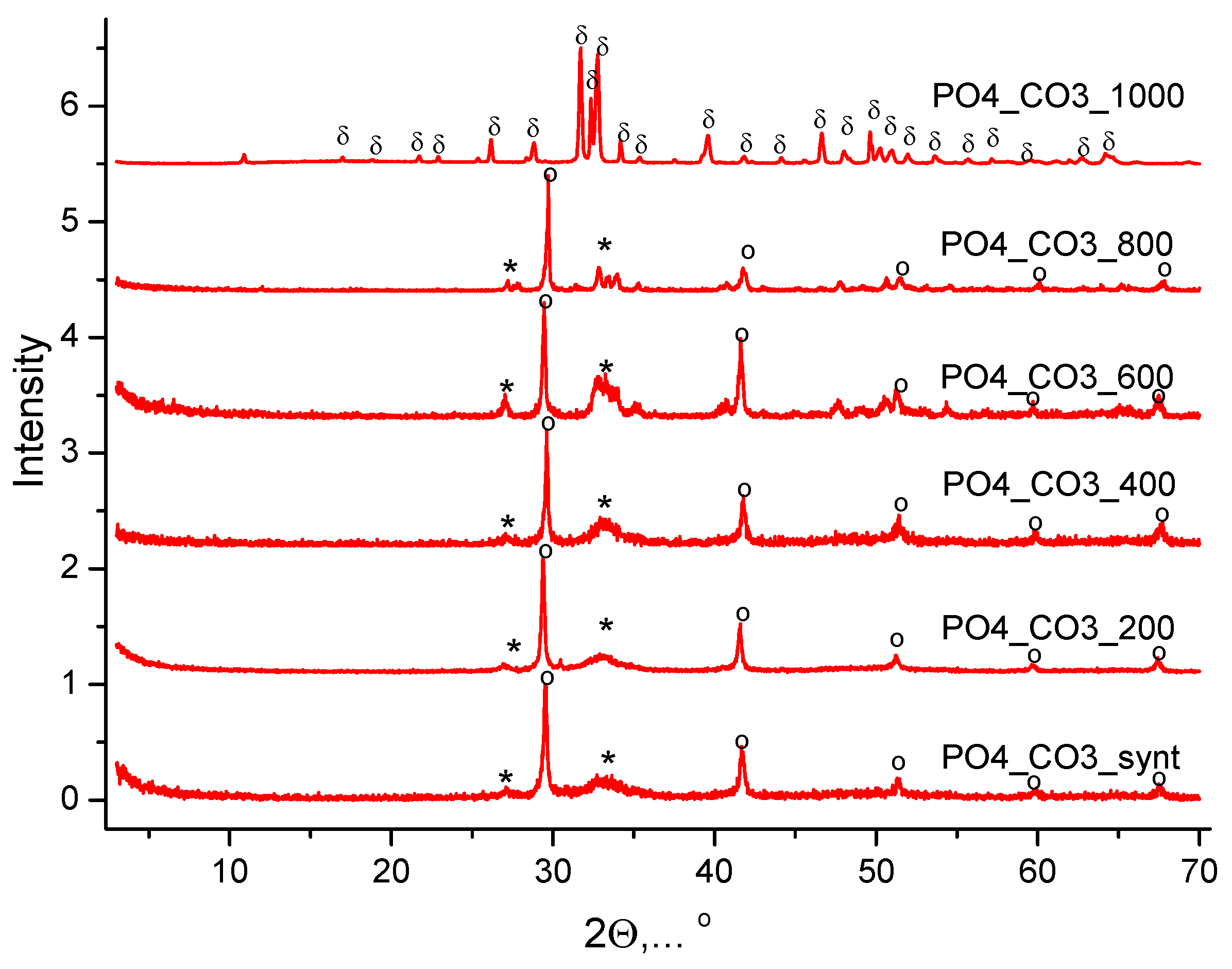
According to the XRD data, the phase composition of the CO3 powder synthesized from aqueous solutions of CaCl2 and K2CO3, after heat treatment at various temperatures in the range of 200-600 °C for 2 hours, was represented by calcite CaCO3. And after heat treatment at 800 °C, the phase composition of the CO3 powder was represented by calcium oxide CaO (reaction 10).
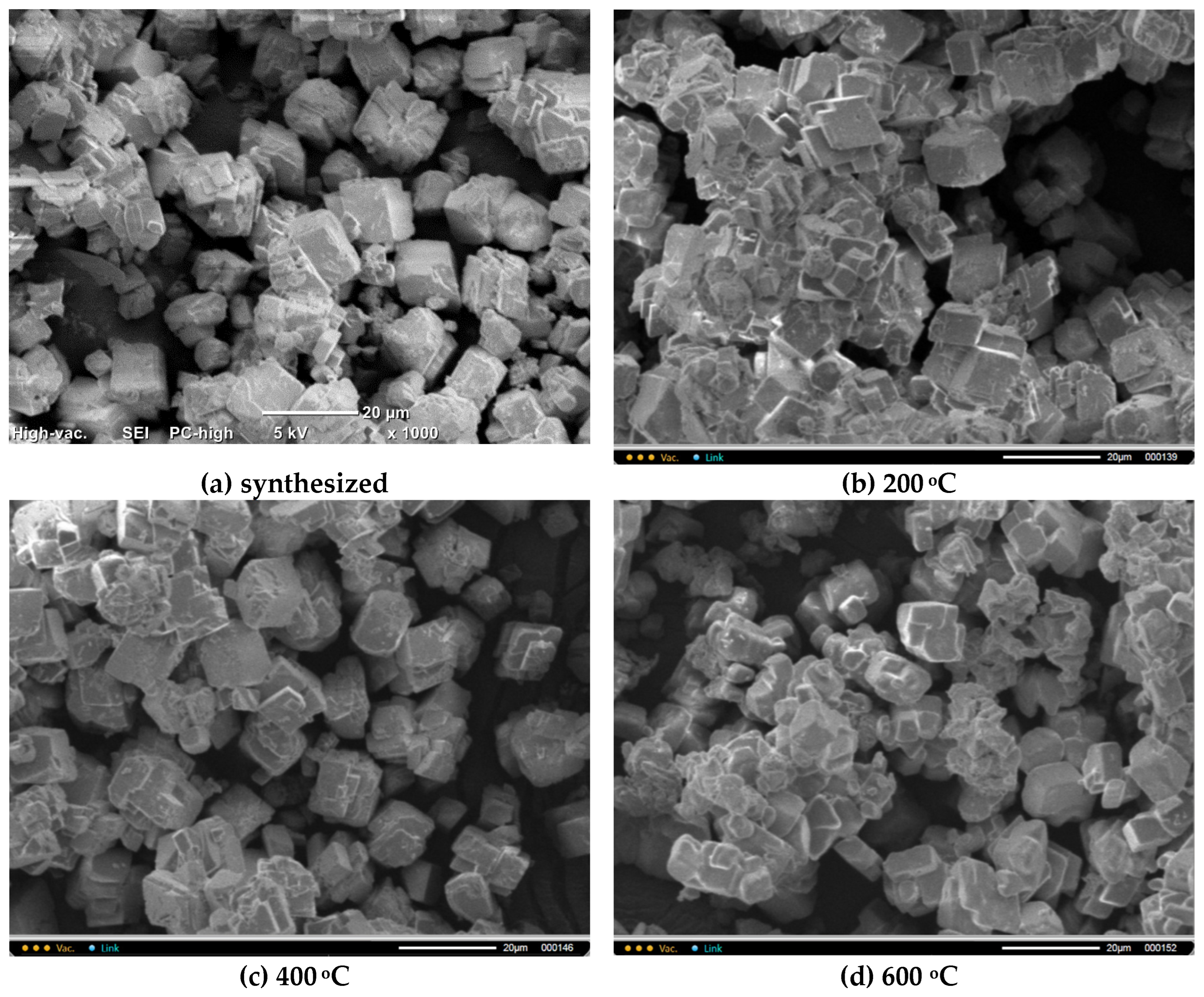
Figure 7 shows micrographs of
CO3 powders after heat treatment for 2 hours at various temperatures in the range of 200-600 °C. The particle size and shape of the powder particles after heat treatment does not significantly differ from the particle size and shape of the synthesized powder. It is difficult to detect particles with dimensions less than 2 μm and more than 20 μm in the micrographs.
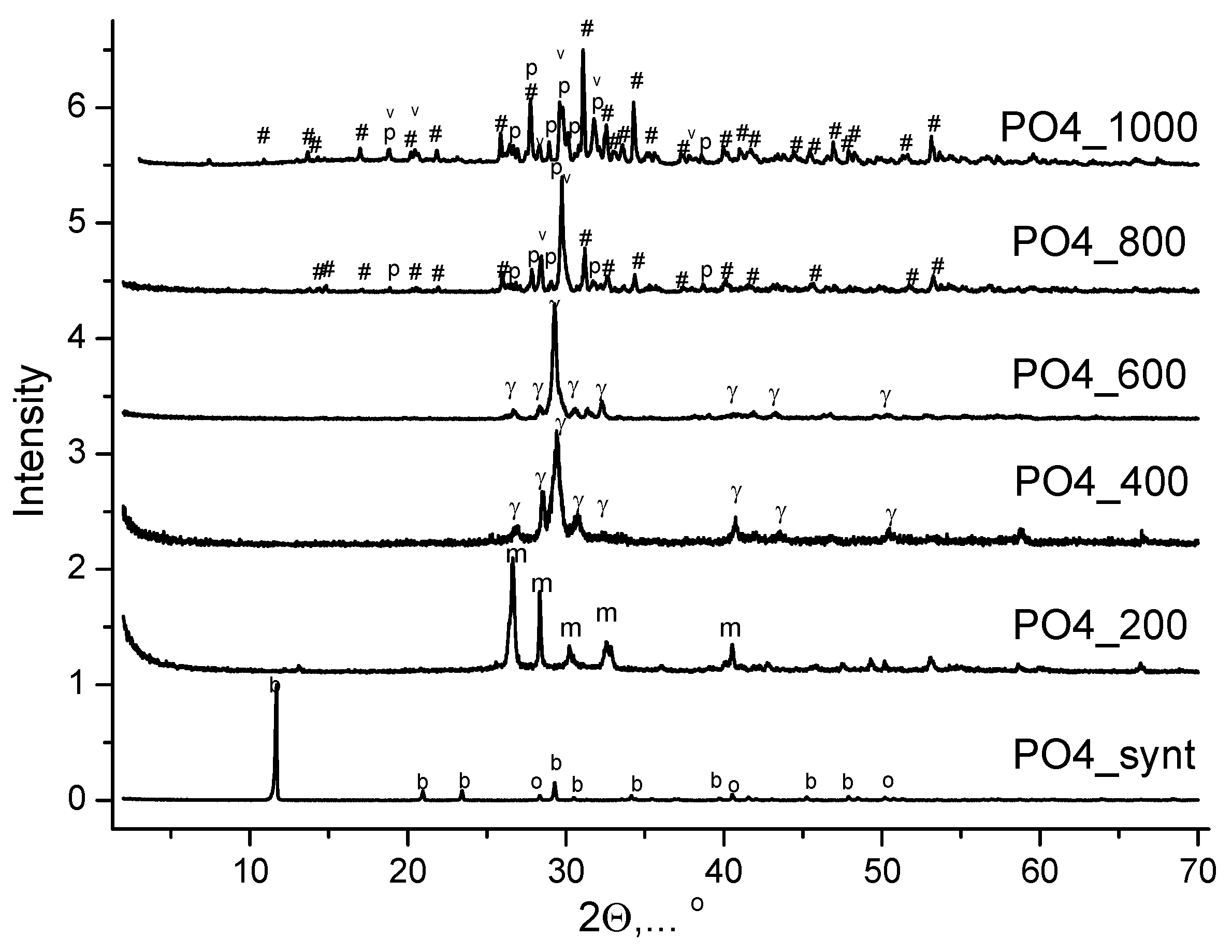
Figure 8 shows the XRD of
PO4 powder synthesized from aqueous solutions of CaCl
2 and K
2HPO
4 after heat treatment at various temperatures. After heat treatment at 200 °C for 2 hours, the phase composition of the
PO4_200 powder is represented by monetite CaHPO
4 (reaction 9). The phase composition of the
PO4_400 and
PO4_600 powders was represented by γ-calcium pyrophosphate γ-Ca
2P
2O
7 (reaction 10) after heat treatment at 400 °C and at 600 °C for 2 hours. The phase composition of the
PO4_800 and
PO4_1000 powders included β-tricalcium phosphate β-Ca
3(PO
4)
2, β-calcium pyrophosphate β-Ca
2P
2O
7, as well as calcium potassium pyrophosphate K
2CaP
2O
7 after heat treatment at 800 °C and at 1000 °C for 2 hours
Reaction (11) may reflect the formation of β-calcium orthophosphate β-Ca
3(PO
4)
2 and calcium potassium pyrophosphate K
2CaP
2O
7:
Figure 9 shows micrographs of
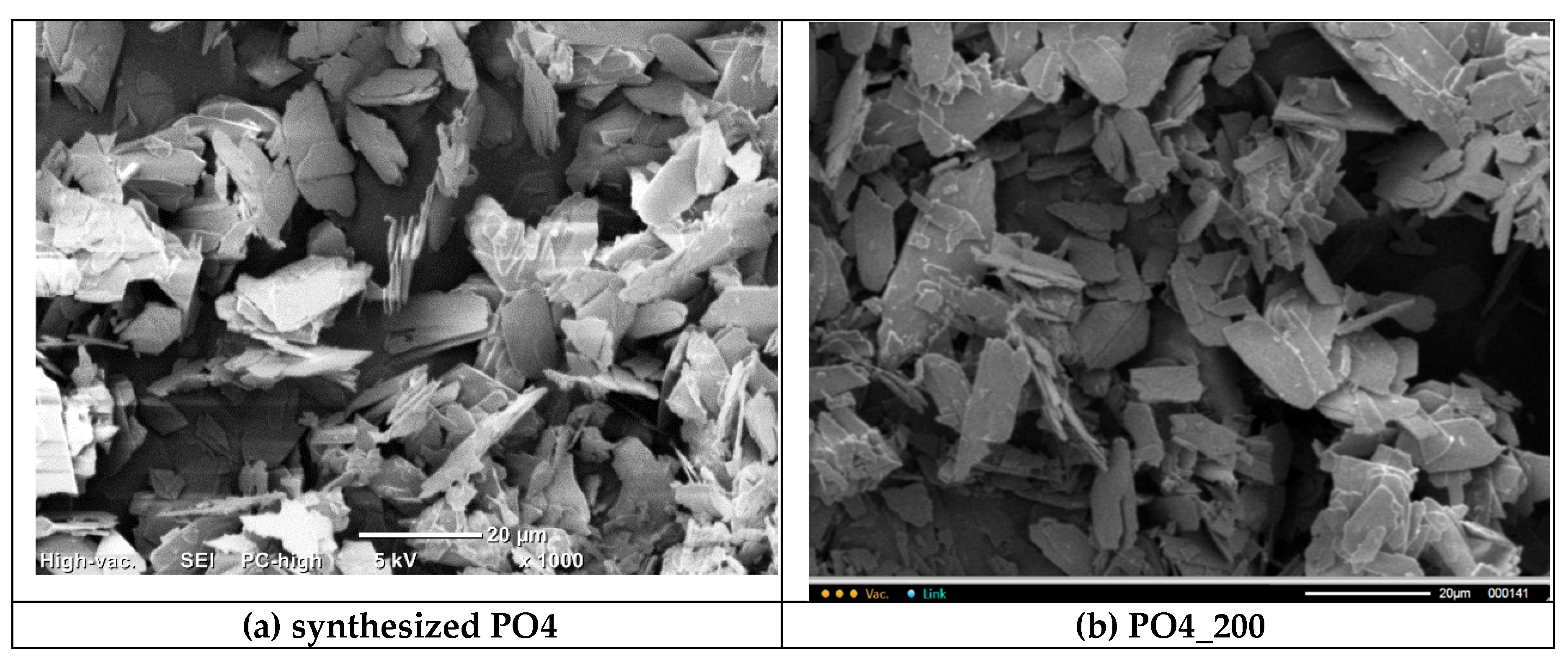
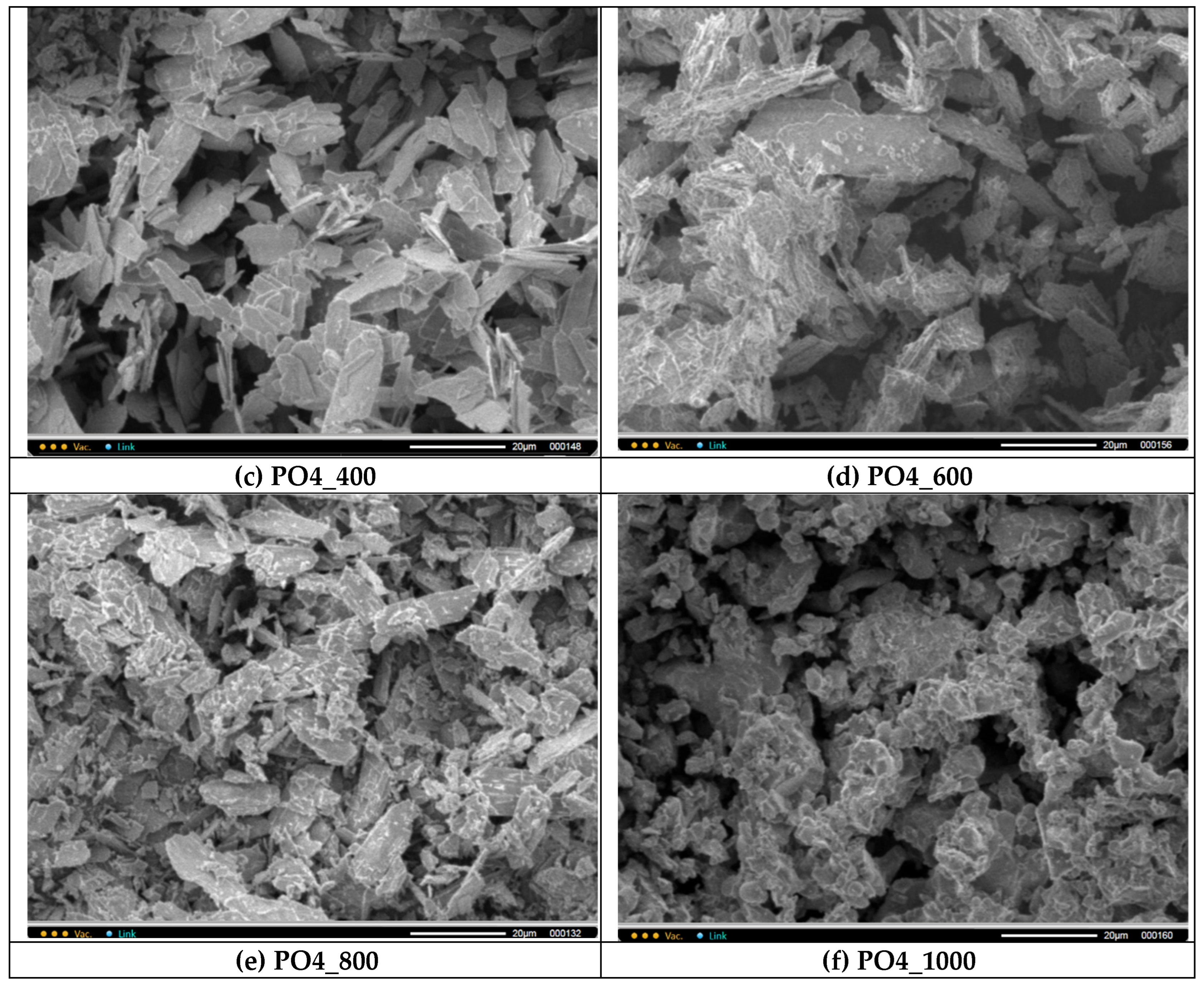
PO4 powders after thermal treatment for 2 hours at various temperatures in the range of 200-1000 °C. The particle size and shape of the powder particles after heat treatment at 200 and 400 ° C practically does not differ from the particle size and shape of the synthesized powder. After heat treatment in the range of 600-1000 °C, as the temperature increases, the powder particles more and more lose their lamellar morphology. And after heat treatment at 1000 °C, the
PO4_1000 powder is composed of conglomerates 5-20 microns in size, consisting of particles 1-3 microns in size, sintered together.
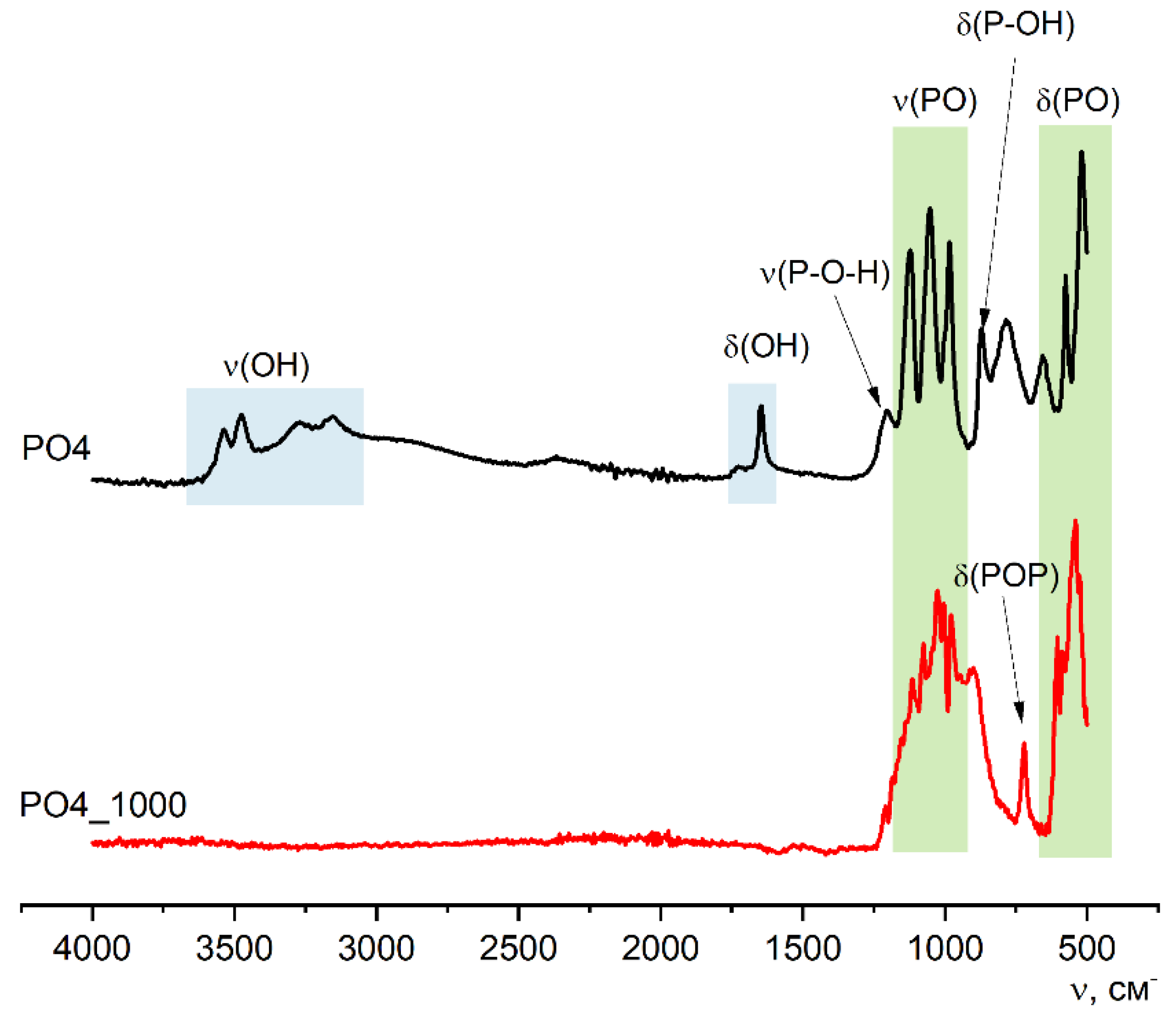
Figure 10 shows the FTIR data for
PO4 and
PO4_1000 powders, along with the XRD and SEM data, confirming the transformation of the synthesized powder after heat treatment at 1000 °C. After heat treatment of the
PO4 powder at 1000 °C, according to the FTIR data, the presence of PO
43- groups remains and a δ(POP) 720 cm
-1 vibration appears, which confirms the presence of the pyrophosphate ion P
2O
74- in both calcium pyrophosphate formed from brushite (reactions 8 and 9) and calcium potassium pyrophosphate (reaction 11).
Figure 11 shows the XRD of the
PO4_CO3 powder synthesized from aqueous solutions of CaCl
2, KHPO
4 and K
2CO
3 after heat treatment at various temperatures. The phase composition of
PO4_CO3 powders after thermal treatment in the range of 200-800 °C is represented by weakly crystallized hydroxyapatite and potassium chloride (sylvine) KCl. After heat treatment at 1000 °C, the phase composition of the powder
PO4_CO3_1000 is represented by chlorapatite Ca
10(PO
4)
6Cl
2. The formation of chlorapatite can be reflected by the reaction (12):
A similar formation of chlorapatite was observed at 1000 °C from weakly crystalline hydroxyapatite of natural origin and sodium chloride NaCl [
55].
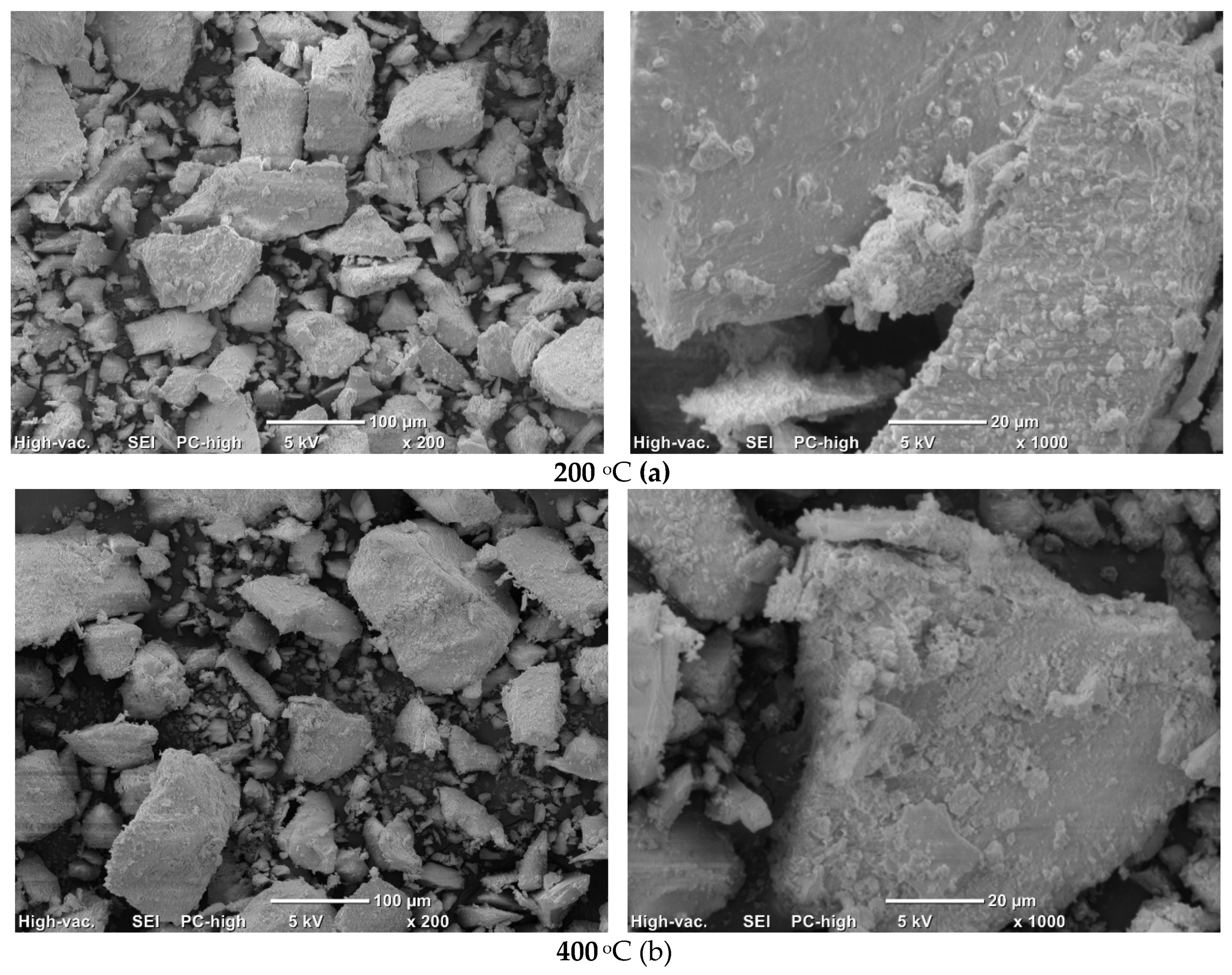
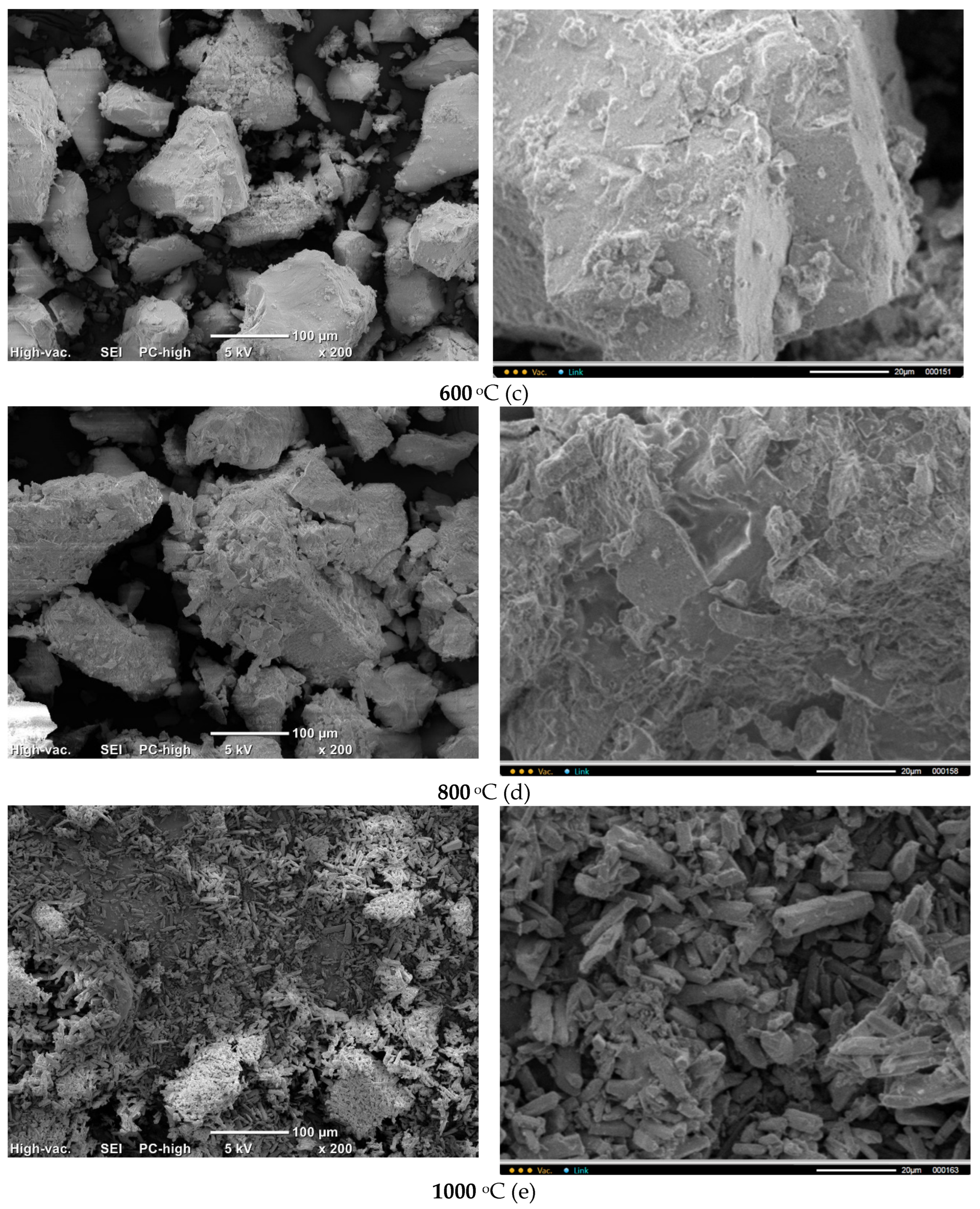
Micrographs of
PO4_CO3 powder after synthesis and after heat treatment at various temperatures are presented in
Figure 12. The microstructure of
PO4_CO3 powders, after thermal treatment in the range of 200-800 °C, does not significantly differ from the microstructure of the powder after synthesis and drying. Powders consist of sufficiently large agglomerates (up to 100-200 μm) consisting of particles of weakly crystallized hydroxyapatite bonded with reaction by-product KCl. The microstructure of the
PO4_CO3_1000 powder after heat treatment at 1000 °C differs significantly from the microstructure of powders after heat treatment in the range of 200-800 °C. The
PO4_CO3_1000 powder after heat treatment at 1000 °C consists of particles with a prismatic shape 5-20 μm long and a transverse dimension of 2-5 μm. In the micrograph (
Figure 12, e, left side) with a lower magnification, one can see loose aggregates up to 100 microns in size, consisting of mentioned above prismatic particles.
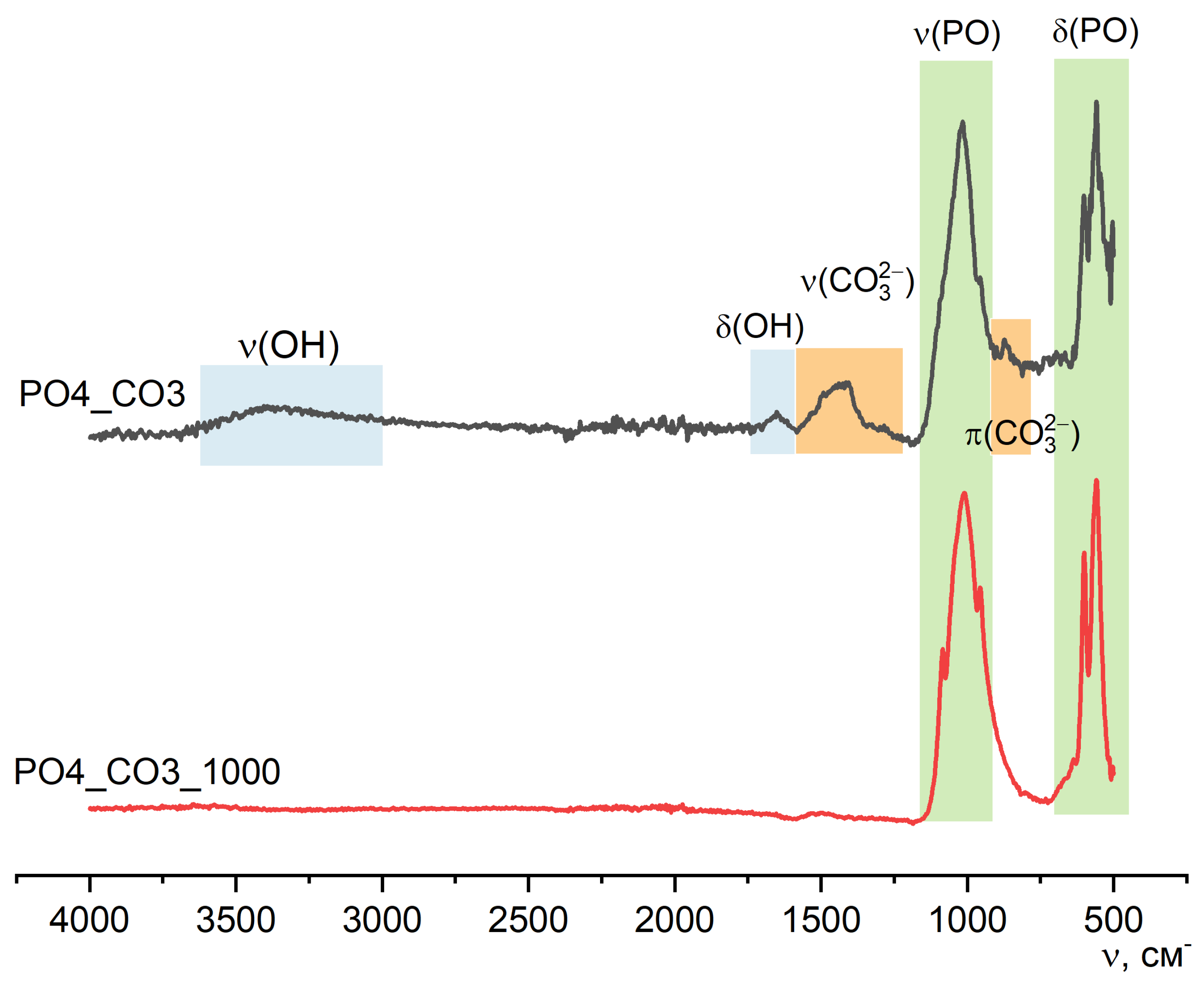
Figure 13 shows the FTIR spectroscopy data for
PO4_CO3 powder after synthesis and after heat treatment at 1000 °C. In the spectrum of the
PO4_CO3_1000 powder, there are no peaks that could be attributed to ν(OH), ν(CO
32-) or π(CO
32-). The FTIR spectrum for the powder
PO4_CO3_1000 corresponds to the reference data for chlorapatite (SpectraBase Compound ID KKRjtSFM3Pp,
https://spectrabase.com/spectrum/KKRjtSFM3Pp).
Figure 1.
XRD data of synthesized powders: c – calcium carbonate (calcite) CaCO3 (PDF card No 5-586; No 96-210-0190); o – potassium chloride (sylvine) KCl (PDF card No 41-1476; No 96-900-8652); * - hydroxyapatite Ca10(PO4)6(OH)2 (PDF card No 9-432, No 96-900-1234); b – brushite CaHPO4·2H2O (PDF card No 9-77; No 96-231-0527).
Figure 1.
XRD data of synthesized powders: c – calcium carbonate (calcite) CaCO3 (PDF card No 5-586; No 96-210-0190); o – potassium chloride (sylvine) KCl (PDF card No 41-1476; No 96-900-8652); * - hydroxyapatite Ca10(PO4)6(OH)2 (PDF card No 9-432, No 96-900-1234); b – brushite CaHPO4·2H2O (PDF card No 9-77; No 96-231-0527).
Figure 2.
XRD data of products isolated from mother liquors after synthesis: o – potassium chloride (sylvine) KCl (PDF card No 41-1476; No 96-900-8652).
Figure 2.
XRD data of products isolated from mother liquors after synthesis: o – potassium chloride (sylvine) KCl (PDF card No 41-1476; No 96-900-8652).
Figure 3.
FTIR spectra of synthesized powders PO4, PO4_CO3, CO3.
Figure 3.
FTIR spectra of synthesized powders PO4, PO4_CO3, CO3.
Figure 4.
Micrographs of synthesized powders PO4 (a), PO4_CO3 (b), CO3 (c).
Figure 4.
Micrographs of synthesized powders PO4 (a), PO4_CO3 (b), CO3 (c).
Figure 5.
TA data of synthesized powders: TGA (a), DSC (b), MS for m/z=44 (CO2).
Figure 5.
TA data of synthesized powders: TGA (a), DSC (b), MS for m/z=44 (CO2).
Figure 6.
XRD data of CO3 powder after synthesis and after heat treatment at different temperatures. c – calcium carbonate (calcite) CaCO3 (PDF card No 5-586; No 96-210-0190); o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); x – calcium oxide CaO (PDF No 37-1497, No 96-101-1096).
Figure 6.
XRD data of CO3 powder after synthesis and after heat treatment at different temperatures. c – calcium carbonate (calcite) CaCO3 (PDF card No 5-586; No 96-210-0190); o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); x – calcium oxide CaO (PDF No 37-1497, No 96-101-1096).
Figure 7.
Microphotographs of CO3 powder after synthesis (a) and after heat treatment at different temperatures 200 oC (b), 400 oC (c), 600 oC (d).
Figure 7.
Microphotographs of CO3 powder after synthesis (a) and after heat treatment at different temperatures 200 oC (b), 400 oC (c), 600 oC (d).
Figure 8.
XRD data of powder PO4 b after synthesis and after heat treatment at different temperatures. o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); b – brushite CaHPO4·2H2O (PDF card No 9-77; No 96-231-0527); m – monetite CaHPO4 (PDF card No 9-80; 96-210-6185); γ - γ-calcium pyrophosphate γ-Ca2P2O7 (PDF card No 17-499); p - β- calcium pyrophosphate β-Ca2P2O7 (PDF card 9-346; No 96-100-1557); # - β-tricalcium phosphate β-Ca3(PO4)2 (PDF card No 9-169; No 96-151-7239) v – K2CaP2O7 (PDF card No 22-805; No 96-220-2941).
Figure 8.
XRD data of powder PO4 b after synthesis and after heat treatment at different temperatures. o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); b – brushite CaHPO4·2H2O (PDF card No 9-77; No 96-231-0527); m – monetite CaHPO4 (PDF card No 9-80; 96-210-6185); γ - γ-calcium pyrophosphate γ-Ca2P2O7 (PDF card No 17-499); p - β- calcium pyrophosphate β-Ca2P2O7 (PDF card 9-346; No 96-100-1557); # - β-tricalcium phosphate β-Ca3(PO4)2 (PDF card No 9-169; No 96-151-7239) v – K2CaP2O7 (PDF card No 22-805; No 96-220-2941).
Figure 9.
Micrographs of PO4 powder after synthesis (a) and after heat treatment at various temperatures: 200 (b), 400 (c), 600 (d), 800 (e), 1000 (f).
Figure 9.
Micrographs of PO4 powder after synthesis (a) and after heat treatment at various temperatures: 200 (b), 400 (c), 600 (d), 800 (e), 1000 (f).
Figure 10.
FTIR spectra of synthesized PO4 powder and powder PO4_1000 after heat treatment at 1000 oC.
Figure 10.
FTIR spectra of synthesized PO4 powder and powder PO4_1000 after heat treatment at 1000 oC.
Figure 11.
XRD data of PO4_CO3 powder after synthesis and after heat treatment at different temperatures. * - hydroxyapatite Ca10(PO4)6(OH)2 (PDF No 96-901-4314) o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); δ – chlorapatite Ca10(PO4)6Cl2 (PDF No 73-1728; No 96-101-0917).
Figure 11.
XRD data of PO4_CO3 powder after synthesis and after heat treatment at different temperatures. * - hydroxyapatite Ca10(PO4)6(OH)2 (PDF No 96-901-4314) o - potassium chloride (sylvine) KCl (PDF No 41-1476; No 96-900-8652); δ – chlorapatite Ca10(PO4)6Cl2 (PDF No 73-1728; No 96-101-0917).
Figure 12.
Micrographs of PO4_CO3 powder after heat treatment at various temperatures: 200 (a), 400 (b), 600 (c), 800 (d), 1000 (e).
Figure 12.
Micrographs of PO4_CO3 powder after heat treatment at various temperatures: 200 (a), 400 (b), 600 (c), 800 (d), 1000 (e).
Figure 13.
FTIR spectra of PO4_CO3 powder after synthesis and after heat treatment at 1000 oC.
Figure 13.
FTIR spectra of PO4_CO3 powder after synthesis and after heat treatment at 1000 oC.
Table 1.
The labeling and synthesis conditions of the powders.
Table 1.
The labeling and synthesis conditions of the powders.
| Labeling of powders |
PO4 |
PO4_CO3 |
CO3 |
| The molar ratio of Ca/(HPO42-+CO32-) |
1 |
1 |
1 |
| CaCl2, mol |
0,25 |
0,25 |
0,25 |
| V of solution CaCl2, l |
0,5 |
0,5 |
0,5 |
| C (CaCl2), mol/l |
0,5 |
0,5 |
0,5 |
| K2HPO4·3H2O, mol |
0,25 |
0,125 |
- |
| K2CO3, moль |
- |
0,125 |
0,25 |
| V of solution, containing anions (HPO42-) and/or (CO32-), l |
0,5 |
0,5 |
0,5 |
| C(K2HPO4·3H2O), mol/l |
0,5 |
0,25 |
- |
| C(K2CO3), mol/l |
- |
0,25 |
0,5 |
Table 2.
Quantities of initial, as well as expected target products and reaction by-product.
Table 2.
Quantities of initial, as well as expected target products and reaction by-product.
| Starting reagents |
Labeling of synthesized powders |
| PO4 |
PO4_CO3 |
CO3 |
| CaCl2, mol |
0,25 |
0,25 |
0,25 |
| K2HPO4·3H2O, mol |
0,25 |
0,125 |
- |
| K2CO3, mol |
- |
0,125 |
0,25 |
| Target products |
|
|
|
| CaHPO4·2H2O, mol |
0,25 |
0,125 |
- |
| Mass of CaHPO4·2H2O, g |
43,0 |
21,5 |
- |
| CaCO3, mol |
- |
0,125 |
0,25 |
| Mass of CaCO3, g |
- |
12,5 |
25,0 |
| By-product |
Labeling of by-products |
|
PO4_by
|
PO4_CO3_by
|
CO3_by
|
| KCl, moль |
0,5 |
0,5 |
0,5 |
| Мacca KCl, г |
37,3 |
37,3 |
37,3 |
| Total mass of expected products* |
80,3 |
73,1 |
62,3 |
Table 3.
Labeling of powders obtained after heat treatment.
Table 3.
Labeling of powders obtained after heat treatment.
| Temperature of heat treatment |
Labeling of synthesized powders |
| PO4 |
PO4_CO3 |
CO3 |
| 200 °C |
PO4_200 |
PO4_CO3_200 |
CO3_200 |
| 400 °C |
PO4_400 |
PO4_CO3_400 |
CO3_400 |
| 600 °C |
PO4_600 |
PO4_CO3_600 |
CO3_600 |
| 800 °C |
PO4_800 |
PO4_CO3_800 |
CO3_800 |
| 1000 °C |
PO4_1000 |
PO4_CO3_1000 |
- |
Table 4.
The expected and obtained masses of synthesized powders and reaction by-products.
Table 4.
The expected and obtained masses of synthesized powders and reaction by-products.
| Labeling |
PO4 |
PO4_CO3 |
CO3 |
| The expected quantities of target products and by-product: |
| CaHPO4·2H2O, g |
43,0 |
- |
- |
| Ca10(PO4)6(OH)2 + CaCO3, g |
- |
25,1 |
- |
| CaCO3, g |
- |
- |
25,0 |
| KCl, g |
37,3 |
37,3 |
37,3 |
| Total mass of expected products *, g |
80,3 |
62,4 |
62,3 |
| The obtained masses of the synthesized powders and the extracted by-products: |
| Mass of the powders after drying, g |
43,0 |
33,5 |
17,4 |
| Mass of the extracted reaction by-product, g |
30,6 |
25,5 |
37,1 |
|
Total mass of prepared products**, g |
73,6 |
59,0 |
54,5 |
| The yield of synthesized products |
91,7% |
94,5% |
87,5% |
| The yield of reaction by-product |
82,0% |
68,4% |
99,4% |
| Mass of by-product, preserved by precipitate (estimation), g |
6,7 |
11,8 |
0,2 |
| Content of by-product in powders (estimation) |
15,6% |
35,2% |
1,1% |
| Content of by-product in powders (estimation according to Match!) |
2.8% |
30.9% |
1,8% |