Submitted:
22 August 2025
Posted:
26 August 2025
You are already at the latest version
Abstract
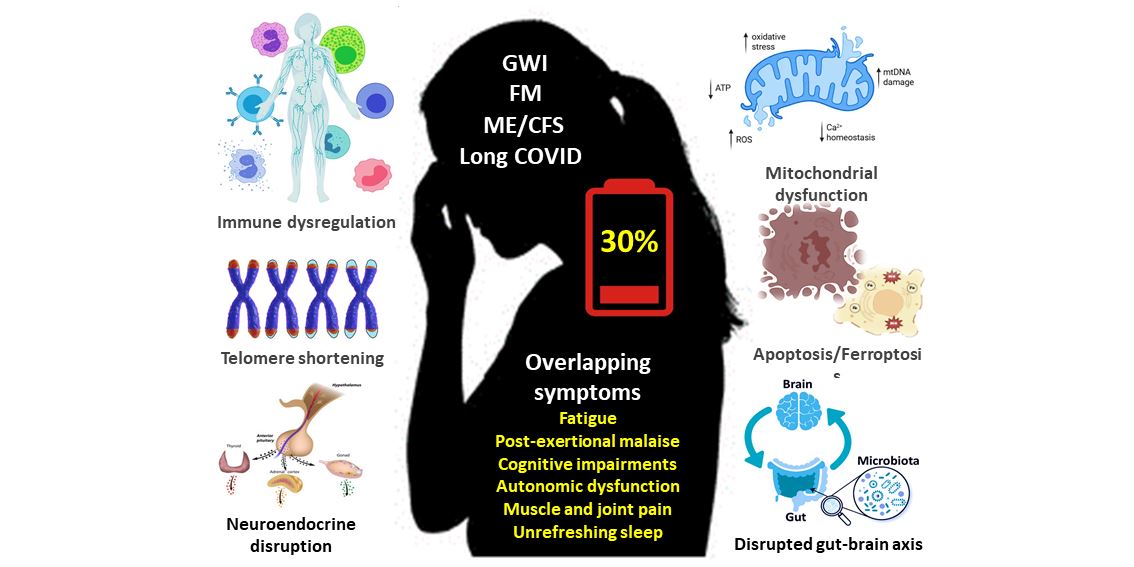
Keywords:
1. Introduction
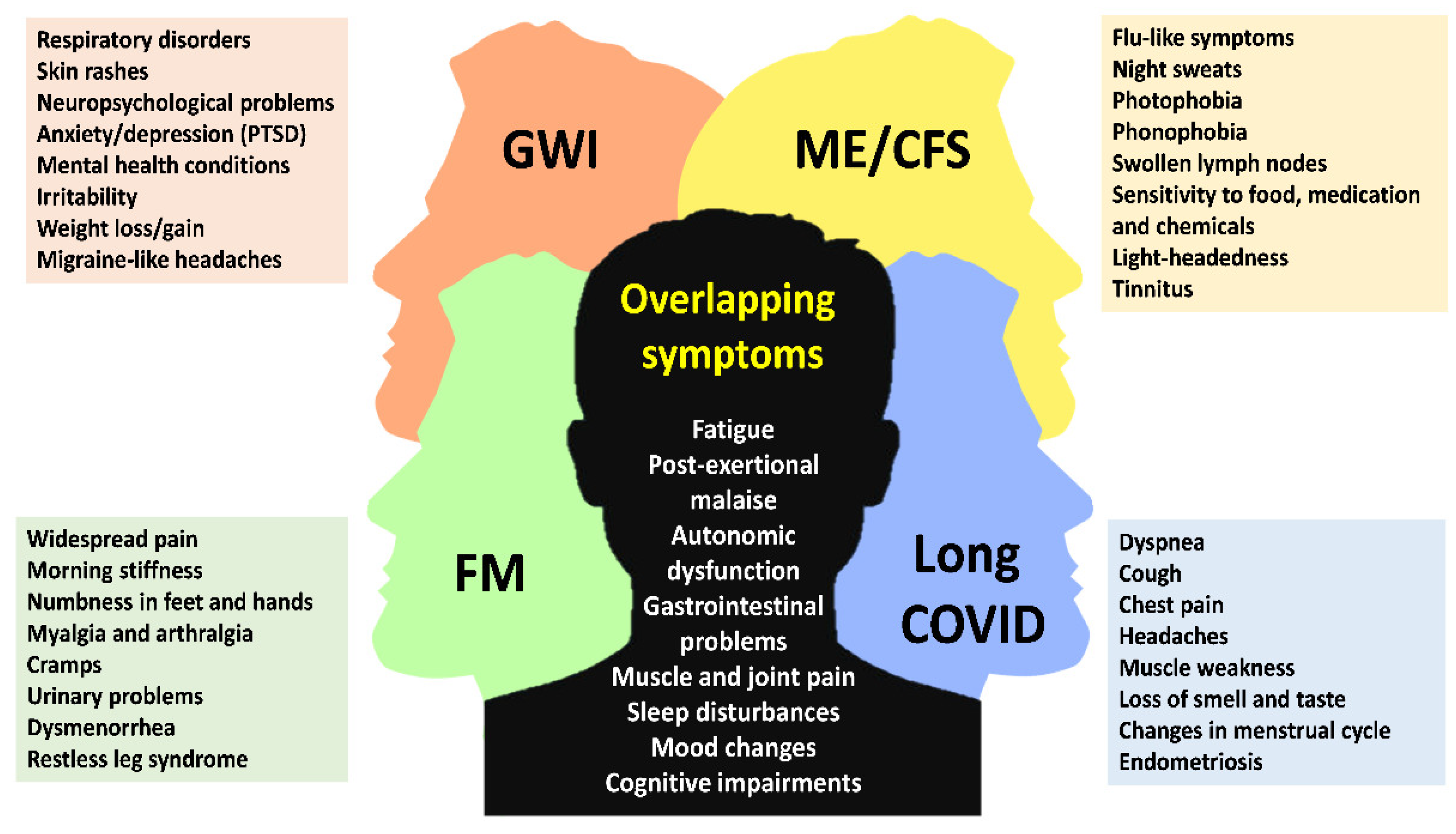
2. Common Symptoms in Low-Energy Associated Disorders
2.1. Fatigue and Post-Exertional Malaise
2.2. Autonomic Dysfunction
2.3. Cognitive Impairment
2.4. Unrefreshing Sleep
2.5. Muscle and Joint Pain
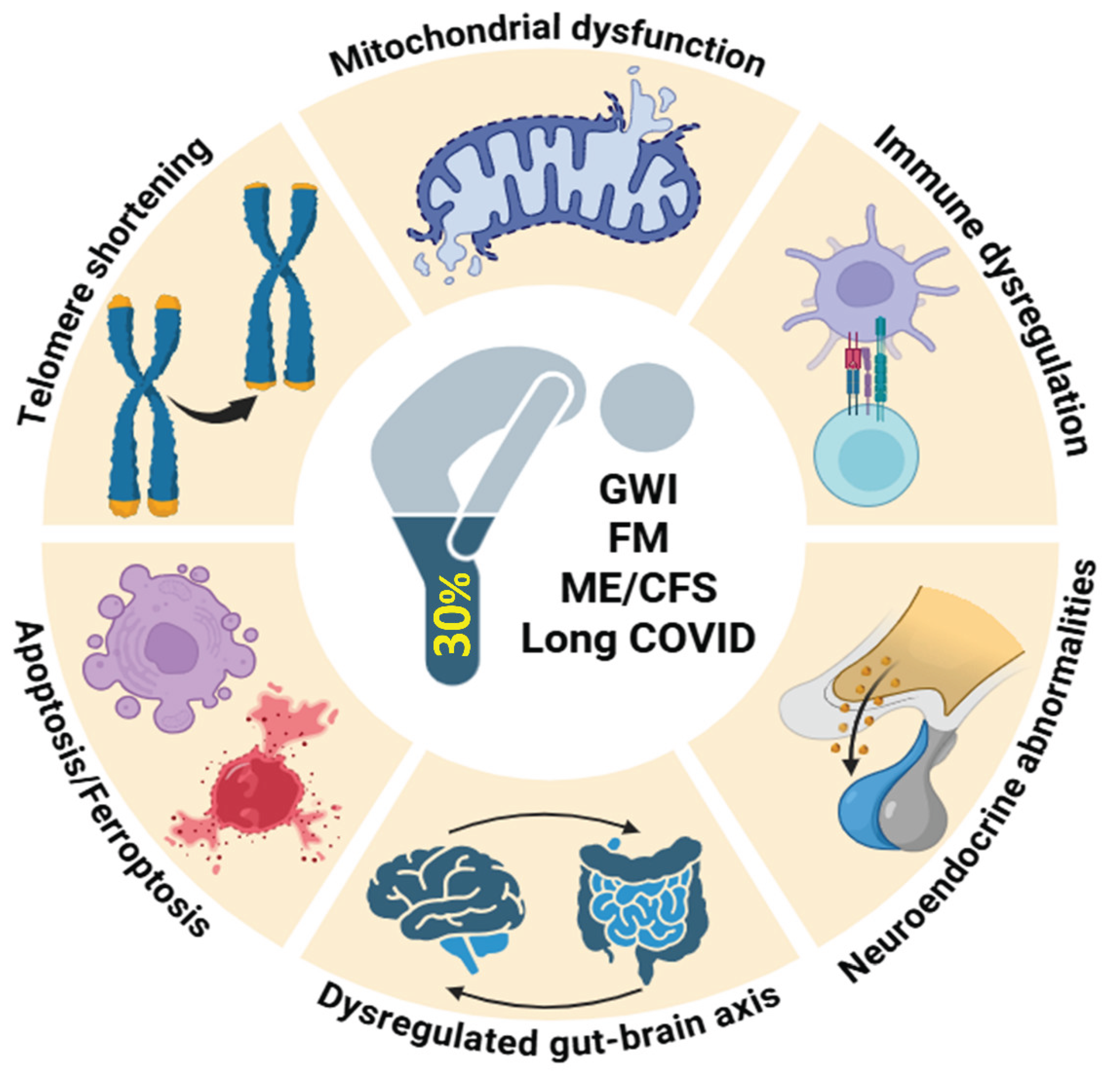
3. Understanding the Shared Biological Mechanisms Between These Conditions
3.1. Mitochondrial Dysfunction, Oxidative Stress and Inflammation
3.2. Immune Dysregulation
3.3. Neuroendocrine Abnormalities
3.4. Dysregulated Gut-Brain Axis
3.5. Apoptosis and Ferroptosis
3.6. Telomere Shortening
4. Implications for Promising Therapeutic Strategies
4.1. Stem Cell Therapy
4.2. Coenzyme Q10 Supplementation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| ATP | Adenosine triphosphate |
| CBT-I | Cognitive behaviour therapy-insomnia |
| CoQ10 | Coenzyme Q10 |
| ENS | Enteric nervous system |
| FM | Fibromyalgia |
| FDA | Food and Drug Administration |
| GWI | Gulf war illness |
| HRV | Heart rate variability |
| HPA | Hypothalamic-pituitary-adrenal axis |
| HO-1 | Heme-oxygenase-1 |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome |
| MPP+ | 1-methyl-4-phenylpyridinium |
| PEM | Post-exertional malaise |
| PESE | Post-exertional symptom exacerbation |
| PI3K | Phosphatidylinositol-3-kinase |
| POTS | Postural orthostatic tachycardia syndrome |
| RCT | Randomized controlled trial |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SLC7A11 | Solute carrier family 7 member 11 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TRPM2 | Transient receptor potential melastatin 2 |
References
- Annesley, S.J.; Missailidis, D.; Heng, B.; Josev, E.K.; Armstrong, C.W. Unravelling shared mechanisms: insights from recent ME/CFS research to illuminate long COVID pathologies. Trends Mol Med 2024, 30, 443–458. [Google Scholar] [CrossRef]
- Bast, E.; Jester, D.J.; Palacio, A.; Krengel, M.; Reinhard, M.; Ashford, J.W. Gulf War Illness: A Historical Review and Considerations of a Post-Viral Syndrome. Mil Med. [CrossRef]
- Goldenberg, D.L. The pivotal role of central sensitization in long COVID, fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome. Expert Rev Neurother 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Steele, L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol 2000, 152, 992–1002. [Google Scholar] [CrossRef]
- Fukuda, K.; Nisenbaum, R.; Stewart, G.; Thompson, W.W.; Robin, L.; Washko, R.M.; Noah, D.L.; Barrett, D.H.; Randall, B.; Herwaldt, B.L. , et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. Jama 1998, 280, 981–988. [Google Scholar] [CrossRef]
- Goldenberg, D.L. How to understand the overlap of long COVID, chronic fatigue syndrome/myalgic encephalomyelitis, fibromyalgia and irritable bowel syndromes. Semin Arthritis Rheum 2024, 67, 152455. [Google Scholar] [CrossRef]
- Khakban, I.; Jain, S.; Gallab, J.; Dharmaraj, B.; Zhou, F.; Lokker, C.; Abdelkader, W.; Zeraatkar, D.; Busse, J.W. Impact of the COVID-19 Pandemic and the 2021 National Institute for Health and Care Excellence Guidelines on Public Perspectives Toward Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Thematic and Sentiment Analysis on Twitter (Rebranded as X). J Med Internet Res 2025, 27, e65087. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Lipkin, W.I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med (Lausanne) 2023, 10, 1187163. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Higgs, M.; Snaith, A.; Lodge, T.A.; Strong, J.; Espejo-Oltra, J.A.; Kujawski, S.; Zalewski, P.; Pretorius, E.; Hoerger, M. , et al. Dysregulation of lipid metabolism, energy production, and oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome, Gulf War Syndrome and fibromyalgia. Front Neurosci 2025, 19, 1498981. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P.; Domingo, J.C.; Castro-Marrero, J. Mitochondrial Dysfunction and Coenzyme Q10 Supplementation in Post-Viral Fatigue Syndrome: An Overview. Int J Mol Sci 2024, 25, 574. [Google Scholar] [CrossRef]
- James, L.M.; Georgopoulos, A.P. At the Root of 3 “Long” Diseases: Persistent Antigens Inflicting Chronic Damage on the Brain and Other Organs in Gulf War Illness, Long-COVID-19, and Chronic Fatigue Syndrome. Neurosci Insights 2022, 17, 26331055221114817. [Google Scholar] [CrossRef]
- León-Moreno, L.C.; Reza-Zaldívar, E.E.; Hernández-Sapiéns, M.A.; Villafaña-Estarrón, E.; García-Martin, M.; Ojeda-Hernández, D.D.; Matias-Guiu, J.A.; Gomez-Pinedo, U.; Matias-Guiu, J.; Canales-Aguirre, A.A. Mesenchymal Stem Cell-Based Therapies in the Post-Acute Neurological COVID Syndrome: Current Landscape and Opportunities. Biomolecules 2023, 14. [Google Scholar] [CrossRef]
- Padda, J.; Khalid, K.; Zubair, U.; Al Hennawi, H.; Yadav, J.; Almanie, A.H.; Mehta, K.A.; Tasnim, F.; Cooper, A.C.; Jean-Charles, G. Stem Cell Therapy and Its Significance in Pain Management. Cureus 2021, 13, e17258. [Google Scholar] [CrossRef]
- Tsilibary, E.C.; Carlson, D.; Georgopoulos, A.P. Protective Effect of Stem Cells from Toxicity Induced by Gulf War Illness (GWI) Serum in N2A Neuroblastoma Cells. J Neurol Neuromedicine 2021, 6, 26–31. [Google Scholar] [CrossRef]
- Hodges, L. Repeated Cardiopulmonary Exercise Testing of ME/CFS Patients. Methods Mol Biol 2025, 2920, 163–172. [Google Scholar] [CrossRef]
- Risbano, M.G. From fatigue to physiology: Submaximal 2-day cardiopulmonary exercise test and emerging standards in long COVID. Exp Physiol. [CrossRef]
- Dehlia, A.; Guthridge, M.A. The persistence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) after SARS-CoV-2 infection: A systematic review and meta-analysis. J Infect 2024, 89, 106297. [Google Scholar] [CrossRef]
- Frank, J.; Tehrani, L.; Gamer, J.; Van Booven, D.J.; Ballarin, S.; Rossman, R.; Edelstein, A.; Uppalati, S.; Reuthebuck, A.; Collado, F. , et al. Gulf War Illness Induced Sex-Specific Transcriptional Differences Under Stressful Conditions. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Vink, M.; Partyka-Vink, K. The PACE Trial’s GET Manual for Therapists Exposes the Fixed Incremental Nature of Graded Exercise Therapy for ME/CFS. Life (Basel) 2025, 15. [Google Scholar] [CrossRef]
- Boruch, A.; Branchaw, G.; O’Connor, P.J.; Cook, D.B. Physical Activity and Fatigue Symptoms: Neurotypical Adults and People with Chronic Multisymptom Illnesses. Curr Top Behav Neurosci 2024, 67, 281–308. [Google Scholar] [CrossRef]
- Di Bari, A.; Demo, G.; Patron, E. Unravelling the relationship between anxiety, autonomic nervous system dysfunction and fibromyalgia: a systematic review. Clin Exp Rheumatol 2025, 43, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, R.M.; Capdevila, L.; Castro, J.R.; Zaragozà, M.C.; Maurel, S.; Alegre, J.; Castro-Marrero, J. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2020, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Haley, R.W.; Dever, J.A.; Kramer, G.; Teiber, J.F. The effect of disease misclassification on the ability to detect a gene-environment interaction: implications of the specificity of case definitions for research on Gulf War illness. BMC Med Res Methodol 2023, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.J.; Mathersul, D.C.; Schulz-Heik, R.J.; Mahoney, L.; Bayley, P.J. Self-Reported Autonomic Dysregulation in Gulf War Illness. Mil Med 2023, 188. [Google Scholar] [CrossRef]
- Garner, R.S.; Rayhan, R.U.; Baraniuk, J.N. Verification of exercise-induced transient postural tachycardia phenotype in Gulf War Illness. Am J Transl Res 2018, 10, 3254–3264. [Google Scholar]
- Verne, Z.T.; Fields, J.Z.; Zhang, B.B.; Zhou, Q. Autonomic dysfunction and gastroparesis in Gulf War veterans. J Investig Med 2023, 71, 7–10. [Google Scholar] [CrossRef]
- Kemp, J.; Sunnquist, M.; Jason, L.A.; Newton, J.L. Autonomic dysfunction in myalgic encephalomyelitis and chronic fatigue syndrome: comparing self-report and objective measures. Clin Auton Res 2019, 29, 475–477. [Google Scholar] [CrossRef]
- Ziaja, C.P.; Young, S.; Stark, M. Dysregulation of the autonomic nervous system in ME/CFS and post-COVID syndrome: insights from 48-h heart rate variability monitoring. J Neurol Sci 2023, 455. [Google Scholar] [CrossRef]
- El-Sawy, N.; El-Tantawi, G.; Achmawi, G.A.H.; Sultan, H.; Younis, S. Autonomic changes in fibromyalgia: Clinical and electrophysiological study. Alexandria J Med 2012, 48, 215–222. [Google Scholar] [CrossRef]
- Kingsley, J.D. Autonomic dysfunction in women with fibromyalgia. Arthritis Res Ther 2012, 14, 103. [Google Scholar] [CrossRef]
- Staud, R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep 2008, 10, 463–466. [Google Scholar] [CrossRef]
- Vincent, A.; Whipple, M.O.; Low, P.A.; Joyner, M.; Hoskin, T.L. Patients With Fibromyalgia Have Significant Autonomic Symptoms But Modest Autonomic Dysfunction. Pm r 2016, 8, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Giordani, C.; De Luca, M.; Olivieri, F. Long-COVID-19 autonomic dysfunction: An integrated view in the framework of inflammaging. Mech Ageing Dev 2024, 218, 111915. [Google Scholar] [CrossRef]
- Fedorowski, A.; Sutton, R. Autonomic dysfunction and postural orthostatic tachycardia syndrome in post-acute COVID-19 syndrome. Nat Rev Cardiol 2023, 20, 281–282. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond) 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.C.; Battistini, F.; Cordobilla, B.; Zaragoza, M.C.; Sanmartin-Sentanes, R.; Alegre-Martin, J.; Cambras, T.; Castro-Marrero, J. Association of circulating biomarkers with illness severity measures differentiates myalgic encephalomyelitis/chronic fatigue syndrome and post-COVID-19 condition: a prospective pilot cohort study. J Transl Med 2024, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A. Oxidative Stress and Mitochondrial Injury in Chronic Multisymptom Conditions: From Gulf War Illness to Autism Spectrum Disorder. Nat Prec. [CrossRef]
- Butterick, T.A.; Trembley, J.H.; Hocum Stone, L.L.; Muller, C.J.; Rudquist, R.R.; Bach, R.R. Gulf War Illness-associated increases in blood levels of interleukin 6 and C-reactive protein: biomarker evidence of inflammation. BMC Res Notes 2019, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Dickey, B.; Madhu, L.N.; Shetty, A.K. Gulf War Illness: Mechanisms Underlying Brain Dysfunction and Promising Therapeutic Strategies. Pharmacol Ther 2021, 220, 107716. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Sullivan, K.; Krengel, M.H.; Killiany, R.J.; Steele, L.; Klimas, N.G.; Koo, B.B. The prevalence of mild cognitive impairment in Gulf War veterans: a follow-up study. Front Neurosci 2023, 17, 1301066. [Google Scholar] [CrossRef]
- Cheng, C.H.; Alshelh, Z.; Guan, Y.; Sullivan, K.; Loggia, M.L.; Koo, B.B. Association of the tissue microstructural diffusivity and translocator protein PET in Gulf War Illness. Brain Behav Immun Health 2021, 18, 100364. [Google Scholar] [CrossRef]
- Cheng, C.H.; Koo, B.B.; Calderazzo, S.; Quinn, E.; Aenlle, K.; Steele, L.; Klimas, N.; Krengel, M.; Janulewicz, P.; Toomey, R. , et al. Alterations in high-order diffusion imaging in veterans with Gulf War Illness is associated with chemical weapons exposure and mild traumatic brain injury. Brain Behav Immun 2020, 89, 281–290. [Google Scholar] [CrossRef]
- Alshelh, Z.; Albrecht, D.S.; Bergan, C.; Akeju, O.; Clauw, D.J.; Conboy, L.; Edwards, R.R.; Kim, M.; Lee, Y.C.; Protsenko, E. , et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav Immun 2020, 87, 498–507. [Google Scholar] [CrossRef]
- Godlewska, B.R.; Sylvester, A.L.; Emir, U.E.; Sharpley, A.L.; Clarke, W.T.; Williams, S.R.; Gonçalves, A.J.; Raman, B.; Valkovič, L.; Cowen, P.J. Brain and muscle chemistry in myalgic encephalitis/chronic fatigue syndrome (ME/CFS) and long COVID: a 7T magnetic resonance spectroscopy study. Mol Psychiatry. [CrossRef]
- Jonsjö, M.A.; Olsson, G.L.; Wicksell, R.K.; Alving, K.; Holmström, L.; Andreasson, A. The role of low-grade inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology 2020, 113, 104578. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Sato, W.; Son, C.G. Brain-regional characteristics and neuroinflammation in ME/CFS patients from neuroimaging: A systematic review and meta-analysis. Autoimmun Rev 2024, 23, 103484. [Google Scholar] [CrossRef]
- Dass, R.; Kalia, M.; Harris, J.; Packham, T. Understanding the Experience and Impacts of Brain Fog in Chronic Pain: A Scoping Review. Can J Pain 2023, 7, 2217865. [Google Scholar] [CrossRef]
- García-Domínguez, M. Fibromyalgia and Inflammation: Unrevealing the Connection. Cells 2025, 14. [Google Scholar] [CrossRef]
- Ferrés, S.; Serrat, M.; Auer, W.; Royuela-Colomer, E.; Almirall, M.; Lizama-Lefno, A.; Nijs, J.; Maes, M.; Luciano, J.V.; Borràs, X. , et al. Immune-inflammatory effects of the multicomponent intervention FIBROWALK in outdoor and online formats for patients with fibromyalgia. Brain Behav Immun 2025, 125, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, P.; Zhao, Y.; Liu, Y.; Hu, Y.; Zhu, Z.; Xiao, H. The Effect of a Remote Network Technology Supervised Exercise Program Combined With Drug Treatment for Fibromyalgia: Randomized, Single-Blind, Controlled Trial. J Med Internet Res 2025, 27, e71624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Martin, E.M.; Reuken, P.A.; Scholcz, A.; Ganse-Dumrath, A.; Srowig, A.; Utech, I.; Kozik, V.; Radscheidt, M.; Brodoehl, S. , et al. Long COVID is associated with severe cognitive slowing: a multicentre cross-sectional study. EClinicalMedicine 2024, 68, 102434. [Google Scholar] [CrossRef]
- Wang, W.; Cui, R.; Leng, L.; Wang, G.; Peng, G. Cognitive Impairment in the Post-Acute Phases of COVID-19 and Mechanisms: An Introduction and Narrative Review. J Alzheimers Dis Rep 2024, 8, 647–658. [Google Scholar] [CrossRef]
- Liew, F.; Efstathiou, C.; Fontanella, S.; Richardson, M.; Saunders, R.; Swieboda, D.; Sidhu, J.K.; Ascough, S.; Moore, S.C.; Mohamed, N. , et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat Immunol 2024, 25, 607–621. [Google Scholar] [CrossRef]
- Lipsky, R.K.; Helmer, D.A.; Beckham, J.C.; Calhoun, P.S.; Pugh, M.J.; Kimbrel, N.A. The association between Gulf War Illness and suicidal thoughts and behaviors among Gulf War Era Veterans. J Psychiatr Res 2025, 183, 302–307. [Google Scholar] [CrossRef]
- Van Wilgen, C.P.; Ucles-Juarez, R.; Krutko, D.; Li, Y.; Polli, A.; Syed, A.; Zampese, S.; Reis, F.J.J.; de Zeeuw, J. Knowledge on cause, clinical manifestation and treatment for fibromyalgia among medical doctors: A worldwide survey. Pain Pract 2024, 24, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Zeron-Rugerio, M.F.; Zaragoza, M.C.; Domingo, J.C.; Sanmartin-Sentanes, R.; Alegre-Martin, J.; Castro-Marrero, J.; Cambras, T. Sleep and circadian rhythm alterations in myalgic encephalomyelitis/chronic fatigue syndrome and post-COVID fatigue syndrome and its association with cardiovascular risk factors: A prospective cohort study. Chronobiol Int 2024, 41, 1104–1115. [Google Scholar] [CrossRef]
- Winograd, D.M.; Sullivan, N.L.; Thien, S.R.; Pigeon, W.R.; Litke, D.R.; Helmer, D.A.; Rath, J.F.; Lu, S.E.; McAndrew, L.M. Veterans with Gulf War Illness perceptions of management strategies. Life Sci 2021, 279, 119219. [Google Scholar] [CrossRef]
- Karabey Aksalli, I.; Baygin, N.; Hagiwara, Y.; Paul, J.K.; Iype, T.; Barua, P.D.; Koh, J.E.W.; Baygin, M.; Dogan, S.; Tuncer, T. , et al. Automated characterization and detection of fibromyalgia using slow wave sleep EEG signals with glucose pattern and D’hondt pooling technique. Cogn Neurodyn 2024, 18, 383–404. [Google Scholar] [CrossRef]
- Lederman, S.; Arnold, L.M.; Vaughn, B.; Engels, J.M.; Kelley, M.; Sullivan, G.M. Pain Relief by Targeting Nonrestorative Sleep in Fibromyalgia: A Phase 3 Randomized Trial of Bedtime Sublingual Cyclobenzaprine. Pain Med. [CrossRef]
- Malluru, N.; Abdullah, Y.; Hackshaw, K.V. Early diagnostics of fibromyalgia: an overview of the challenges and opportunities. Expert Rev Mol Diagn 2025, 25, 21–31. [Google Scholar] [CrossRef]
- Benjamin, N.Z.Y.; Parekh, R.S.; Inban, P.; Sakthi, S.; Tekuru, Y.; Prajjwal, P.; John, J.; Sharma, R. Fibromyalgia: Advances in pathophysiology, diagnostic biomarkers, genetic insights, multisystemic involvement, and treatment updates and multidisciplinary interventions. Dis Mon 2025, 101965. [Google Scholar] [CrossRef] [PubMed]
- Climent-Sanz, C.; Valenzuela-Pascual, F.; Martínez-Navarro, O.; Blanco-Blanco, J.; Rubí-Carnacea, F.; García-Martínez, E.; Soler-González, J.; Barallat-Gimeno, E.; Gea-Sánchez, M. Cognitive behavioral therapy for insomnia (CBT-i) in patients with fibromyalgia: a systematic review and meta-analysis. Disabil Rehabil 2022, 44, 5770–5783. [Google Scholar] [CrossRef]
- Imamura, M.; Robertson, C.; Hudson, J.; Whibley, D.; Aucott, L.; Gillies, K.; Beasley, M.; Stevens, M.J.; Manson, P.; Dulake, D. , et al. Effects of pharmacological and non-pharmacological interventions for the management of sleep problems in people with fibromyalgia: a multi-methods evidence synthesis. Health Technol Assess 2025, 29, 1–228. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Zaragozá, M.C.; González-Garcia, S.; Aliste, L.; Sáez-Francàs, N.; Romero, O.; Ferré, A.; Fernández de Sevilla, T.; Alegre, J. Poor self-reported sleep quality and health-related quality of life in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Sleep Res 2018, 27, e12703. [Google Scholar] [CrossRef]
- Weigel, B.; Eaton-Fitch, N.; Thapaliya, K.; Marshall-Gradisnik, S. Illness presentation and quality of life in myalgic encephalomyelitis/chronic fatigue syndrome and post COVID-19 condition: a pilot Australian cross-sectional study. Qual Life Res 2024, 33, 2489–2507. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.Z.; Andersen, T.; Radovic, S.; Del Fante, P.; Kwiatek, R.; Calhoun, V.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J.; Shan, Z.Y. Objective sleep measures in chronic fatigue syndrome patients: A systematic review and meta-analysis. Sleep Med Rev 2023, 69, 101771. [Google Scholar] [CrossRef]
- Yin, J.; Xu, J.; Ren, T.L. Recent Progress in Long-Term Sleep Monitoring Technology. Biosensors (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Chao, L.L. Examining the current health of Gulf War veterans with the veterans affairs frailty index. Front Neurosci 2023, 17, 1245811. [Google Scholar] [CrossRef]
- Pettersen, P.S.; Haugmark, T.; Berg, I.J.; Hammer, H.B.; Neogi, T.; Zangi, H.; Haugen, I.K.; Provan, S.A. Pain sensitization in fibromyalgia. Cross-sectional associations between quantitative sensory testing of pain sensitization and fibromyalgia disease burden. Eur J Pain 2025, 29, e4771. [Google Scholar] [CrossRef]
- Escalante, A.; Fischbach, M. Musculoskeletal manifestations, pain, and quality of life in Persian Gulf War veterans referred for rheumatologic evaluation. J Rheumatol 1998, 25, 2228–2235. [Google Scholar]
- Castro-Marrero, J.; Cordero, M.D.; Sáez-Francas, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre-Martin, J. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal 2013, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meyer, J.N.; Hill, H.Z.; Lange, G.; Condon, M.R.; Klein, J.C.; Ndirangu, D.; Falvo, M.J. Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLoS One 2017, 12, e0184832. [Google Scholar] [CrossRef]
- Koslik, H.J.; Hamilton, G.; Golomb, B.A. Mitochondrial dysfunction in Gulf War illness revealed by 31Phosphorus Magnetic Resonance Spectroscopy: a case-control study. PLoS One 2014, 9, e92887. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Kelley, R.I.; Han, J.H.; Miller, B.; Bui, L. Gulf war illness: a tale of two genomes. BMC Res Notes 2024, 17, 230. [Google Scholar] [CrossRef]
- Golomb, B.A.; Han, J.H.; Fung, A.; Berg, B.K.; Miller, B.J.; Hamilton, G. Bioenergetic impairment in Gulf War illness assessed via (31)P-MRS. Sci Rep 2024, 14, 7418. [Google Scholar] [CrossRef]
- Golomb, B.A.; Sanchez Baez, R.; Schilling, J.M.; Dhanani, M.; Fannon, M.J.; Berg, B.K.; Miller, B.J.; Taub, P.R.; Patel, H.H. Mitochondrial impairment but not peripheral inflammation predicts greater Gulf War illness severity. Sci Rep 2023, 13, 10739. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.A.; Hattiangady, B.; Upadhya, D.; Bates, A.; Attaluri, S.; Shuai, B.; Kodali, M.; Shetty, A.K. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front Mol Neurosci 2017, 10, 182. [Google Scholar] [CrossRef]
- Cordero, M.D.; de Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol Lett 2010, 31, 169–173. [Google Scholar] [PubMed]
- Assavarittirong, C.; Samborski, W.; Grygiel-Górniak, B. Oxidative Stress in Fibromyalgia: From Pathology to Treatment. Oxid Med Cell Longev 2022, 2022, 1582432. [Google Scholar] [CrossRef]
- Holden, S.; Maksoud, R.; Eaton-Fitch, N.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med 2020, 18, 290. [Google Scholar] [CrossRef]
- Smits, B.; van den Heuvel, L.; Knoop, H.; Küsters, B.; Janssen, A.; Borm, G.; Bleijenberg, G.; Rodenburg, R.; van Engelen, B. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion 2011, 11, 735–738. [Google Scholar] [CrossRef]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS One 2017, 12, e0186802. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial dysfunction in long COVID: mechanisms, consequences, and potential therapeutic approaches. Geroscience 2024, 46, 5267–5286. [Google Scholar] [CrossRef] [PubMed]
- Szögi, T.; Borsos, B.N.; Masic, D.; Radics, B.; Bella, Z.; Bánfi, A.; Ördög, N.; Zsiros, C.; Kiricsi, Á.; Pankotai-Bodó, G. , et al. Novel biomarkers of mitochondrial dysfunction in Long COVID patients. Geroscience 2025, 47, 2245–2261. [Google Scholar] [CrossRef]
- Stufano, A.; Isgrò, C.; Palese, L.L.; Caretta, P.; De Maria, L.; Lovreglio, P.; Sardanelli, A.M. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Goolkasian, P.; Palomo, I.M.; Williams, M.V.; Maloney, S.R.; Ariza, M.E. Reactivation of Latent Herpesviruses and a Faulty Antiviral Response may Contribute to Chronic Multi-Symptom and Multi-System Illnesses in U.S. Military Veterans. J Med Virol 2025, 97, e70400. [Google Scholar] [CrossRef]
- Mettler, J.; Ming-Azevedo, P.; Hügle, T. Fibromyalgia with concomitant immune-mediated rheumatic diseases: an evaluation of clinical characteristics, diagnostic criteria and multimodal treatment outcomes. Adv Rheumatol 2025, 65, 27. [Google Scholar] [CrossRef]
- Burzynski, H.E.; Reagan, L.P. Exposing the latent phenotype of Gulf War Illness: examination of the mechanistic mediators of cognitive dysfunction. Front Immunol 2024, 15, 1403574. [Google Scholar] [CrossRef] [PubMed]
- Trageser, K.J.; Sebastian-Valverde, M.; Naughton, S.X.; Pasinetti, G.M. The Innate Immune System and Inflammatory Priming: Potential Mechanistic Factors in Mood Disorders and Gulf War Illness. Front Psychiatry 2020, 11, 704. [Google Scholar] [CrossRef]
- Whistler, T.; Fletcher, M.A.; Lonergan, W.; Zeng, X.R.; Lin, J.M.; Laperriere, A.; Vernon, S.D.; Klimas, N.G. Impaired immune function in Gulf War Illness. BMC Med Genomics 2009, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R. , et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat Immunol 2024, 25, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W. Immune damage in Long Covid. Science 2024, 383, 262–263. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A. , et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Eaton-Fitch, N.; Rudd, P.; Er, T.; Hool, L.; Herrero, L.; Marshall-Gradisnik, S. Immune exhaustion in ME/CFS and long COVID. JCI Insight 2024, 9. [Google Scholar] [CrossRef]
- Lutz, L.; Rohrhofer, J.; Zehetmayer, S.; Stingl, M.; Untersmayr, E. Evaluation of Immune Dysregulation in an Austrian Patient Cohort Suffering from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- O’Neal, A.J.; Hanson, M.R. The Enterovirus Theory of Disease Etiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Critical Review. Front Med (Lausanne) 2021, 8, 688486. [Google Scholar] [CrossRef] [PubMed]
- Vinker-Shuster, M.; Magen, E.; Green, I.; Merzon, E.; Golan-Cohen, A.; Israel, A. Increased Rates of Infectious Diseases in Fibromyalgia Patients: A Population-Based Case-Control Study. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Findeisen, K.; Guymer, E.; Littlejohn, G. Neuroinflammatory and Immunological Aspects of Fibromyalgia. Brain Sci 2025, 15. [Google Scholar] [CrossRef]
- Clauw, D.; Sarzi-Puttini, P.; Pellegrino, G.; Shoenfeld, Y. Is fibromyalgia an autoimmune disorder? Autoimmun Rev 2024, 23, 103424. [Google Scholar] [CrossRef]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U. , et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Golier, J.A.; Caramanica, K.; Yehuda, R. Neuroendocrine response to CRF stimulation in veterans with and without PTSD in consideration of war zone era. Psychoneuroendocrinology 2012, 37, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Tomic, S.; Brkic, S.; Lendak, D.; Maric, D.; Medic Stojanoska, M.; Novakov Mikic, A. Neuroendocrine disorder in chronic fatigue syndrome. Turk J Med Sci 2017, 47, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Losacco, S.; Giordano, G.; Mucci, V.; Blanchini, F.; Burlando, B. Fibromyalgia pathogenesis explained by a neuroendocrine multistable model. PLoS One 2024, 19, e0303573. [Google Scholar] [CrossRef]
- Sasikumar, S.; Unniappan, S. SARS-CoV-2 Infection and the Neuroendocrine System. Neuroendocrinology 2024, 114, 1158–1175. [Google Scholar] [CrossRef]
- Kimono, D.A. Gastrointestinal problems, mechanisms and possible therapeutic directions in Gulf war illness: a mini review. Mil Med Res 2021, 8, 50. [Google Scholar] [CrossRef]
- Trivedi, A.; Bose, D.; Moffat, K.; Pearson, E.; Walsh, D.; Cohen, D.; Skupsky, J.; Chao, L.; Golier, J.; Janulewicz, P. , et al. Gulf War Illness Is Associated with Host Gut Microbiome Dysbiosis and Is Linked to Altered Species Abundance in Veterans from the BBRAIN Cohort. Int J Environ Res Public Health 2024, 21. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.A.; Shaughnessy, C.; Baubie, K.; Kates, A.E.; Putman-Buehler, N.; Watson, L.; Dominguez, N.; Watson, K.; Cook, D.B.; Rabago, D. , et al. Characterising the gut microbiome in veterans with Gulf War Illness: a protocol for a longitudinal, prospective cohort study. BMJ Open 2019, 9, e031114. [Google Scholar] [CrossRef]
- Nono Djotsa, A.B.S.; Nguyen Wenker, T.H.; Ahmed, S.T.; Ghosh, S.; Malhotra, D.; Boyle, S.H.; Gifford, E.J.; Sims, K.J.; White, D.L.; Steele, L. , et al. Irritable Bowel Syndrome in Veterans With Gulf War Illness Evaluated at VA’s War-Related Illness and Injury Study Center. Mil Med 2024, 189, e2644–e2654. [Google Scholar] [CrossRef]
- Malhotra, D.; Boyle, S.H.; Gifford, E.J.; Sullivan, B.A.; Nguyen Wenker, T.H.; Abs, N.D.; Ahmed, S.T.; Upchurch, J.; Vahey, J.; Stafford, C. , et al. Self-reported gastrointestinal disorders among veterans with gulf war illness with and without posttraumatic stress disorder. Neurogastroenterol Motil 2023, 35, e14548. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bose, D.; Seth, R. Host gut microbiome and potential therapeutics in Gulf War Illness: A short review. Life Sci 2021, 280, 119717. [Google Scholar] [CrossRef]
- Saha, P.; Skidmore, P.T.; Holland, L.A.; Mondal, A.; Bose, D.; Seth, R.K.; Sullivan, K.; Janulewicz, P.A.; Horner, R.; Klimas, N. , et al. Andrographolide Attenuates Gut-Brain-Axis Associated Pathology in Gulf War Illness by Modulating Bacteriome-Virome Associated Inflammation and Microglia-Neuron Proinflammatory Crosstalk. Brain Sci 2021, 11. [Google Scholar] [CrossRef]
- Collier, C.A.; Salikhova, A.; Sabir, S.; Raghavan, S.A. Persistent enteric neuroinflammation chronically impairs colonic motility in a pyridostigmine bromide-induced mouse model of Gulf War illness. Biol Open 2025, 14. [Google Scholar] [CrossRef]
- Shtrozberg, S.; Bazzichi, L.; Sarzi-Puttini, P.; Aloush, V.; Ablin, J.N. Is the gut microbiome of importance in fibromyalgia? A critical review of emerging evidence. Clin Exp Rheumatol 2025, 43, 990–998. [Google Scholar] [CrossRef]
- Martín, F.; Blanco-Suárez, M.; Zambrano, P.; Cáceres, O.; Almirall, M.; Alegre-Martín, J.; Lobo, B.; González-Castro, A.M.; Santos, J.; Domingo, J.C. , et al. Increased gut permeability and bacterial translocation are associated with fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: implications for disease-related biomarker discovery. Front Immunol 2023, 14, 1253121. [Google Scholar] [CrossRef] [PubMed]
- Waterman, A.; Doumas, S.A.; Fischer, M.; Mattar, M.; Charbel, S.; Jennings, J.; Doman, D.B. Uncovering the Hidden Link Between the Aberrant Intestinal Microbiome and Fibromyalgia. Gastroenterol Hepatol (N Y) 2025, 21, 111–121. [Google Scholar]
- Iannuccelli, C.; Favretti, M.; Dolcini, G.; Di Carlo, M.; Pellegrino, G.; Bazzichi, L.; Atzeni, F.; Lucini, D.; Varassi, G.; Leoni, M.L.G. , et al. Fibromyalgia: one year in review 2025. Clin Exp Rheumatol 2025, 43, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Jurek, J.M.; Castro-Marrero, J. A Narrative Review on Gut Microbiome Disturbances and Microbial Preparations in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Implications for Long COVID. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Trautmann, A. Core features and inherent diversity of post-acute infection syndromes. Front Immunol 2025, 16, 1509131. [Google Scholar] [CrossRef]
- Guo, C.; Che, X.; Briese, T.; Ranjan, A.; Allicock, O.; Yates, R.A.; Cheng, A.; March, D.; Hornig, M.; Komaroff, A.L. , et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 2023, 31, 288–304. [Google Scholar] [CrossRef]
- Tian, H.; Wang, L.; Aiken, E.; Ortega, R.J.V.; Hardy, R.; Placek, L.; Kozhaya, L.; Unutmaz, D.; Oh, J.; Yao, X. Fast Targeted Metabolomics for Analyzing Metabolic Diversity of Bacterial Indole Derivatives in ME/CFS Gut Microbiome. bioRxiv 2024. [CrossRef] [PubMed]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-‘omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe 2023, 31, 273–287. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Ng, S.C. Long COVID and gut microbiome: insights into pathogenesis and therapeutics. Gut Microbes 2025, 17, 2457495. [Google Scholar] [CrossRef]
- Oh, S.; An, S.; Park, K.; Lee, S.; Han, Y.M.; Koh, S.J.; Lee, J.; Gim, H.; Kim, D.; Seo, H. Gut Microbial Signatures in Long COVID: Potential Biomarkers and Therapeutic Targets. Infect Dis Ther 2025, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Smail, S.W.; Albarzinji, N.; Salih, R.H.; Taha, K.O.; Hirmiz, S.M.; Ismael, H.M.; Noori, M.F.; Azeez, S.S.; Janson, C. Microbiome dysbiosis in SARS-CoV-2 infection: implication for pathophysiology and management strategies of COVID-19. Front Cell Infect Microbiol 2025, 15, 1537456. [Google Scholar] [CrossRef]
- Yao, L.; Devotta, H.; Li, J.; Lunjani, N.; Sadlier, C.; Lavelle, A.; Albrich, W.C.; Walter, J.; O’Toole, P.W.; O’Mahony, L. Dysrupted microbial tryptophan metabolism associates with SARS-CoV-2 acute inflammatory responses and long COVID. Gut Microbes 2024, 16, 2429754. [Google Scholar] [CrossRef]
- Yu, L.C. Gastrointestinal pathophysiology in long COVID: Exploring roles of microbiota dysbiosis and serotonin dysregulation in post-infectious bowel symptoms. Life Sci 2024, 358, 123153. [Google Scholar] [CrossRef]
- El-Sehrawy, A.; Ayoub, II; Uthirapathy, S. ; Ballal, S.; Gabble, B.C.; Singh, A.; V, K.; Panigrahi, R.; Kamali, M.; Khosravi, M. The microbiota-gut-brain axis in myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review of an emerging field. Eur J Transl Myol 2025, 35. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, D. Monitoring autophagy-dependent ferroptosis. Methods Cell Biol 2021, 165, 163–176. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; James, L.M.; Carpenter, A.F.; Engdahl, B.E.; Leuthold, A.C.; Lewis, S.M. Gulf War illness (GWI) as a neuroimmune disease. Exp Brain Res 2017, 235, 3217–3225. [Google Scholar] [CrossRef]
- Tsilibary, E.C.; Souto, E.P.; Kratzke, M.; James, L.M.; Engdahl, B.E.; Georgopoulos, A.P. Anthrax Protective Antigen 63 (PA63): Toxic Effects in Neural Cultures and Role in Gulf War Illness (GWI). Neurosci Insights 2020, 15, 2633105520931966. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. Dorsal root ganglia: fibromyalgia pain factory? Clin Rheumatol 2021, 40, 783–787. [Google Scholar] [CrossRef]
- Yüksel, E.; Nazıroğlu, M.; Şahin, M.; Çiğ, B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci Rep 2017, 7, 17543. [Google Scholar] [CrossRef]
- Gheita, T.A.; El Sisi, R.W.; Raafat, H.A.; Khalil, H.M. Anti-annexin V antibodies in primary fibromyalgia syndrome: relation to associated Sjögren’s syndrome. J Clin Immunol 2013, 33, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Jiao, W.; Wang, Y.; Wang, X.; Zhao, Y.; Fan, X.; Tian, L.; Li, X.; Mi, J. Ferroptosis and its role in skeletal muscle diseases. Front Mol Biosci 2022, 9, 1051866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhu, Z.; Han, K.; Chen, D.; Huang, L.; Hu, N.; Du, J.; Zhang, B.; Su, Y.; Li, T. , et al. Magnesium hexacyanoferrate nanocatalysts alleviates fibromyalgia syndrome by reversing cellular ferroptosis. J Chem Eng 2024, 498, 155019. [Google Scholar] [CrossRef]
- Kennedy, G.; Khan, F.; Hill, A.; Underwood, C.; Belch, J.J. Biochemical and vascular aspects of pediatric chronic fatigue syndrome. Arch Pediatr Adolesc Med 2010, 164, 817–823. [Google Scholar] [CrossRef]
- Cezar, R.; Kundura, L.; André, S.; Lozano, C.; Vincent, T.; Muller, L.; Lefrant, J.Y.; Roger, C.; Claret, P.G.; Duvnjak, S. , et al. T4 apoptosis in the acute phase of SARS-CoV-2 infection predicts long COVID. Front Immunol 2023, 14, 1335352. [Google Scholar] [CrossRef]
- Sousa, R.A.L.; Yehia, A.; Abulseoud, O.A. Attenuation of ferroptosis as a potential therapeutic target for neuropsychiatric manifestations of post-COVID syndrome. Front Neurosci 2023, 17, 1237153. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Jin, W.; Mo, N.; Ye, G.; Su, Z.; Tang, L.; Wang, Y.; Li, Y.; Du, J. The potential role of ferroptosis in COVID-19-related cardiovascular injury. Biomed Pharmacother 2023, 168, 115637. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, S.; Han, Y.; Zhang, H.; Cao, J.; Dong, S.; Li, D.; Lei, M.; Liu, C.; Gao, Y. Role of Ferroptosis in the Progression of COVID-19 and the Development of Long COVID. Curr Med Chem 2024. [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell Mol Life Sci 2020, 77, 61–79. [Google Scholar] [CrossRef]
- Starkweather, A.R.; Alhaeeri, A.A.; Montpetit, A.; Brumelle, J.; Filler, K.; Montpetit, M.; Mohanraj, L.; Lyon, D.E.; Jackson-Cook, C.K. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res 2014, 63, 36–50. [Google Scholar] [CrossRef]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev Rep 2022, 18, 2315–2327. [Google Scholar] [CrossRef]
- Nassour, J.; Przetocka, S.; Karlseder, J. Telomeres as hotspots for innate immunity and inflammation. DNA Repair (Amst) 2024, 133, 103591. [Google Scholar] [CrossRef]
- Opresko, P.L.; Sanford, S.L.; De Rosa, M. Oxidative Stress and DNA Damage at Telomeres. Cold Spring Harb Perspect Biol 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M. The Role of Telomeres in Human Disease. Annu Rev Genomics Hum Genet 2022, 23, 363–381. [Google Scholar] [CrossRef]
- Howard, J.T.; Janak, J.C.; Santos-Lozada, A.R.; McEvilla, S.; Ansley, S.D.; Walker, L.E.; Spiro, A.; Stewart, I.J. Telomere Shortening and Accelerated Aging in US Military Veterans. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Charatan, F. Nerve gas antidote a possible cause of gulf war illness. Bmj 1999, 319, 1154. [Google Scholar] [CrossRef]
- Mantle, D.; Golomb, B.A. Coenzyme Q10 and Xenobiotic Metabolism: An Overview. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Murray, J.; Oakley, L.; Lin, J.S.; Unger, E.R. Association of chronic fatigue syndrome with premature telomere attrition. J Transl Med 2018, 16, 44. [Google Scholar] [CrossRef]
- Hassett, A.L.; Epel, E.; Clauw, D.J.; Harris, R.E.; Harte, S.E.; Kairys, A.; Buyske, S.; Williams, D.A. Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain 2012, 13, 959–969. [Google Scholar] [CrossRef]
- Dos Reis, G.G.; Silvestre, R.T.; Alves, G.; Delmonico, L.; Chantre-Justino, M.; Moreira, A.D.S.; Müller, B.L.A.; do Nascimento, C.R.; da Silva, D.L.P.; Dos Santos, L.S. , et al. Leukocyte telomere length and telomerase activity in Long COVID patients from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2025, 120, e240129. [Google Scholar] [CrossRef]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A. , et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Godderis, L.; Martens, D.S.; Patil, M.S.; Hendrix, J.; Wyns, A.; Van Campenhout, J.; Richter, E.; Fanning, L.; Vandekerckhove, O. , et al. Exploring DNA methylation, telomere length, mitochondrial DNA, and immune function in patients with Long-COVID. BMC Med 2025, 23, 60. [Google Scholar] [CrossRef]
- Hetta, H.F.; Elsaghir, A.; Sijercic, V.C.; Ahmed, A.K.; Gad, S.A.; Zeleke, M.S.; Alanazi, F.E.; Ramadan, Y.N. Clinical Progress in Mesenchymal Stem Cell Therapy: A Focus on Rheumatic Diseases. Immun Inflamm Dis 2025, 13, e70189. [Google Scholar] [CrossRef]
- Safina, I.; Embree, M.C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater 2022, 143, 26–38. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Z.; Pan, M.; Zhang, L. Stem cell-derived mitochondria transplantation: A promising therapy for mitochondrial encephalomyopathy. CNS Neurosci Ther 2021, 27, 733–742. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Fei, X.; Yi, X.; Li, W.; Wang, Q. The miR-29b-Sirt1 axis regulates self-renewal of mouse embryonic stem cells in response to reactive oxygen species. Cell Signal 2014, 26, 1500–1505. [Google Scholar] [CrossRef]
- Mahrouf-Yorgov, M.; Augeul, L.; Da Silva, C.C.; Jourdan, M.; Rigolet, M.; Manin, S.; Ferrera, R.; Ovize, M.; Henry, A.; Guguin, A. , et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ 2017, 24, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Ennis, W.J.; Sui, A.; Bartholomew, A. Stem Cells and Healing: Impact on Inflammation. Adv Wound Care (New Rochelle) 2013, 2, 369–378. [Google Scholar] [CrossRef]
- Rebelatto, C.L.K.; Boldrini-Leite, L.M.; Daga, D.R.; Marsaro, D.B.; Vaz, I.M.; Jamur, V.R.; de Aguiar, A.M.; Vieira, T.B.; Furman, B.P.; Aguiar, C.O. , et al. Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z. , et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.Q.; Song, L.; Wang, Z.R.; Zhang, Z.Y.; Shi, M.; He, J.; Mo, Q.; Zheng, N.; Yao, W.Q.; Zhang, Y. , et al. Long-term outcomes of mesenchymal stem cell therapy in severe COVID-19 patients: 3-year follow-up of a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther 2025, 16, 94. [Google Scholar] [CrossRef]
- Mokhemer, S.A.; Desouky, M.K.; Abdelghany, A.K.; Ibrahim, M.F.G. Stem cells therapeutic effect in a reserpine-induced fibromyalgia rat model: A possible NLRP3 inflammasome modulation with neurogenesis promotion in the cerebral cortex. Life Sci 2023, 325, 121784. [Google Scholar] [CrossRef]
- Golomb, B.A.; Allison, M.; Koperski, S.; Koslik, H.J.; Devaraj, S.; Ritchie, J.B. Coenzyme Q10 benefits symptoms in Gulf War veterans: results of a randomized double-blind study. Neural Comput 2014, 26, 2594–2651. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Segundo, M.J.; Lacasa, M.; Martinez-Martinez, A.; Sentañes, R.S.; Alegre-Martin, J. Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U. , et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina (Kaunas) 2021, 57. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal 2013, 19, 1356–1361. [Google Scholar] [CrossRef]
- Sawaddiruk, P.; Apaijai, N.; Paiboonworachat, S.; Kaewchur, T.; Kasitanon, N.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, N.; Chattipakorn, S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic Res 2019, 53, 901–909. [Google Scholar] [CrossRef]
- Barletta, M.A.; Marino, G.; Spagnolo, B.; Bianchi, F.P.; Falappone, P.C.F.; Spagnolo, L.; Gatti, P. Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med 2023, 23, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Mogensen, T.H.; Agergaard, J.; Schiøttz-Christensen, B.; Østergaard, L.; Vibholm, L.K.; Leth, S. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur 2023, 24, 100539. [Google Scholar] [CrossRef]
- Opstad, T.B.; Alexander, J.; Aaseth, J.O.; Larsson, A.; Seljeflot, I.; Alehagen, U. Selenium and Coenzyme Q(10) Intervention Prevents Telomere Attrition, with Association to Reduced Cardiovascular Mortality-Sub-Study of a Randomized Clinical Trial. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, B.; Yu, S.; Zhang, C.; Wang, B.; Wang, Y.; Wang, J.; Yuan, Z.; Zhang, L.; Pan, J. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid Med Cell Longev 2015, 2015, 867293. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Ding, J.; Gu, H.; Wang, Q.; Wang, Y.; Zhang, D.; Gao, C. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact Mater 2023, 19, 550–568. [Google Scholar] [CrossRef]
- Li, X.; Zhan, J.; Hou, Y.; Hou, Y.; Chen, S.; Luo, D.; Luan, J.; Wang, L.; Lin, D. Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H(2)O(2) Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury. Oxid Med Cell Longev 2019, 2019, 6493081. [Google Scholar] [CrossRef]
- Saka, O.M.; Dora, D.D.; Kibar, G.; Tevlek, A. Expanding the role of exosomes in drug, biomolecule, and nanoparticle delivery. Life Sci 2025, 368, 123499. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Han, G.; Liang, M.; Yang, Z.; Zhang, C.; Huang, S.; Tai, S.; Yu, S. The Therapeutic Potential and Clinical Significance of Exosomes as Carriers of Drug Delivery System. Pharmaceutics 2022, 15. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Amini, R.; Soleimani Asl, S.; Saidijam, M.; Hashemi, S.M.; Najafi, R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed Pharmacother 2022, 152, 113224. [Google Scholar] [CrossRef]
- Sun, J.; Yang, F.; Wang, L.; Yu, H.; Yang, Z.; Wei, J.; Vasilev, K.; Zhang, X.; Liu, X.; Zhao, Y. Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration. Bioact Mater 2023, 23, 247–260. [Google Scholar] [CrossRef]
- Maruo, Y.; Shiraishi, M.; Hibino, M.; Abe, J.; Takeda, A.; Yamada, Y. Activation of Mitochondria in Mesenchymal Stem Cells by Mitochondrial Delivery of Coenzyme Q(10). Biol Pharm Bull 2024, 47, 1415–1421. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, C.; Wang, J.; Wang, S.; Hu, L. Dysfunction of metabolic activity of bone marrow mesenchymal stem cells in aged mice. Cell Prolif 2022, 55, e13191. [Google Scholar] [CrossRef]
- Park, J.; Park, H.H.; Choi, H.; Kim, Y.S.; Yu, H.J.; Lee, K.Y.; Lee, Y.J.; Kim, S.H.; Koh, S.H. Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res 2012, 1478, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, H.H.; Lee, K.Y.; Choi, N.Y.; Yu, H.J.; Lee, Y.J.; Park, J.; Huh, Y.M.; Lee, S.H.; Koh, S.H. Coenzyme Q10 restores amyloid beta-inhibited proliferation of neural stem cells by activating the PI3K pathway. Stem Cells Dev 2013, 22, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, M.J.; Joo, B.S.; Joo, J.K.; Kim, Y.H.; Yang, S.W.; Kim, C.W.; Kim, K.H. Effects of coenzyme Q10 on ovarian surface epithelium-derived ovarian stem cells and ovarian function in a 4-vinylcyclohexene diepoxide-induced murine model of ovarian failure. Reprod Biol Endocrinol 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Velichkovska, M.; Surnar, B.; Nair, M.; Dhar, S.; Toborek, M. Targeted Mitochondrial COQ(10) Delivery Attenuates Antiretroviral-Drug-Induced Senescence of Neural Progenitor Cells. Mol Pharm 2019, 16, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Jiang, J.; Zhang, Y.; Luo, Y.; Zhao, J.; Xu, J.; Xie, Y.; Liao, W.; Wang, W. , et al. CoQ10 enhances the efficacy of airway basal stem cell transplantation on bleomycin-induced idiopathic pulmonary fibrosis in mice. Respir Res 2022, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, O.R.; Juárez-Navarro, K.J.; Diaz, N.F.; Padilla-Camberos, E.; Beltran-Garcia, M.J.; Cardenas-Castrejon, D.; Corona-Perez, H.; Hernández-Jiménez, C.; Díaz-Martínez, N.E. Biomolecules resveratrol + coenzyme Q10 recover the cell state of human mesenchymal stem cells after 1-methyl-4-phenylpyridinium-induced damage and improve proliferation and neural differentiation. Front Neurosci 2022, 16, 929590. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, X.; Ouyang, L.; Chen, W.; Zhang, L.; Cao, Y. Antioxidants Improve the Proliferation and Efficacy of hUC-MSCs against H(2)O(2)-Induced Senescence. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
| Study refs. | Models | Outcomes |
| Park et al. (2012) [183] | Neural stem cells (rat) | Improved cell viability and intracellular signaling proteins during hypoxia-reperfusion |
| Choi et al. (2013) [184] | Neural stem cells (mouse) | CoQ10 restored amyloid beta-inhibited proliferation by activating the PI3K pathway |
| Zhang et al. (2015) [174] | Mesenchymal stem cells (rat) | Inhibition of oxidative stress and cell aging induced by D-galactose |
| Lee et al. (2021) [185] | Ovarian stem cells (mouse) | Improved stem cell function in vinylcyclohexene-diepoxide induced model of ovarian failure |
| Velichkovska et al. (2019) [186] |
Neural progenitor cells (mouse) | Mitochondrial dysfunction induced by anti-retroviral drugs (tenofovir and emtricitabine) improved |
| Liu et al. (2022) [187] | Airway basal stem cells (human) | Reduced oxidative stress induced by hydrogen peroxide; improved efficiency of transplanted cells in bleomycin-induced model of pulmonary fibrosis |
| Sun et al. (2023) [180] | Mesenchymal stem cells (rat) | Improved efficiency of transplanted cells in rat model of intervertebral disc degeneration |
| Hernández-Perez et al. (2022) [188] | Mesenchymal stem cells (human) | Reduced oxidative stress, improved cell viability and proliferation following exposure to MPP+ |
| Zheng et al. (2023) [189] | Umbilical cord mesenchymal stem cells (human) | Reduced oxidative stress induced by hydrogen peroxide, cell senescence reduced and proliferation capacity improved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





