Submitted:
20 August 2025
Posted:
21 August 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Fibrinaloid Microclot Complexes
Results and Analysis
Comorbidities of Tinnitus and Fibrinaloid Microclot Measurements
Tinnitus, Hearing Loss, Ageing and Endothelial Senescence/Dysfunction
Ototoxic Drugs
Role of Anticoagulants in Treating Tinnitus
Role of Fibrinolytic Enzymes in Treating Tinnitus
Role of Antioxidants in Treating Tinnitus
Dosing
Consonance of Therapeutics Against Microclots
Discussion
- Every disease in which microclots have been measured experimentally demonstrates a comorbidity with tinnitus, making it hard not to suppose that the microclots are causative of each
- There is extensive evidence that ototoxic drugs such as cisplatin can induce clotting
- Activation of Nrf2 and its antioxidant response elements is protective against orotoxicity
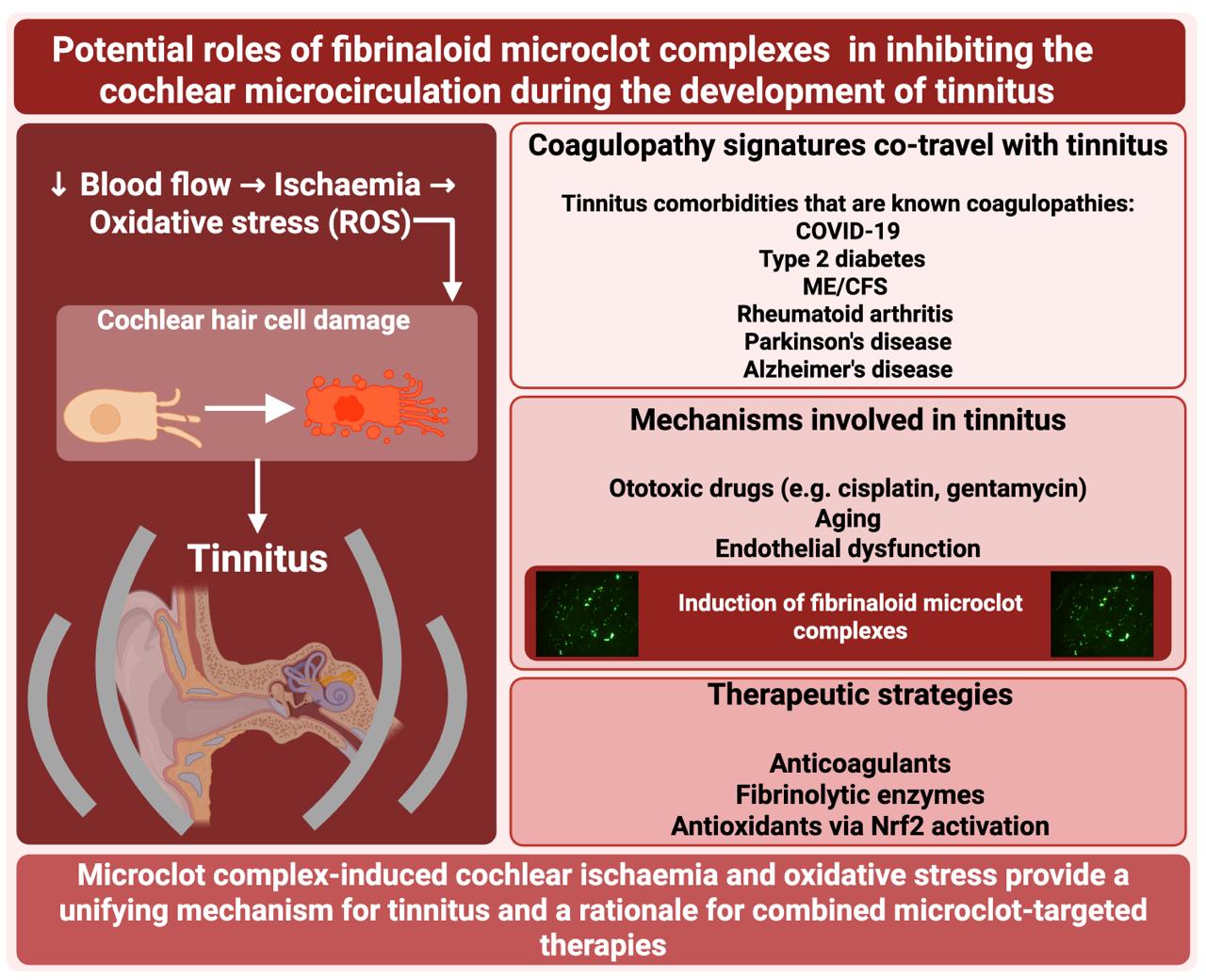
- Microclots provide a straightforward mechanistic explanation for tinnitus by decreasing the cochlear microcirculation
- Stenting also improves tinnitus (by increasing blood flow)
- Three other therapies designed to obviate microclots or their effects have shown promise when applied singly, namely the use of various anticoagulants, the use of fibrinolytic enzymes, and the use of antioxidants
- The transcription factor Nrf2 seems to play an important role.
Conclusions and Prospects
- Fibrinaloid microclot complexes measurements, using fluorescence microscopy or flow clotometry, on platelet-poor plasma of individuals with tinnitus, and an assessment of their relationship to disease severity.
- Assessment of whether known ototoxic drugs can induce fibrinaloid microclot complexes
- In vivo imaging of the cochlear and more general microcirculation of those with tinnitus and controls, using methods [604] such as endoscopy [605], fluorescence microscopy [30,606,607,608], two-photon microscopy [609], and optical microangiography [84,610,611], and especially laser speckle contrast imaging and laser Doppler optical microangiography [99,612,613,614,615,616,617,618,619,620,621,622,623,624,625]; many of these have already demonstrated a lowered blood flow accompanying hearing loss. There is also a role for the more widely (and financially) accessible methods of capillaroscopy [98].
Author Contributions
Funding
Conflicts of Interest
References
- McCormack, A., Edmondson-Jones, M., Somerset, S. and Hall, D. (2016) A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 337, 70-79. [CrossRef]
- Dawes, P., Newall, J., Stockdale, D. and Baguley, D. M. (2020) Natural history of tinnitus in adults: a cross-sectional and longitudinal analysis. BMJ Open. 10, e041290. [CrossRef]
- Nondahl, D. M., Cruickshanks, K. J., Huang, G. H., Klein, B. E., Klein, R., Nieto, F. J. and Tweed, T. S. (2011) Tinnitus and its risk factors in the Beaver Dam offspring study. Int J Audiol. 50, 313-320. [CrossRef]
- Baguley, D., McFerran, D. and Hall, D. (2013) Tinnitus. Lancet. 382, 1600-1607. [CrossRef]
- Tunkel, D. E., Bauer, C. A., Sun, G. H., Rosenfeld, R. M., Chandrasekhar, S. S., Cunningham, E. R., Jr., Archer, S. M., Blakley, B. W., Carter, J. M., Granieri, E. C., Henry, J. A., Hollingsworth, D., Khan, F. A., Mitchell, S., Monfared, A., Newman, C. W., Omole, F. S., Phillips, C. D., Robinson, S. K., Taw, M. B., Tyler, R. S., Waguespack, R. and Whamond, E. J. (2014) Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 151, S1-S40. [CrossRef]
- Harmon, E. D. and Goodman, M. L. (2025) Tinnitus, the phantom sound: A review of history and guidelines for care. Nurse Pract. 50, 18-25. [CrossRef]
- Jarach, C. M., Lugo, A., Scala, M., van den Brandt, P. A., Cederroth, C. R., Odone, A., Garavello, W., Schlee, W., Langguth, B. and Gallus, S. (2022) Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 79, 888-900. [CrossRef]
- Al-Lahham, S., Nazzal, Z., Massarweh, A., Saymeh, D., Al-Abed, S., Muhammad, D., Alawni, R., Bouzya, N., Alqub, M., Ghanim, M. and Ellahham, N. (2022) Prevalence and associated risk factors of tinnitus among adult Palestinians: a cross-sectional study. Sci Rep. 12, 20617. [CrossRef]
- de Gruy, J. A., Laurenzo, W. W., Vu, T. H., Paul, O., Lee, C. and Spankovich, C. (2025) Prevalence and predictors of problematic tinnitus. Int J Audiol. 64, 307-313. [CrossRef]
- Henry, J. A. (2016) "Measurement" of Tinnitus. Otol Neurotol. 37, e276-285. [CrossRef]
- Shargorodsky, J., Curhan, G. C. and Farwell, W. R. (2010) Prevalence and characteristics of tinnitus among US adults. Am J Med. 123, 711-718. [CrossRef]
- McFerran, D. J., Stockdale, D., Holme, R., Large, C. H. and Baguley, D. M. (2019) Why Is There No Cure for Tinnitus? Front Neurosci. 13, 802. [CrossRef]
- Ubbink, S. W. J., Hofman, R., van Dijk, P. and van Dijk, J. M. C. (2024) Sound Measurements in Pulsatile Tinnitus: A Review in 171 Patients. Otol Neurotol. 45, 1186-1191. [CrossRef]
- Beukes, E. W., Manchaiah, V., Allen, P. M., Andersson, G. and Baguley, D. M. (2021) Exploring tinnitus heterogeneity. Prog Brain Res. 260, 79-99. [CrossRef]
- Kang, Y. J. and Zheng, Y. (2024) Current understanding of subjective tinnitus in adults. Eur Arch Otorhinolaryngol. 281, 4507-4517. [CrossRef]
- Alvear, A. S., Limón, G. A., Reyes Martínez, L. M., Gómez, R. S. and Serrano Arias, F. E. (2025) Pulsatile Tinnitus: A Narrative Review. J Int Adv Otol. 21, 1-7. [CrossRef]
- Demoen, S., Cardon, E., Jacquemin, L., Timmermans, A., Van Rompaey, V., Gilles, A. and Michiels, S. (2024) Health-Related Quality of Life in Subjective, Chronic Tinnitus Patients: A Scoping Review. J Assoc Res Otolaryngol. 25, 103-129. [CrossRef]
- Narsinh, K. H., Hui, F., Saloner, D., Tu-Chan, A., Sharon, J., Rauschecker, A. M., Safoora, F., Shah, V., Meisel, K. and Amans, M. R. (2022) Diagnostic Approach to Pulsatile Tinnitus: A Narrative Review. JAMA Otolaryngol Head Neck Surg. 148, 476-483. [CrossRef]
- Tang, D., Li, H. and Chen, L. (2019) Advances in Understanding, Diagnosis, and Treatment of Tinnitus. Adv Exp Med Biol. 1130, 109-128. [CrossRef]
- Langguth, B., Elgoyhen, A. B. and Cederroth, C. R. (2019) Therapeutic Approaches to the Treatment of Tinnitus. Annu Rev Pharmacol Toxicol. 59, 291-313. [CrossRef]
- Chhaya, V., Patel, D., Shethia, F., Manchaiah, V. and Khambholja, K. (2023) Current Therapeutic Trends for Tinnitus Cure and Control: A Scoping Review. Indian J Otolaryngol Head Neck Surg. 75, 1-9. [CrossRef]
- Liu, D., Hu, Y., Wang, D., Han, H., Wang, Y., Wang, X., Zhou, Z., Ma, X. and Dong, Y. (2022) Herbal medicines in the treatment of tinnitus: An updated review. Front Pharmacol. 13, 1037528. [CrossRef]
- Li, P., Che, C., Wu, Y. and Sun, S. (2025) Pharmacotherapy options for the management of subjective tinnitus: a systematic review and network meta-analysis. BMJ Open. 15, e096995. [CrossRef]
- Kim, S. H., Kim, D., Lee, J. M., Lee, S. K., Kang, H. J. and Yeo, S. G. (2021) Review of Pharmacotherapy for Tinnitus. Healthcare (Basel). 9, 779. [CrossRef]
- Haider, H. F., Hoare, D. J., Ribeiro, S. F., Ribeiro, D., Caria, H., Trigueiros, N., Borrego, L. M., Szczepek, A. J., Papoila, A. L., Elarbed, A., da Luz Martins, M., Paço, J. and Sereda, M. (2021) Evidence for biological markers of tinnitus: A systematic review. Prog Brain Res. 262, 345-398. [CrossRef]
- Bulla, J., Brueggemann, P., Wrzosek, M., Klasing, S., Boecking, B., Basso, L., Nyamaa, A., Psatha, S., Rose, M. and Mazurek, B. (2023) Limited Link of Common Blood Parameters with Tinnitus. J Clin Med. 12, 3814. [CrossRef]
- Cederroth, C. R., Hong, M. G., Freydin, M. B., Edvall, N. K., Trpchevska, N., Jarach, C., Schlee, W., Schwenk, J. M., Lopez-Escamez, J. A., Gallus, S., Canlon, B., Bulla, J. and Williams, F. M. K. (2023) Screening for Circulating Inflammatory Proteins Does Not Reveal Plasma Biomarkers of Constant Tinnitus. J Assoc Res Otolaryngol. 24, 593-606. [CrossRef]
- Kang, D. W., Kim, S. S., Park, D. C., Kim, S. H. and Yeo, S. G. (2021) Objective and Measurable Biomarkers in Chronic Subjective Tinnitus. Int J Mol Sci. 22, 6619. [CrossRef]
- Szczepek, A. J., Haupt, H., Klapp, B. F., Olze, H. and Mazurek, B. (2014) Biological correlates of tinnitus-related distress: an exploratory study. Hear Res. 318, 23-30. [CrossRef]
- Arpornchayanon, W., Canis, M., Ihler, F., Settevendemie, C. and Strieth, S. (2013) TNF-alpha inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo. Int J Audiol. 52, 545-552. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev. 93, 1518-1557. [CrossRef]
- Neri, S., Signorelli, S., Pulvirenti, D., Mauceri, B., Cilio, D., Bordonaro, F., Abate, G., Interlandi, D., Misseri, M., Ignaccolo, L., Savastano, M., Azzolina, R., Grillo, C., Messina, A., Serra, A. and Tsami, A. (2006) Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic Res. 40, 615-618. [CrossRef]
- Celik, M. and Koyuncu, I. (2018) A Comprehensive Study of Oxidative Stress in Tinnitus Patients. Indian J Otolaryngol Head Neck Surg. 70, 521-526. [CrossRef]
- Ekinci, A. and Kamasak, K. (2020) Evaluation of serum prolidase enzyme activity and oxidative stress in patients with tinnitus. Braz J Otorhinolaryngol. 86, 405-410. [CrossRef]
- Yang, H., Xie, Y., Yu, J., Shi, M., Li, Y., Cai, Y., Cai, Q., Huang, F., Ye, Z., Wang, H. and Sun, Y. (2025) Nrf2 deficiency enhances oxidative stress and promotes susceptibility to tinnitus in mice. Sci Rep. 15, 16474. [CrossRef]
- Petridou, A. I., Zagora, E. T., Petridis, P., Korres, G. S., Gazouli, M., Xenelis, I., Kyrodimos, E., Kontothanasi, G. and Kaliora, A. C. (2019) The Effect of Antioxidant Supplementation in Patients with Tinnitus and Normal Hearing or Hearing Loss: A Randomized, Double-Blind, Placebo Controlled Trial. Nutrients. 11, 3037. [CrossRef]
- Savastano, M., Brescia, G. and Marioni, G. (2007) Antioxidant therapy in idiopathic tinnitus: preliminary outcomes. Arch Med Res. 38, 456-459. [CrossRef]
- Chen, J. J., Chen, Y. W., Zeng, B. Y., Hung, C. M., Zeng, B. S., Stubbs, B., Carvalho, A. F., Thompson, T., Roerecke, M., Su, K. P., Tu, Y. K., Wu, Y. C., Smith, L., Chen, T. Y., Lin, P. Y., Liang, C. S., Hsu, C. W., Hsu, S. P., Kuo, H. C., Wu, M. K. and Tseng, P. T. (2021) Efficacy of pharmacologic treatment in tinnitus patients without specific or treatable origin: A network meta-analysis of randomised controlled trials. EClinicalMedicine. 39, 101080. [CrossRef]
- Wu, Z., Zhu, Z., Cao, J., Wu, W., Hu, S., Deng, C., Xie, Q., Huang, X. and You, C. (2022) Prediction of network pharmacology and molecular docking-based strategy to determine potential pharmacological mechanism of Liuwei Dihuang pill against tinnitus. Medicine (Baltimore). 101, e31711. [CrossRef]
- Zhang, M., Wang, X., Zhang, S., He, X., Chen, X., Wang, L., Fu, L., Wang, H., Fu, Q., Jiang, Y., Li, X. and Zhang, Q. (2025) Association of 15 common dietary factors with tinnitus: a systematic review and meta-analysis of observational studies. BMJ Open. 15, e091507. [CrossRef]
- Chauhan, B., Arya, S. and Chauhan, K. (2023) Ginkgo biloba Administered Singly and Combined With Antioxidants in Tinnitus Patients. J Audiol Otol. 27, 37-44. [CrossRef]
- Gill, N. B., Dowker-Key, P. D., Hedrick, M. and Bettaieb, A. (2024) Unveiling the Role of Oxidative Stress in Cochlear Hair Cell Death: Prospective Phytochemical Therapeutics against Sensorineural Hearing Loss. Int J Mol Sci. 25, 4272. [CrossRef]
- Bath, P. M. and Butterworth, R. J. (1996) Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 7, 157-161.
- Bath, P., Algert, C., Chapman, N., Neal, B. and PROGRESS Collaborative Group. (2004) Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 35, 622-626. [CrossRef]
- Vizioli, L., Muscari, S. and Muscari, A. (2009) The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 63, 1509-1515. [CrossRef]
- Chu, S. G., Becker, R. C., Berger, P. B., Bhatt, D. L., Eikelboom, J. W., Konkle, B., Mohler, E. R., Reilly, M. P. and Berger, J. S. (2010) Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 8, 148-156. [CrossRef]
- Sansanayudh, N., Muntham, D., Yamwong, S., Sritara, P., Akrawichien, T. and Thakkinstian, A. (2016) The association between mean platelet volume and cardiovascular risk factors. Eur J Intern Med. 30, 37-42. [CrossRef]
- Barale, C., Melchionda, E., Morotti, A. and Russo, I. (2021) Prothrombotic Phenotype in COVID-19: Focus on Platelets. Int J Mol Sci. 22, 13638. [CrossRef]
- Bilir, C., Engin, H. and Bilir, F. (2013) Increased Mean Platelet Volume in Deep Vein Thrombosis Patients With Cancer. J Hematol. 2, 64-68. [CrossRef]
- Choi, D. H., Kang, S. H. and Song, H. (2016) Mean platelet volume: a potential biomarker of the risk and prognosis of heart disease. Korean J Intern Med. 31, 1009-1017. [CrossRef]
- Pafili, K., Penlioglou, T., Mikhailidis, D. P. and Papanas, N. (2019) Mean platelet volume and coronary artery disease. Curr Opin Cardiol. 34, 390-398. [CrossRef]
- Brækkan, S. K., Mathiesen, E. B., Njolstad, I., Wilsgaard, T., Stormer, J. and Hansen, J. B. (2010) Mean platelet volume is a risk factor for venous thromboembolism: the Tromsø Study. J Thromb Haemost. 8, 157-162. [CrossRef]
- Machin, S. J. and Briggs, C. (2010) Mean platelet volume: a quick, easy determinant of thrombotic risk? J Thromb Haemost. 8, 146-147. [CrossRef]
- Gasparyan, A. Y., Ayvazyan, L., Mikhailidis, D. P. and Kitas, G. D. (2011) Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 17, 47-58. [CrossRef]
- Ha, S. I., Choi, D. H., Ki, Y. J., Yang, J. S., Park, G., Chung, J. W., Koh, Y. Y., Chang, K. S. and Hong, S. P. (2011) Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets. 22, 408-414. [CrossRef]
- Karli, R., Alacam, H., Unal, R., Kucuk, H., Aksoy, A. and Ayhan, E. (2013) Mean platelet volume: is it a predictive parameter in the diagnosis of sudden sensorineural hearing loss? Indian J Otolaryngol Head Neck Surg. 65, 350-353. [CrossRef]
- Liang, Q. C., Jin, D., Li, Y. and Wang, R. T. (2014) Mean platelet volume and platelet distribution width in vascular dementia and Alzheimer's disease. Platelets. 25, 433-438. [CrossRef]
- Lai, H. M., Xu, R., Yang, Y. N., Ma, Y. T., Li, X. M., Zhao, Q., Chen, Q. J., Zhai, H., Liu, F. and Chen, B. D. (2015) Association of mean platelet volume with angiographic thrombus burden and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Catheter Cardiovasc Interv. 85 Suppl 1, 724-733. [CrossRef]
- Xu, X. F., Jiang, F. L., Ou, M. J. and Zhang, Z. H. (2015) The association between mean platelet volume and chronic atrial fibrillation and the presence of thrombotic events. Biomed Rep. 3, 388-394. [CrossRef]
- Kovács, S., Csiki, Z., Zsóri, K. S., Bereczky, Z. and Shemirani, A. H. (2019) Characteristics of platelet count and size and diagnostic accuracy of mean platelet volume in patients with venous thromboembolism. A systematic review and meta-analysis. Platelets. 30, 139-147. [CrossRef]
- Sadeghi, F., Kovács, S., Zsóri, K. S., Csiki, Z., Bereczky, Z. and Shemirani, A. H. (2020) Platelet count and mean volume in acute stroke: a systematic review and meta-analysis. Platelets. 31, 731-739. [CrossRef]
- Edvardsen, M. S., Hansen, E. S., Hindberg, K., Morelli, V. M., Ueland, T., Aukrust, P., Brækkan, S. K., Evensen, L. H. and Hansen, J. B. (2021) Combined effects of plasma von Willebrand factor and platelet measures on the risk of incident venous thromboembolism. Blood. 138, 2269-2277. [CrossRef]
- Lin, W., Wu, Y., Lu, X. and Hu, Y. (2021) Association between mean platelet volume and pulmonary embolism: a systematic review and meta-analysis. Aging (Albany NY). 13, 17253-17273. [CrossRef]
- Mi, A. E., Abdallah, N. and Eldars, W. (2021) Mean Platelet Volume and Platelet Distribution Width Correlate with Microvascular Complications in Egyptian People with Type 2 Diabetes Mellitus. Curr Diabetes Rev. 17, e080621193947. [CrossRef]
- Ot, S., Zafar, L., Beg, M. and Siddiqui, O. A. (2021) Association of Mean Platelet Volume with Risk Factors and Functional Outcome in Acute Ischemic Stroke. J Neurosci Rural Pract. 12, 764-769. [CrossRef]
- Kallel, S., Kchaou, K., Kharrat, I., Chaabouni, M. A., Ayedi, S. and Charfeddine, I. (2023) Association between mean platelet volume and cardiovascular disease in obstructive sleep apnea syndrome. SAGE Open Med. 11, 20503121231181634. [CrossRef]
- Rupa-Matysek, J., Gil, L., Wojtasińska, E., Ciepłuch, K., Lewandowska, M. and Komarnicki, M. (2014) The relationship between mean platelet volume and thrombosis recurrence in patients diagnosed with antiphospholipid syndrome. Rheumatol Int. 34, 1599-1605. [CrossRef]
- Rupa-Matysek, J., Gil, L., Kroll-Balcerzak, R., Barańska, M. and Komarnicki, M. (2017) Mean platelet volume as a predictive marker for venous thromboembolism and mortality in patients treated for diffuse large B-cell lymphoma. Hematol Oncol. 35, 456-464. [CrossRef]
- Zheng, M., Chen, S., Zhu, Y. and Gu, X. (2020) Mean platelet volume: a new predictor of ischaemic stroke risk in patients with nonvalvular atrial fibrillation. BMC Cardiovasc Disord. 20, 241. [CrossRef]
- Verdoia, M., Camaro, C., Barbieri, L., Schaffer, A., Marino, P., Bellomo, G., Suryapranata, H. and De Luca, G. (2013) Mean platelet volume and the risk of periprocedural myocardial infarction in patients undergoing coronary angioplasty. Atherosclerosis. 228, 136-141. [CrossRef]
- Kemal, O., Müderris, T., Başar, F., Kutlar, G. and Gül, F. (2016) Prognostic value of mean platelet volume on tinnitus. J Laryngol Otol. 130, 162-165. [CrossRef]
- Yüksel, F. and Karataş, D. (2016) Can Platelet Indices Be New Biomarkers for Subjective Tinnitus? J Craniofac Surg. 27, e420-424. [CrossRef]
- Ulusoy, B., Bozdemir, K., Akyol, M., Mişe, H. I., Kutluhan, A. and Korkmaz, M. H. (2018) Investigation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and mean platelet volume in patients with tinnitus. J Laryngol Otol. 132, 129-132. [CrossRef]
- Sarıkaya, Y., Bayraktar, C., Karataş, M., Doğan, S., Olt, S., Kaskalan, E. and Türkbeyler, I. H. (2016) Increased mean platelet volume in patients with idiopathic subjective tinnitus. Eur Arch Otorhinolaryngol. 273, 3533-3536. [CrossRef]
- Yildiz, S., Karaca, H. and Toros, S. Z. (2022) Mean platelet volume and neutrophil to lymphocyte ratio in patients with tinnitus: a case-control study. Braz J Otorhinolaryngol. 88, 155-160. [CrossRef]
- Ahmed, R., Shadis, A. and Ahmed, R. (2022) Potential inflammatory biomarkers for tinnitus in platelets and leukocytes: a critical scoping review and meta-analysis. Int J Audiol. 61, 905-916. [CrossRef]
- De Ridder, D., Vanneste, S., Adriaenssens, I., Lee, A. P., Plazier, M., Menovsky, T., van der Loo, E., Van de Heyning, P. and Moller, A. (2010) Microvascular decompression for tinnitus: significant improvement for tinnitus intensity without improvement for distress. A 4-year limit. Neurosurgery. 66, 656-660. [CrossRef]
- Donofrio, C. A., Riccio, L., Badaloni, F., Rosellini, E., Servadei, F. and Fioravanti, A. (2023) Endoscope-Assisted Microvascular Decompression of Cochleo-Vestibular Nerve for Treating Unilateral Pulsatile Tinnitus, Sensorineural Hearing Loss, and Paroxysmal Vertigo: 2-Dimensional Operative Video. Oper Neurosurg. 25, e277-e278. [CrossRef]
- Guevara, N., Deveze, A., Buza, V., Laffont, B. and Magnan, J. (2008) Microvascular decompression of cochlear nerve for tinnitus incapacity: pre-surgical data, surgical analyses and long-term follow-up of 15 patients. Eur Arch Otorhinolaryngol. 265, 397-401. [CrossRef]
- Ko, Y. and Park, C. W. (1997) Microvascular decompression for tinnitus. Stereotact Funct Neurosurg. 68, 266-269. [CrossRef]
- Nash, B., Carlson, M. L. and Van Gompel, J. J. (2017) Microvascular decompression for tinnitus: systematic review. J Neurosurg. 126, 1148-1157. [CrossRef]
- van den Berge, M. J. C., van Dijk, J. M. C., Posthumus, I. A., Smidt, N., van Dijk, P. and Free, R. H. (2017) Microvascular decompression of the cochleovestibular nerve for treatment of tinnitus and vertigo: a systematic review and meta-analysis of individual patient data. J Neurosurg. 127, 588-601. [CrossRef]
- Zhang, L., Yu, Y., Yuan, Y., Xu, J., Xu, X. and Zhang, J. (2012) Microvascular decompression of cochleovestibular nerve in patients with tinnitus and vertigo. Neurol India. 60, 495-497. [CrossRef]
- Shi, X. (2011) Physiopathology of the cochlear microcirculation. Hear Res. 282, 10-24. [CrossRef]
- Lin, C. D., Wei, I. H., Tsai, M. H., Kao, M. C., Lai, C. H., Hsu, C. J., Oshima, T. and Tsai, M. H. (2010) Changes in guinea pig cochlea after transient cochlear ischemia. Neuroreport. 21, 968-975. [CrossRef]
- Nuttall, A. L. (1999) Sound-Induced Cochlear Ischemia/Hypoxia as a Mechanism of Hearing Loss. Noise Health. 2, 17-32.
- Maetani, T., Hakuba, N., Taniguchi, M., Hyodo, J., Shimizu, Y. and Gyo, K. (2003) Free radical scavenger protects against inner hair cell loss after cochlear ischemia. Neuroreport. 14, 1881-1884. [CrossRef]
- Maetani, T., Hyodo, J., Takeda, S., Hakuba, N. and Kiyofumi, G. (2009) Prednisolone prevents transient ischemia-induced cochlear damage in gerbils. Acta Otolaryngol Suppl, 24-27. [CrossRef]
- Schweinfurth, J. M. and Cacace, A. T. (2000) Cochlear ischemia induced by circulating iron particles under magnetic control: An animal model for sudden hearing loss. Am J Otol. 21, 636-640.
- Tsuji, S., Tabuchi, K., Hara, A. and Kusakari, J. (2002) Long-term observations on the reversibility of cochlear dysfunction after transient ischemia. Hear Res. 166, 72-81. [CrossRef]
- Tabuchi, K., Nishimura, B., Tanaka, S., Hayashi, K., Hirose, Y. and Hara, A. (2010) Ischemia-reperfusion injury of the cochlea: pharmacological strategies for cochlear protection and implications of glutamate and reactive oxygen species. Curr Neuropharmacol. 8, 128-134. [CrossRef]
- Gyo, K. (2013) Experimental study of transient cochlear ischemia as a cause of sudden deafness. World J Otorhinolaryngol. 3, 1-15. [CrossRef]
- Okada, M., Kawaguchi, A. T., Hakuba, N., Hyodo, J., Hato, N. and Gyo, K. (2013) Liposome-encapsulated hemoglobin alleviates hearing loss after transient cochlear ischemia: An experimental study in the gerbil. Neurosci Lett. 553, 176-180. [CrossRef]
- Tsetsos, N., Poutoglidis, A., Vlachtsis, K., Kilmpasanis, A. and Gougousis, S. (2021) Sudden Sensorineural Hearing Loss Following the Second Dose of COVID-19 Vaccine. Cureus. 13, e17435. [CrossRef]
- Skare, T. L., de Carvalho, J. F., de Medeiros, I. R. T. and Shoenfeld, Y. (2024) Ear abnormalities in chronic fatigue syndrome (CFS), fibromyalgia (FM), Coronavirus-19 infectious disease (COVID) and long-COVID syndrome (PCS), sick-building syndrome (SBS), post-orthostatic tachycardia syndrome (PoTS), and autoimmune/inflammatory syndrome induced by adjuvants (ASIA): A systematic review. Autoimmun Rev. 23, 103606. [CrossRef]
- Buckey, J. C. (2019) Use of Gases to Treat Cochlear Conditions. Front Cell Neurosci. 13, 155. [CrossRef]
- Shi, F., Ye, Z., Zha, B., Wu, W., Zhang, Y., Yu, L., Liu, W., Rong, Y. and Yang, J. (2025) Recent advances on the mechanism of acupuncture in the treatment of subjective tinnitus. Front Syst Neurosci. 19, 1523761. [CrossRef]
- Kell, D. B. and Pretorius, E. (2025) On the utility of nailfold capillaroscopy in detecting the effects of fibrinaloid microclots in diseases involving blood stasis. Preprints, 202505.202356/v202501. [CrossRef]
- Kell, D. B., Zhao, H. and Pretorius, E. (2025) Assessment of the impacts of fibrinaloid microclots on the microcirculation and endothelial function, using laser speckle and laser Doppler imaging. Preprints, 2025062239. [CrossRef]
- Henry, J. A., Roberts, L. E., Caspary, D. M., Theodoroff, S. M. and Salvi, R. J. (2014) Underlying mechanisms of tinnitus: review and clinical implications. J Am Acad Audiol. 25, 5-22; quiz 126. [CrossRef]
- Jastreboff, P. J. and Hazell, J. W. (1993) A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 27, 7-17. [CrossRef]
- Shore, S. E., Roberts, L. E. and Langguth, B. (2016) Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol. 12, 150-160. [CrossRef]
- Shore, S. E. and Wu, C. (2019) Mechanisms of Noise-Induced Tinnitus: Insights from Cellular Studies. Neuron. 103, 8-20. [CrossRef]
- Capaccio, P., Ottaviani, F., Cuccarini, V., Bottero, A., Schindler, A., Cesana, B. M., Censuales, S. and Pignataro, L. (2007) Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 117, 547-551. [CrossRef]
- Suckfüll, M. (2009) Perspectives on the pathophysiology and treatment of sudden idiopathic sensorineural hearing loss. Dtsch Ärztebl Int. 106, 669-675; quiz 676. [CrossRef]
- Goldwin, B., Khan, M. J., Shivapuja, B., Seidman, M. D. and Quirk, W. S. (1998) Sarthran preserves cochlear microcirculation and reduces temporary threshold shifts after noise exposure. Otolaryngol Head Neck Surg. 118, 576-583. [CrossRef]
- Cheng, Y. F., Xirasagar, S., Yang, T. H., Wu, C. S., Kao, Y. W., Shia, B. C. and Lin, H. C. (2020) Increased risk of tinnitus following a trigeminal neuralgia diagnosis: a one-year follow-up study. J Headache Pain. 21, 46. [CrossRef]
- Seidman, M. D., Quirk, W. S. and Shirwany, N. A. (1999) Mechanisms of alterations in the microcirculation of the cochlea. Ann N Y Acad Sci. 884, 226-232. [CrossRef]
- Borghi, C., Cosentino, E. R., Rinaldi, E. R., Brandolini, C., Rimondi, M. C., Veronesi, M., Cicero, A. F. G., Dormi, A. and Pirodda, A. (2011) Tinnitus in elderly patients and prognosis of mild-to-moderate congestive heart failure: a cross-sectional study with a long-term extension of the clinical follow-up. BMC Med. 9, 80. [CrossRef]
- Figueiredo, R. R., de Azevedo, A. A. and Penido Nde, O. (2015) Tinnitus and arterial hypertension: a systematic review. Eur Arch Otorhinolaryngol. 272, 3089-3094. [CrossRef]
- Yamada, S., Kita, J., Shinmura, D., Nakamura, Y., Sahara, S., Misawa, K. and Nakanishi, H. (2022) Update on Findings about Sudden Sensorineural Hearing Loss and Insight into Its Pathogenesis. J Clin Med. 11, 6387. [CrossRef]
- Huang, Y. S., Koo, M., Chen, J. C. and Hwang, J. H. (2017) The association between tinnitus and the risk of ischemic cerebrovascular disease in young and middle-aged patients: A secondary case-control analysis of a nationwide, population-based health claims database. PLoS One. 12, e0187474. [CrossRef]
- Bayraktar, C. and Taşolar, S. (2017) Relationship between increased carotid artery stiffness and idiopathic subjective tinnitus. Eur Arch Otorhinolaryngol. 274, 2125-2130. [CrossRef]
- Abdelhaliem, A., Howard, C., Bashir, M., Elsantawy, H. and Al-Khaffaf, H. (2020) Correlation of carotid artery disease and tinnitus: is it an auditory phantom in vascular surgery practice? A wide evidence-based review. Vessel Plus. 4, 31. [CrossRef]
- Gedikli, Ö., Kemal, O., Yıldırım, U., Çeçen, A. B., Karabulut, H., Akcay, M. and Terzi, O. (2020) Is there an association between the parameters of arterial stiffness and tinnitus? Acta Otolaryngol. 140, 128-132. [CrossRef]
- Aytac, I., Yazici, A., Tunc, O., Gul, R., Inanc, Y. and Tumuklu, K. (2025) Association Between Vertebral Artery Stiffness and Idiopathic Subjective Tinnitus: A Prospective Study. Appl Sci. 15, 7890. [CrossRef]
- Ihn, Y. K., Jung, W. S. and Kim, B. S. (2013) Disappeared pulsatile tinnitus related to petrous segment stenosis of the ICA after relief of the stenosis by stenting. Interv Neuroradiol. 19, 97-101. [CrossRef]
- Cowley, P. O., Jones, R., Tuch, P. and McAuliffe, W. (2009) Pulsatile tinnitus from reversal of flow in an aberrant occipital artery: resolved after carotid artery stenting. AJNR Am J Neuroradiol. 30, 995-997. [CrossRef]
- Fiani, B., Kondilis, A., Doan, T., Runnels, J., Fiani, N. J. and Sarno, E. (2021) Venous sinus stenting for intractable pulsatile tinnitus: A review of indications and outcomes. Surg Neurol Int. 12, 81. [CrossRef]
- Patsalides, A., Santillan, A., Sundararajan, S. H., Michael, M., Suurna, M. and Alexiades, G. (2021) Venous sinus stenting for the treatment of isolated pulsatile tinnitus: Results of a prospective trial. Interv Neuroradiol. 27, 266-274. [CrossRef]
- Cummins, D. D., Caton, M. T., Hemphill, K., Lamboy, A., Tu-Chan, A., Meisel, K., Narsinh, K. H. and Amans, M. R. (2023) Cerebrovascular pulsatile tinnitus: causes, treatments, and outcomes in 164 patients with neuroangiographic correlation. J Neurointerv Surg. 15, 1014-1020. [CrossRef]
- Guédon, A., Checkouri, T., Fantoni, M., Civelli, V., Labeyrie, M. A., Saint-Maurice, J. P., Vallee, F. and Houdart, E. (2023) Blood Flow Velocity: a Decision Tool for Stenting Indication in Venous Pulsatile Tinnitus. Clin Neuroradiol. 33, 729-737. [CrossRef]
- Schartz, D., Finkelstein, A., Akkipeddi, S. M. K., Williams, Z., Vates, E. and Bender, M. T. (2024) Outcomes of Pulsatile Tinnitus After Cerebral Venous Sinus Stenting: Systematic Review and Pooled Analysis of 616 Patients. World Neurosurg. 190, e992-e999. [CrossRef]
- Su, H., Huo, Y., Li, B., Lv, B., Zhang, R., Du, Z., Liu, X., Wang, J., Chen, X. and Cao, X. (2025) Long-Term Outcomes of Stenting for Pulsatile Tinnitus Caused by Cerebral Venous Sinus Stenosis. Otol Neurotol. 46, 112-117. [CrossRef]
- Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. J. and Kell, D. B. (2016) Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface. 123, 20160539. [CrossRef]
- Pretorius, E., Page, M. J., Hendricks, L., Nkosi, N. B., Benson, S. R. and Kell, D. B. (2018) Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface. 15, 20170941. [CrossRef]
- Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J. and Kell, D. B. (2021) Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 20, 172. [CrossRef]
- Pretorius, E., Venter, C., Laubscher, G. J., Kotze, M. J., Oladejo, S., Watson, L. R., Rajaratnam, K., Watson, B. W. and Kell, D. B. (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 21, 148. [CrossRef]
- Turner, S., Khan, M. A., Putrino, D., Woodcock, A., Kell, D. B. and Pretorius, E. (2023) Long COVID: pathophysiological factors and abnormal coagulation. Trends Endocrinol Metab. 34, 321-344. [CrossRef]
- Dalton, C. F., de Oliveira, M. I. R., Stafford, P., Peake, N., Kane, B., Higham, A., Singh, D., Jackson, N., Davies, H., Price, D., Duncan, R., Tattersall, N., Barnes, A. and Smith, D. P. (2024) Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv, 2024.2004.2004.24305318. [CrossRef]
- Kell, D. B., Khan, M. A. and Pretorius, E. (2024) Fibrinaloid microclots in Long COVID: assessing the actual evidence properly. Res Pract Thromb Haemost. 8, 102566. [CrossRef]
- Kruger, A., Joffe, D., Lloyd-Jones, G., Khan, M. A., Šalamon, Š., Laubscher, G. J., Putrino, D., Kell, D. B. and Pretorius, E. (2025) Vascular pathogenesis in acute and long covid: current insights and therapeutic outlook Semin Throm Hemost. 51, 256-271. [CrossRef]
- Pretorius, E., Nunes, M., Pretorius, J. and Kell, D. B. (2024) Flow clotometry: measuring amyloid microclots in ME/CFS, long COVID, and healthy samples with imaging flow cytometry. Research Square. https://www.researchsquare.com/article/rs-4507472/v4507471. [CrossRef]
- Pretorius, E., Thierry, A., Sanchez, C., Ha, T., Pastor, B., Mirandola, A., Pisareva, E., Prevostel, C., Laubscher, G., Usher, T., Venter, C., Turner, S., Waters, M. and Kell, D. B. (2024) Circulating microclots are structurally associated with Neutrophil Extracellular Traps and their amounts are strongly elevated in long COVID patients. Res Square https://www.researchsquare.com/article/rs-4666650/v4666651. [CrossRef]
- Thomas, C., Nunes, M., Pretorius, J. H., Ashton, R. E., Shawa, I. T., Bewick, T., Pretorius, E., Kell, D. B. and Faghy, M. A. (2025) Exercise-induced changes in microclotting and cytokine levels point to vascular injury and inflammation in people with Long COVID. ResearchSquare. https://www.researchsquare.com/article/rs-6717727/v6717721. [CrossRef]
- Turner, S., Naidoo, C. A., Usher, T. J., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. and Pretorius, E. (2024) Increased levels of inflammatory and endothelial biomarkers in blood of long COVID patients point to thrombotic endothelialitis. Semin Thromb Hemost. 50, 288-294. [CrossRef]
- Venter, C., Pretorius, J. H., Kell, D. B. and Pretorius, E. (2025) A novel MetaCyte Metafer Classifier for Platelet Morphology Using Long COVID as a Model. J Thromb Thrombolysis, online. [CrossRef]
- Kell, D. B., Laubscher, G. J. and Pretorius, E. (2022) A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 479, 537-559. [CrossRef]
- Kell, D. B. and Pretorius, E. (2022) The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. 479, 1653-1708. [CrossRef]
- Kell, D. B. and Pretorius, E. (2023) Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J. 480, 1217-1240. [CrossRef]
- Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. and Pretorius, E. (2024) Possible role of fibrinaloid microclots in Postural Orthostatic Tachycardia Syndrome (POTS): focus on Long COVID. J Personalised Medicine. 14, 170. [CrossRef]
- Kell, D. B., Lip, G. Y. H. and Pretorius, E. (2024) Fibrinaloid Microclots and Atrial Fibrillation. Biomedicines. 12, 891. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Potential roles of fibrinaloid microclots in fibromyalgia syndrome. OSF preprint. https://osf.io/9e2y5/. [CrossRef]
- Eckey, M., Li, P., Morrison, B., Davis, R. W. and Xiao, W. (2024) Patient-Reported Treatment Outcomes in ME/CFS and Long COVID. medRxiv, 2024.2011.2027.24317656. [CrossRef]
- Laubscher, G. J., Khan, M. A., Venter, C., Pretorius, J. H., Kell, D. B. and Pretorius, E. (2023) Treatment of Long COVID symptoms with triple anticoagulant therapy. https://www.researchsquare.com/article/rs-2697680/v2697681. [CrossRef]
- Kell, D. B. and Pretorius, E. (2025) The proteome content of blood clots observed under different conditions: successful role in predicting clot amyloid(ogenicity). Molecules. 30, 668. [CrossRef]
- Kell, D. B., Pretorius, E. and Zhao, H. (2025) A direct relationship between ‘blood stasis’ and fibrinaloid microclots in chronic, inflammatory and vascular diseases, and some traditional natural products approaches to treatment. Pharmaceuticals. 18, 712. [CrossRef]
- Kell, D. B., Doyle, K. M., Salcedo-Sora, E., Sekhar, A., Walker, M. and Pretorius, E. (2025) AmyloGram reveals amyloidogenic potential in stroke thrombus proteomes. bioRxiv, 2025.2007.2007.663482. [CrossRef]
- Biancalana, M. and Koide, S. (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 1804, 1405-1412. [CrossRef]
- Gade Malmos, K., Blancas-Mejia, L. M., Weber, B., Buchner, J., Ramirez-Alvarado, M., Naiki, H. and Otzen, D. (2017) ThT 101: a primer on the use of thioflavin T to investigate amyloid formation. Amyloid. 24, 1-16. [CrossRef]
- Kell, D. B. and Pretorius, E. (2017) Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol. 123, 16-41. [CrossRef]
- Xue, C., Lin, T. Y., Chang, D. and Guo, Z. (2017) Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R Soc Open Sci. 4, 160696. [CrossRef]
- Basha, S., Mukunda, D. C., Pai, A. R. and Mahato, K. K. (2025) Assessing amyloid fibrils and amorphous aggregates: A review. Int J Biol Macromol. 311, 143725. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J. and Meiring, J. H. (2010) The changed ultrastructure of fibrin networks during use of oral contraception and hormone replacement. J Thromb Thrombolysis. 30, 502-506. [CrossRef]
- Swanepoel, A. C., Visagie, A., de Lange, Z., Emmerson, O., Nielsen, V. G. and Pretorius, E. (2016) The clinical relevance of altered fibrinogen packaging in the presence of 17beta-estradiol and progesterone. Thromb Res. 146, 23-34. [CrossRef]
- Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 41, BSR20210611. [CrossRef]
- Grobbelaar, L. M., Kruger, A., Venter, C., Burger, E. M., Laubscher, G. J., Maponga, T. G., Kotze, M. J., Kwaan, H. C., Miller, J. B., Fulkerson, D., Huff, W., Chang, E., Wiarda, G., Bunch, C. M., Walsh, M. M., Raza, S., Zamlut, M., Moore, H. B., Moore, E. E., Neal, M. D., Kell, D. B. and Pretorius, E. (2022) Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost. 48, 858-868. [CrossRef]
- Kell, D. B. and Pretorius, E. (2024) Proteomic evidence for amyloidogenic cross-seeding in fibrinaloid microclots. Int J Mol Sci. 25, 10809. [CrossRef]
- Maihoub, S., Mavrogeni, P., Molnár, V. and Molnár, A. (2025) Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships. J Clin Med. 14, 1285. [CrossRef]
- Tziridis, K., Neubert, B., Seehaus, A., Krauss, P., Schilling, A., Bruggemann, P., Mazurek, B. and Schulze, H. (2025) Correlation of non-auditory comorbidities and hearing loss in chronic subjective tinnitus patients: a retrospective database study. Front Neurol. 16, 1596274. [CrossRef]
- Bhatt, I. S., Washnik, N. J., Kingsbury, S., Deshpande, A. K., Kingsbury, H., Bhagavan, S. G., Michel, K., Dias, R. and Torkamani, A. (2023) Identifying health-related conditions associated with tinnitus in young adults. Audiol Res. 13, 546-562. [CrossRef]
- Basso, L., Boecking, B., Brueggemann, P., Pedersen, N. L., Canlon, B., Cederroth, C. R. and Mazurek, B. (2020) Gender-Specific Risk Factors and Comorbidities of Bothersome Tinnitus. Front Neurosci. 14, 706. [CrossRef]
- Mühlmeier, G., Baguley, D., Cox, T., Suckfüll, M. and Meyer, T. (2016) Characteristics and Spontaneous Recovery of Tinnitus Related to Idiopathic Sudden Sensorineural Hearing Loss. Otol Neurotol. 37, 634-641. [CrossRef]
- Ratnayake, S. A., Jayarajan, V. and Bartlett, J. (2009) Could an underlying hearing loss be a significant factor in the handicap caused by tinnitus? Noise Health. 11, 156-160. [CrossRef]
- Brinkmann, P., Kotz, S. A., Smit, J. V., Janssen, M. L. F. and Schwartze, M. (2021) Auditory thalamus dysfunction and pathophysiology in tinnitus: a predictive network hypothesis. Brain Struct Funct. 226, 1659-1676. [CrossRef]
- Cheng, Y. F., Xirasagar, S., Yang, T. H., Wu, C. S., Kao, Y. W. and Lin, H. C. (2021) Risk of early-onset dementia among persons with tinnitus: a retrospective case-control study. Sci Rep. 11, 13399. [CrossRef]
- Chu, H. T., Liang, C. S., Yeh, T. C., Hu, L. Y., Yang, A. C., Tsai, S. J. and Shen, C. C. (2020) Tinnitus and risk of Alzheimer's and Parkinson's disease: a retrospective nationwide population-based cohort study. Sci Rep. 10, 12134. [CrossRef]
- Ruan, Q., Chen, B. and Panza, F. (2023) Which Came First, Age-Related Hearing Loss with Tinnitus or Cognitive Impairment? What are the Potential Pathways? J Integr Neurosci. 22, 109. [CrossRef]
- Yang, D., Zhang, D., Zhang, X. and Li, X. (2024) Tinnitus-associated cognitive and psychological impairments: a comprehensive review meta-analysis. Front Neurosci. 18, 1275560. [CrossRef]
- Machado, M. G., Machado, T. H., Caramelli, P. and Macedo de Resende, L. (2025) Tinnitus and hearing loss in people with dementia. Alz Dement. 20, e095725. [CrossRef]
- Ralli, M., Gilardi, A., Stadio, A. D., Severini, C., Salzano, F. A., Greco, A. and Vincentiis, M. (2019) Hearing loss and Alzheimer's disease: A Review. Int Tinnitus J. 23, 79-85. [CrossRef]
- Lee, H. Y. (2020) Beyond Hearing Loss: Does Tinnitus Cause Cognitive Impairment? Clin Exp Otorhinolaryngol. 13, 2-3. [CrossRef]
- Lee, S. Y., Lee, J. Y., Han, S. Y., Seo, Y., Shim, Y. J. and Kim, Y. H. (2020) Neurocognition of Aged Patients With Chronic Tinnitus: Focus on Mild Cognitive Impairment. Clin Exp Otorhinolaryngol. 13, 8-14. [CrossRef]
- Wu, J., Shi, M. and Wang, C. (2025) Association between tinnitus and cognitive impairment: analysis of National Health and Nutrition Examination Survey 2011:2014. Front Neurol. 16, 1533821. [CrossRef]
- Al-Sallami, D., Aameri, R., Tischkau, S., Rybak, L. P. and Ramkumar, V. (2025) Cochlear Amyloid-beta42 Accumulation Drives Progressive Auditory Neuropathy in 5XFAD Mice: A Potential Biomarker for Early Alzheimer's Disease. Res Sq. [CrossRef]
- Han, S. Y., Kim, H., Yun, Y., Lee, M. J., Lee, J. Y., Park, S. W., Kim, Y. K. and Kim, Y. H. (2024) Comparative study on structural and functional brain differences in mild cognitive impairment patients with tinnitus. Front Aging Neurosci. 16, 1470919. [CrossRef]
- Pretorius, E., Bester, J. and Kell, D. B. (2016) A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis. 53, 1237-1256. [CrossRef]
- Pretorius, E., Bester, J., Page, M. J. and Kell, D. B. (2018) The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Frontiers Aging Neurosci. 10, 257. [CrossRef]
- Pretorius, L., Kell, D. B. and Pretorius, E. (2018) Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer's Type Dementia. Front Neurosci. 12, 851. [CrossRef]
- Grobler, C., van Tongeren, M., Gettemans, J., Kell, D. and Pretorius, E. (2023) Alzheimer-type dementia: a systems view provides a unifying explanation of its development. J Alz Dis. 91, 43-70. [CrossRef]
- Kilic, O., Kalcioglu, M. T., Cag, Y., Tuysuz, O., Pektas, E., Caskurlu, H. and Cetın, F. (2020) Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis. 97, 208-211. [CrossRef]
- Narozny, W., Tretiakow, D. and Skorek, A. (2021) Tinnitus in COVID-19 Pandemic. Ear Nose Throat J. 100, 197S-198S. [CrossRef]
- Ricciardiello, F., Pisani, D., Viola, P., Cristiano, E., Scarpa, A., Giannone, A., Longo, G., Russo, G., Bocchetti, M., Coppola, C., Perrella, M., Oliva, F. and Chiarella, G. (2021) Sudden Sensorineural Hearing Loss in Mild COVID-19: Case Series and Analysis of the Literature. Audiol Res. 11, 313-326. [CrossRef]
- Viola, P., Ralli, M., Pisani, D., Malanga, D., Sculco, D., Messina, L., Laria, C., Aragona, T., Leopardi, G., Ursini, F., Scarpa, A., Topazio, D., Cama, A., Vespertini, V., Quintieri, F., Cosco, L., Cunsolo, E. M. and Chiarella, G. (2021) Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur Arch Otorhinolaryngol. 278, 3725-3730. [CrossRef]
- Beukes, E. W., Baguley, D. M., Jacquemin, L., Lourenco, M., Allen, P. M., Onozuka, J., Stockdale, D., Kaldo, V., Andersson, G. and Manchaiah, V. (2020) Changes in Tinnitus Experiences During the COVID-19 Pandemic. Front Public Health. 8, 592878. [CrossRef]
- McIntyre, K. M., Favre, N. M., Kuo, C. C. and Carr, M. M. (2021) Systematic Review of Sensorineural Hearing Loss Associated With COVID-19 Infection. Cureus. 13, e19757. [CrossRef]
- Thrane, J. F., Britze, A. and Fjaeldstad, A. W. (2022) Incidence and duration of self-reported hearing loss and tinnitus in a cohort of COVID-19 patients with sudden chemosensory loss: A STROBE observational study. Eur Ann Otorhinolaryngol Head Neck Dis. 139, 125-128. [CrossRef]
- Baig, A. M. (2021) Pathways and Pathogenesis of Hearing Deficits, Tinnitus, and Vertigo in COVID-19. ACS Chem Neurosci. 12, 4368-4370. [CrossRef]
- Figueiredo, R. R., de O. Penido, N., de Azevedo, A. A., de Oliveira, P. M., de Siqueira, A. G., Figueiredo, G. M. R., Schlee, W. and Langguth, B. (2022) Tinnitus emerging in the context of a COVID-19 infection seems not to differ in its characteristics from tinnitus unrelated to COVID-19. Front Neurol. 13, 974179. [CrossRef]
- Mao, S., Gu, D., Wang, D., Li, P., Huang, X., Yin, H. and Sun, S. (2024) Prevalence and prognosis of tinnitus in post-COVID-19 patients: a cross-sectional survey. Epidemiol Infect. 152, e137. [CrossRef]
- Tai, Y., Jain, N., Kim, G. and Husain, F. T. (2024) Tinnitus and COVID-19: effect of infection, vaccination, and the pandemic. Front Public Health. 12, 1508607. [CrossRef]
- Meng, X., Wang, J., Sun, J. and Zhu, K. (2022) COVID-19 and Sudden Sensorineural Hearing Loss: A Systematic Review. Front Neurol. 13, 883749. [CrossRef]
- Peron, K. A., Scott, M. C. P., Soeiro, T. L. T., do Amaral, J. B., Chandrasekhar, S. S. and de Oliveira Penido, N. (2024) Sudden sensorineural hearing loss: audiological profile during the COVID-19 pandemic. Front Neurol. 15, 1415068. [CrossRef]
- Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. and Kell, D. B. (2020) Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol. 19, 193. [CrossRef]
- Xia, W., Cui, J., Luo, Y., Xu, J. J., Chen, H., Yin, X., Ma, J. and Wu, Y. (2020) Glucose Control Has an Impact on Cerebral Blood Flow Alterations in Chronic Tinnitus Patients. Front Neurosci. 14, 623520. [CrossRef]
- Gibrin, P. C. D., Melo, J. J. and de Moraes Marchiori, L. L. (2013) Prevalence of tinnitus complaints and probable association with hearing loss, diabetes mellitus and hypertension in elderly. Codas. 25, 176-180. [CrossRef]
- Mousavi, S. H. G., Sajadinejad, B., Khorsandi, S. and Farhadi, A. (2021) Diabetes Mellitus and Tinnitus: an Epidemiology Study. Maedica (Bucur). 16, 580-584. [CrossRef]
- Kumar, P., Singh, N. K., Apeksha, K., Ghosh, V., Kumar, R. R. and Kumar Muthaiah, B. (2022) Auditory and Vestibular Functioning in Individuals with Type-2 Diabetes Mellitus: A Systematic Review. Int Arch Otorhinolaryngol. 26, e281-e288. [CrossRef]
- Luo, S., Wen, J., Bao, Q., Ou, H., Yi, S. and Peng, P. (2025) Association between diabetes mellitus and tinnitus: A meta-analysis. Biomol Biomed. 25, 1937-1948. [CrossRef]
- de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B. and Pretorius, E. (2018) Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus. Sci Rep. 8, 16798. [CrossRef]
- Pretorius, E., Bester, J., Vermeulen, N., Alummoottil, S., Soma, P., Buys, A. V. and Kell, D. B. (2015) Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 134, 30. [CrossRef]
- Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C. and Kell, D. B. (2017) Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol. 16, 141. [CrossRef]
- Campello, C. P., Lemos, C. A. A., Lopes de Andrade, W. T., Fernandes de Melo, L. P., de Santana Nunes, G. R. and Cavalcanti, H. G. (2024) Migraine associated with tinnitus and hearing loss in adults: a systematic review. Int J Audiol. 63, 1-7. [CrossRef]
- de Villiers, S., Bester, J., Kell, D. B. and Pretorius, E. (2019) Erythrocyte health and the possible role of amyloidogenic blood clotting in the evolving haemodynamics of female migraine-with-aura pathophysiology: Results from a pilot study. Frontiers Neurol. 10, 1262. [CrossRef]
- Lechner, J. and Schick, F. (2021) Chronic Fatigue Syndrome and Bone Marrow Defects of the Jaw - A Case Report on Additional Dental X-Ray Diagnostics with Ultrasound. Int Med Case Rep J. 14, 241-249. [CrossRef]
- Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. and Pretorius, E. (2022) The occurrence of hyperactivated platelets and fibrinaloid microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel). 15, 931. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2023) Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev. 60, 101075. [CrossRef]
- Nunes, J. M., Vlok, M., Proal, A., Kell, D. B. and Pretorius, E. (2024) Data-independent LC-MS/MS analysis of ME/CFS plasma reveals a dysregulated coagulation system, endothelial dysfunction, downregulation of complement machinery. Cardiovasc. Diabetol. 23, 254. [CrossRef]
- Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., Kell, D. B. and Pretorius, E. (2019) Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Ag Neurosci. 11, 210. [CrossRef]
- Pretorius, E., Page, M. J., Mbotwe, S. and Kell, D. B. (2018) Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PlosOne. 13, e0192121. [CrossRef]
- van Vuuren, M. J., Nell, T. A., Carr, J. A., Kell, D. B. and Pretorius, E. (2021) Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson’s disease. Biomolecules. 11, 30. [CrossRef]
- Davis, H. E., McCorkell, L., Vogel, J. M. and Topol, E. J. (2023) Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 21, 133-146. [CrossRef]
- Degen, C. V., Mikuteit, M., Niewolik, J., Schroder, D., Vahldiek, K., Mucke, U., Heinemann, S., Muller, F., Behrens, G. M. N., Klawonn, F., Dopfer-Jablonka, A. and Steffens, S. (2022) Self-reported Tinnitus and Vertigo or Dizziness in a Cohort of Adult Long COVID Patients. Front Neurol. 13, 884002. [CrossRef]
- Africa, R. E., Westenhaver, Z. K., Zimmerer, R. E. and McKinnon, B. J. (2023) Evaluation of Disturbances in Hearing, Tinnitus, and Dizziness as Signs of COVID-19 Infection. Otol Neurotol. 44, 126-133. [CrossRef]
- Fritz, C. G., Choi, J. S., Conway, R. M., Casale, G. G., Bojrab, D. I., 2nd and Babu, S. C. (2023) Characterizing the most Popular Tinnitus Inquiries: Is Tinnitus Incidence on the Rise Since COVID-19? Otol Neurotol. 44, e435-e442. [CrossRef]
- Nocini, R., Lippi, G. and Mattiuzzi, C. (2023) Impact of COVID-19 pandemic on the worldwide burden of tinnitus. Eur Arch Otorhinolaryngol. 280, 945-946. [CrossRef]
- Rozbicki, P., Krzywdzińska, S., Kaczmarczyk, M., Usowski, J., Lubas, A. and Jurkiewicz, D. (2023) Patogenesis of tinnitus in patients with post-COVID syndrome - preliminary report. Otolaryngol Pol. 77, 18-22. [CrossRef]
- Wang, D., Li, P., Huang, X., Liu, Y., Mao, S., Yin, H., Wang, N., Luo, Y. and Sun, S. (2024) Exploring the Prevalence of Tinnitus and Ear-Related Symptoms in China After the COVID-19 Pandemic: Online Cross-Sectional Survey. JMIR Form Res. 8, e54326. [CrossRef]
- Aydogan, Z., Can, M., Soylemez, E., Karakoc, K., Buyukatalay, Z. C. and Yilmaz, S. T. (2025) The Effects of COVID-19 on Tinnitus Severity and Quality of Life in Individuals With Subjective Tinnitus. Brain Behav. 15, e70317. [CrossRef]
- Jafari, Z., Kolb, B. E., Aiken, S. and Wilson, S. (2025) Updates on Auditory Outcomes of COVID-19 and Vaccine Side Effects: An Umbrella Review. J Speech Lang Hear Res. 68, 1311-1332. [CrossRef]
- Kruger, A., Vlok, M., Turner, S., Venter, C., Laubscher, G. J., Kell, D. B. and Pretorius, E. (2022) Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 21, 190. [CrossRef]
- Pretorius, E., Venter, C., Laubsher, G. J., Kotze, M. J., Moremi, K., Oladejo, S., Watson, L. R., Rajaratnam, K., Watson, B. W. and Kell, D. B. (2021) Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Research Square rs-1205453/v1205451.
- Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. and Pretorius, E. (2023) Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon. 9, e19605. [CrossRef]
- Kim, H. J., Lee, H. J., An, S. Y., Sim, S., Park, B., Kim, S. W., Lee, J. S., Hong, S. K. and Choi, H. G. (2015) Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 10, e0127578. [CrossRef]
- Schubert, N. M. A., Rosmalen, J. G. M., van Dijk, P. and Pyott, S. J. (2021) A retrospective cross-sectional study on tinnitus prevalence and disease associations in the Dutch population-based cohort Lifelines. Hear Res. 411, 108355. [CrossRef]
- Torere, B. E., Chittipolu, S., Alugba, G., Aiwuyo, H. O. and Kennard, J. L. (2023) Sudden-Onset Sensorineural Hearing Loss and Tinnitus in a Patient With Rheumatoid Arthritis: A Case Report and Literature Review. Cureus. 15, e38739. [CrossRef]
- Bezuidenhout, J., Venter, C., Roberts, T., Tarr, G., Kell, D. and Pretorius, E. (2020) The Atypical Fibrin Fibre Network in Rheumatoid Arthritis and its Relation to Autoimmunity, Inflammation and Thrombosis. bioRxiv, 2020.2005.2028.121301v121301. [CrossRef]
- Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. and Soma, P. (2012) Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int. 32, 1611-1615. [CrossRef]
- Pretorius, E., Akeredolu, O.-O., Soma, P. and Kell, D. B. (2017) Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med. 242, 355-373. [CrossRef]
- Pretorius, E., Bester, J., Vermeulen, N., Lipinski, B., Gericke, G. S. and Kell, D. B. (2014) Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One. 9, e85271. [CrossRef]
- Kell, D. B. and Pretorius, E. (2018) To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Semin Thromb Hemost. 44, 224-238. [CrossRef]
- Schofield, J., Abrams, S. T., Jenkins, R., Lane, S., Wang, G. and Toh, C. H. (2024) Microclots, as defined by amyloid-fibrinogen aggregates, predict risks of disseminated intravascular coagulation and mortality. Blood Adv. 8, 2499-2508. [CrossRef]
- Crummer, R. W. and Hassan, G. A. (2004) Diagnostic approach to tinnitus. Am Fam Physician. 69, 120-126.
- Dalrymple, S. N., Lewis, S. H. and Philman, S. (2021) Tinnitus: Diagnosis and Management. Am Fam Physician. 103, 663-671.
- Tanaka, L. S., de Moraes Marchiori, L. L., de Almeida Soares Ciquinato, D., de Castro Teixeira, D., de Moraes Marchiori, G., Branco, B. H. M. and Poli-Frederico, R. C. (2024) Inflammatory Biomarkers and Tinnitus in Older Adults. Noise Health. 26, 535-542. [CrossRef]
- Joo, Y. H., Han, K. D. and Park, K. H. (2015) Association of Hearing Loss and Tinnitus with Health-Related Quality of Life: The Korea National Health and Nutrition Examination Survey. PLoS One. 10, e0131247. [CrossRef]
- Lee, D. Y. and Kim, Y. H. (2018) Risk factors of pediatric tinnitus: Systematic review and meta-analysis. Laryngoscope. 128, 1462-1468. [CrossRef]
- Aazh, H. and Salvi, R. (2019) The Relationship between Severity of Hearing Loss and Subjective Tinnitus Loudness among Patients Seen in a Specialist Tinnitus and Hyperacusis Therapy Clinic in UK. J Am Acad Audiol. 30, 712-719. [CrossRef]
- Waechter, S. (2021) Association between hearing status and tinnitus distress. Acta Otolaryngol. 141, 381-385. [CrossRef]
- Waechter, S. and Brännstrom, K. J. (2023) Magnitude of extended high frequency hearing loss associated with auditory related tinnitus distress, when controlling for magnitude of hearing loss at standard frequenciesa). J Acoust Soc Am. 154, 2821-2827. [CrossRef]
- Schmutzhard, J., Glueckert, R., Pritz, C., Blumer, M. J., Bitsche, M., Lackner, P., Fille, M., Riechelmann, H., Harkamp, M., Sitthisak, T. and Schrott-Fischer, A. (2013) Sepsis otopathy: experimental sepsis leads to significant hearing impairment due to apoptosis and glutamate excitotoxicity in murine cochlea. Dis Model Mech. 6, 745-754. [CrossRef]
- Cheng, C. G., Chien, W. C., Lin, H. C., Lin, H. C., Chung, C. H. and Cheng, C. A. (2020) Hearing impairment in young and middle-aged septicemia survivors. Medicine (Baltimore). 99, e21050. [CrossRef]
- Cheng, C. G., Chen, Y. H., Chang, Y. H., Lin, H. C., Chin, P. W., Lin, Y. Y., Yung, M. C. and Cheng, C. A. (2023) Underestimated Subsequent Sensorineural Hearing Loss after Septicemia. Medicina (Kaunas). 59, 1897. [CrossRef]
- Yüksel, F., Karataş, D., Türkdoğan, F. T. and Yuksel, Ö. (2018) Increased Atherosclerosis Correlates with Subjective Tinnitus Severity. Indian J Otolaryngol Head Neck Surg. 70, 119-124. [CrossRef]
- Hou, Y., Yang, H., Xu, Y., Wang, K., Fu, Y. and Lu, Z. (2024) Hearing disorders, genetic predisposition, and risk of new-onset atrial fibrillation: A prospective cohort study in the UK biobank. Int J Cardiol. 401, 131829. [CrossRef]
- Hahad, O., Hackenberg, B., Doge, J., Bahr-Hamm, K., Kerahrodi, J. G., Tüscher, O., Michal, M., Kontohow-Beckers, K., Schuster, A. K., Schmidtmann, I., Lackner, K. J., Schattenberg, J. M., Konstantinides, S., Wild, P. S. and Münzel, T. (2025) Tinnitus is not associated with cardiovascular risk factors or mortality in the Gutenberg Health Study. Clin Res Cardiol. [CrossRef]
- Cheng, Y. F., Xirasagar, S., Kuo, N. W., Chung, S. D. and Lin, H. C. (2021) Association of erectile dysfunction with tinnitus: a nationwide population-based study. Sci Rep. 11, 6982. [CrossRef]
- Kedia, G. T., Ückert, S., Tsikas, D., Becker, A. J., Kuczyk, M. A. and Bannowsky, A. (2020) The Use of Vasoactive Drugs in the Treatment of Male Erectile Dysfunction: Current Concepts. J Clin Med. 9, 2987. [CrossRef]
- Skare, T. L. and de Carvalho, J. F. (2024) Ear Complaints in Fibromyalgia: A Narrative Review. Rheumatol Ther. 11, 1085-1099. [CrossRef]
- Chung, C. H., Jang, G. and Lee, C. H. (2021) The Impact of Tinnitus on Fibromyalgia. J Rheum Dis. 28, 31-37. [CrossRef]
- Gupta, A. and Schikler, K. (2024) Prevalence of tinnitus in juvenile fibromyalgia patients visiting a pediatric rheumatology clinic. Int J Pediatr Otorhinolaryngol. 176, 111837. [CrossRef]
- Ausland, J. H., Engdahl, B., Oftedal, B., Hopstock, L. A., Johnsen, M. and Krog, N. H. (2024) Tinnitus and cardiovascular disease: the population-based Tromsø Study (2015-2016). BMJ Public Health. 2, e000621. [CrossRef]
- Arai, M., Takada, T. and Nozue, M. (2003) Orthostatic tinnitus: an otological presentation of spontaneous intracranial hypotension. Auris Nasus Larynx. 30, 85-87. [CrossRef]
- Boari, L., Chaves, A. G., Ganança, F. F. and Munhoz, M. S. L. (2008) Vestibular disorder and autonomic dysfunction. Braz J Otorhinolaryngol. 74, 797. [CrossRef]
- Weinstock, L. B., Brook, J. B., Blasingame, K. E., Kaleem, Z., Afrin, L. B. and Molderings, G. J. (2021) Tinnitus in Mast Cell Activation Syndrome: A Prospective Survey of 114 Patients. J Otolaryngol Neurotol Res. 4, 92-96.
- Amor-Dorado, J. C., Barreira-Fernandez, M. P., Pina, T., Vázquez-Rodríguez, T. R., Llorca, J. and González-Gay, M. A. (2014) Investigations into audiovestibular manifestations in patients with psoriatic arthritis. J Rheumatol. 41, 2018-2026. [CrossRef]
- Borgia, F., Ciodaro, F., Guarneri, F., Bartolotta, A., Papaianni, V., Guarneri, C., Catalano, N., Galletti, F. and Cannavo, S. P. (2018) Auditory System Involvement in Psoriasis. Acta Derm Venereol. 98, 655-659. [CrossRef]
- Choi, H. G., Park, B., Hong, S. M., Park, I. S. and Kim, S. K. (2020) Psoriasis Increases the Risk of Sudden Sensorineural Hearing Loss: A Longitudinal Follow Up Study Using a National Sample Cohort. Int J Environ Res Public Health. 17, 9310. [CrossRef]
- Jeong, S. S., Shih, M. C., Rizk, H. G. and Lambert, P. R. (2022) Otologic Manifestations of Psoriasis: A Systematic Review and Meta-Analysis. Otol Neurotol. 43, 742-752. [CrossRef]
- Palmer, K. T., Griffin, M. J., Syddall, H. E., Pannett, B., Cooper, C. and Coggon, D. (2002) Raynaud's phenomenon, vibration induced white finger, and difficulties in hearing. Occup Environ Med. 59, 640-642. [CrossRef]
- Brydøy, M., Oldenburg, J., Klepp, O., Bremnes, R. M., Wist, E. A., Wentzel-Larsen, T., Hauge, E. R., Dahl, O. and Fosså, S. D. (2009) Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 101, 1682-1695. [CrossRef]
- Piltcher, O., Cigana, L., Friedriech, J., Ribeiro, F. A. and da Costa, S. S. (2000) Sensorineural hearing loss among sickle cell disease patients from southern Brazil. Am J Otolaryngol. 21, 75-79. [CrossRef]
- Saito, N., Watanabe, M., Liao, J., Flower, E. N., Nadgir, R. N., Steinberg, M. H. and Sakai, O. (2011) Clinical and radiologic findings of inner ear involvement in sickle cell disease. AJNR Am J Neuroradiol. 32, 2160-2164. [CrossRef]
- Bois, E., Francois, M., Benkerrou, M., Van Den Abbeele, T. and Teissier, N. (2019) Hearing loss in children with sickle cell disease: A prospective French cohort study. Pediatr Blood Cancer. 66, e27468. [CrossRef]
- Alabssi, H., Alfares, M., AlMulhim, N. F., Latif, R., Rafique, N., Almulhim, N. F., Alismail, M. A., Algheryafi, S. S., Bokhari, D. R., Alzayyat, R. T., Sulaiman, A. A. and Alwaheed, A. J. (2024) Prevalence and risk factors of hearing loss and otological symptoms among Sickle Cell Disease patients in Saudi Arabia: a single center experience. Acta Biomed. 95, e2024028. [CrossRef]
- Maciaszczyk, K., Waszczykowska, E., Pajor, A., Bartkowiak-Dziankowska, B. and Durko, T. (2011) Hearing organ disorders in patients with systemic sclerosis. Rheumatol Int. 31, 1423-1428. [CrossRef]
- Silva, M. M., Araujo, R. P. C., Araujo, F., Valente, J. S. and Corona, A. P. (2019) Hearing alterations in systemic sclerosis. Codas. 31, e20170119. [CrossRef]
- Bobeica, C., Niculet, E., Craescu, M., Parapiru, E. L., Musat, C. L., Dinu, C., Chiscop, I., Nechita, L., Stefanescu, V., Stefanopol, I. A., Pelin, A. M., Nechifor, A., Balan, G. and Tatu, A. L. (2022) Hearing Loss Secondary to Systemic Sclerosis Vasculopathy: Case Study with a Short Review. Clin Cosmet Investig Dermatol. 15, 967-973. [CrossRef]
- Turan, K., Yayla, M. E., Arslan, M., Tokgoz Yilmaz, S., Okatan, E., Turgay, M. and Meco, C. (2022) Audiological involvement in patients with systemic sclerosis. Mod Rheumatol. 32, 1094-1101. [CrossRef]
- Mazeda, C., Silva, S. P., Romao, J., Matias, D., Azevedo, L. and Barcelos, A. (2024) Audiovestibular Involvement in Patients With Systemic Sclerosis. J Clin Rheumatol. 30, 276-282. [CrossRef]
- Salvador, C. D., Keith, B. A., Ward, C., Nguyen, S. A., Gordis, T., Chidarala, S., Brennan, E. and Rizk, H. (2025) Audiovestibular symptoms in systemic sclerosis: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 282, 1147-1157. [CrossRef]
- Braverman, I. M. (1972) Electron microscopic studies of the microcirculation in psoriasis. J Invest Dermatol. 59, 91-98. [CrossRef]
- Braverman, I. M. and Sibley, J. (1982) Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol. 78, 12-17. [CrossRef]
- Fuga, G. C., Marmo, W., Acierno, F., Bassetti, F., Leonetti, F., Pampanelli, L. M. and Palermi, G. (1994) Cutaneous microcirculation in psoriasis. A videocapillaroscopic morphofunctional study. Acta Derm Venereol Suppl (Stockh). 186, 138.
- Hendriks, A. G. M., Steenbergen, W., Hondebrink, E., van Hespen, J. C. G., van de Kerkhof, P. C. M. and Seyger, M. M. B. (2014) Whole field laser Doppler imaging of the microcirculation in psoriasis and clinically unaffected skin. J Dermatolog Treat. 25, 18-21. [CrossRef]
- Alba, B. K., Greaney, J. L., Ferguson, S. B. and Alexander, L. M. (2018) Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol. 314, H343-H349. [CrossRef]
- Bernardino, V. R., Rodrigues, A. C. and Panarra, A. (2019) Raynaud's phenomenon and inflammatory bowel disease: The possible role of microcirculation. Eur J Intern Med. 62, e16. [CrossRef]
- Csiki, Z., Garai, I., Varga, J., Szucs, G., Galajda, Z., Andras, C., Zeher, M. and Galuska, L. (2005) Microcirculation of the fingers in Raynaud's syndrome: (99m)Tc-DTPA imaging. Nuklearmedizin. 44, 29-32. [CrossRef]
- Gregorczyk-Maga, I., Frołow, M., Kaczmarczyk, P. and Maga, P. (2019) Microcirculation disorders of the oral cavity in patients with primary Raynaud phenomenon. Pol Arch Intern Med. 129, 36-42. [CrossRef]
- Ingegnoli, F., Ughi, N., Dinsdale, G., Orenti, A., Boracchi, P., Allanore, Y., Foeldvari, I., Sulli, A., Cutolo, M., Smith, V., Herrick, A. L. and EULAR Study Group on Microcirculation in Rheumatic Diseases. (2017) An international SUrvey on non-iNvaSive tecHniques to assess the mIcrocirculation in patients with RayNaud's phEnomenon (SUNSHINE survey). Rheumatol Int. 37, 1879-1890. [CrossRef]
- Latuskiewicz-Potemska, J., Chmura-Skirlinska, A., Gurbiel, R. J. and Smolewska, E. (2016) Nailfold capillaroscopy assessment of microcirculation abnormalities and endothelial dysfunction in children with primary or secondary Raynaud syndrome. Clin Rheumatol. 35, 1993-2001. [CrossRef]
- Mosdósi, B., Bölcskei, K. and Helyes, Z. (2018) Impairment of microcirculation and vascular responsiveness in adolescents with primary Raynaud phenomenon. Pediatr Rheumatol Online J. 16, 20. [CrossRef]
- Radić, M., Snow, M., Frech, T. M., Saketkoo, L. A., Cutolo, M. and Smith, V. (2020) Consensus-based evaluation of dermatoscopy versus nailfold videocapillaroscopy in Raynaud's phenomenon linking USA and Europe: a European League against Rheumatism study group on microcirculation in rheumatic diseases project. Clin Exp Rheumatol. 38 Suppl 125, 132-136.
- Szabo, N., Csiki, Z., Szanto, A., Danko, K., Szodoray, P. and Zeher, M. (2008) Functional and morphological evaluation of hand microcirculation with nailfold capillaroscopy and laser Doppler imaging in Raynaud's and Sjogren's syndrome and poly/dermatomyositis. Scand J Rheumatol. 37, 23-29. [CrossRef]
- Correa, M. J., Andrade, L. E. and Kayser, C. (2010) Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis Res Ther. 12, R157. [CrossRef]
- Della Rossa, A., Cazzato, M., d'Ascanio, A., Tavoni, A., Bencivelli, W., Pepe, P., Mosca, M., Baldini, C., Rossi, M. and Bombardieri, S. (2013) Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: a laser speckle contrast analysis. Clin Exp Rheumatol. 31, 109-114.
- Mandujano, A. and Golubov, M. (2022) Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life (Basel). 12, 703. [CrossRef]
- Mugii, N., Hasegawa, M., Hamaguchi, Y., Tanaka, C., Kaji, K., Komura, K., Ueda-Hayakawa, I., Horie, S., Ikuta, M., Tachino, K., Ogawa, F., Sato, S., Fujimoto, M. and Takehara, K. (2009) Reduced red blood cell velocity in nail-fold capillaries as a sensitive and specific indicator of microcirculation injury in systemic sclerosis. Rheumatology (Oxford). 48, 696-703. [CrossRef]
- Picart, C., Carpentier, P. H., Brasseur, S., Galliard, H. and Piau, J. M. (1998) Systemic sclerosis: blood rheometry and laser Doppler imaging of digital cutaneous microcirculation during local cold exposure. Clin Hemorheol Microcirc. 18, 47-58.
- Visser, M. J. E., Venter, C., Roberts, T. J., Tarr, G. and Pretorius, E. (2021) Psoriatic disease is associated with systemic inflammation, endothelial activation, and altered haemostatic function. Sci Rep. 11, 13043. [CrossRef]
- Cohen, B. E., Durstenfeld, A. and Roehm, P. C. (2014) Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 18. [CrossRef]
- Mattia, J. G., Vandy, M. J., Chang, J. C., Platt, D. E., Dierberg, K., Bausch, D. G., Brooks, T., Conteh, S., Crozier, I., Fowler, R. A., Kamara, A. P., Kang, C., Mahadevan, S., Mansaray, Y., Marcell, L., McKay, G., O'Dempsey, T., Parris, V., Pinto, R., Rangel, A., Salam, A. P., Shantha, J., Wolfman, V., Yeh, S., Chan, A. K. and Mishra, S. (2016) Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis. 16, 331-338. [CrossRef]
- Xu, M. J., Stanford-Moore, G. and Czechowicz, J. A. (2019) Association of Ebola Virus Infection With Hearing Loss in Regions Where Ebola Virus Infection Is Endemic: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 145, 669-675. [CrossRef]
- Fappiano, C. M. and Baraniuk, J. N. (2020) Gulf War Illness Symptom Severity and Onset: A Cross-Sectional Survey. Mil Med. 185, e1120-e1127. [CrossRef]
- van der Westhuizen, Y., Swanepoel, d. W., Heinze, B. and Hofmeyr, L. M. (2013) Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol. 52, 37-43. [CrossRef]
- de Jong, M. A., Luder, A. and Gross, M. (2019) Main Aspects of Peripheral and Central Hearing System Involvement in Unexplained HIV-Related Hearing Complaints. Front Neurol. 10, 845. [CrossRef]
- Moscatello, A. L., Worden, D. L., Nadelman, R. B., Wormser, G. and Lucente, F. (1991) Otolaryngologic aspects of Lyme disease. Laryngoscope. 101, 592-595. [CrossRef]
- Sowula, K., Skladzien, J., Szaleniec, J. and Gawlik, J. (2018) Otolaryngological symptoms in patients treated for tick-borne diseases. Otolaryngol Pol. 72, 30-34. [CrossRef]
- Coulson, S., Croxson, G. R., Adams, R. and Oey, V. (2011) Prognostic factors in herpes zoster oticus (Ramsay Hunt syndrome). Otol Neurotol. 32, 1025-1030. [CrossRef]
- Petersen, P. T., Bodilsen, J., Jepsen, M. P. G., Larsen, L., Storgaard, M., Helweg-Larsen, J., Wiese, L., Hansen, B. R., Lüttichau, H. R., Andersen, C. Ø., Nielsen, H., Brandt, C. T. and Danish Study Group of Infections of the Brain (DASGIB). (2023) Ramsay Hunt syndrome and concurrent varicella-zoster virus meningitis in Denmark: A nationwide cohort study. J Med Virol. 95, e29291. [CrossRef]
- Ansari, D., Ansari, D., Andersson, R. and Andren-Sandberg, A. (2015) Pancreatic cancer and thromboembolic disease, 150 years after Trousseau. Hepatobiliary Surg Nutr. 4, 325-335. [CrossRef]
- Falanga, A., Schieppati, F. and Russo, D. (2015) Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 41, 756-764. [CrossRef]
- Mrozinska, S., Cieslik, J., Broniatowska, E., Malinowski, K. P. and Undas, A. (2019) Prothrombotic fibrin clot properties associated with increased endogenous thrombin potential and soluble P-selectin predict occult cancer after unprovoked venous thromboembolism. J Thromb Haemost. 17, 1912-1922. [CrossRef]
- Ząbczyk, M. and Undas, A. (2024) Fibrin Clot Properties in Cancer: Impact on Cancer-Associated Thrombosis. Semin Thromb Hemost. 50, 402-412. [CrossRef]
- Cheung, S., Henderson-Sabes, J., Mastick, J., Abrams, G., Snowberg, K., Alfaro, E., Quinn, M., Paul, S., Cooper, B., Wallhagen, M., Conley, Y., Levine, J. and Miaskowski, C. (2023) Cancer survivors and neurotoxic chemotherapy: hearing loss and tinnitus. BMJ Support Palliat Care. 13, 345-353. [CrossRef]
- Strebel, S., Baust, K., Grabow, D., Byrne, J., Langer, T., Am Zehnhoff-Dinnesen, A., Kuonen, R., Weiss, A., Kepak, T., Kruseova, J., Berger, C., Calaminus, G., Sommer, G., Kuehni, C. E. and PanCareLIFE Consortium. (2025) Auditory complications among childhood cancer survivors and health-related quality of life: a PanCareLIFE study. J Cancer Surviv. 19, 162-173. [CrossRef]
- Meijer, A. J. M., Clemens, E., Hoetink, A. E., van Grotel, M. and van den Heuvel-Eibrink, M. M. (2019) Tinnitus during and after childhood cancer: A systematic review. Crit Rev Oncol Hematol. 135, 1-7. [CrossRef]
- Meijer, A. J. M., Fiocco, M. F., Janssens, G. O., Clemens, E., Tissing, W. J. E., Loonen, J. J., van Dulmen-den Broeder, E., de Vries, A. C. H., Bresters, D., Versluys, B., Ronckers, C. M., Kremer, L. C. M., van der Pal, H. J., Neggers, S., van der Heiden-van der Loo, M., Stokroos, R. J., Hoetink, A. E., van Grotel, M., van den Heuvel-Eibrink, M. M. and DCOG-LATER Consortium. (2020) Risk factors associated with tinnitus in 2948 Dutch survivors of childhood cancer: a Dutch LATER questionnaire study. Neurooncol Adv. 2, vdaa122. [CrossRef]
- Janowiak-Majeranowska, A., Abdulaziz-Opiela, G., Osowski, J. and Mikaszewski, B. (2024) Prevalence of platinum-induced ototoxicity among patients suffering from hematological malignancies - a systematic review. Contemp Oncol (Pozn). 28, 98-104. [CrossRef]
- Su, S. Y., Chien, W. C., Chung, C. H., Su, W. F. and Fu, E. (2022) Association of periodontitis with tinnitus: A population-based cohort study in Taiwan. J Clin Periodontol. 49, 970-979. [CrossRef]
- Pretorius, E., Mbotwe, S. and Kell, D. B. (2017) Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities. Sci Rep. 7, 9680. [CrossRef]
- Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., Kell, D. B. and Pretorius, E. (2019) Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. bioRxiv, 646307v646301. [CrossRef]
- Nunes, J. M., Fillis, T., Page, M. J., Venter, C., Lancry, O., Kell, D. B., Windberger, U. and Pretorius, E. (2020) Gingipain R1 and lipopolysaccharide from Porphyromonas gingivalis have major effects on blood clot morphology and mechanics. Front Immunol. 11, 1551. [CrossRef]
- Narsinh, K. H., Hui, F., Duvvuri, M., Meisel, K. and Amans, M. R. (2022) Management of vascular causes of pulsatile tinnitus. J Neurointerv Surg. 14, 1151-1157. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2024) The clots removed from ischaemic stroke patients by mechanical thrombectomy are amyloid in nature. medRxiv, 10.1101/2024.1111.1101.24316555v24316551. [CrossRef]
- Grixti, J. M., Chandran, A., Pretorius, J. H., Walker, M., Sekhar, A., Pretorius, E. and Kell, D. B. (2025) Amyloid presence in acute ischemic stroke thrombi: observational evidence for fibrinolytic resistance. Stroke. 56, e165-e167. [CrossRef]
- Jafari, Z., Kolb, B. E. and Mohajerani, M. H. (2019) Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 56, 100963. [CrossRef]
- Oosterloo, B. C., Croll, P. H., Baatenburg de Jong, R. J., Ikram, M. K. and Goedegebure, A. (2021) Prevalence of Tinnitus in an Aging Population and Its Relation to Age and Hearing Loss. Otolaryngol Head Neck Surg. 164, 859-868. [CrossRef]
- Reisinger, L., Schmidt, F., Benz, K., Vignali, L., Roesch, S., Kronbichler, M. and Weisz, N. (2023) Ageing as risk factor for tinnitus and its complex interplay with hearing loss-evidence from online and NHANES data. BMC Med. 21, 283. [CrossRef]
- Reisinger, L. and Weisz, N. (2024) Chronic tinnitus is associated with aging but not dementia. Hear Res. 453, 109135. [CrossRef]
- Wang, P., Konja, D., Singh, S., Zhang, B. and Wang, Y. (2024) Endothelial Senescence: From Macro- to Micro-Vasculature and Its Implications on Cardiovascular Health. Int J Mol Sci. 25, 1978. [CrossRef]
- Casale, M., Mangiacapra, F., Bressi, E., Pace, A., Sabatino, L., Moffa, A., Abbate, A., Di Sciascio, G. and Salvinelli, F. (2016) Idiopathic sensorineural hearing loss is associated with endothelial dysfunction. Int J Cardiol Heart Vasc. 12, 32-33. [CrossRef]
- Quaranta, N., De Ceglie, V. and D'Elia, A. (2016) Endothelial Dysfunction in Idiopathic Sudden Sensorineural Hearing Loss: A Review. Audiol Res. 6, 151. [CrossRef]
- Ungvari, Z., Tarantini, S., Sorond, F., Merkely, B. and Csiszar, A. (2020) Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J Am Coll Cardiol. 75, 931-941. [CrossRef]
- Cavallaro, G., Pantaleo, A., Pontillo, V., Barbara, F., Murri, A. and Quaranta, N. (2023) Endothelial Dysfunction and Metabolic Disorders in Patients with Sudden Sensorineural Hearing Loss. Medicina (Kaunas). 59, 1718. [CrossRef]
- Rissatto-Lago, M. R., Salles, C., Campos de Pinho, F. G., Menezes Lyra, I., Terse-Ramos, R., Teixeira, R. and Ladeia, A. M. (2017) Association between endothelial dysfunction and otoneurological symptoms in children with sickle cell disease. Hematology. 22, 299-303. [CrossRef]
- Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp`, J., Laubscher, J., Lourens, P. J., Steenkamp, J., Kell, D. B. and Pretorius, E. (2020) COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci. 21, 5168. [CrossRef]
- Laubscher, G. J., Lourens, P. J., Venter, C., Kell, D. B. and Pretorius, E. (2021) TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med. 10, 5381. [CrossRef]
- Durstenfeld, M. S., Weiman, S., Holtzman, M., Blish, C., Pretorius, R. and Deeks, S. G. (2024) Long COVID and post-acute sequelae of SARS-CoV-2 pathogenesis and treatment: A Keystone Symposia report. Ann N Y Acad Sci. 1535, 31-41. [CrossRef]
- Nunes, J. M., Kell, D. B. and Pretorius, E. (2024) Herpesvirus Infection of Endothelial Cells as a Systemic Pathological Axis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Viruses. 16, 572. [CrossRef]
- Nunes, M., Kell, L., Slaghekke, A., Wüst, R., Fielding, B., Kell, D. B. and Pretorius, E. (2025) Virus-induced endothelial senescence as a cause and driving factor for ME/CFS and Long COVID: mediated by a dysfunctional immune system. Preprints, 202505.201875.v202501. [CrossRef]
- Cianfrone, G., Pentangelo, D., Cianfrone, F., Mazzei, F., Turchetta, R., Orlando, M. P. and Altissimi, G. (2011) Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. 15, 601-636.
- Altissimi, G., Colizza, A., Cianfrone, G., de Vincentiis, M., Greco, A., Taurone, S., Musacchio, A., Ciofalo, A., Turchetta, R., Angeletti, D. and Ralli, M. (2020) Drugs inducing hearing loss, tinnitus, dizziness and vertigo: an updated guide. Eur Rev Med Pharmacol Sci. 24, 7946-7952. [CrossRef]
- Lin, C. D., Kao, M. C., Tsai, M. H., Lai, C. H., Wei, I. H., Tsai, M. H., Tang, C. H., Lin, C. W., Hsu, C. J. and Lin, C. Y. (2011) Transient ischemia/hypoxia enhances gentamicin ototoxicity via caspase-dependent cell death pathway. Lab Invest. 91, 1092-1106. [CrossRef]
- Abd-Elhafiz, H. I., Faried, M. A., Khodir, S. A., Moaty, A. S. and Sweed, E. M. (2024) Ezetimibe protects against Gentamycin-induced ototoxicity in rats by antioxidants, anti-inflammatory mechanisms, and BDNF upregulation. Immunopharmacol Immunotoxicol. 46, 635-650. [CrossRef]
- Kros, C. J. and Steyger, P. S. (2019) Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb Perspect Med. 9. [CrossRef]
- Dille, M. F., Konrad-Martin, D., Gallun, F., Helt, W. J., Gordon, J. S., Reavis, K. M., Bratt, G. W. and Fausti, S. A. (2010) Tinnitus onset rates from chemotherapeutic agents and ototoxic antibiotics: results of a large prospective study. J Am Acad Audiol. 21, 409-417. [CrossRef]
- Clemens, E., de Vries, A. C., Pluijm, S. F., Am Zehnhoff-Dinnesen, A., Tissing, W. J., Loonen, J. J., van Dulmen-den Broeder, E., Bresters, D., Versluys, B., Kremer, L. C., van der Pal, H. J., van Grotel, M., van den Heuvel-Eibrink, M. M. and DCOG-LATER The Netherlands. (2016) Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. Eur J Cancer. 69, 77-85. [CrossRef]
- Paken, J., Govender, C. D., Pillay, M. and Sewram, V. (2016) Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J Toxicol. 2016, 1809394. [CrossRef]
- Wang, X., Zhou, Y., Wang, D., Wang, Y., Zhou, Z., Ma, X., Liu, X. and Dong, Y. (2023) Cisplatin-induced ototoxicity: From signaling network to therapeutic targets. Biomed Pharmacother. 157, 114045. [CrossRef]
- Lanvers-Kaminsky, C., Zehnhoff-Dinnesen, A. A., Parfitt, R. and Ciarimboli, G. (2017) Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin Pharmacol Ther. 101, 491-500. [CrossRef]
- Waissbluth, S. and Daniel, S. J. (2013) Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hear Res. 299, 37-45. [CrossRef]
- Paken, J., Govender, C. D., Pillay, M. and Sewram, V. (2019) A Review of Cisplatin-Associated Ototoxicity. Semin Hear. 40, 108-121. [CrossRef]
- Rybak, L. P., Mukherjea, D. and Ramkumar, V. (2019) Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Semin Hear. 40, 197-204. [CrossRef]
- Attar, M., Alqarni, M. S., Alsinnari, Y. M., Bukhari, Z. M., Alshegifi, H., Alzhrani, A., Alshaikh, K., Alsubaie, H., Muqat, M., Alhakami, H. and Algarni, M. (2022) The Incidence and Risk Factors of Cisplatin and Carboplatin Ototoxicity in Pediatric Oncology Patients at Tertiary Oncology Center. Indian J Surg Oncol. 13, 925-930. [CrossRef]
- Chattaraj, A., Syed, M. P., Low, C. A. and Owonikoko, T. K. (2023) Cisplatin-Induced Ototoxicity: A Concise Review of the Burden, Prevention, and Interception Strategies. JCO Oncol Pract. 19, 278-283. [CrossRef]
- Correa-Morales, J. E., Giraldo-Moreno, S., Mantilla-Manosalva, N., Cuellar-Valencia, L., Borja-Montes, O. F., Bedoya-Muñoz, L. J., Iriarte-Aristizábal, M. F., Quintero-Munoz, E. and Zuluaga-Liberato, A. M. (2024) Prevention and treatment of cisplatin-induced ototoxicity in adults: A systematic review. Clin Otolaryngol. 49, 1-15. [CrossRef]
- Orasan, A., Negru, M. C., Morgovan, A. I., Fleser, R. C., Sandu, D., Sitaru, A. M., Motofelea, A. C. and Balica, N. C. (2025) Strategies to Mitigate Cisplatin-Induced Ototoxicity: A Literature Review of Protective Agents, Mechanisms, and Clinical Gaps. Audiol Res. 15, 22. [CrossRef]
- Chen, Y., Bielefeld, E. C., Mellott, J. G., Wang, W., Mafi, A. M., Yamoah, E. N. and Bao, J. (2021) Early Physiological and Cellular Indicators of Cisplatin-Induced Ototoxicity. J Assoc Res Otolaryngol. 22, 107-126. [CrossRef]
- Tan, W. J. T. and Vlajkovic, S. M. (2023) Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int J Mol Sci. 24, 16545. [CrossRef]
- Salah, R. S., Mahmoud, A. A., El-Shiekh, R. A., El-Dessouki, A. M., Hassan, A. G. A. and Khalaf, S. S. (2025) A comprehensive review of the impact of natural products in preventing drug-induced ototoxicity. Inflammopharmacology. [CrossRef]
- Al Aameri, R. F. H., Alanisi, E. M. A., Al Sallami, D., Alberts, I., Tischkau, S., Rybak, L. P. and Ramkumar, V. (2025) Role of RGS17 in cisplatin-induced cochlear inflammation and ototoxicity via caspase-3 activation. Front Immunol. 16, 1470625. [CrossRef]
- Han, S., Sun, J., Li, W., Li, J., Yu, H., Wang, S. and Chi, Y. (2025) Studies on the mechanism of ototoxic action of cisplatin and the antagonistic effect of polyphenolic compounds. Front Pharmacol. 16, 1586243. [CrossRef]
- Iaţentiuc, A., Iaţentiuc, I. M., Frăsinariu, O. E., Cozma, S. R., Bitere-Popa, O. R., Olariu, R., Rădulescu, L. M., Ioniuc, I., Cuciureanu, M., Alecsa, M., Guma, C. and Miron, I. C. (2025) The Role of Genetic and Non-Genetic Factors in the Occurrence of Cisplatin-Associated Ototoxicity. Int J Mol Sci. 26, 4787. [CrossRef]
- Lee, J. J. W., Latif, A., Scott, E. N., Thakral, A., Mahler, M. B., Brooks, B., Hueniken, K., Billfalk-Kelly, A., Espin-Garcia, O., Zhan, L. J., Rassekh, S. R., Pecheux, L., Spavor, M., Li, Y., Goldstein, D., Hope, A., Ross, C. J., Liu, G., Carleton, B. C. and Bhavsar, A. P. (2025) TLR4 downregulation protects against cisplatin-induced ototoxicity in adult and pediatric patients with cancer. J Pharmacol Exp Ther. 392, 100057. [CrossRef]
- Ouyang, L., Ma, L. and Feng, Y. (2025) Protective effects of MET channels on aminoglycosides- and cisplatin-induced ototoxicity. Int J Med Sci. 22, 732-744. [CrossRef]
- Briggs, E. E., Kallenberger, E. M., Nguyen, S. A., Dixon, P. R., Drawdy, A. V., Kejner, A. E., Kaczmar, J. M., Newman, J. G. and Albergotti, W. G. (2025) Preventing Cisplatin-Induced Hearing Loss in Adults: A Systematic Review and Meta-Analysis. Otol Neurotol. 46, 351-357. [CrossRef]
- Dobson, P. D. and Kell, D. B. (2008) Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Disc. 7, 205-220. [CrossRef]
- Giacomini, K. M., Huang, S. M., Tweedie, D. J., Benet, L. Z., Brouwer, K. L., Chu, X., Dahlin, A., Evers, R., Fischer, V., Hillgren, K. M., Hoffmaster, K. A., Ishikawa, T., Keppler, D., Kim, R. B., Lee, C. A., Niemi, M., Polli, J. W., Sugiyama, Y., Swaan, P. W., Ware, J. A., Wright, S. H., Wah Yee, S., Zamek-Gliszczynski, M. J. and Zhang, L. (2010) Membrane transporters in drug development. Nat Rev Drug Discov. 9, 215-236. [CrossRef]
- Kell, D. B. and Oliver, S. G. (2014) How drugs get into cells: tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion. Front Pharmacol. 5, 231. [CrossRef]
- Superti-Furga, G., Lackner, D., Wiedmer, T., Ingles-Prieto, A., Barbosa, B., Girardi, E., Ulrich Goldman, Gürtl, B., Klavins, K., Klimek, C., Lindinge, S., Liñeiro-Retes, E., Müller, A. C., Onstein, S., Gregor Redinge, Reil, D., Sedlyarov, V., Wolf, G., Crawford, M., Everley, R., Hepworth, D., Liu, S., Noell, S., Piotrowski, M., Stanton, R., Zhang, H., Corallino, S., Faedo, A., Insidioso, M., Maresca, G., Redaelli, L., Sassone, F., Scarabottolo, L., Stucchi, M., Tarroni, P., Tremolada, S., Batoulis, H., Becker, A., Bender, E., Chang, Y.-N., Ehrmann, A., Müller-Fahrnow, A., Pütter, V., Zindel, D., Hamilton, B., Lenter, M., Santacruz, D., Viollet, C., Whitehurst, C., Johnsson, K., Leippe, P., Baumgarten, B., Chang, L., Ibig, Y., Pfeifer, M., Reinhardt, J., Schönbett, J., Selzer, P., Seuwen, K., Bettembourg, C., Biton, B., Czech, J., de Foucauld, H., Didier, M., Licher, T., Mikol, V., Pommereau, A., Puech, F., Yaligara, V., Edwards, A., Bongers, B. J., Heitman, L. H., IJzerman, A. P., Sijben, H. J., van Westen, G. J. P., Grixti, J., Kell, D. B., Mughal, F., Swainston, N., Wright Muelas, M., Bohstedt, T., Burgess-Brown, N., Carpenter, L., Dürr, K., Jesper Hanse, Scacioc, A., Banci, G., Colas, C., Daniela Digle, Ecker, G., Füzi, B., Gamsjäger, V., Grandits, M., Martini, R., Troger, F., Altermatt, P., Doucerain, C., Dürrenberger, F., Manolova, V., Steck, A.-L., Sundström, H., Wilhelm, M. and Steppan, C. M. (2020) The RESOLUTE consortium: unlocking SLC transporters for drug discovery. Nat Rev Drug Discov. 19, 429-430. [CrossRef]
- Dvorak, V., Wiedmer, T., Ingles-Prieto, A., Altermatt, P., Batoulis, H., Bärenz, F., Bender, E., Digles, D., Dürrenberger, F., Heitman, L. H., Ijzerman, A. P., Kell, D. B., Kickinger, S., Körzö, D., Leippe, P., Licher, T., Manolova, V., Rizzetto, R., Sassone, F., Scarabottolo, L., Schlessinger, A., Schneider, V., Sijben, H. J., Steck, A.-L., Sundström, H., Tremolada, S., Wilhelm, M., Wright Muelas, M., Zindel, D., Steppan, C. M. and Superti-Furga, G. (2021) An overview of cell-based assay platforms for the solute carriers family of transporters. Front Pharmacol. 12, 722889. [CrossRef]
- Kell, D. B. (2021) The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: how and why phospholipid bilayer transport is negligible in real biomembranes. Molecules. 26, 5629. [CrossRef]
- Ciarimboli, G. (2012) Membrane Transporters as Mediators of Cisplatin Effects and Side Effects. Scientifica. 2012, 473829. [CrossRef]
- Ciarimboli, G. (2014) Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 34, 547-550.
- Lanvers-Kaminsky, C. and Ciarimboli, G. (2017) Pharmacogenetics of drug-induced ototoxicity caused by aminoglycosides and cisplatin. Pharmacogenomics. 18, 1683-1695. [CrossRef]
- Waissbluth, S., Martinez, A. D., Figueroa-Cares, C., Sánchez, H. A. and Maass, J. C. (2023) MATE1 expression in the cochlea and its potential involvement in cisplatin cellular uptake and ototoxicity. Acta Otolaryngol. 143, 242-249. [CrossRef]
- Hucke, A., Kantauskaite, M., Kopp, T. N., Wehe, C. A., Karst, U., Nedvetsky, P. I. and Ciarimboli, G. (2024) Modulating the Activity of the Human Organic Cation Transporter 2 Emerges as a Potential Strategy to Mitigate Unwanted Toxicities Associated with Cisplatin Chemotherapy. Int J Mol Sci. 25. [CrossRef]
- Langer, T., Clemens, E., Broer, L., Maier, L., Uitterlinden, A. G., de Vries, A. C. H., van Grotel, M., Pluijm, S. F. M., Binder, H., Mayer, B., von dem Knesebeck, A., Byrne, J., van Dulmen-den Broeder, E., Crocco, M., Grabow, D., Kaatsch, P., Kaiser, M., Spix, C., Kenborg, L., Winther, J. F., Rechnitzer, C., Hasle, H., Kepak, T., van der Kooi, A. F., Kremer, L. C., Kruseova, J., Bielack, S., Sorg, B., Hecker-Nolting, S., Kuehni, C. E., Ansari, M., Kompis, M., van der Pal, H. J., Parfitt, R., Deuster, D., Matulat, P., Tillmanns, A., Tissing, W. J. E., Beck, J. D., Elsner, S., Am Zehnhoff-Dinnesen, A., van den Heuvel-Eibrink, M. M., Zolk, O. and PanCareLIFE consortium. (2020) Association of candidate pharmacogenetic markers with platinum-induced ototoxicity: PanCareLIFE dataset. Data Brief. 32, 106227. [CrossRef]
- Fetoni, A. R., Eramo, S. L., Rolesi, R., Troiani, D. and Paludetti, G. (2012) Antioxidant treatment with coenzyme Q-ter in prevention of gentamycin ototoxicity in an animal model. Acta Otorhinolaryngol Ital. 32, 103-110.
- Le, T. A., Hiba, T., Chaudhari, D., Preston, A. N., Palowsky, Z. R., Ahmadzadeh, S., Shekoohi, S., Cornett, E. M. and Kaye, A. D. (2023) Aminoglycoside-Related Nephrotoxicity and Ototoxicity in Clinical Practice: A Review of Pathophysiological Mechanism and Treatment Options. Adv Ther. 40, 1357-1365. [CrossRef]
- Maniu, A., Perde-Schrepler, M. and Cosgarea, M. (2011) Protective effect of L-N-acetylcysteine against gentamycin ototoxicity in the organ cultures of the rat cochlea. Rom J Morphol Embryol. 52, 159-164.
- Ramkumar, V., Mukherjea, D., Dhukhwa, A. and Rybak, L. P. (2021) Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants (Basel). 10, 1919. [CrossRef]
- Katanić Stanković, J. S., Selaković, D. and Rosić, G. (2023) Oxidative Damage as a Fundament of Systemic Toxicities Induced by Cisplatin-The Crucial Limitation or Potential Therapeutic Target? Int J Mol Sci. 24, 14574. [CrossRef]
- Domarecka, E., Skarzynska, M., Szczepek, A. J. and Hatzopoulos, S. (2020) Use of zebrafish larvae lateral line to study protection against cisplatin-induced ototoxicity: A scoping review. Int J Immunopathol Pharmacol. 34, 2058738420959554. [CrossRef]
- Li, Y., Zhang, T., Song, Q., Gao, D., Li, Y., Jie, H., Huang, P., Zheng, G., Yang, J. and He, J. (2023) Cisplatin ototoxicity mechanism and antagonistic intervention strategy: a scope review. Front Cell Neurosci. 17, 1197051. [CrossRef]
- Rose, O., Croonenberg, T., Clemens, S., Hinteregger, T., Eppacher, S., Huber-Cantonati, P., Garcia-Miralles, M., Liuni, R. and Dossena, S. (2024) Cisplatin-Induced Hearing Loss, Oxidative Stress, and Antioxidants as a Therapeutic Strategy-A State-of-the-Art Review. Antioxidants (Basel). 13, 1578. [CrossRef]
- Tawalbeh, M., Ibrahim, R. M., Al-Saraireh, T., Khreesha, L. and Rawashdeh, B. A. (2025) Intratympanic N-acetylcysteine in the prevention of cisplatin-induced ototoxicity: a systematic review and meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. 26, 26. [CrossRef]
- Rosic, G., Srejovic, I., Zivkovic, V., Selakovic, D., Joksimovic, J. and Jakovljevic, V. (2015) The effects of N-acetylcysteine on cisplatin-induced cardiotoxicity on isolated rat hearts after short-term global ischemia. Toxicol Rep. 2, 996-1006. [CrossRef]
- Lateef Al-Awsi, G. R., Arshed, U., Arif, A., Ramirez-Coronel, A. A., Alhassan, M. S., Mustafa, Y. F., Rahman, F. F., Zabibah, R. S., Gupta, J., Iqbal, M. S., Iswanto, A. H. and Farhood, B. (2024) The Chemoprotective Potentials of Alpha-lipoic Acid against Cisplatin-induced Ototoxicity: A Systematic Review. Curr Med Chem. 31, 3588-3603. [CrossRef]
- Kim, K. H., Lee, B., Kim, Y. R., Kim, M. A., Ryu, N., Jung, D. J., Kim, U. K., Baek, J. I. and Lee, K. Y. (2018) Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis. 9, 827. [CrossRef]
- Gu, J., Wang, X., Chen, Y., Xu, K., Yu, D. and Wu, H. (2022) An enhanced antioxidant strategy of astaxanthin encapsulated in ROS-responsive nanoparticles for combating cisplatin-induced ototoxicity. J Nanobiotechnology. 20, 268. [CrossRef]
- Xiong, M., He, Q., Wang, J. and Lai, H. (2011) Astragalosides reduce cisplatin ototoxicity in guinea pigs. ORL J Otorhinolaryngol Relat Spec. 73, 131-136. [CrossRef]
- Zhao, W., Fan, W., Hu, R., Lou, J., Chen, G., Cai, Z. and Chen, S. J. (2025) The antioxidant ergothioneine alleviates cisplatin-induced hearing loss through the Nrf2 pathway. Antioxid Redox Signal. 42, 97-114. [CrossRef]
- Tan, X., Zhou, Y., Agarwal, A., Lim, M., Xu, Y., Zhu, Y., O'Brien, J., Tran, E., Zheng, J., Gius, D. and Richter, C. P. (2020) Systemic application of honokiol prevents cisplatin ototoxicity without compromising its antitumor effect. Am J Cancer Res. 10, 4416-4434.
- Fetoni, A. R., Paciello, F. and Troiani, D. (2022) Cisplatin Chemotherapy and Cochlear Damage: Otoprotective and Chemosensitization Properties of Polyphenols. Antioxid Redox Signal. 36, 1229-1245. [CrossRef]
- Yumusakhuylu, A. C., Yazici, M., Sari, M., Binnetoglu, A., Kosemihal, E., Akdas, F., Sirvanci, S., Yuksel, M., Uneri, C. and Tutkun, A. (2012) Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 76, 404-408. [CrossRef]
- Alsaikhan, F., Jasim, S. A., Margiana, R., Opulencia, M. J. C., Yasin, G., Hammid, A. T., Nasretdinova, M. T., Mahdi, A. B., Farhood, B., Abedi-Firouzjah, R., Jamialahmadi, T. and Sahebkar, A. (2024) Recent Update on the Protective Potentials of Resveratrol against Cisplatin-induced Ototoxicity: A Systematic Review. Curr Med Chem. 31, 4850-4866. [CrossRef]
- Freyer, D. R., Chen, L., Krailo, M. D., Knight, K., Villaluna, D., Bliss, B., Pollock, B. H., Ramdas, J., Lange, B., Van Hoff, D., VanSoelen, M. L., Wiernikowski, J., Neuwelt, E. A. and Sung, L. (2017) Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 18, 63-74. [CrossRef]
- Brock, P. R., Maibach, R., Childs, M., Rajput, K., Roebuck, D., Sullivan, M. J., Laithier, V., Ronghe, M., Dall'Igna, P., Hiyama, E., Brichard, B., Skeen, J., Mateos, M. E., Capra, M., Rangaswami, A. A., Ansari, M., Rechnitzer, C., Veal, G. J., Covezzoli, A., Brugieres, L., Perilongo, G., Czauderna, P., Morland, B. and Neuwelt, E. A. (2018) Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med. 378, 2376-2385. [CrossRef]
- Chen, C. H., Huang, C. Y., Lin, H. H., Wang, M. C., Chang, C. Y. and Cheng, Y. F. (2021) Association of Sodium Thiosulfate With Risk of Ototoxic Effects From Platinum-Based Chemotherapy: A Systematic Review and Meta-analysis. JAMA Netw Open. 4, e2118895. [CrossRef]
- Abrams, J., Hill, R., Pizer, B., Bate, J. and Group, C. S. C. S. I. (2025) Sodium Thiosulfate for Ototoxicity Prevention: A National Review of UK Practice. Pediatr Blood Cancer. 72, e31536. [CrossRef]
- Kallenberger, E. M., Briggs, E. E., Nguyen, S. A., Dixon, P. R., Drawdy, A. V., Labadie, R. F., Meyer, T. A. and White, D. R. (2025) Preventing Hearing Loss in Children Receiving Cisplatin: A Systematic Review and Meta-Analysis. Laryngoscope. 135, 2662-2669. [CrossRef]
- Ramot, Y. and Nyska, A. (2007) Drug-induced thrombosis--experimental, clinical, and mechanistic considerations. Toxicol Pathol. 35, 208-225. [CrossRef]
- Ramot, Y., Nyska, A. and Spectre, G. (2013) Drug-induced thrombosis: an update. Drug Saf. 36, 585-603. [CrossRef]
- Al-Nouri, Z. L., Reese, J. A., Terrell, D. R., Vesely, S. K. and George, J. N. (2015) Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 125, 616-618. [CrossRef]
- Girolami, A., Cosi, E., Tasinato, V., Santarossa, C., Ferrari, S. and Girolami, B. (2017) Drug-Induced Thrombophilic or Prothrombotic States: An Underestimated Clinical Problem That Involves Both Legal and Illegal Compounds. Clin Appl Thromb Hemost. 23, 775-785. [CrossRef]
- Mazzierli, T., Allegretta, F., Maffini, E. and Allinovi, M. (2023) Drug-induced thrombotic microangiopathy: An updated review of causative drugs, pathophysiology, and management. Front Pharmacol. 13, 1088031. [CrossRef]
- Doll, D. C., List, A. F., Greco, F. A., Hainsworth, J. D., Hande, K. R. and Johnson, D. H. (1986) Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 105, 48-51. [CrossRef]
- Czaykowski, P. M., Moore, M. J. and Tannock, I. F. (1998) High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 160, 2021-2024. [CrossRef]
- Li, S. H., Chen, W. H., Tang, Y., Rau, K. M., Chen, Y. Y., Huang, T. L., Liu, J. S. and Huang, C. H. (2006) Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg. 108, 150-156. [CrossRef]
- Meattini, I., Scotti, V., Pescini, F., Livi, L., Sulprizio, S., Palumbo, V., Sarti, C. and Biti, G. (2010) Ischemic stroke during cisplatin-based chemotherapy for testicular germ cell tumor: case report and review of the literature. J Chemother. 22, 134-136. [CrossRef]
- Fernandes, D. D., Louzada, M. L., Souza, C. A. and Matzinger, F. (2011) Acute aortic thrombosis in patients receiving cisplatin-based chemotherapy. Curr Oncol. 18, e97-e100. [CrossRef]
- Ito, S., Nakamura, Y., Noumi, T. and Sasaki, Y. (2013) Acute aortic thrombosis during cisplatin based chemotherapy for gastric cancer. Intern Med. 52, 973-975. [CrossRef]
- Heidegger, I., Porres, D., Veek, N., Heidenreich, A. and Pfister, D. (2017) Predictive Factors for Developing Venous Thrombosis during Cisplatin-Based Chemotherapy in Testicular Cancer. Urol Int. 99, 104-109. [CrossRef]
- Muto, J., Kishimoto, H., Kaizuka, Y., Kinjo, M., Higashi, H. and Kishihara, F. (2017) Thrombotic Microangiopathy Following Chemotherapy with S-1 and Cisplatin in a Patient with Gastric Cancer: A Case Report. In Vivo. 31, 439-441. [CrossRef]
- Cameron, A. C., McMahon, K., Hall, M., Neves, K. B., Rios, F. J., Montezano, A. C., Welsh, P., Waterston, A., White, J., Mark, P. B., Touyz, R. M. and Lang, N. N. (2020) Comprehensive Characterization of the Vascular Effects of Cisplatin-Based Chemotherapy in Patients With Testicular Cancer. JACC CardioOncol. 2, 443-455. [CrossRef]
- Kanjanapan, Y., Gilbourd, D. and Pranavan, G. (2020) Acute ischaemic stroke following cisplatin-based chemotherapy for testicular cancer. BMJ Case Rep. 13, e235005. [CrossRef]
- Haugnes, H. S., Negaard, H. F., Jensvoll, H., Wilsgaard, T., Tandstad, T. and Solberg, A. (2021) Thromboembolic Events During Treatment with Cisplatin-based Chemotherapy in Metastatic Testicular Germ-cell Cancer 2000-2014: A Population-based Cohort Study. Eur Urol Open Sci. 32, 19-27. [CrossRef]
- Valiyaveettil, D., Jilla, S., Krishna, J. M., Kollu, R., Patil, C. and Gupta, R. (2021) Cisplatin induced cerebral sinus venous thrombosis in cervical cancer patients treated with concurrent chemoradiation: a case series. Ecancermedicalscience. 15, 1320. [CrossRef]
- Aoki, R., Kato, S., Nakajima, K., Sakai, J., Yoshida, K., Masui, H., Ikeda, S., Yoshigi, J. and Utsunomiya, D. (2023) Superior mesenteric artery embolism associated with Cisplatin-induced aortic thrombosis. BJR Case Rep. 9, 20220149. [CrossRef]
- Anders, J. C., Grigsby, P. W. and Singh, A. K. (2006) Cisplatin chemotherapy (without erythropoietin) and risk of life-threatening thromboembolic events in carcinoma of the uterine cervix: the tip of the iceberg? A review of the literature. Radiat Oncol. 1, 14. [CrossRef]
- Moore, R. A., Adel, N., Riedel, E., Bhutani, M., Feldman, D. R., Tabbara, N. E., Soff, G., Parameswaran, R. and Hassoun, H. (2011) High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 29, 3466-3473. [CrossRef]
- Proverbs-Singh, T., Chiu, S. K., Liu, Z., Seng, S., Sonpavde, G., Choueiri, T. K., Tsao, C. K., Yu, M., Hahn, N. M., Oh, W. K. and Galsky, M. D. (2012) Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 104, 1837-1840. [CrossRef]
- Seng, S., Liu, Z., Chiu, S. K., Proverbs-Singh, T., Sonpavde, G., Choueiri, T. K., Tsao, C. K., Yu, M., Hahn, N. M., Oh, W. K. and Galsky, M. D. (2012) Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol. 30, 4416-4426. [CrossRef]
- Lee, Y. G., Lee, E., Kim, I., Lee, K. W., Kim, T. M., Lee, S. H., Kim, D. W. and Heo, D. S. (2015) Cisplatin-Based Chemotherapy Is a Strong Risk Factor for Thromboembolic Events in Small-Cell Lung Cancer. Cancer Res Treat. 47, 670-675. [CrossRef]
- Gizzi, M., Oberic, L., Massard, C., Poterie, A., Le Teuff, G., Loriot, Y., Albiges, L., Baciarello, G., Michels, J., Bossi, A., Blanchard, P., Escudier, B. and Fizazi, K. (2016) Predicting and preventing thromboembolic events in patients receiving cisplatin-based chemotherapy for germ cell tumours. Eur J Cancer. 69, 151-157. [CrossRef]
- Solari, L., Kronig, M., Ihorst, G., Drognitz, K., Heinz, J., Jilg, C. A., Schultze-Seemann, W., Engelhardt, M. and Waller, C. F. (2016) High Rates of Thromboembolic Events in Patients with Germ Cell Cancer Undergoing Cisplatin-Based Polychemotherapy. Urol Int. 96, 399-405. [CrossRef]
- Zahir, M. N., Shaikh, Q., Shabbir-Moosajee, M. and Jabbar, A. A. (2017) Incidence of Venous Thromboembolism in cancer patients treated with Cisplatin based chemotherapy - a cohort study. BMC Cancer. 17, 57. [CrossRef]
- Abdel-Razeq, H., Mansour, A., Abdulelah, H., Al-Shwayat, A., Makoseh, M., Ibrahim, M., Abunasser, M., Rimawi, D., Al-Rabaiah, A., Alfar, R., Abufara, A., Ibrahim, A., Bawaliz, A. and Ismael, Y. (2018) Thromboembolic events in cancer patients on active treatment with cisplatin-based chemotherapy: another look! Thromb J. 16, 2. [CrossRef]
- Mitani, A., Jo, T., Yasunaga, H., Sakamoto, Y., Hasegawa, W., Urushiyama, H., Yamauchi, Y., Matsui, H., Fushimi, K. and Nagase, T. (2018) Venous thromboembolic events in patients with lung cancer treated with cisplatin-based versus carboplatin/nedaplatin-based chemotherapy. Anticancer Drugs. 29, 560-564. [CrossRef]
- May, B. M., Soldatelli, M. D. and Torres, F. S. (2019) Pulmonary thromboembolism and coronary thrombosis after chemotherapy with cisplatin: simultaneous diagnosis with non-ECG gated computed tomography. Radiol Bras. 52, 198-199. [CrossRef]
- Shields, L. B. E., Daniels, M. W., Mar, N. and Rezazadeh Kalebasty, A. (2021) Thromboembolic events in metastatic testicular cancer treated with cisplatin-based chemotherapy. World J Clin Oncol. 12, 183-194. [CrossRef]
- Cho, H., Choi, J. H., Kang, S. Y., Lee, H. W., Choi, Y. W., Kim, T. H., Ahn, M. S., Kim, C. H., Shin, Y. S., Jang, J. Y., Oh, Y. T., Heo, J. and Sheen, S. S. (2022) Analysis of thromboembolic events in head and neck cancer patients who underwent concurrent chemoradiotherapy with cisplatin. Korean J Intern Med. 37, 653-659. [CrossRef]
- Herrmann, J., Yang, E. H., Iliescu, C. A., Cilingiroglu, M., Charitakis, K., Hakeem, A., Toutouzas, K., Leesar, M. A., Grines, C. L. and Marmagkiolis, K. (2016) Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 133, 1272-1289. [CrossRef]
- Cerrud-Rodriguez, R. C., Quinteros, M. G. and Azam, M. (2017) Internal carotid artery occlusion and stroke as a complication of cisplatin-based chemotherapy for metastatic testicular germ cell tumour. BMJ Case Rep. 2017. [CrossRef]
- Soultati, A., Mountzios, G., Avgerinou, C., Papaxoinis, G., Pectasides, D., Dimopoulos, M. A. and Papadimitriou, C. (2012) Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 38, 473-483. [CrossRef]
- Cameron, A. C., Touyz, R. M. and Lang, N. N. (2016) Vascular Complications of Cancer Chemotherapy. Can J Cardiol. 32, 852-862. [CrossRef]
- Gupta, A., Long, J. B., Chen, J., Gross, C. P., Feldman, D. R. and Steingart, R. M. (2016) Risk of Vascular Toxicity with Platinum Based Chemotherapy in Elderly Patients with Bladder Cancer. J Urol. 195, 33-40. [CrossRef]
- Herrmann, J. (2020) Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 17, 503-522. [CrossRef]
- Grover, S. P., Hisada, Y. M., Kasthuri, R. S., Reeves, B. N. and Mackman, N. (2021) Cancer Therapy-Associated Thrombosis. Arterioscler Thromb Vasc Biol. 41, 1291-1305. [CrossRef]
- Pantazi, D. and Tselepis, A. D. (2022) Cardiovascular toxic effects of antitumor agents: Pathogenetic mechanisms. Thromb Res. 213 Suppl 1, S95-S102. [CrossRef]
- Nagy, A., Börzsei, D., Hoffmann, A., Török, S., Veszelka, M., Almási, N., Varga, C. and Szabó, R. (2024) A Comprehensive Overview on Chemotherapy-Induced Cardiotoxicity: Insights into the Underlying Inflammatory and Oxidative Mechanisms. Cardiovasc Drugs Ther. [CrossRef]
- Orszaghova, Z., Mladosievicova, B., Mego, M. and Chovanec, M. (2025) Cardiovascular toxicity in testicular germ cell tumor survivors. Front Oncol. 15, online. [CrossRef]
- Waissbluth, S., Garnier, D., Akinpelu, O. V., Salehi, P. and Daniel, S. J. (2017) The impact of erdosteine on cisplatin-induced ototoxicity: a proteomics approach. Eur Arch Otorhinolaryngol. 274, 1365-1374. [CrossRef]
- El Beaino, M., McCaskey, M. K. and Eter, E. (2018) Sulodexide Monotherapy in Chronic Idiopathic Subjective Tinnitus: A Randomized Controlled Trial. Otolaryngol Head Neck Surg. 158, 1107-1112. [CrossRef]
- Neri, G., De Stefano, A., Baffa, C., Kulamarva, G., Di Giovanni, P., Petrucci, G., Poliandri, A., Dispenza, F., Citraro, L. and Croce, A. (2009) Treatment of central and sensorineural tinnitus with orally administered Melatonin and Sulodexide: personal experience from a randomized controlled study. Acta Otorhinolaryngol Ital. 29, 86-91.
- Ferrari, G., Agnese, A., Cavallero, A., Delehaye, E., Rocchetti, O., Rossi, W. and Tombolini, A. (2015) Medical and surgical treatments for tinnitus: the efficacy of combined treatment with sulodexide and melatonin. J Neurosurg Sci. 59, 1-9.
- Mora, R., Salami, A., Barbieri, M., Mora, F., Passali, G. C., Capobianco, S. and Magnan, J. (2003) The use of sodium enoxaparin in the treatment of tinnitus. Int Tinnitus J. 9, 109-111.
- Mora, R., Dellepiane, M., Mora, F. and Jankowska, B. (2006) Sodium enoxaparin and venovenous hemofiltration in treating sudden sensorineural hearing loss and tinnitus. Int Tinnitus J. 12, 83-86.
- Arif, T. and Gull, H. (2022) Anticoagulation in the Treatment of Pulsatile Tinnitus Caused by Internal Jugular Vein Stenosis: A Rare Case Report. J Med Res Surg. 3, 68. [CrossRef]
- Gonzalez-Ochoa, A. J., Raffetto, J. D., Hernández, A. G., Zavala, N., Gutiérrez, O., Vargas, A. and Loustaunau, J. (2021) Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb Haemost. 121, 944-954. [CrossRef]
- Charfeddine, S., Ibnhadjamor, H., Jdidi, J., Torjmen, S., Kraiem, S., Bahloul, A., Makni, A., Kallel, N., Moussa, N., Boudaya, M., Touil, I., Ghrab, A., Elghoul, J., Meddeb, Z., Thabet, Y., Ben Salem, K., Addad, F., Bouslama, K., Milouchi, S., Hammami, R., Abdessalem, S. and Abid, L. (2022) Sulodexide Significantly Improves Endothelial Dysfunction and Alleviates Chest Pain and Palpitations in Patients With Long-COVID-19: Insights From TUN-EndCOV Study. Front Cardiovasc Med. 9, 866113. [CrossRef]
- Wright, C., Kell, D. B., Pretorius, E. and Putrino, D. (2025) Treatment of Long Covid with enoxaparin. Cardiopulmonary Phys Ther J. 36, 70-73. [CrossRef]
- Chen, H., McGowan, E. M., Ren, N., Lal, S., Nassif, N., Shad-Kaneez, F., Qu, X. and Lin, Y. (2018) Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomarker Insights. 13, 1177271918785130. [CrossRef]
- Fujita, M., Nomura, K., Hong, K., Ito, Y., Asada, A. and Nishimuro, S. (1993) Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. 197, 1340-1347. [CrossRef]
- Sumi, H., Hamada, H., Tsushima, H., Mihara, H. and Muraki, H. (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 43, 1110-1111. [CrossRef]
- Sumi, H., Hamada, H., Nakanishi, K. and Hiratani, H. (1990) Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 84, 139-143. [CrossRef]
- Sumi, H., Yanagisawa, Y., Yatagai, C. and Saito, J. (2004) Natto bacillus as an oral fibrinolytic agent: Nattokinase activity and the ingestion effect of Bacillus subtilis natto. Food Science and Technology Research. 10, 17-20.
- Takagaki, S., Suzuki, M., Suzuki, E. and Hasumi, K. (2020) Unsaturated fatty acids enhance the fibrinolytic activity of subtilisin NAT (nattokinase). J Food Biochem. 44, e13326. [CrossRef]
- Granito, M., Alvarenga, L., Ribeiro, M., Carvalhosa, P., Andrade, T., Mesquita, C. T., Stockler-Pinto, M. B., Mafra, D. and Cardozo, L. F. (2024) Nattokinase as an adjuvant therapeutic strategy for non-communicable diseases: a review of fibrinolytic, antithrombotic, anti-inflammatory, and antioxidant effects. Expert Rev Cardiovasc Ther. 22, 565-574. [CrossRef]
- Li, Y., Tang, X., Chen, L., Ma, A., Zhu, W., Huang, W. and Li, J. (2023) Improvement of the fibrinolytic activity, acid resistance and thermostability of nattokinase by surface charge engineering. Int J Biol Macromol. 253, 127373. [CrossRef]
- Liu, Z., He, Y., Zhang, H. and Ma, X. (2024) Layer-by-layer self-assembly embedding of nattokinase in chitosan/gamma-polyglutamic acid: Preparation, fibrinolytic activity, stability, and in vitro digestion study. Eur J Pharm Biopharm. 199, 114281. [CrossRef]
- Takabayashi, T., Imoto, Y., Sakashita, M., Kato, Y., Tokunaga, T., Yoshida, K., Narita, N., Ishizuka, T. and Fujieda, S. (2017) Nattokinase, profibrinolytic enzyme, effectively shrinks the nasal polyp tissue and decreases viscosity of mucus. Allergol Int. 66, 594-602. [CrossRef]
- Grixti, J. M., Theron, C. W., Salcedo-Sora, J. E., Pretorius, E. and Kell, D. B. (2024) Automated microscopic measurement of fibrinaloid microclots and their degradation by nattokinase, the main natto protease. J Exp Clin Appl Chin Med. 5, 30-55. [CrossRef]
- Pinontoan, R., Elvina, Sanjaya, A. and Jo, J. (2021) Fibrinolytic characteristics of Bacillus subtilis G8 isolated from natto. Biosci Microbiota Food Health. 40, 144-149. [CrossRef]
- Fadl, N. N., Ahmed, H. H., Booles, H. F. and Sayed, A. H. (2013) Serrapeptase and nattokinase intervention for relieving Alzheimer's disease pathophysiology in rat model. Hum Exp Toxicol. 32, 721-735. [CrossRef]
- Ahmed, H. H., Nevein, N. F., Karima, A. E.-S. and Hamza, A. H. (2014) Miracle enzymes serrapeptase and nattokinase mitigate neuroinflammation and apoptosis associated with Alzheimer’s disease in experimental model. World J Pharm Pharm Sci. 3, 876-891.
- Kumar, S. S. and Sabu, A. (2019) Fibrinolytic Enzymes for Thrombolytic Therapy. Adv Exp Med Biol. 1148, 345-381. [CrossRef]
- Mei, J. f., Cai, S. f., Yi, Y., Wang, X. D. and Ying, G. Q. (2022) Study of the fibrinolytic activity of serrapeptase and its in vitro thrombolytic effects. Braz J Pharm Sci. 58, e201004. [CrossRef]
- Altaf, F., Wu, S. R. and Kasim, V. (2021) Role of Fibrinolytic Enzymes in Anti-Thrombosis Therapy. Frontiers in Molecular Biosciences. 8.
- Cao, Y. J., Zhang, X., Wang, W. H., Zhai, W. Q., Qian, J. F., Wang, J. S., Chen, J., You, N. X., Zhao, Z., Wu, Q. Y., Xu, Y., Yuan, L., Li, R. X. and Liu, C. F. (2013) Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: results from a multicenter, randomized, parallel-group and controlled clinical trial. Chin Med J (Engl). 126, 4060-4065. [CrossRef]
- Metkar, S. K., Girigoswami, A., Murugesan, R. and Girigoswami, K. (2017) Lumbrokinase for degradation and reduction of amyloid fibrils associated with amyloidosis. Journal of Applied Biomedicine. 15, 96-104.
- Metkar, S. K., Girigoswami, A., Bondage, D. D., Shinde, U. G. and Girigoswami, K. (2024) The potential of lumbrokinase and serratiopeptidase for the degradation of Aβ 1–42 peptide – an in vitro and in silico approach. Int J Neurosci. 134, 112-123. [CrossRef]
- Wang, K. Y., Tull, L., Cooper, E., Wang, N. and Liu, D. (2013) Recombinant protein production of earthworm lumbrokinase for potential antithrombotic application. Evid Based Complement Alternat Med. 2013, 783971. [CrossRef]
- Metkar, S. K., Girigoswami, A., Vijayashree, R. and Girigoswami, K. (2020) Attenuation of subcutaneous insulin induced amyloid mass in vivo using Lumbrokinase and Serratiopeptidase. Int J Biol Macromol. 163, 128-134. [CrossRef]
- Pham, P. T., Han, B. and Hoang, B. X. (2020) Nattospes as Effective and Safe Functional Supplements in Management of Stroke. Journal of Medicinal Food. 23, 879-885. [CrossRef]
- Morimitsu, T. (2017) The treatment of tinnitus cerebri with a fibrinolytic enzyme. Jibi To Rinsho (in Japanese). 63, 138-144. [CrossRef]
- Hosseinzadeh, A., Kamrava, S. K., Moore, B. C. J., Reiter, R. J., Ghaznavi, H., Kamali, M. and Mehrzadi, S. (2019) Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr Drug Targets. 20, 1112-1128. [CrossRef]
- Lazzeri, G., Biagioni, F., Ferrucci, M., Puglisi-Allegra, S., Lenzi, P., Busceti, C. L., Giannessi, F. and Fornai, F. (2023) The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes. Int J Mol Sci. 24, 16664. [CrossRef]
- Xiong, M., Feng, X., Tang, L., Li, C. and Yu, L. (2021) Butylphthalide enhances recovery from sudden deafness. Am J Otolaryngol. 42, 102891. [CrossRef]
- Nan, B., Gu, X. and Huang, X. (2019) The Role of the Reactive Oxygen Species Scavenger Agent, Astaxanthin, in the Protection of Cisplatin-Treated Patients Against Hearing Loss. Drug Des Devel Ther. 13, 4291-4303. [CrossRef]
- Nan, B., Zhao, Z., Jiang, K., Gu, X., Li, H. and Huang, X. (2022) Astaxanthine attenuates cisplatin ototoxicity in vitro and protects against cisplatin-induced hearing loss in vivo. Acta Pharm Sin B. 12, 167-181. [CrossRef]
- Abbasi, R., Emami, F., Atighechi, S. and Sadeghi, Z. (2025) The Effect of Coenzyme Q10 on Tinnitus Severity and Sleep Quality in Patients with Presbycusis. Iran J Otorhinolaryngol. 37, 33-39. [CrossRef]
- Jiang, S., Yang, Y., Li, T., Ma, Z., Hu, W., Deng, C., Fan, C., Lv, J., Sun, Y. and Yi, W. (2016) An overview of the mechanisms and novel roles of Nrf2 in cardiovascular diseases. Expert Opin Ther Targets. 20, 1413-1424. [CrossRef]
- Kloska, D., Kopacz, A., Piechota-Polanczyk, A., Nowak, W. N., Dulak, J., Jozkowicz, A. and Grochot-Przeczek, A. (2019) Nrf2 in aging - Focus on the cardiovascular system. Vascul Pharmacol. 112, 42-53. [CrossRef]
- Syed, A. M., Ram, C., Murty, U. S. and Sahu, B. D. (2021) A review on herbal Nrf2 activators with preclinical evidence in cardiovascular diseases. Phytother Res. 35, 5068-5102. [CrossRef]
- Roberts, J. A., Rainbow, R. D. and Sharma, P. (2023) Mitigation of Cardiovascular Disease and Toxicity through NRF2 Signalling. Int J Mol Sci. 24. [CrossRef]
- Khan, S. U., Khan, S. U., Suleman, M., Khan, M. U., Khan, M. S., Arbi, F. M., Hussain, T., Mohammed Alsuhaibani, A. and M, S. R. (2024) Natural Allies for Heart Health: Nrf2 Activation and Cardiovascular Disease Management. Curr Probl Cardiol. 49, 102084. [CrossRef]
- Wu, Q., Yao, J., Xiao, M., Zhang, X., Zhang, M. and Xi, X. (2024) Targeting Nrf2 signaling pathway: new therapeutic strategy for cardiovascular diseases. J Drug Target. 32, 874-883. [CrossRef]
- Kell, D. B., Kell, L., Kenny, L. C., Merriel, A., Moore, J. B. and Pretorius, E. (2025) The roles of placental senescence, autophagy and senotherapeutics in the development and prevention of pre-eclampsia: a focus on ergothioneine. J Reprod Immunol. 171, 104621. [CrossRef]
- Wu, X., Wei, J., Yi, Y., Gong, Q. and Gao, J. (2022) Activation of Nrf2 signaling: A key molecular mechanism of protection against cardiovascular diseases by natural products. Front Pharmacol. 13, 1057918. [CrossRef]
- Kavian, N., Mehlal, S., Jeljeli, M., Saidu, N. E. B., Nicco, C., Cerles, O., Chouzenoux, S., Cauvet, A., Camus, C., Ait-Djoudi, M., Chereau, C., Kerdine-Romer, S., Allanore, Y. and Batteux, F. (2018) The Nrf2-Antioxidant Response Element Signaling Pathway Controls Fibrosis and Autoimmunity in Scleroderma. Front Immunol. 9, 1896. [CrossRef]
- Mundal, S. B., Rakner, J. J., Silva, G. B., Gierman, L. M., Austdal, M., Basnet, P., Elschot, M., Bakke, S. S., Ostrop, J., Thomsen, L. C. V., Moses, E. K., Acharya, G., Bjørge, L. and Iversen, A. C. (2022) Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. Int J Mol Sci. 23, 1966. [CrossRef]
- Robertson, R. P. (2023) Nrf2 and Antioxidant Response in Animal Models of Type 2 Diabetes. Int J Mol Sci. 24, 3082. [CrossRef]
- Tamaru, E., Kokubu, D., Ushida, Y. and Itoh, K. (2024) Nrf2 induction potency of plant-derived compounds determined using an antioxidant response element luciferase reporter and conventional NAD(P)H-quinone acceptor oxidoreductase 1 activity assay. BMC Res Notes. 17, 373. [CrossRef]
- Vomhof-Dekrey, E. E. and Picklo, M. J., Sr. (2012) The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 23, 1201-1206. [CrossRef]
- Vriend, J. and Reiter, R. J. (2015) The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome. Mol Cell Endocrinol. 401, 213-220. [CrossRef]
- Shen, Y., Wang, Y. and Zhang, G. (2014) Applying bioinformatic technique to discovery molecular mechansim of Banxia and Tianma combination treating tinnitus. IEEE Int Conf Bioinfo Biomed. 1, 160-163. [CrossRef]
- Li, D., Zhao, H., Cui, Z. K. and Tian, G. (2021) The Role of Nrf2 in Hearing Loss. Front Pharmacol. 12, 620921. [CrossRef]
- Li, D., Zhao, H., Xu, P., Lin, Q., Zhao, T., Li, C., Cui, Z. K. and Tian, G. (2022) Polydatin activates the Nrf2/HO-1 signaling pathway to protect cisplatin-induced hearing loss in guinea pigs. Front Pharmacol. 13, 887833. [CrossRef]
- So, H., Kim, H., Kim, Y., Kim, E., Pae, H. O., Chung, H. T., Kim, H. J., Kwon, K. B., Lee, K. M., Lee, H. Y., Moon, S. K. and Park, R. (2008) Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol. 9, 290-306. [CrossRef]
- Kim, S. J., Park, C., Han, A. L., Youn, M. J., Lee, J. H., Kim, Y., Kim, E. S., Kim, H. J., Kim, J. K., Lee, H. K., Chung, S. Y., So, H. and Park, R. (2009) Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear Res. 251, 70-82. [CrossRef]
- Honkura, Y., Matsuo, H., Murakami, S., Sakiyama, M., Mizutari, K., Shiotani, A., Yamamoto, M., Morita, I., Shinomiya, N., Kawase, T., Katori, Y. and Motohashi, H. (2016) NRF2 Is a Key Target for Prevention of Noise-Induced Hearing Loss by Reducing Oxidative Damage of Cochlea. Sci Rep. 6, 19329. [CrossRef]
- Zhang, W., Xiong, H., Pang, J., Su, Z., Lai, L., Lin, H., Jian, B., He, W., Yang, H. and Zheng, Y. (2020) Nrf2 activation protects auditory hair cells from cisplatin-induced ototoxicity independent on mitochondrial ROS production. Toxicol Lett. 331, 1-10. [CrossRef]
- Lou, J., Wu, F., He, W., Hu, R., Cai, Z., Chen, G., Zhao, W., Zhang, Z. and Si, Y. (2024) Hesperidin activates Nrf2 to protect cochlear hair cells from cisplatin-induced damage. Redox Rep. 29, 2341470. [CrossRef]
- Adachi, M., Yanagizono, K., Okano, Y., Koizumi, H., Uemaetomari, I. and Tabuchi, K. (2023) Estradiol protects hair cells from cisplatin-induced ototoxicity via Nrf2 activation. Redox Rep. 28, 2161224. [CrossRef]
- Mirzaei, S., Mohammadi, A. T., Gholami, M. H., Hashemi, F., Zarrabi, A., Zabolian, A., Hushmandi, K., Makvandi, P., Samec, M., Liskova, A., Kubatka, P., Nabavi, N., Aref, A. R., Ashrafizadeh, M., Khan, H. and Najafi, M. (2021) Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol Res. 167, 105575. [CrossRef]
- Borodina, I., Kenny, L. C., McCarthy, C. M., Paramasivan, K., Pretorius, R., Roberts, T. J., van der Hoek, S. A. and Kell, D. B. (2020) The biology of ergothioneine, an antioxidant nutraceutical. Nutr Res Rev. 33, 190-217. [CrossRef]
- Bernardo, V. S., Torres, F. F., de Paula, C. P., de Oliveira da Silva, J. P. M., de Almeida, E. A., da Cunha, A. F. and da Silva, D. G. H. (2022) Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells. Genes (Basel). 13, 2368. [CrossRef]
- Dare, A., Elrashedy, A. A., Channa, M. L. and Nadar, A. (2022) Cardioprotective Effects and in-silico Antioxidant Mechanism of L-Ergothioneine in Experimental Type-2 Diabetic Rats. Cardiovasc Hematol Agents Med Chem. 20, 133-147. [CrossRef]
- Halliwell, B., Cheah, I. K. and Tang, R. M. Y. (2018) Ergothioneine - a diet-derived antioxidant with therapeutic potential. FEBS Lett. 592, 3357-3366. [CrossRef]
- Halliwell, B. and Cheah, I. (2022) Ergothioneine, where are we now? FEBS Lett. 596, 1227-1230. [CrossRef]
- Halliwell, B., Tang, R. M. Y. and Cheah, I. K. (2023) Diet-derived antioxidants: the special case of ergothioneine. Annu Rev Food Sci Technol. 14, 323-345. [CrossRef]
- Hseu, Y. C., Lo, H. W., Korivi, M., Tsai, Y. C., Tang, M. J. and Yang, H. L. (2015) Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic Biol Med. 86, 102-117. [CrossRef]
- Jeong, J. Y., Cai, L., Kim, M., Choi, H., Oh, D., Jawad, A., Kim, S., Zheng, H., Lee, E., Lee, J. and Hyun, S. H. (2023) Antioxidant effect of ergothioneine on in vitro maturation of porcine oocytes. J Vet Sci. 24, e24. [CrossRef]
- Kell, D. B., Kell, L., Kenny, L. C., Merriel, A., Moore, J. B. and Pretorius, E. (2025) The roles of placental senescence, autophagy and senotherapeutics in the development and prevention of pre-eclampsia: a focus on ergothioneine. Preprints. https://www.preprints.org/manuscript/202504.201261/v202501. [CrossRef]
- Ko, H. J., Kim, J., Ahn, M., Jin Hwa, K., Lee, G. S. and Shin, T. (2021) Ergothioneine alleviates senescence of fibroblasts induced by UVB damage of keratinocytes via activation of the Nrf2/HO-1 pathway and HSP70 in keratinocytes. Exp Cell Res, 112516. [CrossRef]
- Leow, D. M., Cheah, I. K., Fong, Z. W., Halliwell, B. and Ong, W. Y. (2023) Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. Int J Mol Sci. 24. [CrossRef]
- Tian, X., Thorne, J. L. and Moore, J. B. (2023) Ergothioneine: an underrecognised dietary micronutrient required for healthy ageing? Br J Nutr. 129, 104-114. [CrossRef]
- Ferreira, F. S., Biasibetti-Brendler, H., Pierozan, P., Schmitz, F., Bertó, C. G., Prezzi, C. A., Manfredini, V. and Wyse, A. T. S. (2018) Kynurenic Acid Restores Nrf2 Levels and Prevents Quinolinic Acid-Induced Toxicity in Rat Striatal Slices. Mol Neurobiol. 55, 8538-8549. [CrossRef]
- Turska, M., Paluszkiewicz, P., Turski, W. A. and Parada-Turska, J. (2022) A review of the health benefits of food enriched with kynurenic acid. Nutrients. 14, 4182. [CrossRef]
- Gao, Y., Guo, X., Zhou, Y., Du, J., Lu, C., Zhang, L., Sun, S., Wang, S. and Li, Y. (2023) Kynurenic acid inhibits macrophage pyroptosis by suppressing ROS production via activation of the NRF2 pathway. Mol Med Rep. 28, 211. [CrossRef]
- Alves, L. d. F., Moore, J. B. and Kell, D. B. (2024) The biology and biochemistry of kynurenic acid, a potential nutraceutical with multiple biological effects. Int J Mol Sci. 25, 9082. [CrossRef]
- Mazurek, B., Olze, H., Haupt, H. and Szczepek, A. J. (2010) The more the worse: the grade of noise-induced hearing loss associates with the severity of tinnitus. Int J Environ Res Public Health. 7, 3071-3079. [CrossRef]
- Bauer, M. A., Bazard, P., Acosta, A. A., Bangalore, N., Elessaway, L., Thivierge, M., Chellani, M., Zhu, X., Ding, B., Walton, J. P. and Frisina, R. D. (2024) L-Ergothioneine slows the progression of age-related hearing loss in CBA/CaJ mice. Hear Res. 446, 109004. [CrossRef]
- Bobbin, R. P. and Ceasar, G. (1987) Kynurenic acid and gamma-D-glutamylaminomethylsulfonic acid suppress the compound action potential of the auditory nerve. Hear Res. 25, 77-81. [CrossRef]
- Kell, D. B. (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom. 2, 2 . [CrossRef]
- Reiter, R. J., Paredes, S. D., Manchester, L. C. and Tan, D. X. (2009) Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 44, 175-200.
- Janjetovic, Z., Jarrett, S. G., Lee, E. F., Duprey, C., Reiter, R. J. and Slominski, A. T. (2017) Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 7, 1274. [CrossRef]
- Ahmadi, Z. and Ashrafizadeh, M. (2020) Melatonin as a potential modulator of Nrf2. Fundam Clin Pharmacol. 34, 11-19. [CrossRef]
- Kim, E. H., Ridlo, M. R., Lee, B. C. and Kim, G. A. (2020) Melatonin-Nrf2 Signaling Activates Peroxisomal Activities in Porcine Cumulus Cell-Oocyte Complexes. Antioxidants (Basel). 9, 1080. [CrossRef]
- Tang, Y., Wang, Z., Chen, Y., Wang, J., Wang, H., Li, B., Liu, B. and Zheng, P. (2024) Melatonin Improves H(2)O(2)-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway. Genes (Basel). 15, 1544. [CrossRef]
- Liu, Y., Wang, X., Li, Z., Gao, X., Wu, X., Pi, J., Wang, X., Wang, Q., Zhou, F. and Wang, X. (2025) Melatonin attenuates brain edema via the PI3K/Akt/Nrf2 pathway in rats with cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis. 34, 108299. [CrossRef]
- Rosenberg, S. I., Silverstein, H., Rowan, P. T. and Olds, M. J. (1998) Effect of melatonin on tinnitus. Laryngoscope. 108, 305-310. [CrossRef]
- Megwalu, U. C., Finnell, J. E. and Piccirillo, J. F. (2006) The effects of melatonin on tinnitus and sleep. Otolaryngol Head Neck Surg. 134, 210-213. [CrossRef]
- Pirodda, A., Raimondi, M. C. and Ferri, G. G. (2010) Exploring the reasons why melatonin can improve tinnitus. Med Hypotheses. 75, 190-191. [CrossRef]
- Reiter, R. J., Tan, D. X., Korkmaz, A. and Fuentes-Broto, L. (2011) Drug-mediated ototoxicity and tinnitus: alleviation with melatonin. J Physiol Pharmacol. 62, 151-157.
- Lasisi, A. O., Fehintola, F. A. and Lasisi, T. J. (2012) The role of plasma melatonin and vitamins C and B12 in the development of idiopathic tinnitus in the elderly. Ghana Med J. 46, 152-157.
- Albu, S. and Chirtes, F. (2014) Intratympanic dexamethasone plus melatonin versus melatonin only in the treatment of unilateral acute idiopathic tinnitus. Am J Otolaryngol. 35, 617-622. [CrossRef]
- Merrick, L., Youssef, D., Tanner, M. and Peiris, A. N. (2014) Does melatonin have therapeutic use in tinnitus? South Med J. 107, 362-366. [CrossRef]
- Abtahi, S. H., Hashemi, S. M., Mahmoodi, M. and Nilforoush, M. H. (2017) Comparison of Melatonin and Sertraline Therapies on Tinnitus: A Randomized Clinical Trial. Int J Prev Med. 8, 61. [CrossRef]
- Aytaç, I. (2005) Complementary and Alternative Treatments for Tinnitus. IntechOpen. https://www.intechopen.com/chapters/65321. [CrossRef]
- Bai, Y., Wang, X., Zhao, S., Ma, C., Cui, J. and Zheng, Y. (2015) Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid Med Cell Longev. 2015, 407580. [CrossRef]
- Calabrese, E. J. and Kozumbo, W. J. (2021) The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharmacol Res. 163, 105283. [CrossRef]
- Dinkova-Kostova, A. T., Fahey, J. W., Kostov, R. V. and Kensler, T. W. (2017) KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol. 69, 257-269. [CrossRef]
- Houghton, C. A., Fassett, R. G. and Coombes, J. S. (2016) Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician's Expectation Be Matched by the Reality? Oxid Med Cell Longev. 2016, 7857186. [CrossRef]
- Kleszczyński, K., Ernst, I. M. A., Wagner, A. E., Kruse, N., Zillikens, D., Rimbach, G. and Fischer, T. W. (2013) Sulforaphane and phenylethyl isothiocyanate protect human skin against UVR-induced oxidative stress and apoptosis: role of Nrf2-dependent gene expression and antioxidant enzymes. Pharmacol Res. 78, 28-40. [CrossRef]
- Liebman, S. E. and Le, T. H. (2021) Eat Your Broccoli: Oxidative Stress, NRF2, and Sulforaphane in Chronic Kidney Disease. Nutrients. 13. [CrossRef]
- Kubo, E., Chhunchha, B., Singh, P., Sasaki, H. and Singh, D. P. (2017) Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 7, 14130. [CrossRef]
- Russo, M., Spagnuolo, C., Russo, G. L., Skalicka-Woźniak, K., Daglia, M., Sobarzo-Sánchez, E., Nabavi, S. F. and Nabavi, S. M. (2018) Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit Rev Food Sci Nutr. 58, 1391-1405. [CrossRef]
- Lu, Z., Ren, Y., Yang, L., Jia, A., Hu, Y., Zhao, Y., Zhao, W., Yu, B., Zhao, W., Zhang, J. and Hou, G. (2021) Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting NRF2 in esophageal squamous cell carcinoma. Acta Pharm Sin B. 11, 1246-1260. [CrossRef]
- Shah, A., Varma, M. and Bhandari, R. (2024) Exploring sulforaphane as neurotherapeutic: targeting Nrf2-Keap & Nf-Kb pathway crosstalk in ASD. Metab Brain Dis. 39, 373-385. [CrossRef]
- Su, X., Jiang, X., Meng, L., Dong, X., Shen, Y. and Xin, Y. (2018) Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid Med Cell Longev. 2018, 5438179. [CrossRef]
- Uddin, M. S., Mamun, A. A., Jakaria, M., Thangapandiyan, S., Ahmad, J., Rahman, M. A., Mathew, B., Abdel-Daim, M. M. and Aleya, L. (2020) Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci Total Environ. 707, 135624. [CrossRef]
- Park, J. Y., Sohn, H. Y., Koh, Y. H. and Jo, C. (2021) Curcumin activates Nrf2 through PKCdelta-mediated p62 phosphorylation at Ser351. Sci Rep. 11, 8430. [CrossRef]
- Ghafouri-Fard, S., Shoorei, H., Bahroudi, Z., Hussen, B. M., Talebi, S. F., Taheri, M. and Ayatollahi, S. A. (2022) Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules. 12, 82. [CrossRef]
- Huang, X., Chu, Y., Ren, H. and Pang, X. (2022) Antioxidation Function of EGCG by Activating Nrf2/HO-1 Pathway in Mice with Coronary Heart Disease. Contrast Media Mol Imaging. 2022, 8639139. [CrossRef]
- Pouremamali, F., Pouremamali, A., Dadashpour, M., Soozangar, N. and Jeddi, F. (2022) An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun Signal. 20, 100. [CrossRef]
- Wei, Z., Jing, Z., Pinfang, K., Chao, S. and Shaohuan, Q. (2022) Quercetin Inhibits Pyroptosis in Diabetic Cardiomyopathy through the Nrf2 Pathway. J Diabetes Res. 2022, 9723632. [CrossRef]
- Zamanian, M. Y., Soltani, A., Khodarahmi, Z., Alameri, A. A., Alwan, A. M. R., Ramirez-Coronel, A. A., Obaid, R. F., Abosaooda, M., Heidari, M., Golmohammadi, M. and Anoush, M. (2023) Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam Clin Pharmacol. 37, 1050-1064. [CrossRef]
- Zardak, M. Y., Keshavarz, F., Mahyaei, A., Gholami, M., Moosavi, F. S., Abbasloo, E., Abdollahi, F., Hossein Rezaei, M., Madadizadeh, E., Soltani, N., Bejeshk, F., Salehi, N., Rostamabadi, F., Bagheri, F., Jafaraghae, M., Ranjbar Zeydabadi, M., Baghgoli, M., Sepehri, G. and Bejeshk, M. A. (2024) Quercetin as a therapeutic agent activate the Nrf2/Keap1 pathway to alleviate lung ischemia-reperfusion injury. Sci Rep. 14, 23074. [CrossRef]
- Ungvari, Z., Bagi, Z., Feher, A., Recchia, F. A., Sonntag, W. E., Pearson, K., de Cabo, R. and Csiszar, A. (2010) Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 299, H18-24. [CrossRef]
- Farkhondeh, T., Folgado, S. L., Pourbagher-Shahri, A. M., Ashrafizadeh, M. and Samarghandian, S. (2020) The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 127, 110234. [CrossRef]
- Alavi, M., Farkhondeh, T., Aschner, M. and Samarghandian, S. (2021) Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int. 21, 579. [CrossRef]
- Pourbagher-Shahri, A. M., Farkhondeh, T., Jafari-Nozad, A. M., Darroudi, M., Naseri, K., Amirian, M. and Samarghandian, S. (2024) Nrf2 Mediates Effect of Resveratrol in Ischemia-reperfusion Injury. Curr Mol Pharmacol. 17, e18761429246578. [CrossRef]
- Mimura, J., Inose-Maruyama, A., Taniuchi, S., Kosaka, K., Yoshida, H., Yamazaki, H., Kasai, S., Harada, N., Kaufman, R. J., Oyadomari, S. and Itoh, K. (2019) Concomitant Nrf2- and ATF4-activation by Carnosic Acid Cooperatively Induces Expression of Cytoprotective Genes. Int J Mol Sci. 20. [CrossRef]
- Satoh, T., Trudler, D., Oh, C. K. and Lipton, S. A. (2022) Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer's Disease, Parkinson's Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants (Basel). 11, 124. [CrossRef]
- Reisman, S. A., Aleksunes, L. M. and Klaassen, C. D. (2009) Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol. 77, 1273-1282. [CrossRef]
- Similie, D., Minda, D., Bora, L., Kroskins, V., Luginina, J., Turks, M., Dehelean, C. A. and Danciu, C. (2024) An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants (Basel). 13, 952. [CrossRef]
- Divya, T., Dineshbabu, V., Soumyakrishnan, S., Sureshkumar, A. and Sudhandiran, G. (2016) Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem Biol Interact. 246, 52-62. [CrossRef]
- An, N., Wang, R., Li, L., Wang, B., Wang, H., Peng, G., Zhou, H. and Chen, G. (2024) Celastrol alleviates diabetic vascular injury via Keap1/Nrf2-mediated anti-inflammation. Front Pharmacol. 15, 1360177. [CrossRef]
- Schiavoni, V., Di Crescenzo, T., Membrino, V., Alia, S., Fantone, S., Salvolini, E. and Vignini, A. (2025) Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications. Pharmaceuticals (Basel). 18, 966. [CrossRef]
- Guo, F. F., Meng, F. G., Zhang, X. N. and Zeng, T. (2022) Spermidine inhibits LPS-induced pro-inflammatory activation of macrophages by acting on Nrf2 signaling but not autophagy. J Funct Foods. 94, 105115. [CrossRef]
- Jiang, T., Harder, B., Rojo de la Vega, M., Wong, P. K., Chapman, E. and Zhang, D. D. (2015) p62 links autophagy and Nrf2 signaling. Free Radical Bio Med. 88, 199-204. [CrossRef]
- Komatsu, M., Kurokawa, H., Waguri, S., Taguchi, K., Kobayashi, A., Ichimura, Y., Sou, Y. S., Ueno, I., Sakamoto, A., Tong, K. I., Kim, M., Nishito, Y., Iemura, S., Natsume, T., Ueno, T., Kominami, E., Motohashi, H., Tanaka, K. and Yamamoto, M. (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 12, 213-223. [CrossRef]
- Wai, K. W., Low, L. E., Goh, B. H. and Yap, W. H. (2024) Nrf2 Connects Cellular Autophagy and Vascular Senescence in Atherosclerosis: A Mini-Review. J Lipid Atheroscler. 13, 292-305. [CrossRef]
- Zhang, W., Feng, C. and Jiang, H. (2021) Novel target for treating Alzheimer's Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res Rev. 65, 101207. [CrossRef]
- Kanninen, K., Malm, T. M., Jyrkkanen, H. K., Goldsteins, G., Keksa-Goldsteine, V., Tanila, H., Yamamoto, M., Yla-Herttuala, S., Levonen, A. L. and Koistinaho, J. (2008) Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 39, 302-313. [CrossRef]
- Osama, A., Zhang, J., Yao, J., Yao, X. and Fang, J. (2020) Nrf2: a dark horse in Alzheimer's disease treatment. Ageing Res Rev. 64, 101206. [CrossRef]
- Uruno, A., Kadoguchi-Igarashi, S., Saito, R., Koiso, S., Saigusa, D., Chu, C. T., Suzuki, T., Saito, T., Saido, T. C., Cuadrado, A. and Yamamoto, M. (2024) The NRF2 inducer CDDO-2P-Im provokes a reduction in amyloid beta levels in Alzheimer's disease model mice. J Biochem. 176, 405-414. [CrossRef]
- Zhang, J., Song, N., Liu, Y. and Guo, J. (2021) Platycodin D Inhibits beta-Amyloid-Induced Inflammation and Oxidative Stress in BV-2 Cells Via Suppressing TLR4/NF-kappaB Signaling Pathway and Activating Nrf2/HO-1 Signaling Pathway. Neurochem Res. 46, 638-647. [CrossRef]
- Xiang, C., Lu, Y., Hao, R., Wei, Y., Hu, Y. and Yu, G. (2024) Catalpol alleviates amyloid- generation and neuronal oxidative stress injury via activating the Keap1-Nrf2/ARE signaling pathway in the immortalized lymphocytes from patients with late-onset Alzheimer's disease and SKNMC cells co-culture model. Iran J Basic Med Sci. 27, 1547-1557. [CrossRef]
- Ahmad, S., Shah, S. A., Nishan, U., Khan, N., Almutairi, M. H., Fozia, F., Jamila, N., Almutairi, B. O. and Ullah, Z. (2023) 6-Aminoflavone Activates Nrf2 to Inhibit the Phospho-JNK/TNF-alpha Signaling Pathway to Reduce Amyloid Burden in an Aging Mouse Model. ACS Omega. 8, 26955-26964. [CrossRef]
- Lee, N., Youn, K., Yoon, J. H., Lee, B., Kim, D. H. and Jun, M. (2023) The Role of Fucoxanthin as a Potent Nrf2 Activator via Akt/GSK-3beta/Fyn Axis against Amyloid-beta Peptide-Induced Oxidative Damage. Antioxidants (Basel). 12. [CrossRef]
- Rong, H., Liang, Y. and Niu, Y. (2018) Rosmarinic acid attenuates beta-amyloid-induced oxidative stress via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic Biol Med. 120, 114-123. [CrossRef]
- Taheri, S., Khalifeh, S., Shajiee, H. and Ashabi, G. (2021) Dietary uptake of Salvia macilenta extract improves Nrf2 antioxidant signaling pathway and diminishes inflammation and apoptosis in amyloid beta-induced rats. Mol Biol Rep. 48, 7667-7676. [CrossRef]
- De Plano, L. M., Calabrese, G., Rizzo, M. G., Oddo, S. and Caccamo, A. (2023) The Role of the Transcription Factor Nrf2 in Alzheimer's Disease: Therapeutic Opportunities. Biomolecules. 13, 549. [CrossRef]
- Chu, C. T., Uruno, A., Katsuoka, F. and Yamamoto, M. (2024) Role of NRF2 in Pathogenesis of Alzheimer's Disease. Antioxidants (Basel). 13. [CrossRef]
- Rapaka, D., Bitra, V. R., Ummidi, R. and Akula, A. (2021) Benincasa hispida alleviates amyloid pathology by inhibition of Keap1/Nrf2-axis: Emphasis on oxidative and inflammatory stress involved in Alzheimer's disease model. Neuropeptides. 88, 102151. [CrossRef]
- Williams, R. J. (1956) Biochemical Individuality. John Wiley, New York.
- Kell, D. B. (2012) Scientific discovery as a combinatorial optimisation problem: how best to navigate the landscape of possible experiments? Bioessays. 34, 236-244. [CrossRef]
- Grossi, M. G., Belcaro, G., Cesarone, M. R., Dugall, M., Hosoi, M., Cacchio, M., Ippolito, E. and Bavera, P. (2010) Improvement in cochlear flow with Pycnogenol(R) in patients with tinnitus: a pilot evaluation. Panminerva Med. 52, 63-67.
- Luzzi, R., Belcaro, G., Hu, S., Dugall, M., Hosoi, M., Cacchio, M., Ippolito, E. and Corsi, M. (2014) Improvement in symptoms and cochlear flow with pycnogenol in patients with Meniere's disease and tinnitus. Minerva Med. 105, 245-254.
- D'Andrea, G. (2010) Pycnogenol: a blend of procyanidins with multifaceted therapeutic applications? Fitoterapia. 81, 724-736. [CrossRef]
- Grossi, M. G., Belcaro, G., Cesarone, M. R., Dugall, M., Hosoi, M., Cacchio, M., Ippolito, E. and Bavera, P. (2011) Improvement in cochlear flow in patients with tinnitus with the complex supplement Acustop: a product evaluation. Panminerva Med. 53, 89-93.
- Thagard, P. and Verbeurgt, K. (1998) Coherence as constraint satisfaction. Cogn Sci. 22, 1-24.
- Thagard, P. (1998) Explaining disease: Correlations, causes, and mechanisms. Minds and Machines. 8, 61-78.
- Thagard, P. (1999) How scientists explain disease. Princeton University Press, Princeton, NJ.
- Thagard, P. (2007) Coherence, truth, and the development of scientific knowledge. Philosophy of Science. 74, 28-47.
- Thagard, P. (2008) Explanatory Coherence. Reasoning: Studies of Human Inference and Its Foundations, 471-513.
- Becker, L., Keck, A., Rohleder, N. and Müller-Voggel, N. (2022) Higher Peripheral Inflammation Is Associated With Lower Orbitofrontal Gamma Power in Chronic Tinnitus. Front Behav Neurosci. 16, 883926. [CrossRef]
- Mennink, L. M., Aalbers, M. W., van Dijk, P. and van Dijk, J. M. C. (2022) The Role of Inflammation in Tinnitus: A Systematic Review and Meta-Analysis. J Clin Med. 11, 1000. [CrossRef]
- Mennink, L. M., Albakri, L. B. M., Aalbers, M. W., Dijk, P. V. and van Dijk, J. M. C. (2024) Cross-sectional screening for inflammation in tinnitus with near-normal hearing. Hear Res. 453, 109124. [CrossRef]
- Shulman, A., Wang, W., Luo, H., Bao, S., Searchfield, G. and Zhang, J. (2021) Neuroinflammation and Tinnitus. Curr Top Behav Neurosci. 51, 161-174. [CrossRef]
- Kell, D. B. (2012) Reviews turn facts into understanding. Nature. 490, 37. [CrossRef]
- Scherbakov, N., Szklarski, M., Hartwig, J., Sotzny, F., Lorenz, S., Meyer, A., Grabowski, P., Doehner, W. and Scheibenbogen, C. (2020) Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 7, 1064-1071. [CrossRef]
- Woudstra, J., Mourmans, S. G. J., Vink, C. E. M., Marques, K. M. J., de Jong, E. A. M., Haddad, R. Y. R., van de Hoef, T. P., Chamuleau, S. A. J., Damman, P., Beijk, M. A. M., van Empel, V. P. M., Serne, E. H., Appelman, Y., Eringa, E. C. and consortium, I. (2024) Relationship between peripheral and intracoronary blood flow in patients with angina and nonobstructive coronary arteries. Am J Physiol Heart Circ Physiol. 327, H1086-H1097. [CrossRef]
- Langguth, B. and De Ridder, D. (2023) Minimal Clinically Important Difference of Tinnitus Outcome Measurement Instruments-A Scoping Review. J Clin Med. 12, 7117. [CrossRef]
- Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., Myers, P. J., Newman, C. W., Sandridge, S., Turk, D. C., Folmer, R. L., Frederick, E. J., House, J. W., Jacobson, G. P., Kinney, S. E., Martin, W. H., Nagler, S. M., Reich, G. E., Searchfield, G., Sweetow, R. and Vernon, J. A. (2012) The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 33, 153-176. [CrossRef]
- Henry, J. A., Griest, S., Thielman, E., McMillan, G., Kaelin, C. and Carlson, K. F. (2016) Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear Res. 334, 58-64. [CrossRef]
- Zeman, F., Koller, M., Figueiredo, R., Aazevedo, A., Rates, M., Coelho, C., Kleinjung, T., de Ridder, D., Langguth, B. and Landgrebe, M. (2011) Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngol Head Neck Surg. 145, 282-287. [CrossRef]
- Henry, J. A., Griest, S., Zaugg, T. L., Thielman, E., Kaelin, C., Galvez, G. and Carlson, K. F. (2015) Tinnitus and hearing survey: a screening tool to differentiate bothersome tinnitus from hearing difficulties. Am J Audiol. 24, 66-77. [CrossRef]
- Nakashima, T., Naganawa, S., Sone, M., Tominaga, M., Hayashi, H., Yamamoto, H., Liu, X. and Nuttall, A. L. (2003) Disorders of cochlear blood flow. Brain Res Brain Res Rev. 43, 17-28. [CrossRef]
- Monfared, A., Blevins, N. H., Cheung, E. L., Jung, J. C., Popelka, G. and Schnitzer, M. J. (2006) In vivo imaging of mammalian cochlear blood flow using fluorescence microendoscopy. Otol Neurotol. 27, 144-152. [CrossRef]
- Shi, X., Zhang, F., Urdang, Z., Dai, M., Neng, L., Zhang, J., Chen, S., Ramamoorthy, S. and Nuttall, A. L. (2014) Thin and open vessel windows for intra-vital fluorescence imaging of murine cochlear blood flow. Hear Res. 313, 38-46. [CrossRef]
- Carrasco, V. N., Prazma, J., Faber, J. E., Triana, R. J. and Pillsbury, H. C. (1990) Cochlear microcirculation. Effect of adrenergic agonists on arteriole diameter. Arch Otolaryngol Head Neck Surg. 116, 411-417. [CrossRef]
- Arpornchayanon, W., Canis, M., Suckfuell, M., Ihler, F., Olzowy, B. and Strieth, S. (2011) Modeling the measurements of cochlear microcirculation and hearing function after loud noise. Otolaryngol Head Neck Surg. 145, 463-469. [CrossRef]
- Ihler, F., Bertlich, M., Weiss, B., Dietzel, S. and Canis, M. (2015) Two-photon microscopy allows imaging and characterization of cochlear microvasculature in vivo. Biomed Res Int. 2015, 154272. [CrossRef]
- Subhash, H. M., Davila, V., Sun, H., Nguyen-Huynh, A. T., Shi, X., Nuttall, A. L. and Wang, R. K. (2011) Volumetric in vivo imaging of microvascular perfusion within the intact cochlea in mice using ultra-high sensitive optical microangiography. IEEE Trans Med Imaging. 30, 224-230. [CrossRef]
- Abdelrahman, M. S. I., Saleh, M. G. A., Aly, M. A. A. and Salah, S. (2025) Analysis of sensorineural hearing loss and its relation to Optical Coherence Tomography Angiography findings in Behcet's disease. Eur Arch Otorhinolaryngol. [CrossRef]
- Miller, J. M., Marks, N. J. and Goodwin, P. C. (1983) Laser Doppler measurements of cochlear blood flow. Hear Res. 11, 385-394. [CrossRef]
- Short, S. O., Goodwin, P. C., Kaplan, J. N. and Miller, J. M. (1985) Measuring cochlear blood flow by laser Doppler spectroscopy. Otolaryngol Head Neck Surg. 93, 786-793. [CrossRef]
- Ren, T., Brechtelsbauer, P. B., Miller, J. M. and Nuttall, A. L. (1994) Cochlear blood flow measured by averaged laser Doppler flowmetry (ALDF). Hear Res. 77, 200-206. [CrossRef]
- Scheibe, F., Haupt, H. and Grunert, H. (1997) Laser Doppler measurements of inner ear blood flow during experimental thrombosis of cochlear blood vessels in the guinea pig. Eur Arch Otorhinolaryngol. 254, 86-90. [CrossRef]
- Tran, Y. H., Ohsaki, K., Houchi, H., Ogawa, T., Zhu, C. S., Fushitani, S. and Minakuchi, K. (2001) The effect of tranexamic acid on cochlear blood flow in guinea pigs measured by laser Doppler flowmetry. Auris Nasus Larynx. 28, 215-218. [CrossRef]
- Didier, A., Droy-Lefaix, M. T., Aurousseau, C. and Cazals, Y. (1996) Effects of Ginkgo biloba extract (EGb 761) on cochlear vasculature in the guinea pig: morphometric measurements and laser Doppler flowmetry. Eur Arch Otorhinolaryngol. 253, 25-30. [CrossRef]
- Ohlsén, A., Hultcrantz, E., Larsen, H. C. and Angelborg, C. (1994) The cochlear blood flow: a comparison between the laser Doppler and the microsphere surface methods. Acta Otolaryngol. 114, 4-10. [CrossRef]
- Levine, R. A., Bu-Saba, N. and Brown, M. C. (1993) Laser-Doppler measurements and electrocochleography during ischemia of the guinea pig cochlea: implications for hearing preservation in acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 102, 127-136. [CrossRef]
- LaRouere, M. J., Sillman, J. S., Nuttall, A. L. and Miller, J. M. (1989) A comparison of laser Doppler and intravital microscopic measures of cochlear blood flow. Otolaryngol Head Neck Surg. 101, 375-384. [CrossRef]
- Dziennis, S., Reif, R., Zhi, Z., Nuttall, A. L. and Wang, R. K. (2012) Effects of hypoxia on cochlear blood flow in mice evaluated using Doppler optical microangiography. J Biomed Opt. 17, 106003. [CrossRef]
- Reif, R., Qin, J., Shi, L., Dziennis, S., Zhi, Z., Nuttall, A. L. and Wang, R. K. (2012) Monitoring hypoxia induced changes in cochlear blood flow and hemoglobin concentration using a combined dual-wavelength laser speckle contrast imaging and Doppler optical microangiography system. PLoS One. 7, e52041. [CrossRef]
- Chen, W., Wang, J., Chen, J., Chen, J. and Chen, Z. (2013) Relationship between changes in the cochlear blood flow and disorder of hearing function induced by blast injury in guinea pigs. Int J Clin Exp Pathol. 6, 375-384.
- Reif, R., Zhi, Z., Dziennis, S., Nuttall, A. L. and Wang, R. K. (2013) Changes in cochlear blood flow in mice due to loud sound exposure measured with Doppler optical microangiography and laser Doppler flowmetry. Quant Imaging Med Surg. 3, 235-242. [CrossRef]
- Kong, T. H., Yu, S., Jung, B., Choi, J. S. and Seo, Y. J. (2018) Monitoring blood-flow in the mouse cochlea using an endoscopic laser speckle contrast imaging system. PLoS One. 13, e0191978. [CrossRef]
- Kell, D. B. and Westerhoff, H. V. (1986) Metabolic control theory: its role in microbiology and biotechnology. FEMS Microbiol. Rev. 39, 305-320. [CrossRef]
- Cornish-Bowden, A., Hofmeyr, J.-H. S. and Cárdenas, M. L. (1995) Strategies for manipulating metabolic fluxes in biotechnology. Bioorg Chem. 23, 439-449. [CrossRef]
- Fell, D. A. (1996) Understanding the control of metabolism. Portland Press, London.
- Fell, D. A. (1998) Increasing the flux in metabolic pathways: A metabolic control analysis perspective. Biotechnol. Bioeng. 58, 121-124. [CrossRef]
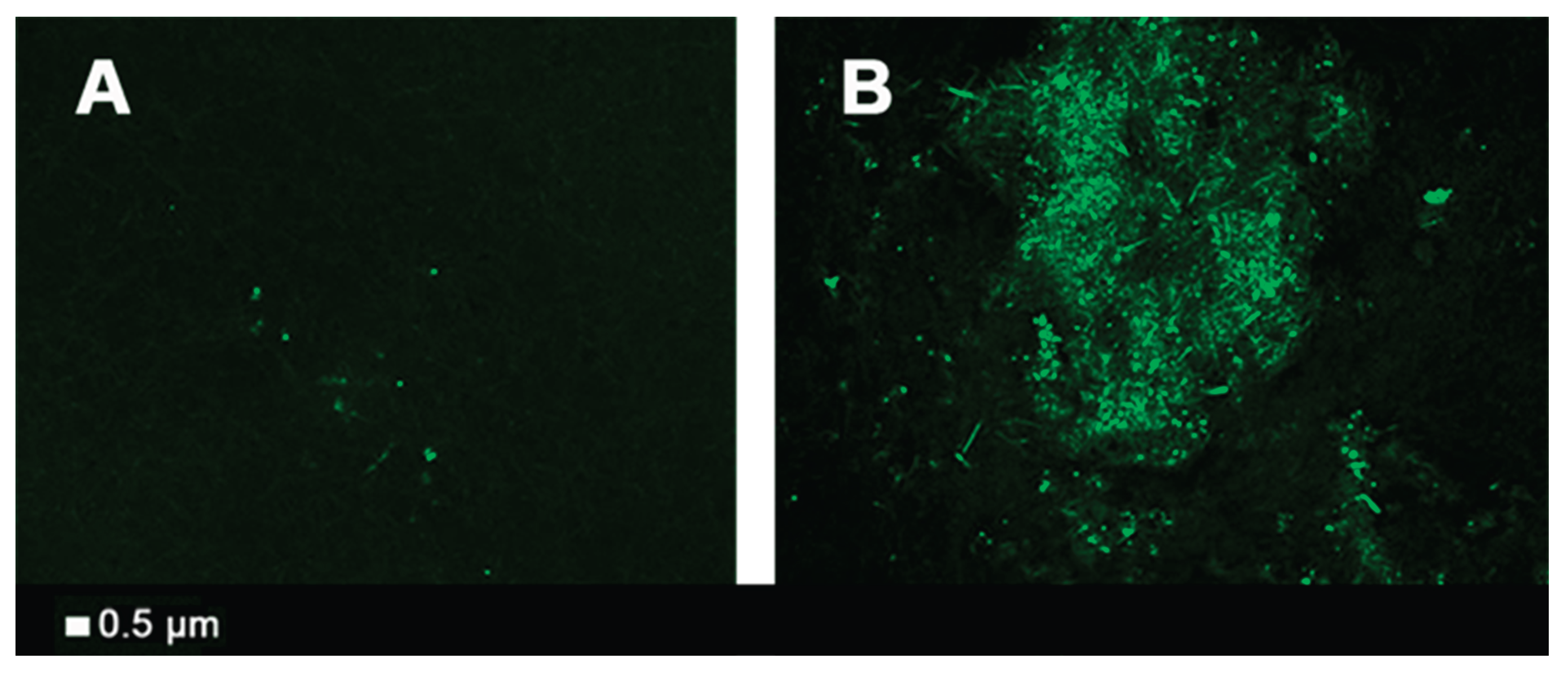
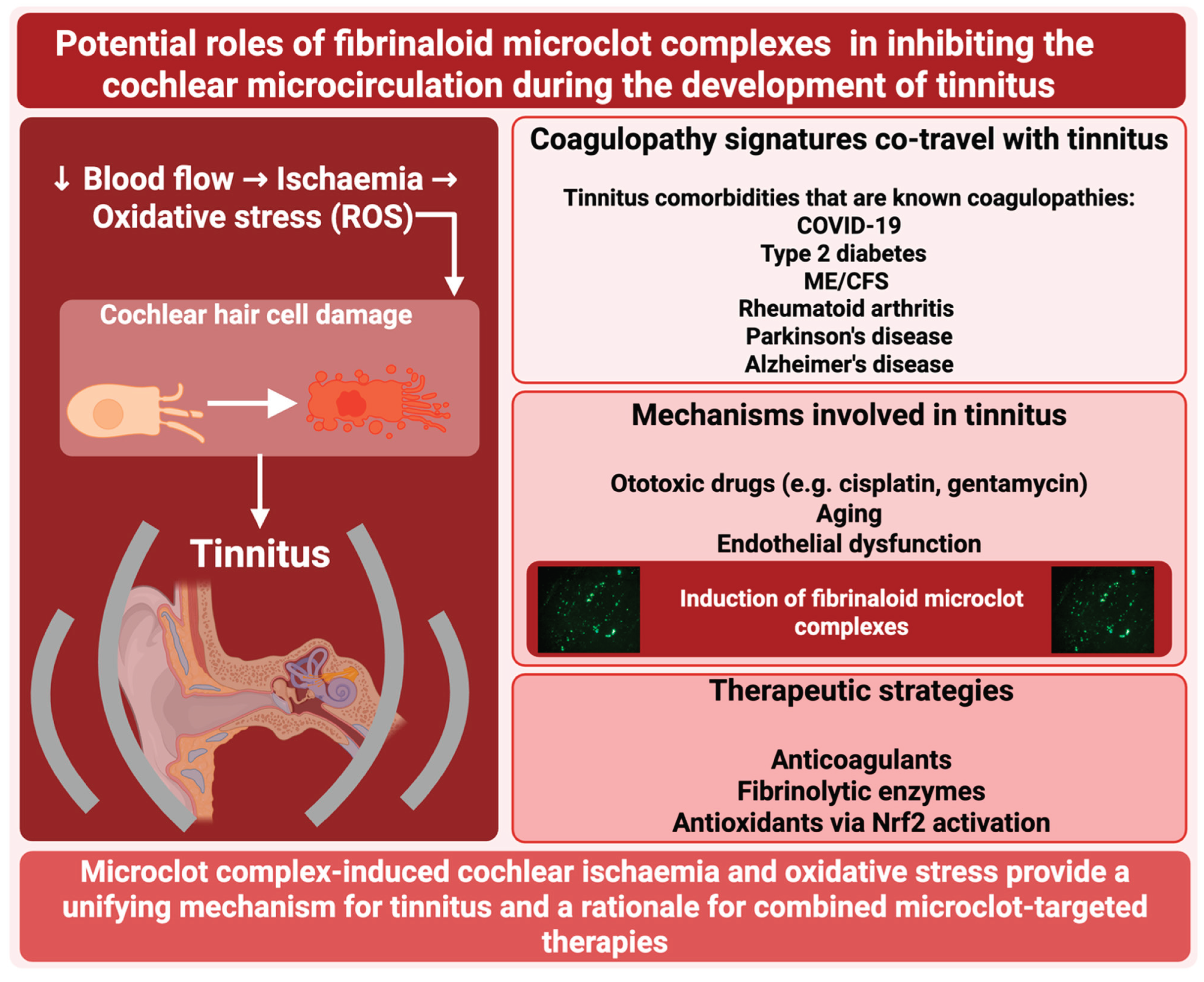
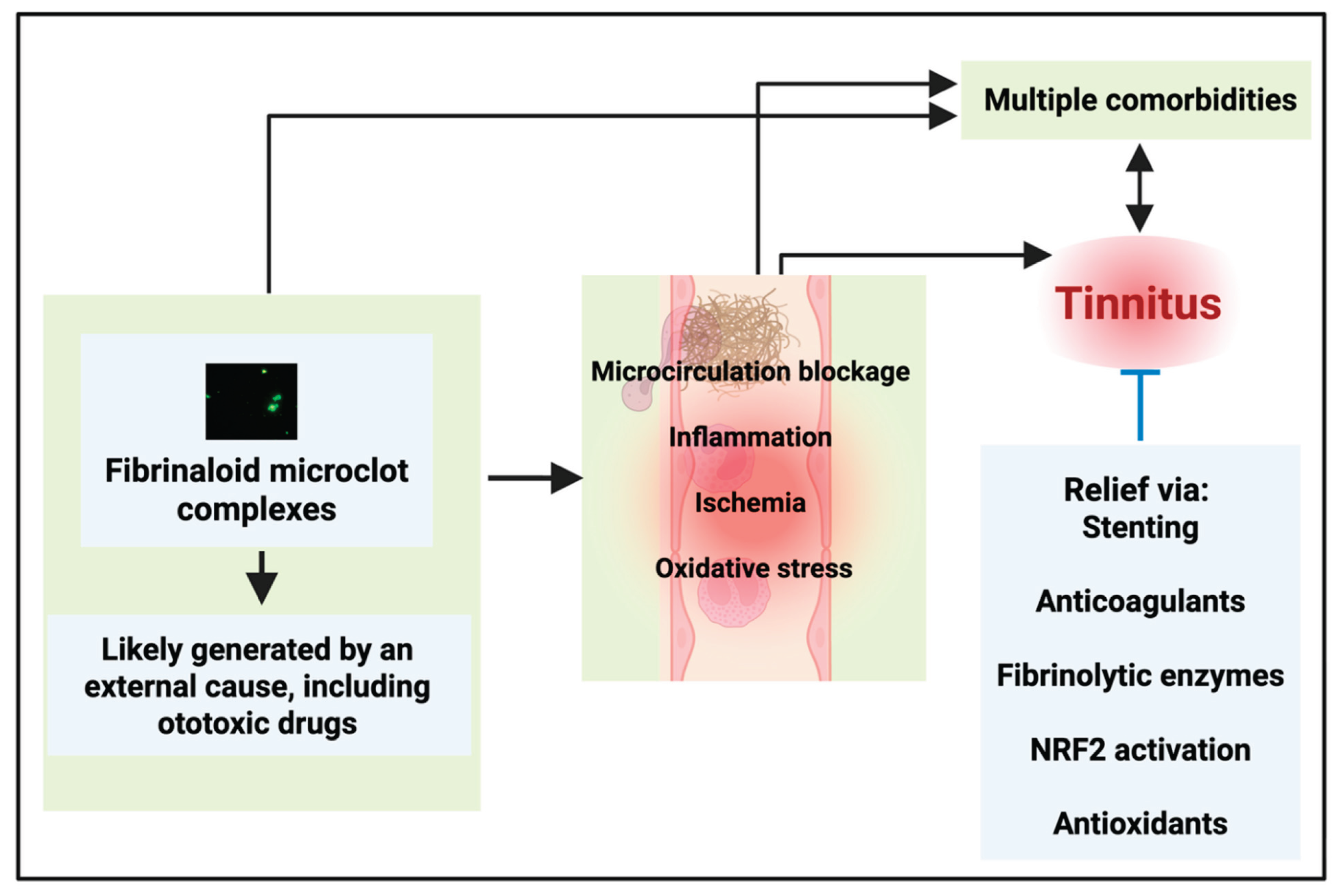
| Disease or syndrome | References indicating tinnitus as a comorbidity | References showing the presence of fibrinaloid microclot complexes |
| Alzheimer’s dementia incl cognitive impairment. Clear amyloid involvement |
[166,167,168,169,170,171,172,173,174] [175,176] |
[31,177,178,179,180] |
| COVID19 | [95,111,181,182,183,184,185,186,187,188,189,190,191,192,193] | [127,128,156,157,194] |
| Diabetes, type 2 | [9,195,196,197,198,199] | [194,200,201,202] |
| Migraine | [203] | [204] |
| Myalgic encephalomyelitis/ chronic fatigue syndrome (ME/CFS) | [95,205] | [206,207,208] |
| Parkinson’s disease | [167] | [200,209,210,211] |
| Post-COVID syndromes | [95,212,213,214,215,216,217,218,219,220] | [127,128,129,133,134,221,222,223] |
| Rheumatoid arthritis | [9,224,225,226] | [139,227,228,229] |
| Disease or syndrome | Selected references | Comments |
| Atherosclerosis | [244] | Atherosclerotic plaques frequently display amyloid |
| Atrial fibrillation (AF) | [245,246] | Fibrinaloid microclot complexes provide a ready explanation as being a cause rather than an effect of AF [142] |
| Congestive heart failure | [109] | |
| Erectile dysfunction | [247] | This is well known to be overcome by vasodilators [248] |
| Fibromyalgia | [95,249,250,251] | Microclots are also strongly implicated [143] |
| Myocardial infarction | [252] | |
| Postural orthostatic tachycardia syndrome (POTS) | [95,253,254,255] | Fibrinaloid microclot complexes provide a ready explanation for POTS [141] |
| Psoriasis | [256,257,258,259] | |
| Raynaud’s phenomenon | [260,261] | |
| Sickle cell disease | [262,263,264,265] | Clear example in which a primary cause that leads to a lowered microcirculation is associated with tinnitus |
| Sjögren’s syndrome | [167] | |
| Systemic sclerosis | [266,267,268,269,270,271] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





