Submitted:
01 August 2025
Posted:
04 August 2025
You are already at the latest version
Abstract
Keywords:
0. Scope of the Review
1. Introduction
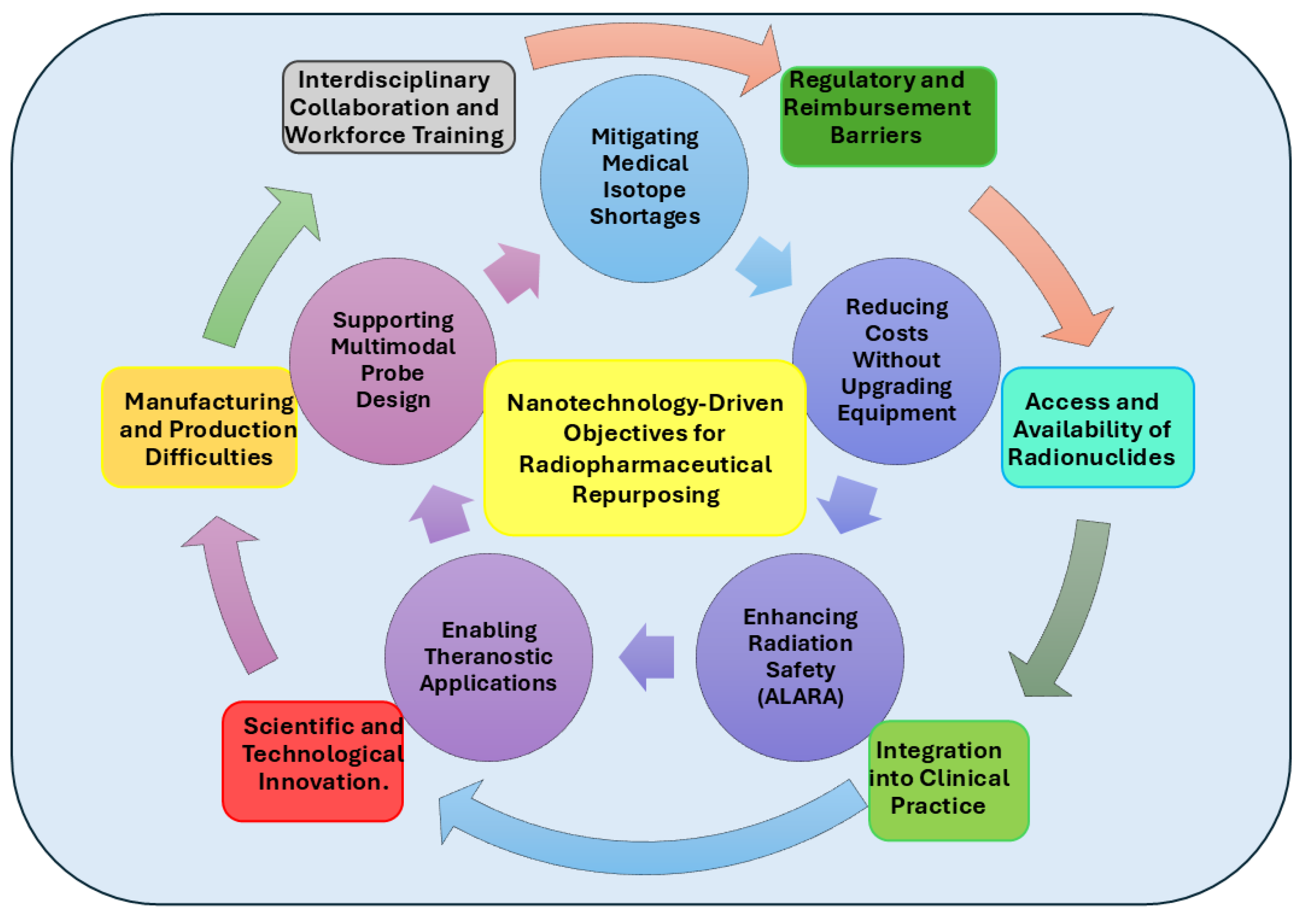
2. Current Challenges in Radiopharmacy
2.1. Manufacturing and Production Difficulties
2.2. Regulatory and Reimbursement Barriers
2.3. Access and Availability of Radionuclides
2.4. Integration into Clinical Practice
2.5. Interdisciplinary Collaboration and Workforce Training
2.6. Scientific and Technological Innovation
- Improve production efficiency and formulation robustness, enhancing stability, scalability, and handling of radioconjugates and nano-radiocarriers.
- Optimize biodistribution and targeting, reducing off-target effects and increasing the signal-to-background ratio for imaging and therapeutic efficacy.
- Enhance radiolabeling stability and chelation (especially for emerging radionuclides), reducing radionuclide leaching or recoil effects.
- Enable multimodal platforms and improved dosimetry by integrating both imaging and therapeutic components within the same nanocarrier, allowing real-time monitoring and precise quantification.
- Overcome biological barriers (e.g., tumor microenvironment, blood-brain barrier), which have historically limited uniform tumor uptake and led to the abandonment of otherwise promising agents.
- High diagnostic sensitivity is critical (e.g., for amyloid plaques, infections, or microthrombi),
- Targeting specificity reduces false positives and off-target toxicity,
- Radiobiological safety margins are especially important (e.g., pediatric or vulnerable populations),
- and multimodal imaging can accelerate clinical decision-making.
- Despite its promise, nanotechnology cannot independently solve:
- Fragmented regulatory and reimbursement frameworks
- Insufficient radionuclide production infrastructure,
- Gaps in workforce training and multidisciplinary collaboration.
3. Functional Objectives of Nanotechnology-Enabled Drug Repurposing in Radiopharmacy
- (1)
- Alleviating the Medical Isotope Shortage
- (2)
- Reducing Costs Without Infrastructure Investment
- (3)
- Enabling ALARA Compliance Through Dose Minimization
- (4)
- Facilitating Theragnostic Design and Implementation
- (5)
- Designing Multimodal Imaging Probes
4. Challenges in the Clinical Translation of Nanotechnology-Enabled Drug Repurposing in Radiopharmacy
4.1. Limited Clinical Translation Despite Extensive Preclinical Promise
4.2. Technical and Biological Constraints of Radiopharmaceutical Nanocarriers
4.3. Regulatory and Translational Outlook
5. Conclusions and Future Directions
Conflicts of Interest
Abbreviations
| ALARA: | As Low As Reasonably Achievable |
| CT: | Computed Tomography |
| DOTA: | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| EMA: | European Medicines Agency |
| EPR: | Enhanced Permeability and Retention |
| EUNCL: | European Nanomedicine Characterisation Laboratory |
| FDA: | Food and Drug Administration |
| GMP: | Good Manufacturing Practices |
| MRI: | Magnetic Resonance Imaging |
| NCL: | Nanomedicine Characterization Laboratory |
| NOTA: | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| PD: | Pharmacodynamics |
| PEG: | Polyethylene glycol |
| PET: | Positron Emission Tomography |
| PK: | Pharmacokinetics |
| SaMD: | Software-as-a-medical-device |
| SPECT: | Single Photon Emission Tomography |
References
- Cañellas CO, Salgueiro MJ, Zubillaga M. Radiofármacos: Del laboratorio al paciente. 1° Ed. 2017. CJP Ediciones. Ciudad Autónoma de Buenos Aires, Argentina. URL: http://www.phd-online.com.ar/radiofarmacos/.
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; Bushnell, D.; O’Dorisio, T.M.; Baum, R.P.; Kulkarni, H.R.; Caplin, M.; Lebtahi, R.; Hobday, T.; Delpassand, E.; Van Cutsem, E.; Benson, A.; Srirajaskanthan, R.; Pavel, M.; Mora, J.; Berlin, J.; Grande, E.; Reed, N.; Seregni, E.; Öberg, K.; Lopera Sierra, M.; Santoro, P.; Thevenet, T.; Erion, J.L.; Ruszniewski, P.; Kwekkeboom, D.; Krenning, E. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; Park, C.H.; Beer, T.M.; Armour, A.; Pérez-Contreras, W.J.; DeSilvio, M.; Kpamegan, E.; Gericke, G.; Messmann, R.A.; Morris, M.J.; Krause, B.J.; VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021, 16, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; Francis, R.J.; Gedye, C.; Rutherford, N.K.; Weickhardt, A.; Scott, A.M.; Lee, S.T.; Kwan, E.M.; Azad, A.A.; Ramdave, S.; Redfern, A.D.; Macdonald, W.; Guminski, A.; Hsiao, E.; Chua, W.; Lin, P.; Zhang, A.Y.; McJannett, M.M.; Stockler, M.R.; Violet, J.A.; Williams, S.G.; Martin, A.J.; Davis, I.D. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet. 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Miederer, M. Alpha emitting nuclides in nuclear medicine theranostics. Nuklearmedizin. 2022, 61, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. Alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J Nucl Med. 2018, 59, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 2. J Nucl Med. 2018, 59, 1020–1027. [Google Scholar] [CrossRef]
- Borgna, F.; Haller, S.; Rodriguez, J.M.M.; Ginj, M.; Grundler, P.V.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P.; Müller, C. Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2022, 49, 1113–1126. [Google Scholar] [CrossRef]
- Funkhouser, J. Reinventing pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- Kuge, Y.; Shiga, T.; Tamaki, N. Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy. Springer Nature. 2016. [CrossRef]
- Lapi, S.E.; Scott, P.J.H.; Scott, A.M.; Windhorst, A.D.; Zeglis, B.M.; Abdel-Wahab, M.; Baum, R.P.; Buatti, J.M.; Giammarile, F.; Kiess, A.P.; Jalilian, A.; Knoll, P.; Korde, A.; Kunikowska, J.; Lee, S.T.; Paez, D.; Urbain, J.L.; Zhang, J.; Lewis, J.S. Recent advances and impending challenges for the radiopharmaceutical sciences in oncology. Lancet Oncol. 2024, 25, e236–e249. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Ocampo-García, B.; Pasanphan, W.; Sakr, T.M.; Melendez-Alafort, L.; Grasselli, M.; Lugao, A.B.; Yousefnia, H.; Dispenza, C.; Janib, S.M.; Khan, I.U.; Maurin, M.; Ulański, P.; Loo, S.C.J.; Safrany, A.; Osso, J.A., Jr.; Duatti, A.; Katti, K.V. IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery. Pharmaceutics. 2022, 14, 1060. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, M.; Morales-Avila, E.; Cruz-Nova, P.; Katti, K.V.; Ocampo-García, B. Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications. Pharmaceutics. 2021, 13, 1123. [Google Scholar] [CrossRef]
- Klain, M.; Zampella, E.; Nappi, C.; Nicolai, E.; Ambrosio, R.; Califaretti, E.; Lamartina, L.; Schlumberger, M.; Deandreis, D.; Salvatore, D. Advances in Functional Imaging of Differentiated Thyroid Cancer. Cancers. 2021, 13, 4748. [Google Scholar] [CrossRef]
- Schwenck, J.; Sonanini, D.; Cotton, J.M.; Rammensee, H.G.; la Fougere, C.; Zenber, L.; Pichler, B.J. Advances in PET imaging of Cancer. Nat Rev Cancer 2023, 23, 474–490. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell. 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell. 2000, 1, 57–70. [Google Scholar] [CrossRef]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat Rev Clin Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.S.M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A.; Anderson, G.; Badawi, R.D.; Barthel, H.; Bengel, F.; Bodei, L.; Buvat, I.; DiCarli, M.; Graham, M.M.; Grimm, J.; Herrmann, K.; Kostakoglu, L.; Lewis, J.S.; Mankoff, D.A.; Peterson, T.E.; Schelbert, H.; Schöder, H.; Siegel, B.A.; Strauss, H.W. The future of nuclear medicine, molecular imaging, and theranostics. J Nucl Med. 2020, 61 (Suppl 2), 263S–272S. [Google Scholar] [CrossRef] [PubMed]
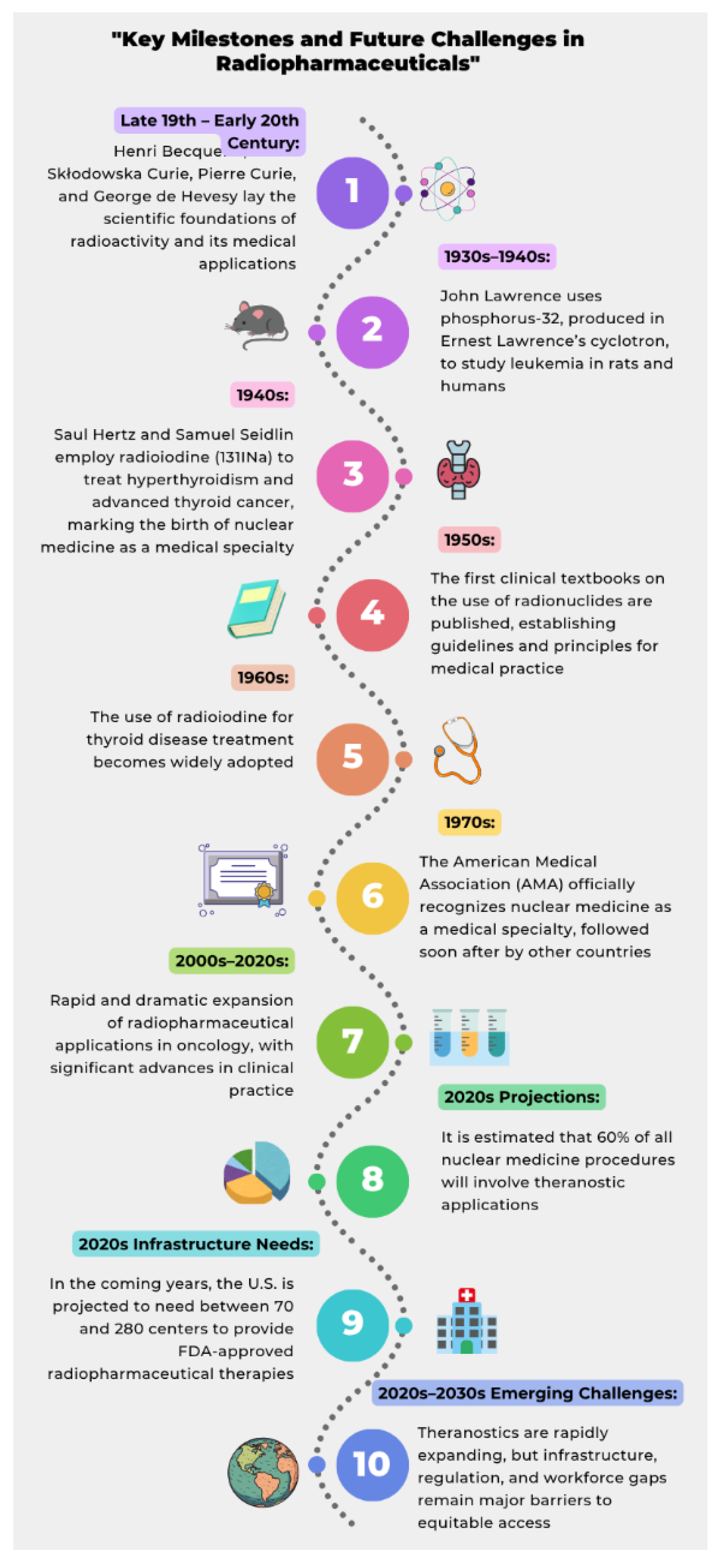
- Radvanyi, P.; Villain, J. The discovery of radioactivity. Comptes Rendus Physique. 2017, 18, 9–10. [Google Scholar] [CrossRef]
- Hevesy, G. The absorption and translocation of lead by plants: A contribution to the application of the method of radioactive indicators in the investigation of the change of substance in plants. Biochem J. 1923, 17, 439–445. [Google Scholar] [CrossRef]
- Myers, W.G. Georg Charles de Hevesy: The father of nuclear medicine. J Nucl Med Technol. 1996, 24, 291–294. [Google Scholar]
- Lawrence, J.; Tuttle, L.; Scott, K.; Connor, C. Studies on neoplasms with the aid of radioactive phosphorus. I. The total phosphorus metabolism of normal and leukemic mice. J Clin Invest. 1940, 19, 267–271. [Google Scholar]
- Tuttle, L.W.; Erf, L.A.; Lawrence, J.H. Studies on neoplasms with the aid of radioactive phosphorus. II. The phosphorus metabolism of the nucleoprotein, phospholipid and acid soluble fractions of normal and leukemic mice. J Clin Invest. 1941, 20, 57–61. [Google Scholar] [CrossRef]
- Chamberlain, R.H.; Low-Beer, B.V.A.; Thomas, C.C. The clinical use of radioactive isotopes. Science. 1950, 112, 794. [Google Scholar] [CrossRef]
- Beierwaltes, W.H.; Johnson, P.C.; Solari, A.J. Clinical Use of Radioisotopes. Philadelphia: W.B. Saunders Company; 1957.
- Czernin, J.; Calais, J. How many theranostics centers will we need in the united states? J Nucl Med. 2022, 63, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Chen, X.; Cai, W.; Mahmood, U. Radiotheranostics in cancer diagnosis and management. Radiology. 2018, 286, 388–400. [Google Scholar] [CrossRef]
- Cutler, C.S.; Bailey, E.; Kumar, V.; Schwarz, S.W.; Bom, H.S.; Hatazawa, J.; Paez, D.; Orellana, P.; Louw, L.; Mut, F.; Kato, H.; Chiti, A.; Frangos, S.; Fahey, F.; Dillehay, G.; Oh, S.J.; Lee, D.S.; Lee, S.T.; Nunez-Miller, R.; Bandhopadhyaya, G.; Pradhan, P.K.; Scott, A.M. Global Issues of Radiopharmaceutical Access and Availability: A Nuclear Medicine Global Initiative Project. J Nucl Med. 2021, 62, 422–430. [Google Scholar] [CrossRef]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; Mindt, T.L.; Neels, O.; Ocak, M.; Patt, M.; Todde, S. Guideline on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. EJNMMI Radiopharm Chem. 2021, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy - The Bad Berka Experience. Theranostics. 2012, 2, 437–447. [Google Scholar] [CrossRef]
- Turner, J.H. Recent advances in theranostics and challenges for the future. Br J Radiol. 2018, 91, 20170893. [Google Scholar] [CrossRef]
- Poot, A.J.; Lam, M.G.E.H.; van Noesel, M.M. The Current Status and Future Potential of Theranostics to Diagnose and Treat Childhood Cancer. Front Oncol. 2020, 10, 578286. [Google Scholar] [CrossRef]
- Herrmann, K.; Giovanella, L.; Santos, A.; Gear, J.; Kiratli, P.O.; Kurth, J.; Denis-Bacelar, A.M.; Hustinx, R.; Patt, M.; Wahl, R.L.; Paez, D.; Giammarile, F.; Jadvar, H.; Pandit-Taskar, N.; Ghesani, M.; Kunikowska, J. Joint EANM, SNMMI and IAEA enabling guide: How to set up a theranostics centre. Eur J Nucl Med Mol Imaging. 2022, 49, 2300–2309. [Google Scholar] [CrossRef]
- Urbain, J.L.; Scott, A.M.; Lee, S.T.; Buscombe, J.; Weston, C.; Hatazawa, J.; Kinuya, S.; Singh, B.; Haidar, M.; Ross, A.; et al. Theranostics Radiopharmaceuticals: A Universal Challenging Educational Paradigm in Nuclear Medicine. J Nucl Med. 2023, 64, 986–991. [Google Scholar] [CrossRef]
- Fahey, F.H.; Goodkind, A.; MacDougall, R.D.; Oberg, L.; Ziniel, S.I.; Cappock, R.; Callahan, M.J.; Kwatra, N.; Treves, S.T.; Voss, S.D. Operational and Dosimetric Aspects of Pediatric PET/CT. J Nucl Med. 2017, 58, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, H.; Kiss, O.; Kunikowska, J.; Wadsak, W.; Decristoforo, C.; Patt, M. EANM position on the in-house preparation of radiopharmaceuticals. Eur J Nucl Med Mol Imaging. 2022, 49, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Database of cyclotrons for radionuclide production. https://nucleus.iaea.org/sites/accelerators/Pages/Cyclotron.aspx.
- International Atomic Energy Agency. Disposal of radioactive waste. IAEA safety standards series No. SSR-5, Vienna 2011. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1449_web.pdf.
- Hricak, H.; Abdel-Wahab, M.; Atun, R.; Lette, M.M.; Paez, D.; Brink, J.A.; Donoso-Bach, L.; Frija, G.; Hierath, M.; Holmberg, O.; Khong, P.L.; Lewis, J.S.; McGinty, G.; Oyen, W.J.G.; Shulman, L.N.; Ward, Z.J.; Scott, A.M. Medical imaging and nuclear medicine: A Lancet Oncology Commission. Lancet Oncol. 2021, 22, e136–e172. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Delgado Bolton, R.C.; El-Haj, N.; Freudenberg, L.S.; Herrmann, K.; Mikhail, M.; Morozova, O.; Orellana, P.; Pellet, O.; Estrada, L.E.; Vinjamuri, S.; Gnanasegaran, G.; Pynda, Y.; Navarro-Marulanda, M.C.; Choudhury, P.S.; Paez, D. Changes in the global impact of COVID-19 on nuclear medicine departments during 2020: An international follow-up survey. Eur J Nucl Med Mol Imaging. 2021, 48, 4318–4330. [Google Scholar] [CrossRef] [PubMed]
- Delgado Bolton, R.C.; Calapaquí Terán, A.K.; Erba, P.A.; Giammarile, F. Medical imaging in times of pandemic: Focus on the cornerstones of successful imaging. Eur J Nucl Med Mol Imaging. 2021, 48, 1724–1725. [Google Scholar] [CrossRef]
- Paez, D.; Mikhail-Lette, M.; Gnanasegaran, G.; Dondi, M.; Estrada-Lobato, E.; Bomanji, J.; Vinjamuri, S.; El-Haj, N.; Morozova, O.; Alonso, O.; Pellet, O.; Orellana, P.; Navarro, M.C.; Delgado Bolton, R.C.; Giammarile, F. Nuclear Medicine Departments in the Era of COVID-19. Semin Nucl Med. 2022, 52, 41–47. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Research reactor database (RRDB). https://nucleus.iaea.org/rrdb/#/home.
- International Atomic Energy Agency. Manual for reactor produced radioisotopes, IAEA-TECDOC-1340, Vienna, 2003. https://www-pub.iaea.org/MTCD/publications/PDF/te_1340_web.pdf.
- International Atomic Energy Agency. Predisposal Management of Radioactive Waste from the Use of Radioactive Material in Medicine, Industry, Agriculture, Research and Education. IAEA Safety Standards Series No. SSG-45, Vienna 2019. https://www.iaea.org/publications/11087/predisposal-management-of-radioactive-waste-from-the-use-of-radioactive-material-in-medicine-industry-agriculture-research-and-education.
- International Atomic Energy Agency. Management of Radioactive Waste from the Use of Radionuclides in Medicine, IAEA-TECDOC-1805, Vienna, 2017. https://www-pub.iaea.org/MTCD/Publications/PDF/te_1183_prn.pdf.
- International Atomic Energy Agency. Radiation Protection and Safety in Medical Uses of Ionizing Radiation, IAEA Safety Standards Series No. SSG-46, Vienna 2018. https://www.iaea.org/publications/11102/radiation-protection-and-safety-in-medical-uses-of-ionizing-radiation.
- Kleynhans, J.; Grobler, A.F.; Ebenhan, T.; Sathekge, M.M.; Zeevaart, J.R. Radiopharmaceutical enhancement by drug delivery systems: A review. J Control Release. 2018, 287, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Chess, R. Economics of drug delivery. Pharm. Res. 1998, 15, 172–174. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science. 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Mishra, N.; Pant, P.; Porwal, A.; Jaiswal, J.; Samad, M.A.; Tiwari, S. Targeted drug delivery: A review. Am. J. Pharmatech. Res. 2016, 6, 1–24. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.A.; Larson, R.G.; Kotov, N.A. Nonadditivity of nanoparticle interaction. Science 2015, 350, 1242477. [Google Scholar] [CrossRef]
- Sahil, K.; Akanksha, M.; Premjeet, S.; Bilandi, A.; Kapoor, B. Microsphere: A review. Int. J. Res. Pharm. Chem. 2011, 1, 1184–1189. [Google Scholar]
- Valliant, J.F. A bridge not too far: Linking disciplines through molecular imaging probes, J. Nucl. Med. Tech. 2016, 44, 176–183. [Google Scholar] [CrossRef]
- Duvall, W.L.; Croft, L.B.; Ginsberg, E.S.; Einstein, A.J.; Guma, K.A.; George, T.; Henzlova, M.J. Reduced isotope dose and imaging time with a high-efficiency CZT SPECT camera. J. Nucl. Cardiol. 2011, 18, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Ross, C.; Wells, R.G. Reduce, reuse and recycle: A green solution to Canada’s medical isotope shortage. Appl. Radiat. Isot. 2014, 87, 148–151. [Google Scholar] [CrossRef]
- Hoedl, S.A.; Updegraff, W.D. The production of medical isotopes without nuclear reactors or uranium enrichment. Sci. Global Security. 2015, 23, 121–125. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy, Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar]
- Kim, T.H.; Lee, S.; Chen, X. Nanotheranostics for personalized medicine, Expert. Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef]
- Kaul, A.; Chaturvedi, S.; Attri, A.; Kalra, M.; Mishra, A.K. Targeted theranostic liposomes: Rifampicin and ofloxacin loaded pegylated liposomes for theranostic application in mycobacterial infections. RSC Adv. 2016, 6, 28919–28926. [Google Scholar] [CrossRef]
- Wang, S.J.; Lin, W.J.; Chen, M.N.; Chi, C.S.; Chen, J.T.; Ho, W.L.; Hsieh, B.T.; Shen, L.H.; Tsai, Z.T.; Ting, G.; Mirzadeh, S.; Knapp, F.F., Jr. Intratumoral injection of rhenium-188 microspheres into an animal model of hepatoma. J. Nucl. Med. 1998, 39, 1752–1757. [Google Scholar] [PubMed]
- Wunderlich, G.; Pinkert, J.; Stintz, M.; Kotzerke, J. Labelling and biodistribution of different particle materials for radioembolization therapy with 188Re, Appl. Radiat. Isot. 2005, 62, 745–750. [Google Scholar] [CrossRef]
- Blankespoor, S.C.; Wu, X.; Kalki, J.K.; Brown, H.R.; Tang, H.R.; Cann, C.E.; Hasegawa, B.H. Attenuation correction of SPECT using x-ray CT on an Emission-Transmission CT System: Myocardial perfusion assessment. IEEE T Nucl. Sci. 1996, 43, 2263–2274. [Google Scholar] [CrossRef]
- Shao, Y.; Cherry, S.R.; Farahani, K.; Meadors, K.; Siegel, S.B.; Silwerman, R.W.; Marsden, P.K. Simultaneous PET and MR imaging, Phys. Med. Biol. 1997, 42, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Townsend, W.D.; Burn, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology, J. Nucl. Med. 2000. 41, 1369–1379.
- Townsend, W.D. Dual-modality imaging: Combining anatomy and function. J. Nucl. Med. 2008, 49, 938–955. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Allen-Auerbach, M.; Schelbert, H.R. Improvements in cancer staging with PET/CT: Literature-based evidence as of September 2006, J. Nucl. Med. 2007, 48, 78S–88S. [Google Scholar]
- Judenhofer, M.S.; Wehrl, H.F.; Newport, D.F.; Catana, C.; Siegel, S.B.; Becker, M.; Thielscher, A.; Kneilling, M.; Lichy, M.P.; Eichner, M.; Klingel, K.; Reischl, G.; Widmaier, S.; Röcken, M.; Nutt, R.E.; Machulla, H.J.; Uludag, K.; Cherry, S.R.; Claussen, C.D.; Pichler, B.J. Simultaneous PET-MRI: A new approach for functional and morphological imaging, Nat. Med. 2008, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, J.H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology, Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef]
- Wehrl, H.F.; Sauter, A.W.; Judenhofer, M.S.; Pichler, B.J. Combined PET/MR imaging- technology and applications, Tech. Cancer Res. Treat. 2010, 9, 5–20. [Google Scholar] [CrossRef]
- Delso, G.; Fust, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef]
- Tan, I.C.; Darne, C.; Lu, Y.; Yan, S.; Smith, A.; Rasmussen, J.; Azhdarinia, A.; Sevick, E. Hybrid fluorescence, PET, and CT for small animal imaging, J. Nucl. Med. 2012, 53 (suppl 1), 493. [Google Scholar]
- Committee on State of Molybdenum-99 Production and Utilization and Progress Toward Eliminating Use of Highly Enriched Uranium; Nuclear and Radiation Studies Board; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Molybdenum-99 for Medical Imaging. Washington (DC): National Academies Press (US); 2016 Oct 28. Available from: https://www.ncbi.nlm.nih.gov/books/NBK396169/. [CrossRef]
- Man, F.; Gawne, P.J.; TMde Rosales, R. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv Drug Deliv Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Mushtaq, S.; Bibi, A.; Park, J.E.; Jeon, J. Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy. Nanomaterials (Basel). 2021, 11, 3022. [Google Scholar] [CrossRef]
- Toro-González, M.; Akingbesote, N.; Bible, A.; Pal, D.; Sanders, B.; Ivanov, A.S.; Jansone-Popova, S.; Popovs, I.; Benny, P.; Perry, R.; Davern, S. Development of 225Ac-doped biocompatible nanoparticles for targeted alpha therapy. J Nanobiotechnology. 2024, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Toro-González, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Encapsulation and retention of 225Ac, 223Ra, 227Th, and decay daughters in zircon-type gadolinium vanadate nanoparticles. Radiochimica Acta. 2020, 108, 967–977. [Google Scholar] [CrossRef]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconjug Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef]
- Trusova, V.; Karnaukhov, I.; Zelinsky, A.; Borts, B.; Ushakov, I.; Sidenko, L.; Gorbenko, G. Radiolabeling of bionanomaterials with technetium 99m: Current state and future prospects. Nanomedicine (Lond). 2024, 19, 1569–1580. [Google Scholar] [CrossRef]
- Engström, A.; Isaksson, M.; Javid, R.; Lundh, C.; Båth, M. A case study of cost-benefit analysis in occupational radiological protection within the healthcare system of Sweden. J Appl Clin Med Phys. 2021, 22, 295–304. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chemical Society Reviews. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Pomykala, K.L.; Würker, M.; Herrmann, K. Tackling the Last Mile: A Major Component to Successfully Establish Radioligand Therapy. J Nucl Med. 2023, 64, 347–348. [Google Scholar] [CrossRef]
- Scott, A.M.; Zeglis, B.M.; Lapi, S.E.; Scott, P.J.H.; Windhorst, A.D.; Abdel-Wahab, M.; Giammarile, F.; Piaez, D.; Jalilian, A.; Knoll, P.; Korde, A.; Vichare, S.; Ayati, N.; Lee, S.T.; Lyashchenko, S.K.; Zhang, J.; Urbain, J.L.; Lewis, J.S. Trends in nuclear medicine and the radiopharmaceutical sciences in oncology: Workforce challenges and training in the age of theranostics. Lancet Oncol. 2024, 25, e250–e259, Erratum in: Lancet Oncol. 2024, 25, e336. doi: 10.1016/S1470-2045(24)00378-4. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.; Eberlein, U.; Holm, S.; Hustinx, R.; Konijnenberg, M.; Strigari, L.; van Leeuwen, F.W.B.; Glatting, G.; Lassmann, M. EANM position paper on the role of radiobiology in nuclear medicine. Eur J Nucl Med Mol Imaging. 2021, 48, 3365–3377. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Béguin, F.; Damien, P.; Floch, R.; Kerleguer, K.; Hennebicq, S.; Robin, P.; Salaün, P.-Y.; Le Roux, P.-Y. Radiation exposure to nuclear medicine technologists performing a V/Q PET: Comparison with conventional V/Q scintigraphy, [18F]FDG PET and [68Ga]Ga DOTATOC PET procedures. Front. Med. 2022, 9, 1051249. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, E.O.; Barwick, T.D.; Haberkorn, U. Radiotheranostics in oncology: Making precision medicine possible. CA Cancer J Clin. 2023, 73, 255–274. [Google Scholar] [CrossRef]
- Thakral, P.; Sen, I.; Simecek, J.; Marx, S.; Kumari, J.; Kumar, S.; Tandon, P.; Dureja, S.; Pant, V. Radiation Exposure to the Nuclear Medicine Personnel During Preparation and Handling of 213Bi-Radiopharmaceuticals. J Nucl Med Technol. 2020, 48, 68–72. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano. 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Aminolroayaei, F.; Shahbazi-Gahrouei, D.; Shahbazi-Gahrouei, S.; Rasouli, N. Recent nanotheranostics applications for cancer therapy and diagnosis: A review. IET Nanobiotechnol. 2021, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent nanotheranostic approaches in cancer research. Clin Exp Med. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Goins, B.; Bao, A.; Phillips, W.T. Techniques for loading technetium-99m and rhenium-186/188 radionuclides into preformed liposomes for diagnostic imaging and radionuclide therapy. Methods Mol. Biol. 2017, 1522, 155–178. [Google Scholar] [PubMed]
- Belhaj-Tayeb, H.; Briane, D.; Vergote, J.; Kothan, S.; Leger, G.; Bendada, S.E.; Tofighi, M.; Tamgac, F.; Cao, A.; Moretti, J.L. In vitro and in vivo study of 99mTc-MIBI encapsulated in PEG-liposomes: A promising radiotracer for tumour imaging. EJNMMI 2003, 30, 502–509. [Google Scholar] [CrossRef]
- Goins, B.; Klipper, R.; Rudolph, A.S.; Phillips, W.T. Use of technetium-99m-liposomes in tumour imaging, J. Nucl. Med. 1994, 35, 1491–1498. [Google Scholar]
- Dams, E.T.; Oyen, W.J.; Boerman, O.C.; Storm, G.; Laverman, P.; Kok, P.J.; Buijs, W.C.; Bakker, H.; van der Meer, J.W.; Corsten, F.H. 99mTc-PEG liposomes for the scintigraphic detection of infection and inflammation: Clinical evaluation, J. Nucl. Med. 2000, 41, 622–630. [Google Scholar]
- Dagar, S.; Krishnadas, A.; Rubinstein, I.; Blend, M.J.; Onyuksel, H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: In vivo studies, J. Control. Release. 2003, 91, 123–133. [Google Scholar] [CrossRef]
- Kleiter, M.M.; Yu, D.; Mohammadian, L.A.; Niehaus, N.; Spasojevic, I.; Sanders, L.; Viglianti, B.L.; Yarmolenko, P.S.; Hauck, M.; Petry, N.A.; Wong, T.Z.; Dewhirst, M.W.; Thrall, D.E. A tracer dose of technetium-99m-labeled liposomes can estimate the effect of hyperthermia on intratumoral doxil extravasation, Clin. Cancer Res. 2006, 15, 6800–6807. [Google Scholar]
- Chen, M.H.; Chang, C.H.; Chang, Y.J.; Chen, L.C.; Yu, C.Y.; Wu, Y.H.; Lee, W.C.; Yeh, C.H.; Lin, F.H.; Lee, T.W.; Yang, C.S.; Ting, G. MicroSPECT/CT imaging and pharmacokinetics of 188Re-(DXR)-liposome n human colorectal adenocarcinoma-bearing mice. Anticancer Res. 2010, 30, 65–72. [Google Scholar]
- Tsai, C.C.; Chang, C.H.; Chen, L.C.; Chang, Y.J.; Lan, K.L.; Wu, Y.H.; Hsu, C.W.; Liu, I.H.; Ho, C.L.; Lee, W.C.; Ni, H.C.; Chang, T.J.; Ting, G.; Lee, T.W. Biodistribution and pharmacokinetics of 188Re-liposomes and their comparative therapeutic efficacy with 5-fluorouracil in a C26 colonic peritoneal carcinomatosis mice, Int. J. Nanomedicine. 2011, 6, 2607–2619. [Google Scholar]
- Liu, C.M.; Chang, C.H.; Chang, Y.J.; Hsu, C.W.; Chen, L.C.; Chen, H.L.; Ho, C.L.; Yu, C.Y.; Chang, T.J.; Chiang, T.C.; Lee, T.W. Preliminary evaluation of acute toxicity of 188Re-BMEDA-liposome in rats, J. Appl. Toxicol. 2010, 30, 680–687. [Google Scholar] [CrossRef]
- Chi-Mou, L.; Chia-Che, T.; Chia-Yu, Y.; Wan-Chi, L.; Chung-Li, H.; Tsui-Jung, C.; Chih-Hsien, C.; Te-Wei, L. Extended acute toxicity study of 188Re-liposome in rats. J. Appl. Toxicol. 2013, 33, 886–893. [Google Scholar] [CrossRef]
- Hsu, W.H.; Liu, S.Y.; Chang, Y.J.; Chang, C.H.; Ting, G.; Lee, T.W. The PEGylated liposomal doxorubicin improves the delivery and therapeutic efficiency of 188Re-Liposome by modulating phagocytosis in C26 murine colon carcinoma tumour mode. , Nucl. Med. Biol. 2014, 41, 765–771. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, S.Y.; Chi, C.W.; Yu, H.L.; Chang, T.J.; Tsai, T.H.; Lee, T.W.; Chen, Y.J. External beam radiotherapy synergizes 188Re-liposome against human oesophageal cancer xenograft and modulates 188Re-liposome pharmacokinetics. Int. J. Nanomedicine. 2015, 10, 3641–3649. [Google Scholar]
- Allard, E.; Hindre, F.; Passirani, C.; Lemaire, L.; Lepareur, N.; Noiret, N.; Menei, P.; Benoit, J.P. 188Re-loaded lipid nanocapsules as a promising radiopharmaceutical carrier for internal radiotherapy of malignant gliomas. EJNMMI. 2008, 35, 1838–1846. [Google Scholar] [CrossRef]
- Wang, S.X.; Bao, A.; Herrera, S.J.; Phillips, W.T.; Goins, B.; Santoyo, C.; Miller, F.R.; Otto, R.A. Intraoperative 186Re-liposome radionuclide therapy in a head and neck squamous cell carcinoma xenograft positive surgical margin model. Clin. Cancer Res. 2008, 14, 3975–3983. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, C.L.; Goins, B.A.; Bao, A.; McManus, L.M.; McMahan, C.A.; Phillips, W.T. Imaging of 186Re-liposome therapy in ovarian cancer xenograft model of peritoneal carcinomatosis. J. Drug Target. 2008, 16, 626–637. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yu, C.Y.; Hsu, C.W.; Lee, W.C.; Chen, S.J.; Chang, C.H.; Lee, T.W. Molecular imaging and therapeutic efficacy of 188Re-(DXR)-liposome-BNN in AR42J pancreatic tumour-bearing mice. Oncol. Rep. 2012, 28, 1736–1742. [Google Scholar] [CrossRef]
- Chen, L.C.; Wu, Y.H.; Liu, I.H.; Ho, C.L.; Lee, W.C.; Chang, C.H.; Lan, K.L.; Ting, G.; Lee, T.W.; Shien, J.H. Pharmacokinetics, dosimetry and comparative efficacy of 188Re-liposome and 5-Fu in a CT26-luc lung-metastatic mice model. Nucl. Med. Biol. 2012, 39, 35–43. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.; Bao, A.; Vargas, D.; Guttierez, J.E.; Trevino, A.; Miller, J.R.; Henry, J.; Zuniga, R.; Vecil, G.; Brenner, A.J. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro-oncology. 2012, 14, 416–425. [Google Scholar] [CrossRef]
- Liu, C.M.; Lee, W.C.; Yu, C.Y.; Lan, K.L.; Chang, C.H.; Ting, G.; Lee, T.W. Comparison of the therapeutic efficacy of 188Rhenium-liposomes and liposomal doxurbicin in a 4T1 murine orthotopic breast cancer model. Oncol. Rep. 2012, 5, 678–684. [Google Scholar]
- Shen, Y.A.; Lan, K.L.; Chang, C.H.; Lin, L.T.; He, C.L.; Chen, P.H.; Lee, T.W.; Lee, Y.J.; Chuang, C.M. Intraperitoneal (188)Re-liposome delivery switches ovarian cancer metabolism from glycolysis to oxidative phosphorylation and effectively controls ovarian tumour growth in mice. Radiother. Oncol. 2016, 119, 282–290. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Yu, J.; Xia, J.; Zang, C.; Yin, D.; Häfeli, U.O. Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. J. Magn. Magn. Mater. 2004, 277, 165–174. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Y.; Zang, C.; Liu, X. Synthesis of amino-modified magnetite nanoparticles coated with Hepama-1 and radiolabeled with 188Re for bio-magnetically targeted radiotherapy. J. Radioanal. Nucl. Ch. 2006, 269, 3–7. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega. 2023, 8, 14290–14320. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Sig Transduct Target Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Azimizonuzi, H.; Ghayourvahdat, A.; Ahmed, M.H.; Kareem, R.A.; Zrzor, A.J.; Mansoor, A.S.; Athab, Z.H.; Kalavi, S. A state-of-the-art review of the recent advances of theranostic liposome hybrid nanoparticles in cancer treatment and diagnosis. Cancer Cell Int. 2025, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, M.A.; Bouziotis, P.; Stiliaris, E.; Stamopoulos, D. Radiolabeled Iron Oxide Nanoparticles as Dual Modality Contrast Agents in SPECT/MRI and PET/MRI. Nanomaterials. 2023, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Thorek, D.L.; Ramos, N.N.; Pinkse, M.W.; Wolterbeek, H.T.; Carlin, S.D.; Beattie, B.J.; Lewis, J.S. 89Zr-labeled paramagnetic octreotide-liposomes for PET-MR imaging of cancer. Pharm. Res. 2013, 30, 878–888. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Radiolabeled iron oxide nanomaterials for multimodal nuclear imaging and positive contrast MRI. ACS Applied Nano Materials 2023, 6, 20523–20538. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Y.; Shen, L. Preparation and in vivo imaging of a novel potential αvβ3 targeting PET/MRI dual-modal imaging agent. J Radioanal Nucl Chem. 2022, 331, 3485–3494. [Google Scholar] [CrossRef]
- Glaus, C.; Rossin, R.; Welch, M.J.; Bao, G. In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjug. Chem. 2010, 21, 715–722. [Google Scholar] [CrossRef]
- Kim, J.; Pandya, D.N.; Lee, W.; Park, J.W.; Kim, Y.J.; Kwak, W.; Ha, Y.S.; Chang, Y.; An, G.I.; Yoo, J. Vivid tumour imaging utilizing liposome-carried bimodal radiotracer, ACS Med. Chem. Lett. 2015, 5, 390–394. [Google Scholar]
- Li, S.; Goins, B.; Zhang, L.; Bao, A. A novel multifunctional theranostic liposome drug delivery system: Construction, characterization, and multimodality MR, Near-infrared fluorescent and nuclear imaging, Bioconjug. Chem. 2012, 23, 1322–1332. [Google Scholar] [CrossRef]
- Zielhuis, S.W.; Seppenwoolde, J.H.; Mateus, V.A.; Bakker, C.J.; Krijger, G.C.; Storm, G.; Zonnenberg, B.A.; van het Schip, A.D.; Koning, G.A.; Nijsen, J.F. Lanthanide-loaded liposomes for multimodality imaging and therapy. Cancer Biother. Radiopharm. 2006, 21, 520–527. [Google Scholar] [CrossRef]
- Zang, J.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments, Clin. Pharm. Therap. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchillin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS. 2012, 14, 303–315. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Buiting, A.M.J.; Van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes, Biochim. Biophys. Acta. 1994, 1190, 99–107. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future, Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S.K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J. Control. Release. 2016, 231, 77–85. [Google Scholar] [CrossRef]
- Petrak, K. Essential properties of drug-targeting delivery systems, Drug Discov. Today. 2006, 10, 1667–1673. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical application. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Meada, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Uriely, B.; Jeffers, S.; Isacson, R.; Kutch, K.; Wei-Tsao, D.; Yehoshua, Z.; Libson, E.; Muggia, F.M.; Lasic, D.D. Liposomal doxorubicin: Antitumor activity and unique toxicities during two complementarty phase I studies. J. Clin. Oncol. 1995, 13, 1777–1785. [Google Scholar]
- Yamashita, F.; Hashida, M. Pharmacokinetic considerations for targeted drug delivery. Adv Drug Del Rev. 2013, 65, 139–147. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Paez, D.; Zimmermann, R.; Cutler, C.S.; Jalilian, A.; Korde, A.; Knoll, P.; Ayati, N.; Lewis, J.S.; Lapi, S.E.; Delgado Bolton, R.C.; Kunikowska, J.; Estrada Lobato, E.; Urbain, J.L.; Holmberg, O.; Abdel-Wahab, M.; Scott, A.M. Production and regulatory issues for theranostics. Lancet Oncol. 2024, 25, e260–e269. [Google Scholar] [CrossRef] [PubMed]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J.; Decuzzi, P. New Opportunities and Old Challenges in the Clinical translation of Nanotheranostics. Nat Rev Mater. 2023, 8, 783–798. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




