Submitted:
29 July 2025
Posted:
30 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
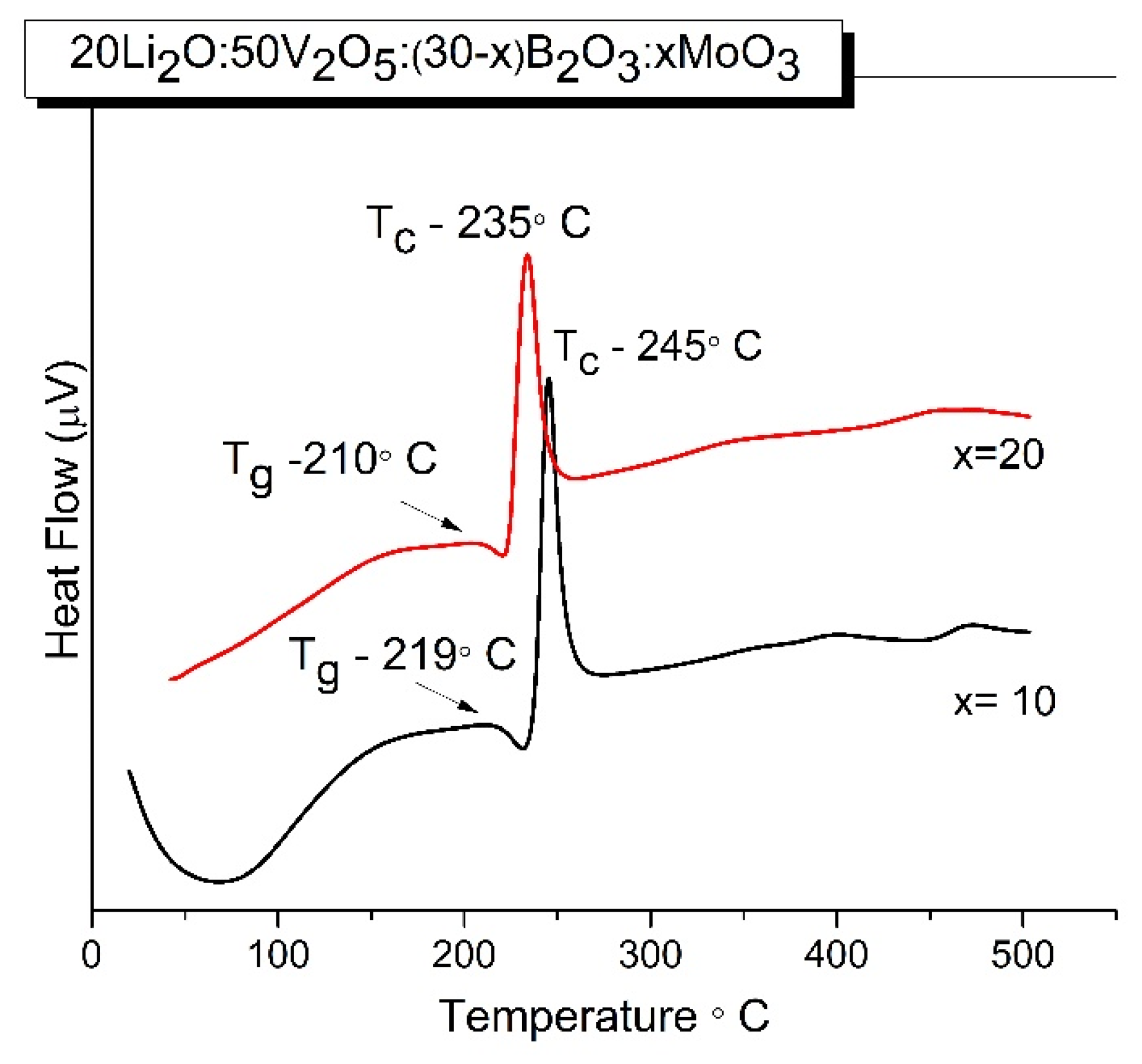
2.1. XRD and DCS Studies
2.2. IR Spectral Analysis
2.3. Density, Molar Volume, Oxygen Packing Density and Oxygen Molar Volume
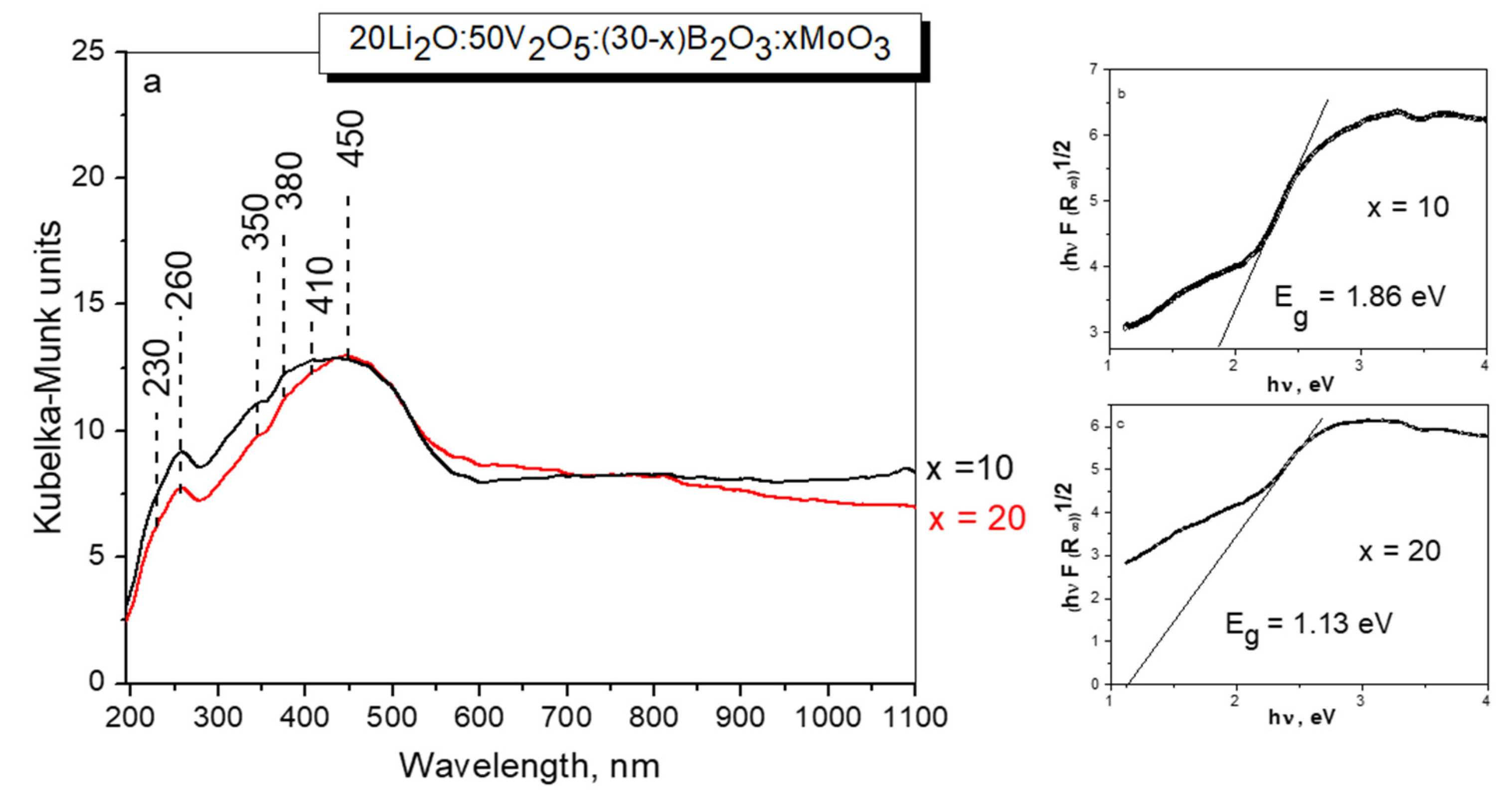
2.4. Diffuse Reflectance Spectra (DRS)
2.5. Electrical Properties
2.6. All-Solid-State Battery
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermeer, W.; Mouli, G.R.C.; Bauer, P. A Comprehensive Review on the Characteristics and Modeling of Lithium-Ion Battery Aging. IEEE Trans. Transp. Electrif. 2022, 8, 2205–2232. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S. Recent Advances on Materials for Lithium-Ion Batteries, Review. Energies 2021, 14, 1–36. [Google Scholar] [CrossRef]
- Afyon, S.; Krumeich, F.; Mensing, C.; Borgschulte, A.; Nesper, R. New High Capacity Cathode Materials for Rechargeable Li-ion Batteries: Vanadate-Borate Glasses. Sci. rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.Y.; Salem, S.M.; Moustafa, M.G. Study of nanostructure and ionic conductivity of Li1.3Nb0.3V1.7(PO4)3 glass ceramics used as cathode material for solid batteries. J. Non-Cryst Solids 2014, 391, 6–11. [Google Scholar] [CrossRef]
- Zhao, E.L.; Zhao, S.X.; Wu, X.; Li, J.W.; Yu, L.Q.; Nan, C.W.; Cao, G. Electrochemical performance of Li2O-V2O5-SiO2-B2O3 glass as cathode material for lithium ion batteries. J. Materiomics 2019, 5, 663–669. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, K.; Zhu, C.; Zhang, X.; Zhang, D. ; Co-Estimation of State-of-Charge and State-of- Health for Lithium-Ion Batteries Using an Enhanced Electrochemical Model. IEEE Trans. Ind. Electron. 2021, 69, 2684–2696. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Wang, L.; Liao, C.; Zhang, Y. Joint State-of-Charge and State-of-Available-Power Estimation Based on the Online Parameter Identification of Lithium-Ion Battery Model. IEEE Trans. Ind. Electron 2021, 69, 3677–3688. [Google Scholar] [CrossRef]
- Wei, Z.; Quan, Z.; Wu, J.; Li, Y.; Pou, J.; Zhong, H. Deep Deterministic Policy Gradient-DRL Enabled Multiphysics-Constrained Fast Charging of Lithium-Ion Battery. IEEE Trans. Ind. Electron. 2022, 69, 2588–2598. [Google Scholar] [CrossRef]
- Ni, Z.; Yang, Y. A Combined Data-Model Method for State-of-Charge Estimation of Lithium-Ion Batteries. IEEE Trans. Instrum. Meas. 2022, 71, 2503611. [Google Scholar] [CrossRef]
- Mustafa, J.; Alqaed, S.; Husain, S.; Jamil, B.; Sharifpur, M.; Cheraghian, G. Effect of Phase Change Materials on Lithium-Ion Plate Batteries. Batteries 2023, 9, 1–18. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, J.; He, H.; Yu, Y.; Marco, J. Embedded Distributed Temperature Sensing Enabled Multistate Joint Observation of Smart Lithium-Ion Battery. IEEE Trans. Ind. Electron. 2023, 70, 555–565. [Google Scholar] [CrossRef]
- Kittaneh, O. On the Theory of the Arrhenius-Normal Model with Applications to the Life Distribution of Lithium-Ion Batteries. Batteries 2023, 9, 1–10. [Google Scholar] [CrossRef]
- Shui, Z.Y.; Li, X.H.; Feng, Y.; Wang, B.C.; Wang, Y. Combining Reduced-Order Model with Data-Driven Model for Parameter Estimation of Lithium-Ion Battery. IEEE Trans. Ind. Electronics 2023, 70, 1521–1531. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Ayyavu, P.G.; Panchal, H.; Shanmugam, D.; Balasubramani, S.; Al-rubaie, A.J.; Al-khaykan, A.; Oza, A.D.; Hembrom, S.; Patel, T.; Vizureanu, P.; Burduhos-Nergis, D.P. Efficient Battery Models for Performance Studies-Lithium Ion and Nickel Metal Hydride Battery. Batteries 2023, 9, 1–14. [Google Scholar] [CrossRef]
- Delmas, C.; Cognac-Auradou, H.; Cocciantelli, J.M.; Menetrier, M.; Doumerc, J.P. The LixV2O5 system: an overview of the structure modifications induced by the lithium intercalation. Solid State Ionics. 1994, 69, 257–264. [Google Scholar] [CrossRef]
- Attos, O.; Massot, M.; Mavi, H.S.; Julien, C. Spectroscopic investigations of the network structure in borovanadate glasses, Mat. Res. Soc. Symp. Proc. 1997, 455, 477–482. [Google Scholar] [CrossRef]
- Bih, L.; Omari, M. El; Reau, J.M.; Haddad, M.; Boudlich, D.; Yacoubi, A.; Nadiri, A. Electronic and ionic conductivity of glasses inside the Li2O–MoO3–P2O5 system. Solid State Ionics 2000, 132, 71–85. [Google Scholar] [CrossRef]
- Maniu, D.; Iliescu, T.; Ardelead, I.; Bratu, I.; Dem, C. Studies of borate vanadate glasses using Raman and IR spectroscopy. Stud. U. Babes-Bol. Phys (Special issue) 2001, 366–371. [Google Scholar]
- Bih, L.; Nadiri, A.; El Omari, M.; Yacoubi, A.; Haddad, M. FTIR, EPR and X-ray investigation of mixed valence molybdenum phosphate A2O–(MoO3)2–P2O5 (A=Li,Na) glasses. Phys Chem Glasses 2002, 43, 153–157. [Google Scholar]
- Jozwiak, P.; Garbarczyk, J.E. Mixed electronic–ionic conductivity in the glasses of the Li2O–V2O5–P2O5 system. Solid State Ionics 2005, 176, 2163–2166. [Google Scholar] [CrossRef]
- Al-Hajry, A.; Al-Shahrani, A.; El-Desoky, M.M. Structural and other physical properties of barium vanadate glasses. Mater. Chem. Phys. 2006, 95, 300–306. [Google Scholar] [CrossRef]
- Bih, L.; Abbas, L.; Nadiri, A.; Amraoui, Y.E.; Mezzane, D.; Khemakhem, H. DC and AC conductivities of the yLi2O–(1-y)[0.35(MoO3)2–0.65(P2O5)] glasses. M.J. Condenced Mater. 2006, 7, 70–73. [Google Scholar]
- Rao, L.S.; Reddy, M.S.; Reddy, M.R.; Veeraiah, N. Dielectric dispersion in Li2O–MoO3–B2O3 glass system doped with V2O5. J. Alloys Compd. 2008, 464, 472–482. [Google Scholar] [CrossRef]
- Takahashi, H.; Karasawa, T; Sakuma, T.; Garbarczyk, J.E. Electrical conduction in the vitreous and crystallized Li2O–V2O5–P2O5 system. Solid State Ionics 2010, 81, 27–32. [Google Scholar] [CrossRef]
- Barczyński, R.J.; Król, P.; Murawski, L. AC and DC conductivities in V2O5–P2O5 glasses containing alkaline ions. J. Non-Cryst Solids 2010, 356, 1965–1967. [Google Scholar] [CrossRef]
- Gowda, V.C.; Chethana, B.K.; Reddy, C.N. Ion transport studies in lithium phospho-molybdate glasses containing Cl− ion. Mat Sci Eng B-Adv. 2013, 178, 826–833. [Google Scholar] [CrossRef]
- Saetova, N. S.; Raskovalov, A.A.; Antonov, B.D.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Zabolotskaya, E.V.; Kadyrova, N.I.; Telyatnikova, A.A. Conductivity and spectroscopic studies of Li2O-V2O5-B2O3 glasses. Ionics 2018, 24, 1929–1938. [Google Scholar] [CrossRef]
- Kindle, M.; Kmiec, S.; d'Anciães, I.; Silva, A.; Eckert, H.; Martin, S.W.; Song, M.K.; McCloy, J.S. Structural properties of alumina-doped lithium borovanadate glasses and glass-ceramics. J. Non-Cryst. Solids 2019, 521, 119551. [Google Scholar] [CrossRef]
- Saetova, N.S.; Raskovalov, A.A.; Antonov, B.D.; Denisova, T.A.; Zhuravlev, N.A. Structural features of Li2O–V2O5–B2O3 glasses: Experiment and molecular dynamics simulation, J. Non-Cryst. Solids 2020, 545, 120253. [Google Scholar] [CrossRef]
- Banagar, A.V.; Kumar, M.P.; Nagaraja, N. Effect of Mixed Transition Metal Ions in B2O3-V2O5-MoO3 Glass System. J. Electron. Mater. 2020, 49, 7370–7378. [Google Scholar] [CrossRef]
- Ori, G.; Montorsi, M.; Pedone, A.; Siligardi, C. Insight into the structure of vanadium containing glasses: a molecular dynamics study. J. Non-Cryst. Solids 2011, 357, 2571–2579. [Google Scholar] [CrossRef]
- Attos, O.; Massot, M.; Mavi, H.S.; Julien, C. Spectroscopic investigations of the network structure in borovanadate glasses. Mat. Res. Soc. Symp. Proc. 1997, 455, 477–482. [Google Scholar] [CrossRef]
- Boora, M.; Malik, S.; Kumar, V.; Bala, M.; Arora, S.; Rohilla, S.; Kumar, A.; Dalal, J. Investigation of structural and impedance spectroscopic properties of borate glasses with high Li+ concentration. Solid State Ionics 2021, 368, 115704. [Google Scholar] [CrossRef]
- Ukpong, A.M. Controlling the crystallization of lithium borovanadate phases in an oxide glass composite using the CALPHAD approach. Mater. Today Proc. 2021, 38, 1059–1070. [Google Scholar] [CrossRef]
- Swapna, G.; Upender, M.P. ; Raman, FTIR, thermal and optical properties of TeO2 -Nb2O5 -B2O3 -V2O5 quaternary glass system. JTUSCI 2017, 11, 583–592. [Google Scholar] [CrossRef]
- Ray, N.H. Composition-properties relationship in Inorganic Oxide Glasses. J. Non-Cryst. Solids 1974, 15, 423–434. [Google Scholar] [CrossRef]
- Saddeek, Y.; Azooz, M.; Saddek, A. Ultrasonic investigations of some bismuth borate glasses doped with Al2O3. Bull. Mater. Sci. 2015, 38, 241–246. [Google Scholar] [CrossRef]
- Saddeek, Y. Effject of B2O3 on the structure and properties of tungsten-tellurite glasses. Philos. Mag. 2009, 89, 41–54. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, T.F.; Nie, Q.H.; Shen, X. Spectral properties and thermal stability of erbium TeO2 -WO3 -La2O3 glass. J. Inorg. Mater. 2006, 21, 351–356. [Google Scholar]
- Hamad, A.H.; Marzouk, M.A.; ElBatal, H.A. The Effect of Bi2O3 on Optical, FTIR and Thermal Properties of SrO-B2O3 glasses. Silicon 2016, 8, 121–131. [Google Scholar] [CrossRef]
- Soliman, A.A.; Kashif, I. Copper oxide content dependence of crystallization behavior, glass forming ability, glass stability and fragility of lithium borate glasses. Phys. B 2010, 405, 247–253. [Google Scholar] [CrossRef]
- Varsamis, C.P.; Makris, E.N.; Valvi, C.; Kamitsos, E.I. Short-range structure, the role of bismuth and property-structure correlations in bismuth borate glasses. Phys. Chem. Chem Phys. 2021, 23, 10006–1020. [Google Scholar] [CrossRef]
- Milanova, M.; Aleksandrov, L.; Yordanova, A.; Iordanova, R.; Tagiara, N.S.; Herrmann, A.; Gao, G.; Wondraczek, L.; Kamitsos, E.I. Structural and luminescence behavior of Eu3+ ions in ZnO-B2O3-WO3 glasses. J. Non-Cryst. Solids 2023, 600, 122006. [Google Scholar] [CrossRef]
- Iordanova, R.; Milanova, M.; Aleksandrov, L.; Khanna, A. Structural study of glasses in the system B2O3-Bi2O3-La2O3-WO3. J. Non-Cryst. Solids 2018, 481, 254–259. [Google Scholar] [CrossRef]
- Markova-Velichkova, M.; Iordanova, R.; Dimitriev, Y. Glass formation in the V2O5-MoO3-ZnO system. Phys. Status Solidi C 2011, 8, 3159–3162. [Google Scholar] [CrossRef]
- Khan, S.; Singhm, K. Structual, optical, thermal and conducting properties of V2-xLixO5-δ (0.15≤x≤0.30) system. Sci. Rep. 2020, 10, 1089–11. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kostov, K.L.; Ganev, V.; Yordanov, St.; Dimitriev, Y. Synthesis and structural chatacterization of a glass in the Ag2O-SeO2-MoO3 system. J. Non-Cryst. Solids 2018, 481, 138–147. [Google Scholar] [CrossRef]
- Milanova, M.; Iordanova, R.; Tatsumisago, M.; Hayashi, A.; Tzvetkov, P.; Nihtianova, D.; Markov, P.; Dimitriev, Y. Soft mechanochemical synthesis and electrochemical behavior of LiVMoO6 for all-solid-state lithium batteries. J Mater Sci 2016, 51, 3574–3584. [Google Scholar] [CrossRef]
- Milanova, M.; Iordanova, R.; Aleksandrov, L.; Hassan, M.; Dimitriev, Y. Glass formation and structure of glasses in the ZnO―Bi2O3―WO3―MoO3 system. J. Non-Cryst. Solids 2011, 357, 2713–2718. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Azooz, M.A.; Saddek, A.B. Ultrasonic investigations of some bismuth borate glasses doped with Al2O3. Bull. Mater. Sci. 2015, 38, 241–246. [Google Scholar] [CrossRef]
- Aryal, P.; Kesavulu, C.R.; Kim, H.J.; Lee, S.W.; Kang, S.J.; Kaewkhao, J.; Chanthima, N.; Damdee, B. Optical and luminescence characteristics of Eu3+-doped B2O3: SiO2: Y2O3: CaO glasses for visible red laser and scintillation material applications. J. Rare Earths 2018, 36, 482–491. [Google Scholar] [CrossRef]
- Villegas, M.A.; Fernández Navarro, J.M. Physical and structural properties of glasses in the TeO2–TiO2–Nb2O5 system. J. Eur. Ceram. Soc. 2007, 27, 2715–2723. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Trifiro, F.; Aboukals, A.; Aissi, C.F.; Guelton, M. Physicochemiacl Characterizationof V-Silicates. J. Phus. Chem. 1992, 96, 2617–2629. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Iordanova, R.; Dimitriev, Y.; Georgiev, N.; Komatsu, T. Eu3+ doped 1La2O3:2WO3:1B2O3 glass and glass–ceramic. Opt. Mater. 2014, 36, 1366–1372. [Google Scholar] [CrossRef]
- Margha, F.H.; El-Bassyouni, G.T.; Turky, G.M. Enhancing the electrical conductivity of vanadate glass system (Fe2O3, B2O3, V2O5) via doping with sodium or strontium cations. Ceram Int. 2018, 45, 11838–11843. [Google Scholar] [CrossRef]
- Arunkumar, V.; Banagar, M.; Kumar, P.; Nagaraja, N. Effect of Mixed Transition Metal Ions in B2O3-V2O5-MoO3 Glass System. J. Electron. Mater. 2020, 49, 7370–7378. [Google Scholar]
- Srinivasa Rao, L. AC conductivity and polarization phenomenon of Li2O-MoO3-B2O3:V2O5 glasses. J. Alloys Compd. 2019, 787, 1280–1289. [Google Scholar] [CrossRef]
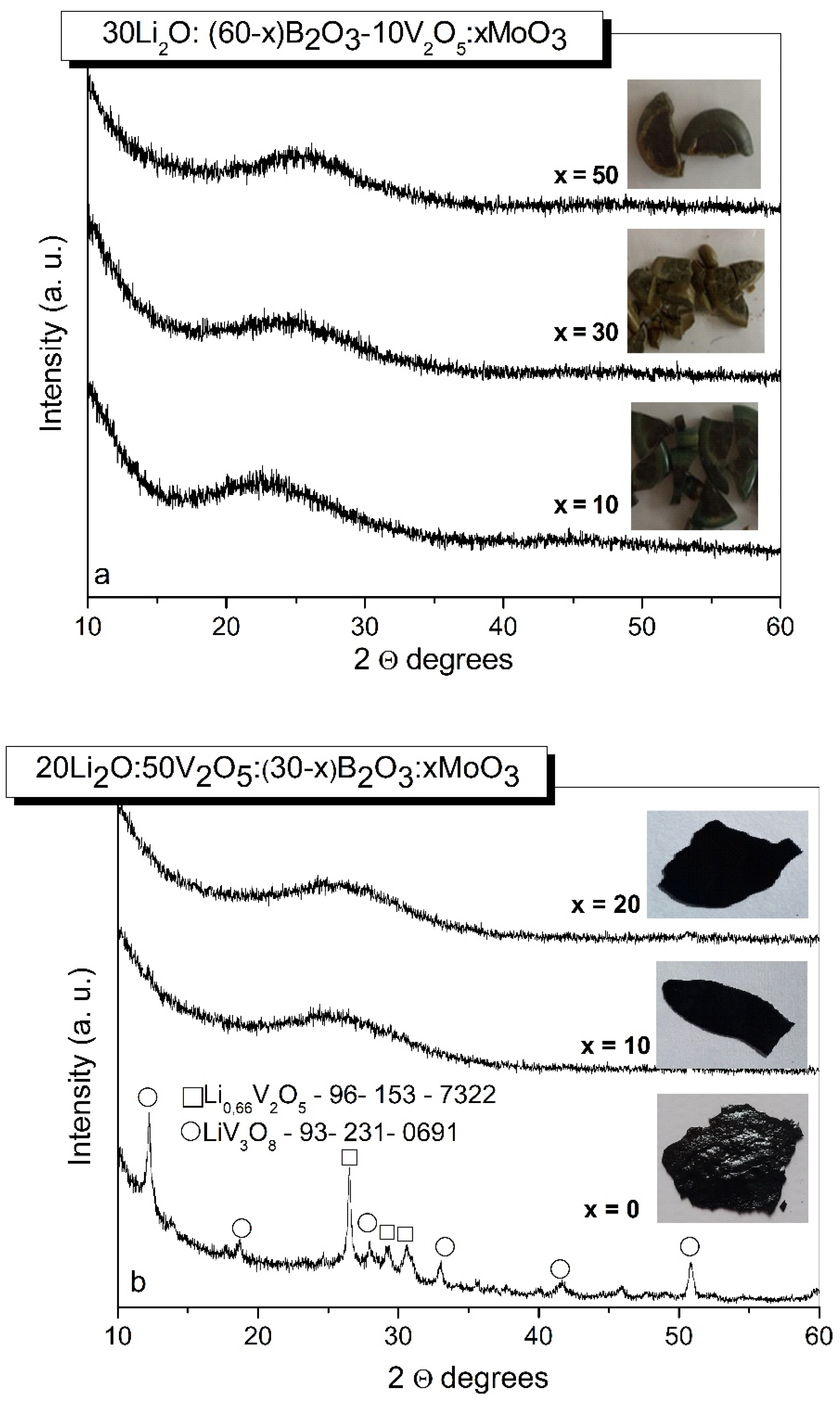
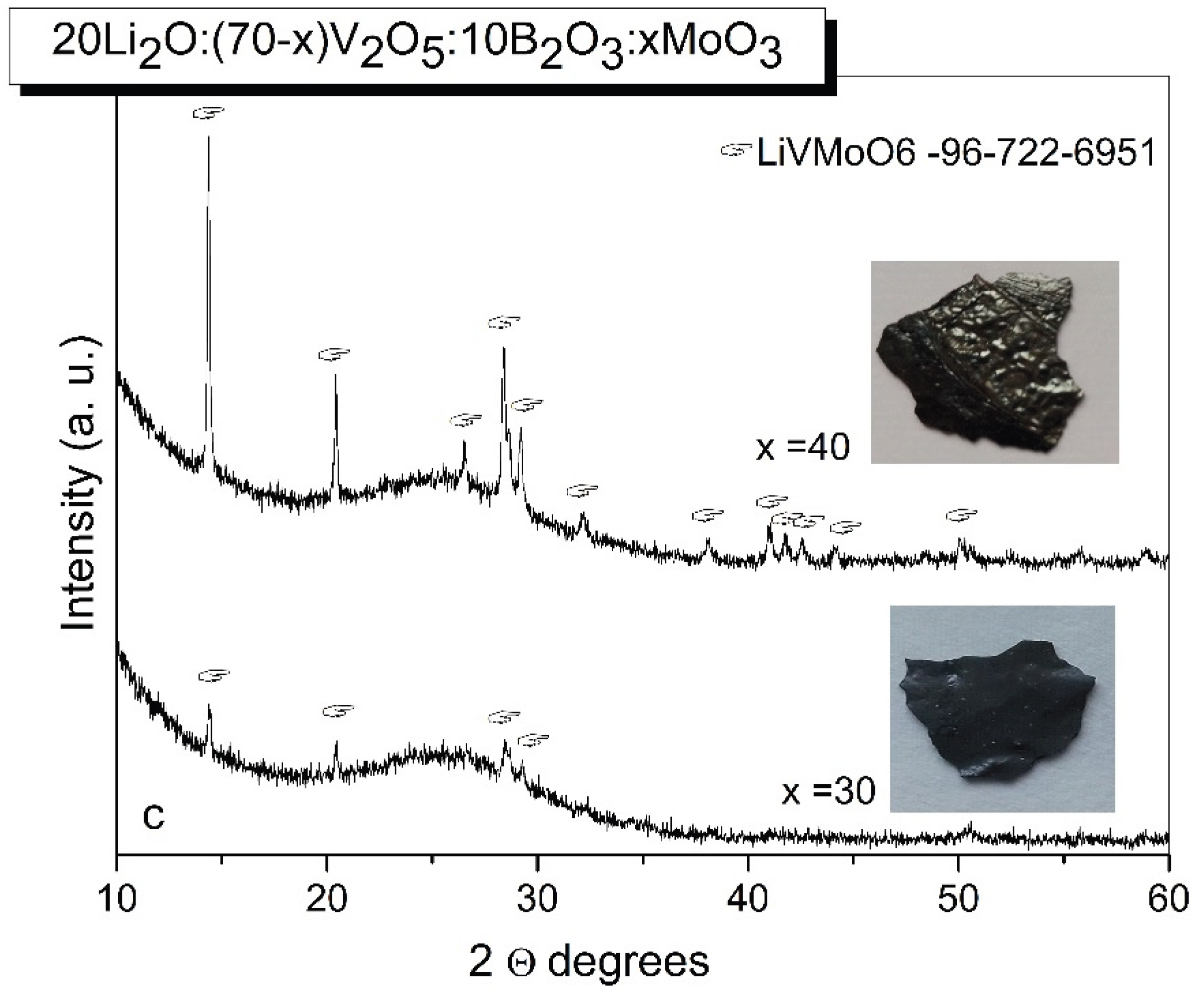
| Sample | Li2O (mol.%) |
B2O3 (mol.%) |
V2O5 (mol.%) |
MoO3 (mol.%) |
Melting temperature (° С) |
Colling rate К/s |
Visual assessment |
| 1. | 30 | 10 | 10 | 50 | 850 | ≤101-102 | Hygroscopic/Non homogeneous glass |
| 2. | 30 | 30 | 10 | 30 | 850 | ≤101-102 | Hygroscopic/Non homogeneous glass |
| 3. | 30 | 50 | 10 | 10 | 850 | ≤101-102 | Hygroscopic/Non homogeneous glass |
| Sample | Li2O (mol.%) |
B2O3 (mol.%) |
V2O5 (mol.%) |
MoO3 (mol.%) |
Melting temperature (° С) |
Colling rate К/s |
Visual assessment |
| 1. | 20 | 20 | 50 | - | 950 | 101-102 | Glass+crystals |
| 2. | 20 | 20 | 50 | 10 | 950 | 101-102 | Non hygroscopic/Homogeneous glass |
| 3. | 20 | 10 | 50 | 20 | 950 | 101-102 | Non hygroscopic/Homogeneous glass |
| 4. | 20 | 10 | 40 | 30 | 950 | 101-102 | Glass+crystals |
| 5. | 20 | 10 | 30 | 40 | 950 | 101-102 | Glass+crystalls |
| Sample ID | Tg/°C | Tc/°C | ΔT/°C | EB/KJ mol-1 |
| x = 10 | 219 | 245 | 24 | 612 |
| x = 20 | 210 | 235 | 25 | 592 |
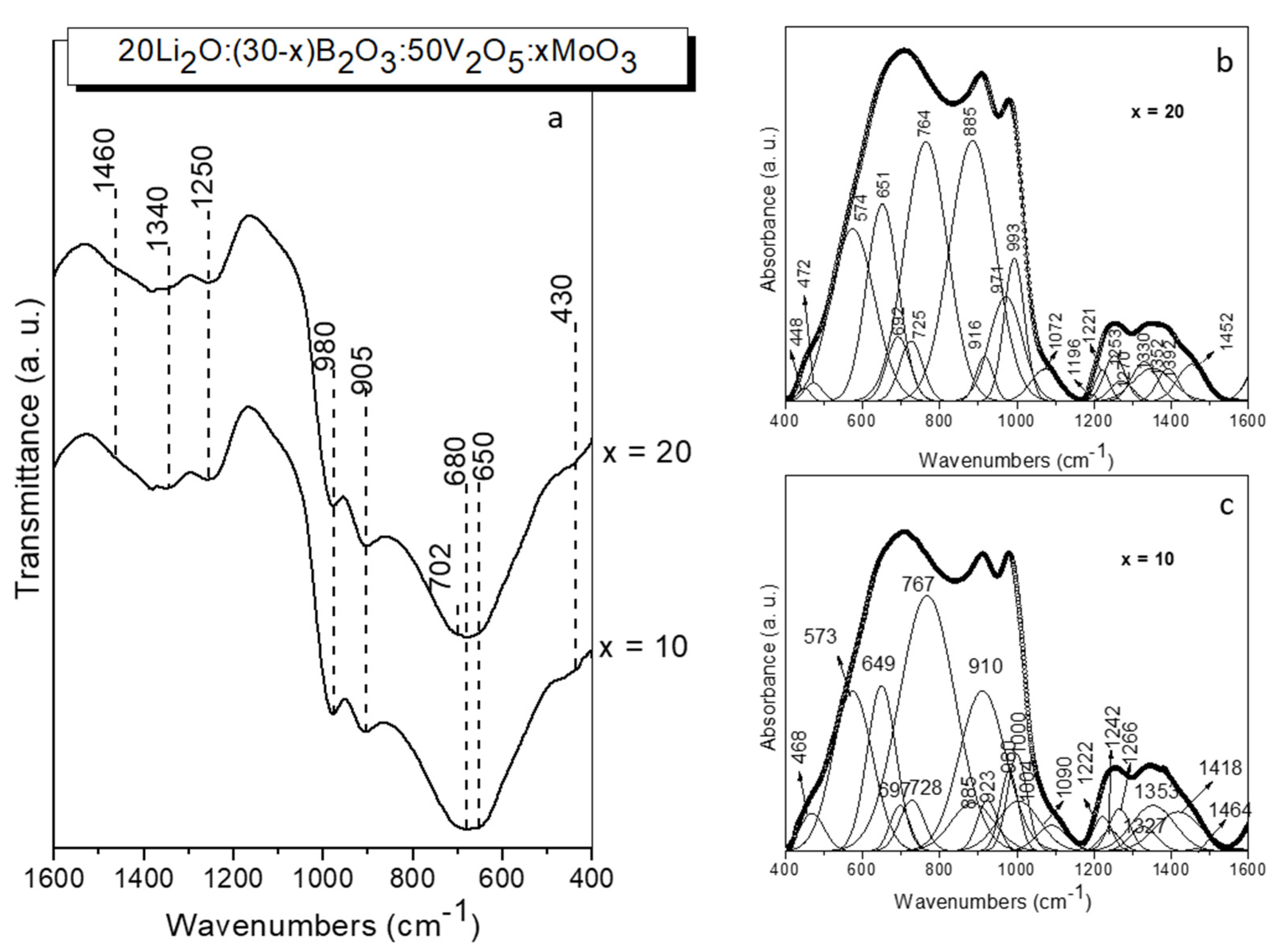
| Infrared bands position (cm-1) | Assignment | Ref. |
| 468-472 | δ V2O2 (VO5) | 46 |
| 573 | νs (Me2O8), Me= V/Mo | 48 |
| 649-651 | νas (VO4) | 45 |
| 697 | ν3 (MoO4) | 49 |
| 728 | νs (Me2O8), Me= V/Mo | 48 |
| 767-764 | ν (V - O - V) | 46 |
| 885 | ν (Me - O - Me), (Mo/VO6) Me= V/Mo |
48 |
| 910 | ν1 (MoO4) | 49 |
| 923-916 | ν (Me = O), (Mo/VO6) Me= V/Mo |
45, 49 |
| 980-971 | ν (Mo = O), MoO6 | 47 |
| 1000-993 | ν (V=O), (VO5) | 46 |
| 1090-1072 | ν BØ4- | 44 |
| 1196; 1242-1253 | ν3 BO33- | 42 |
| 1221; 1237-1330 | B-O-B stretch in pyroborate units, BØO22- ;B-O- stretch in in pyroborate units, BØO22- | 42 |
| 1266-1270; 1352, 1392-1418; 1452-1464 |
BØ stretch in metaborate unuts, BØ2O+ νas(B–O–B); B–O–B bridges connect BO3 units ν(B–O-) stretch in BØ2O- units |
42, 43 42, 43 |
| Sample ID | ρ (g/cm3) | Vm (cm3/mol) | Vo (cm3/mol) | OPD (g atom/l) |
| x = 10 | 2.964±0.006 | 39.90 | 11.08 | 90.22 |
| x = 20 | 3.051±0.002 | 43.48 | 12.08 | 82.80 |
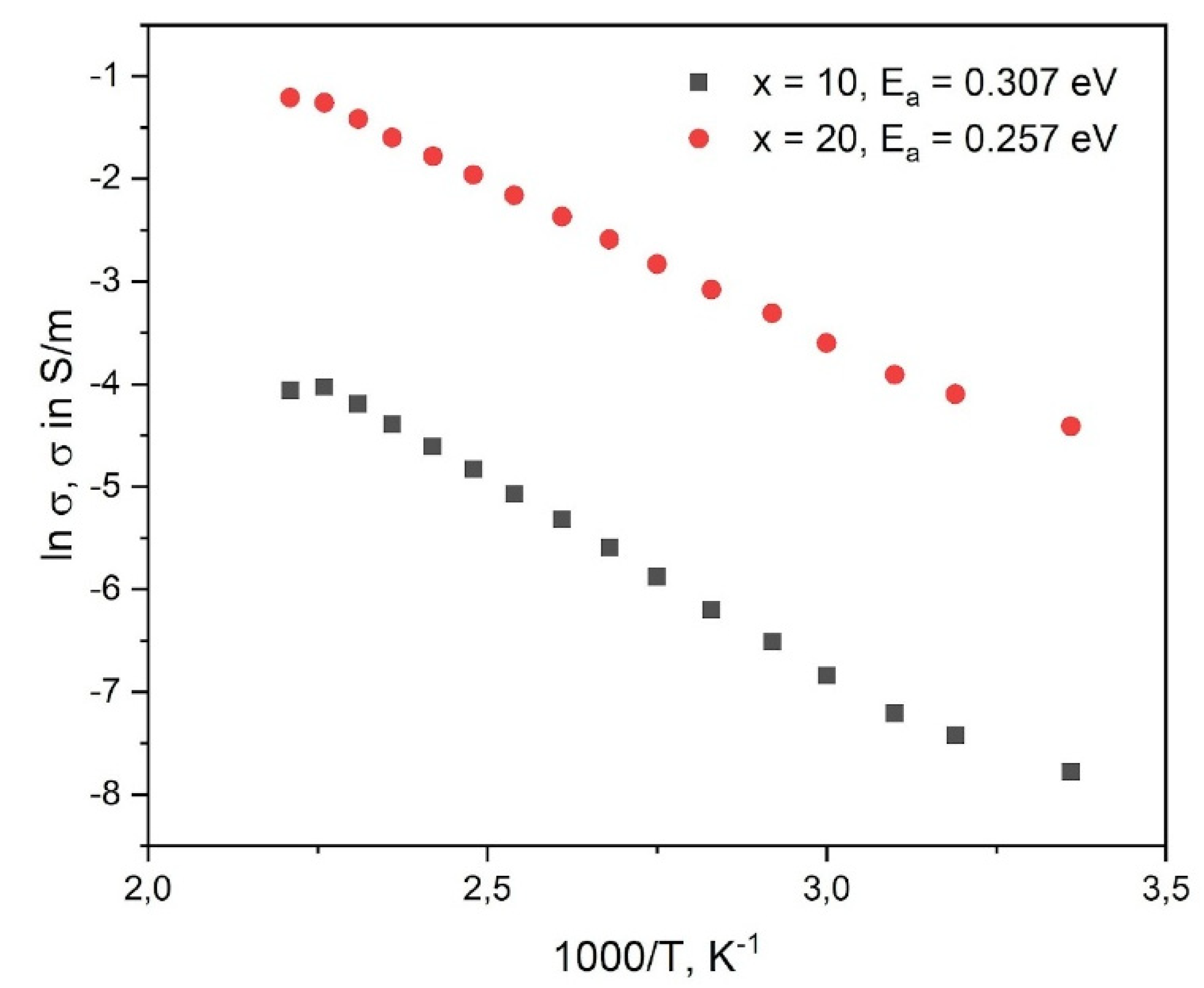
| x (mol%) | t (°C) | |Z| (Ω) | φ (°) | σ (S/m) | Ea (eV) |
|
20 |
RT | 1.0699e+03 | - 0.03 | 0,0121 |
0.257±0.001 |
| 40 | 7.8543e+02 | - 0.03 | 0,0165 | ||
| 50 | 6.4880e+02 | - 0.03 | 0,02 | ||
| 60 | 4.7676e+02 | - 0.04 | 0,0273 | ||
| 70 | 3.5601e+02 | - 0.05 | 0,0365 | ||
| 80 | 2.8176e+02 | - 0.05 | 0,0461 | ||
| 90 | 2.1960e+02 | - 0.06 | 0,0592 | ||
| 100 | 1.7293e+02 | - 0.05 | 0,0751 | ||
| 110 | 1.3925e+02 | - 0.05 | 0,0933 | ||
| 120 | 1.1285e+02 | - 0.06 | 0,115 | ||
| 130 | 9.2586e+01 | - 0.06 | 0,14 | ||
| 140 | 7.7259e+01 | - 0.07 | 0,168 | ||
| 150 | 6.4038e+01 | - 0.07 | 0,203 | ||
| 160 | 5.3605e+01 | - 0.08 | 0,242 | ||
| 170 | 4.5850e+01 | - 0.10 | 0,283 | ||
| 180 | 4.3496e+01 | - 0.11 | 0,299 | ||
| 10 | RT | 3.0617e+04 | - 0.05 | 4,16E-4 | 0.307±0.002 |
| 40 | 2.1214e+04 | - 0.03 | 6E-4 | ||
| 50 | 1.7194e+04 | - 0.03 | 7,41E-4 | ||
| 60 | 1.1905e+04 | - 0.03 | 0,00107 | ||
| 70 | 8.5299e+03 | - 0.03 | 0,00149 | ||
| 80 | 6.2686e+03 | - 0.03 | 0,00203 | ||
| 90 | 4.5696e+03 | - 0/03 | 0,00279 | ||
| 100 | 3.4185e+03 | - 0.04 | 0,00373 | ||
| 110 | 2.6089e+03 | - 0.04 | 0,00488 | ||
| 120 | 2.0284e+03 | - 0.04 | 0,00628 | ||
| 130 | 1.5909e+03 | - 0.05 | 0,00801 | ||
| 140 | 1.2780e+03 | - 0.05 | 0,00997 | ||
| 150 | 1.0293e+03 | - 0.06 | 0,0124 | ||
| 160 | 8.4183e+02 | - 0.06 | 0,0151 | ||
| 170 | 7.1487e+02 | - 0.08 | 0,0178 | ||
| 180 | 7.3508e+02 | - 0.11 | 0,0173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





