Submitted:
21 July 2025
Posted:
22 July 2025
You are already at the latest version
Abstract
Keywords:
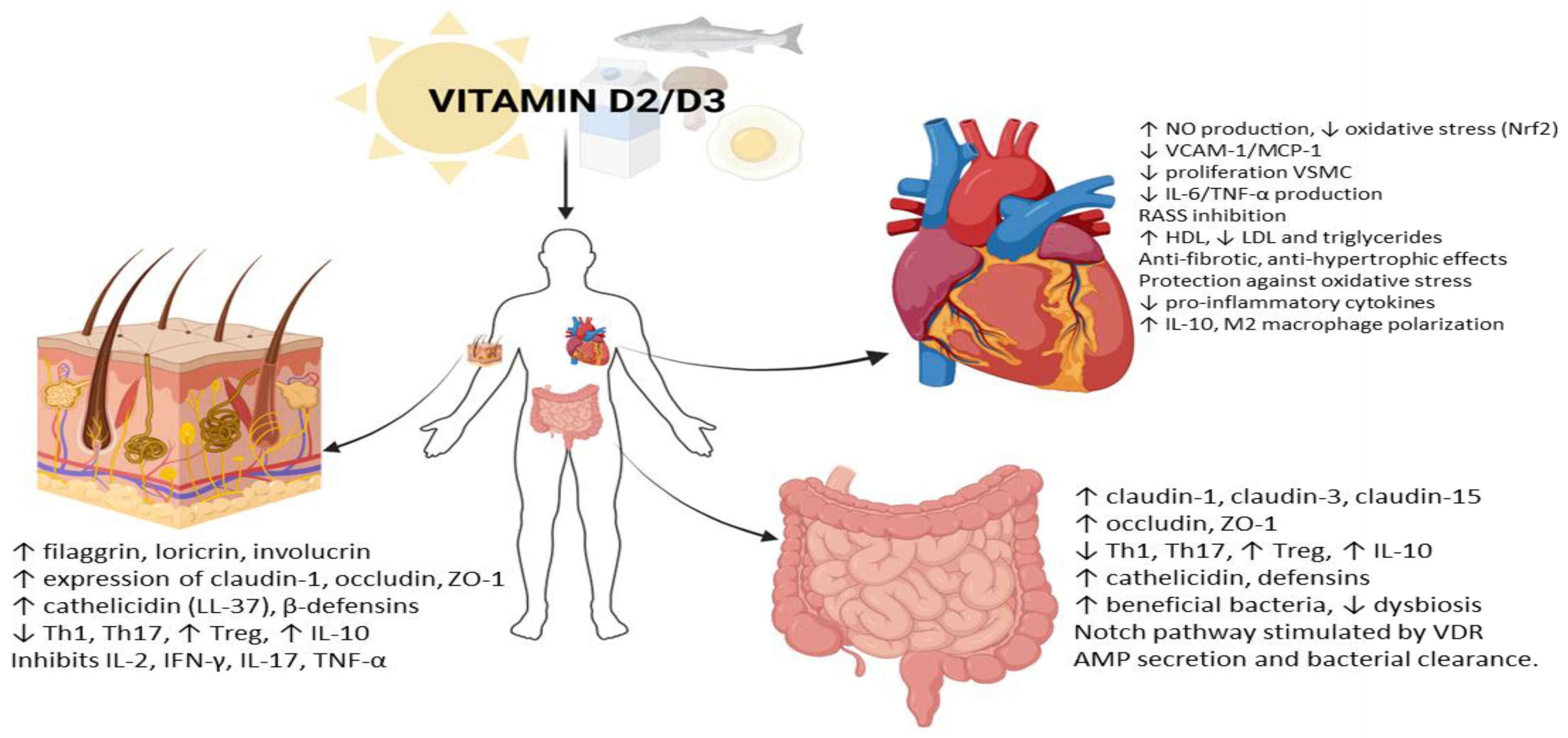
1. Introduction
2. Vitamin D and the Skin Barrier: Atopic Dermatitis and Psoriasis
2.1. The Regulatory Role of Vitamin D in Atopic Dermatitis
2.2. Clinical and Epidemiological Evidence Linking Vitamin D to AD
2.3. The Regulatory Role of Vitamin D in Psoriasis
2.4. Clinical and Epidemiological Evidence Linking Vitamin D to Psoriasis
3. Vitamin D and Cardiovascular Health
3.1. Pathogenesis of Cardiovascular Disease and Molecular Actions of Vitamin D
3.2. Clinical Implications of Vitamin D Deficiency in Cardiovascular Disease: Conditions and Evidence
4. Vitamin D and Intestinal Bowel Disease
4.1. Modulation of Gut Barrier Function by Vitamin D: Epithelial, Immune, and Microbial Interactions
4.2. Vitamin D and Inflammatory Bowel Disease and Celiac Disease: Evidence from Clinical Trials and Meta-analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R. Emerging Evidence of Thresholds for Beneficial Effects from Vitamin D Supplementation. Nutrients 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Grieco, T.; Paolino, G.; Moliterni, E.; Chello, C.; Sernicola, A.; Egan, C.G.; Morelli, M.; Nannipieri, F.; Battaglia, S.; Accoto, M.; et al. Differential Expression of Proteins Involved in Skin Barrier Maintenance and Vitamin D Metabolism in Atopic Dermatitis: A Cross-Sectional, Exploratory Study. Int. J. Mol. Sci. 2024, 26, 211. [Google Scholar] [CrossRef] [PubMed]
- Trasciatti, S.; Piras, F.; Bonaretti, S.; Marini, S.; Nencioni, S.; Biasci, E.; Egan, C.G.; Nannipieri, F. Effect of Oral Cholecalciferol in a Murine Model of Celiac Disease: A Dose Ranging Study. J. Steroid Biochem. Mol. Biol. 2022, 220, 106083. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Giusti, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; Sinigaglia, L. The Immunologic Profile of Vitamin D and Its Role in Different Immune-Mediated Diseases: An Expert Opinion. Nutrients 2022, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Vergatti, A.; Abate, V.; Iannuzzo, G.; Barbato, A.; De Filippo, G.; Rendina, D. The Bone-Heart Axis in the Pathogenesis of Cardiovascular Diseases: A Narrative Review. Nutr. Metab. Cardiovasc. Dis. NMCD 2025, 35, 103872. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Marini, F.; Parri, S.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; et al. Association of Vitamin D and Bisphenol A Levels with Cardiovascular Risk in an Elderly Italian Population: Results from the InCHIANTI Study. GeroScience 2024, 46, 6141–6156. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Fusaro, M.; Arcidiacono, G.; Sella, S.; Giannini, S. Evaluating Benefit from Vitamin D Supplementation: Defining the Area for Treatment. Osteoporos. Int. 2023, 34, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Moliterni, E.; Didona, D.; Garelli, V.; Corsetti, P.; Lopez, T.; Richetta, A.G.; Cantisani, C.; Bottoni, U.; Calvieri, S. Clinicopathological Features, Vitamin D Serological Levels and Prognosis in Cutaneous Melanoma of Shield-Sites: An Update. Med. Oncol. Northwood Lond. Engl. 2015, 32, 451. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Panetta, C.; Cota, C.; Didona, D.; Moliterni, E.; Di Mattia, C.; De Vita, G.; Bottoni, U.; Donati, P.; Calvieri, S. Vitamin D Receptor Immunohistochemistry Variability in Sun-Exposed and Non-Sun-Exposed Melanomas. Melanoma Res. 2017, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Moliterni, E.; Corsetti, P.; Didona, D.; Bottoni, U.; Calvieri, S.; Mattozzi, C. Vitamin D and Melanoma: State of the Art and Possible Therapeutic Uses. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2019, 154, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the Skin: Physiology and Pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Houdek, P.; Fromm, M.; Moll, I.; Brandner, J.M. Tight Junctions Form a Barrier in Human Epidermis. Eur. J. Cell Biol. 2010, 89, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Paez, J.V.; Peng, G.; Le Thanh Nguyen, H.; Nakamura, M.; Umehara, Y.; Yue, H.; Ikutama, R.; Takahashi, M.; Ikeda, S.; Ogawa, H.; et al. Calcitriol Modulates Epidermal Tight Junction Barrier Function in Human Keratinocytes. J. Dermatol. Sci. 2024, 114, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S. Vitamin D Regulation of Cathelicidin in the Skin: Toward a Renaissance of Vitamin D in Dermatology? J. Invest. Dermatol. 2008, 128, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Médica Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 1: The Role of Filaggrin in the Stratum Corneum Barrier and Atopic Skin. J. Clin. Aesthetic Dermatol. 2013, 6, 16–22. [Google Scholar]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Le Lamer, M.; Pellerin, L.; Reynier, M.; Cau, L.; Pendaries, V.; Leprince, C.; Méchin, M.-C.; Serre, G.; Paul, C.; Simon, M. Defects of Corneocyte Structural Proteins and Epidermal Barrier in Atopic Dermatitis. Biol. Chem. 2015, 396, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed]
- Borzutzky, A.; and Camargo Jr, C.A. Role of Vitamin D in the Pathogenesis and Treatment of Atopic Dermatitis. Expert Rev. Clin. Immunol. 2013, 9, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Mihály, J.; Gericke, J.; Törőcsik, D.; Rühl, R. Vitamin D Signaling in a Mouse Allergic Sensitization Model. Int. J. Vitam. Nutr. Res. Int. Z. Vitam.- Ernahrungsforschung J. Int. Vitaminol. Nutr. 2020, 90, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.; Geisler, C.; von Essen, M. The Vitamin D Receptor and T Cell Function. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D Signaling, Infectious Diseases, and Regulation of Innate Immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the Immune System: New Perspectives on an Old Theme. Endocrinol. Metab. Clin. North Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the Innate Epithelial Antimicrobial Response Is Cell-Type Specific and Dependent on Relevant Microenvironmental Stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Dimitroff, C.J.; Fuhlbrigge, R.C.; Kakeda, M.; Kurokawa, I.; Mizutani, H.; Kupper, T.S. Vitamins A and D Are Potent Inhibitors of Cutaneous Lymphocyte-Associated Antigen Expression. J. Allergy Clin. Immunol. 2008, 121, 148–157.e3. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.L.; Tawfik, S.S.; Theocharopoulos, I.; Atkar, R.; McDonald, B.; Dhoat, S.; Hughes, A.; Thomas, B.R.; O’Toole, E.A. Vitamin D Deficiency and Atopic Dermatitis Severity in a Bangladeshi Population Living in East London: A Cross-Sectional Study. Skin Health Dis. 2024, 4, e358. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of Serum 25-Hydroxyvitamin d Levels in Children with Atopic Dermatitis: Correlation with SCORAD Index. Dermat. Contact Atopic Occup. Drug 2013, 24, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Bae, J.-H. Vitamin D and Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutr. Burbank Los Angel. Cty. Calif 2016, 32, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.Y.; Høj, S.; Thomsen, S.F.; Meteran, H. Vitamin D Supplementation for Treating Atopic Dermatitis in Children and Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4128. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.R.; Shin, J.E.; Kim, Y.J.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Relationship between Serum 25-Hydroxyvitamin D and Interleukin-31 Levels, and the Severity of Atopic Dermatitis in Children. Korean J. Pediatr. 2015, 58, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Munawwarah, L.; Evalina, R.; Sofyani, S. Serum 25-Hydroxyvitamin-D Level and Atopic Dermatitis Severity in Children. Paediatr. Indones. 2017, 57, 234–238. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Dairy Food, Calcium and Vitamin D Intake in Pregnancy, and Wheeze and Eczema in Infants. Eur. Respir. J. 2010, 35, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord Blood 25-Hydroxyvitamin D3 and Allergic Disease during Infancy. Pediatrics 2012, 130, e1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Bäck, O.; Blomquist, H.K.S.; Hernell, O.; Stenberg, B. Does Vitamin D Intake during Infancy Promote the Development of Atopic Allergy? Acta Derm. Venereol. 2009, 89, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Heede, N.G.; Tang, L.; Skaaby, T.; Thyssen, J.P.; Friedrich, N.; Linneberg, A. No Association between Vitamin D and Atopy, Asthma, Lung Function or Atopic Dermatitis: A Prospective Study in Adults. Allergy 2015, 70, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Hoefer, N.; Franke, A.; Nöthling, U.; Schumann, R.R.; Hamann, L.; Worm, M. Association of Vitamin D Receptor Gene Polymorphisms with Severe Atopic Dermatitis in Adults. Br. J. Dermatol. 2013, 168, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Ecin, S.; Taskin, E.; Sen, A.; Kara, M. The Vitamin D Receptor Gene Polymorphisms in Asthmatic Children: A Case-Control Study. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Grieco, T.; Moliterni, E.; Paolino, G.; Chello, C.; Sernicola, A.; Egan, C.G.; Nannipieri, F.; Battaglia, S.; Accoto, M.; Tirotta, E.; et al. Association between Vitamin D Receptor Polymorphisms, Tight Junction Proteins and Clinical Features of Adult Patients with Atopic Dermatitis. Dermatol. Pract. Concept. 2024, 14, e2024214. [Google Scholar] [CrossRef] [PubMed]
- Hallau, J.; Hamann, L.; Schumann, R.R.; Worm, M.; Heine, G. A Promoter Polymorphism of the Vitamin D Metabolism Gene Cyp24a1 Is Associated with Severe Atopic Dermatitis in Adults. Acta Derm. Venereol. 2016, 96, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef] [PubMed]
- Benhadou, F.; Mintoff, D.; del Marmol, V. Psoriasis: Keratinocytes or Immune Cells – Which Is the Trigger? Dermatology 2018, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Priyadarssini, M.; Divya Priya,D. ; Indhumathi,S.; Rajappa,Medha; Chandrashekar,Laxmisha; and Thappa, D.M. Immunophenotyping of T Cells in the Peripheral Circulation in Psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Jørgensen, T.N. Immunological Effects of Vitamin D and Their Relations to Autoimmunity. J. Autoimmun. 2019, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, N.; van Spriel, A.B.; Looman, M.W.G.; Chen, S.; Spilgies, L.M.; Lieben, L.; Carmeliet, G.; Ansems, M.; Adema, G.J. Vitamin D Controls Murine and Human Plasmacytoid Dendritic Cell Function. J. Invest. Dermatol. 2014, 134, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Dyring-Andersen, B.; Bonefeld, C.M.; Bzorek, M.; Løvendorf, M.B.; Lauritsen, J.P.H.; Skov, L.; Geisler, C. The Vitamin D Analogue Calcipotriol Reduces the Frequency of CD8+ IL-17+ T Cells in Psoriasis Lesions. Scand. J. Immunol. 2015, 82, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Ito, T.; Umayahara, T.; Ikeya, S.; Tatsuno, K.; Funakoshi, A.; Hashizume, H.; Tokura, Y. Topical Application of a Vitamin D3 Analogue and Corticosteroid to Psoriasis Plaques Decreases Skin Infiltration of TH17 Cells and Their Ex Vivo Expansion. J. Allergy Clin. Immunol. 2016, 138, 517–528.e5. [Google Scholar] [CrossRef] [PubMed]
- Sato-Deguchi, E.; Imafuku, S.; Chou, B.; Ishii, K.; Hiromatsu, K.; Nakayama, J. Topical Vitamin D₃ Analogues Induce Thymic Stromal Lymphopoietin and Cathelicidin in Psoriatic Skin Lesions. Br. J. Dermatol. 2012, 167, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Schiattarella, M.; Lembo, S.; Mattii, M.; Prevete, N.; Balato, N.; Ayala, F. Interleukin-1 Family Members Are Enhanced in Psoriasis and Suppressed by Vitamin D and Retinoic Acid. Arch. Dermatol. Res. 2013, 305, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D Analog Calcipotriol Suppresses the Th17 Cytokine-Induced Proinflammatory S100 “Alarmins” Psoriasin (S100A7) and Koebnerisin (S100A15) in Psoriasis. J. Invest. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Datta Mitra, A.; Raychaudhuri, S.P.; Abria, C.J.; Mitra, A.; Wright, R.; Ray, R.; Kundu-Raychaudhuri, S. 1α,25-Dihydroxyvitamin-D3-3-Bromoacetate Regulates AKT/mTOR Signaling Cascades: A Therapeutic Agent for Psoriasis. J. Invest. Dermatol. 2013, 133, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Komuves, L.; Yu, Q.-C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Yoshizawa, T.; Kato, S.; et al. Lack of the Vitamin D Receptor Is Associated with Reduced Epidermal Differentiation and Hair Follicle Growth. J. Invest. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Terminal Differentiation of Cultured Mouse Epidermal Cells by 1 Alpha,25-Dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Visconti, B.; Paolino, G.; Carotti, S.; Pendolino, A.L.; Morini, S.; Richetta, A.G.; Calvieri, S. Immunohistochemical Expression of VDR Is Associated with Reduced Integrity of Tight Junction Complex in Psoriatic Skin. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Novelli, M.; De Matteis, A.; Verrone, A.; Bernengo, M.G. Effects of Topical Calcipotriol on the Expression of Adhesion Molecules in Psoriasis. J. Cutan. Pathol. 1998, 25, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Richetta, A.; Silvestri, V.; Giancristoforo, S.; Rizzolo, P.; D’Epiro, S.; Graziano, V.; Mattozzi, C.; Navazio, A.; Campoli, M.; D’Amico, C.; et al. A-1012G Promoter Polymorphism of Vitamin D Receptor Gene Is Associated with Psoriasis Risk and Lower Allele-Specific Expression. DNA Cell Biol. 2014, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L.; Li, Y. The Association of Polymorphisms of the Vitamin D Receptor Gene with Psoriasis in the Han Population of Northeastern China. J. Dermatol. Sci. 2014, 73, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Polić, M.V.; Rucević, I.; Barisić-Drusko, V.; Miskulin, M.; Glavas-Obrovac, L.; Stefanić, M.; Karner, I.; Lipozencić, J.; Bacun, T.; Mihaljević, I. Polymorphisms of Vitamin D Receptor Gene in the Population of Eastern Croatia with Psoriasis Vulgaris and Diabetes Mellitus. Coll. Antropol. 2012, 36, 451–457. [Google Scholar] [PubMed]
- Rucevic, I.; Stefanic, M.; Tokic, S.; Vuksic, M.; Glavas-Obrovac, L.; Barisic-Drusko, V. Lack of Association of Vitamin D Receptor Gene 3’-Haplotypes with Psoriasis in Croatian Patients. J. Dermatol. 2012, 39, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zuel-Fakkar, N.M.; Kamel, M.M.; Asaad, M.K.; Mahran, M.Z.; Shehab, A.A. A Study of ApaI and TaqI Genotypes of the Vitamin D Receptor in Egyptian Patients with Psoriasis. Clin. Exp. Dermatol. 2011, 36, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Renfro, L.; Collins, P.; Kirby, B.; Rogers, S. Clinical and Genetic Predictors of Response to Narrowband Ultraviolet B for the Treatment of Chronic Plaque Psoriasis. Br. J. Dermatol. 2010, 163, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Halsall, J.A.; Osborne, J.E.; Pringle, J.H.; Hutchinson, P.E. Vitamin D Receptor Gene Polymorphisms, Particularly the Novel A-1012G Promoter Polymorphism, Are Associated with Vitamin D3 Responsiveness and Non-Familial Susceptibility in Psoriasis. Pharmacogenet. Genomics 2005, 15, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Asano, N.; Tsunemi, Y.; Takekoshi, T.; Kishimoto, M.; Mitsui, H.; Tada, Y.; Torii, H.; Komine, M.; Asahina, A.; et al. Polymorphisms of Vitamin D Receptor Gene in Japanese Patients with Psoriasis Vulgaris. J. Dermatol. Sci. 2002, 30, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Nahidi, Y.; Azizahari, S.; Meibodi, N.T.; Hadianfar, A. Serum 25-OH Vitamin D Level in Psoriatic Patients and Comparison With Control Subjects. J. Cutan. Med. Surg. 2016, 20, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Bergler-Czop, B.; Brzezińska-Wcisło, L. Serum Vitamin D Level - the Effect on the Clinical Course of Psoriasis. Postepy Dermatol. Alergol. 2016, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Association between Circulating 25-Hydroxyvitamin D Levels and Psoriasis, and Correlation with Disease Severity: A Meta-Analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral Vitamin D3 Supplementation for Chronic Plaque Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Dermatol. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, L.; Kumarit, G.R.K.; Rajappa, M.; Revathy, G.; Munisamy, M.; Thappa, D.M. 25-Hydroxy Vitamin D and Ischaemia-Modified Albumin Levels in Psoriasis and Their Association with Disease Severity. Br. J. Biomed. Sci. 2015, 72, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, R.; Agrawal, S.; Pandey, P.; Lamsal, M. Assessment of Vitamin D Level in Patients with Psoriasis and Its Correlation with Disease Severity: A Case–Control Study. Psoriasis Targets Ther. 2022, 12, 251–258. [Google Scholar] [CrossRef]
- Wilson, P.B. Serum 25-Hydroxyvitamin D Status in Individuals with Psoriasis in the General Population. Endocrine 2013, 44, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A Pilot Study Assessing the Effect of Prolonged Administration of High Daily Doses of Vitamin D on the Clinical Course of Vitiligo and Psoriasis. Dermatoendocrinol. 2013, 5, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Camargo Jr,Carlos Arturo; Coomarasamy,Christin; and Scragg, R. A Randomized, Double-Blind, Placebo-Controlled Trial of the Effect of Monthly Vitamin D Supplementation in Mild Psoriasis*. J. Dermatol. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB Phototherapy and Oral Calcitriol (1,25-Dihydroxyvitamin D3) on Vitamin D Photosynthesis in Patients with Psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Gumowski-Sunek, D.; Rizzoli, R.; Saurat, J.H. Effects of Topical Calcipotriol on Calcium Metabolism in Psoriatic Patients: Comparison with Oral Calcitriol. Dermatologica 1991, 183, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N. Diagnosis and Treatment of Pediatric Psoriasis: Current and Future. Am. J. Clin. Dermatol. 2013, 14, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Kokelj, F.; Lavaroni, G.; Guadagnini, A. UVB versus UVB plus Calcipotriol (MC 903) Therapy for Psoriasis Vulgaris. Acta Derm. Venereol. 1995, 75, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Altmeyer, P.; Kaufmann, R.; Ring, J.; Christophers, E.; Pavel, S.; Ziegler, J. Topical Calcipotriol plus Oral Fumaric Acid Is More Effective and Faster Acting than Oral Fumaric Acid Monotherapy in the Treatment of Severe Chronic Plaque Psoriasis Vulgaris. Dermatology 2002, 205, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kokelj, F.; Torsello, P.; Plozzer, C. Calcipotriol Improves the Efficacy of Cyclosporine in the Treatment of Psoriasis Vulgaris. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Green, L. j.; Selmer, J.; Praestegaard, M.; Gold, L. s.; Augustin, M.; Group, T.T.I. A Pooled Analysis of Randomized, Controlled, Phase 3 Trials Investigating the Efficacy and Safety of a Novel, Fixed Dose Calcipotriene and Betamethasone Dipropionate Cream for the Topical Treatment of Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Aggarwal,Kamal; and Jain, V. K. Tacalcitol: A Useful Adjunct to Narrow Band Ultraviolet B Phototherapy in Psoriasis. J. Dermatol. Treat. 2016, 27, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Ashton, R.E.; Marks, R.; Harris, R.I.; Berth-Jones, J. Topical Maxacalcitol for the Treatment of Psoriasis Vulgaris: A Placebo-Controlled, Double-Blind, Dose-Finding Study with Active Comparator. Br. J. Dermatol. 1999, 141, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Furuhashi, T.; Matsumoto, K.; Morita, A. Safety Profiles of Topical Vitamin D3 in Psoriasis Patients: A Retrospective Large-Scale Study. Psoriasis Targets Ther. 2012, 2, 81–88. [Google Scholar] [CrossRef]
- Segaert, S.; Ropke, M. The Biological Rationale for Use of Vitamin d Analogs in Combination with Corticosteroids for the Topical Treatment of Plaque Psoriasis. J. Drugs Dermatol. JDD 2013, 12, e129–137. [Google Scholar] [PubMed]
- Bagel, J.; Levi, E.; Tyring, S.; Knuckles, M.L.F. Real-Life Treatment Profile of Calcipotriene and Betamethasone Dipropionate Topical Suspension in Patients with Psoriasis Vulgaris. J. Drugs Dermatol. JDD 2014, 13, 1374–1379. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Ganslandt, C.; Kurvits, M.; Schlessinger, J. Safety and Efficacy of Calcipotriene plus Betamethasone Dipropionate Topical Suspension in the Treatment of Extensive Scalp Psoriasis in Adolescents Ages 12 to 17 Years. Pediatr. Dermatol. 2015, 32, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Franken, S.M.; Witte, B.; Pavel, S.; Rustemeyer, T. Psoriasis and Daily Low-Emission Phototherapy: Effects on Disease and Vitamin D Level. Photodermatol. Photoimmunol. Photomed. 2015, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases: Implications for Management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Coppi, F.; Severino, P.; Penna, C.; Pagliaro, P.; Dei Cas, A.; Bucciarelli, V.; Madonna, R.; Tarperi, C.; Schena, F.; et al. A Personalized Approach to Vitamin D Supplementation in Cardiovascular Health Beyond the Bone: An Expert Consensus by the Italian National Institute for Cardiovascular Research. Nutrients 2025, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Richetta, A.G.; Grassi, S.; Moliterni, E.; Chello, C.; Calvieri, C.; Carnevale, R.; Peruzzi, M.; Violi, F.; Calvieri, S. Increased Intestinal Barrier Permeability in Patients with Moderate to Severe Plaque-Type Psoriasis. J. Dermatol. 2020, 47, e366–e368. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-Derived Low-Grade Endotoxaemia, Atherothrombosis and Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Janubová, M.; Žitňanová, I. The Effects of Vitamin D on Different Types of Cells. Steroids 2024, 202, 109350. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D Protects Human Endothelial Cells from Oxidative Stress through the Autophagic and Survival Pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Rizzi, M.; Squarzanti, D.F.; Pittarella, P.; Vacca, G.; Renò, F. 1α,25-Dihydroxycholecalciferol (Vitamin D3) Induces NO-Dependent Endothelial Cell Proliferation and Migration in a Three-Dimensional Matrix. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 31, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Laera, N.; Malerba, P.; Vacanti, G.; Nardin, S.; Pagnesi, M.; Nardin, M. Impact of Immunity on Coronary Artery Disease: An Updated Pathogenic Interplay and Potential Therapeutic Strategies. Life 2023, 13, 2128. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, L.; Izadpanah, P.; Asadi, S. The Effect of Calcitriol and Cholecalciferol on Inflammatory Markers in Periprocedural Myocardial Injury: A Randomized Controlled Trial. Medicine (Baltimore) 2025, 104, e42103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lyons, C.J.; Ayu, C.; O’Brien, T. Recent Advances in Endothelial Colony-Forming Cells: From the Transcriptomic Perspective. J. Transl. Med. 2024, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D Cell Signalling in Health and Disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Zhou, T.B.T.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho Is Mediated via Regulation of Members of the Redox System *. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho, Phosphate and FGF-23 in Ageing and Disturbed Mineral Metabolism. Nat. Rev. Nephrol. 2013, 9, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Al-Oanzi, Z.H.; Alenazy, F.O.; Alhassan, H.H.; Alruwaili, Y.; Alessa, A.I.; Alfarm, N.B.; Alanazi, M.O.; Alghofaili, S.I. The Role of Vitamin D in Reducing the Risk of Metabolic Disturbances That Cause Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Izzo, M.; Carrizzo, A.; Izzo, C.; Cappello, E.; Cecere, D.; Ciccarelli, M.; Iannece, P.; Damato, A.; Vecchione, C.; Pompeo, F. Vitamin D: Not Just Bone Metabolism but a Key Player in Cardiovascular Diseases. Life 2021, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and Cardiovascular Health. Clin. Nutr. Edinb. Scotl. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D Status and Arterial Hypertension: A Systematic Review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A Negative Endocrine Regulator of the Renin-Angiotensin System and Blood Pressure. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ajabshir, S.; Asif, A.; Nayer, A. The Effects of Vitamin D on the Renin-Angiotensin System. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, X.-J.; Zhang, J.-S.; Liu, H.-M. Association between Vitamin D Deficiency and Levels of Renin and Angiotensin in Essential Hypertension. Int. J. Clin. Pract. 2022, 2022, 8975396. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Price, R.J.G.; Struthers, A.D.; Donnan, P.T.; Messow, C.-M.; Ford, I.; McMurdo, M.E.T. Cholecalciferol Treatment to Reduce Blood Pressure in Older Patients with Isolated Systolic Hypertension: The VitDISH Randomized Controlled Trial. JAMA Intern. Med. 2013, 173, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Theiler-Schwetz, V.; Trummer, C.; Grübler, M.R.; Keppel, M.H.; Zittermann, A.; Tomaschitz, A.; Karras, S.N.; März, W.; Pilz, S.; Gängler, S. Effects of Vitamin D Supplementation on 24-Hour Blood Pressure in Patients with Low 25-Hydroxyvitamin D Levels: A Randomized Controlled Trial. Nutrients 2022, 14, 1360. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Namazi, S.; Rostami-Yalmeh, J.; Sahebi, E.; Khalili, N.; Jamialahmadi, T.; Sahebkar, A. Effect of Vitamin D Supplementation on the Regulation of Blood Pressure in Iranian Patients with Essential Hypertension: A Clinical Trial. Adv. Exp. Med. Biol. 2021, 1328, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Nardin, S.; Cao, D.; Chiarito, M.; Kedhi, E.; Galasso, G.; Condorelli, G.; De Luca, G. Vitamin D and Cardiovascular Diseases: From Physiology to Pathophysiology and Outcomes. Biomedicines 2024, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, S.; Hirani, S.; Mishra, A.; Vardhan, S.; Hirani, S.; Prasad, R.; Wanjari, M. Exploring the Role of Vitamin D in Atherosclerosis and Its Impact on Cardiovascular Events: A Comprehensive Review. Cureus 2023, 15, e42470. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, M.; Ning, H.; A, L.; Li, Y.; Sun, C. Lipoprotein Lipase Links Vitamin D, Insulin Resistance, and Type 2 Diabetes: A Cross-Sectional Epidemiological Study. Cardiovasc. Diabetol. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Salekzamani, S.; Bavil, A.S.; Mehralizadeh, H.; Jafarabadi, M.A.; Ghezel, A.; Gargari, B.P. The Effects of Vitamin D Supplementation on Proatherogenic Inflammatory Markers and Carotid Intima Media Thickness in Subjects with Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Endocrine 2017, 57, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Säidifard, N.; Tangestani, H.; Djafarian, K.; Shab-Bidar, S. Serum Vitamin D Level and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Control Trials. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2020, 52, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin Resistance: The Link Between Obesity and Cardiovascular Disease. Med. Clin. North Am. 2011, 95, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 Alpha (OH)-Vitamin D3 on Insulin Secretion in Diabetes Mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar] [PubMed]
- Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired Insulin Secretory Capacity in Mice Lacking a Functional Vitamin D Receptor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Wolden-Kirk, H.; Overbergh, L.; Gysemans, C.; Brusgaard, K.; Naamane, N.; Van Lommel, L.; Schuit, F.; Eizirik, D.L.; Christesen, H.; Mathieu, C. Unraveling the Effects of 1,25OH2D3 on Global Gene Expression in Pancreatic Islets. J. Steroid Biochem. Mol. Biol. 2013, 136, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Dávila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D Response Element in the Human Insulin Receptor Gene Promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Rhoten, W.B. 1,25-Dihydroxyvitamin D3 Evokes Oscillations of Intracellular Calcium in a Pancreatic Beta-Cell Line. Endocrinology 1995, 136, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and Pancreas: The Role of Sunshine Vitamin in the Pathogenesis of Diabetes Mellitus and Pancreatic Cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-Analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. Vitamin D Supplementation, Glycemic Control, and Insulin Resistance in Prediabetics: A Meta-Analysis. J. Endocr. Soc. 2018, 2, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Morucci, G.; Branca, J.J.V.; Aterini, S.; Amato, M.; Gulisano, M.; Ruggiero, M. Effects of Vitamin D3 and Paricalcitol on Immature Cardiomyocytes: A Novel Role for Vitamin D Analogs in the Prevention of Cardiovascular Diseases. Nutrients 2013, 5, 2076–2092. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, E.; Musazadeh, V.; Kavyani, Z.; Naghsh, N.; Shoura, S.M.S.; Dehghan, P. Efficacy of Vitamin D Supplementation as an Adjunct Therapy for Improving Inflammatory and Oxidative Stress Biomarkers: An Umbrella Meta-Analysis. Pharmacol. Res. 2022, 186, 106484. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Appelbaum, E.; Pritchett, Y.; Chang, Y.; Wenger, J.; Tamez, H.; Bhan, I.; Agarwal, R.; Zoccali, C.; Wanner, C.; et al. Vitamin D Therapy and Cardiac Structure and Function in Patients with Chronic Kidney Disease: The PRIMO Randomized Controlled Trial. JAMA 2012, 307, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Gnudi, L.; Fountoulakis, N.; Panagiotou, A.; Corcillo, A.; Maltese, G.; Rife, M.F.; Ntalas, I.; Franks, R.; Chiribiri, A.; Ayis, S.; et al. Effect of Active Vitamin-D on Left Ventricular Mass Index: Results of a Randomized Controlled Trial in Type 2 Diabetes and Chronic Kidney Disease. Am. Heart J. 2023, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Arabi, A. Association between Vitamin D and Cardiovascular Health: Myth or Fact? A Narrative Review of the Evidence. Womens Health 2023, 19, 17455057231158222. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, G.E.; Schnell, D.M.; Thomas, D.T.; Bollinger, L.M. Calcitriol Concomitantly Enhances Insulin Sensitivity and Alters Myocellular Lipid Partitioning in High Fat-Treated Skeletal Muscle Cells. J. Physiol. Biochem. 2017, 73, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Krul-Poel, Y.H.M.; Ter Wee, M.M.; Lips, P.; Simsek, S. MANAGEMENT OF ENDOCRINE DISEASE: The Effect of Vitamin D Supplementation on Glycaemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2017, 176, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of Cardiovascular Risk Factors and the Serum Levels of 25-Hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Sun, X.; Wang, L.; Wang, A. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Meta-Analysis of Interventional Studies. Medicine (Baltimore) 2019, 98, e14970. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes After 5 Years: Results from a National, Population-Based Prospective Study (the Australian Diabetes, Obesity and Lifestyle Study). Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, S.; St Peter, J.V.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 Supplementation Improves Insulin Sensitivity in Subjects with Impaired Fasting Glucose. Transl. Res. J. Lab. Clin. Med. 2011, 158, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Fallah, A.A.; Barani, A. Effects of Vitamin D on Serum Lipid Profile in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. Edinb. Scotl. 2016, 35, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ghayour-Mobarhan, M.; Ferns, G.; Taghizadeh, M.; Badehnoosh, B.; Mirzaei, H.; Asemi, Z. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 25 201–210. [CrossRef] [PubMed]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The Impacts of Vitamin D Supplementation in Adults with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Sukik, A.; Alaayesh, H.; Rasoulinejad, H.; Shraim, M. Serum Vitamin D Concentrations Are Inversely Related to Prevalence of Metabolic Syndrome in Qatari Women. BioFactors Oxf. Engl. 2020, 46, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, C.; Chen, X.; Wan, H.; Chen, Y.; Chen, C.; Han, B.; Lu, Y. Vitamin D, Prediabetes and Type 2 Diabetes: Bidirectional Mendelian Randomization Analysis. Eur. J. Nutr. 2020, 59, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Sharp, S.J.; Day, F.R.; Imamura, F.; Gundersen, T.E.; Lotta, L.A.; Sluijs, I.; Stewart, I.D.; et al. The Association between Circulating 25-Hydroxyvitamin D Metabolites and Type 2 Diabetes in European Populations: A Meta-Analysis and Mendelian Randomisation Analysis. PLOS Med. 2020, 17, e1003394. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 13 3–10. [CrossRef] [PubMed]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D Levels and Microvascular Complications in Type 2 Diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537. [Google Scholar] [CrossRef] [PubMed]
- Shehab, D.; Al-Jarallah, K.; Mojiminiyi, O.A.; Al Mohamedy, H.; Abdella, N.A. Does Vitamin D Deficiency Play a Role in Peripheral Neuropathy in Type 2 Diabetes? Diabet. Med. 2012, 29, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Assy, M.H.; Draz, N.A.; Fathy, S.E.; Hamed, M.G. Impact of Vitamin D Level in Diabetic People with Peripheral Neuropathy. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 117. [Google Scholar] [CrossRef]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Levels of Vitamin D and Cardiometabolic Disorders: Systematic Review and Meta-Analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J. Serum Vitamin D Status and Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis. Nutr. Res. Pract. 2021, 15, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Park, C.H. Low Serum Vitamin D Level Is Associated with High Risk of Metabolic Syndrome in Post-Menopausal Women. J. Endocrinol. Invest. 2013, 36, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent Association between 1,25-Dihydroxyvitamin D, 25-Hydroxyvitamin D and the Renin-Angiotensin System: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Williams, J.S.; Fisher, N.D.L. Plasma 25-Hydroxyvitamin D and Regulation of the Renin-Angiotensin System in Humans. Hypertens. Dallas Tex 1979 2010, 55, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Beierwaltes, W.H. The Role of Calcium in the Regulation of Renin Secretion. Am. J. Physiol. Renal Physiol. 2010, 298, F1–F11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-D.; Jia, J.-J.; Dong, P.-S.; Zhao, D.; Yang, X.-M.; Li, D.-L.; Zhang, H.-F. Effect of Vitamin D on Ventricular Remodelling in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, M.P.; Nemerovski, C.W.; Ellingrod, V.L.; Cowger, J.A.; Dyke, D.B.; Koelling, T.M.; Wu, A.H.; Aaronson, K.D.; Simpson, R.U.; Bleske, B.E. Vitamin D Receptor Genetics on Extracellular Matrix Biomarkers and Hemodynamics in Systolic Heart Failure. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The Peculiar Role of Vitamin D in the Pathophysiology of Cardiovascular and Neurodegenerative Diseases. Life Sci. 2022, 289, 120193. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Brown, J.M.; Williams, J.S. The Renin–Angiotensin–Aldosterone System and Calcium-Regulatory Hormones. J. Hum. Hypertens. 2015, 29, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Zhao, S.; Brock, G.; Kline, D.; Echouffo-Tcheugui, J.B.; Effoe, V.S.; Bertoni, A.G.; Michos, E.D.; de Boer, I.H.; Kestenbaum, B.; et al. Vitamin D, Parathyroid Hormone, Glucose Metabolism and Incident Diabetes in the Multiethnic Study of Atherosclerosis. BMJ Open Diabetes Res. Care 2022, 10, e002931. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Ostadmohammadi, V.; Doosti-Irani, A.; Ghayour-Mobarhan, M.; Ferns, G.; Akbari, H.; Ghaderi, A.; Talari, H.R.; Asemi, Z. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2018, 50, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Khosroshahi, M.Z.; Kashkooli, S.; Abbasnezhad, A. Effect of Calcium-Vitamin D Co-Supplementation on Insulin, Insulin Sensitivity, and Glycemia: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Horm. Metab. Res. 2019, 51, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; Giusti, R.; Egan, C.G.; et al. Association between Vitamin D and Bisphenol A Levels in an Elderly Italian Population: Results from the InCHIANTI Study. Endocr. Connect. 2022, 11, e210571. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Rozing, J.; Sapone, A.; Lammers, K.; Fasano, A. Tight Junctions, Intestinal Permeability, and Autoimmunity Celiac Disease and Type 1 Diabetes Paradigms. Ann. N. Y. Acad. Sci. 2009, 1165, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Atreya, R.; Neurath, M.F. A Spotlight on Intestinal Permeability and Inflammatory Bowel Diseases. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease—Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Brandt, A.; Koidl, L.; Bergheim, I. The Intestinal Barrier Dysfunction as Driving Factor of Inflammaging. Nutrients 2022, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Moens, E.; Veldhoen, M. Epithelial Barrier Biology: Good Fences Make Good Neighbours. Immunology 2012, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between Tight Junctions & Adherens Junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of Cytokine Modulation of Epithelial Tight Junction Barrier. Front. Biosci.-Landmark 2009, 14, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Trasciatti, S.; Grizzi, F. Vitamin D and Celiac Disease. Adv. Food Nutr. Res. 2024, 109, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Geng, C.; Song, S.; Yang, W.; Li, X.; Wang, C. Vitamin D/Vitamin D Receptor Protects Intestinal Barrier against Colitis by Positively Regulating Notch Pathway. Front. Pharmacol. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Singh, T.P.; Wei, X.; Yao, H.; Wang, H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin-Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Dig. Dis. Sci. 2018, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Munem, F.; Thianhlun, P.C.K.; Anderson, P.H.; Stringer, A.M. Vitamin D Is a Potential Treatment for the Management of Gastrointestinal Mucositis. Curr. Opin. Support. Palliat. Care 2023, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 Affects Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells1. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D Metabolism and Signaling in the Immune System. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef] [PubMed]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The Dynamic Shifts of IL-10-Producing Th17 and IL-17-Producing Treg in Health and Disease: A Crosstalk between Ancient “Yin-Yang” Theory and Modern Immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 Down-Regulates Intracellular Toll-like Receptor 9 Expression and Toll-like Receptor 9-Induced IL-6 Production in Human Monocytes. Rheumatology 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal Epithelial Vitamin D Receptor Deletion Leads to Defective Autophagy in Colitis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-Wide Association Analysis Identifies Variation in Vitamin D Receptor and Other Host Factors Influencing the Gut Microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, Y.; Xia, Y.; Zhang, J.; Kaser, A.; Blumberg, R.; Sun, J. Paneth Cell Alertness to Pathogens Maintained by Vitamin D Receptors. Gastroenterology 2021, 160, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate-Induced Colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Mehigan, G.A.; Villegas, F.; Mitsuhashi, S.; Longhi, M.S.; Malvar, G.; Csizmadia, E.; Robson, S.; Moss, A.C. Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells. Inflamm. Bowel Dis. 2020, 26, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.H.; Puchner, T.; Korkmaz, H.I.; Vos, M.; Soeters, M.R.; Vries, C.J.M. de Lithocholic Acid Controls Adaptive Immune Responses by Inhibition of Th1 Activation through the Vitamin D Receptor. PLOS ONE 2017, 12, e0176715. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, R.; Weinstein, T.; Pettei, M.J. Vitamin D in Pediatric Gastrointestinal Disease. Curr. Opin. Pediatr. 2017, 29, 122. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou,Weihua; He,Xinjue; Zhou,Xinxin; and Yu, C. Vitamin D Status and Vitamin D Receptor Genotypes in Celiac Disease: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Ciacci, C. The Value and Significance of 25(OH) and 1,25(OH) Vitamin D Serum Levels in Adult Coeliac Patients: A Review of the Literature. Dig. Liver Dis. 2018, 50, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Barera, G.; Maruca, K.; Sgaramella, P.; Di Stefano, M.; Mora, S. Short-Term, Low Dose Vitamin D Supplementation in Young Patients with Celiac Disease: A Pilot Study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 663. [Google Scholar] [CrossRef] [PubMed]
- Blazina, Š.; Bratanič, N.; Čampa, A.Š.; Blagus, R.; Orel, R. Bone Mineral Density and Importance of Strict Gluten-Free Diet in Children and Adolescents with Celiac Disease. Bone 2010, 47, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Hassanian, S.M.; Mottaghi-Moghaddam, A.; Ghazaghi, A.; Ghandehari, M.; Alizade-Noghani, M.; Khazaei, M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Vitamin D in Inflammatory Bowel Disease: From Biology to Clinical Implications. Complement. Ther. Med. 2019, 47, 102189. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; di Filippo, L.; Allora, A.; Bikle, D.D.; Cavestro, G.M.; Feldman, D.; Latella, G.; Minisola, S.; Napoli, N.; Trasciatti, S.; et al. Vitamin D and Malabsorptive Gastrointestinal Conditions: A Bidirectional Relationship? Rev. Endocr. Metab. Disord. 2023, 24, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeizadeh, S.-A.; Tafazoli, N.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. Vitamin D, the Gut Microbiome and Inflammatory Bowel Disease. J. Res. Med. Sci. 2018, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Ham, M.; Longhi, M.S.; Lahiff, C.; Cheifetz, A.; Robson, S.; Moss, A.C. Vitamin D Levels in Adults with Crohn’s Disease Are Responsive to Disease Activity and Treatment. Inflamm. Bowel Dis. 2014, 20, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Rigterink, T.; Appleton, L.; Day, A.S. Vitamin D Therapy in Children with Inflammatory Bowel Disease: A Systematic Review. World J. Clin. Pediatr. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The Efficacy of Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review with Meta-Analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.; Magistroni, M.; Cesaro, N.; Carlino, G.; Monaco, S.; Fabiani, S.; Vinci, A.; Vernia, F.; Viscido, A.; Latella, G. Effectiveness of Vitamin D Supplementation on Disease Course in Inflammatory Bowel Disease Patients: Systematic Review With Meta-Analysis. Inflamm. Bowel Dis. 2024, 30, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, T.; Wang, Y.; Liu, R.; Chang, M.; Wang, X. Effects of Oral Vitamin D Supplementation on Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Food Funct. 2021, 12, 7588–7606. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.W.; Collins, E.; Cao, B.; Carrellas, M.; Crowell, A.M.; Korzenik, J.R. Higher 25-Hydroxyvitamin D Levels Are Associated with Greater Odds of Remission with Anti-Tumour Necrosis Factor-α Medications among Patients with Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Potential Role of Vitamin D Supplementation as a Gut Microbiota Modifier in Healthy Individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limón,Patricia; Pilo,Jesús; Lisbona-Montañez,José Manuel; Tinahones,Francisco J. ; Moreno Indias,Isabel; and Macías-González, M. Linking Serum Vitamin D Levels with Gut Microbiota after 1-Year Lifestyle Intervention with Mediterranean Diet in Patients with Obesity and Metabolic Syndrome: A Nested Cross-Sectional and Prospective Study. Gut Microbes 2023, 15, 2249150. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
| Organ/System | Effects of Vitamin D | Key Markers/Proteins | Main Clinical/Epidemiological Evidence |
| Skin (AD, Psoriasis) |
Strengthens epidermal barrier via TJs; promotes keratinocyte differentiation; boosts antimicrobial peptides; modulates immune response | Claudin-1, Occludin, ZO-1, Filaggrin, Cathelicidin, VDR, CYP27B1, CYP24A1 | Lower severity in AD/PSO patients; supplementation (1500–1600 IU/day) improves SCORAD/PASI; beneficial in genetic variants |
| Intestine (IBD, CeD) |
Maintains epithelial barrier; regulates TJs; modulates immunity and microbiota; reduces epithelial apoptosis; promotes Notch-1–mediated regeneration | Claudin-1/2/3/15, Occludin, ZO-1, VDR, Cathelicidin, CD3+ T cells, Notch-1 | Supplementation reduces disease activity, relapse; improves mucosal healing and microbiota; better response in patients with <30 ng/mL Supplementation reduces disease activity, relapse; improves mucosal healing and microbiota; better response in patients with <30 ng/mL |
| Cardiovascular system (CVD) |
Reduces inflammation and oxidative stress; regulates RAAS and endothelial function; modulates lipid/glucose metabolism; protects cardiomyocytes | NO, VCAM-1, MCP-1, Nrf2, Klotho, PMCA, TRPV5/6, ABCA1, SOD, IL-6, TNF-α | Observational studies show lower CVD risk with higher vitamin D; interventional data mixed |
| Disease/Condition | Study Design/Reference | Subjects/Sample Size | Primary Outcome/ Endpoints | Main Findings on Vitamin D |
| Atopic Dermatitis (AD) | Cross-sectional (McCarthy 2024) | 681 children/young adults | Serum 25(OH)D vs AD severity (EASI) | 84% of AD patients were vitamin D deficient; levels inversely correlated with severity; <25 nmol/L = 3x risk severe AD |
| Meta-analysis (Hattangdi-Haridas 2019, Kim 2016) | 11 RCTs, n=686 | SCORAD, EASI scores | Supplementation (1500–1600 IU/day ≥12 wks) significantly reduced severity | |
| RCT (Hata 2014) | 107 children | AD severity after vitamin D or placebo | Supplementation improved SCORAD vs placebo | |
| Observational (Peroni 2012) | 106 children | Serum 25(OH)D and AD severity | Lower 25(OH)D in moderate-severe AD; negative correlation | |
| Psoriasis (PSO) | Cross-sectional (Chandrashekar 2015, Maleki 2016) | 100–300 | Serum 25(OH)D vs PASI | Lower vitamin D in psoriasis; inverse correlation with PASI |
| RCT (Finamor 2013) | 25 patients | Oral vitamin D3 (35,000 IU/d, 6mo), PASI | Significant PASI improvement, ↑25(OH)D | |
| RCT (Ingram 2010, Jarrett 2018) | >200 total | High-dose vitamin D3 (monthly/weekly) | No significant clinical improvement vs placebo | |
| Meta-analysis (Upala 2017) | 8 studies, n=4349 | Serum 25(OH)D and psoriasis risk | Low vitamin D associated with increased risk | |
| IBD (CD + UC) | Meta-analysis (Giustina 2023) | 8316 IBD patients | Disease activity, relapse, QOL | Low vitamin D = ↑activity, relapse, worse QOL |
| Cohort (Tabatabaeizadeh 2018) | 470 IBD patients | Serum 25(OH)D and flares | 25(OH)D ≥27.5 ng/mL predicted fewer flares | |
| RCT (Jørgensen 2010, Wang 2024) | 94 CD patients | Vit D3 (2000 IU/d) vs placebo, relapse | Supplementation reduced relapse risk | |
| Cross-sectional (Wang 2024) | 182 CD; 50 UC | 25(OH)D vs CDAI, Mayo | Higher vitamin D in remission; lower in active disease | |
| Meta-analysis (Rigterink 2019) | 10 trials, pediatric IBD | 25(OH)D, CRP, ESR, activity | Safe supplementation; ↓CRP/ESR, trend to benefit | |
| Meta-analysis (Wang 2024) | 27 studies, n=8316 | Disease activity, relapse, QOL | Low vit D: ↑activity (OR 1.53), relapse (OR 1.23), poor QOL | |
| Celiac Disease (CeD) | Meta-analysis (Giustina 2023) | 24 studies; 1,137 CeD; 2,613 ctrl | Serum 25(OH)D, effect GFD | Mean 25(OH)D lower by 3.3 ng/mL; improved with GFD |
| Prospective (Barera 2020) | 33 pediatric CeD | Vitamin D (400 IU/d) + Ca, 6mo | Improved symptoms and bone metabolism with supplementation | |
| Animal (Trasciatti 2022) | Mouse celiac model | Cholecalciferol, villus/TJ, inflammation | High-dose vitamin D improved mucosal structure/TJs | |
| Cardiovascular Disease (CVD) | Meta-analysis (Upala 2017; Manson 2019) | >80,000 (multiple studies) | CVD risk, MI, stroke, MACE, BP | Low vit D = ↑CVD risk (obs. studies); supplementation effect on MACE inconsistent, some benefit in subgroups |
| Cohort (InCHIANTI Study, Brandi 2024) | >1000 elderly | 25(OH)D/1,25(OH)2D vs CVD risk | Low vit D linked to higher CVD risk, obesity, inflammation | |
| Meta-analysis (Ahmadieh 2023; Argano 2023) | >17 RCTs, >8000 subjects | Lipids, BP, HOMA-IR, CRP | Mixed results: some ↓CRP, fasting glucose, HOMA-IR; limited effect on major CVD outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





