Background
Caffeine and nicotine are common CNS stimulants. Their interaction affects multiple systems, including dopaminergic and cholinergic pathways, inflammatory cytokine profiles, and the hypothalamic-pituitary-adrenal (HPA) axis. Excessive doses may provoke systemic responses mimicking autoimmune or psychosomatic syndromes.
Case Description
Caffeine intake (approx. 382 mg) was achieved through two espresso shots and one americano, consumed over a 90-minute period. Nicotine (approx. 9 mg) was delivered via a closed-system vape device (18 mg/ml nicotine solution), totaling approx. 25–30 puffs.
This self-experiment was initially designed to provoke vasospastic sensitivity (Raynaud-like response) and was formally described as the “Dimitriev Caffeine-Nicotine Provocation Test” [12]. Unexpectedly, a distinct biphasic symptom pattern emerged, leading to the formulation of the present PSSP hypothesis.
Blood pressure and heart rate were measured every 15 minutes using an Omron M3 CE-certified monitor. Body temperature was recorded with a standard mercury thermometer. Symptom severity was self-rated using a 10-point VAS (0 = no symptom, 10 = maximal).
A healthy 20-year-old male with suspected autoimmune connective tissue disease performed a self-provocation test involving 382 mg of caffeine and 9 mg of nicotine. Psychological state rapidly deteriorated: intense anxiety, derealization, fear of death, and cold extremities were reported. Somatic signs included mottled skin, tremor, sweating, ocular myokymia, and impaired gait.
Blood pressure monitoring post-stimulation revealed progressive sympathetic activation. Hemodynamic parameters during the post-stimulant phase is summarized in Table 1.
Table 1. Blood Pressure and Heart Rate monitoring.
A transient subfebrile state (T = 37.0°C) was recorded. Subjective hyperesthesia and photophobia suggested central sensitization. After 3 hours of stimulation, the subject experienced throat tightness and coughing, followed by a hypertensive response.
Medications:
- Paroxetine 20 mg (11:00)
- Lamotrigine 100 mg (15:00)
- Sulpiride 100 mg (19:00) — minor anxiolytic and mood-elevating effects were noted
Pathophysiological Hypothesis
The phenomenon likely arises from synergistic overactivation of dopaminergic and cholinergic systems, leading to overstimulation of limbic circuits and brainstem autonomic centers [
1,
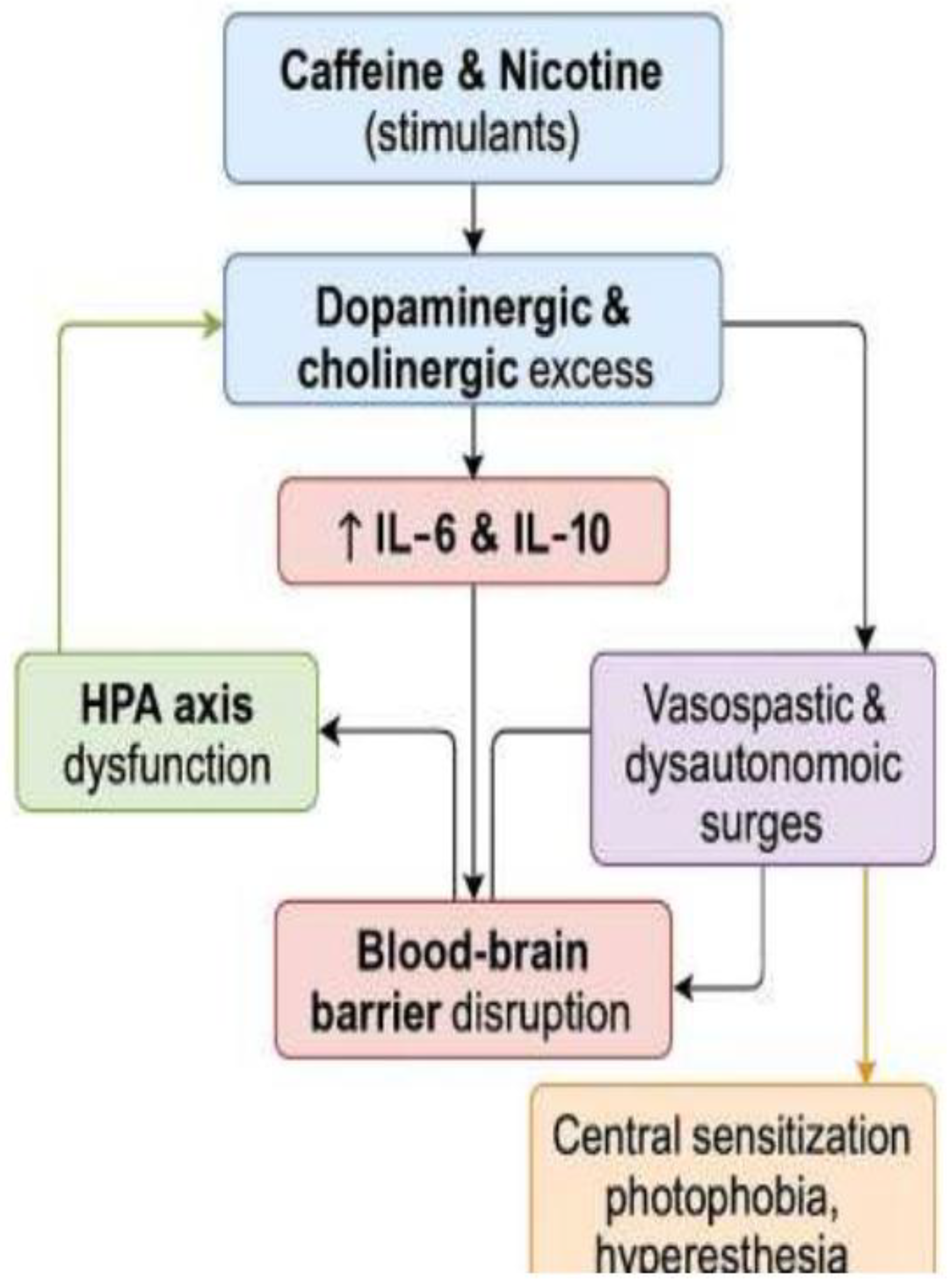
2]. This proposed pathophysiological cascade is illustrated in Fig. 1. Both caffeine and nicotine transiently increase dopamine and acetylcholine, amplifying arousal, anxiety, and sympathetic drive [
1,
2].
Figure 1.
Pathophysiological cascade of PSSP.
Figure 1.
Pathophysiological cascade of PSSP.
Proposed mechanism underlying the post-stimulant neurovascular-psychosomatic reaction (PSSP). Dopaminergic and cholinergic hyperactivation initiates a cascade involving limbic stimulation, glial sensitization, neuroinflammation, and HPA axis dysregulation. Dashed arrows indicate hypothetical connections. This is a theoretical model based on a self-experimental case.
Caffeine and nicotine increase IL-6 and IL-10, acting as acute pro-inflammatory triggers [
3,
4]. The HPA axis may fail to adequately suppress this due to paradoxical glucocorticoid action in certain individuals [
5]. Elevated cortisol may even exacerbate neuroinflammation and blood-brain barrier permeability [
6].
Central sensitization, via NMDA receptor facilitation and dopaminergic-cholinergic crosstalk, may explain hyperesthesia and photophobia [
7]. Hypothalamic-pituitary imbalance, vagal withdrawal, and impaired glymphatic drainage may further worsen the clinical state [
8].
A transient vasospasm and dysautonomia with impaired thermoregulation and neuroimmune crosstalk results in a syndrome mimicking psychosomatic crisis or withdrawal.
This reaction may particularly affect individuals with heightened sensitivity to stimulants, latent neurovegetative instability, or underlying affective disorders. The combination of dopamine and acetylcholine receptor activation can disproportionately engage limbic and autonomic circuits in predisposed individuals, provoking psychomotor agitation, transient affective shifts, and somatic hypersensitivity.
The subsequent rebound phase may include central fatigue, depressive mood, nausea, appetite suppression, and migraine-like headache, potentially linked to transient neurotransmitter depletion, inflammatory activation, and hypothalamic dysregulation. Observed muscle microtwitches (ocular and shoulder) and heaviness in the head and eyes may represent transient dysregulation of motor thresholds. These responses, though reversible, may signal a low threshold for stress-induced neuroinflammatory responses.
Testable Predictions for Future Research
1. PSSP subjects may show elevated serum IL-6 and cortisol levels at 2 hours post-stimulant exposure, compared to matched controls (p<0.05, ELISA).
2. Low-dose ketamine (0.3 mg/kg IV) may reduce photophobia VAS scores by >30% in PSSP patients (randomized double-blind placebo-controlled trial).
3. Autonomic testing using HRV may reveal persistent sympathetic overactivation during PSSP episodes (LF/HF ratio > 3.0).
4. Acute D2 antagonism (e.g., amisulpride 100 mg) will reduce anxiety VAS by >50% in PSSP, without improving photophobia or headache (p<0.01)."
Discussion
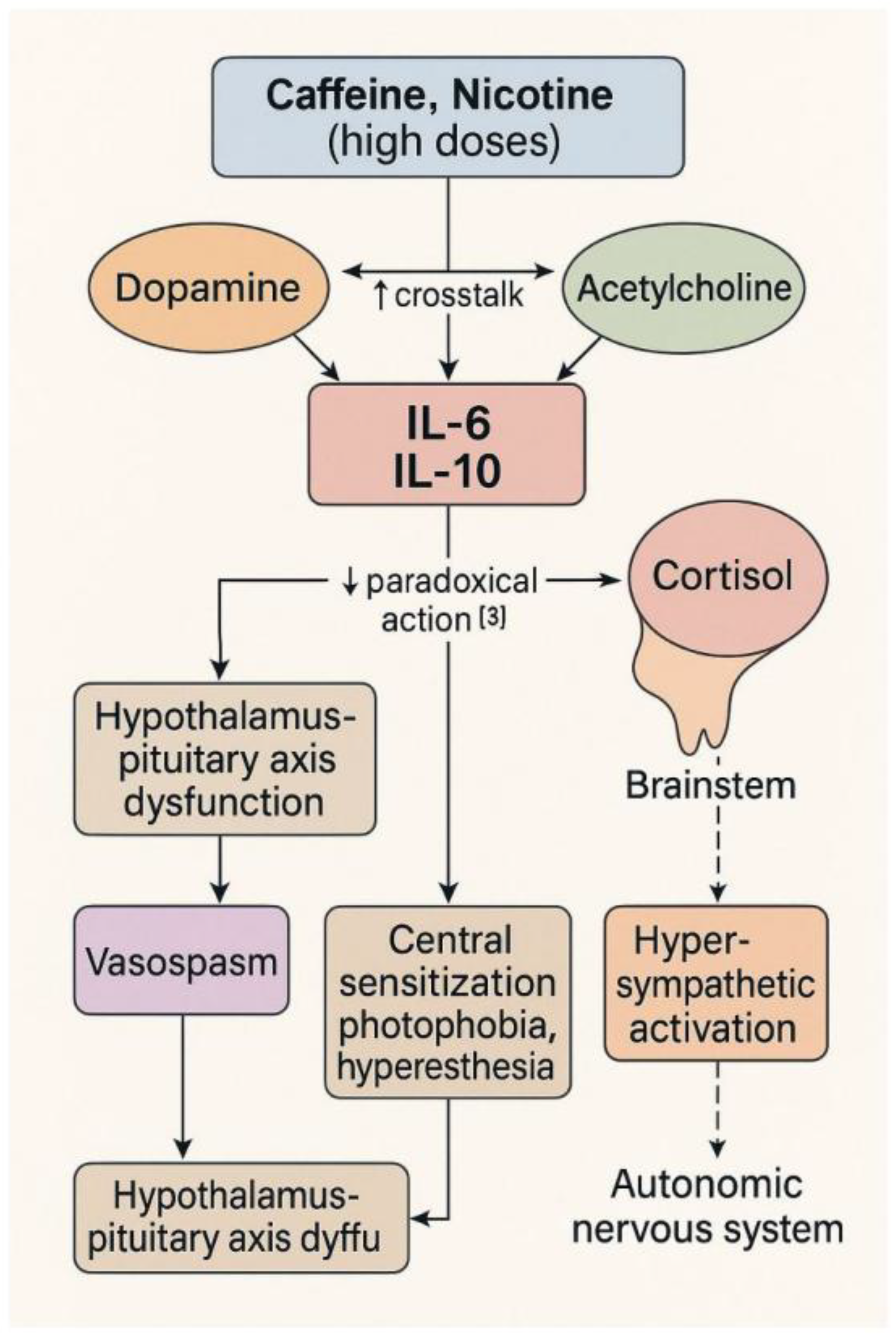
Notably, PSSP symptoms resemble caffeine-induced anxiogenic response [10], but with a delayed migraine phase, implying a neurovascular component not typical of pure anxiety states [12]. Although PSSP shares features with serotonin syndrome—particularly autonomic activation—it lacks hallmark signs such as hyperreflexia, myoclonus, and clonus [11]. The multisystemic pattern we observed aligns with previous hypotheses of dopaminergic-cholinergic dissociation [14] and emerged unexpectedly during a self-provocation experiment aimed at provoking Raynaud’s syndrome [13]. The hypothesized neurotransmitter and vascular interactions contributing to the delayed phase of PSSP are visualized in
Figure 2.
Hypothesized modulation of blood–brain barrier integrity and neuroinflammation by dopaminergic (D2), nicotinic (nAChR), and adenosinergic (A2A) receptor systems. Solid arrows represent known pathways; dashed arrows denote speculative or indirect mechanisms. The model illustrates proposed cross-talk contributing to delayed PSSP symptoms.
This report presents a conceptual hypothesis derived from a single self-experiment, aiming to delineate a possible neurovascular-psychosomatic response to stimulant exposure. This case-based observation describes a distinct neurovascular and psychosomatic response following combined intake of caffeine and nicotine in a sensitive individual with a suspected autoimmune background. The onset of symptoms was acute and included anxiety, derealization, cold extremities, tremor, ocular myokymia, heaviness in the eyes, and later — a flu-like state with hyperesthesia and photophobia. Cognitive functions remained preserved, supporting the integrity of higher cortical control during the episode.The administration of sulpiride (100 mg), a selective dopamine D2 receptor antagonist, led to a noticeable reduction in anxiety and obsessive thinking, while migraine and motor impairments persisted. This selective effect supports the hypothesis that dopaminergic overactivation contributes primarily to the affective and psychomotor components of the proposed neurovascular-psychosomatic cascade. The persistence of non-affective symptoms suggests that cholinergic, vascular, and potentially neuroimmune mechanisms are also involved. This observation highlights the multisystemic nature of the post-stimulant response and the differential neurotransmitter involvement in its expression. Notably, paroxetine and lamotrigine—taken hours before and during the episode—did not prevent or significantly alter the core features of PSSP. The patient had been taking both medications chronically for over two months, which reduces the likelihood that early side effects such as SSRI-induced anxiety contributed to the episode. Lamotrigine, while a mood stabilizer with glutamatergic modulation, is not known to provoke autonomic or sensory exacerbation in therapeutic doses. This supports the view that the observed phenomenon was not a pharmacological artifact, but rather a distinct response to stimulant synergy.
Several hours post-ingestion, the subject reported vegetative instability, fatigue, palmar dysregulation, and a transient subfebrile state. A short episode of daytime sleep relieved some symptoms, but re-administration of nicotine led to an intensification of headache with migraine-like characteristics. We propose the term Post-Stimulant Neurovascular-Psychosomatic Phenomenon (PSSP) to describe this constellation of signs and symptoms. Unlike withdrawal, PSSP begins within 1–3 hours after acute stimulant intake and may include both central (sensory hypersensitivity, emotional lability) and peripheral (vasomotor and autonomic) manifestations.
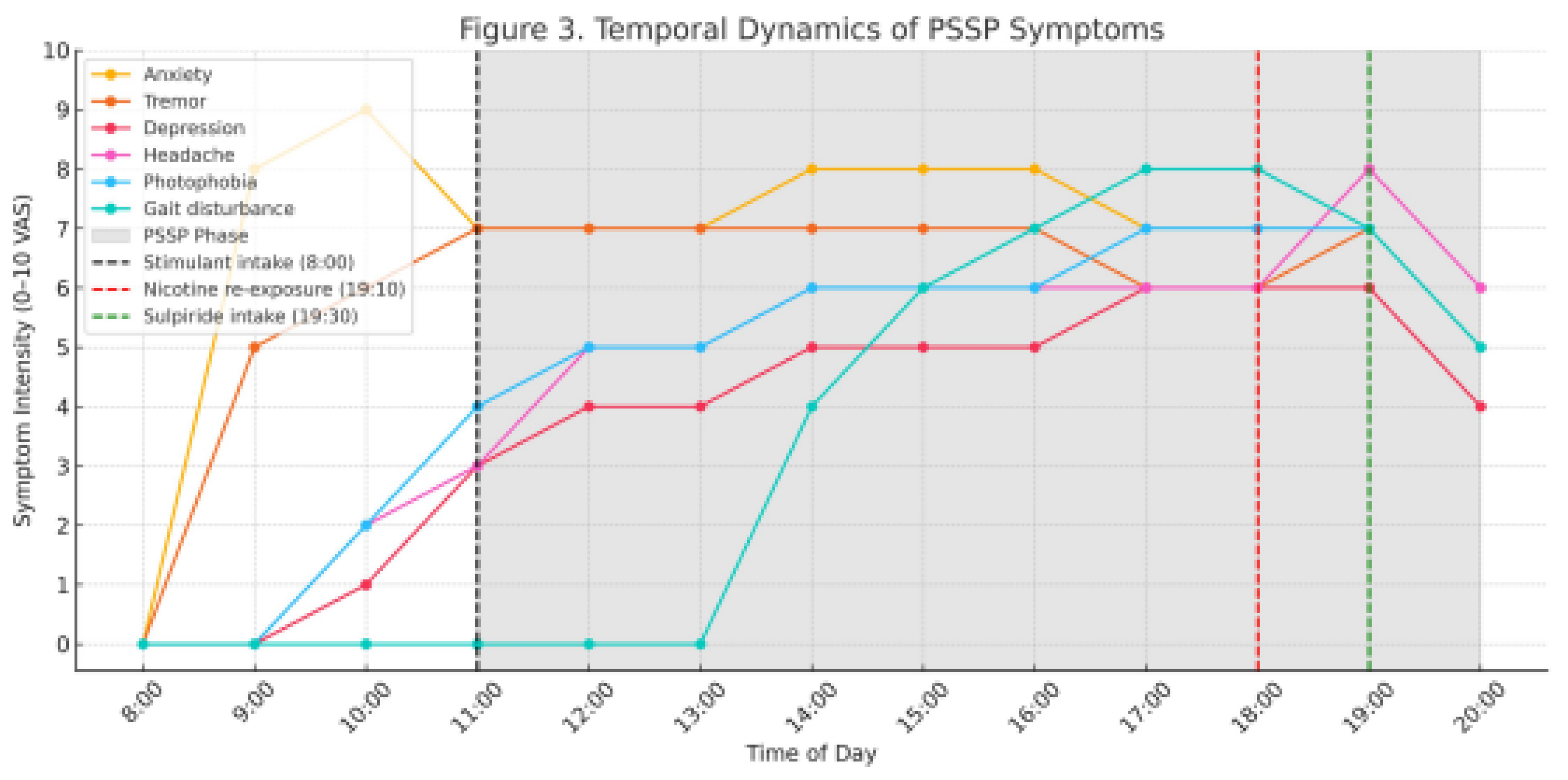
Figure 3 illustrates the temporal progression of symptoms, highlighting the delayed migraine phase and its exacerbation after nicotine re-exposure. Appetite suppression and mild nausea were also noted. Importantly, these symptoms differed in nature and timing from classic stimulant withdrawal and do not meet the criteria for panic attack or psychosomatic conversion disorder. Table 2 compares the clinical trajectory of PSSP with nicotine withdrawal, highlighting divergent responses to re-exposure.
Self-reported symptom intensity on a 0–10 Visual Analogue Scale (VAS) over 12 hours. Vertical dashed lines represent key events:
Caffeine + nicotine intake at 8:00
Nicotine re-exposure at 19:10
Sulpiride administration at 19:30
Shaded region denotes the hypothesized PSSP phase. Notable post-re-exposure exacerbation of headache and motor symptoms is marked.
Table 2. Differential features of PSSP and nicotine withdrawal.
Comparative overview highlighting clinical onset, symptom progression, and response to re-exposure. PSSP displays paradoxical worsening after stimulant re-exposure, unlike typical withdrawal.
This phenomenon may be particularly relevant in individuals with heightened neuroimmune sensitivity, where even a single stimulant exposure may provoke a dysautonomic cascade. The reproducibility of symptoms upon re-exposure supports the existence of a sensitized state. To clarify the unique features of PSSP, a comparative matrix with similar syndromes is presented in Table 3.
Table 3. Differential diagnostic features of PSSP compared to clinically overlapping conditions.
Comparison with serotonin syndrome, panic attack, and psychovegetative crisis (autonomic storm) across multiple domains. PSSP is distinguished by its biphasic pattern, delayed somatic phase, and partial dopaminergic response.
This report describes a single self-monitored case without laboratory biomarkers or instrument-based validation. Further exploration is needed using inflammatory biomarkers (e.g., IL-6, CRP, cortisol), cognitive testing, and objective tools such as thermal imaging or capillaroscopy.
Conclusion
This case-based hypothesis describes a novel post-stimulant neurovascular-psychosomatic response (PSSP) induced by the combined intake of caffeine and nicotine. The proposed mechanism suggests a multi-system cascade involving dopaminergic, cholinergic, and neurovascular pathways. The partial relief of symptoms by dopaminergic modulation supports the role of dopamine in the affective and psychomotor components, while the persistence of migraine and motor impairments points to other regulatory systems. This report highlights the potential value of PSSP as a reproducible model for studying individualized reactivity to stimulant combinations and autonomic dysregulation.
Competing interests
The author declares no competing financial interests.
Non-financial
A.D. has personal experience with stimulant sensitivity, which motivated this theoretical and self-experimental exploration.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets used are available from the corresponding author on request.
Competing interests
The author declares no competing interests.
Clinical trial registration
Clinical trial number: not applicable.
Authors' contributions
Alexander Dimitriev (AD): Conceptualization, investigation, writing.
Acknowledgements
Not applicable.
Description
This self-observed case-based report introduces the concept of Post-Stimulant Neurovascular-Psychosomatic Phenomenon (PSSP), triggered by the combined intake of caffeine and nicotine. The report describes a reproducible biphasic syndrome characterized by anxiety, vasomotor instability, photophobia, and central sensitization, hypothesized to involve dopaminergic-cholinergic dysregulation. The manuscript is currently under peer review at *Progress in Psychopharmacology and Biological Psychiatry*. It is published here as a preprint to ensure open scientific visibility. No derivative works or commercial use is permitted under the license chosen by the author.
References
- Ferré, S. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology 2016, 233, 1963–1979. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Rodas, L.; et al. Caffeine intake increases IL-6 and IL-10 in response to running. J Int Soc Sports Nutr. 2020, 17, 40. [Google Scholar] [CrossRef]
- Abbotts, K.S.; et al. Caffeine increases IL-6 and lactate during moderate exercise. Med Sci Sports Exerc. 2023, 55, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Sapolsky, R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007, 21, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Archie, S.R.; et al. Role of BBB in nicotine-induced neuroinflammation. Fluids Barriers CNS. 2023, 20, 16. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: a generator of pain hypersensitivity. J Pain. 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Zhang, C.; Hu, Z.; Tang, J.; Xue, J.; Lu, W. Caffeine intake and anxiety: a meta-analysis. Front Psychol. 2024, 15, 1270246. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N Engl J Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B.; Andreou, A.P. Caffeine and Primary (Migraine) Headaches—Friend or Foe? Front Neurol. 2019, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, A. The Dimitriev Caffeine-Nicotine Provocation Test: A Self-Experiment Model for Vasospastic Sensitivity. Zenodo 2025. [Google Scholar] [CrossRef]
- Dimitriev, A. Nicotine and Caffeine as a Compensatory Strategy for Early Antipsychotic-Induced Dysfunction: A Case-Based Hypothesis. Zenodo 2025. [Google Scholar] [CrossRef]
- Ferré, S.; Fredholm, B.B.; Morelli, M.; Popoli, P.; Fuxe, K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997, 20, 482–487. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).