3. Results
Cohort Demographics
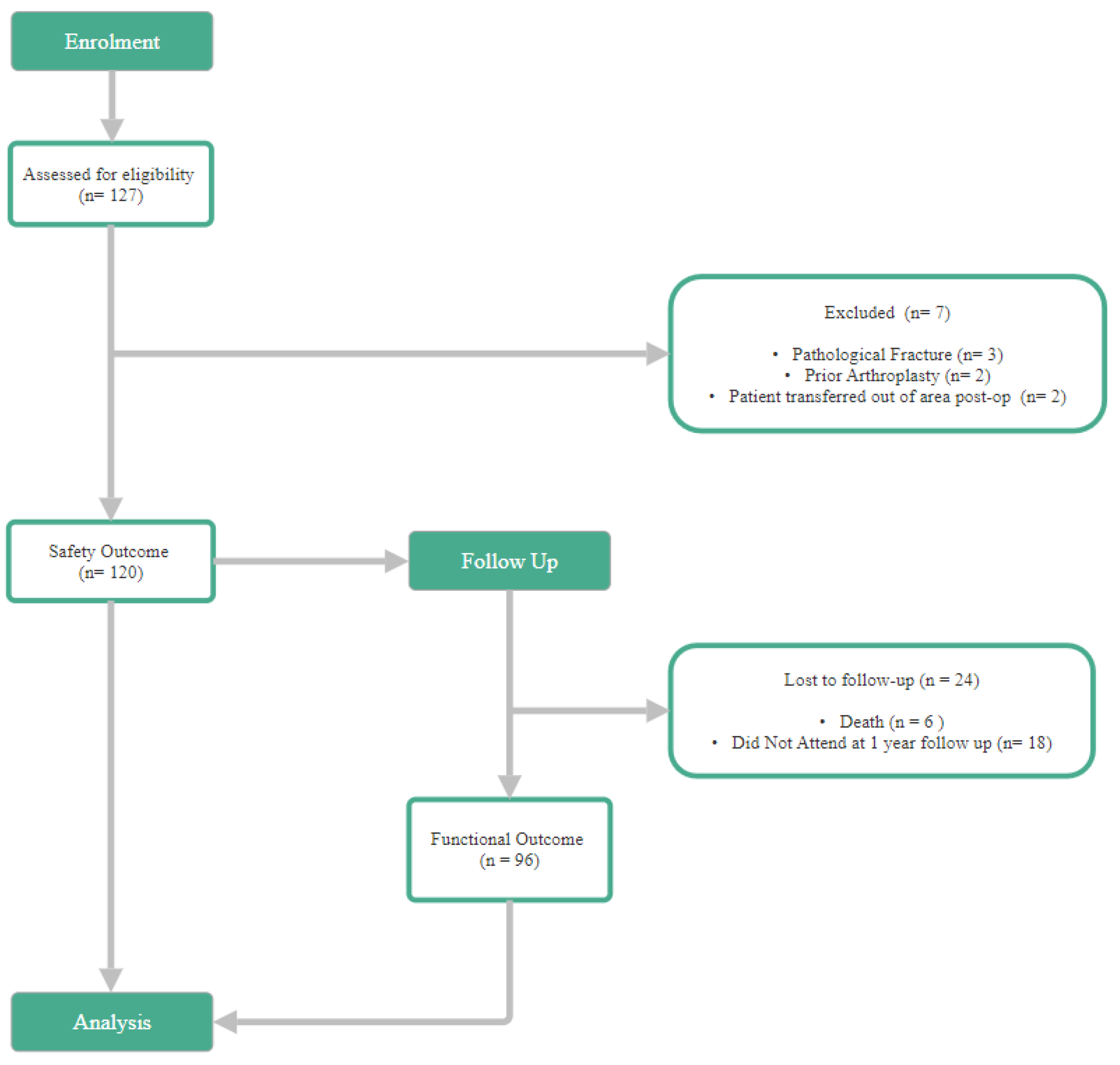
The study analysed 120 patients (Safety Cohort) who underwent G7® DM-THA for femoral neck fractures. Of these, 96 patients (90.1%) had completed 1-year follow-up (Functional cohort). Outcome of the 120 patients, the mean age was 71.6 ± 9.4 years (range 43-92), with 74.2% (n = 89) female and 25.8% (n = 31) male. Most patients were classified as ASA grade II (61.7%) or III (33.3%), reflecting a relatively high comorbidity burden typical of this trauma cohort.
Table 1.
Age Distribution.
Table 1.
Age Distribution.
| Mean Age |
Standard Deviation |
Range |
| 71.6 |
9.4 |
43-92 |
Table 2.
Gender Distribution.
Table 2.
Gender Distribution.
| Sex |
Count |
% |
| Female |
89 |
(74.2%) |
| Male |
31 |
(25.8%) |
| Total |
120 |
|
Table 3.
ASA Scores.
| ASA |
Count |
% |
| I |
5 |
(4.2%) |
| II |
74 |
(61.7%) |
| III |
40 |
(33.3%) |
| IV |
1 |
(0.83%) |
| Total |
120 |
|
Implant Distribution
All patients received the 3rd-generation Zimmer Biomet G7® dual mobility acetabular cup. Femoral implant selection was based on intraoperative bone quality and patient comorbidities, with cemented stems more frequently used in older or osteoporotic patients. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Table 4.1.
Stem Type – Safety Cohort.
Table 4.1.
Stem Type – Safety Cohort.
| Stem Type |
Count |
% |
Notes |
| Cemented |
93 |
77.5 |
CPT/C-Stem |
| |
2 |
1.7 |
C-Stem |
| |
91 |
75.8 |
CPT |
| Uncemented |
24 |
20.0 |
Corail Stems |
| Unknown |
3 |
2.5 |
Op note didn’t specify - excluded from stem comparisons. |
| Total |
120 |
|
|
Table 4.2.
Stem Type – Functional Cohort
Table 4.2.
Stem Type – Functional Cohort
| Stem Type |
Count |
% |
Notes |
| Cemented |
73 |
76.0 |
CPT/C-Stem |
| |
1 |
1.0 |
C-Stem |
| |
72 |
75.0 |
CPT |
| Uncemented |
20 |
20.8 |
Corail Stems |
| Unknown |
3 |
3.1 |
Op note didn’t specify - excluded from stem comparisons. |
| Total |
96 |
|
|
Functional Outcomes
Overall, at one-year follow-up there was no case of dislocation (0.0 %), demonstrating excellent stability. All radiographs demonstrated appropriate component positioning and no radiographic loosening (AP/lateral views) of the implant.
Table 5.
Dislocation incidents upon at 1 year follow-up.
Table 5.
Dislocation incidents upon at 1 year follow-up.
| Dislocation |
Count |
% |
|
| Cemented |
0 |
0 |
|
| Uncemented |
0 |
0 |
|
| Total |
0 |
|
|
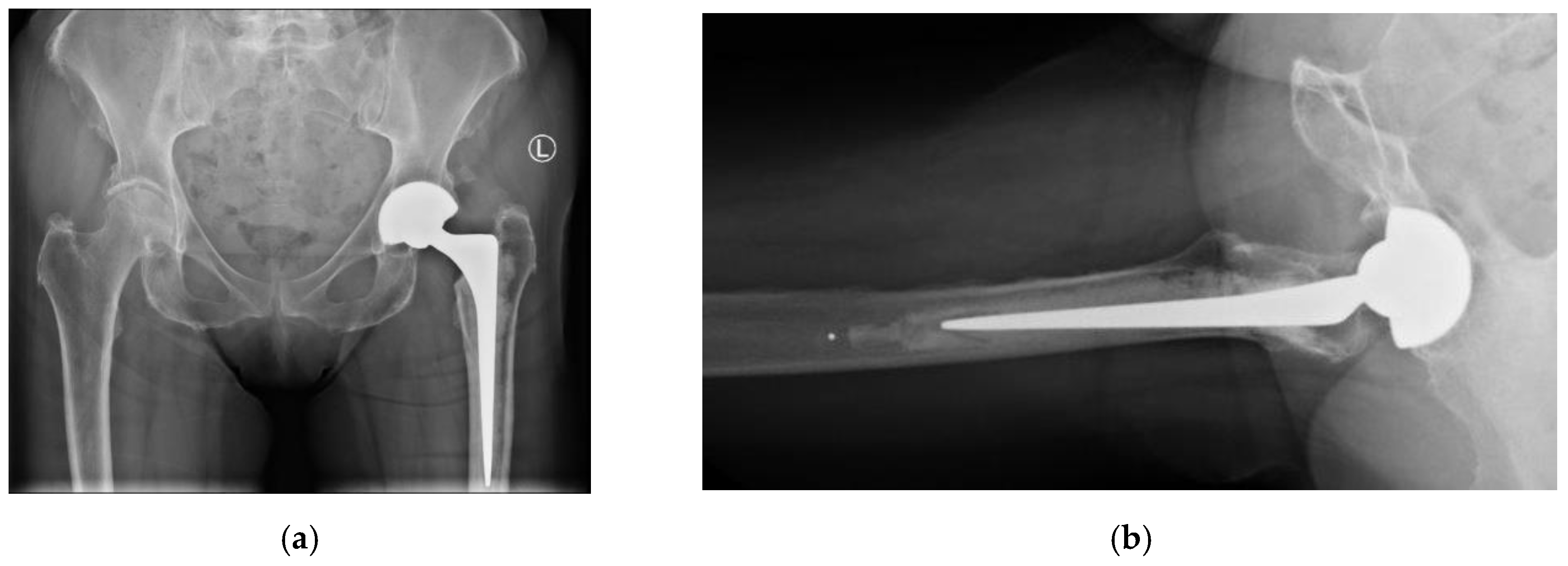
Figure 1.
Postoperative radiographs at 1 year follow-up. 1a) AP view showing cemented CPT stem and neutral cup positioning (Left). 1b) Lateral view confirming absence of radiolucency around the acetabular component.
Figure 1.
Postoperative radiographs at 1 year follow-up. 1a) AP view showing cemented CPT stem and neutral cup positioning (Left). 1b) Lateral view confirming absence of radiolucency around the acetabular component.
At one-year follow-up, the mean OHS was 41.0 ± 8.3 (range: 5-48), with 68.8 % achieving excellent outcomes (OHS ≥40). There is no statistically significant difference in OHS was observed between cemented and uncemented stem groups (p=0.125, Mann-Whitney U test)
Table 6.
Mean Oxford Hip Score at 1-year follow up.
Table 6.
Mean Oxford Hip Score at 1-year follow up.
| Fixation Type |
Mean OHS |
SD |
Range |
95% Confidence Interval for meanLower Bound Upper Bound |
| Cemented |
40.2 |
8.9 |
5-48 |
38.1 |
|
42.3 |
| Uncemented |
43.9 |
4.5 |
34-38 |
41.8 |
|
46.0 |
| Total |
41.0 |
8.3 |
5-48 |
38.3 |
42.7 |
Table 7.
Incidents of Peri-prosthetic Fracture (PPF) at 1-year follow up.
Table 7.
Incidents of Peri-prosthetic Fracture (PPF) at 1-year follow up.
| Peri-prosthetic Fracture |
Count |
% |
|
| Cemented |
1 |
1.0 |
|
| Uncemented |
0 |
0 |
|
| Total |
1 |
|
|
Safety Outcomes
Complications analyses within the Safety Cohort (n=120) were stratified to separate early post-op (≤6 weeks) and long-term (>6 weeks) events, with clear definitions and clinical context. This is so we can identify different targets to assist with recovery at different phases. Overall, no dislocations were observed during follow-up, reinforcing the enhanced stability profile of the G7® dual mobility system.
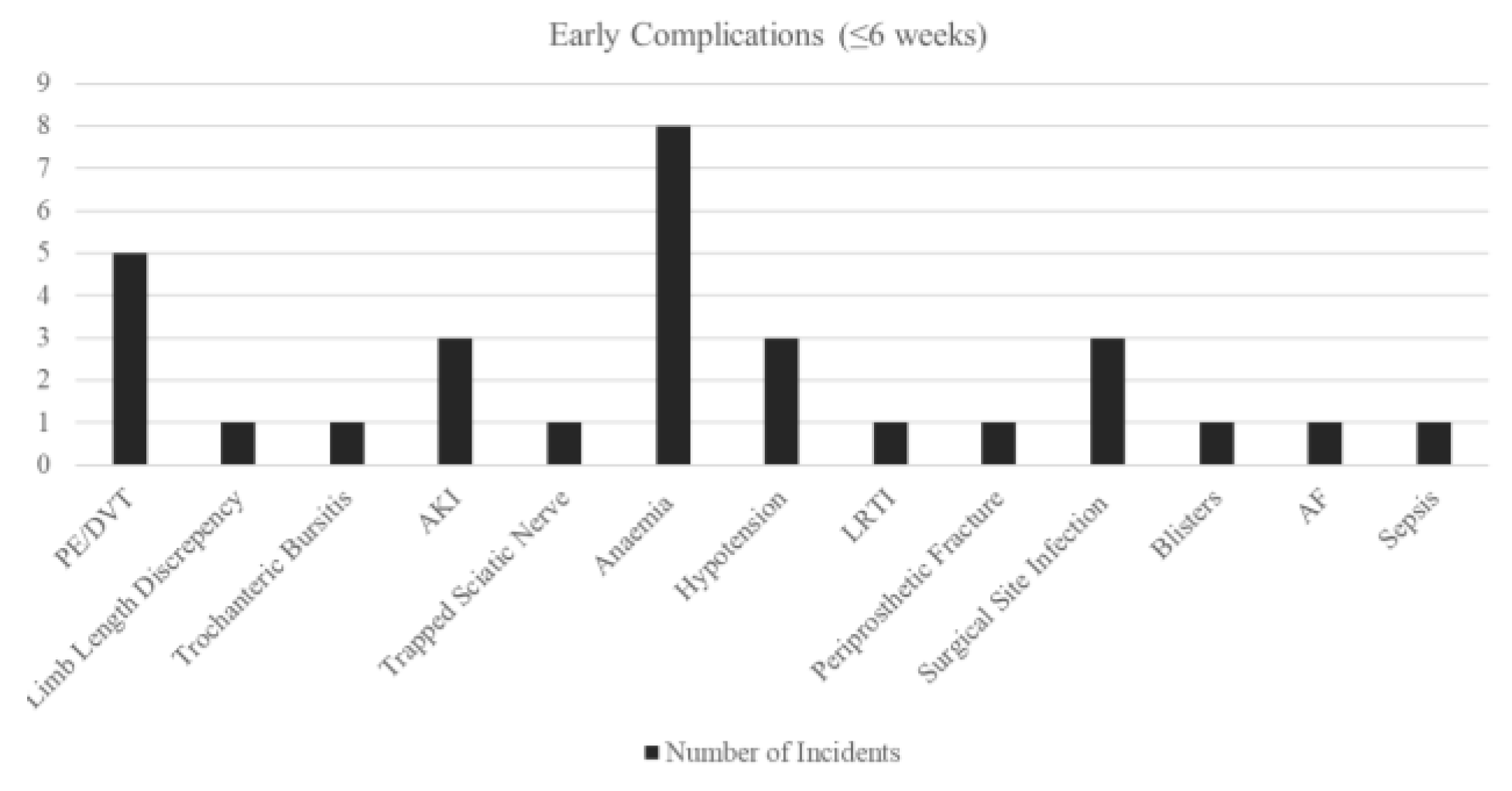
Early complications (≤6 weeks) were primarily medical (6.6% Anaemia, 4.2% VTE), rather than implant related. There was one case of PPF (0.8%), which resulted in the revision of the acetabulum. There are no dislocations or early mechanical failures identified at this phase. All radiographs post-operative demonstrated appropriate component positioning and no radiographic loosening (AP/lateral views) of the implant.
Figure 2.
Bar Chart of Early Complications - Overall.
Figure 2.
Bar Chart of Early Complications - Overall.
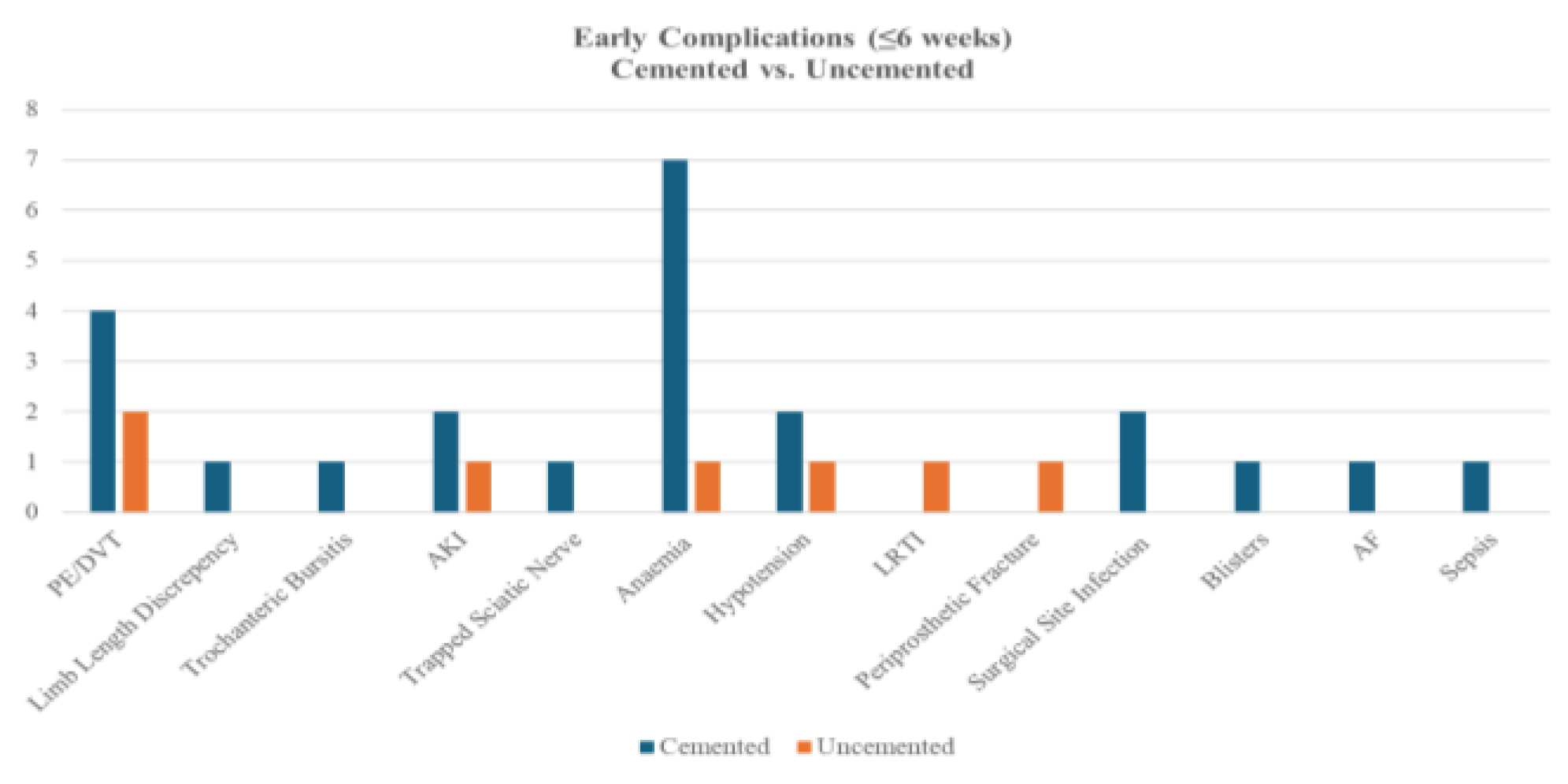
Figure 3.
Bar Chart of Early Complications - by Cement Types.
Figure 3.
Bar Chart of Early Complications - by Cement Types.
A comparison of early complications between cemented (n=93) and uncemented (n=24) procedures revealed no statistically significant differences in overall or individual complication rates (all p>0.05, Fisher’s Exact test). Cemented fixation showed non-significantly higher rates of anaemia (7.5% vs. 4.2%), PE/DVT (4.3% vs. 8.3%), and surgical site infection (2.2% vs. 0%), while uncemented fixation had a numerically higher incidence of PPF (4.2% vs. 0%).
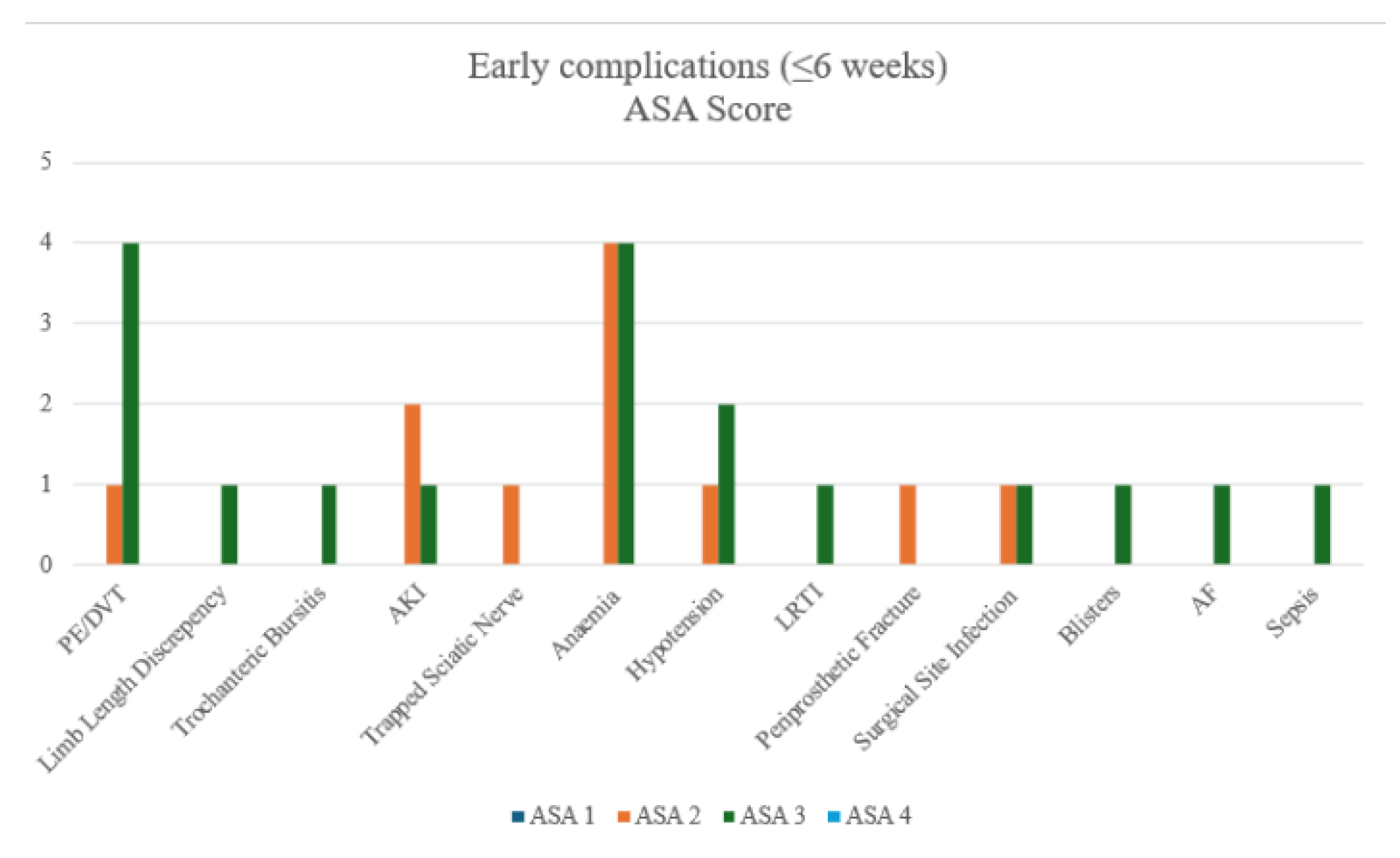
Figure 4.
Bar Chart of Early Complications - by ASA groups.
Figure 4.
Bar Chart of Early Complications - by ASA groups.
After merging ASA groups for analysis, High-risk group (ASA 3+4) patients had significantly higher PE/DVT rates than Low-risk groups (ASA 1+2) (9.8% vs. 1.3%, p=0.0459). No other complications differed significantly, though anaemia occurred most frequently in both high-risk and low-risk ASA groups.
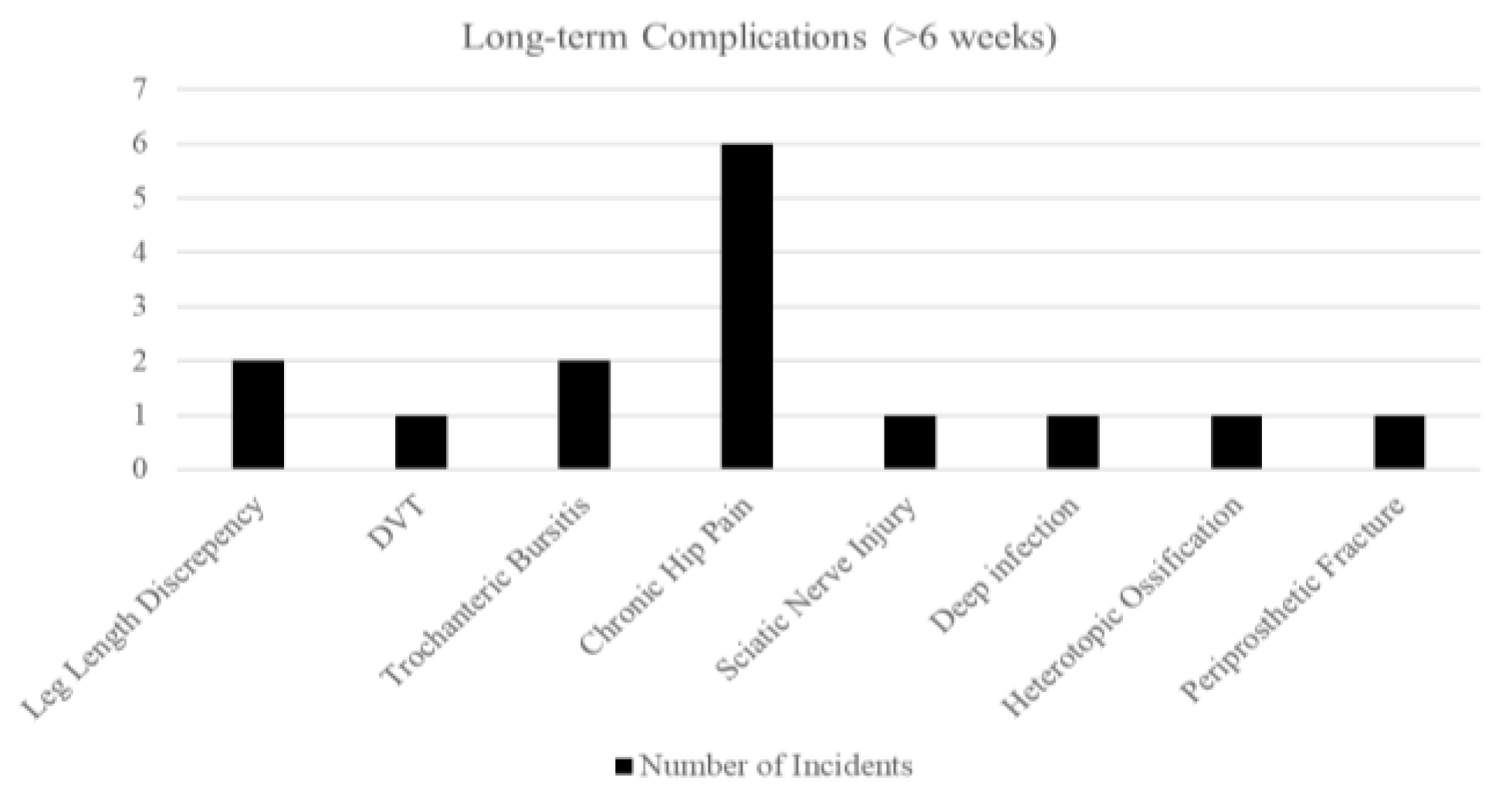
Long-term issues (>6 weeks) were dominated by chronic hip pain (5.0%). The table also includes ones from the early stage developed into chronic. There was one PPF (0.8%) at 8 months, post-fall. All radiographs at follow up demonstrated appropriate component positioning. There were no cases of cup migration, subsidence, or aseptic loosening during the follow-up period. The dual mobility articulation showed no signs of polyethene wear, dissociation, or intra-prosthetic dislocation.
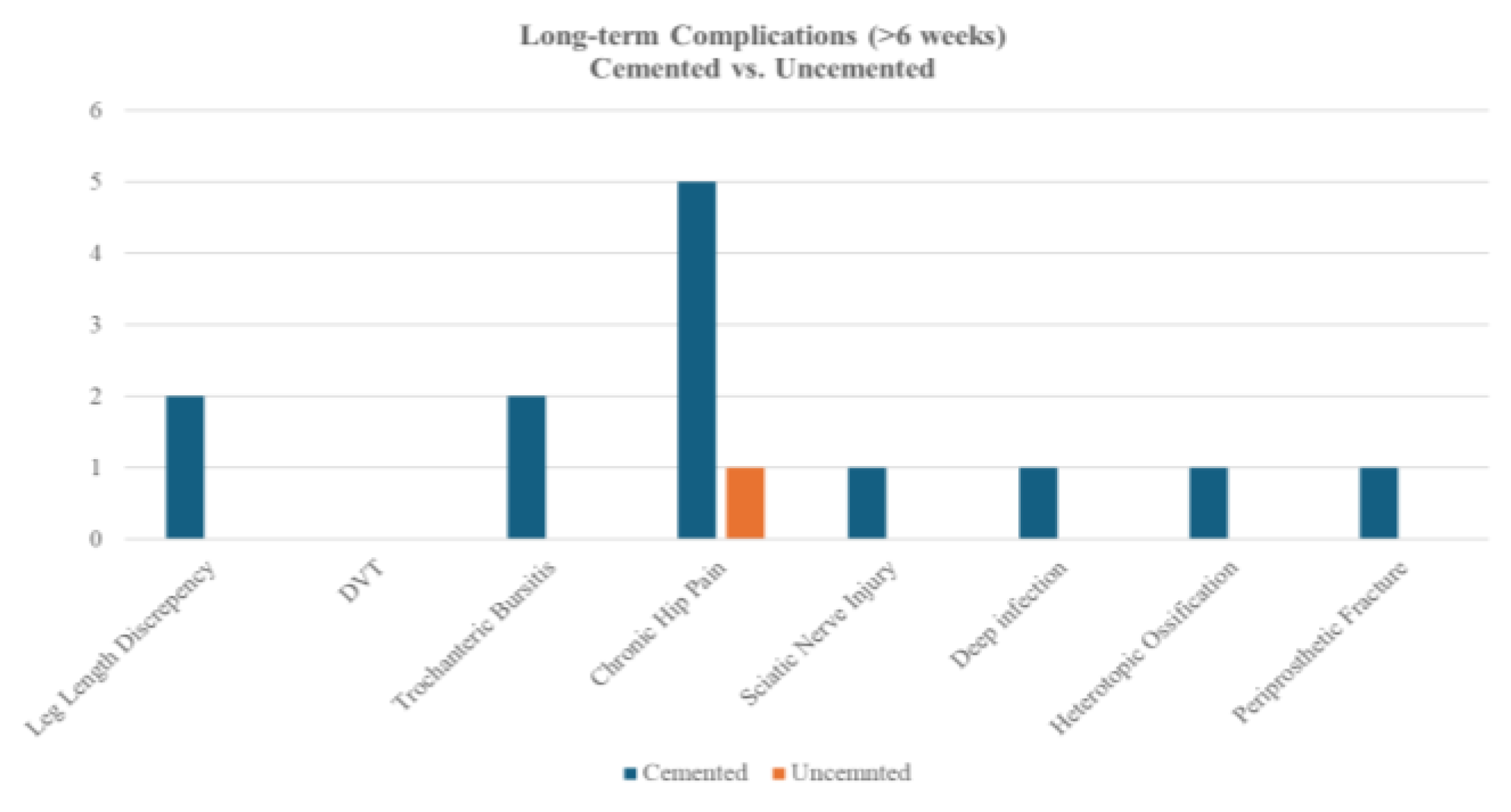
Long-term complications occurred in both cemented (n=93) and uncemented (n=24) cohorts, with no statistically significant differences detected (all p>0.05, Fisher’s exact test). Cemented procedures demonstrated numerically higher rates of chronic hip pain (5.4% vs. 4.2%), trochanteric bursitis (2.2% vs. 0%), and leg length discrepancy (2.2% vs. 0%). Uncemented procedures reported no cases of sciatic nerve injury, deep infection, heterotopic ossification, or PPF, though event rates were low overall.
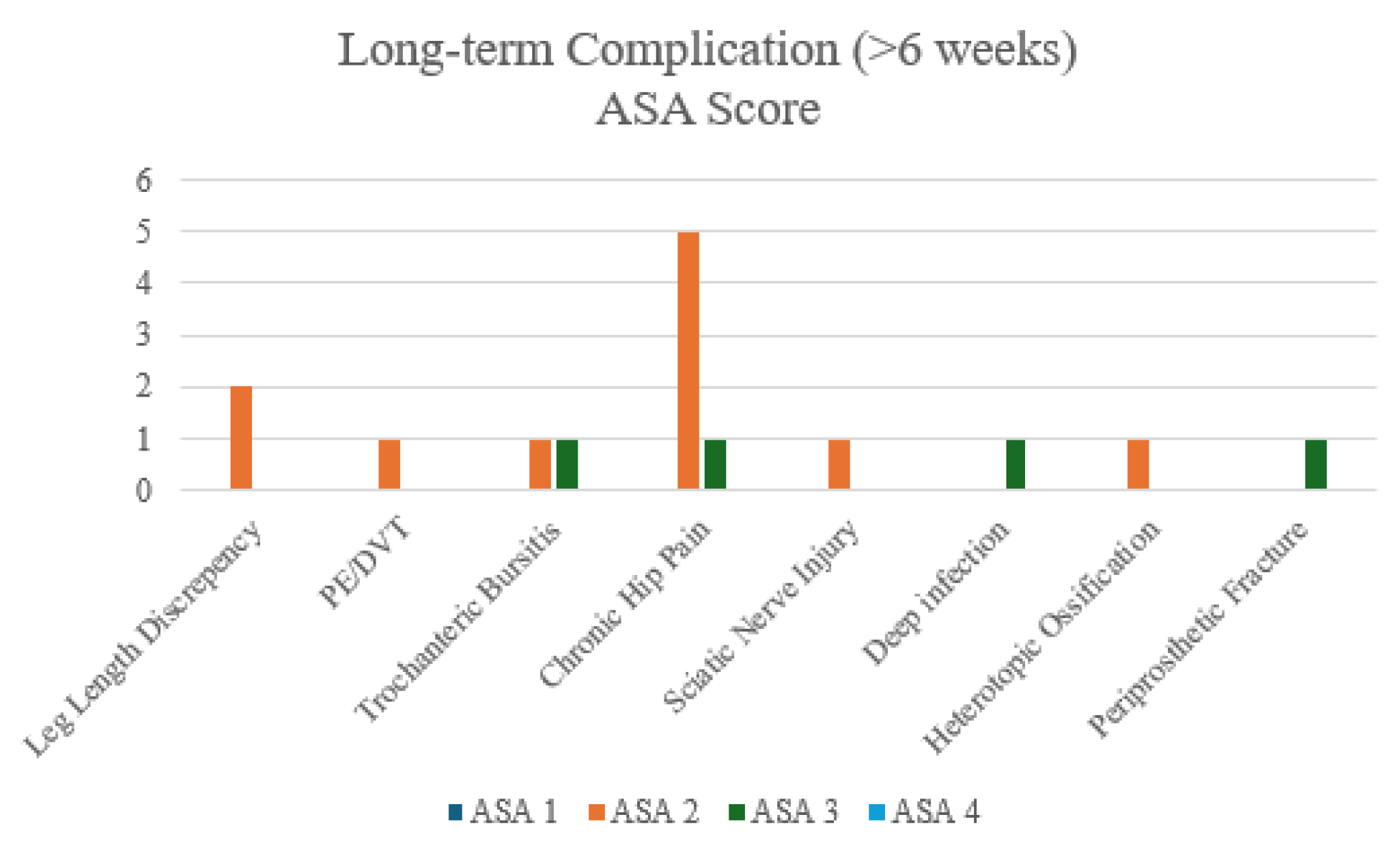
After merging ASA groups, no complications differed significantly. Though chronic hip pain shown to be the most prominent long-term complications after DM-THA
Figure 5.
Bar Chart of Long-term Complications - Overall.
Figure 5.
Bar Chart of Long-term Complications - Overall.
Figure 6.
Bar Chart of Long-term Complications - by Cement Types.
Figure 6.
Bar Chart of Long-term Complications - by Cement Types.
Figure 7.
Bar Chart of Long-term Complications - by ASA groups.
Figure 7.
Bar Chart of Long-term Complications - by ASA groups.
Benchmarking against national outcomes
Thirty-day mortality in the study cohort was 0.8% (1/120), compared to 6.0% (4,314/71,901) in the UK National Hip Fracture Database (NHFD 2024. A two-sample Z-test for proportions demonstrated a statistically significant difference between the two groups (p=0.017).
Table 8.
Comparison if adjusted thirty-days mortality rate.
Table 8.
Comparison if adjusted thirty-days mortality rate.
| Adjusted 30-day Mortality Rate |
This study |
NHFD 2024 |
| Count |
1 |
4314 |
| % |
0.8 |
6.0 |
4. Discussion
Principle Findings
This study demonstrates that the third-generation Zimmer Biomet G7® dual mobility (DM) system provides excellent early outcomes in patients undergoing total hip arthroplasty (THA) for femoral neck fractures (FNF).
Intra-prosthetic Dislocation
The most prominent finding with the dual mobility THA in FNF patients was the 0% dislocation rate, an outcome that emphasises the enhanced stability offered by the dual mobility design. Radiographic evaluations confirmed satisfactory implant positioning with no evidence of loosening, migration, or intra-prosthetic dislocation, further supporting the mechanical reliability of the G7® DM system in this high-risk trauma population. In a high-risk population, traditionally prone to instability (dislocation rates of 4-10% are commonly cited for standard THA after FNF, with some series reporting rates as high as 22% [
10,
11]), achieving zero dislocations is noteworthy. Even the large HEALTH trial (NEJM 2019) found a 4.7% dislocation rate in the THA group (versus 2.4% for hemiarthroplasty) despite the strict inclusion of healthier patients [
12].
This finding aligns with a growing body of evidence that dual mobility implants dramatically reduce dislocation risk compared to conventional designs [
11,
13,
14]. The dual articulation and enlarged effective head size (“head-neck ratio”) of the DM design increase the jump distance required for the head to dislocate [
11], thereby resisting prosthetic dislocation even under extremes of motion and/or less-than-ideal positioning. A comparative series by Zagorov et al. found no dislocations with primary dual mobility THA in femoral neck fracture patients, versus an 11.1% dislocation rate in those with a standard cup [
15]. Large registry data support this stability benefit, reporting a 60% reduction in dislocation risk with dual mobility relative to fixed-bearing implants [
16]. Similarly, a 2021 meta-analysis by Mufarrih et al [
17] noted an average dislocation rate of only 1.87% with DM-THA for fractures, significantly lower than previously reported rates with single-bearing cups.
The surgical approach may have contributed: a posterior approach if used, demands meticulous soft tissue repair to mitigate its higher baseline instability risk (3-8% dislocation with the posterior approach [
11] vs 0.5-0.6% with lateral approach [
18]). A recent UK multicentre study of THA for fractures (n=295) reported a 0% dislocation rate with DM components vs 5.7% with conventional cups when using a posterior approach [
10]. That study found DM cups lowered dislocation risk by more than 4-fold without increasing other complications. In our institution, posterior approach is primarily employed which is known to cut dislocation rates to ~1% [
11]. Notably, no trade-off in function was evident - as discussed below - suggesting that any approach-related limp was minimal.
Moreover, DM may enable a more liberal rehabilitation (with immediate full weight-bearing and fewer motion restrictions), converting into better early stability through restored muscle tone and patient confidence in the new hip. Even typically high-risk patients (e.g. those with cognitive impairment or poor compliance) seem to benefit from the forgiving nature of dual mobility bearings - prior studies have reported zero dislocations in dementia patients managed with DM-THA for hip fractures [
19].
Our findings reinforce that the DM designs, combined with an optimised surgical approach and technique, can eliminate early dislocations in the frail population, a major improvement in safety and a reduction in one of the most feared complications of hip arthroplasty.
Oxford Hip Score (OHS)
Beyond stability, functional recovery in this cohort has been excellent, as evidenced by a mean Oxford Hip OHS of 41.0 ± 8.3 and 68.8% of patients achieving excellent outcomes (OHS ≥ 40) at 1-year follow-up. This corresponds to patients experiencing only mild residual symptoms in daily life - an outcome on a par with, if not exceeding, typical post-THA recovery for osteoarthritis patients [
20]. For context, registry data indicate that the average OHS about 3 months after elective primary THA is ~34 points, improving to ~40 by one year [
20]. Verhaegen et al. found that hip fracture patients treated with THA by arthroplasty specialists achieved a mean OHS of ~43 at final follow-up, statistically equivalent to the outcomes of matched THA patients treated for elective osteoarthritis [
21]. In their study the dislocation rate was 1.7% (with no dual mobility used), suggesting that when arthroplasty is performed under optimal conditions, fracture patients can attain functional scores on par with elective cases. Our OHS results, while slightly lower, remain within the “excellent” range and underscore the benefits of total hip arthroplasty in restoring function for displaced neck fractures.
Achieving an OHS in the 40s so early after surgery suggests that these hip fracture patients rapidly regained mobility and quality of life comparable to elective-surgery patients. Such robust patient-reported outcomes likely stem from multiple factors.
First, DM implants do not appear to compromise the range of motion or hip function; by design, they allow a large jump distance and arc of motion before impingement [
22]. The greater range of safe motion and intrinsic stability may allow patients to move without fear, facilitating more aggressive rehabilitation and return to activities. In our study, patients were mobilised as early as the first postoperative day under the supervision of physiotherapists, utilising the DM cup’s freedom of movement to encourage early functional use of the limb. This is consistent with reports that DM-THA can confer superior range-of-motion and functional scores versus conventional THA in elderly fracture patients [
11]. Agarwala et al., for example, demonstrated significantly higher Harris hip scores and the ability to perform high-flexion activities (such as squatting or sitting cross-legged) in a dual mobility group compared to a standard THR group [
11]. Mechanistically, the large effective head of a dual mobility implant delays impingement and permits a wider arc of motion before instability, which not only prevents dislocation but also enables patients to resume routine movements (like bending to dress or cutting toenails) with greater ease.
Secondly, the surgical approach and soft tissue handling influence functional recovery. If a lateral approach was utilised, one might expect some abductor insufficiency. In our study, a posterior approach was primarily used, which generally has less impact on the hip abductors. This is confirmed by our high OHS, which suggests that any limp or muscle weakness was minimal and transient. Indeed, dual mobility technology has been cited as an enabler for surgeons to use familiar approaches (like posterior) in fracture patients without incurring the usual penalty of higher dislocation rates [
23].
In summary, the combination of implant stability and tailored surgical technique in our study allowed patients to achieve rapid and meaningful functional restoration after what is often a life-altering injury. Our findings suggest that leveraging a dual mobility construct to minimise instability, we achieved both the low re-operation benefits of THA and excellent patient-reported function (mean OHS ~41) in an elderly fracture cohort.
Peri-prosthetic Fractures (PPF)
The rate of Peri-prosthetic Fracture (PPF) in total was 1.7% and early postoperative (first 90 days) PPF was 0.8%, which is in line with or lower than rates reported elsewhere. Large registry-based studies on primary THA note that early postoperative PPF occurs in 1-2% of cases [
24].
Intraoperative or early postoperative femoral fractures are a known hazard in osteoporotic bone, particularly if uncemented stems or forceful impaction techniques are used [
24]. For example, a recent single-centre report (6,788 THAs) documented a 1.9% incidence of PPF within 90 days, with significantly more fractures occurring when using a cementless “compaction” technique as opposed to broaching (2.3% vs 1.3%) [
24]. Our low fracture rate possibly reflects careful surgical technique and appropriate implant choice for this vulnerable population; most patients in our study received cemented femoral implants. In the context of hip fracture, adherence to cemented fixation has been emphasised to reduce PPF risk in osteoporotic bone [
25]. Cementing the stem improves implant fit in poor-quality bone and has been shown to reduce PPF risks relative to press-fit designs, though with an acceptable trade-off of a small risk of cementation syndrome. However, the absence of stem-type association in our study (both PPFs occurred in cemented stems) challenges fracture theories, instead, implicating bone quality and fall dynamics as primary determinants.
It is also noteworthy that dual mobility cups themselves have not been associated with any increase in PPF risk. In a UK multicentre study, the PPF rate was ~2% overall and did not differ between the dual mobility and conventional bearing cohorts [
10]. Likewise, our 0.8% PPF in the functional cohort, incidence suggests that adopting the dual mobility system combined with CPT the stem did not introduce new fracture complications compared to historical controls. However, it also underscores that while rare, vigilance is required to prevent and address this complication.
The incidences of other surgical complications were low (2.5% superficial infection and 0.83% deep infection). There was also no deep infection in the 30-day postoperative window - an indication of stringent antiseptic protocols and appropriate antibiotic prophylaxis. Other complication rates in our series were low, and importantly we recorded no intra-prosthetic dissociations of the dual mobility liner - a rare complication unique to these implants (typically <1% in literatures [
25]). All radiographs (postoperative and at 1-year follow-up) demonstrated appropriate component positioning and no radiographic loosening (AP/lateral views) of the implant. These findings further indicate the robustness of the surgical and institutional protocols.
In summary, the low surgical complication profile observed suggests that a DM-THA can be introduced into the hip fracture pathway without incurring excessive early risks if surgeons adhere to geriatric-specific surgical principles and the hospital system supports the patient’s perioperative needs.
Early Complications
The early medical complications were a noteworthy concern. Postoperative anaemia occurred in 6.6% of cases, reflecting perioperative blood loss and the limited physiological reserve in this frail, elderly fracture population. Although this rate is comparatively modest, given that transfusion rates up to 22.2% have been reported after hip arthroplasty in some centres [
26], precautions are needed for the optimisation of management. Interestingly, there was no significant difference between low-risk and high-risk ASA groups, contradicting the mainstream findings of high ASA class (III/IV) being identified as an independent predictor of postoperative transfusion and medical complications in arthroplasty cohorts [
27]. However, the relatively high incidence rate still underscores the importance of vigilant perioperative blood conservation, consideration of preoperative optimisation (e.g. treating pre-existing anaemia) and timely transfusion for high-risk patients whenever feasible.
Venous thromboembolism (VTE) was another significant early complication, with an incidence of 4.2% in our cohort. Encouragingly, no fatal pulmonary embolism (PE) occurred. Cases of deep vein thrombosis (DVT) and PE were concentrated in the ASA III/IV cohort. This observation aligns with reports in the arthroplasty literature that patients with higher ASA scores - indicative of greater comorbidity burden - have elevated risks of VTE after total hip or knee replacement [
28]. In the context of femoral neck fractures, several factors compound the thromboembolic risk: advanced age, acute trauma, immobilisation before surgery, and medical comorbidities (e.g. cardiac failure, malignancy, or coagulation disorders common in ASA III/IV). Our VTE rate is slightly higher than those reported in elective primary THA series with rigorous prophylaxis, which is understandable given the acute fracture setting. It highlights the need for aggressive thromboprophylaxis (such as early chemical prophylaxis and mechanical compression) and early postoperative mobilisation in this vulnerable group. Therefore, identifying ASA III/IV patients as elevated risk allows clinicians to tailor prophylactic strategies and monitoring (including extended prophylaxis post-discharge) to mitigate VTE incidence.
Long-term Complication
At one-year follow-up, 5.0% of the cohort reported chronic hip pain, which dominated the long-term issues. This aligns with and is much lower than some reports from large cohorts (e.g. a Danish registry study noted at least 12.1% of patients with significant chronic hip pain [
29]). Although not significant, ASA I/II patients reported more persistent hip pain, compared to ASA III/IV patients following G7® dual mobility THA for femoral neck fracture. This contradicts the theory of greater comorbidity burdens has worse pain outcomes [
30]. These findings have important implications for rehabilitation strategies: patients may benefit from tailored postoperative care, with enhanced multimodal analgesia, vigilant monitoring, and specialised physiotherapy input to mitigate chronic pain and optimise functional recovery [
31].
Mortality Rate and Benchmarking
Our study’s 30-day mortality of 0.8% is significantly low when benchmarked against The UK National Hip Fracture Database (NHFD). The UK NHFD reports a risk-adjusted 30-day mortality of approximately 6% for hip fracture patients [
32]. Historically, hip fracture carries high early mortality; other cohorts have documented roughly 5-13% mortality by 30 days in this population [
33,
34]. This is especially encouraging given that THA in fractures is a more extensive procedure than hemiarthroplasty; our results indicate that with proper patient selection and optimised care, the broader surgery (THA with a DM cup) can be safely tolerated even by elderly, frail patients. We also acknowledge that patient selection might play a role - candidates for THA (as opposed to hemiarthroplasty) are often the fitter hip fracture patients (good cognition, ambulatory baseline), which could inherently confer a survival advantage.
Against this background, the 0.8% thirty-day mortality in our series potentially implies that our protocol successfully mitigated the usual drivers of hip fracture mortality - namely perioperative medical complications like cardiac events, venous thromboembolism, and infections [
34]. Several institutional practices likely contributed to improved survival. We employ a coordinated multidisciplinary perioperative pathway, consisting of orthopaedic surgeons, geriatricians, anaesthetists, physiotherapists, and nursing staff who collaborate from admission through discharge. This orthogeriatric co-management model has demonstrated improvements in survival and early outcomes in many studies [
35]. In our study, nearly all patients received their THA within a day of presentation, often after preoperative optimisation by the geriatric team (addressing anaemia, hydration, heart failure, etc.). Additionally, modern anaesthesia and enhanced recovery protocols (including regional nerve blocks, judicious fluid management, and early mobilisation on Day 1 post-op) have likely contributed to the survival benefit.
Reassuringly, the use of a DM implant itself does not appear to adversely affect mortality; prior studies have found no significant difference in early or medium-term mortality between DM versus standard implants in fracture patients [
10]. Thus, our outcomes suggest that a DM-THA can be safely adopted in this high-risk group without incurring the higher early mortality commonly associated with hip fractures when system-level best practices are in place, including multidisciplinary care and fast-track management.
Health Economics
Finally, it is worth considering the broader implications and cost of using DM-THA for hip fractures. Dual mobility implants are more expensive upfront than standard THA bearings or hemiarthroplasty; however, our results indicate a potential cost offset through complication avoidance. A single dislocation event in an elderly patient can incur significant costs - hospital readmission, revision surgery, rehabilitation, and the morbidity of prolonged immobility. By achieving a zero-dislocation rate, our series likely avoided several such costly events. Health-economic analyses from other centres support this trade-off: for example, a Markov modelling based on a large registry found that routine use of dual mobility in primary THA would save roughly €28 million per 100,000 cases by preventing dislocations and their downstream costs [
16]. Barlow et al. similarly reported that a dual mobility implant could remain cost-effective even if its price were up to
$1000 more than a conventional implant, given the expense of treating dislocation and revision is so high [
36]. Additionally, implant longevity must be considered when interpreting early success: dual mobility cups have evolved to address earlier problems (like polyethene wear or intra-prosthetic dislocation seen in first-generation designs). Modern highly cross-linked polyethene, improved locking mechanisms, and refined implant geometry have greatly lowered the risk of intra-prosthetic dislocation and wear-related failures in contemporary DM systems [
11]. This bodes well for the cost-effectiveness of DM-THA in the long run, as implant survivorship appears excellent -for instance, Neri et al. reported 25-year survivorship with no dislocations using a dual mobility construct [
37]. Thus, when considering system-level outcomes, the dual mobility THA strategy in FNF patients seems to offer a superior early outcome (stability and function) which likely reduces downstream healthcare utilisation, and any initial cost premium of the implant is justified by the reduction in complication-related expenditures. Our early results suggest that, in the setting of a comprehensive care model, dual mobility THA not only yields outstanding clinical outcomes for femoral neck fracture patients but also may be a prudent choice from a health economics perspective, benefiting both patients and the healthcare system.
Strength of the Study
Standardised Implant Selection: A key strength is the uniform use of the Zimmer Biomet G7® dual mobility acetabular system for all patients. This standardisation eliminates inter-prosthetic variability and ensures that outcome differences are not confounded by differing implant designs. By focusing on a single, modern dual mobility (DM) implant throughout the cohort, our study avoids the heterogeneity seen in prior series that combined various implant types, thereby improving the internal validity of the comparisons. Unlike some previous reports limited by mixed implant methodologies or small sample sizes (e.g., Alberio et al [
38]) our consistent implant strategy strengthens the interpretability of our results in attributing outcomes directly to the G7® system.
Consistent Surgical Technique and Expertise: All procedures were performed by experienced arthroplasty surgeons following a uniform surgical approach and standardised technique. This high level of surgical consistency minimises technical variability across cases, thereby enhancing the reliability of outcome assessment. Notably, high surgeon volume and experience are known to correlate with lower complication rates -for example, surgeons performing fewer than ~35 hip arthroplasties per year face higher dislocation and revision risks [
39]. In our study, operations were conducted at a high-volume arthroplasty centre by surgeons likely above this volume threshold, which is conducive to optimising patient outcomes. The consistent use of the same approach, fixation method, and post-operative protocols across all patients further reduces confounders that could bias the early outcome evaluation.
Multidisciplinary Perioperative Care Protocols: We implemented a comprehensive multidisciplinary care pathway for femoral neck fracture patients undergoing THA, involving close collaboration between orthopaedic surgeons, geriatric medicine, anaesthesiology, nursing, and rehabilitation services. This standardised “shared care” protocol ensured timely surgery, optimised medical management, and coordinated rehabilitation for all patients. Such an approach is supported by literature showing that coordinated co-management programs (e.g. “Code Hip”) significantly improve perioperative metrics and patient outcomes in hip fracture care [
40]. Indeed, multidisciplinary protocols have been associated with faster time-to-surgery, improved early mobilisation, reduced complication rates, and even lower mortality in geriatric hip fracture populations [
41]. The use of these protocols in our study likely contributed to more uniform and improved early recovery, thereby enhancing the generalisability of our findings to real-world clinical practice where similar protocols are increasingly advocated.
Comprehensive Outcome Assessment: The study’s evaluation framework was broad in scope, capturing clinical, radiographic, and functional outcomes for a holistic assessment of the dual mobility THA’s performance. Clinically, we monitored not only dislocation and revision rates but also other complications (e.g. Peri-prosthetic Fractures, infections) and patient survival, providing a thorough safety profile. Radiographically, all patients underwent scheduled postoperative imaging to assess component positioning, osseointegration signs, and the presence of radiolucent lines or heterotopic ossification, based on standardised criteria. This allowed us to confirm robust implant fixation and identify any early hardware issues (none of which were significant in our series, as no cases of component loosening were detected and osseointegration signs were high at 1 year). The inclusion of validated functional indices -alongside objective measures -is a considerable strength, as it gauges the quality of recovery from the patient’s perspective. Many large-scale investigations into DM-THA focus predominantly on hard endpoints like dislocation or revision [
42], often lacking granular data on postoperative function or radiographic healing. Furthermore, even when functional outcomes are assessed in the literature, the evidence has often been of at longer follow-ups (e.g., Santiago et al [
43]). By capturing high-quality functional outcome data out to the early follow-up interval and combining it with clinical and radiographic analyses, our study offers a richly detailed picture of how the G7® dual mobility system performs in practice. This comprehensive approach provides confidence that improvements (or issues) were not missed and that our conclusions about the efficacy of the implant are well-rounded and patient centred.
Collectively, these strengths underscore the methodological robustness of our study and distinguish it from earlier investigations that often-had narrower scopes or less controlled designs. Our use of a homogenous implant and surgical approach, within a multidisciplinary care framework, and diligent follow-up with multi-faceted outcome collection, enhances the internal validity and reliability of the findings. In addition, by aligning our outcome measures with common clinical benchmarks (NHFD), we ensure that our results are directly comparable to real-world data and existing literature. This alignment with real-world benchmarks means that the early outcomes reported here for the G7® dual mobility THA can serve as a meaningful point of reference for both clinicians and researchers. In summary, the study’s rigorous design and execution strengthen the credibility of the conclusions and provide a high-quality evidence base regarding the use of dual mobility THA in femoral neck fracture patients. The comprehensive and standardised approach we adopted can inform future research and support the generalisability of our findings to broader clinical practice.
Limitation of the Study
Single-centre design: This study was conducted at a single institution, which inherently limits the external validity of the findings. Results from one centre may not fully generalize to other hospitals or populations, as differences in patient demographics, surgical techniques, and perioperative protocols across institutions could lead to different outcomes. Therefore, caution is needed when extrapolating our results to broader settings.
Short-to-mid-term follow-up: Our follow-up was restricted to the short-to-mid-term, so long-term outcomes remain unknown. This limited follow-up means we could not assess the durability of the G7® dual mobility implant or detect complications that might manifest only in the longer term. The lack of long-term data introduces uncertainty about the sustained benefits of the implant and the possibility of delayed adverse events or failures that could emerge over time. Consequently, any conclusions about implant longevity or late complication rates should be viewed as preliminary.
Limited sample size in subgroups: While the overall sample size provides insight into early outcomes, the number of patients in certain subgroups was small. Only a few patients in our cohort received uncemented femoral stems, which limits the power to analyse outcomes for that subgroup. Such small subgroup numbers can undermine statistical power, increase the risk of type II error (missing a true effect) and warrant caution in interpreting any apparent lack of differences. As a result, findings related to these sub-cohorts (e.g. the performance of uncemented stems) should be considered exploratory rather than definitive.
Lack of blinding: The study did not employ blinding of patients, surgeons, or outcome assessors, which introduces potential bias. Knowledge of the implant type by the care team and patients could have influenced postoperative management or subjective outcome reporting (performance and response bias). Similarly, outcome assessors aware of the treatment may have unconscious expectations that skew the evaluation of results. Because no blinding was in place, there is an inherent risk that positive outcomes may have been overestimated or certain negative outcomes under-recognised, so the results must be interpreted with appropriate caution.
Potential confounding from patient selection: Finally, as an observational (non-randomised) study, our findings are subject to possible selection and confounding bias. Patients were not randomly assigned to treatment; instead, the inclusion relied on clinical decisions and eligibility criteria, which may have favoured enrolling relatively healthier or more active patients for THA. If those selected patients had inherently better prognoses, it could confound the association between the dual mobility implant and the outcomes observed. In other words, differences in baseline characteristics or unmeasured variables might partly explain the favourable early outcomes. We attempted to control for known factors, but residual confounding may remain due to the study design. This limitation means that one should be cautious in ascribing all observed benefits solely to the implant, as some effects could stem from the patient population selected.
Future Directions
Long-Term Outcomes: Extended follow-up studies are needed to determine the durability of the G7® dual mobility construct. The current evidence is limited to short-term results, so the sustained benefits of dual mobility (e.g. continued low dislocation rates) and any late complications (such as polyethene wear or PPF) remain uncertain. Long-term surveillance (5-10 years and beyond) will clarify implant survivorship and safety, ensuring that early advantages persist over timepubmed.ncbi.nlm.nih.gov. Such data are especially critical given the high life expectancy variance in this patient population and the need to anticipate revision rates in older adults.
Randomised Trials and Comparative Studies: High-quality comparative research is crucial to validate the benefits of dual mobility THA in hip fractures. A sufficiently powered RCT with dislocation as the primary endpoint would definitively quantify the reduction in instability afforded by dual mobility components versus standard THA or hemiarthroplasty. Additionally, comparative trials should evaluate functional recovery, complication rates, and mortality, to ensure that the stability gains do not come at the expense of other outcomes (noting that some meta-analyses have found worse short-term outcomes [
43]). Rigorous head-to-head evidence will guide surgeons in choosing the optimal arthroplasty approach for displaced femoral neck fractures.
Optimal Implant Fixation Strategies: Investigations are needed to identify the best fixation and surgical techniques for dual mobility THA in fracture patients. Elderly fracture populations often have poor bone quality, raising questions about cemented versus uncemented fixation for both acetabular cups and femoral stems. Our data contradicted early data and suggested no significant differences in outcomes (such as dislocations, early complications and PPF) between cemented and uncemented stem fixation. Future research should explore how these findings translate to dual mobility systems.
Health Economic Evaluation: The cost-effectiveness of adopting dual mobility THA for hip fractures must be rigorously assessed. Dual mobility implants are typically more expensive upfront, but they may avert downstream costs by preventing dislocations and reoperations. A recent modelling study suggests that using dual mobility in displaced neck fractures can be cost-effective in patients under 80, provided the dislocation risk is substantially reduced [
44]. However, these analyses rely on assumptions that need validation in real-world settings. Future research should incorporate health economic evaluations alongside clinical trials - measuring quality-adjusted life years (QALYs), implant costs, complication costs, and overall healthcare utilisation. Particularly, if dual mobility THA indeed lowers revision or institutional care needs (due to fewer complications), it could offer long-term cost savings despite higher implant costs. Confirming this through prospective economic studies or registry data will be important for policymakers and hospital systems. Ultimately, demonstrating value for money will facilitate wider adoption if the clinical benefits are borne out.