Submitted:
11 July 2025
Posted:
14 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathology
3. Diagnosis
3.1. Biochemestry
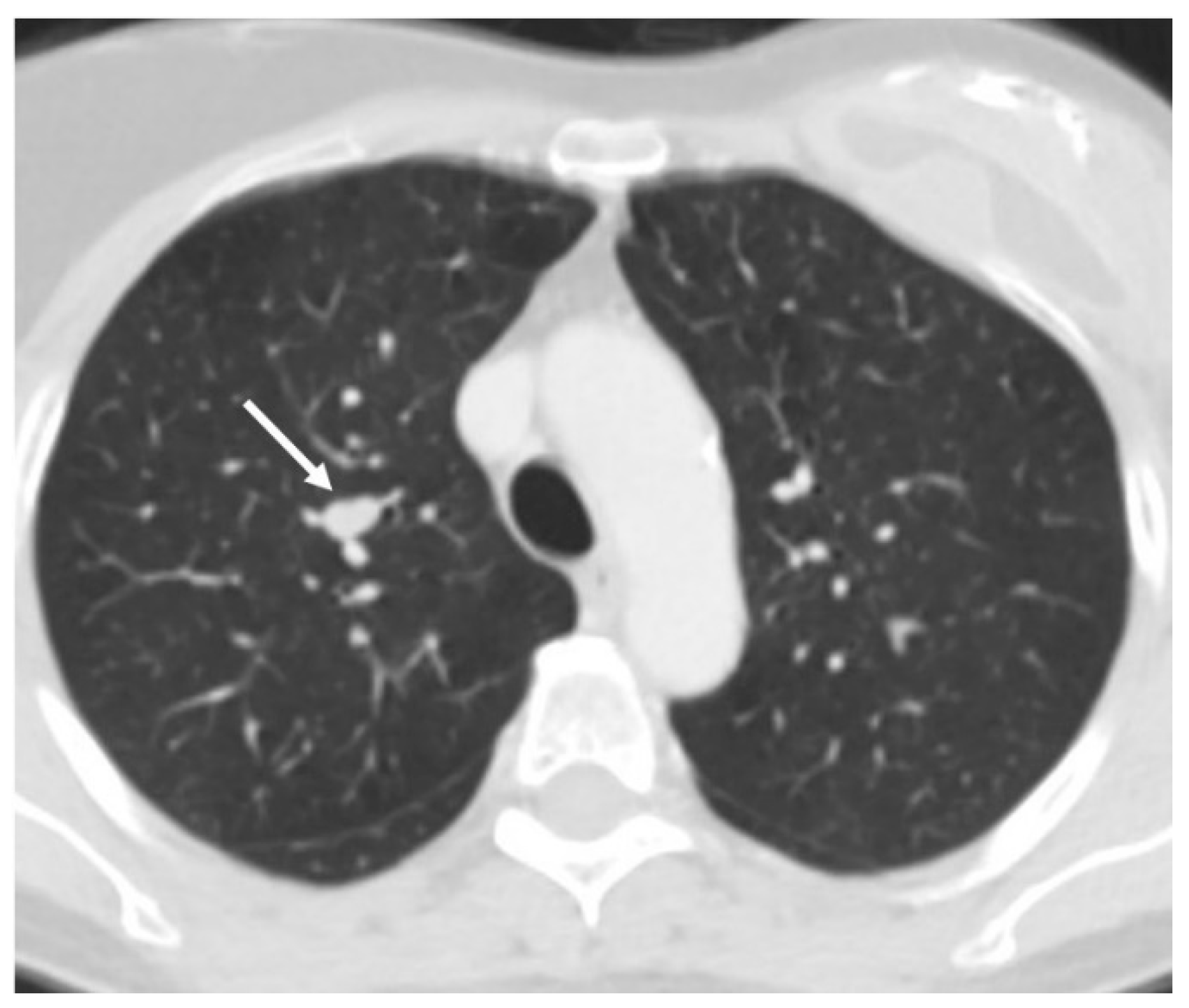
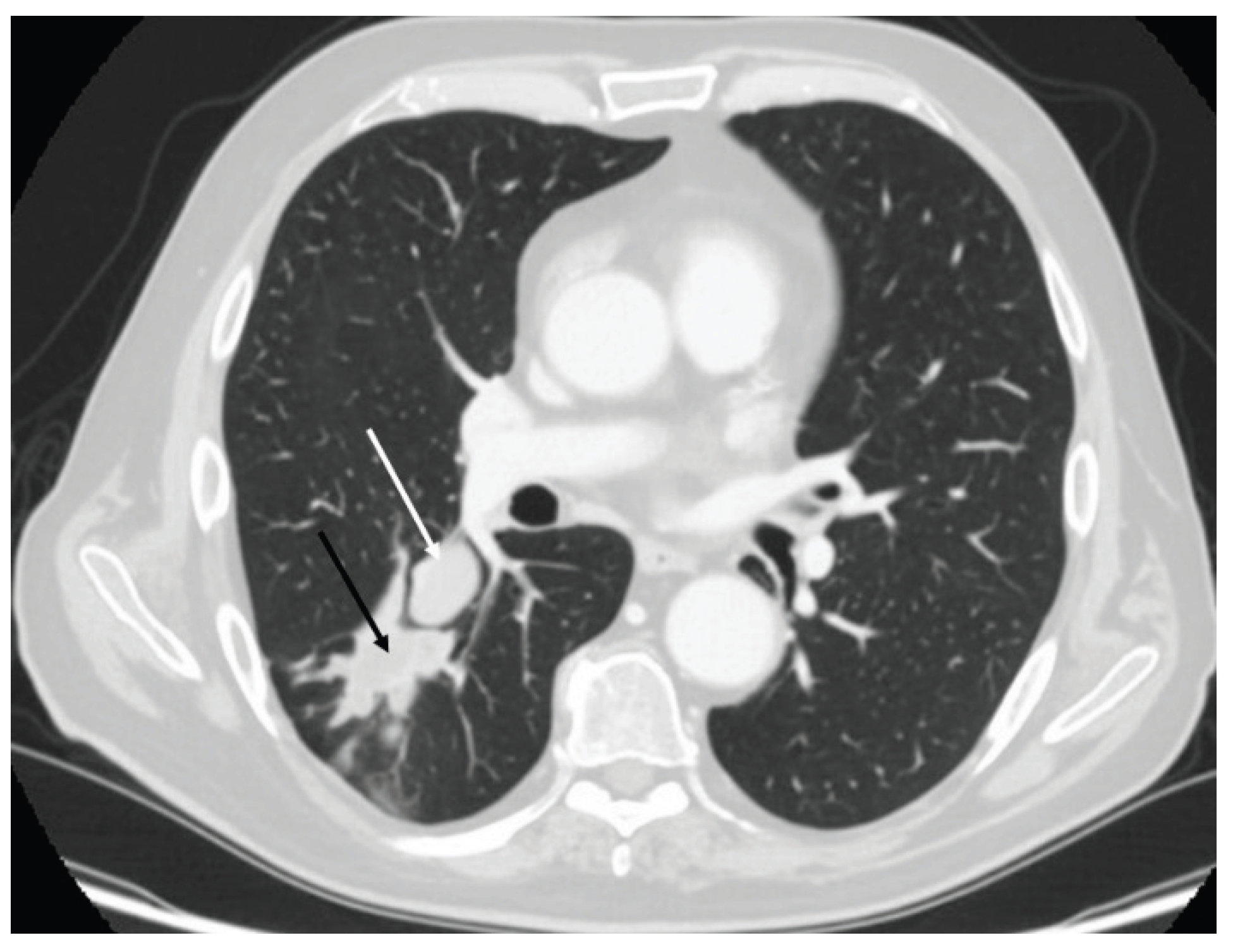
3.2. Radiology
3.3. Nuclear Medicine
3.4. Bronchoscopy
4. Treatment
4.1. Surgery
4.2. Advanced Stages: Medical Management and Therapeutic Strategies
5. A Brief Overview of Other Neuroendocrine Non-Carcinoid Lung Tumors
5.1. Small Cell Lung Cancer (SCLC)
5.2. Large Cell Neuroendocrine Carcinoma (LCNEC)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PC | Pulmonay Carcinoid |
| TC | Typical carcinoid |
| AC | Atypical Carcinoid |
| CT | Computed tomography |
| MEN1 | Multiple endocrine neoplasia type 1 |
| SCLC | Small cell lung cancer |
| LCNEC | Large cell neuroendocrine carcinoma |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| ACTH | adrenocorticotropic hormone |
| SIADH | syndrome of inappropriate antidiuretic hormone secretion |
| DIPNECH | Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia |
| PRRT | peptide receptor radionuclide therapy |
| SRS | somatostatin receptor scintigraphy |
| SPECT | single-photon emission computed tomography |
| FDG-PET | Positron emission tomography with fludeoxyglucose |
| EBUS-FNA | endobronchial ultrasound-guided fine-needle aspiration |
| EUS | endoscopic ultrasound |
| DOTA | Gallium-68-labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| TAE | transarterial embolization |
| TACE | transarterial chemoembolization |
| SSA | Somatostatin analogue |
| VEGF | Vascular endothelial growth factor |
| PDGFR | platelet-derived growth factor receptors |
| EGF | epidermal growth factor |
| SNP | single nucleotide polymorphism |
References
- Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, Travis WD; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015 Aug;26(8):1604-20. [CrossRef]
- Brisset C, Roumy M, Lacour B, Hescot S, Bras ML, Dijoud F, Brisse H, Delehaye F, Desandes E, Philippe-Chomette P, Sarnacki S, Irtan S, Drabent P, Pellier I, Fresneau B, Pire A, Réguerre Y, Orbach D, Mallebranche C. Bronchial Carcinoid Tumors in Children and Adolescents. Pediatr Blood Cancer. 2025 May 30:e31822. [CrossRef]
- Hemminki K, Li X. Familial carcinoid tumors and subsequent cancers: a nationwide epidemiologic study from Sweden. Int J Cancer 2001; 94: 444–448.
- Bini A, Grazia M, Petrella F, Chittolini M. Multiple chondromatous hamartomas of the lung. Interact Cardiovasc Thorac Surg. 2002 Dec;1(2):78-80. [CrossRef]
- Li Y, Linnoila I. Multi-directional differentiation of Ascl1-defined progenitors in lung development and injury repair. Am J Respir Cell Mol Biol 2012; 47:768–775.
- Ahuja G, Iyer A, Harwood R, Balata H, Craig C, Crosbie PAJ, Hewitt K, Peplow K, Hutchings D, Sharman A, Bishop P, Joseph L, Paiva-Correia A, Chaturvedi A, Barr J, Leek A, Backen A, Nuttall C, Kennedy O, Williamson A, Weaver J, Mansoor W, Evison M. Pathological & radiological variables in the diagnosis of bronchopulmonary carcinoids (BPCs) with a focus on Antigen Kiel 67 (Ki-67) proliferation index. Lung Cancer. 2025 Apr;202:108493. [CrossRef]
- Öberg K, Hellman P, Ferolla P, Papotti M, ESMO Guidelines Working Group. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(S7): vii120–3.
- Aung NH, Hlaing WY, Thwe H, Aung HA. The Importance of Advanced Imaging in Diagnosing and Differentiating Lung Carcinoid Tumors From Pulmonary TB and Upper Respiratory Infections. Cureus. 2024 Oct 22;16(10):e72158. [CrossRef]
- Reznek, RH. CT/MRI of neuroendocrine tumours. Cancer Imaging. 2006 Oct 31;6(Spec No A):S163-77. [CrossRef]
- Meisinger QC, Klein JS, Butnor KJ, Gentchos G, Leavitt BJ. CT features of peripheral pulmonary carcinoid tumors. AJR Am J Roentgenol. 2011 Nov;197(5):1073-80. [CrossRef]
- Strange CD, Strange TA, Erasmus LT, Patel S, Ahuja J, Shroff GS, Agrawal R, Truong MT. Imaging in Lung Cancer Staging. Clin Chest Med. 2024 Jun;45(2):295-305. [CrossRef]
- Baettig E, Molina-Centelles MF, Amr-Rey A, Mancheño-Franch N, Muñoz-Núñez C. Pulmonary neuroendocrine cells: Spectrum of diseases and their radiological-pathological correlations. Radiologia (Engl Ed). 2025 May-Jun;67(3):357-364. [CrossRef]
- Genovese E, Canì A, Rizzo S, Angeretti MG, Leonardi A, Fugazzola C. Comparison between MRI with spin-echo echo-planar diffusion-weighted sequence (DWI) and histology in the diagnosis of soft-tissue tumours. Radiol Med. 2011 Jun;116(4):644-56. English, Italian. [CrossRef]
- Rizzo S, Summers P, Raimondi S, Belmonte M, Maniglio M, Landoni F, Colombo N, Bellomi M. Diffusion-weighted MR imaging in assessing cervical tumour response to nonsurgical therapy. Radiol Med. 2011 Aug;116(5):766-80.
- Druskin SC, Macura KJ. MR Imaging for Prostate Cancer Screening and Active Surveillance. Radiol Clin North Am. 2018 Mar;56(2):251-261. [CrossRef]
- Park GE, Jee WH, Lee SY, Sung JK, Jung JY, Grimm R, Son Y, Paek MY, Min CK, Ha KY. Differentiation of multiple myeloma and metastases: Use of axial diffusion-weighted MR imaging in addition to standard MR imaging at 3T. PLoS One. 2018 Dec 17;13(12):e0208860. [CrossRef]
- Kurihara Y, Matsuoka S, Yamashiro T, Fujikawa A, Matsushita S, Yagihashi K, Nakajima Y. MRI of pulmonary nodules. AJR Am J Roentgenol. 2014 Mar;202(3):W210-6. [CrossRef]
- Aggarwal P, Satapathy S, Kaur G, Sood A, Bhadada SK, Walia R, Gupta R, Mittal BR. Safety and Efficacy of Peptide Receptor Radionuclide Therapy in Multiple Endocrine Neoplasia Syndrome: A Single-center Experience. Clin Nucl Med. 2025 Jul 1;50(7):605-611. [CrossRef]
- Fanti S, Farsad M, Battista G, Monetti F, Montini GC, Chiti A, Savelli G, Petrella F, Bini A, Nanni C, Romeo A, Franchi R, Bombardieri E, Canini R, Monetti N. Somatostatin receptor scintigraphy for bronchial carcinoid follow-up. Clin Nucl Med. 2003 Jul;28(7):548-52. [CrossRef]
- Pattenden H, Beddow E, Dusmet M et al. Test performance of PET-CT for mediastinal lymph node staging of pulmonary carcinoid tumors. J Clin Oncol 2013; 31: (suppl; abstr 7544).
- Guarize J, Casiraghi M, Donghi S, Diotti C, Vanoni N, Romano R, Casadio C, Brambilla D, Maisonneuve P, Petrella F, Spaggiari L. Endobronchial Ultrasound Transbronchial Needle Aspiration in Thoracic Diseases: Much More than Mediastinal Staging. Can Respir J. 2018 Mar 4;2018:4269798. [CrossRef] [PubMed]
- Ambrosini V, Nanni C, Zompatori M et al. (68)Ga-DOTA-NOC PET/CT in comparison with CT for the detection of bone metastasis in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2010; 37: 722–727.
- Pelosi G, Petrella F, Sandri MT, Spaggiari L, Galetta D, Viale G. A primary pure yolk sac tumor of the lung exhibiting CDX-2 immunoreactivity and increased serum levels of alkaline phosphatase intestinal isoenzyme. Int J Surg Pathol. 2006 Jul;14(3):247-51. [CrossRef]
- Pfeifer A, Knigge U, Mortensen J et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med 2012; 53: 1207–1215.
- Brisset C, Roumy M, Lacour B, Hescot S, Bras ML, Dijoud F, Brisse H, Delehaye F, Desandes E, Philippe-Chomette P, Sarnacki S, Irtan S, Drabent P, Pellier I, Fresneau B, Pire A, Réguerre Y, Orbach D, Mallebranche C. Bronchial Carcinoid Tumors in Children and Adolescents. Pediatr Blood Cancer. 2025 Aug;72(8):e31822. [CrossRef]
- Petrella F, Borri A, Casiraghi M, Cavaliere S, Donghi S, Galetta D, Gasparri R, Guarize J, Pardolesi A, Solli P, Tessitore A, Venturino M, Veronesi G, Spaggiari L. Operative rigid bronchoscopy: indications, basic techniques and results. Multimed Man Cardiothorac Surg. 2014 May 27;2014:mmu006. [CrossRef]
- Messina G, Pica DG, Vicario G, Bove M, Natale G, Di Filippo V, Capasso F, Mirra R, Panini D'Alba F, Conzo G, Posta TD, Giorgiano NM, Vicidomini G, Capaccio D, Peritore V, Teodonio L, Andreetti C, Rendina EA, Fiorelli A. Advances in Endoscopic Management of Endobronchial Carcinoid. J Clin Med. 2023 Aug 16;12(16):5337. [CrossRef]
- Torii A, Oki M, Ishii Y, Yamada A, Shigematsu F, Ishida A, Niwa H, Kogure Y, Kitagawa C, Saka H. The Role of Rigid Bronchoscopic Intervention for Bronchial Carcinoid. Tohoku J Exp Med. 2021 Oct;255(2):105-110. [CrossRef]
- Boyd M, Sahebazamani M, Ie S, Rubio E. The safety of cryobiopsy in diagnosing carcinoid tumors. J Bronchology Interv Pulmonol. 2014 Jul;21(3):234-6. [CrossRef]
- Gao Y, Moua T, Midthun DE, Mullon JJ, Decker PA, Ryu JH. Diagnostic Yield and Bleeding Complications Associated With Bronchoscopic Biopsy of Endobronchial Carcinoid Tumors. J Bronchology Interv Pulmonol. 2020 Jul;27(3):184-189. [CrossRef]
- Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010 Aug;12(6):427-33. [CrossRef]
- Peri M, Botteri E, Pisa E, De Marinis F, Ungaro A, Spada F, Grana CM, Gasparri R, Spaggiari L, Romentz N, Badalamenti G, Russo A, Fazio N. A single-institution retrospective analysis of metachronous and synchronous metastatic bronchial neuroendocrine tumors. J Thorac Dis. 2018 Jul;10(7):3928-3939. [CrossRef]
- Reuling EMBP, Dickhoff C, Plaisier PW, Coupé VMH, Mazairac AHA, Lely RJ, Bonjer HJ, Daniels JMA. Endobronchial Treatment for Bronchial Carcinoid: Patient Selection and Predictors of Outcome. Respiration. 2018;95(4):220-227. [CrossRef]
- Petrella F, Guarize J, Spaggiari L. The Role of Endobronchial Treatment for Bronchial Carcinoid: Considerations from the Thoracic Surgeon's Point of View. Respiration. 2018;96(2):204. [CrossRef]
- van der Heijden EHFM. Bronchial Carcinoid? Interventional Pulmonologist First! Respiration. 2018;95(4):217-219. [CrossRef]
- Guarino C, Mazzarella G, De Rosa N, Cesaro C, La Cerra G, Grella E, Perrotta F, Curcio C, Guerra G, Bianco A. Pre-surgical bronchoscopic treatment for typical endobronchial carcinoids. Int J Surg. 2016 Sep;33 Suppl 1:S30-5. [CrossRef]
- Cetinkaya E, Aras G, Sökücü SN, Ozgül A, Altin S. Treatment of endoluminal typical carcinoid tumor with bronchoscopic techniques. Tuberk Toraks. 2009;57(4):427-30.
- Bertoletti L, Elleuch R, Kaczmarek D, Jean-François R, Vergnon JM. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest. 2006 Nov;130(5):1405-11. [CrossRef]
- Aronowitz DI, Hyman K. Emerging therapies and current standards in pulmonary carcinoid management. Curr Opin Pulm Med. 2025 Jul 1;31(4):321-325. [CrossRef]
- Vaca R, Shah NA. Finding the Culprit: Cushing Syndrome Secondary to Lung Carcinoid Tumor. AACE Clin Case Rep. 2024 Sep 12;11(1):10-13. [CrossRef]
- Aldrete K, Shahla L. Severe Ectopic Adrenocorticotropic Hormone Syndrome Due to Pulmonary Carcinoid Tumor: A Case Report and Literature Review. AACE Clin Case Rep. 2024 Aug 13;10(6):232-235. [CrossRef]
- Lauricella E, Vilisova S, Chaoul N, Giglio A, D'Angelo G, Porta C, Cives M. The current status of somatostatin analogs in the treatment of neuroendocrine tumors and future perspectives. Expert Rev Neurother. 2025 Feb;25(2):245-258. [CrossRef]
- Elsheikh A, Harbuz-Miller I, Vates E, Nead M, Shafiq I. Complete Tumor Resection and Radical Lymphadenectomy: Potential Cure for Adrenocorticotropic Hormone (ACTH)-Dependent Pulmonary Carcinoid. Cureus. 2024 Nov 11;16(11):e73438. [CrossRef]
- Liu C, Cong Z, Luo J, Wang Q, Qiang Y, Wu H, Shen Y. Implications of surgical intervention in patients with metastatic pulmonary carcinoid tumors: a SEER-based population study and propensity score matching comparative analysis. J Thorac Dis. 2025 Apr 30;17(4):2248-2264. [CrossRef]
- Grozinsky-Glasberg S, Kaltsas G, Kaltsatou M, Lev-Cohain N, Klimov A, Vergadis V, Uri I, Bloom AI, Gross DJ. Hepatic intra-arterial therapies in metastatic neuroendocrine tumors: lessons from clinical practice. Endocrine. 2018 Jun;60(3):499-509. [CrossRef]
- Pelage JP, Fohlen A, Mitry E, Lagrange C, Beauchet A, Rougier P. Chemoembolization of Neuroendocrine Liver Metastases Using Streptozocin and Tris-acryl Microspheres: Embozar (EMBOsphere + ZAnosaR) Study. Cardiovasc Intervent Radiol. 2017 Mar;40(3):394-400. Epub 2016 Dec 29. Erratum in: Cardiovasc Intervent Radiol. 2017 Mar;40(3):480. [CrossRef]
- Hou S, Deng M, Hou Z, Wang Z, Wang H, Fan H. Y-90 Selective Internal Radiation Therapy for Inoperable, Chemotherapy-Resistant Liver Metastases: A Meta-analysis. Acad Radiol. 2025 Mar 26:S1076-6332(25)00099-6. [CrossRef]
- Ward C, Scott S, Wesson W, Mazurek J, Kozlowski I, Werner G, Dehbozorgi A, Phadnis M, Walter C, Rohr A, Collins Z. Dosimetry Assessment in Predicting Treatment Outcomes Following Yttrium-90 Transarterial Radioembolization of Hepatic Tumors. Cancer Biother Radiopharm. 2025 May;40(4):244-253. [CrossRef]
- Ronot M, Loffroy R, Arnold D, Greget M, Sengel C, Pinaquy JB, Pellerin O, Maleux G, Peynircioglu B, Pelage JP, Schaefer N, Sangro B, de Jong N, Zeka B, Urdaniz M, Helmberger T, Vilgrain V. Transarterial Radioembolisation with Y90 Resin Microspheres and the Effect of Reimbursement Criteria in France: Final Results of the CIRT-FR Prospective Observational Study. Cardiovasc Intervent Radiol. 2025 Feb;48(2):205-220. [CrossRef]
- Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, Mäcke HR, Rochlitz C, Müller-Brand J, Walter MA. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011 Jun 10;29(17):2416-23. [CrossRef]
- Sun W, Lipsitz S, Catalano P et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005; 23: 4897–4904.
- Brizzi MP, Berruti A, Ferrero A et al. Continuous 5-fluorouracil infusion plus long acting octreotide in advanced well-differentiated neuroendocrine carcinomas. A phase II trial of the Piemonte oncology network. BMC Cancer 2009; 9: 388.
- Bajetta E, Catena L, Procopio G et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumors? Cancer Chemother Pharmacol 2007; 59: 637–642.
- Pavel M, Grossman A, Arnold R et al. ENETS consensus guidelines for the management of brain, cardiac and ovarian metastases from neuroendocrine tumors. Neuroendocrinology 2010; 91: 326–332.
- Turner NC, Strauss SJ, Sarker D et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer 2010; 102:1106–1112.
- Meyer T, Qian W, Caplin ME et al. Capecitabine and streptozocin ± cisplatin in advanced gastro-entero-pancreatic neuroendocrine tumours. Eur J Cancer 2014;50: 902–911.
- Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005; 8:179–183.
- Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364(6): 514–523.
- Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016 Mar 5;387(10022):968-977. [CrossRef]
- Raymond E, Dahan L, Raoul JL et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364: 501–513.
- Kulke MH, Lenz HJ, Meropol NJ et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008; 26: 3403–3410.
- Grande E, Castellano D, García-Carbonero R et al. PAZONET: a phase II trial of pazopanib as a sequencing treatment in progressive metastatic neuroendocrine tumors (NETs) patients ( pts), on behalf of the Spanish Task Force for NETs (GETNE). In Paper Presented at ESMO, Vienna, 2012. Abstract 11570.
- Yao JC, Phan A, Hoff PM et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 2008; 26: 1316–1323.
- Rickman OB, Vohra PK, Sanyal B, Vrana JA, Aubry MC, Wigle DA, Thomas CF Jr. Analysis of ErbB receptors in pulmonary carcinoid tumors. Clin Cancer Res. 2009 May 15;15(10):3315-24. [CrossRef]
- Karpathakis A, Caplin M, Thirlwell C. Hitting the target: where do molecularly targeted therapies fit in the treatment scheduling of neuroendocrine tumours? Endocr Relat Cancer 2012; 19: R73–R92.
- Petrella F, Bardoni C, Casiraghi M, Spaggiari L. The Role of Surgery in High-Grade Neuroendocrine Cancer: Indications for Clinical Practice. Front Med (Lausanne). 2022 Mar 25;9:869320. [CrossRef]
- Network NCC. Small Cell Lung Cancer - NCCN Guidelines, 2020 (2020). Available online at: https://www.nccn.org/professionals/physician_gls/ (accessed on 9 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




