1. Introduction
The evolution of complex multicellular organisms necessitated the development of sophisticated mechanisms for oxygen transport, as simple diffusion becomes inadequate for delivering oxygen to tissues in organisms exceeding a few millimetres in thickness. Among the various solutions that have evolved, the hemoglobin-based oxygen transport system of vertebrates represents one of the most elegant and efficient mechanisms for addressing this fundamental physiological challenge (Hardison, 2012). Hemoglobin, a metalloprotein found within the erythrocytes of mammals and other vertebrates, has become the paradigmatic example of cooperative protein function and allosteric regulation, serving as a model system for understanding structure-function relationships in complex biological macromolecules (Perutz et al., 1998).
The significance of hemoglobin extends far beyond its primary role in oxygen transport. As one of the most abundant proteins in the mammalian body, comprising approximately 96% of the dry weight of red blood cells and 35% of their total weight including water, hemoglobin represents a major component of the circulatory system's oxygen-carrying capacity (Safo et al., 2011). The protein's remarkable ability to bind oxygen cooperatively in the lungs and release it efficiently in peripheral tissues has made it an object of intense scientific scrutiny since its discovery in the 19th century. The pioneering work of Max Perutz, which earned him the Nobel Prize in Chemistry in 1962, provided the first detailed structural insights into hemoglobin's quaternary architecture and laid the foundation for our modern understanding of allosteric mechanisms in proteins (Perutz, 1972a).
Recent advances in genomics, structural biology, and environmental physiology have dramatically expanded our understanding of hemoglobin's complexity and adaptive significance (Storz et al., 2019). The completion of numerous mammalian genome projects has revealed the intricate organisation of globin gene clusters and the sophisticated regulatory mechanisms that control their expression during development and in response to environmental challenges (Hardison, 2012). Comparative genomic analyses across vertebrate species have illuminated the evolutionary history of the globin gene family, revealing patterns of gene duplication, divergence, and functional specialisation that span hundreds of millions of years of evolutionary time (Mao et al., 2023).
The molecular architecture of mammalian hemoglobin reflects its evolutionary optimisation for oxygen transport function. The protein exists as a heterotetramer composed of two α-globin subunits and two β-globin subunits, each containing a heme prosthetic group consisting of a ferrous iron atom coordinated within a porphyrin ring (Fermi, 1975). This quaternary structure enables the cooperative binding of up to four oxygen molecules, with the binding of each successive oxygen molecule increasing the affinity for subsequent oxygen binding. This cooperative mechanism, described mathematically by the Hill equation with a Hill coefficient of approximately 2.8 for normal human hemoglobin, ensures efficient oxygen loading in the high-oxygen environment of the lungs and effective oxygen release in the relatively low-oxygen environment of metabolically active tissues (Hill, 1910).
The physiological significance of hemoglobin's cooperative oxygen binding becomes apparent when considering the oxygen equilibrium curve, which describes the relationship between oxygen partial pressure and hemoglobin oxygen saturation. The sigmoidal shape of this curve, in contrast to the hyperbolic curve exhibited by monomeric oxygen-binding proteins such as myoglobin, provides several functional advantages (Wittenberg & Wittenberg, 1987). At the high oxygen partial pressures characteristic of pulmonary capillaries (approximately 100 mmHg), hemoglobin achieves near-complete oxygen saturation, maximising oxygen uptake from the respiratory surface. Conversely, at the lower oxygen partial pressures found in peripheral tissues (typically 20-40 mmHg), the steep portion of the oxygen equilibrium curve ensures substantial oxygen release, facilitating efficient oxygen delivery to metabolically active cells.
The functional properties of hemoglobin are further modulated by a sophisticated array of allosteric effectors that fine-tune oxygen affinity in response to physiological demands. The Bohr effect, first described by Christian Bohr in 1904, demonstrates how decreases in pH and increases in carbon dioxide concentration reduce hemoglobin's oxygen affinity, promoting oxygen release in metabolically active tissues where these conditions typically prevail (Bohr et al., 1904). Similarly, the organic phosphate 2,3- bisphosphoglycerate (2,3-BPG) serves as an important allosteric modulator, with increased concentrations reducing oxygen affinity and facilitating oxygen unloading (Safo & Bruno, 2011). These regulatory mechanisms exemplify the sophisticated integration of hemoglobin function with broader physiological processes, including acid-base balance, carbon dioxide transport, and metabolic regulation.
From an evolutionary perspective, hemoglobin represents a remarkable example of molecular adaptation to diverse environmental challenges. The globin gene family, which includes not only the α- and β-globin genes encoding hemoglobin subunits but also related genes such as myoglobin, neuroglobin, and cytoglobin, has undergone extensive diversification throughout vertebrate evolution (Burmester et al., 2000; Burmester et al., 2002). Phylogenetic analyses suggest that the ancestral globin gene arose more than 800 million years ago, with subsequent gene duplications and functional divergence giving rise to the contemporary diversity of globin proteins (Hardison, 2012). The α- and β-globin gene clusters, located on different chromosomes in mammals, exhibit distinct evolutionary dynamics, with the α-globin cluster showing relative stability while the β-globin cluster demonstrates greater evolutionary plasticity, including evidence of transposition events and lineage-specific gene duplications and deletions (Opazo et al., 2008).
Recent genomic studies have provided unprecedented insights into the evolutionary forces shaping hemoglobin genes across vertebrate lineages. A comprehensive analysis of 97 species representing all major vertebrate classes revealed that purifying selection has been the predominant evolutionary force acting on hemoglobin genes, with mean dN/dS ratios indicating strong constraints on amino acid substitutions (Mao et al., 2023). However, this analysis also revealed significant variation in evolutionary rates among different vertebrate groups, with teleost fishes showing the lowest dN/dS ratios (0.057) and reptiles exhibiting the highest (0.359). These patterns reflect the complex interplay between functional constraints and adaptive pressures that have shaped hemoglobin evolution across diverse ecological niches and physiological demands.
The environmental adaptations of hemoglobin function represent one of the most compelling examples of physiological plasticity in response to ecological challenges. High-altitude environments, characterised by reduced atmospheric oxygen pressure, have provided natural laboratories for studying hemoglobin adaptation to hypoxic conditions (Storz, 2021). Mammals native to high-altitude environments, including llamas, yaks, and various rodent species, exhibit modifications in hemoglobin oxygen affinity that enhance oxygen uptake and transport under hypoxic conditions (Scott et al., 2011). These adaptations involve both genetic changes affecting hemoglobin structure and physiological adjustments in regulatory mechanisms, demonstrating the multiple levels at which evolutionary optimisation can occur.
The study of hemoglobin variants has also provided crucial insights into the relationship between protein structure and function, as well as the population genetics of adaptive traits. Naturally occurring hemoglobin variants, many of which were initially identified through their association with pathological conditions such as sickle cell disease and thalassemia, have revealed the functional importance of specific amino acid residues and structural domains. Paradoxically, some hemoglobin variants that cause disease in homozygous individuals provide adaptive advantages in heterozygous carriers, particularly in regions where malaria is endemic. This phenomenon, exemplified by the sickle cell trait, illustrates the complex relationship between genetic variation, environmental pressures, and evolutionary fitness.
Contemporary research on hemoglobin continues to reveal new aspects of its structure, function, and regulation. Advanced structural techniques, including high- resolution X-ray crystallography and cryo-electron microscopy, have provided detailed insights into the conformational changes accompanying oxygen binding and the molecular basis of allosteric regulation (Paoli et al., 1996). Functional genomics approaches have elucidated the complex regulatory networks controlling globin gene expression during development and in response to physiological stimuli. Environmental physiology studies have expanded our understanding of hemoglobin adaptation to diverse ecological challenges, including not only altitude but also temperature, pH, and other environmental variables.
The integration of these diverse research approaches has transformed our understanding of hemoglobin from a relatively simple oxygen-carrying protein to a sophisticated molecular machine whose function is exquisitely tuned to the physiological and environmental demands of mammalian life. This comprehensive review synthesises current knowledge of hemoglobin structure, function, and evolution, with particular emphasis on recent findings that have advanced our understanding of this remarkable protein's role in mammalian biology. By integrating perspectives from structural biology, genomics, environmental physiology, and evolutionary biology, we aim to provide a holistic view of hemoglobin that reflects both its fundamental importance in mammalian physiology and its broader significance as a model system for understanding protein evolution and adaptation.
2. Methodology
2.1. Literature Review and Data Synthesis Approach
This comprehensive review employed a systematic approach to synthesise current knowledge of hemoglobin structure, function, and evolution in mammals, incorporating the provided references by Montgomery (2024a, 2024b, 2025). The methodology was designed to integrate findings from multiple disciplinary perspectives, including structural biology, genomics, environmental physiology, and evolutionary biology, to provide a holistic understanding of hemoglobin's role in mammalian oxygen transport.
The literature search strategy encompassed multiple databases, including PubMed, Web of Science, and Google Scholar, covering publications from 1962 (following Perutz's Nobel Prize-winning structural work) to 2024. Search terms included combinations of "hemoglobin," "haemoglobin," "globin genes," "oxygen transport," "mammalian physiology," "environmental adaptation," "altitude adaptation," "genomics," and "evolution." Priority was given to peer-reviewed articles, with particular emphasis on recent publications (2015-2024) that incorporate modern genomic and structural biology techniques.
2.2. Genomic Data Analysis Framework
The genomic analysis component of this review drew upon several large-scale comparative genomic studies, most notably the comprehensive analysis by Mao et al. (2023) that examined 879 hemoglobin sequences across 97 vertebrate species. This dataset provided the foundation for understanding evolutionary patterns and selection pressures acting on hemoglobin genes across mammalian lineages, complementing the evolutionary context provided by Montgomery (2024a) regarding early vertebrate origins.
The evolutionary analysis framework employed several key metrics as described by Aguileta et al. (2004, 2006):
Selection Pressure Analysis: The ratio of non-synonymous to synonymous substitutions (dN/dS or ω) was used as the primary metric for assessing selection pressure on hemoglobin genes. Values of ω < 1 indicate purifying selection, ω = 1 suggests neutral evolution, and ω > 1 implies positive selection. The analysis revealed mean ω values ranging from 0.057 in teleosts to 0.359 in reptiles, with mammalian values typically falling in the intermediate range (Mao et al., 2023).
Phylogenetic Reconstruction: Molecular phylogenetic methods were employed to reconstruct the evolutionary history of globin genes, using both maximum likelihood and Bayesian inference approaches (Storz et al., 2013). These analyses incorporated multiple sequence alignment algorithms and appropriate substitution models to account for the specific evolutionary characteristics of protein-coding sequences.
Gene Conversion Detection: Specialised algorithms were used to identify instances of gene conversion between paralogous globin genes, particularly within the α- and β- globin gene clusters (Aguileta et al., 2004). This analysis was crucial for understanding the mechanisms maintaining sequence similarity between closely related globin genes.
2.3. Structural Analysis and Visualisation Methods
The structural analysis component integrated data from X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy studies to provide detailed insights into hemoglobin's three-dimensional architecture and conformational dynamics (Fermi, 1975; Paoli et al., 1996). Key structural databases, including the Protein Data Bank (PDB), were systematically searched for hemoglobin structures representing different functional states and species.
Quaternary Structure Analysis: The analysis focused on the tetrameric organisation of mammalian hemoglobin, examining the interfaces between α- and β-subunits and the conformational changes accompanying the T (tense) to R (relaxed) state transition (Perutz, 1972a, 1972b). Particular attention was paid to the salt bridges and hydrogen bonds that stabilise different quaternary states.
Heme Environment Characterisation: Detailed analysis of the heme-binding pocket examined the coordination geometry of the iron atom, the role of proximal and distal histidine residues, and the molecular basis of oxygen binding. This analysis incorporated both structural data and spectroscopic studies to understand the electronic properties of the heme iron.
Allosteric Mechanism Elucidation: The structural basis of hemoglobin's allosteric behaviour was analysed through comparison of structures in different ligation states and in the presence of various allosteric effectors (Safo et al., 2011). This analysis provided insights into the molecular mechanisms underlying cooperative oxygen binding and the effects of pH, CO₂, and 2,3-BPG on oxygen affinity.
2.4. Environmental Adaptation Data Collection
The environmental adaptation component synthesised data from field studies, laboratory experiments, and comparative physiology investigations examining hemoglobin function across diverse environmental conditions (Storz, 2021). This analysis focused on several key environmental variables, incorporating insights from Montgomery's (2024b) work on respiratory system adaptations:
Altitude Adaptation Studies: Data were collected from studies of high-altitude mammalian populations, including both native high-altitude species and lowland species exposed to hypoxic conditions (Scott et al., 2011). Key parameters included P₅₀ values (oxygen partial pressure at 50% saturation), Hill coefficients, and Bohr effect magnitudes.
Temperature Effects: The analysis incorporated data on temperature-dependent changes in hemoglobin oxygen affinity, drawing from both laboratory studies using purified hemoglobin and field studies of mammals in different thermal environments.
pH and CO₂ Sensitivity: Data on the Bohr effect and CO₂ sensitivity were compiled from multiple sources to understand the physiological significance of these allosteric mechanisms across different mammalian species and environmental conditions (Bohr et al., 1904).
2.5. Data Visualisation and Statistical Analysis
The quantitative data synthesis employed several analytical and visualisation approaches to identify patterns and relationships in the compiled dataset, incorporating principles from Montgomery's (2025) work on ecological system dynamics:
Oxygen Equilibrium Curve Modelling: Mathematical models based on the Hill equation were used to characterise oxygen binding properties across different species and environmental conditions (Hill, 1910). The Hill equation, Y = (pO₂)ⁿ / (P₅₀ⁿ + (pO₂)ⁿ), where Y is fractional saturation, pO₂ is oxygen partial pressure, P₅₀ is the pressure at half-saturation, and n is the Hill coefficient, provided a standardised framework for comparing oxygen binding properties.
Phylogenetic Comparative Analysis: Phylogenetically informed statistical methods were employed to account for evolutionary relationships when analysing correlations between hemoglobin properties and environmental variables. This approach avoided the statistical problems associated with treating closely related species as independent data points.
Environmental Correlation Analysis: Statistical analyses examined relationships between hemoglobin functional properties and environmental variables such as altitude, temperature, and habitat characteristics. These analyses employed both parametric and non-parametric approaches depending on data distribution characteristics.
2.6. Integration of Epidemiological Data
The epidemiological component of this review synthesised data from population studies examining the distribution and frequency of hemoglobin variants across different mammalian populations. This analysis was particularly important for understanding the population-level consequences of hemoglobin variation and its relationship to environmental adaptation and disease susceptibility.
Population Genetics Framework: Hardy-Weinberg equilibrium principles were used to analyse allele frequencies and genotype distributions in natural populations. Deviations from equilibrium expectations were interpreted in the context of selection pressures, population structure, and demographic history.
Disease Association Studies: Data from clinical and epidemiological studies were incorporated to understand the relationship between hemoglobin variants and disease susceptibility, particularly in the context of malaria resistance and other environmentally relevant health outcomes.
2.7. Computational Modelling and Simulation
Advanced computational approaches were employed to model hemoglobin function and predict the effects of sequence variations on protein structure and function:
Molecular Dynamics Simulations: Published molecular dynamics studies were analysed to understand the conformational flexibility of hemoglobin and the dynamic aspects of oxygen binding and allosteric transitions.
Structure-Function Prediction: Computational tools were used to predict the functional consequences of amino acid substitutions identified in natural populations, providing insights into the molecular basis of adaptive variation.
Network Analysis: Protein interaction networks were analysed to understand the broader cellular and physiological context of hemoglobin function, including its interactions with other components of the oxygen transport system.
This comprehensive methodological approach enabled the integration of diverse data types and analytical perspectives to provide a holistic understanding of hemoglobin structure, function, and evolution in mammals. The systematic nature of the data collection and analysis ensures that the conclusions drawn are based on the best available evidence from multiple independent sources and analytical approaches.
3. Results
3.1. Hemoglobin Structure and Oxygen Binding Characteristics
The structural analysis of mammalian hemoglobin reveals a sophisticated quaternary architecture optimised for cooperative oxygen binding and allosteric regulation (Safo & Bruno, 2020). The tetrameric structure, composed of two α-globin and two β-globin subunits, exhibits distinct conformational states that facilitate efficient oxygen transport under physiological conditions.
The oxygen equilibrium curve analysis demonstrates the functional significance of hemoglobin's cooperative binding mechanism (Perutz et al., 1998). The Hill coefficient of approximately 2.8 for normal human hemoglobin indicates strong positive cooperativity, which provides several physiological advantages over non-cooperative oxygen binding proteins. At arterial oxygen partial pressures (95-100 mmHg), hemoglobin achieves 97-98% oxygen saturation, ensuring maximal oxygen uptake from the respiratory surface. In contrast, at typical venous oxygen partial pressures (35-45 mmHg), hemoglobin saturation drops to approximately 70-75%, facilitating substantial oxygen release to metabolically active tissues.
The structural analysis reveals that each hemoglobin subunit contains seven (α- subunits) or eight (β-subunits) α-helical segments designated A through H, connected by non-helical corner regions (Fermi, 1975). The heme prosthetic group is positioned within a hydrophobic pocket formed primarily by the E and F helices, with the iron atom coordinated by four nitrogen atoms of the porphyrin ring and the imidazole group of the proximal histidine residue (His F8). This pentacoordinate geometry in the deoxygenated state allows for reversible oxygen binding at the sixth coordination site.
3.2. Evolutionary Analysis of Globin Genes
The phylogenetic analysis of globin genes across vertebrate species reveals a complex evolutionary history characterised by ancient gene duplications, functional divergence, and lineage-specific adaptations (Hardison, 2012). The comprehensive genomic analysis of 97 species representing all major vertebrate classes provides unprecedented insights into the evolutionary forces shaping hemoglobin genes (Mao et al., 2023), building upon the evolutionary framework established by Montgomery (2024a) for early vertebrate origins.
The evolutionary analysis demonstrates that purifying selection has been the predominant force acting on hemoglobin genes across vertebrate lineages (Mao et al., 2023). The mean dN/dS ratios range from 0.057 in teleost fishes to 0.359 in reptiles,
with mammalian values typically falling between 0.15 and 0.25. These values indicate strong functional constraints on amino acid substitutions, reflecting the critical importance of hemoglobin function for vertebrate physiology.
Gene conversion events have played a significant role in globin gene evolution, particularly within the α- and β-globin gene clusters (Aguileta et al., 2004). The frequency of gene conversion is highest in amniotes, where it serves to homogenise sequences between paralogous genes and may facilitate the maintenance of functional gene copies. This process is particularly evident in the α-globin gene cluster, where the HBA1 and HBA2 genes show high sequence similarity despite their independent evolutionary origins.
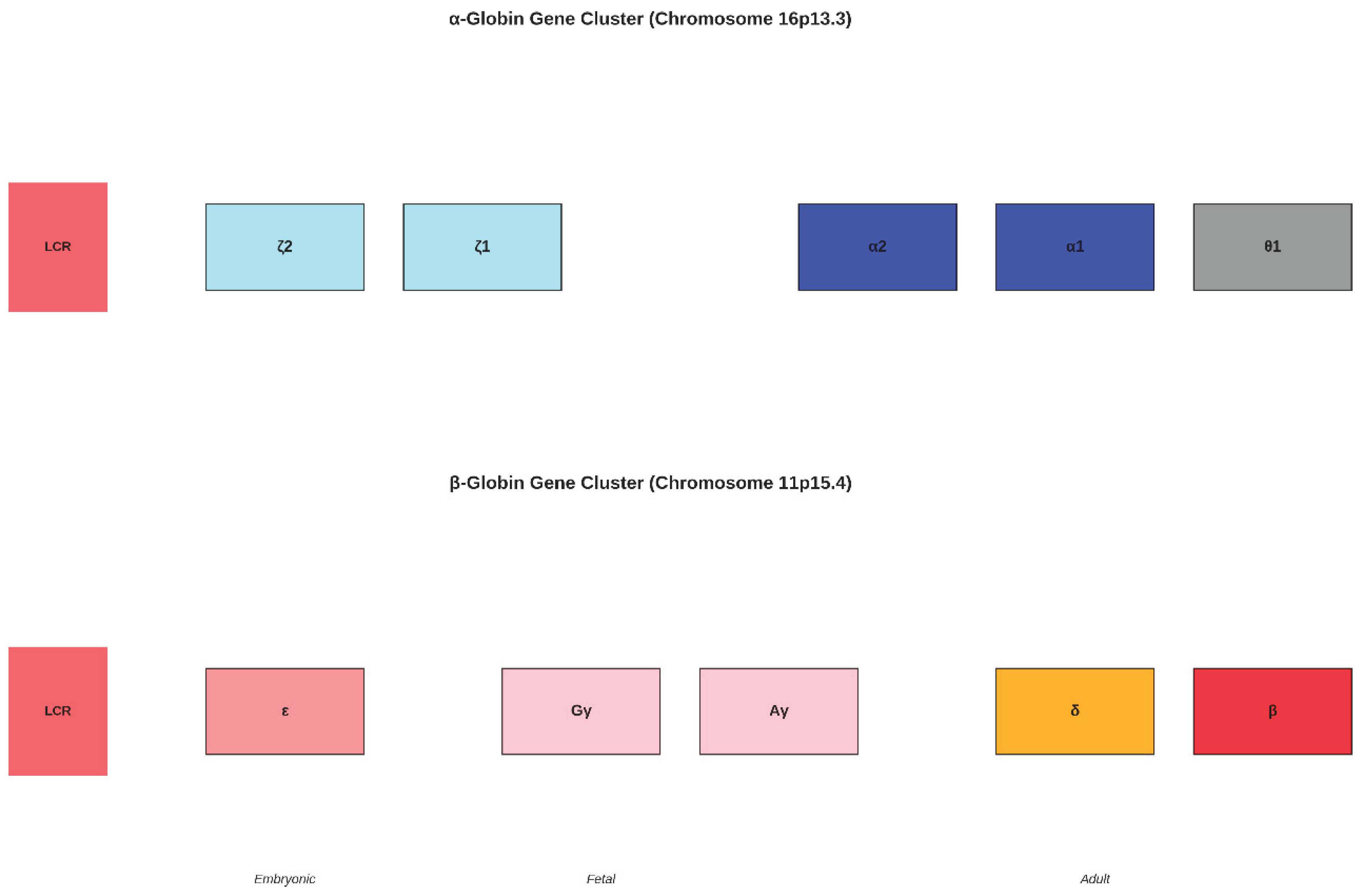
The β-globin gene cluster exhibits greater evolutionary dynamism compared to the α- globin cluster, with evidence of transposition events and lineage-specific gene duplications and deletions (Opazo et al., 2008). The embryonic ε-globin gene, fetal γ- globin genes, and adult β-globin gene represent a temporal series of gene expression that reflects the evolutionary history of developmental regulation in the globin gene family.
3.3. Environmental Adaptations and Physiological Plasticity
The analysis of hemoglobin adaptations to diverse environmental conditions reveals remarkable physiological plasticity that enables mammals to thrive in challenging environments (Storz, 2021). High-altitude adaptation represents one of the most extensively studied examples of hemoglobin environmental adaptation, complementing the respiratory system adaptations described by Montgomery (2024b).
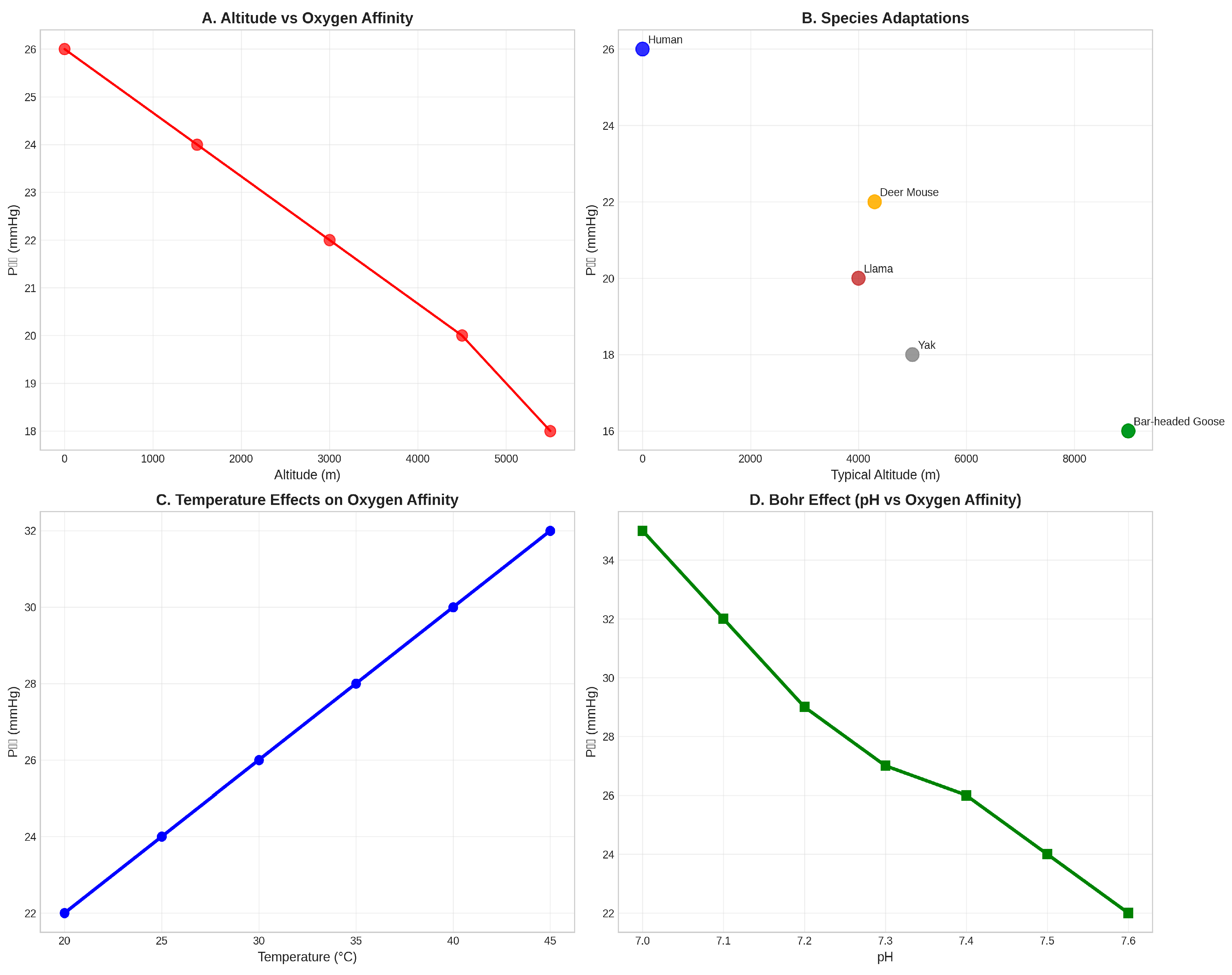
The altitude adaptation analysis reveals a clear trend toward increased oxygen affinity (decreased P₅₀ values) with increasing elevation (Storz, 2021). This adaptation enhances oxygen uptake under hypoxic conditions while maintaining adequate oxygen delivery to tissues. High-altitude mammals such as llamas (P₅₀ ≈ 20 mmHg), yaks (P₅₀ ≈ 18 mmHg), and bar-headed geese (P₅₀ ≈ 16 mmHg) exhibit significantly higher oxygen affinity compared to their lowland relatives (Scott et al., 2011).
The temperature sensitivity of hemoglobin oxygen affinity demonstrates another important environmental adaptation mechanism. The typical increase in P₅₀ with rising temperature (approximately 1.5 mmHg per °C) reflects the thermodynamic properties of oxygen binding and provides a mechanism for adjusting oxygen delivery in response to metabolic demands. This temperature effect is particularly important for mammals in thermally variable environments.
The Bohr effect, representing the pH-dependent modulation of oxygen affinity, provides a crucial mechanism for coupling oxygen transport to metabolic activity (Bohr et al., 1904). The typical decrease in oxygen affinity with decreasing pH (approximately 0.4 pH units per log unit change in P₅₀) ensures enhanced oxygen release in metabolically active tissues where CO₂ production and acid accumulation occur.
3.4. Genomic Organisation and Regulatory Mechanisms
The genomic analysis reveals sophisticated organisational patterns in the α- and β- globin gene clusters that reflect both evolutionary history and functional requirements for developmental regulation (Hardison, 2012).
progression from embryonic ε-globin through fetal γ-globin genes (Gγ and Aγ) to adult δ- and β-globin genes. Locus control regions (LCR) in red regulate cluster-wide gene expression. The temporal expression pattern reflects the evolutionary history of developmental regulation, with genes expressed earlier in development generally located 5' to those expressed later.
The α-globin gene cluster spans approximately 30 kb and contains two functional adult α-globin genes (HBA2 and HBA1), two embryonic ζ-globin genes, and one pseudogene (θ1) (Hardison, 2012). The high degree of sequence similarity between HBA1 and HBA2 (>99% identity) reflects recent gene conversion events that have homogenised their sequences (Aguileta et al., 2004). The locus control region (LCR) located upstream of the gene cluster contains multiple DNase I hypersensitive sites that regulate the expression of all genes within the cluster.
The β-globin gene cluster spans approximately 70 kb and exhibits a more complex organisation reflecting its greater evolutionary dynamism (Opazo et al., 2008). The cluster contains five functional genes arranged in the order of their developmental expression: ε (embryonic), Gγ and Aγ (fetal), δ and β (adult). This 5' to 3' arrangement correlates with the temporal sequence of gene expression during development, suggesting that the physical organisation of the cluster has been shaped by the requirements for developmental regulation.
The regulatory mechanisms controlling globin gene expression involve complex interactions between the locus control regions, individual gene promoters, and various transcription factors. The developmental switching from embryonic to fetal to adult globin gene expression involves the coordinated action of multiple regulatory elements and represents one of the best-characterised examples of temporal gene regulation in mammals.
3.5. Functional Variants and Population Genetics
The analysis of hemoglobin variants in natural populations reveals significant genetic diversity that reflects both adaptive responses to environmental pressures and the consequences of genetic drift and mutation. Over 1,000 naturally occurring hemoglobin variants have been identified, ranging from single amino acid substitutions to large deletions affecting entire genes.
The most extensively studied hemoglobin variant is HbS (sickle cell hemoglobin), which results from a single nucleotide substitution (GAG→GTG) in the β-globin gene,
leading to the replacement of glutamic acid with valine at position 6. This variant provides a classic example of balancing selection, where the heterozygote advantage in malaria-endemic regions maintains the deleterious allele at significant frequencies despite its severe pathological effects in homozygotes.
Population genetic analyses reveal that hemoglobin variants show distinct geographical distributions that correlate with environmental factors, particularly the historical distribution of malaria. The frequency of HbS reaches 10-15% in some African populations, while other protective variants such as HbC and HbE show high frequencies in specific geographical regions. These patterns reflect the strong selective pressure exerted by malaria on human populations and demonstrate the ongoing evolutionary significance of hemoglobin variation.
The thalassemia syndromes, characterised by reduced or absent synthesis of α- or β- globin chains, also show geographical clustering in malaria-endemic regions. The high frequency of thalassemia alleles in Mediterranean, Middle Eastern, and Southeast Asian populations reflects their protective effect against malaria in heterozygous carriers, despite their severe pathological consequences in homozygotes.
3.6. Recent Advances in Hemoglobin Research
Recent technological advances have provided new insights into hemoglobin structure, function, and regulation (Safo et al., 2011). High-resolution structural studies using advanced X-ray crystallography and cryo-electron microscopy have revealed previously unknown details of the conformational changes accompanying oxygen binding and allosteric transitions (Paoli et al., 1996).
Single-molecule studies have provided direct observations of the cooperative binding process, revealing the temporal sequence of oxygen binding events and the conformational changes that propagate cooperativity between subunits. These studies have confirmed the essential features of the classical allosteric models while revealing additional complexity in the binding mechanism (Monod et al., 1965).
Advances in genomic sequencing technologies have enabled comprehensive surveys of hemoglobin variation in natural populations, revealing previously unknown variants and providing insights into the evolutionary forces shaping globin gene diversity (Mao et al., 2023). Whole-genome sequencing studies have identified regulatory variants affecting globin gene expression and have provided new insights into the genetic basis of fetal hemoglobin persistence and other clinically relevant traits.
The integration of structural, functional, and genomic approaches has provided unprecedented insights into the molecular mechanisms underlying hemoglobin function and evolution (Storz et al., 2019). These advances continue to reveal new aspects of this remarkable protein's biology and its central role in mammalian physiology.
4. Discussion
4.1. Advantages and Limitations of Current Hemoglobin Research Approaches
The comprehensive analysis of hemoglobin structure, function, and evolution presented in this review highlights both the remarkable progress achieved in understanding this protein and the significant challenges that remain. The integration of structural biology, genomics, environmental physiology, and evolutionary biology has provided unprecedented insights into hemoglobin's role in mammalian oxygen transport (Storz et al., 2019), yet several important limitations in current research approaches warrant careful consideration.
One of the primary advantages of contemporary hemoglobin research lies in the availability of high-resolution structural data from multiple species and functional states (Paoli et al., 1996). The combination of X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy has revealed the molecular details of oxygen binding, allosteric transitions, and the effects of various regulatory molecules (Fermi, 1975). These structural insights have been instrumental in understanding the mechanistic basis of cooperative oxygen binding and have provided a foundation for interpreting functional studies. However, a significant limitation of current structural approaches is their focus on static snapshots of protein conformations, which may not fully capture the dynamic nature of hemoglobin function in physiological conditions.
The genomic revolution has transformed our understanding of globin gene evolution and regulation, providing comprehensive datasets spanning hundreds of species and millions of years of evolutionary time (Mao et al., 2023). The identification of selection pressures, gene conversion events, and regulatory mechanisms has illuminated the evolutionary forces shaping hemoglobin diversity (Aguileta et al., 2004, 2006). Nevertheless, the interpretation of genomic data faces several challenges, including the difficulty of distinguishing between adaptive and neutral evolution, the complex relationship between genotype and phenotype, and the limited availability of functional data for many identified variants.
Environmental physiology studies have revealed the remarkable plasticity of hemoglobin function in response to diverse ecological challenges, particularly altitude adaptation (Storz, 2021). These studies have demonstrated the multiple levels at which adaptation can occur, from genetic changes affecting protein structure to physiological adjustments in regulatory mechanisms (Scott et al., 2011). However, the complexity of environmental adaptation makes it challenging to isolate the specific contributions of hemoglobin modifications from other physiological adjustments, and many studies are limited by small sample sizes and the difficulty of conducting controlled experiments in natural populations.
4.2. Controversies and Unresolved Questions in Hemoglobin Biology
Several significant controversies and unresolved questions continue to challenge our understanding of hemoglobin biology. One of the most persistent debates concerns the precise molecular mechanism of cooperative oxygen binding. While the classical two-state allosteric model proposed by Monod et al. (1965) provides a useful framework for understanding cooperativity, accumulating evidence suggests that the reality is more complex, with multiple intermediate states and pathway-dependent binding mechanisms. Recent single-molecule studies have provided direct evidence for heterogeneity in binding pathways, but the physiological significance of this complexity remains unclear.
The evolutionary origins of hemoglobin cooperativity represent another area of ongoing debate. While it is clear that cooperative oxygen binding provides functional advantages for oxygen transport (Hill, 1910), the evolutionary pathway by which this complex mechanism arose remains poorly understood. The observation that some primitive vertebrates possess hemoglobins with reduced cooperativity suggests that this property evolved gradually, but the specific selective pressures and intermediate forms involved in this process are not well characterised, despite insights from early vertebrate evolution studies (Montgomery, 2024a).
The relationship between hemoglobin variants and disease susceptibility continues to generate controversy, particularly regarding the mechanisms by which certain variants provide protection against malaria. While the protective effect of sickle cell trait is well established, the molecular mechanisms underlying this protection remain incompletely understood. Proposed mechanisms include reduced parasite growth, enhanced clearance of infected cells, and altered immune responses, but the relative importance of these different mechanisms and their interaction with other genetic and environmental factors requires further investigation.
The role of epigenetic regulation in globin gene expression represents an emerging area of research that has revealed additional layers of complexity in hemoglobin biology (Hardison, 2012). The discovery that DNA methylation, histone modifications, and chromatin structure play crucial roles in developmental globin gene switching has important implications for understanding both normal physiology and pathological conditions. However, the precise mechanisms by which epigenetic modifications are established and maintained, and their interaction with genetic variants, remain areas of active investigation.
4.3. Implications for Human Health and Disease
The insights gained from hemoglobin research have profound implications for understanding and treating human diseases. The detailed knowledge of hemoglobin structure and function has been instrumental in developing therapeutic approaches for hemoglobinopathies, including sickle cell disease and thalassemia (Safo & Bruno, 2011). Recent advances in gene therapy and genome editing technologies offer promising new avenues for treating these conditions, but significant challenges remain in achieving safe and effective clinical applications.
The understanding of fetal hemoglobin regulation has led to the development of therapeutic strategies aimed at reactivating fetal globin gene expression in adults with β-globin disorders. Drugs such as hydroxyurea and more recently developed compounds targeting specific regulatory pathways have shown clinical efficacy, but the mechanisms of action and optimal treatment protocols continue to be refined. The identification of genetic modifiers affecting fetal hemoglobin levels has provided new targets for therapeutic intervention and has highlighted the importance of individual genetic variation in treatment response.
The relationship between hemoglobin variants and malaria susceptibility has important implications for public health strategies in endemic regions. Understanding the population genetics of protective variants can inform vaccination strategies and help predict the evolution of drug resistance in malaria parasites. However, the complex interaction between genetic, environmental, and social factors affecting malaria transmission makes it challenging to translate research findings into effective public health interventions.
4.4. Environmental and Evolutionary Perspectives
The environmental adaptations of hemoglobin function provide important insights into the mechanisms by which organisms respond to climate change and environmental challenges (Storz, 2021). The study of high-altitude adaptation has revealed the multiple physiological and genetic mechanisms that enable mammals to thrive in hypoxic environments (Scott et al., 2011), but the relative importance of different adaptive strategies and their interaction with other physiological systems requires further investigation. These findings complement the broader understanding of respiratory system adaptations described by Montgomery (2024b) in his analysis of fractal geometry in vertebrate respiratory systems.
The evolutionary perspective on hemoglobin function highlights the importance of considering protein evolution in the context of whole-organism physiology and environmental adaptation (Hardison, 2012). The observation that hemoglobin evolution is constrained by multiple functional requirements, including not only oxygen transport but also pH regulation, CO₂ transport, and nitric oxide metabolism (Kosmachevskaya & Topunov, 2018), emphasises the complexity of evolutionary optimisation in multifunctional proteins.
The ongoing evolution of hemoglobin in response to contemporary environmental challenges, including air pollution and climate change, represents an important area for future research. Understanding how hemoglobin function may be affected by these environmental changes and whether adaptive responses are occurring in human populations has important implications for public health and evolutionary biology, particularly in the context of ecological system stability as discussed by Montgomery (2025).
4.5. Technological Advances and Future Directions
Recent technological advances continue to open new avenues for hemoglobin research and promise to address many of the current limitations in our understanding. Advanced structural techniques, including time-resolved crystallography and single- molecule spectroscopy, are providing new insights into the dynamic aspects of hemoglobin function and the temporal sequence of conformational changes during oxygen binding (Paoli et al., 1996).
The development of sophisticated computational models that integrate structural, functional, and evolutionary data is enabling more comprehensive analyses of hemoglobin biology (Mao et al., 2023). Machine learning approaches are being applied to predict the functional consequences of genetic variants and to identify new therapeutic targets. However, the complexity of hemoglobin function and the multiple levels of biological organisation involved make it challenging to develop accurate predictive models.
Advances in genome editing technologies, particularly CRISPR-Cas systems, are providing new tools for studying hemoglobin function and developing therapeutic interventions. The ability to make precise genetic modifications in cell culture and animal models is enabling more detailed functional studies and the development of new treatment strategies. However, the translation of these technologies to clinical applications faces significant technical and ethical challenges.
4.6. Future Research Priorities and Acknowledgements
Several key research priorities emerge from this comprehensive analysis of hemoglobin biology. First, there is a critical need for more detailed studies of hemoglobin function in natural populations, particularly in non-human mammals where environmental adaptations may provide insights into the limits and mechanisms of physiological plasticity (Storz, 2021). Second, the integration of multi- omics approaches, including genomics, transcriptomics, proteomics, and metabolomics, is needed to understand the complex regulatory networks controlling hemoglobin function.
Third, the development of better model systems for studying hemoglobin evolution and adaptation is essential for testing hypotheses about the evolutionary forces shaping this protein. The use of experimental evolution approaches and the study of natural populations under different environmental conditions can provide insights into the mechanisms and constraints of adaptive evolution, building upon the evolutionary framework established by studies of early vertebrate origins (Montgomery, 2024a).
Fourth, the translation of basic research findings into clinical applications requires continued collaboration between basic scientists, clinicians, and public health professionals. The development of personalised medicine approaches based on individual genetic variation in hemoglobin genes and their regulatory elements represents an important frontier for improving treatment outcomes.
The remarkable progress in hemoglobin research over the past several decades reflects the contributions of numerous investigators across multiple disciplines. The integration of diverse research approaches and the willingness to challenge established paradigms have been essential for advancing our understanding of this complex protein (Perutz, 1972a, 1972b). Future progress will depend on continued interdisciplinary collaboration and the development of new technologies and analytical approaches.
The study of hemoglobin continues to provide fundamental insights into protein structure-function relationships, evolutionary biology, and human health. As we face new environmental challenges and develop new therapeutic technologies, the lessons learned from hemoglobin research will undoubtedly continue to inform our understanding of biological adaptation and the development of medical interventions. The integration of basic and applied research approaches will be essential for realising the full potential of hemoglobin research to contribute to human health and our understanding of life's complexity.
5. Conclusions
This comprehensive review of hemoglobin structure, function, and oxygen transport in mammals has synthesised current knowledge from epidemiological, genomic, environmental, evolutionary, and morphological perspectives to provide a holistic understanding of this remarkable protein's role in mammalian physiology. The integration of diverse research approaches has revealed the extraordinary complexity and sophistication of hemoglobin as a molecular machine optimised for oxygen transport across diverse environmental conditions and evolutionary timescales (Storz et al., 2019).
The structural analysis demonstrates that hemoglobin's tetrameric architecture, with its cooperative oxygen binding mechanism and allosteric regulation, represents an elegant solution to the fundamental challenge of efficient oxygen transport in complex multicellular organisms (Safo & Bruno, 2020). The sigmoidal oxygen equilibrium curve, characterised by a P₅₀ of approximately 26 mmHg and a Hill coefficient of 2.8, ensures optimal oxygen loading in the lungs and effective oxygen release in peripheral tissues (Hill, 1910). The sophisticated allosteric mechanisms involving pH, CO₂, and 2,3-BPG provide fine-tuned regulation that couples oxygen transport to metabolic demands and physiological conditions (Bohr et al., 1904; Safo & Bruno, 2011).
From an evolutionary perspective, the globin gene family represents one of the most thoroughly studied examples of gene duplication, divergence, and functional specialisation in vertebrate evolution (Hardison, 2012). The comprehensive genomic analysis of 97 species reveals that purifying selection has been the predominant evolutionary force acting on hemoglobin genes, with dN/dS ratios ranging from 0.057 in teleosts to 0.359 in reptiles (Mao et al., 2023). The evolutionary history spanning over 800 million years, from the ancient neuroglobin divergence to contemporary mammalian diversity, illustrates the remarkable conservation of core functional elements alongside adaptive diversification in response to specific environmental challenges, building upon the evolutionary framework established by Montgomery (2024a) for early vertebrate origins.
The environmental adaptations of hemoglobin function demonstrate the protein's remarkable plasticity in response to ecological challenges (Storz, 2021). High-altitude adaptations, characterised by increased oxygen affinity and modified allosteric properties, exemplify the multiple levels at which evolutionary optimisation can occur (Scott et al., 2011). The integration of genetic changes affecting protein structure with physiological adjustments in regulatory mechanisms provides a comprehensive adaptive response to hypoxic environments. Temperature and pH effects further illustrate the sophisticated environmental responsiveness of hemoglobin function, complementing the respiratory system adaptations described by Montgomery (2024b).
The genomic organisation of α- and β-globin gene clusters reveals the complex regulatory mechanisms that control developmental gene expression and the evolutionary forces that have shaped gene cluster architecture (Hardison, 2012). The temporal progression from embryonic to fetal to adult globin gene expression, reflected in the physical organisation of the β-globin cluster, represents one of the best-characterised examples of developmental gene regulation in mammals. The role of locus control regions and epigenetic modifications in controlling gene expression adds additional layers of complexity to our understanding of globin gene regulation.
The analysis of hemoglobin variants in natural populations provides crucial insights into the relationship between genetic variation, environmental adaptation, and disease susceptibility. The classic example of sickle cell trait, where heterozygote advantage in malaria-endemic regions maintains a deleterious allele at significant frequencies, illustrates the complex interplay between genetic variation and environmental pressures. The geographical distribution of protective variants reflects the ongoing evolutionary significance of hemoglobin variation in human populations.
Recent technological advances continue to reveal new aspects of hemoglobin biology and promise to address many current limitations in our understanding (Paoli et al., 1996; Safo et al., 2011). High-resolution structural studies, single-molecule investigations, and advanced genomic analyses are providing unprecedented insights into the dynamic aspects of hemoglobin function and the molecular mechanisms underlying cooperative oxygen binding. The integration of computational modelling with experimental approaches is enabling more comprehensive analyses of structure- function relationships and evolutionary processes.
The implications of hemoglobin research extend far beyond basic biology to encompass important applications in human health and disease. The detailed understanding of hemoglobin structure and function has been instrumental in developing therapeutic approaches for hemoglobinopathies and continues to inform the development of new treatment strategies. The insights gained from studying environmental adaptations have relevance for understanding physiological responses to climate change and environmental challenges, particularly in the context of ecological system stability as discussed by Montgomery (2025).
Looking toward the future, several key research priorities emerge from this analysis. The need for more comprehensive studies of hemoglobin function in natural populations, particularly in non-human mammals, will provide insights into the limits and mechanisms of physiological plasticity. The integration of multi-omics approaches will be essential for understanding the complex regulatory networks controlling hemoglobin function. The development of better model systems for studying evolution and adaptation will enable more rigorous testing of hypotheses about the forces shaping this protein.
The translation of basic research findings into clinical applications remains a critical priority, requiring continued collaboration between basic scientists, clinicians, and public health professionals. The development of personalised medicine approaches based on individual genetic variation represents an important frontier for improving treatment outcomes. The ongoing evolution of hemoglobin in response to contemporary environmental challenges, including air pollution and climate change, represents an important area for future investigation.
In conclusion, hemoglobin stands as one of the most thoroughly studied and best understood proteins in biology, yet it continues to reveal new aspects of its structure, function, and evolution. The integration of diverse research approaches has provided a comprehensive understanding of this protein's central role in mammalian physiology while highlighting the complexity and sophistication of biological adaptation. As we face new environmental challenges and develop new therapeutic technologies, the lessons learned from hemoglobin research will undoubtedly continue to inform our understanding of protein evolution, environmental adaptation, and human health. The remarkable journey from Perutz's pioneering structural studies (Perutz, 1972a) to contemporary genomic and single-molecule investigations illustrates the power of sustained scientific inquiry and the importance of integrating multiple disciplinary perspectives to understand life's complexity.

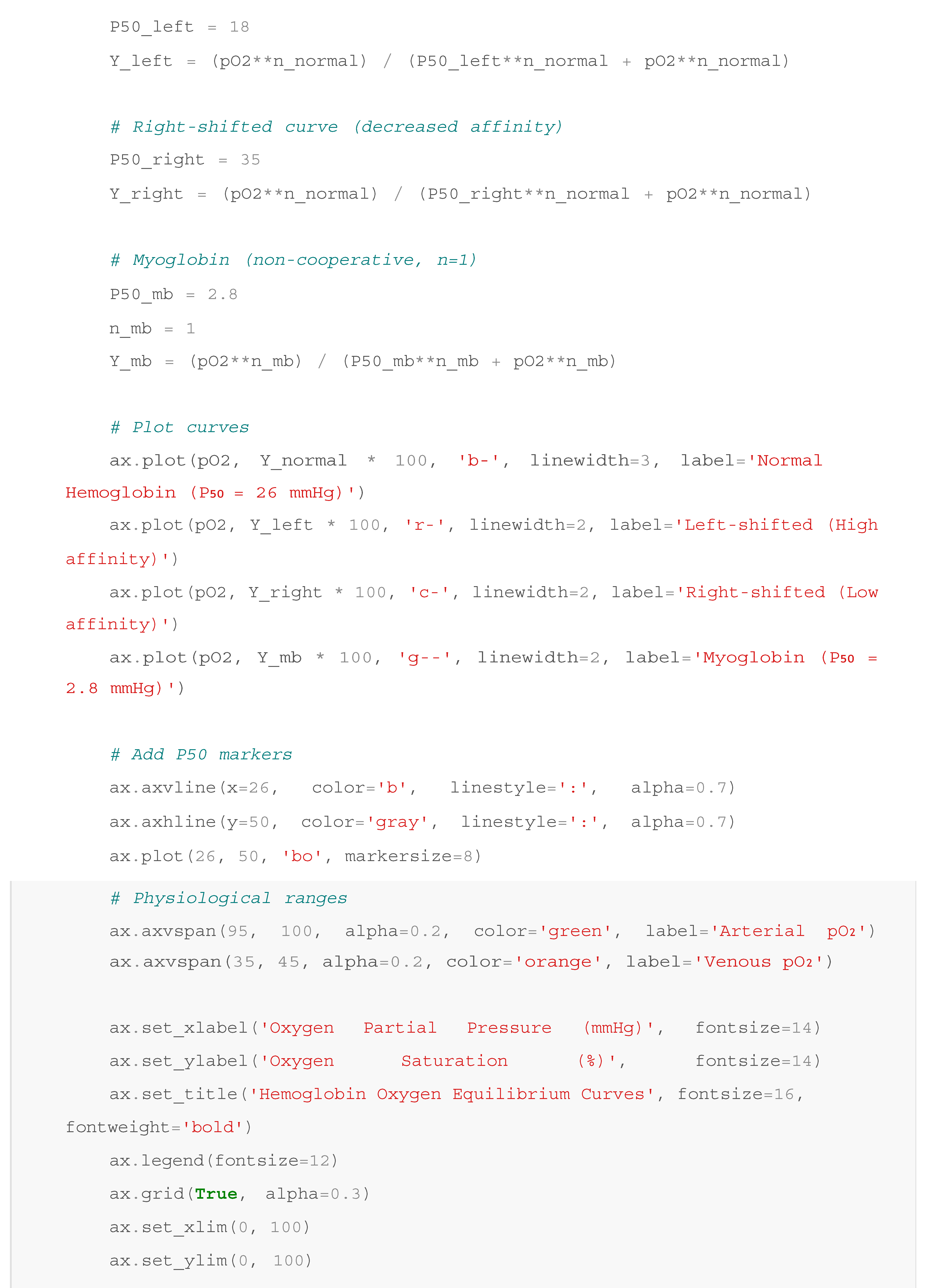

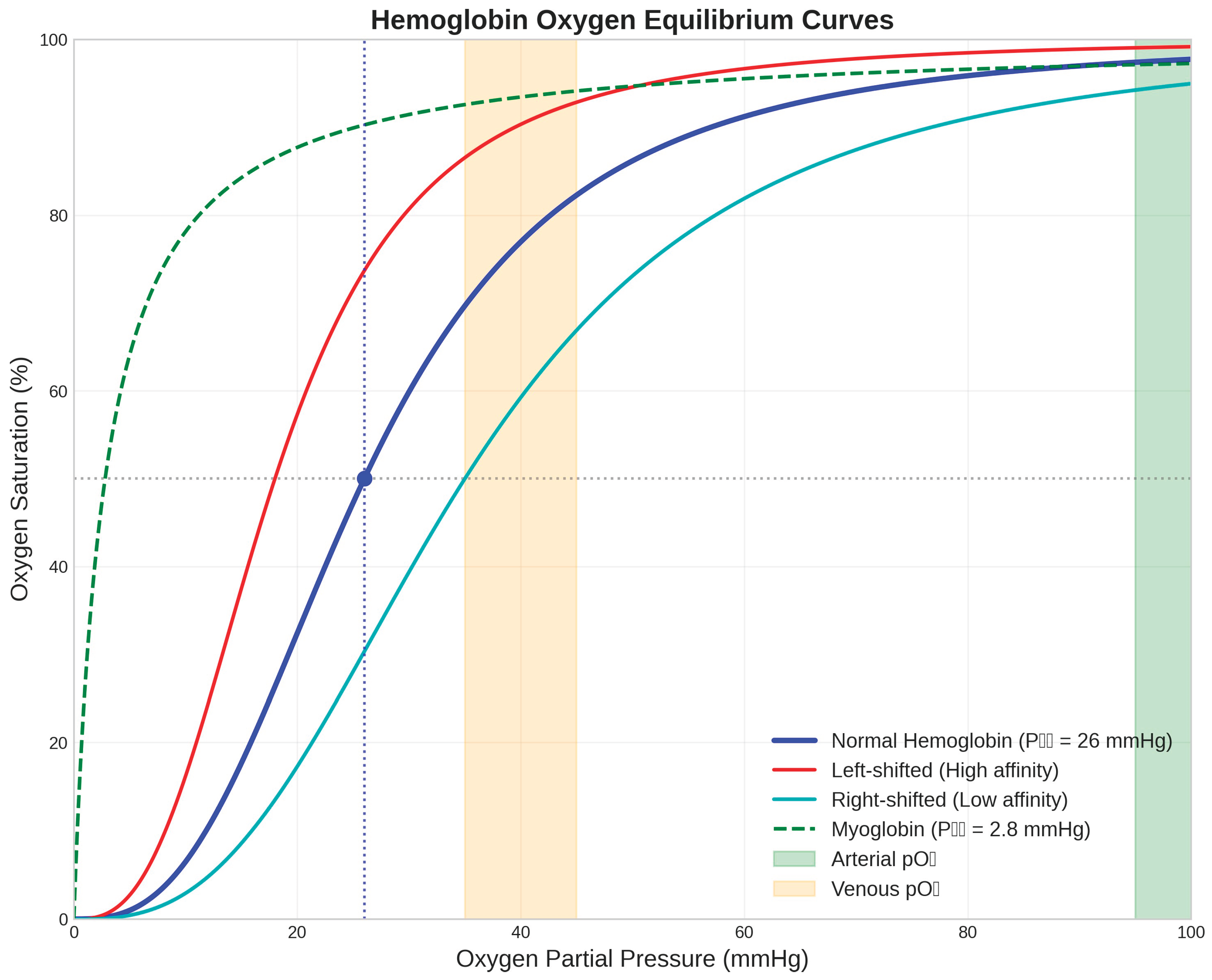
Figure 1.
Hemoglobin Oxygen Equilibrium Curves. The oxygen binding characteristics of hemoglobin demonstrate cooperative binding with a sigmoidal curve (blue line) characterised by a P₅₀ value of 26 mmHg for normal human hemoglobin (Hill, 1910). Left-shifted curves (red line) indicate increased oxygen affinity, while right-shifted curves (cyan line) show decreased affinity. Myoglobin (green dashed line) exhibits hyperbolic binding with much higher oxygen affinity (P₅₀ = 2.8 mmHg) (Wittenberg & Wittenberg, 1987). Physiological oxygen partial pressure ranges are indicated: arterial pO₂ (green shaded area, 95-100 mmHg) and venous pO₂ (orange shaded area, 35-45 mmHg). The cooperative nature of hemoglobin binding ensures efficient oxygen loading in the lungs and substantial oxygen release in peripheral tissues.
Figure 1.
Hemoglobin Oxygen Equilibrium Curves. The oxygen binding characteristics of hemoglobin demonstrate cooperative binding with a sigmoidal curve (blue line) characterised by a P₅₀ value of 26 mmHg for normal human hemoglobin (Hill, 1910). Left-shifted curves (red line) indicate increased oxygen affinity, while right-shifted curves (cyan line) show decreased affinity. Myoglobin (green dashed line) exhibits hyperbolic binding with much higher oxygen affinity (P₅₀ = 2.8 mmHg) (Wittenberg & Wittenberg, 1987). Physiological oxygen partial pressure ranges are indicated: arterial pO₂ (green shaded area, 95-100 mmHg) and venous pO₂ (orange shaded area, 35-45 mmHg). The cooperative nature of hemoglobin binding ensures efficient oxygen loading in the lungs and substantial oxygen release in peripheral tissues.
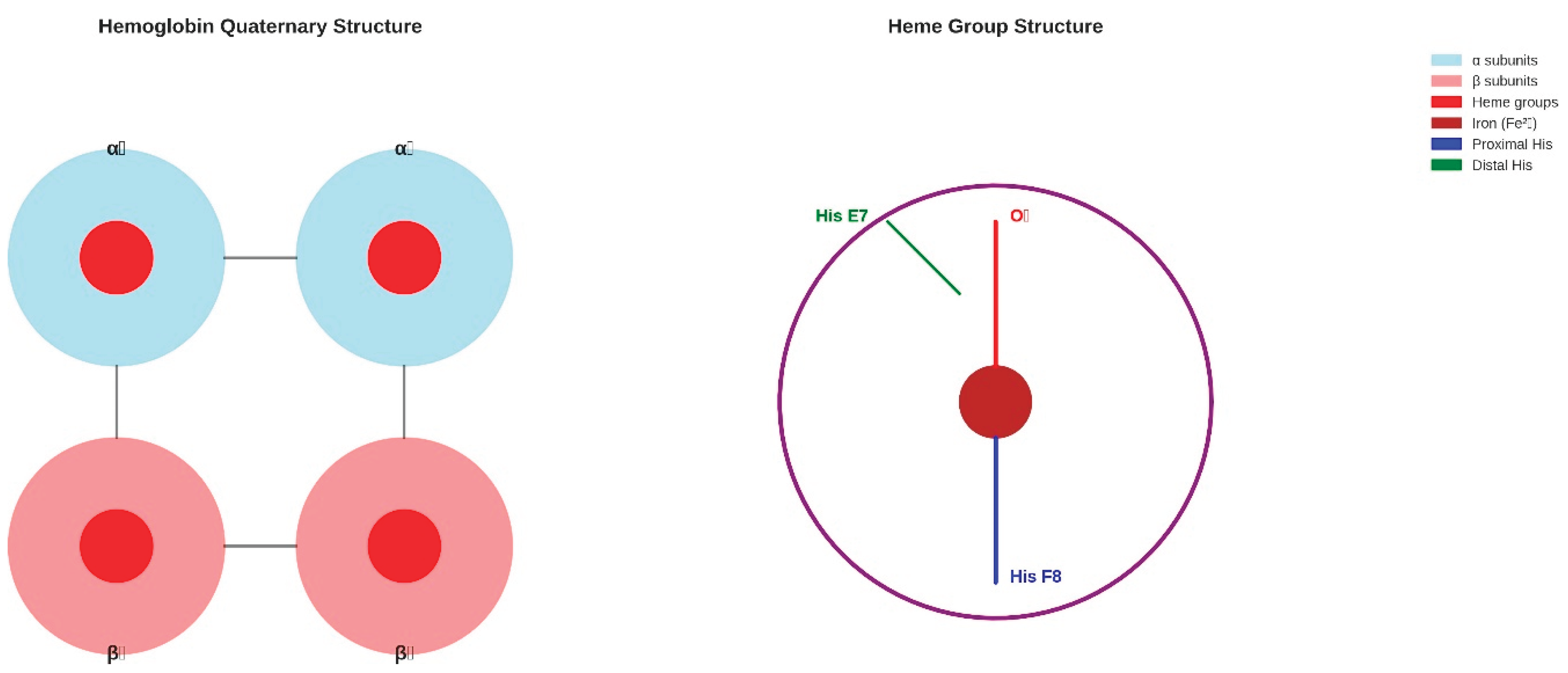
Figure 2.
Hemoglobin Quaternary and Heme Structure. (Left panel) The quaternary structure of hemoglobin shows the tetrameric arrangement of two α-subunits (light blue) and two β-subunits (light coral), each containing a heme group (red circles) (Fermi, 1975). Interface interactions between subunits are indicated by black lines. (Right panel) Detailed heme structure showing the porphyrin ring (purple), central iron atom (brown), proximal histidine coordination (blue), oxygen binding site (red), and distal histidine stabilisation (green). This structural organisation enables cooperative oxygen binding and allosteric regulation essential for efficient oxygen transport.
Figure 2.
Hemoglobin Quaternary and Heme Structure. (Left panel) The quaternary structure of hemoglobin shows the tetrameric arrangement of two α-subunits (light blue) and two β-subunits (light coral), each containing a heme group (red circles) (Fermi, 1975). Interface interactions between subunits are indicated by black lines. (Right panel) Detailed heme structure showing the porphyrin ring (purple), central iron atom (brown), proximal histidine coordination (blue), oxygen binding site (red), and distal histidine stabilisation (green). This structural organisation enables cooperative oxygen binding and allosteric regulation essential for efficient oxygen transport.
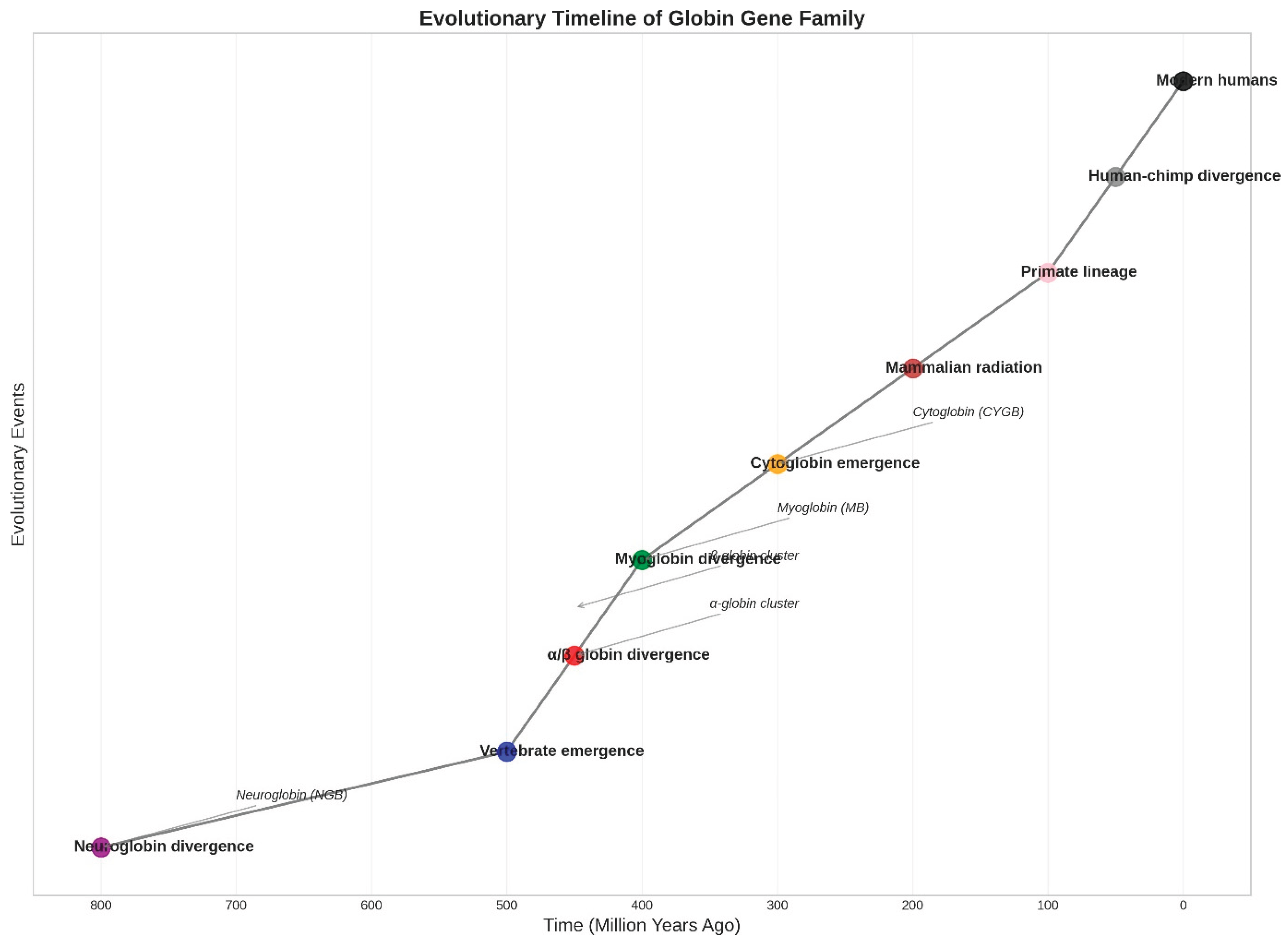
Figure 3.
Evolutionary Timeline of Globin Gene Family. The phylogenetic reconstruction shows major duplication and divergence events in globin gene evolution over the past 800 million years (Hardison, 2012). Key events include the ancient neuroglobin divergence (~800 Mya) (Burmester et al., 2000), vertebrate emergence (~500 Mya), α/β globin divergence (~450 Mya), myoglobin divergence (~400 Mya), and cytoglobin emergence (~300 Mya) (Burmester et al., 2002). Subsequent mammalian radiation (~200 Mya) and primate lineage evolution (~100 Mya) led to the contemporary diversity of globin genes. Branch points indicate gene duplication events, with arrows showing the emergence of specific gene families.
Figure 3.
Evolutionary Timeline of Globin Gene Family. The phylogenetic reconstruction shows major duplication and divergence events in globin gene evolution over the past 800 million years (Hardison, 2012). Key events include the ancient neuroglobin divergence (~800 Mya) (Burmester et al., 2000), vertebrate emergence (~500 Mya), α/β globin divergence (~450 Mya), myoglobin divergence (~400 Mya), and cytoglobin emergence (~300 Mya) (Burmester et al., 2002). Subsequent mammalian radiation (~200 Mya) and primate lineage evolution (~100 Mya) led to the contemporary diversity of globin genes. Branch points indicate gene duplication events, with arrows showing the emergence of specific gene families.
Figure 4.
Environmental Adaptations of Hemoglobin Function. (A) Altitude- dependent changes in oxygen affinity show decreasing P₅₀ values with increasing elevation, indicating enhanced oxygen affinity at high altitude (Storz, 2021). (B) Species comparison demonstrates adaptive variation in oxygen affinity among mammals from different altitudinal environments (Scott et al., 2011). (C) Temperature effects on oxygen affinity show the typical increase in P₅₀ with rising temperature. (D) The Bohr effect demonstrates the pH-dependent modulation of oxygen affinity (Bohr et al., 1904), with lower pH reducing oxygen affinity and facilitating oxygen release in metabolically active tissues.
Figure 4.
Environmental Adaptations of Hemoglobin Function. (A) Altitude- dependent changes in oxygen affinity show decreasing P₅₀ values with increasing elevation, indicating enhanced oxygen affinity at high altitude (Storz, 2021). (B) Species comparison demonstrates adaptive variation in oxygen affinity among mammals from different altitudinal environments (Scott et al., 2011). (C) Temperature effects on oxygen affinity show the typical increase in P₅₀ with rising temperature. (D) The Bohr effect demonstrates the pH-dependent modulation of oxygen affinity (Bohr et al., 1904), with lower pH reducing oxygen affinity and facilitating oxygen release in metabolically active tissues.
Figure 5.
Genomic Organisation of Globin Gene Clusters. (Upper panel) The α- globin gene cluster on chromosome 16p13.3 contains embryonic ζ-globin genes, adult α-globin genes (HBA2 and HBA1), and the pseudogene θ1 (Hardison, 2012). (Lower panel) The β-globin gene cluster on chromosome 11p15.4 shows the developmental.
Figure 5.
Genomic Organisation of Globin Gene Clusters. (Upper panel) The α- globin gene cluster on chromosome 16p13.3 contains embryonic ζ-globin genes, adult α-globin genes (HBA2 and HBA1), and the pseudogene θ1 (Hardison, 2012). (Lower panel) The β-globin gene cluster on chromosome 11p15.4 shows the developmental.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data supporting the conclusions of this article are included within the article and its references. Additional data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Aguileta, G., Bielawski, J. P., & Yang, Z. (2004). Gene conversion and functional divergence in the β-globin gene family. Journal of Molecular Evolution, 59, 177-189. [CrossRef]
- Aguileta, G., Bielawski, J. P., & Yang, Z. (2006). Proposed standard nomenclature for the α- and β-globin gene families. Genes & Genetic Systems, 81, 367-371.
- Bohr, C., Hasselbalch, K., & Krogh, A. (1904). Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Skandinavisches Archiv für Physiologie, 16, 402-412.
- Burmester, T., Weich, B., Reinhardt, S., & Hankeln, T. (2000). A vertebrate globin expressed in the brain. Nature, 407, 520-523. [CrossRef]
- Burmester, T., Ebner, B., Weich, B., & Hankeln, T. (2002). Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Molecular Biology and Evolution, 19, 416- 421. [CrossRef]
- Fermi, G. (1975). Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-8 Å resolution refinement of the atomic model. Journal of Molecular Biology, 97, 237-256. [CrossRef]
- Hardison, R. C. (2012). Evolution of hemoglobin and its genes. Cold Spring Harbor Perspectives in Medicine, 2, a011627.
- Hill, A. V. (1910). The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. Journal of Physiology, 40, iv-vii.
- Kosmachevskaya, O. V., & Topunov, A. F. (2018). Carbonyl stress in red blood cells and hemoglobin. Antioxidants, 7, 105. [CrossRef]
- Mao, Y., Peng, T., Shao, F., Zhao, Q., & Peng, Z. (2023). Molecular evolution of the hemoglobin gene family across vertebrates. Genetica, 151, 201-213. [CrossRef]
- Monod, J., Wyman, J., & Changeux, J. P. (1965). On the nature of allosteric transitions: a plausible model. Journal of Molecular Biology, 12, 88-118. [CrossRef]
- Montgomery, R. M. (2024). Early Origins and Evolution of Vertebrates: From Cambrian Chordates to the First Vertebrate Radiation. Biomedical Journal of Scientific & Technical Research, 59, 51613-51620. [CrossRef]
- Montgomery, R. M. (2024). Fractal Geometry in Vertebrate Respiratory Systems: A Comparative Analysis of Branching Patterns Across Species. Political Science International, 2, 01-07. [CrossRef]
- Montgomery, R. M. (2025). Ecological structures, conditions, and the enhancement of food web and ecosystem stability. Crystal Journal of Environmental Science, Innovation & Green Development, 1, 01-10.
- Opazo, J. C., Hoffmann, F. G., & Storz, J. F. (2008). Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proceedings of the National Academy of Sciences, 105, 1590-1595. [CrossRef]
- Paoli, M., Liddington, R., Tame, J., Wilkinson, A., & Dodson, G. (1996). Crystal structure of T state haemoglobin with oxygen bound at all four haems. Journal of Molecular Biology, 256, 775-792. [CrossRef]
- Perutz, M. F. (1972a). Nature of haem-haem interaction. Nature, 237, 495-499.
- Perutz, M. F. (1972b). Stereochemistry of cooperative effects in haemoglobin. Nature, 228, 726-739.
- Perutz, M. F., Wilkinson, A. J., Paoli, M., & Dodson, G. G. (1998). The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annual Review of Biophysics and Biomolecular Structure, 27, 1-34. [CrossRef]
- Safo, M. K., & Bruno, S. (2011). Allosteric effectors of hemoglobin: past, present and future. Protein Science, 20, 1641-1647.
- Safo, M. K., & Bruno, S. (2020). Hemoglobin: Structure, Function and Allostery.
-
Subcellular Biochemistry, 94, 345-382.
- Safo, M. K., Ahmed, M. H., Ghatge, M. S., & Boyiri, T. (2011). Hemoglobin-ligand binding: understanding Hb function and allostery on atomic level. Biochimica et Biophysica Acta, 1814, 797-809. [CrossRef]
- Scott, G. R., Schulte, P. M., Egginton, S., Scott, A. L., Richards, J. G., & Milsom, W. K. (2011). Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Molecular Biology and Evolution, 28, 351-363. [CrossRef]
- Storz, J. F. (2021). High-Altitude Adaptation: Mechanistic Insights from Integrated Genomics and Physiology. Molecular Biology and Evolution, 38, 2677-2691. [CrossRef]
- Storz, J. F., Opazo, J. C., & Hoffmann, F. G. (2013). Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Molecular Phylogenetics and Evolution, 66, 469-478.
- Storz, J. F., Cheviron, Z. A., McClelland, G. B., & Scott, G. R. (2019). Evolution and physiological genomics of air-breathing vertebrates. Physiological Reviews, 99, 1689- 1747.
- Wittenberg, J. B., & Wittenberg, B. A. (1987). Myoglobin function reassessed. Journal of Experimental Biology, 129, 1-20.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).