1. Introduction
1.1. Background
Fluconazole, a widely used antifungal agent, has long been a cornerstone in the treatment of
Candida infections[
11]. However, the emergence of Fluconazole-resistant strains poses a significant challenge in clinical management, necessitating innovative approaches to combat antifungal resistance[
12]. Fluconazole resistance calls for the need of use of alternative antifungal drugs like Voriconazole, Posaconazole, Ravuconazole, Amphotericin B, and Echinocandins[
13]. However, usage of these agents are limited due to high cost or adverse effects associated with these agents[
14]. Recent research has shown promising results in reversing Fluconazole resistance by combining this antifungal agent with efflux pump inhibitors (EPIs) such as Aspirin or Ibuprofen[
15,
16]. This article explores the role of efflux pumps in mediating Fluconazole resistance, and the potential of EPIs to enhance the efficacy of Fluconazole in combating resistant
Candida isolates.
1.2. Understanding Fluconazole Resistance and Synergistic Effects of Fluconazole and EPIs
Fluconazole belongs to the azole class of antifungal drugs and acts by inhibiting the synthesis of ergosterol, an essential component of the fungal cell membrane[
17]. Resistance to Fluconazole can arise through various mechanisms, including alterations in the target enzyme (lanosterol 14-alpha-demethylase), upregulation of drug efflux pumps, and changes in the fungal cell membrane composition[
18]. Among these, overexpression of efflux pumps, particularly ATP-binding cassette (ABC) transporters such as CDR1p and CDR2p in
Candida species, is a major mechanism of Fluconazole resistance[
19].
Efflux pumps play a crucial role in multidrug resistance by actively pumping antifungal agents out of the fungal cell, thereby reducing intracellular drug concentrations. Efflux pump inhibitors (EPIs) are compounds that inhibit the activity of these pumps, restoring intracellular drug levels and enhancing the susceptibility of resistant strains to antifungal agents. While EPIs have been extensively studied in the context of antibacterial resistance, their potential to reverse antifungal resistance is a burgeoning area of research.
Recent studies have demonstrated the synergistic effects of combining Fluconazole with EPIs such as Aspirin or Ibuprofen in reversing Fluconazole resistance in
Candida species[
20,
21]. By inhibiting efflux pump activity, EPIs potentiate the antifungal activity of Fluconazole, leading to increased intracellular drug concentrations and improved efficacy against resistant strains[
22]. Moreover, the use of EPIs may help overcome cross-resistance to other azole antifungals, thereby expanding the therapeutic options for managing resistant
Candida infections.
In this study, we aimed to determine whether efflux pump inhibitors such as Aspirin and Ibuprofen could reverse Fluconazole resistance by blocking the efflux of the drug and thereby increasing its intracellular accumulation. This may help in instituting new policies to curb the menace of drug resistance thereby offering novel breakthrough.
2. Materials and Methods
This was an interventional study conducted at a tertiary care hospital in eastern India, involving any culture plate showing pure growth of Candida sp. as the study participants. The study duration spanned from October 2021 to June 2023, with blood samples collected and laboratory workup performed from October 2021 to January 2023, followed by data analysis from January 2023 to June 2023. The sampling population comprised Fluconazole-resistant Candida species, and the total sample size was 18 Candida sp. resistant to Fluconazole, calculated using the Formula for Comparing Paired Proportions. The sampling technique employed was consecutive sampling.
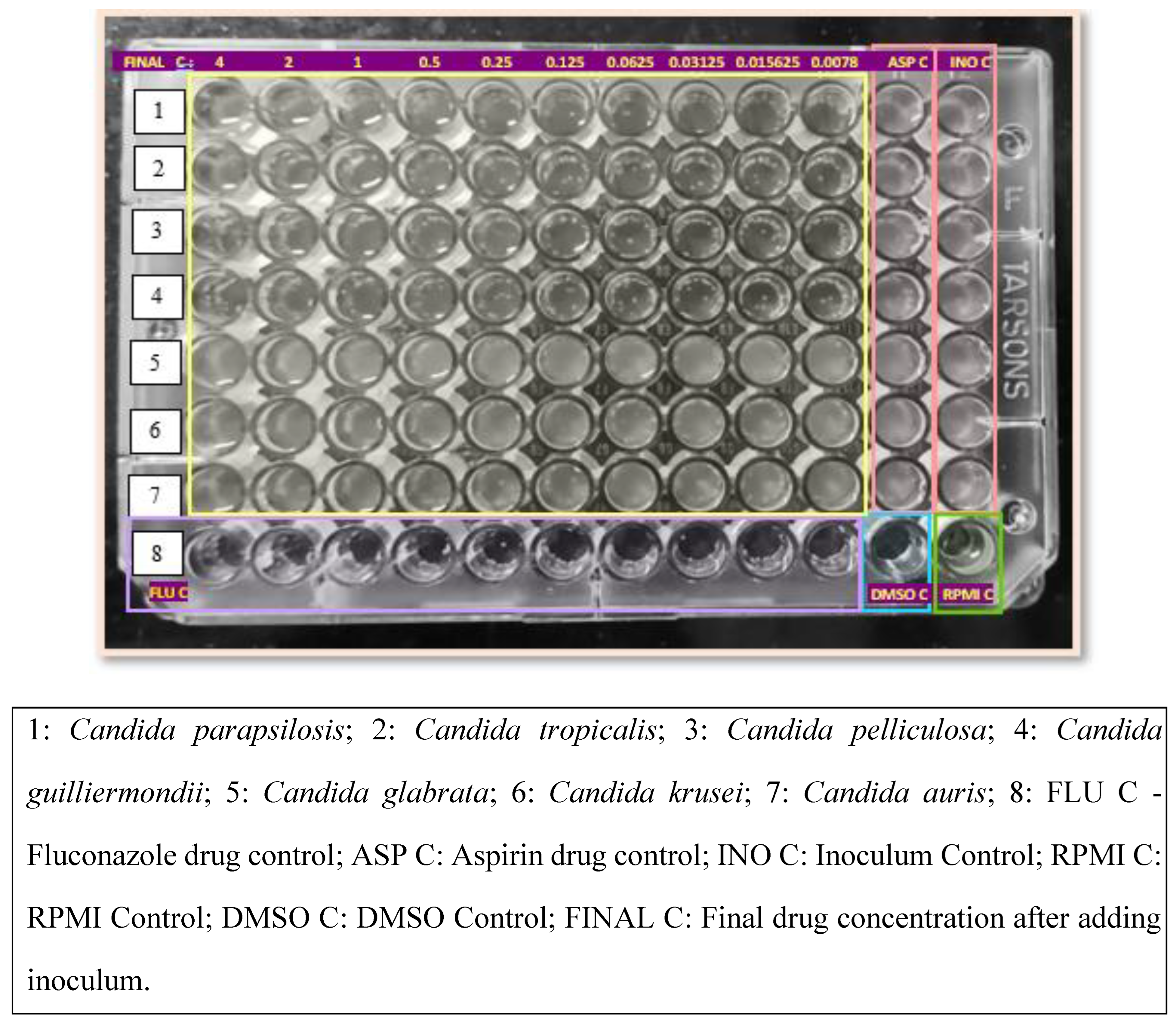
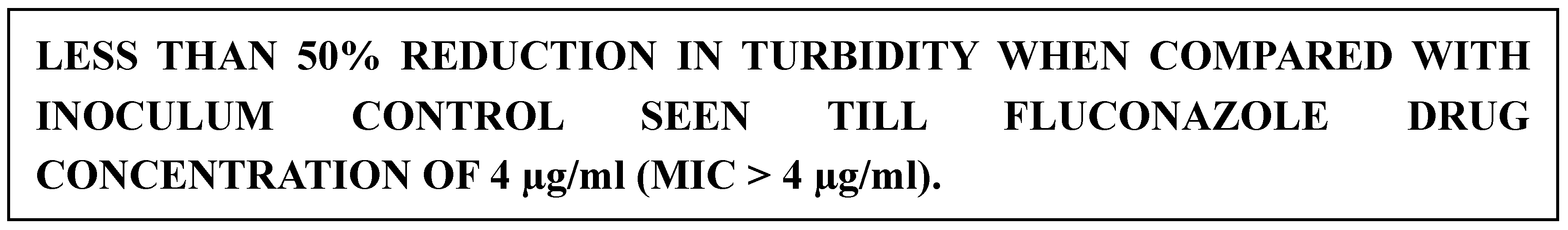
Antifungal Susceptibility Testing of Yeasts by Broth Microdilution was performed for Fluconazole following the M27/A4 protocol of CLSI[
23]. The broth microdilution test was performed using sterile, disposable, multiwell microdilution plates (96 U-shaped wells). Antifungal stock solutions were prepared at concentrations of at least 100 times the highest concentration to be tested using Dimethyl sulfoxides (DMSO) as the solvent. The inoculum was prepared by picking five colonies of 1 mm in diameter from a 24-hour-old culture of Candida species and suspending them in 5 mL of sterile saline. The resulting suspension was vortexed for 15 seconds, and the turbidity was matched to a 0.5 McFarland standard. This procedure yielded a yeast stock suspension of 1 x 10^6 to 5 x 10^6 cells per mL. A working suspension was made by a 1:50 dilution followed by a 1:20 dilution of the stock suspension with RPMI 1640 broth medium, resulting in 5.0 x 10^2 to 2.5 x 10^3 cells per mL. The 2x drug concentrations were dispensed into the wells of rows 1 to 10 of the microdilution plates in 100 μL volumes, with row 1 containing the highest drug concentration and row 10 the lowest drug concentration. Each well of a microdilution tray was inoculated with 100 μL of the 2x diluted inoculum suspension. The microdilution plates were incubated at 35°C for 24 to 48 hours and observed for the presence or absence of visible growth.
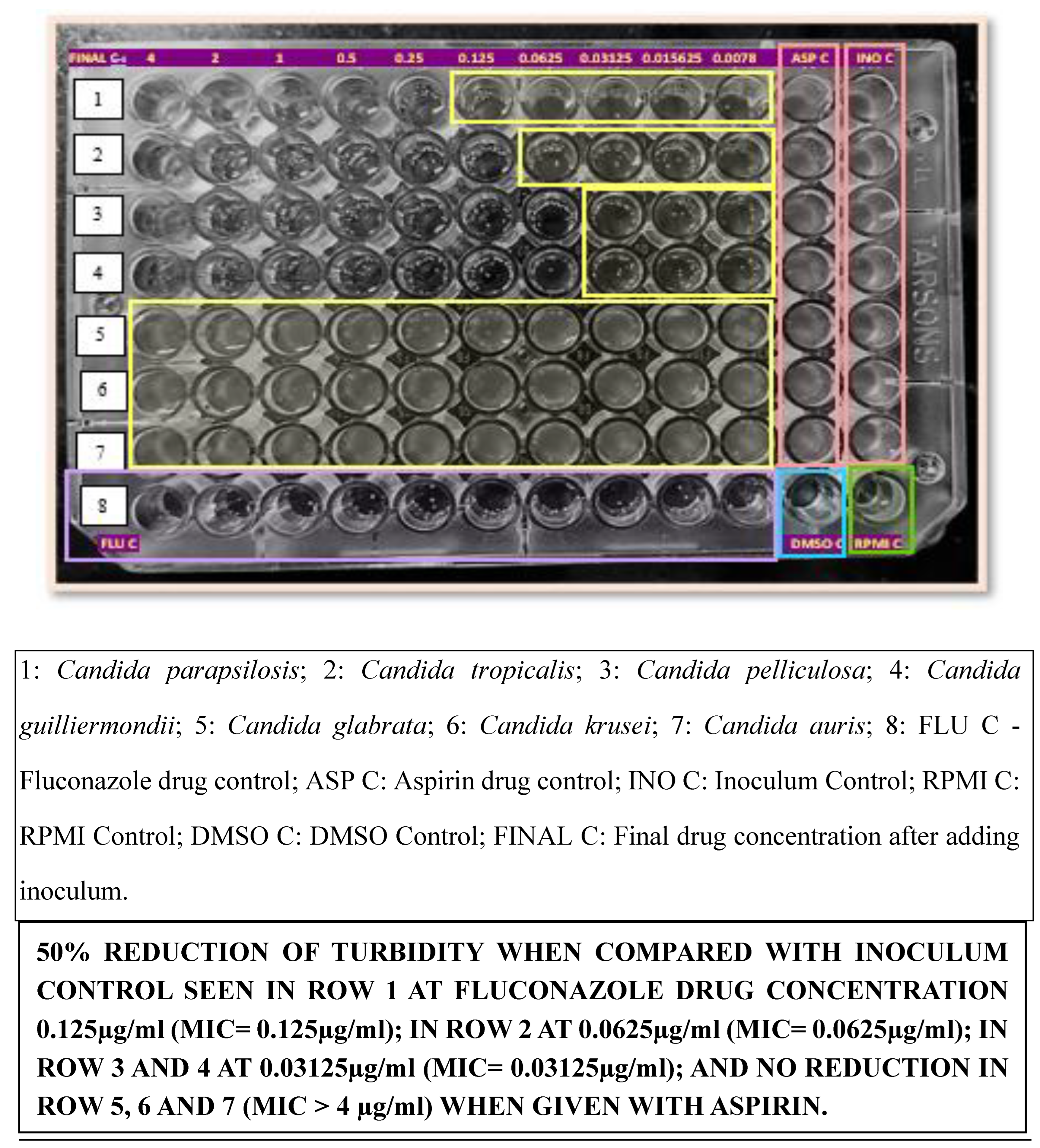
To evaluate the effect of Aspirin or Ibuprofen, 2x drug concentrations of Fluconazole were dispensed into the wells of rows 1 to 10 of the microdilution plates in 100 μL volumes. Each well of a microdilution tray was inoculated with 100 μL of the 2x diluted inoculum suspension. Aspirin or Ibuprofen drug solution was dispensed immediately after the addition of the fungal inoculum. The microdilution plates were incubated at 35°C for 24 to 48 hours and observed for the presence or absence of visible growth[
24].
3. Results
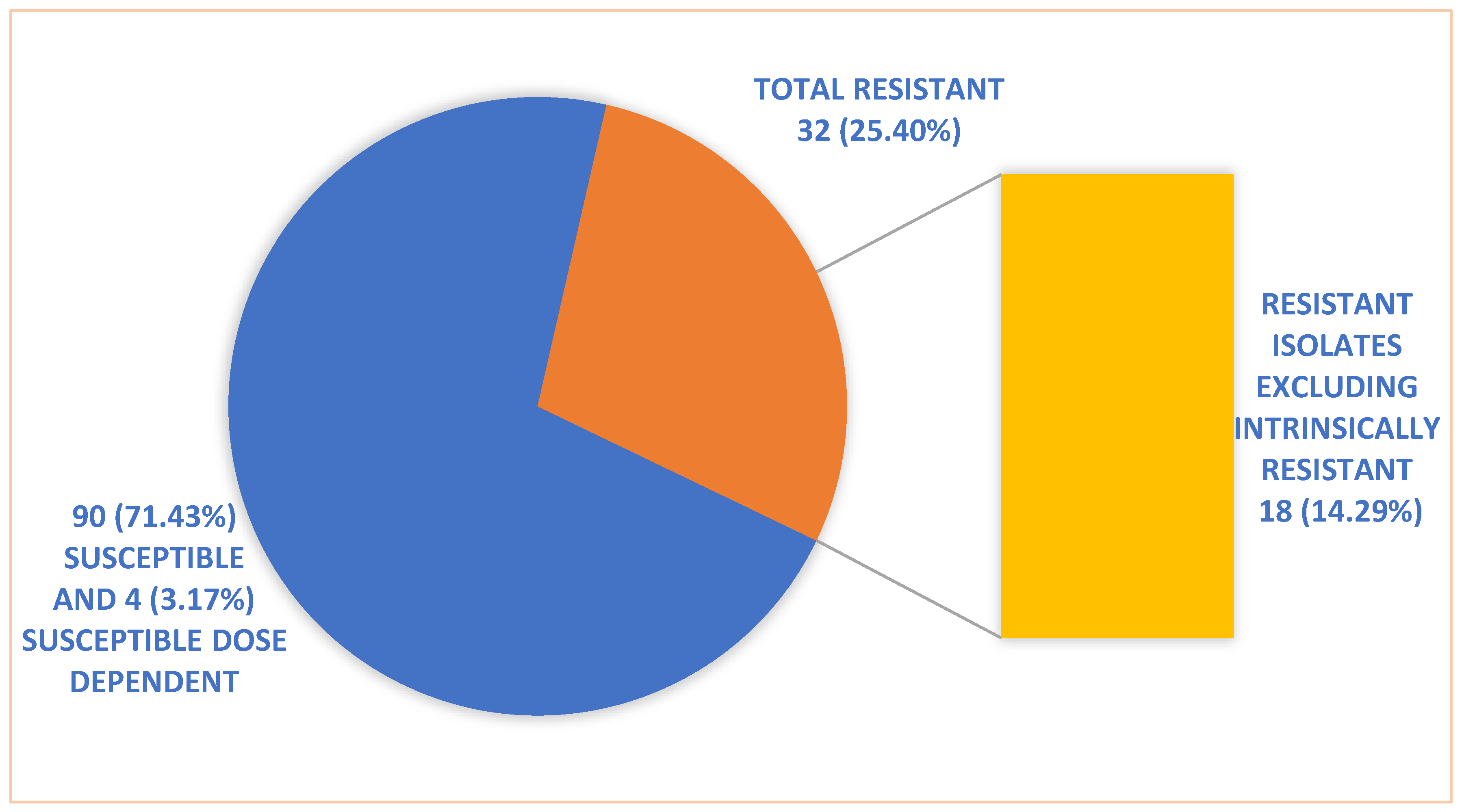
A total of 126
Candida species were isolated from patients with candidemia, out of which 90 were susceptible, 4 susceptible dose dependent, and 32 isolates were resistant to Fluconazole.
Figure 1 shows the susceptibility pattern of
Candida isolates to Fluconazole. Of these 32 isolates, only 18 were included for testing the reversal of resistance when given in combination with Aspirin or Ibuprofen. These 18 isolates comprised 10
Candida parapsilosis with a minimum inhibitory concentration (MIC) ≥8µg/ml, 3
Candida pelliculosa with MIC ≥4µg/ml, 3
Candida guilliermondii with MIC ≥4µg/ml, and 2
Candida tropicalis with MIC ≥8µg/ml. The remaining 14 isolates, comprising 10 Candida auris, 3
Candida glabrata, and 1
Candida krusei, were intrinsically resistant to Fluconazole and were excluded from further resistance reversal testing in combination with Aspirin or Ibuprofen.
Of the 18 resistant isolates of
Candida species other than the intrinsically resistant isolates, concurrent use of Aspirin or Ibuprofen with Fluconazole led to the reversal of Fluconazole resistance in 16 isolates (except 1
Candida parapsilosis and 1
Candida pelliculosa) with a considerable decrease in MIC (≤0.125µg/ml), suggesting an 88.89% conversion rate as shown in
Table 1. Specifically, the concurrent use of Aspirin or Ibuprofen with Fluconazole led to the reversal of Fluconazole resistance in 9 out of 10
Candida parapsilosis (90%), 2 out of 3
Candida pelliculosa (66.67%), and all 3
Candida guilliermondii (100%) and 2
Candida tropicalis (100%) as shown in
Table 2. Fluconazole MIC of isolates pre and post intervention with Aspirin or Ibuprofen are shown in
Figure 2 and
Figure 3.
4. Discussion
Within the limited antifungal arsenal, the azole antifungals are the most often used class of antifungals to treat
Candida infections. Due to their low cost, low toxicity, and oral availability, Fluconazole and other azole antifungals are usually preferred over other therapies for diverse
Candida infections. However, it has been demonstrated that a variety of
Candida species possess both inherent and acquired resistance to azole antifungals[
25]. As per several studies lower rates of azole resistance (0–5%) are found in
Candida albicans isolates from candidemic patients.
Candida glabrata demonstrates intrinsically lower susceptibility to the azole class of antifungals and has the greatest incidence of azole resistance among clinical isolates of
Candida. Fluconazole resistance in
Candida tropicalis in the Asia-Pacific region ranges from 0% to 83%. The worldwide incidence of Fluconazole resistance in
Candida parapsilosis disseminated infections ranges between 2 and 5% . Given that
Candida krusei demonstrates intrinsic resistance to Fluconazole, it is debatable whether the higher infection rate is a result of prophylactic Fluconazole use or previous therapy
In this study out of 126 isolates of Candida, 32 (25.40%) isolates were resistant to Fluconazole of which 14 (11.11%) were inherently resistant. Only 63% of the Candida parapsilosis samples in this study were susceptible to Fluconazole, and the remaining 27% were resistant. Also, Fluconazole resistance was noticed in 25% of Candida guilliermondii, 16.67% of Candida pelliculosa and in 8% of Candida tropicalis. This Fluconazole resistance may be caused by a variety of mechanisms, including overexpression of the ERG11 gene, mutations in the genes encoding membrane transport proteins of the ABC transporter (like CDR-1/CDR-2) or the major facilitator superfamilies (like MDR1), changes in sterol biosynthesis, and mutations in the drug target enzyme and sterol 14 alpha-demethylase (14DM). CDR-1 and CDR-2 genes from the ATP-binding cassette superfamily and MDR-1 genes from the major facilitator superfamily are examples of efflux pumps encoded by two carrier gene families.
Since azoles like Fluconazole is one of the most frequently used antifungal medications for the treatment of candidiasis, resistance to the azole group of antifungal medications is a matter for concern. Since Fluconazole is the most popular azole used to treat widespread candidiasis, including candidemia, resistance to it is a serious problem. Despite being effective against most strains of Candida sp., Amphotericin B is not the first-line treatment for candidemia because of its nephro-toxicity. In order to conserve and advance the azole class of antifungals for the treatment of Candida infections, it is crucial to explain the causes of such resistance as the frequency of azole resistant Candida isolates in the clinical context rises.
In order to deal with treatment failures, several methods have been put forth to make Candida sp. more susceptible to Fluconazole, one of which is the combination of Fluconazole with various classes of non-antifungal agents like anti-bacterials, calcineurin inhibitors, heat shock protein 90 inhibitors, calcium homeostasis regulators, and traditional Chinese medicine drugs. The primary mechanisms of these synergistic effects seem to be increasing membrane permeability, decreasing antifungal drug efflux, interfering with intracellular ion homeostasis, inhibiting the activity of proteins and enzymes necessary for fungal survival, and inhibiting the formation of biofilms.
The reversal of Fluconazole resistance with Aspirin and Ibuprofen, as found by us, hints at the likely participation of efflux pumps mediating azole resistance in majority of our isolates, in line with past research revealing Aspirin and Ibuprofen to be a potential efflux pump inhibitor[
26]. Similar to our findings, a few other publications have also noted an in-vitro synergistic impact of Ibuprofen and Fluconazole in the pathogenic yeast
Candida. It was shown that the CDR1, CDR2 and MDR1 efflux pump genes (the former two related to azole cross-resistance, and the third associated with selective resistance to Fluconazole) were overexpressed in resistant isolates that turned susceptible after being exposed to Ibuprofen. In contrast, strains that did not revert showed a substantial increase in the expression of CDR genes and the azole target gene, ERG11. The transmembrane proteins CDR1p and CDR2p are members of the ABC family, ATP-dependent; their substrates are diverse and include unrelated substances including steroids, lipids, and antifungal drugs like azoles, although its specific mode of action is still not well understood. MDR1p is a member of the Major Facilitator protein family, which derives its energy from the proton motive force. As previously mentioned, hyper-susceptibility to azoles is conferred by a loss in any one of these genes. Point mutations in the ERG11 gene that change the protein’s amino acid sequence reduce the azole’s affinity for its molecular target are another resistance mechanism mentioned. One of the most prevalent lipids in fungal cell membranes, ergosterol, is produced by the cytochrome P450 enzyme lanosterol 14a-demethylase, which is encoded by the ERG11 gene. A rise in its expression may also lead to an excess of proteins that could counteract the effects of the azole concentration and its consequences. Additionally, given the significance of prostaglandins in fungus colonisation, Ibuprofen’s inhibitory action on prostaglandin formation may result in additional in-vivo therapeutic benefits[
27].
Clinical isolates of Candida are becoming resistant to commonly used antifungal Fluconazole. A common mechanism for azole resistance is reduction of intracellular antifungal concentration by overexpression of efflux pump encoded by CDR1, CDR2 and MDR1 genes. In majority of our resistant isolates efflux pumps may contribute to azole resistance, hence we observed Aspirin and Ibuprofen two well-known efflux pump inhibitors can reverse Fluconazole resistance when used concomitantly.
Clinical Implications and Future Directions
The potential of EPIs to reverse Fluconazole resistance holds significant promise for clinical practice, offering a novel strategy to combat antifungal resistance in Candida infections. However, further research is needed to optimize EPIs’ dosing regimens, evaluate their safety profiles, and elucidate their mechanisms of action in combination with Fluconazole. Additionally, clinical trials are warranted to assess the efficacy of Fluconazole-EPI combinations in treating Fluconazole-resistant Candida infections in human subjects.
5. Conclusions
The emergence of Fluconazole-resistant
Candida strains poses a formidable challenge in clinical management, necessitating innovative approaches to combat antifungal resistance. Efflux pump inhibitors (EPIs), such as Aspirin or Ibuprofen, show promise in reversing Fluconazole resistance by inhibiting the activity of drug efflux pumps. Combining Fluconazole with EPIs represents a novel therapeutic strategy to enhance the efficacy of Fluconazole against resistant
Candida isolates and warrants further investigation in clinical settings[
28]. As our understanding of the interplay between antifungal resistance mechanisms grows, leveraging synergistic drug combinations may pave the way for more effective management of
Candida infections in the future.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org,
Table S1: Effect of Aspirin/Ibuprofen on Fluconazole Resistant Clinical Isolates of Candida species. Table S2: Species Wise Distribution of Clinical Isolates of
Candida Demonstrating Reversal of Fluconazole Resistance with Aspirin or Ibuprofen.
Figure S1: Fluconazole Susceptibility of
Candida Isolates.
Figure S2: Fluconazole MIC by Broth Microdilution Method Pre-Intervention.
Figure S3: Fluconazole MIC in Combination with Aspirin by Broth Microdilution Method.
Author Contributions
Dr. KP Anirima: Investigation, Methodology, Writing - Original Draft; Dr. Asim Sarfraz: Methodology, Conceptualization, Writing - Review & Editing; Dr. Bhaskar Thakuria: Conceptualization, Writing - Review & Editing; Dr. Binod Kumar Pati: Visualization, Supervision; Dr. Prathyusha K : Visualization, Supervision.
Institutional Review Board Statement
In This study was conducted on laboratory isolates of Candida species and did not involve human participants, human data and therefore did not require ethical approval, in accordance with the Declaration of Helsinki and the policies of Institutional Ethics Committee, All India Institute of Medical Sciences Patna (Ref. No. AIIMS/Pat/IEC/PGTh/Jan21/29).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABC |
ATP-binding cassette |
| CDR |
Candida drug resistance |
| CLSI |
Clinical and Laboratory Standards Institute |
| DMSO |
Dimethyl sulfoxide |
| EPI |
Efflux pump inhibitor |
| MIC |
Minimum inhibitory concentration |
| RPMI |
Roswell Park Memorial Institute (culture medium) |
References
- Pincus, D.H.; Orenga, S.; Chatellier, S. Yeast identification--past, present, and future methods. Med Mycol. 2007, 45, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. Journal of Medical Microbiology. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Melhem, M.S.C.; Bertoletti, A.; Lucca, H.R.L.; Silva, R.B.O.; Meneghin, F.A.; Szeszs, M.W. Use of the VITEK 2 system to identify and test the antifungal susceptibility of clinically relevant yeast species. Braz J Microbiol. 2013, 44, 1257–1266. [Google Scholar] [CrossRef]
- Rex, J.H. Clinical and Laboratory Standards Institute, editors. Reference method for broth dilution antifungal susceptibility testing of yeats: Approved standard. 3. ed. Wayne, Pa: CLSI; 2008. 25 p. (Clinical and Laboratory Standards Institute).
- Procop, G.W. Performance standards for antifungal susceptibility testing of yeasts. 2nd edition. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2020.
- eucast: Breakpoints for antifungals [Internet]. [cited 2023 May 16]. Available from: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals.
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Biswas, D.; Kotwal, A.; Thakuria, B.; Kakati, B.; Chauhan, B.S.; et al. Ibuprofen-Mediated Reversal of Fluconazole Resistance in Clinical Isolates of Candida. J Clin Diagn Res. 2015, 9, DC20–2. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J.; Ma, Y.; Xi, Z.; Ji, Y.; Ren, Q.; et al. Cotreatment with Aspirin and Azole Drugs Increases Sensitivity of Candida albicans in vitro. Infect Drug Resist. 2021, 14, 2027–2038. [Google Scholar] [CrossRef]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Non-albicans Candida Infection: An Emerging Threat. Interdiscip Perspect Infect Dis. 2014, 2014, 615958. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Dhakad, M.S.; Goyal, R.; Kumar, R. Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients. Asian Pacific Journal of Tropical Biomedicine. 2016, 6, 455–460. [Google Scholar] [CrossRef]
- Bhattacharjee, P. Epidemiology and antifungal susceptibility of Candida species in a tertiary care hospital, Kolkata, India. Curr Med Mycol. 2016, 2, 20–27. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar Verma, R.; Sarswat, S.; Saraswat, S. Non-Candida albicans Candida species: Virulence factors and species identification in India. Curr Med Mycol. 2021, 7, 8–13. [Google Scholar]
- Debruyne, D.; Ryckelynck, J.P. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.A.; Perfect, J.; Colombo, A.L.; Cornely, O.A.; Groll, A.H.; Seidel, D.; et al. Global guideline for the diagnosis and management of rare yeast infections: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis. 2021, 21, e375–86. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, O.; Moreillon, P.; Entenza, J.M.; Vouillamoz, J.; Glauser, M.P.; Bille, J.; et al. Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob Agents Chemother. 2003, 47, 1565–1570. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Chen, X.; Gao, Y.; Li, H.; Sun, S. Combination of fluconazole with non-antifungal agents: A promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int J Antimicrob Agents. 2014, 43, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. The Lancet Infectious Diseases. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Khosravi Rad, K.; Falahati, M.; Roudbary, M.; Farahyar, S.; Nami, S. Overexpression of MDR-1 and CDR-2 genes in fluconazole resistance of Candida albicans isolated from patients with vulvovaginal candidiasis. Curr Med Mycol. 2016, 2, 24–29. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez-DE-Oliveira, J.; Fonseca, A.F.; Mårdh, P.A. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J Med Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.G.; Costa-de-Oliveira, S.; Ricardo, E.; Mårdh, P.A. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J Antimicrob Chemother. 2005, 56, 678–685. [Google Scholar] [CrossRef]
- Ricardo, E.; Costa-de-Oliveira, S.; Dias, A.S.; Guerra, J.; Rodrigues, A.G.; Pina-Vaz, C. Ibuprofen reverts antifungal resistance on Candida albicans showing overexpression of CDR genes. FEMS Yeast Res. 2009, 9, 618–625. [Google Scholar] [CrossRef]
- Alexander, B.D.; Procop, G.W.; Dufresne, P. Clinical and Laboratory Standards Institute, editors. Performance standards for antifungal susceptibility testing of yeasts. Wayne, Pa: CLSI; 2017. 14 p. (Clinical and Laboratory Standards Institute).
- Elena, R.; Simona, E.S.; Oana, N.; Vassu, T. The conventional identification and effect of diclofenac and aspirin on the Candida species. Romanian Biotechnological Letters. 2009, 14. [Google Scholar]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7. Available online: http://journal.frontiersin.org/article/10.3389/fmicb.2016.02173/full (accessed on 1 June 2023). [CrossRef] [PubMed]
- Chakrabarti, A.; Chatterjee, S.S.; Rao, K.L.N.; Zameer, M.M.; Shivaprakash, M.R.; Singhi, S.; et al. Recent experience with fungaemia: Change in species distribution and azole resistance. Scand J Infect Dis. 2009, 41, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Tariq, V.N.; McCrory, R.M. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob Agents Chemother. 1995, 39, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Misseri, G.; Chowdhary, A. What’s new on emerging resistant Candida species. Intensive Care Med. 2019, 45, 512–515. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).