1. Introduction
Pancreatic adenocarcinoma is a cancer with a poor prognosis. In the United States, it is the third leading cause of cancer death, after lung and colon cancer, although its incidence is much lower. The 5-year survival rate is less than 8%. The structure of registered incidence of cancer cases in Poland in men in Poland in 2022 was 2.2% and deaths 4.7%, respectively in women 5.6%. Registered cancer incidence cases of the leading cancer sites by frequency in Poland 2022 are in males 1938 and in females 1955. Deaths from the leading cancer sites in males by frequency in Poland 2022 is 2424 and in women 2464. The most common risk factors of this cancer are: age, smoking, obesity, diabetes and alcohol abuse.
Improved patient survival can be associated with earlier detection of neoplastic lesions. It depends on a faster pathological diagnosis for patients with pancreatic tumors, improvement of surgical technique along with an increase in the number of centers performing “aggressive” resections, with particular emphasis on vascular resections and an increase in the number of resectable cases due to neoadjuvant treatment. In patients with a primary resectable lesion based on imaging studies, it is advisable to attempt resection as soon as possible. In patients with a borderline resectable or non-resectable lesion, preoperative chemotherapy improves the possibility of performing surgery.
The pancreas is an organ consisting of an exocrine and endocrine part. The choice of surgical treatment method, pancreatoduodenectomy or distal resection, depends on the localization of the tumor. The size of the tumor, degree of local advancement and clinical stage according to cTNM (AJCC/UICC) decide on the method of treatment. Decision on surgical treatment or preoperative chemotherapy based on the radiological examination. Computer tomography (CT) and magnetic resonance (MR) imaging are the main methods on which the surgeon’s decision is based. CT is also the basis for selection of the traditional or da Vinci robot-assisted surgery method. Sometimes the radiological examination is supported by endoscopic ultrasonography with endoscopic ultrasound (EUS)-guided tissue sampling including the techniques of fine needle aspiration (FNA) and fine needle biopsy (FNB) [
1,
2]. Preoperative diagnosis of pancreatic tumors is difficult due to the multitude of macro- and microscopic features of the tumors. Obtaining representative material for histopathological examination is, however, difficult, especially in small solid tumors. Therefore, the diagnosis of the lesion developing in the pancreas can be established on the basis of the pathomorphological examination of the surgical material.
The landscape of pancreatic lesions includes solid tumors, solid cystic tumors or cysts[
3,
4]. The classification of cystic lesions based firstly on the neoplastic and nonneoplastic epithelium, secondary according to type of epithelial lining. The WHO 2019 classification divided pancreatic tumors into categories of types, precursors as intraepithelial neoplasia, benign or malignant neoplasm. Cystic lesions form a particularly complex group among them.[
5,
6] Their diagnosis is based on the assessment of the epithelial type. Pre-invasive neoplastic precursor lesions include mucinous cystic neoplasm (MCN), intraductal papillary neoplasm (IPMN) and Pancreatic Intraepithelial Neoplasia (PanIN) with Low-Grade and/or High-Grade Dysplasia [
7]. In a large group of mucinous cystic neoplasms important for diagnosis is recognition of ovarian-type stroma in MCN and focal or multifocal lesion of IPMN or PanIn which may be or not identifiable macroscopically. The second group of neoplasm consists of benign tumors such as serous cystic neoplasm (SCN) and acinar cell cystadenoma. A special third group forms the malignant neoplasms with cystic degenerative changes. They include solid pseudopapillary neoplasm (SPN), pancreatic neuroendocrine neoplasm (pNEN), cystic pancreatic ductal adenocarcinoma, cystic acinar cell carcinoma, malignant cystic teratoma, cystic pancreatoblastoma and cystic metastasis. The category of cystic lesions lined with non neoplastic epithelium includes; congenital cyst, duplication (foregut) cyst, choledochal cyst, cystic hamartoma, lymphoepithelial cyst, mucinous non neoplastic cyst, retention cyst, paraduodenal pancreatitis and endometrial cyst. Special types are cysts with non neoplastic non-epithelium as pseudocyst and parasitic cyst.
The aim of the study was to verify pathomorphological diagnoses in patients treated with the da Vinci robot-assisted surgery method in one center. To our knowledge, this is the first summary of results based on a large number of patients from one center.
2. Materials and Methods
In the year 2021 – 2024, 141 distal resections of the body and tail of the pancreas were performed using the da Vinci robot-assisted surgery method at the PIM MSWiA in Warsaw. The pathomorphological examination was performed at the Pathomorphology Center of the Ministry of Interior and Administration.The operative specimens were fixed in 10% buffered formalin, embedded in paraffin and sectioned. The histopathological examination in each case included macro- and microscopic assessment in accordance with the College of American Pathologists (CAP) guidelines. In the macroscopic examination, the number, size and appearance of the tumor were evaluated taking into account the solid, cystic or solid-cystic appearance. The circumferential margin marked by the surgeon. Representative samples were taken from the surgical material for evaluation. The surgical margins were identified as closest margin(s) to invasive carcinoma and verified as distance from invasive carcinoma to closest margin. The sections were routinely stained with hematoxylin and eosin. In cases of neuroendocrine tumours, immunohistochemical tests for Synaptophysin, Chromogranin A and Ki-67 proliferation index were additionally performed. The histological type of the tumor was identified by microscopic examination. In case of neoplasms, the histological type and degree of differentiation (Grading, G) were evaluated according to 2019 WHO classification, the degree of pathological advancement based on pTNM AJCC/UICC and the presence of vascular and nerve invasion. The surgical margin was evaluated according to resectability classification (R classification) by degree R0 (complete, macroscopic and microscopic resection, without tumor invasion at the surgical margins), R1 (the neoplastic infiltration remains in the surgical margins in microscopic evaluation) and R2 (the neoplastic infiltration remains in the primary tumor in the macroscopic assessment and has not been surgically removed, as well as beyond the surgical margins). Margin Status for Dysplasia and Intraepithelial Neoplasia, regarding the extent of high-grade dysplasia in PanIN, noninvasive IPMN and MCN can be recorded but does not currently have clinical relevance.
The pathomorphological diagnosis in the cases examined was made in the form of an electronic pathomorphological report based on the CAP guidelines.
3. Results
3.1. Clinical Data
The study material consisted of 141 surgical specimens after distal pancreatic resection performed using da Vinci robot-assisted surgery method. The preoperative diagnosis of the patients was established on the basis of radiological examination; ultrasound and computed tomography, revealing a resectable solid, solid-cystic or cystic tumor of the body or tail of the pancreas. In 6/141 patients, EUS with BCI or BGI was additionally performed. Only in 2/6 patients with SPN and pancreatic ductal adenocarcinoma, a preoperative diagnosis was established based on representative biopsy material. In the remaining 4 cases, no histopathological diagnosis was made before surgery based on EUS/FNA, FNB. Epithelial cells without atypia were found in 2 cases of IPMN and few lymphocytes in the case of ductal adenocarcinoma and SCN.
3.2. Pathomorphological Data
3.2.1. Pathomorphological Diagnosis of Specimen after da Vinci Robot-Assisted Surgery Method
Based on pathological examination, patients were divided into three main groups. The first and second groups consisted of patients with primary and secondary pancreatic malignancies. The third group consisted of patients with benign tumors, precursors of pancreatic adenocarcinoma and non-cancer lesions.
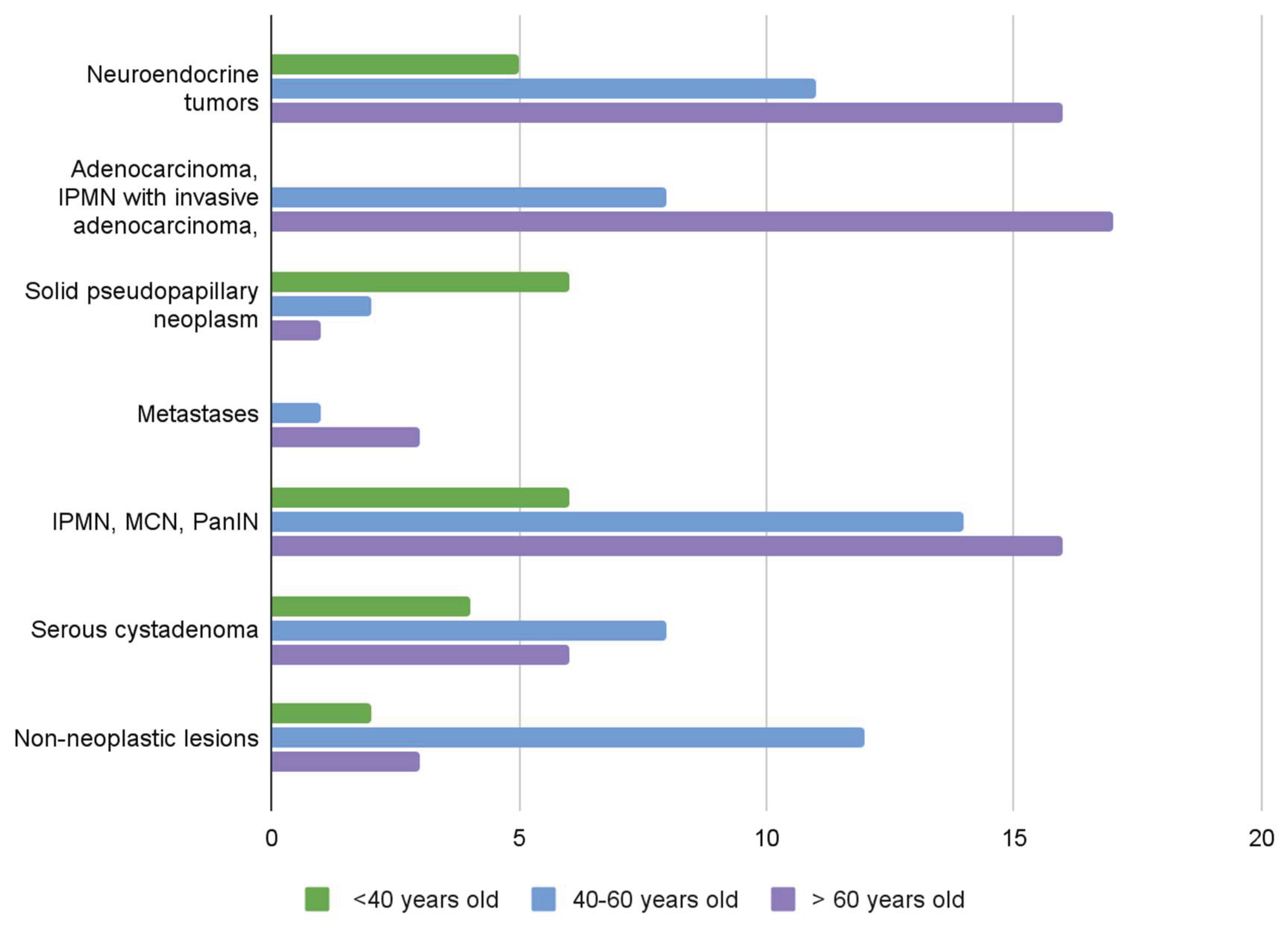
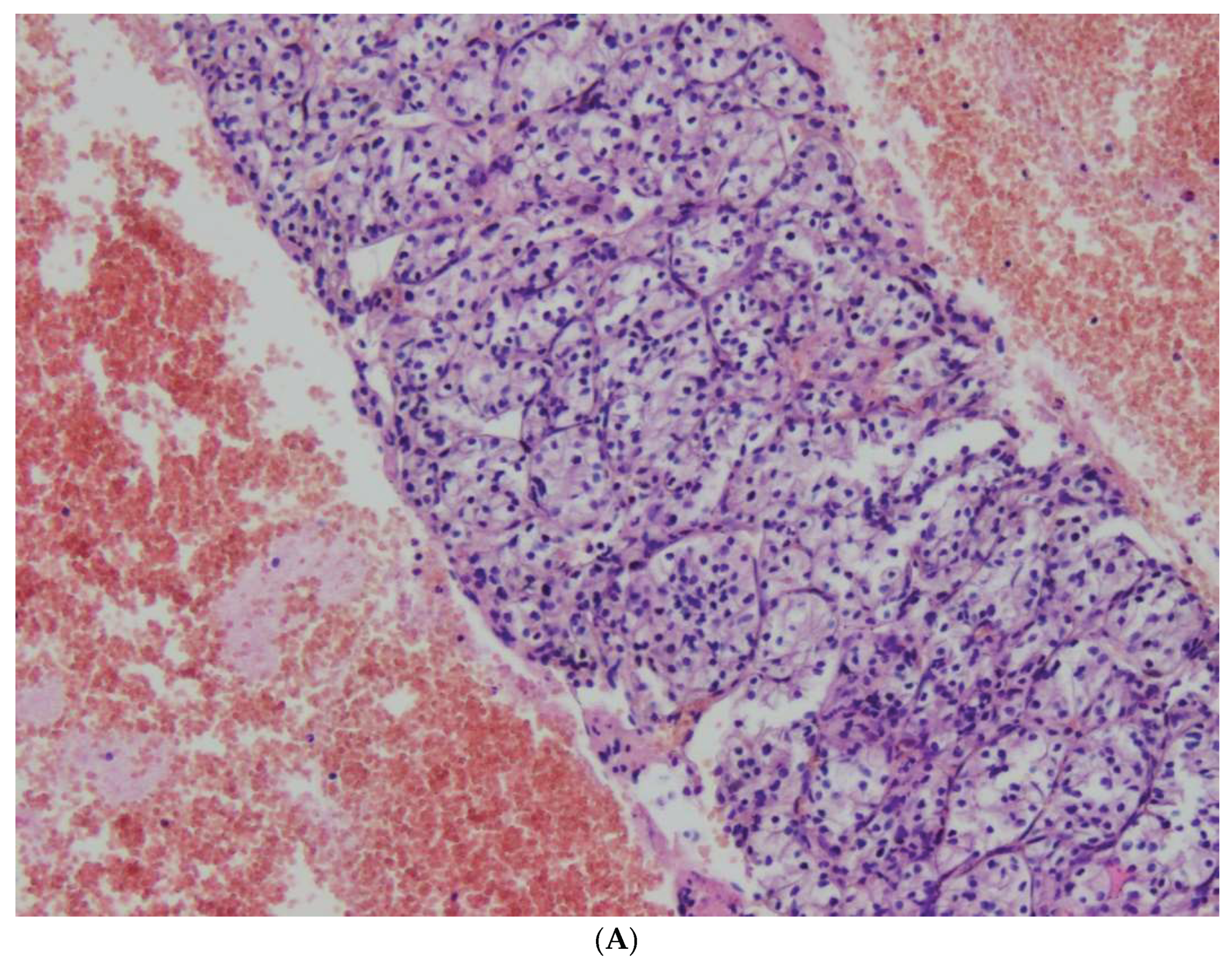
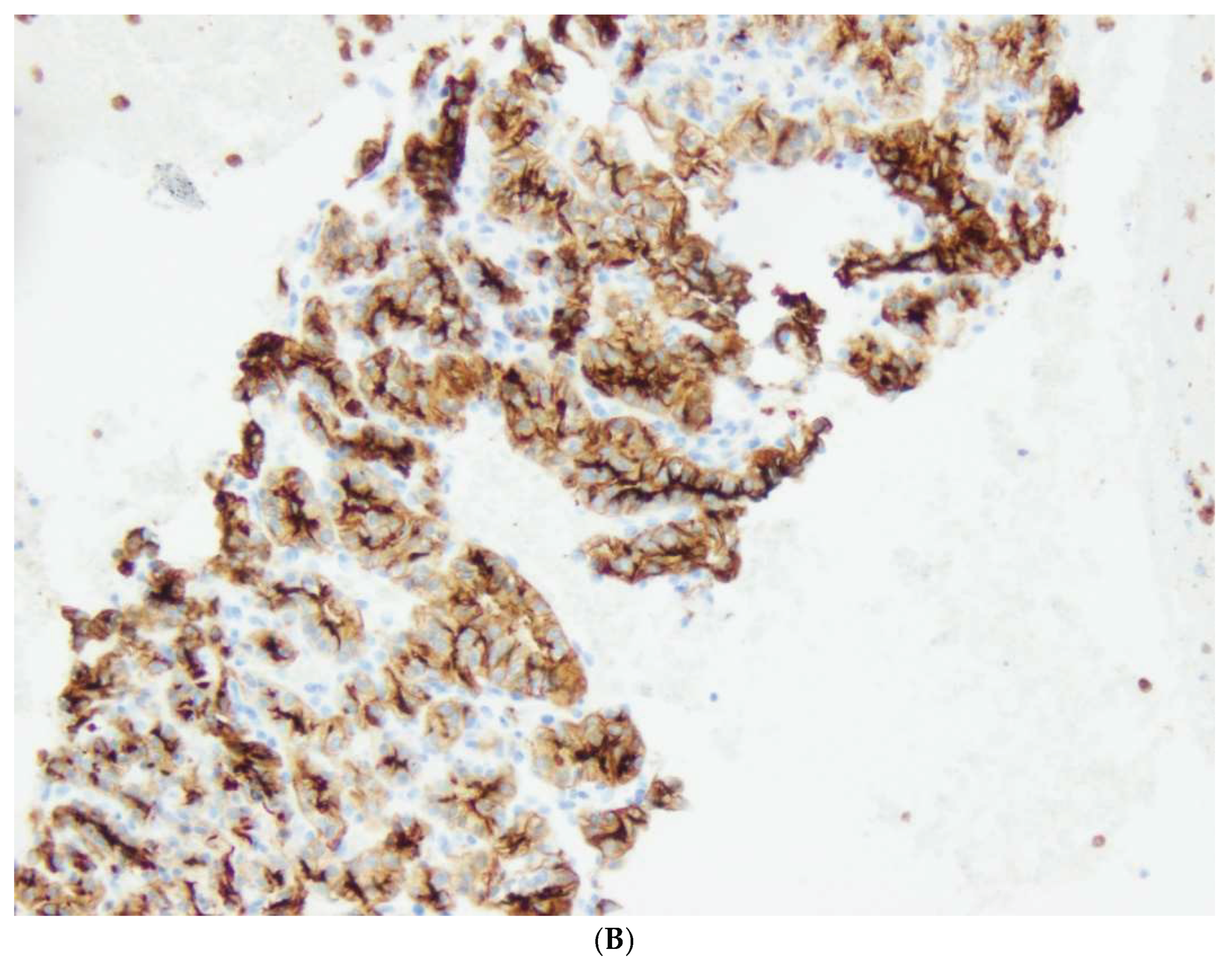
The first group (group I) consisted of 32/141 patients (23%), 22 women and 10 men, with well-differentiated neuroendocrine tumors, NETs, aged 30 to 80 years. NET G1 was diagnosed in 20 patients , NET G2 in 10 cases and NET G3 in 2 cases. In patients with NETs, 35 nodules ranging in diameter from 0.1 to 7 cm (median 2.3 cm) were found. R0 resection was performed in 28 patients (87,5%). R1 resection was observed in 4 patients; in one patient with multiple trifocal nodules NET G1 size of 0.9, 0.5 and 0.2 cm in diameter, in the second patient with NET G2 diameter 7 cm, in the third patient with NET G3 diameter 4.7 cm and in the fourth patient with NET G1 diameter 0.7 cm located at the cutting line. No cases of pancreatic neuroendocrine cancer were detected.
The second group (Group II) consisted of 38 patients (27%) with 34 primary malignant and 4 secondary neoplasms. Primary malignant tumors were diagnosed in 25 patients , 12 men and 13 women, 20 cases of pancreatic ductal adenocarcinomas (PDAC), 4 cases of invasive cancer coexisting with IPMN, one case of acinar carcinoma and 9 cases of Solid Pseudopapillary Neoplasm (SPN) in 1 male and 8 female. Secondary neoplasms were diagnosed in 4 cases, including metastases of clear cell renal carcinoma in three patients and metastases of melanoma in one patient. The tumor size in 20 patients with pancreatic adenocarcinoma ranged from 1.2 to 7.5 cm (median 4 cm), in one case of lobular carcinoma it was 8 cm and in 9 cases SPN ranged from 0.9 to 5 cm (medina 2,6 cm). In most patients with pancreatic cancer, R0 resection was performed in 11/20 cases (55%), and R1 in 8/20 patients (40%). R2 resection was found in 1 patient with cancer not forming a demarcated tumor but spreading diffusely over a distance of more than 6 cm. Similarly, R0 resections cases predominated in patients with Solid SPN; R0 was found in 6 of 9 patients, R1 in one patient and R2 in the remaining two.
A significant prognostic factor in groups I and II was the stage according to pTN advancement. It was found that pancreatic NETs were neoplasms of a less advanced stage than carcinomas. In 26/32 cases (86%) NETs were of the pT1 and pT2 stage (diameter up to 4 cm), whereas adenocarcinomas of the pT1 and T2 stage were detected in 15/25 cases (60%) and pT3 in 10/25 (40%) patients. No patient had stage pT4 Tumor (involves the celiac axis or the superior mesenteric artery, and / or common hepatic artery, regardless of size). When examining the status of lymph nodes, metastases were found in 4/32 (12.5%) cases of NET and in 12/25 cases (48%) of ductal adenocarcinoma.
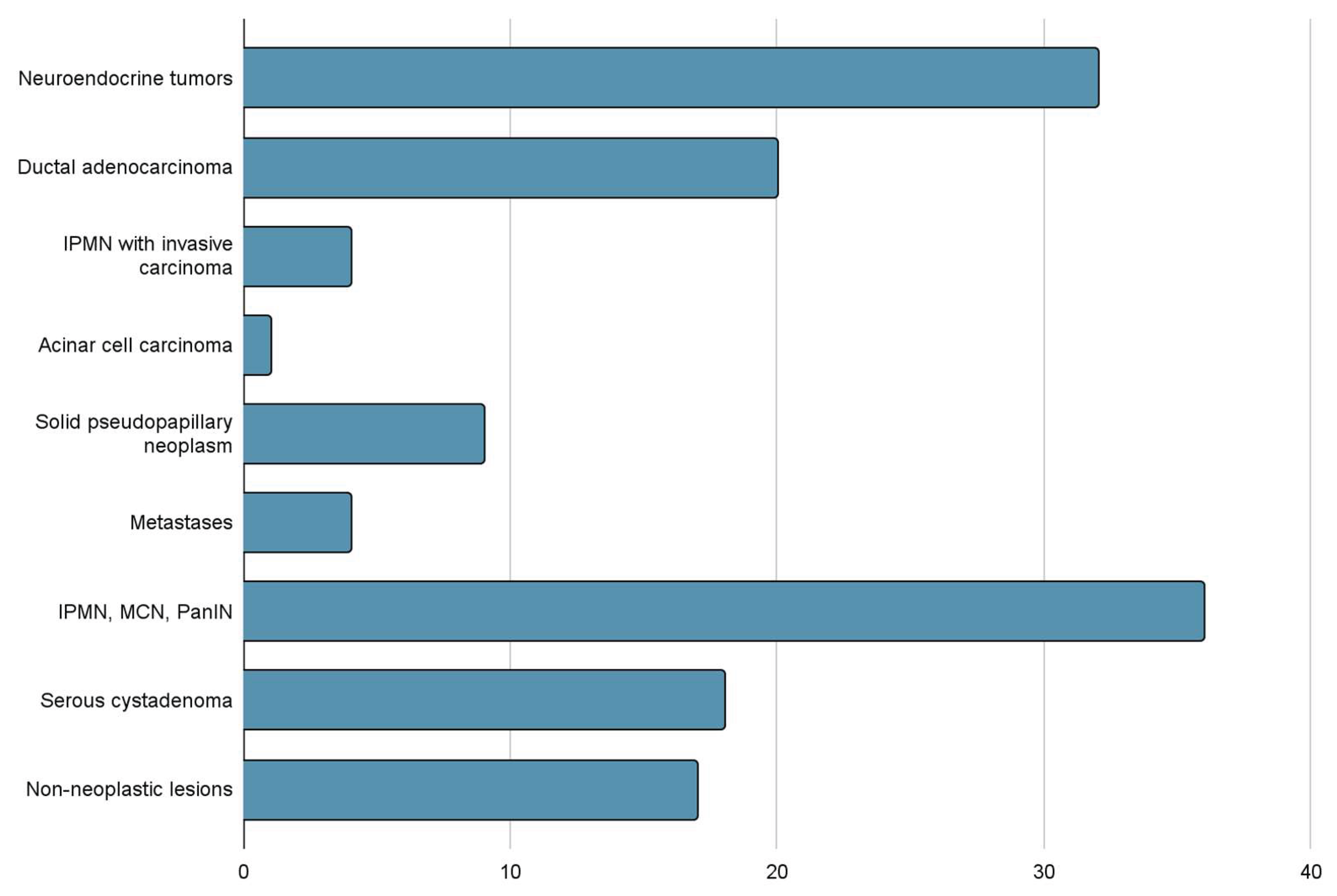
The third group (Group III) consisted of 71 patients (50%). In 18 of them (13%) benign tumors of the Serous Cystic Neoplasm (SCN) type were diagnosed, in 36 (25%) ductal adenocarcinoma precursors of the IPMN Low Grade and IPMN High Grade, MCN Low Grade and MCN High Grade and PanIN were found. Non-neoplastic changes were detected in 12 patients (13%), including pancreatitis, retention cysts and an additional spleen. Results are shown in
Table 1 and
Table 2,
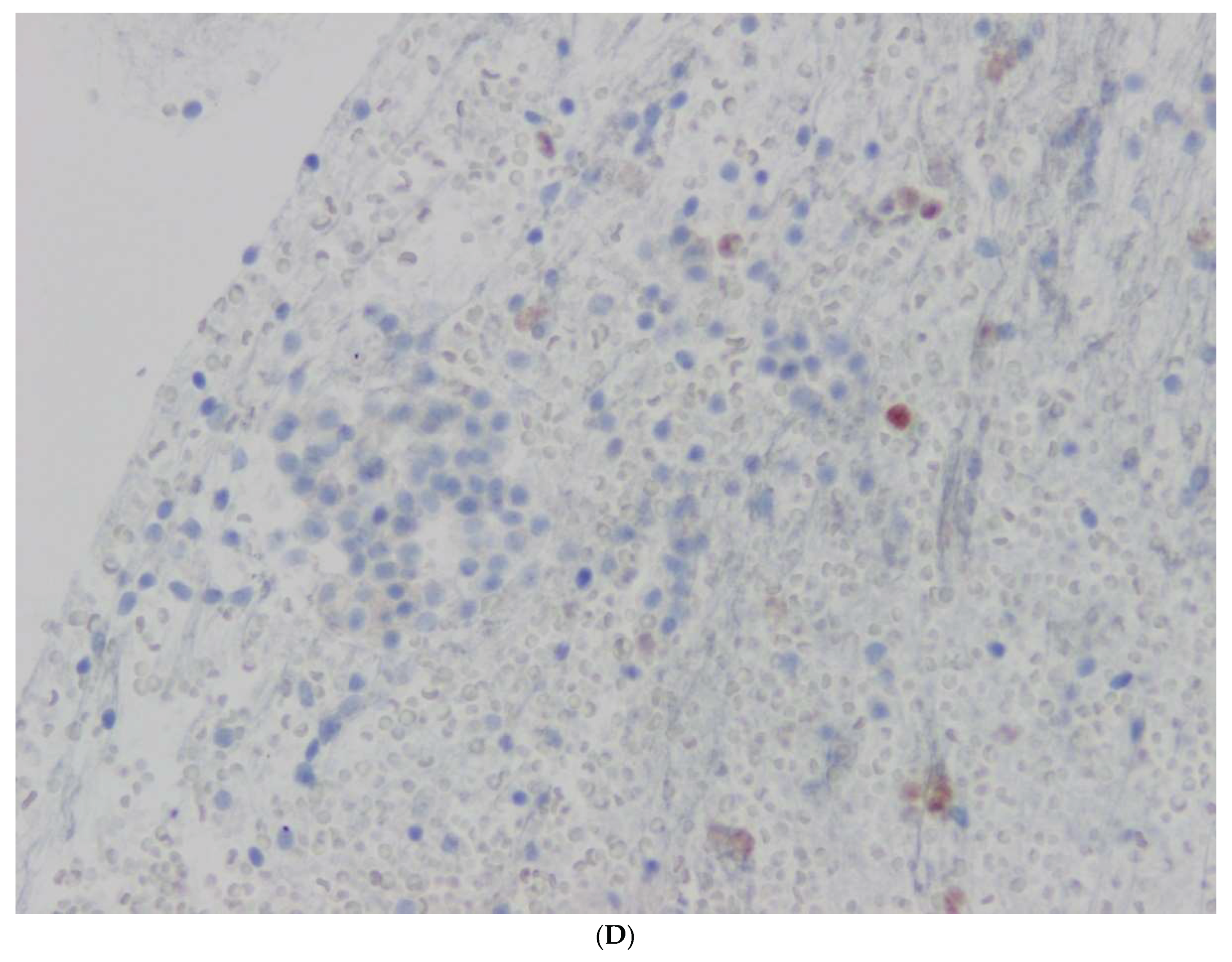
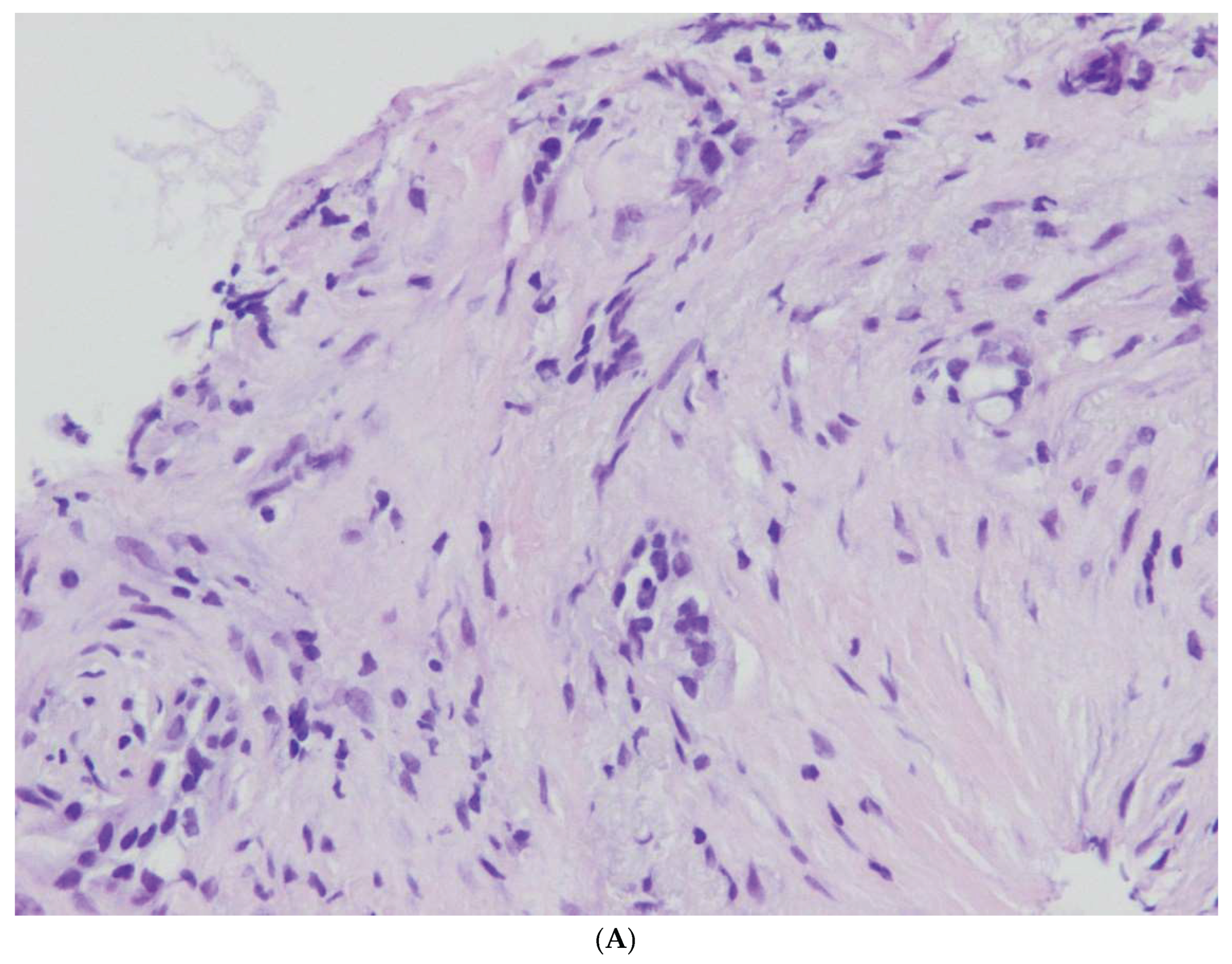
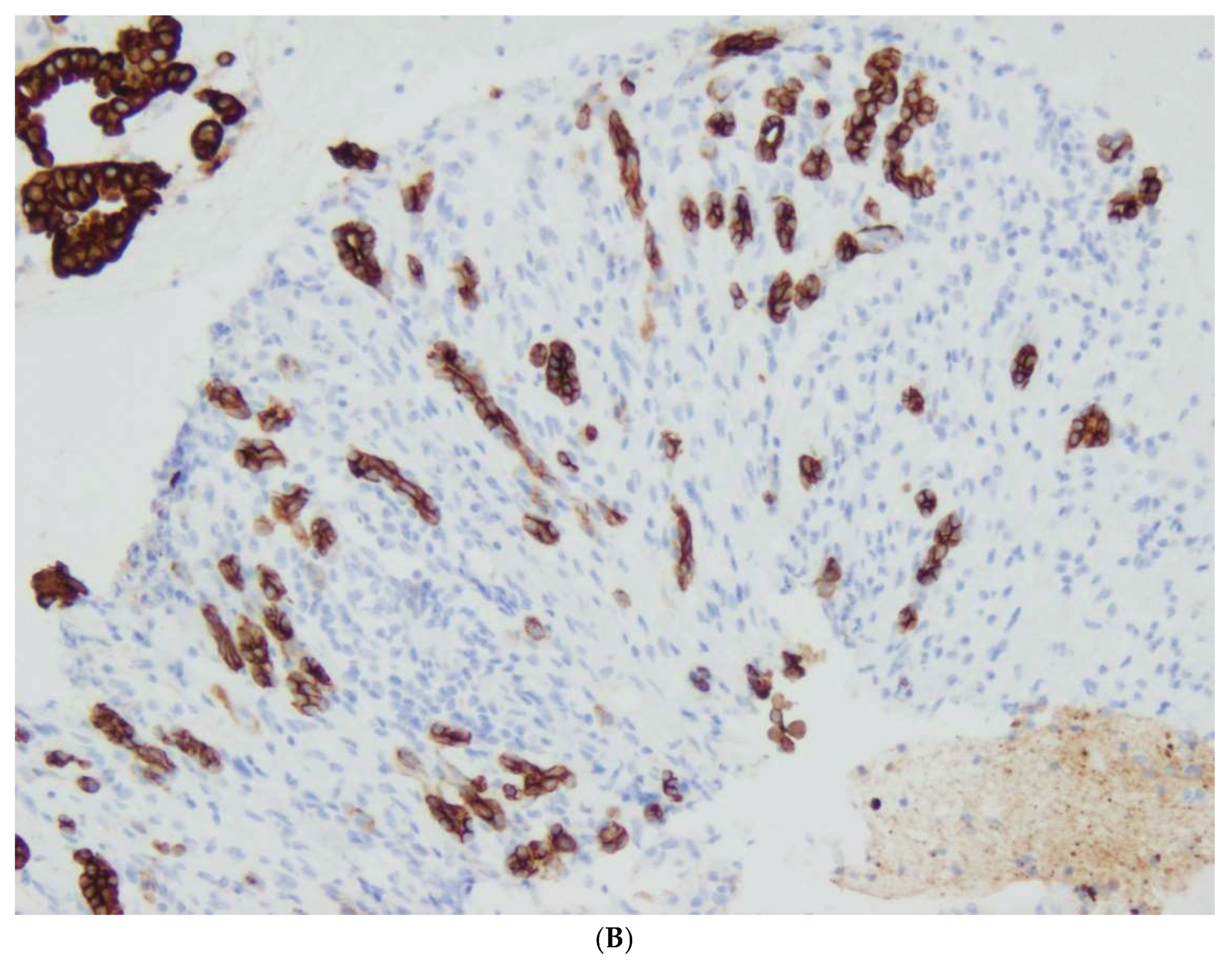
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
3.2.2. Pancreatic Cancer Precursors
In surgical specimens after da Vinci robot-assisted surgery, PDAC precursors constituted a significant group of 40/141 cases (28%). There were 18 cases of MCN, 16 cases of IPMN without invasive carcinoma, 4 cases of IPMN with small foci of invasive carcinoma, and 2 PanIN lesions. MCN and IPMN are relatively uncommon and account for approximately 10% and 20% of resected cystic neoplasm of the pancreas. MCN has been referred to previously as mucinous cystadenoma and mucinous cystadenocarcinoma (non-invasive or invasive). In contrast to IPMN, MCN is not associated with pancreatitis.
In our MCN cases, a solitary large cystic mass, mean size 5 – 9 cm, were found. The cysts were lined by tall, columnar, mucin-producing epithelial cells resembling gastric type epithelium or intestinal differentiation with goblet cells and paneth cells. MCNs with Low Grade Dysplasia were characterized by tall columnar epithelium with small basally located nuclei or pseudostratified nuclei. MCN with High Grade Dysplasia were characterized by typical architecture and cytological atypia with the formation of papillae with irregular branching, nuclear stratification and pleomorphism with frequent mitotic figures. In our 18 cases of MCN, no associated invasive carcinoma was observed.
IPMN cases in our study involved the main pancreatic duct of Wirsung or brunch-ducts or a combination of the two. IPMN showed three epithelial subtypes, namely gastric, intestinal and pancreatobiliary type . PAS histochemical examination and immunohistochemical profiles of the mucin glycoproteins helped to distinguish the epithelial types. Low Grade or High Grade Dysplasia was found in all of our 16 cases of IPMN. The two-tiered grading system included in the 2019 WHO classification of tumors of the pancreas based on the degree of architectural and cytological atypia. Low-grade dysplasia was characterized by a single layer of uniform, columnar cells with basal nuclei showing minimal atypia or by cells showing nuclear crowding, nuclear polymorphism and occasional mitotic figures. A complex papillary architecture and budding off of neoplastic cells into the duct lumen were seen in the areas of high-grade dysplasia. The epithelial cells showed complete loss of polarity, nuclear polymorphism and prominent mitotic figures. Progression from low-grade dysplasia to high-grade dysplasia to invasive carcinoma appears to take several years. In our study, 4 cases of IPMN with associated invasive carcinoma were diagnosed.
Pancreatic Intraepithelial Neoplasia (PanIN) is one of the three main precursors of pancreatic invasive adenocarcinoma[
5,
6,
7]. They are not detected clinically. Two cases of PanIN of 1.4 and 1.5 cm of diameter were diagnosed in this study. PanIN may arise in any part of the pancreatic duct system. The two-tiered system has been included in the 2019 WHO classification of tumors of the pancreas. Low-grade PanIN showed epithelial lesions composed of tall columnar cells with varying amounts of supranuclear mucin and mild-to-moderate cytological atypia. The epithelium may be flat or have a papillary or micropapillary architecture. The nuclei showed some nuclear abnormalities such as loss of nuclear polarity, nuclear crowding and pseudostratification. The case with high-grade PanIN showed a papillary or micropapillary pattern of epithelium with significant cytological atypia with loss of nuclear polarity, nuclear pleomorphism with mitotic figures.
4. Discussion
A prospective review of maintained database on all robotic distal pancreatic procedures at the National Medical Institute of MSWiA in Warsaw was performed. The study of the single-center cohort was based on a histopathological analysis of prognostic factors in 141 operated patients. The procedure resulted in tumor-free surgical margins. Complete R0 resection was observed in 47/66 (71%) diagnosed as NET, PDAC, IPMN with invasive carcinoma and SPN. R1 resection verified in 15/66 (23%) and R2 in 4 cases. The use of the Da Vinci system facilitated a safe and effective robot-assisted corpo-caudal pancreatectomy and splenectomy[
8,
9,
10]. Chen at al. in international, multicenter, retrospective, cohort study, including 542 consecutive patients undergoing robotic distal pancreatectomy (RDP) and laparoscopic distal pancreatectomy (LDP) for resectable pancreatic cancer in 33 experienced centers from 11 countries (2010–2019). They evaluated 103 RDP (19%) patients and 439 LDP (81%) cases. The R0-resection was diagnosed in 75,7% cases after RDP and 69,3% cases after LDP.[
11]
Meta analysis based on 7 observational studies with a total of 4212 patients showed that minimally invasive total pancreatectomy is safe and feasible compared to open pancreatectomy. The primary endpoints of this systematic review were overall postoperative complication and resection margin involvement rates. Secondary endpoints included operating time, estimated blood loss, postoperative complication rate, postoperative pancreatic fistula rate, rate of delayed gastric emptying, surgical site infection rate, reoperation rate, length of hospital stay, and number of lymph nodes harvested [
11,
12,
13].
Robotic distal pancreatectomy (RDP), with or without spleen preservation, has been previously described in detail. The procedure is performed using the da Vinci Robotic Surgical System (Intuitive Surgical, Inc.)[
14,
15,
16]. Six ports are typically employed, including four robotic trocars and two assistant ports. Pneumoperitoneum is established and maintained at 12 mmHg. The camera port (12 mm) is inserted in the paraumbilical position, followed by placement of three 8-mm robotic ports arranged in a curved line. A 12-mm assistant port for suction and linear stapler insertion is positioned in the left lower abdominal quadrant along the midclavicular line. Second assistant port 12-mm AirSeal
® is placed laterally in the left anterior axillary line to facilitate precise delivery of suture needles and hemostatic materials. Before docking the robotic system, a thorough inspection of the peritoneal cavity is performed to exclude metastatic disease in the liver or peritoneum. For the da Vinci Xi system, the typical port configuration includes: the camera port placed to the right of the umbilicus, the Cadiere forceps port in the right anterior axillary line near the subcostal margin, the bipolar forceps port in the right midclavicular line, and the hook/vessel sealer port in the left midclavicular line. The pancreatic neck is first identified, and the superior mesenteric vein-portal vein confluence is carefully exposed. Management of the splenic vessels depends on the surgical plan. In spleen-preserving techniques, the splenic artery and vein are precisely isolated and preserved. When splenectomy is indicated, these vessels are ligated near their origins. Pancreatic transection is performed using a linear stapler that provides graduated compression of the pancreatic tissue. The pancreatic body and tail are then carefully mobilized using a hook cautery. The specimen is always extracted using an endobag through the extended assistant port site in the lower abdomen, which is performed as the final step of the procedure. A closed suction drain is routinely placed near the pancreatic stump for postoperative monitoring. Prior to closure, hemostasis is confirmed. Advances in robotic-assisted surgery, particularly with the Da Vinci system, have revolutionized these complex procedures, offering enhanced precision, reduced blood loss, and faster recovery. Postoperative specimen of distal pancreas presented in
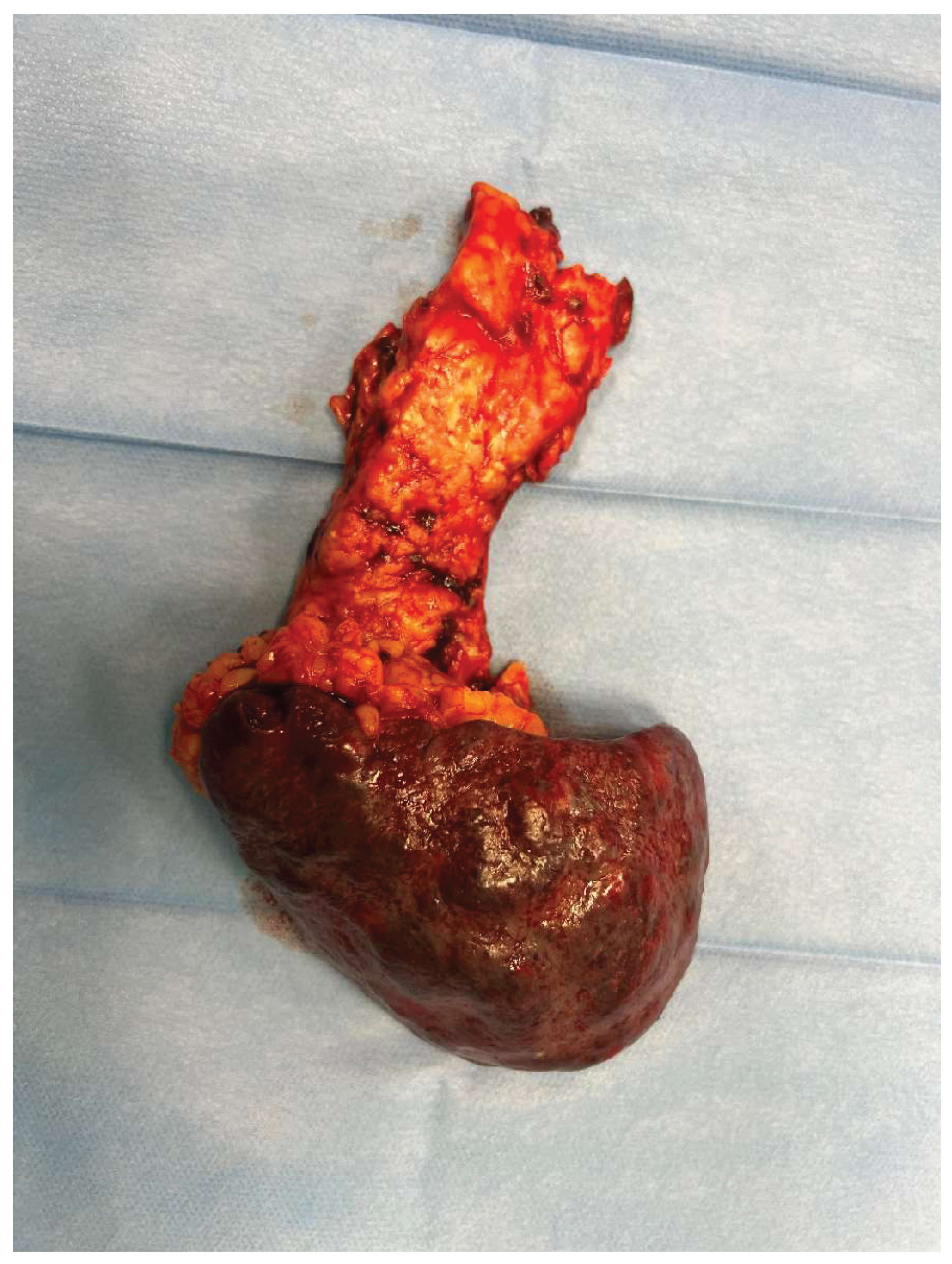
Figure 6 .
From the clinical point of view, an important group were patients with precursors of pancreatic adenocarcinoma . There is no reliable method of discerning between low-risk and high-risk before the surgery. In the study, 30% of patients had cancer precursors, 36 without invasive cancer and 4 IPMN patients with invasive cancer. Despite the research conducted to identify risk factors for the transformation of low-risk and high-risk lesions into cancer, there are no clinically useful markers. In this situation, minimally invasive surgery is useful, improves patient survival and reduces the risk of developing cancer [
16,
17,
18,
19].
Similarly, robotic surgery is clinically justified in cases of well-differentiated nonfunctioning pancreatic neuroendocrine tumours (NF-Pan-NET)[
20]. Curative resection for NF-Pan-NET is associated with 5-year survival rates of 70%–80%. In patients undergoing curative resection, a shorter survival was observed in patients with lymphatic invasion, and size >10 cm, while a shorter disease-free survival (DFS) was associated with advanced pT-stage (pT3–4), size >5 cm and histological grade (G) 2–3. Ki-67 > 10% predicted a poorer prognosis. In our study, 28 out of 32 patients underwent complete excision with R0 resection. Minimally invasive surgery is beneficial for these patients [
13]. It is particularly useful in resectable tumors where a preoperative diagnosis cannot be obtained. Transabdominal ultrasound with biopsy has limited sensitivity for Pan-NET.
The results of pathomorphological examinations of benign and malignant tumors conducted in our study demonstrated microscopic resectability of locally advanced malignant tumors and their precursors as well as benign cystic tumors . The Da Vinci robot-assisted surgery method in these patients proved to be safe, diagnostic and therapeutic at the same time, and improved the quality of life of the patients. Radical surgical treatment of pancreatic cancer precursors and locally advanced tumors improves the prognosis and survival of patients. This applies especially to cancer where there is now reliable method of discerning between low-risk and high-risk IPMNs or MCN with current laboratory, endoscopic, cytologic, and imaging modalities. Surgery remains the mainstay of treatment in eligible patients.
5. Conclusions
Preoperative diagnosis of cystic, solid-cystic and solid neoplasms is based on computed tomography and only in individual cases on endoscopic ultrasonography with biopsy. Resectable cases are treated surgically. The final pathomorphological diagnosis is made based on the examination of the surgical material. In the study group of patients from a single center after robotic distal pancreatectomy, locally advanced neuroendocrine tumors and ductal carcinomas, SPNs, SCNs, and low- and high-risk cancer precursors were found. A significant prognostic factor determined by the degree of resection was studied. R0 was found in 87.5% of neuroendocrine tumors and comparable R0 in 55% and R1 in 40% of ductal pancreatic cancers. In addition to the above-mentioned malignant tumors, a significant research group (28%) consisted of low- and high-risk cancer precursors (IPMN, MCN, PanIN). Removal of the above-mentioned changes has a particular prognostic significance, reducing the risk of developing cancer.
Minimally invasive distal pancreatectomy (MIDP) has become the preferred approach for most resectable lesions in the pancreatic body and tail. Beneficiaries are patients with resectable and locally advanced tumors with R0 resection and precursor lesions. Robotic distal pancreatectomy in these patients improves the quality of life and reduces postoperative complications. The presented single-center study is the first presentation of a large collection of pancreatic ductal carcinoma and its precursors, neuroendocrine tumors, and secondary and benign tumors treated with the da Vinci robot-assisted surgery method.
Author Contributions
Anna Nasierowska-Guttmejer; Conceptualization, Methodology, Investigation, Resources, Writing – Original Draft Preparation, Project Administration, Funding Acquisition, Maria Maliniwska; Development of a patient database, Software, Methodology, Writing – Original Draft Preparation; Natalia Wiśniewska; Formal Analysis, Writing - Original Draft Preparation; Marek Durlik; Writing – Review & Editing, Supervision, Project Administration.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SPN |
Solid Pseudopapillary Neoplasm |
| IPMN |
Intraductal Papillary Mucinous Neoplasm |
| MCN |
Mucinous Cystic Neoplasm |
| PanIN |
Pancreatic Intraepithelial Neoplasia |
| SCN |
Serous Cystic Neoplasm |
| pNEN |
Pancreatic Neuroendocrine Neoplasm |
| PDAC |
Pancreatic Ductal Adenocarcinoma |
References
- Dhar, J.; Samanta, J.; Nabi, Z.; Aggarwal, M.; Conti Bellocchi, M.C.; Facciorusso, A.; Frulloni, L.; Crinò, S.F. Endoscopic Ultrasound-Guided Pancreatic Tissue Sampling: Lesion Assessment, Needles, and Techniques. Medicina (Mex.) 2024, 60, 2021. [CrossRef]
- Hamada, T.; Oyama, H.; Takahara, N.; Nakai, Y.; Fujishiro, M. Role of Endoscopy in Clinical Management of Intraductal Papillary Mucinous Neoplasms. J. Gastroenterol. Hepatol. 2025, 40, 1045–1058. [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [CrossRef]
- Basturk, O.; Adsay, N.V. Early Cancerous Lesions of the Pancreas and Ampulla. Gastroenterol. Clin. North Am. 2024, 53, 57–84. [CrossRef]
- Moris, D.; Liapis, I.; Gupta, P.; Ziogas, I.A.; Karachaliou, G.-S.; Dimitrokallis, N.; Nguyen, B.; Radkani, P. An Overview for Clinicians on Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas. Cancers 2024, 16, 3825. [CrossRef]
- Lucocq, J.; Haugk, B.; Parkinson, D.; Darne, A.; Joseph, N.; Hawkyard, J.; White, S.; Mownah, O.; Menon, K.; Furukawa, T.; et al. Precursor Epithelial Subtypes of Adenocarcinoma Arising from Intraductal Papillary Mucinous Neoplasms (A-IPMN): Clinicopathological Features, Recurrence and Response to Adjuvant Chemotherapy. Ann. Surg. Oncol. 2024, 31, 7023–7032. [CrossRef]
- Park, M.A.; Gumpper-Fedus, K.; Krishna, S.G.; Genilo-Delgado, M.C.; Brantley, S.; Hart, P.A.; Dillhoff, M.E.; Gomez, M.F.; Basinski, T.L.; Mok, S.R.; et al. Molecular Pathway and Immune Profile Analysis of IPMN-Derived Versus PanIN-Derived Pancreatic Ductal Adenocarcinomas. Int. J. Mol. Sci. 2024, 25, 13164. [CrossRef]
- Luis, M.A.; Francisco, N. Robot-Assisted Corpo-Caudal Pancreatectomy and Splenectomy for Pancreatic Acinar Cell Carcinoma: A Case Report. J. Surg. Case Rep. 2025, 2025, rjaf112. [CrossRef]
- Liu, R.; Abu Hilal, M.; Besselink, M.G.; Hackert, T.; Palanivelu, C.; Zhao, Y.; He, J.; Boggi, U.; Jang, J.-Y.; Panaro, F.; et al. International Consensus Guidelines on Robotic Pancreatic Surgery in 2023. Hepatobiliary Surg. Nutr. 2024, 13, 89–104. [CrossRef]
- Celotto, F.; Ramacciotti, N.; Mangano, A.; Danieli, G.; Pinto, F.; Lopez, P.; Ducas, A.; Cassiani, J.; Morelli, L.; Spolverato, G.; et al. Da Vinci Single-Port Robotic System Current Application and Future Perspective in General Surgery: A Scoping Review. Surg. Endosc. 2024, 38, 4814–4830. [CrossRef]
- Chen, J.W.; van Ramshorst, T.M.E.; Lof, S.; Al-Sarireh, B.; Bjornsson, B.; Boggi, U.; Burdio, F.; Butturini, G.; Casadei, R.; Coratti, A.; et al. Robot-Assisted Versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International, Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 3023–3032. [CrossRef]
- Dreifuss, N.H.; Cubisino, A.; Schlottmann, F.; Giulianotti, P.C. Robotic-Assisted Central Pancreatectomy: A Minimally Invasive Approach for Benign and Low-Grade Lesions. Surg. Oncol. 2022, 41, 101736. [CrossRef]
- Zureikat, A.H.; Moser, A.J.; Boone, B.A.; Bartlett, D.L.; Zenati, M.; Zeh, H.J. 250 Robotic Pancreatic Resections: Safety and Feasibility. Ann. Surg. 2013, 258, 554–559; discussion 559-562. [CrossRef]
- Wei, K.; Cheng, L.; Zheng, Q.; Tian, J.; Liu, R.; Hackert, T. Minimally Invasive Surgery versus Open Surgery for Total Pancreatectomy: A Bibliometric Review and Meta-Analysis. HPB 2023, 25, 723–731. [CrossRef]
- Brada, L.J.H.; Schouten, T.J.; Daamen, L.A.; Seelen, L.W.F.; Walma, M.S.; van Dam, R.; de Hingh, I.H.; Liem, M.S.L.; de Meijer, V.E.; Patijn, G.A.; et al. Evaluation of Short- and Long-Term Outcomes After Resection in Patients with Locally Advanced versus (Borderline) Resectable Pancreatic Cancer. Ann. Surg. 2024, 281, 1026–1031. [CrossRef]
- Farrarons, S.S.; Van Bodegraven, E.A.; Sauvanet, A.; Hilal, M.A.; Besselink, M.G.; Dokmak, S. Minimally Invasive versus Open Central Pancreatectomy: Systematic Review and Meta-Analysis. Surgery 2022, 172, 1490–1501. [CrossRef]
- Aaquist, T.; Fristrup, C.W.; Hasselby, J.P.; Hamilton-Dutoit, S.; Eld, M.; Pfeiffer, P.; Mortensen, M.B.; Detlefsen, S. Prognostic Significance of Margin Clearance in Pancreaticoduodenectomy Specimens with Pancreatic Ductal Adenocarcinoma in a Danish Population-Based Nationwide Study. HPB 2023, 25, 826–835. [CrossRef]
- Mas, L.; Lupinacci, R.M.; Cros, J.; Bachet, J.-B.; Coulet, F.; Svrcek, M. Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. Int. J. Mol. Sci. 2021, 22, 6756. [CrossRef]
- Ishizuka, M.; Shibuya, N.; Hachiya, H.; Nishi, Y.; Kono, T.; Takayanagi, M.; Nemoto, T.; Ihara, K.; Shiraki, T.; Matsumoto, T.; et al. Robotic Surgery Is Associated with a Decreased Risk of Circumferential Resection Margin Positivity Compared with Conventional Laparoscopic Surgery in Patients with Rectal Cancer Undergoing Mesorectal Excision: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2024, 50, 108538. [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; De Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society ( ENETS ) 2023 Guidance Paper for Nonfunctioning Pancreatic Neuroendocrine Tumours. J. Neuroendocrinol. 2023, 35, e13343. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).