1. Introduction
The advent of immune checkpoint inhibitors (ICIs) has revolutionized cancer therapy, significantly improving survival outcomes and quality of life in patients with advanced malignancies[
1]. Despite their clinical benefits, ICIs are associated with a broad spectrum of immune-related adverse events (irAEs), most commonly affecting the skin, thyroid, gastrointestinal tract, liver, and pituitary gland[
2]. Among these, immune-related colitis (ir-colitis) presents a particular concern. While the overall incidence of ir-colitis is estimated at approximately 11%, a meta-analysis of PD-1 inhibitor monotherapy reported a much lower incidence of around 0.9%, with grade 3–4 events occurring in only about 0.6% of patients[
3,
4]. Clinically, ir-colitis typically presents with diarrhea (with or without mucus or bloody stools), abdominal pain, and weight loss, with endoscopic findings including ulceration, erythema, and loss of vascular architecture. However, its insidious onset and nonspecific gastrointestinal symptoms often overlap with infectious or chemotherapy-induced colitis, contributing to delayed diagnosis and suboptimal management. This delay increases the risk of refractory inflammation, impairs quality of life, and imposes a substantial healthcare burden.
Sintilimab, a novel PD-1 inhibitor, has demonstrated potent antitumor efficacy across multiple solid tumors. Nevertheless, it is also associated with various irAEs, including immune-mediated diabetes mellitus[
5], thyroid dysfunction[
6], dermatologic toxicities[
7], and urologic adverse events[
8]. Notably, sintilimab-induced colitis is rarely reported, with an estimated incidence of <1%, and severe (grade ≥3) cases occurring in fewer than 0.5% of treated patients[
9]. This underreporting, combined with the lack of standardized diagnostic and therapeutic protocols, may contribute to delayed clinical recognition, leading to treatment interruptions, disease progression, and diminished patient autonomy.
From a clinical pharmacist’s and pharmacotherapeutic management perspective, this case represents a detailed report of sintilimab-induced colitis in an elderly patient with advanced endometrial cancer. By integrating insights from immunology, pharmacology, and clinical practice, and through a multidisciplinary team (MDT) approach, we highlight key aspects of ir-colitis pathogenesis, early detection, and evidence-based treatment strategies. This case highlights the crucial role of clinical pharmacists within the multidisciplinary team (MDT) in facilitating timely pharmacovigilance to optimize the safety and effectiveness of ICI- based immunotherapy.
2. Case Report
2.1. The Patient’s History and Clinical Presentation
A 68-year-old woman (156 cm, 51 kg, BMI 20.96 kg/m²) with stage IIIA moderately to poorly differentiated endometrial adenocarcinoma underwent laparoscopic radical surgery in September 2018. Following the surgery, she received 20 cycles of chemotherapy with paclitaxel (270 mg) and carboplatin (600 mg), experiencing nausea and vomiting but no abdominal pain or diarrhea.
In August 2023, tumor recurrence was documented, leading to the initiation of camrelizumab (200 mg) plus chemotherapy. Ten days post-administration, the patient developed grade 1 immune-related diarrhea, characterized by approximately three episodes daily and a fecal volume of about 400 mL, which resolved spontaneously without intervention. We hypothesized that this adverse effect was related to camrelizumab, as this humanized anti-PD-1 monoclonal antibody has a broader tissue distribution in the intestine, potentially triggering excessive activation of the local immune microenvironment[
10]. To reduce the risk of further immune-mediated intestinal toxicity, her subsequent immunotherapy cycle (September 14, 2023) switched to sintilimab (200 mg), a fully human PD-1 monoclonal antibody, which is anticipated to be less immunogenic, thereby reducing immune-mediated intestinal inflammation.
However, 40 days after sintilimab administration (October 23, 2023), the patient developed worsening diarrhea of uncertain cause. Initially, she experienced yellow, formed stools (5–10 times per day), which progressed over the next six days to yellow, watery stools (10–15 times per day) of variable volume, accompanied by intermittent fever (up to 37.7°C) and periumbilical colicky pain (VAS score of 3). Treatment with montmorillonite powder and Bacillus licheniformis provided no relief.
Ten days later, the patient presented with purulent and bloody stools (without mucus) and persistent abdominal pain. Levofloxacin and symptomatic therapy at an external facility failed to yield significant improvement. Seventeen days later, her diarrhea worsened, characterized by watery, bloody stools with a blood volume of approximately 10 mL, occurring 10–16 times per day, and a total daily volume ranging from 1000 to 1500 mL. The patient experienced intermittent diarrhea for about 20 days, during which anti-diarrheal agents and empirical antibiotic therapy proved ineffective, and her condition continued to worsen. The patient had no history of long-term use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). The patient’s surgical and prior medication history is summarized in
Supplementary Table S1.
2.2. The Patient’s Treatment Process
On November 13, 2023, the patient was admitted for further evaluation and management. Upon admission, her vital signs were stable (temperature: 36.4°C, pulse: 68 bpm, respiratory rate: 18 breaths/min, blood pressure: 118/64 mmHg), but the physical examination revealed hyperactive bowel sounds (6 beats/min). Laboratory investigations demonstrated markedly elevated fecal calprotectin (183.23 μg/g), C-reactive protein (18 mg/L), IL-8 (36.65 pg/mL), IL-9 (1.81 pg/mL), G-CSF (12.38 pg/mL), indicative of active intestinal inflammation, while inflammatory markers, including white blood cell count, procalcitonin, and cytomegalovirus, remained within normal limits. Tests for Clostridium difficile toxins and antigens, antinuclear antibodies (ANA), and T-SPOT were negative, and the patient had no history of lactose intolerance. Whole blood biochemical parameters, electrolyte levels before and during treatment, cytokine and PCT levels are presented in the
Supplementary Table S2 and
Figures S1–S3.
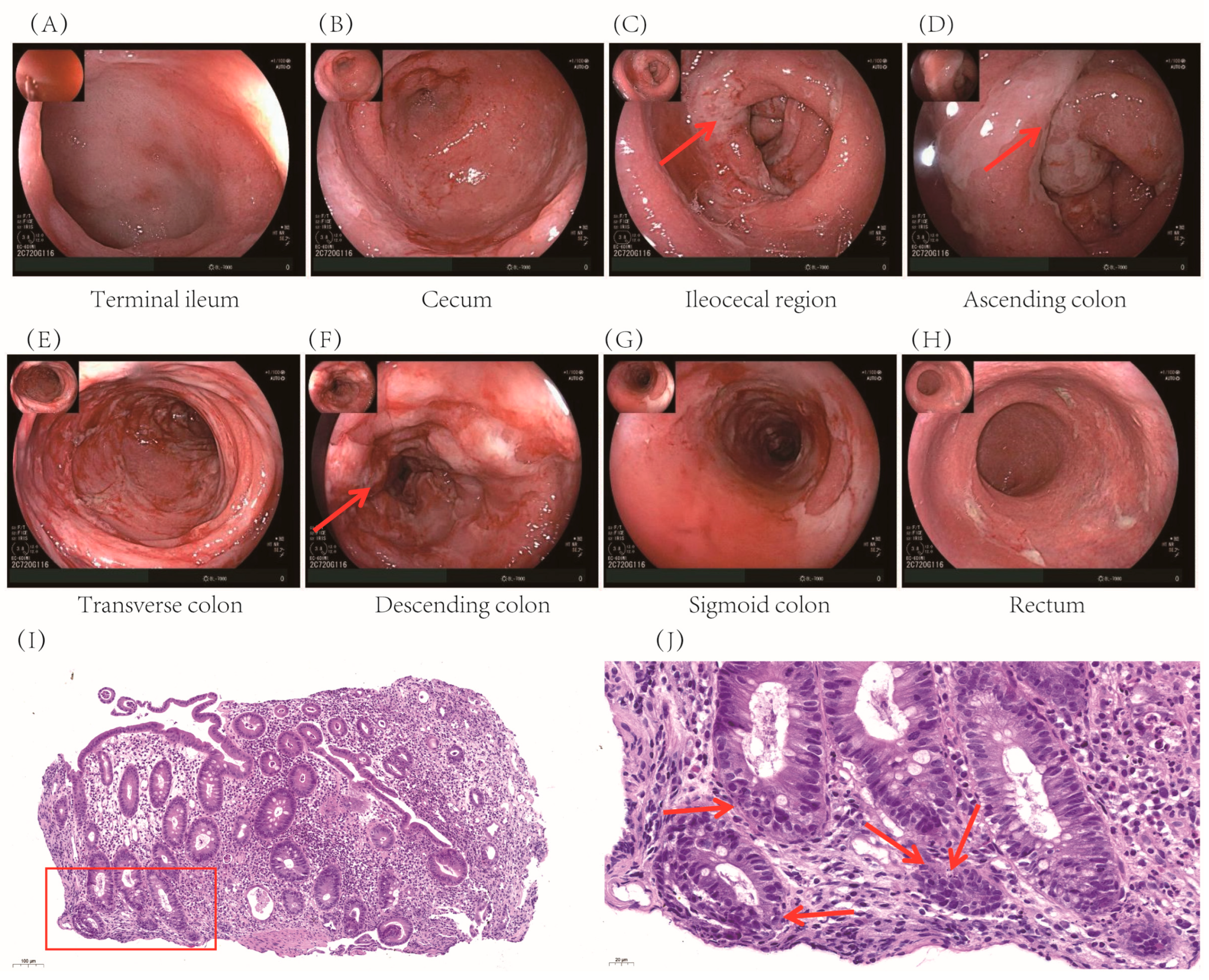
Colonoscopy revealed extensive mucosal edema from the terminal ileum to the colon, along with spasm, pseudomembrane formation, and friable mucosa prone to hemorrhage (
Figure 1 A-H). Histopathological examination confirmed significant colonic inflammation, characterized by mucosal erosion, tissue necrosis, and inflammatory exudates, findings consistent with immune-related colitis (
Figure 1 I-J).
The patient was initially treated with intravenous cefoperazone (1 g, 3 times daily) and metronidazole (0.5 g, 2 times daily) for broad-spectrum antibiotic coverage, oral montmorillonite powder (3 g, 3 times daily) for symptomatic control of diarrhea, and bifidobacterium triple viable capsules (840 mg, 2 times daily) to regulate the gut microbiota. Despite treatment, the patient experienced persistent diarrhea (15–20 episodes/day). On November 17, intravenous methylprednisolone (40 mg/day) was initiated; however, after 3 days, stool frequency remained at 10–20 times/day.
Given the refractory nature of the symptoms, a multidisciplinary team (MDT) consultation, including gastroenterologists, oncologists, a pathologist, and a clinical pharmacist, was conducted to reassess the patient’s clinical history, disease progression, and differential diagnosis. From the perspective of pharmacotherapy management, the clinical pharmacist reviewed the patient’s medication history, evaluated the clinical trajectory, and strongly suspected grade 3 ir-colitis. Based on the NCCN Guidelines for Management of Immune-Related Adverse Events, the pharmacist recommended escalating methylprednisolone to 1–2 mg/kg/day[
11]. Consequently, the dose was increased to 40 mg IV BID (total 80 mg/day) on November 20, with a taper planned over 4–6 weeks. The patient was maintained on a nil per os (NPO) status and received nutritional support with a caloric target of 1,560 kcal/day and protein intake of 1–1.5 g/kg/day. Nutritional supplementation consisted of 27 scoops per day of oral enteral nutrition powder, IV medium- and long-chain triglyceride (MCT/LCT) fat emulsion (250 mL), and a compound amino acid solution (18AA-7).
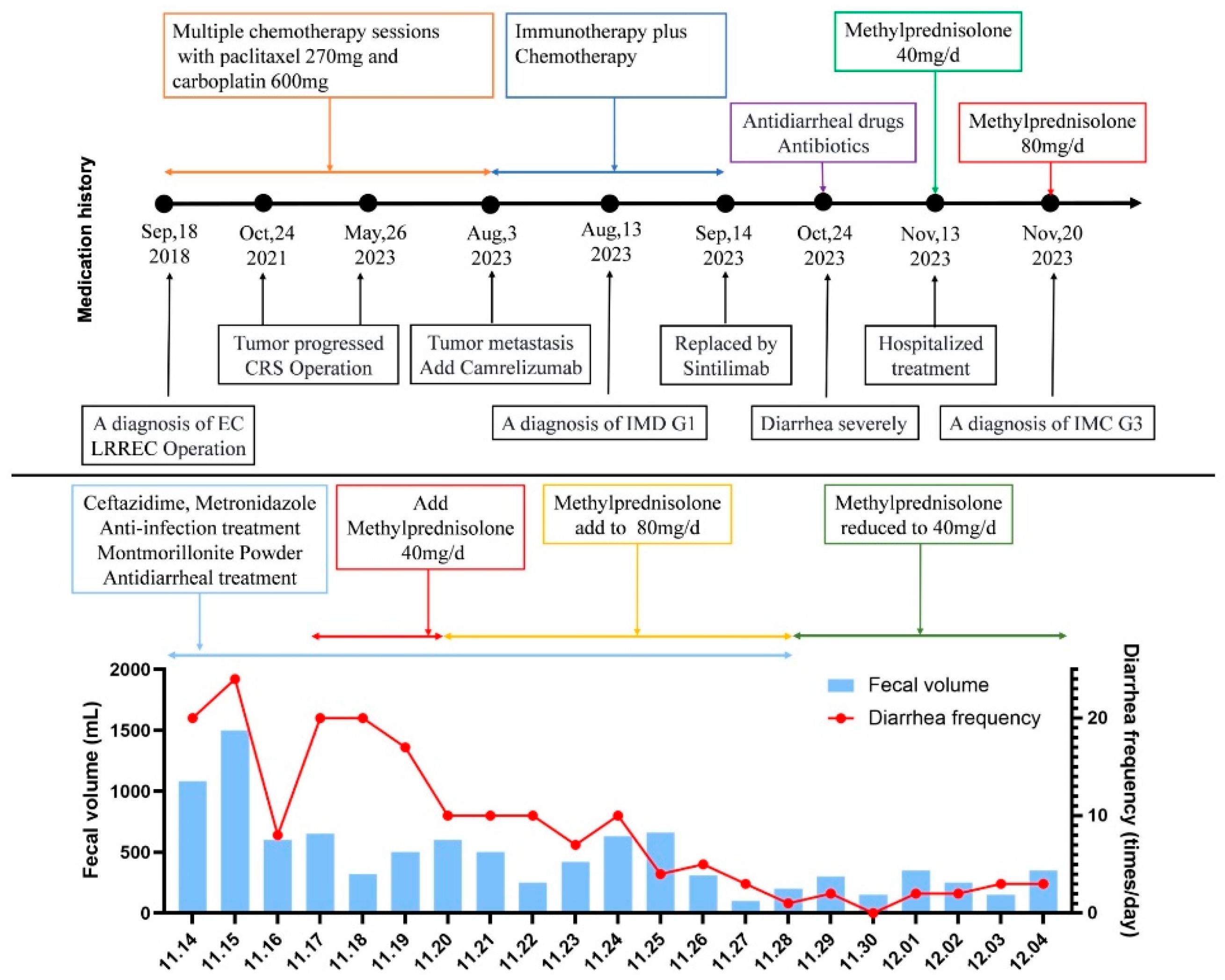
Given the expected exposure to high-dose corticosteroids (≥20 mg/day prednisone equivalent for ≥4 weeks), Pneumocystis jirovecii pneumonia (PJP) prophylaxis was initiated with oral co-trimoxazole (800 mg twice daily), as advised by the clinical pharmacist. Remarkably, one day after steroid escalation, the patient’s stool frequency decreased to <10 times/day, and by day 6, to 0–5 soft, formed stools/day (total volume: 0–500 mL/day). By December 5, 2023, the diarrhea had fully resolved, and the patient’s vital signs remained stable. Following a corticosteroid taper, the patient was discharged. The patient’s medication history and treatment responses are summarized in
Figure 2.
Due to the severity of ir-colitis, ICI therapy was discontinued in accordance with guidelines. Reinitiation was deferred until toxicity resolved to grade ≤1. At the 6-week post-discharge follow-up, the patient reported 3–4 episodes of diarrhea per day, characterized by yellow, pasty stools, with fecal occult blood testing (FOBT) remaining positive. At the 14-week follow-up, the frequency of diarrhea had decreased to 1–2 times per day, with yellow, soft stools; however, the FOBT continued to be positive. At the 1-year follow-up, the patient reported 0–2 normal bowel movements per day, a negative FOBT, and no recurrence of colitis symptoms.
3. Discussion
3.1. Assessment of Adverse Drug Reactions
The patient developed severe, progressive diarrhea on October 23, 2023, which was not observed during previous chemotherapy cycles involving paclitaxel liposomes and carboplatin, agents known to disrupt gut microbiota and sensitize intestinal mucosa. Infectious causes and tumor progression were ruled out, strengthening the likelihood of an immune-related etiology. Notably, symptoms emerged 40 days after the initiation of sintilimab, aligning well with the reported onset times of sintilimab-induced colitis, which range from 6 to 374 days, with a median onset of 112 days[
12]. Based on this timeframe, sintilimab was considered the most probable causative agent.
To quantitatively assess the causality of sintilimab-induced colitis, we applied the Naranjo Adverse Drug Reaction Probability Scale, a widely accepted tool for evaluating drug-related adverse events[
13,
14]. The patient’s total score was 6, indicating a “probable” association between sintilimab and the adverse event (details in
Supplementary Table S3). The clinical pharmacist diagnosed the patient with grade 3 immune-related colitis (ir-colitis) induced by sintilimab, which was further supported by the prompt resolution of diarrhea following the discontinuation of sintilimab and methylprednisolone therapy (40 mg IV, twice daily). Of note, the patient had previously developed grade 1 self-limiting diarrhea after receiving camrelizumab on August 3, 2023, suggesting a predisposition to ICI-induced gastrointestinal toxicity.
3.2. Mechanisms of Immune-Related Colitis
Sintilimab is a fully humanized IgG4 monoclonal antibody that targets PD-1 with high specificity and affinity for its FG loop region[
15]. Under physiological conditions, engagement of PD-1 by its ligand PD-L1 negatively regulates T cell receptor (TCR)-mediated signaling pathways, suppressing the expression of proinflammatory cytokines including IL-2 and IFN-γ[
16]. These signaling cascades are essential for maintaining peripheral immune tolerance and preventing autoreactive immune responses. Although the fully human structure of sintilimab is associated with a lower risk of irAEs[
15], its potent immunostimulatory activity can override immune homeostasis, increasing the susceptibility to ir-colitis.
PD-1 blockade enhances the proliferation and activation of effector T cells (Teff) while concurrently impairing the suppressive function of regulatory T cells (Tregs)[
17]. In patients with ir-colitis, histological analyses reveal significant infiltration of CD8⁺ tissue-resident memory T cells (Trm) in the colonic mucosa, which further differentiate into cytotoxic T lymphocytes (CTLs) capable of secreting high levels of IFN-γ and TNF-α[
18]. At the same time, Tregs dysfunction leads to diminished secretion of essential immunosuppressive cytokines, including IL-10 and TGF-β. This imbalance favors the expansion of proinflammatory Th1 and Th17 cells, resulting in increased levels of IL-17 in the gut mucosa and upregulation of chemokines such as TNF-α, IL-6, CXCL9, and CXCL10[
19,
20]. These inflammatory mediators facilitate the recruitment of additional T cells and neutrophils to inflamed tissues, amplifying the mucosal immune response. In some instances, Tregs undergo phenotypic plasticity, acquiring an IFN-γ–producing Th1-like profile[
21], thereby losing their immunosuppressive capacity and further promoting mucosal inflammation.
Histopathologically, one of the defining features of ir-colitis is the increased rate of apoptosis in colonic crypt epithelial cells[
22]. This apoptosis is primarily mediated by activated CD8⁺ CTLs through both extrinsic and intrinsic pathways, including Fas/FasL signaling leading to caspase-8–dependent extrinsic apoptosis, and the perforin-granzyme B axis, which induces epithelial cell lysis and caspase-3–dependent intrinsic apoptosis[
23]. These processes contribute to crypt architectural distortion and disruption of the epithelial barrier. Additionally, PD-1 inhibitors may induce the production of autoantibodies targeting the integrin αVβ6, an epithelial adhesion molecule, thereby further impairing epithelial integrity and exacerbating tissue damage[
24,
25].
As the intestinal barrier becomes increasingly compromised, microbial translocation of luminal bacteria and their metabolites into the lamina propria ensues, activating innate immune cells and promoting diffuse mucosal inflammation[
26]. Clinical studies have demonstrated that patients with ICI-induced colitis exhibit significantly reduced gut microbial diversity[
27]. In particular, a marked decrease in the relative abundance of
Faecalibacterium prausnitzii and
Bacteroides fragilis has been consistently observed[
28].
F. prausnitzii is known for its critical role in maintaining colonic mucosal integrity, primarily through the production of butyrate and other short-chain fatty acids that support epithelial barrier function and modulate local immune responses[
29,
30]. Meanwhile,
B. fragilis exerts potent anti-inflammatory effects by inducing IL-10 production via its capsular polysaccharide A[
31], thereby contributing to the attenuation of mucosal inflammation and the preservation of immune homeostasis.
The combination of immune dysregulation, epithelial damage, and microbial translocation results in impaired absorptive function, increased vascular permeability, and microvascular injury[
32]. Clinically, these alterations manifest as acute watery diarrhea, often accompanied by hematochezia, mucus-containing stools, and crampy abdominal pain—hallmarks of sintilimab-induced colitis. (
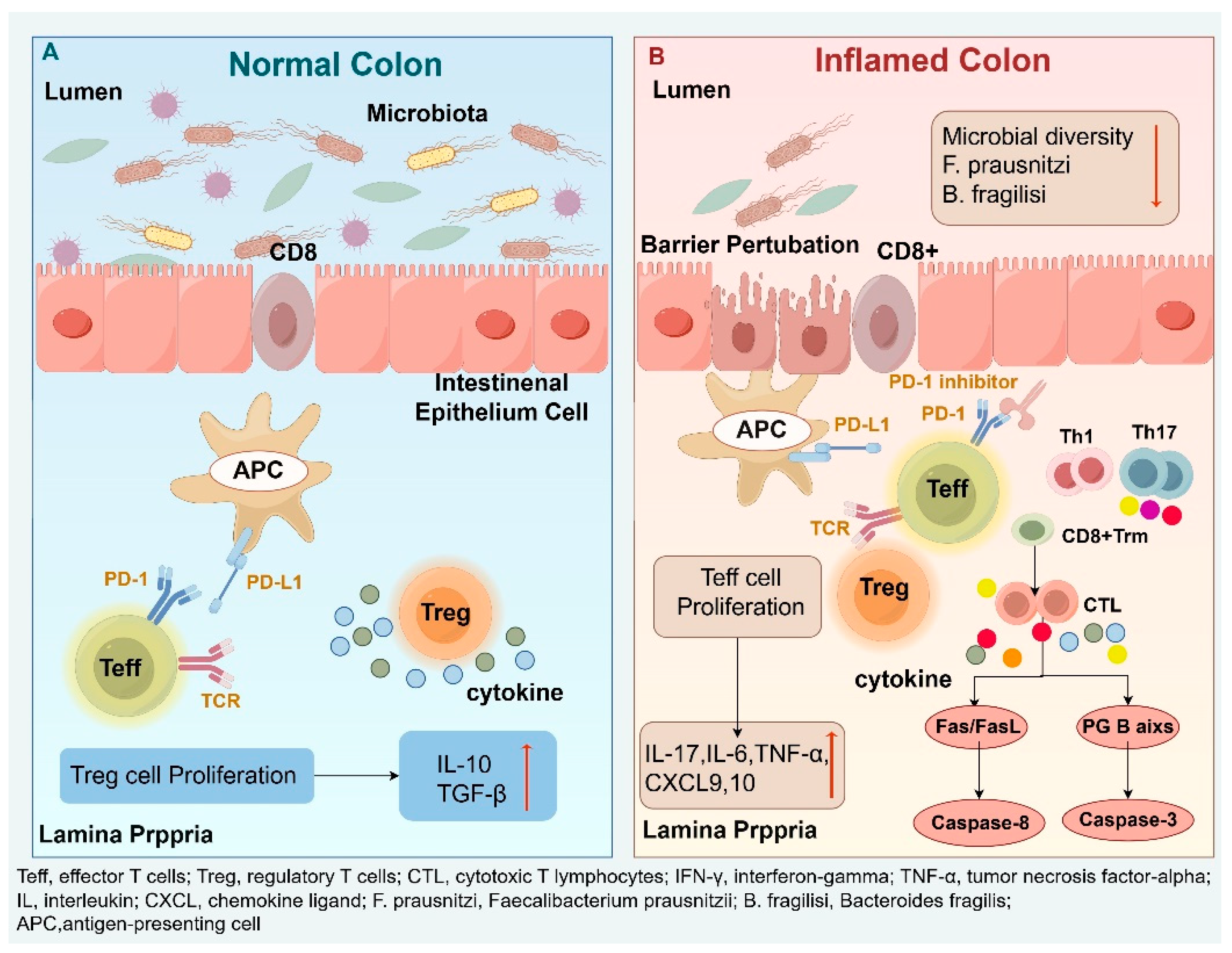
Figure 3. Mechanisms of ir-colitis provides a schematic overview of the pathophysiology, including checkpoint blockade, T-cell dysregulation, epithelial damage, and microbiota-associated factors.)
3.3. Risk Factors for ir-Colitis Development
The risk of ir-colitis associated with PD-1 blockade is influenced by the specific agent used, treatment regimen, underlying malignancy, and patient-level comorbidities. Combination therapy with PD-1 and CTLA-4 inhibitors significantly increases the risk, with an overall incidence of up to 30%[
33]. In a real-world cohort of 362 lung cancer patients, the incidence of ir-colitis varied across PD-1 inhibitors: sintilimab (3.1%), camrelizumab (3.2%), pembrolizumab (5.0%), and tislelizumab (3.6%). Grade 3–4 colitis occurred in 1.0% and 0.8% of patients treated with camrelizumab and pembrolizumab, respectively, while no high-grade events were reported in the sintilimab group[
34]. The risk appears to be dose-dependent, with ir-colitis most commonly emerging after the third treatment cycle[
35].
Additional risk factors include tumor biology and patient characteristics. Patients with melanoma have higher ir-colitis rates compared to those with non-small cell lung cancer (NSCLC) or renal cell carcinoma, likely due to distinct tumor–immune microenvironment interactions between the gut microbiome and the tumor-immune microenvironment[
36]. Individuals with pre-existing autoimmune diseases, particularly inflammatory bowel disease (IBD), face heightened susceptibility, with colitis incidence rates reaching 40% [
37,
38]. Gender differences have also been observed: data from the FDA Adverse Event Reporting System indicate that 53.5% of irritable colitis cases occurred in males versus 33.2% in females, although this disparity warrants further study[
39].
3.4. Therapeutic Management of ir-Colitis
Gastrointestinal irAEs are among the most common toxicities associated with PD-1 inhibitors, second only to dermatologic events, and more frequent than endocrine toxicities[
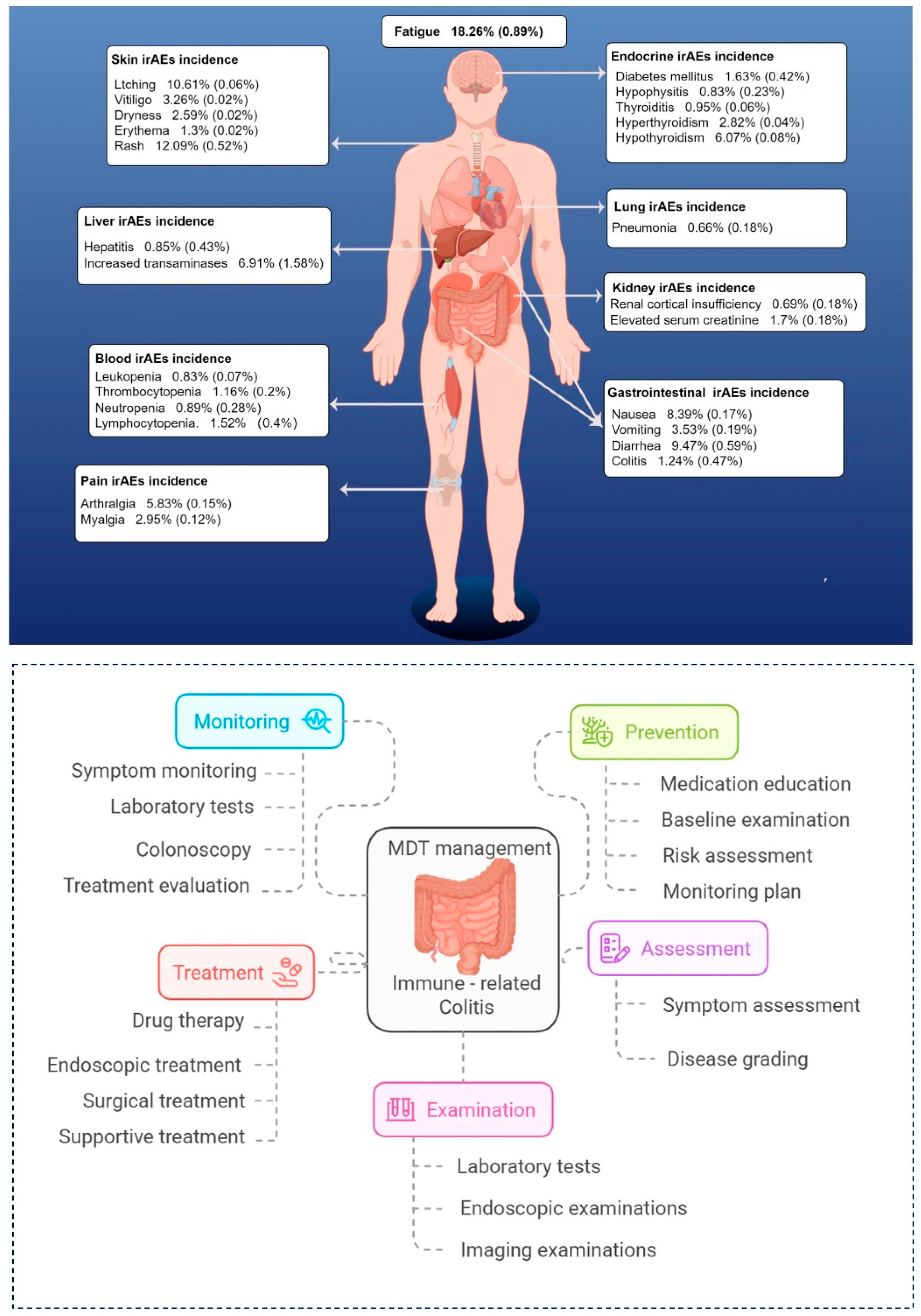
40]. However, their nonspecific presentation—diarrhea, abdominal pain—can lead to diagnostic delays. Given these challenges, an early MDT intervention is essential, involving oncologists, gastroenterologists, radiologists, pathologists, pharmacists, nutritionists, and psychologists to deliver comprehensive care. Timely identification and intervention not only improve clinical outcomes but also enhance patients’ quality of life and treatment adherence (see
Figure 4).
Management strategies are guided by evidence-based recommendations from major oncology societies, including the Society for Immunotherapy of Cancer (SITC), American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO), and Chinese Society of Clinical Oncology (CSCO)[
11,
41,
42,
43,
44]. A comparative overview is presented in
Supplementary Table S4.
For grade 1 ir-colitis, symptomatic management with loperamide, atropine, dietary modifications, and possibly mesalazine is sufficient[
11,
41,
42]. Immunotherapy can typically be continued. In grade 2 ir-colitis, infections should be ruled out, and topical budesonide (9 mg/day) is the preferred initial treatment by NCCN for its enteric-coated formulation allows targeted release in the terminal ileum and right colon, making it theoretically advantageous for localized mucosal inflammation while minimizing systemic corticosteroid exposure, particularly in cases with mild to moderate symptoms[
11]. A retrospective study by Machado et al. demonstrated that budesonide achieves remission rates comparable to systemic corticosteroids[
45]. However, budesonide has significant limitations. Its efficacy in moderate to severe or extensive colitis remains uncertain, and it is generally not recommended as monotherapy for grade 2 ir-colitis in both international guidelines and expert consensus in China[
41,
42,
43,
44], initial management still involves the oral corticosteroids, typically prednisone or methylprednisolone at a dose of 1 mg/kg/day. If symptoms fail to improve within 3–5 days, escalation to intravenous corticosteroids or biologics may be necessary.
For grade ≥3 ir-colitis, immediate discontinuation of ICIs is mandatory. Patients require hospitalization, continuous monitoring, and urgent IV corticosteroid therapy (methylprednisolone 1–2 mg/kg/day). For steroid-refractory cases, occurring in up to 60% of patients, escalation to biologics is necessary. Infliximab (5 mg/kg)[
46]or vedolizumab (α4β7 integrin antagonist) are first-line agents[
47]. Additionally, ustekinumab (an IL-12/IL-23 inhibitor) is also effective in select refractory cases[
48].
Emerging treatment strategies include Janus kinase (JAK) inhibitors and microbiota-targeted interventions. Tofacitinib (10 mg twice daily) has achieved a 75% clinical remission rate in steroid-refractory IBD[
49,
50], while upadacitinib (45 mg/day) has demonstrated rapid efficacy in severe cases[
51]. Fecal microbiota transplantation (FMT) has demonstrated a 58% clinical remission rate within four weeks and is being investigated as a salvage therapy[
52].
While novel biologics and small molecules offer hope, their use is limited by potential risks, including infection reactivation (e.g., hepatitis B, tuberculosis), systemic immunosuppression, and incomplete safety profiles. JAK inhibitors, in particular, show promise due to oral administration and rapid onset but require further validation. Future trials should evaluate the safety, efficacy, and durability of these interventions in oncologic populations[
53].
4. Conclusions
Sintilimab-induced ir-colitis represents a serious but underrecognized complication of cancer immunotherapy, underscoring the need for timely diagnosis and evidence-based management. Advancing our understanding of its immunopathogenesis, refining risk stratification, and developing targeted therapeutic approaches will be pivotal for optimizing patient outcomes and maximizing the clinical benefits of PD-1 inhibitors. Future prospective studies are warranted to validate emerging treatments and to refine clinical guidelines for managing immune-mediated gastrointestinal toxicities.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing, H.L.; Data curation, Formal analysis, Visualization, Writing – original draft, Y.P.; Investigation, Supervision, W.L.; Visualization, H.Z.; Data curation Investigation, Supervision, X.T.; Writing – review & editing, R.Z.; Funding acquisition, Writing – review & editing, Y.Z.
Funding
This research was funded by Key Clinical Projects of Peking University Third Hospital, grant number BYSYZD2022022; Peking University Medicine Sailing Program for Young Scholars’ Scientific & Technological Innovation BMU2024YFJHPY040; Fund for Returned Overseas Talents of Peking University Third Hospital BYSYLXHG2024010. Macau Science and Technology Development fund (FDCT (0012/2021/AMJ, 0001/2024/RDP,0001/2024/AKP,0092/2022/A2,0144/2022/A3)). Shenzhen-Hong Kong-Macao Science and Technology Fund (Category C: SGDX20220530111203020)
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements.
Informed Consent Statement
The participant provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/
Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bogani, G., B. J. Monk, M. A. Powell, S. N. Westin, B. Slomovitz, K. N. Moore, R. N. Eskander, F. Raspagliesi, M. P. Barretina-Ginesta, N. Colombo, and M. R. Mirza. “Adding Immunotherapy to First-Line Treatment of Advanced and Metastatic Endometrial Cancer.” Annals of Oncology : Official Journal of the European Society For Medical Oncology 35, no. 5 (2024): 414-28. [CrossRef]
- Keam, Synat, Naimah Turner, Fernanda G. Kugeratski, Rene Rico, Jocelynn Colunga-Minutti, Rayansh Poojary, Sayan Alekseev, Anisha B. Patel, Yuanteng Jeff Li, Ajay Sheshadri, Monica E. Loghin, Karin Woodman, Ashley E. Aaroe, Sarah Hamidi, Priyanka Chandrasekhar Iyer, Nicolas L. Palaskas, Yinghong Wang, and Roza Nurieva. “Toxicity in the Era of Immune Checkpoint Inhibitor Therapy.” Frontiers In Immunology 15 (2024): 1447021. [CrossRef]
- Nielsen, Dorte Lisbet, Carsten Bogh Juhl, Inna Markovna Chen, Lauge Kellermann, and Ole Haagen Nielsen. “Immune Checkpoint Inhibitor-Induced Diarrhea and Colitis: Incidence and Management. A Systematic Review and Meta-Analysis.” Cancer Treatment Reviews 109 (2022): 102440. [CrossRef]
- Bishay, K., P. Tandon, S. Bourassa-Blanchette, S. A. Laurie, and J. D. McCurdy. “The Risk of Diarrhea and Colitis in Patients with Lung Cancer Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis.” Current Oncology (Toronto, Ont.) 27, no. 5 (2020): e486-e94. [CrossRef]
- Wen, Liang, Xiuwen Zou, Yiwen Chen, Xueli Bai, and Tingbo Liang. “Sintilimab-Induced Autoimmune Diabetes in a Patient with the Anti-Tumor Effect of Partial Regression.” Frontiers In Immunology 11 (2020): 2076. [CrossRef]
- Wang, Chunliang, Ye Cai, and Pei Feng. “Case Report: A Case of Sintilimab-Induced Recurrent Diabetic Ketoacidosis and Thyroid Dysfunction in a Patient with Advanced Cervical Carcinoma.” Frontiers In Immunology 15 (2024): 1405856. [CrossRef]
- Li, Xiang, Li-Xin Qu, Yu-Mei Ren, and Chang Hu. “Case Report: A Case Report and Literature Review on Severe Bullous Skin Reaction Induced by Anti-Pd-1 Immunotherapy in a Cervical Cancer Patient.” Frontiers In Pharmacology 12 (2021): 707967. [CrossRef]
- Ji, Jiaxiang, Chin-Hui Lai, Xiaowei Zhang, and Hao Hu. “Immune-Related Adverse Events with Renal Colic as the Main Manifestation: A Case Report of Sintilimab-Induced Ureteritis/Cystitis Treated by Ureteral Stent and Review of the Literature.” Frontiers In Immunology 15 (2024): 1501415. [CrossRef]
- Suijkerbuijk, Karijn P. M., Mick J. M. van Eijs, Femke van Wijk, and Alexander M. M. Eggermont. “Clinical and Translational Attributes of Immune-Related Adverse Events.” Nature Cancer 5, no. 4 (2024): 557-71. [CrossRef]
- Ren, Shengxiang, Jianhua Chen, Xingxiang Xu, Tao Jiang, Ying Cheng, Gongyan Chen, Yueyin Pan, Yong Fang, Qiming Wang, Yunchao Huang, Wenxiu Yao, Rui Wang, Xingya Li, Wei Zhang, Yanjun Zhang, Sheng Hu, Renhua Guo, Jianhua Shi, Zhiwu Wang, Peiguo Cao, Donglin Wang, Jian Fang, Hui Luo, Yi Geng, Chunyan Xing, Dongqing Lv, Yiping Zhang, Junyan Yu, Shundong Cang, Zeyu Yang, Wei Shi, Jianjun Zou, and Caicun Zhou. “Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous Nsclc (Camel-Sq): A Phase 3 Trial.” Journal of Thoracic Oncology : Official Publication of the International Association For the Study of Lung Cancer 17, no. 4 (2022): 544-57. [CrossRef]
- Thompson, John A., Bryan J. Schneider, Julie Brahmer, Mohammad Abu Zaid, Amaka Achufusi, Philippe Armand, Meghan K. Berkenstock, Bonnie Bermas, Tawnie Braaten, Lihua E. Budde, Saurin Chokshi, Zachary D. Crees, Marianne Davies, Changchun Deng, Yaron Gesthalter, Michael Jain, Prantesh Jain, Andrew Jallouk, Benjamin H. Kaffenberger, Maya Khalil, Melissa G. Lechner, Tianhong Li, Alissa Marr, Suzanne McGettigan, Jordan McPherson, Theresa Medina, Nisha A. Mohindra, Anthony J. Olszanski, Olalekan Oluwole, Sandip P. Patel, Jason Prosek, Sunil Reddy, Pankti Reid, John Ryan, Mabel Ryder, Huda Salman, Bianca Santomasso, Scott Shofer, Jeffrey A. Sosman, Yinghong Wang, Vlad G. Zaha, Stephen Zucker, Megan Lyons, Ajibola Awotiwon, and Lisa Hang. “Nccn Guidelines® Insights: Management of Immunotherapy-Related Toxicities, Version 2.2024.” Journal of the National Comprehensive Cancer Network : JNCCN 22, no. 9 (2024): 582-92. [CrossRef]
- Hoy, Sheridan M. “Sintilimab: First Global Approval.” Drugs 79, no. 3 (2019): 341-46. [CrossRef]
- Wang, Ming-Xing, Ai-Xin Liu, Qing-Ming Sun, and Wan-Hui Dong. “Sintilimab for the Treatment of Lung Adenocarcinoma-Induced Immune-Related Hypophysitis: A Case Report.” Frontiers In Immunology 16 (2025): 1534179. [CrossRef]
- Liang, Gang, Yongmei Han, Haiyan He, Ci Lu, and Chen Zhu. “Case Report and Brief Literature Review: Possible Association of Secukinumab with Guillain-Barré Syndrome in Psoriasis.” Frontiers In Immunology 15 (2024): 1412470. [CrossRef]
- Wu, Yuan-Yuan, and Hua Shao. “Research Progress of Sintilimab in the Treatment of Cancer (Review).” Oncol Lett 29, no. 5 (2025): 240. [CrossRef]
- Tang, L., J. Wang, N. Lin, Y. Zhou, W. He, J. Liu, and X. Ma. “Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management.” Frontiers In Immunology 12 (2021): 800879. [CrossRef]
- Som, Aniruddh, Rohan Mandaliya, Dana Alsaadi, Maham Farshidpour, Aline Charabaty, Nidhi Malhotra, and Mark C. Mattar. “Immune Checkpoint Inhibitor-Induced Colitis: A Comprehensive Review.” World Journal of Clinical Cases 7, no. 4 (2019): 405-18. [CrossRef]
- Luoma, Adrienne M., Shengbao Suo, Hannah L. Williams, Tatyana Sharova, Keri Sullivan, Michael Manos, Peter Bowling, F. Stephen Hodi, Osama Rahma, Ryan J. Sullivan, Genevieve M. Boland, Jonathan A. Nowak, Stephanie K. Dougan, Michael Dougan, Guo-Cheng Yuan, and Kai W. Wucherpfennig. “Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy.” Cell 182, no. 3 (2020). [CrossRef]
- Lu, Yuming, Yifan Wang, Tiantian Ruan, Yihan Wang, Linling Ju, Mengya Zhou, Luyin Liu, Dengfu Yao, and Min Yao. “Immunometabolism of Tregs: Mechanisms, Adaptability, and Therapeutic Implications in Diseases.” Frontiers In Immunology 16 (2025): 1536020. [CrossRef]
- Alissafi, T., A. Hatzioannou, A. I. Legaki, A. Varveri, and Panayotis Verginis. “Balancing Cancer Immunotherapy and Immune-Related Adverse Events: The Emerging Role of Regulatory T Cells.” Journal of Autoimmunity 104 (2019): 102310. [CrossRef]
- Thomas, Molly Fisher, Kamil Slowikowski, Kasidet Manakongtreecheep, Pritha Sen, Nandini Samanta, Jessica Tantivit, Mazen Nasrallah, Leyre Zubiri, Neal P. Smith, Alice Tirard, Swetha Ramesh, Benjamin Y. Arnold, Linda T. Nieman, Jonathan H. Chen, Thomas Eisenhaure, Karin Pelka, Yuhui Song, Katherine H. Xu, Vjola Jorgji, Christopher J. Pinto, Tatyana Sharova, Rachel Glasser, PuiYee Chan, Ryan J. Sullivan, Hamed Khalili, Dejan Juric, Genevieve M. Boland, Michael Dougan, Nir Hacohen, Bo Li, Kerry L. Reynolds, and Alexandra-Chloé Villani. “Single-Cell Transcriptomic Analyses Reveal Distinct Immune Cell Contributions to Epithelial Barrier Dysfunction in Checkpoint Inhibitor Colitis.” Nature Medicine 30, no. 5 (2024): 1349-62. [CrossRef]
- Chen, J. H., M. K. Pezhouh, G. Y. Lauwers, and R. Masia. “Histopathologic Features of Colitis Due to Immunotherapy with Anti-Pd-1 Antibodies.” Am J Surg Pathol 41, no. 5 (2017): 643-54. [CrossRef]
- Lo, Jonathan W., Domenico Cozzetto, James L. Alexander, Nathan P. Danckert, Matthew Madgwick, Naomi Knox, Jillian Yong Xin Sieh, Marton Olbei, Zhigang Liu, Hajir Ibraheim, Jesus Miguens Blanco, Hiromi Kudo, Rocio Castro Seoane, Lucia A. Possamai, Robert Goldin, Julian Marchesi, Tamas Korcsmaros, Graham M. Lord, and Nick Powell. “Immune Checkpoint Inhibitor-Induced Colitis Is Mediated by Polyfunctional Lymphocytes and Is dependent on An il23/Ifnγ axis.” Nature Communications 14, no. 1 (2023): 6719. [CrossRef]
- Yokode, Masataka, Masahiro Shiokawa, Hisato Kawakami, Takeshi Kuwada, Yoshihiro Nishikawa, Yuya Muramoto, Hiroki Kitamoto, Makoto Okabe, Hajime Yamazaki, Norihiro Okamoto, Toshihiro Morita, Kazuya Ohno, Risa Nakanishi, Ikuhisa Takimoto, Muneji Yasuda, Koki Chikugo, Shimpei Matsumoto, Hiroyuki Yoshida, Sakiko Ota, Takeharu Nakamura, Hirokazu Okada, Tomonori Hirano, Nobuyuki Kakiuchi, Tomoaki Matsumori, Shuji Yamamoto, Norimitsu Uza, Makoto Ooi, Yuzo Kodama, Tsutomu Chiba, Hidetoshi Hayashi, and Hiroshi Seno. “Anti-Integrin Avβ6 Autoantibodies Are a Potential Biomarker for Ulcerative Colitis-Like Immune Checkpoint Inhibitor-Induced Colitis.” British Journal of Cancer 130, no. 9 (2024): 1552-60. [CrossRef]
- Bloemen, Hannah, Alexandra E. Livanos, Adrielly Martins, Richard Dean, Ana Catarina Bravo, Arno R. Bourgonje, Michael Tankelevich, Jake Herb, Judy Cho, André Anastácio Santos, Cecília M. P. Rodrigues, Francesca Petralia, Jean-Frederic Colombel, Christopher L. Bowlus, Thomas Schiano, Joana Torres, Cynthia Levy, and Saurabh Mehandru. “Anti-Integrin Avβ6 Autoantibodies Are Increased in Primary Sclerosing Cholangitis Patients with Concomitant Inflammatory Bowel Disease and Correlate with Liver Disease Severity.” Clinical Gastroenterology and Hepatology : the Official Clinical Practice Journal of the American Gastroenterological Association (2024). [CrossRef]
- Wang, Juhong, Yannan Yang, Fei Shao, Ying Meng, Dong Guo, Jie He, and Zhimin Lu. “Acetate Reprogrammes Tumour Metabolism and Promotes Pd-L1 Expression and Immune Evasion by Upregulating C-Myc.” Nature Metabolism 6, no. 5 (2024): 914-32. [CrossRef]
- Wu, Di, Luni Hu, Mengwei Han, Yichen Deng, Yime Zhang, Guanqun Ren, Xingyu Zhao, Zongxian Li, Peng Li, Yinlian Zhang, Shanwen Chen, Jun Li, Yanyan Shi, Jianxin Xue, Pengyuan Wang, and Chao Zhong. “Pd-1 Signaling Facilitates Activation of Lymphoid Tissue Inducer Cells by Restraining Fatty Acid Oxidation.” Nature Metabolism 4, no. 7 (2022): 867-82. [CrossRef]
- Kaźmierczak-Siedlecka, Karolina, Karolina Skonieczna-Żydecka, Theodore Hupp, Renata Duchnowska, Natalia Marek-Trzonkowska, and Karol Połom. “Next-Generation Probiotics - Do They Open New Therapeutic Strategies for Cancer Patients?” Gut Microbes 14, no. 1 (2022): 2035659. [CrossRef]
- Gao, Yaqi, Pingping Xu, Danfeng Sun, Yi Jiang, Xiao-Lin Lin, Ting Han, Jun Yu, Chunquan Sheng, Haoyan Chen, Jie Hong, Yingxuan Chen, Xiu-Ying Xiao, and Jing-Yuan Fang. “Faecalibacterium Prausnitzii Abrogates Intestinal Toxicity and Promotes Tumor Immunity to Increase the Efficacy of Dual Ctla4 and Pd-1 Checkpoint Blockade.” Cancer Research 83, no. 22 (2023): 3710-25. [CrossRef]
- Wang, Ying, Linjie Li, Shuze Chen, Zonglin Yu, Xuefeng Gao, Xiaojie Peng, Qiujuan Ye, Zitong Li, Weihao Tan, and Ye Chen. “Faecalibacterium Prausnitzii-Derived Extracellular Vesicles Alleviate Chronic Colitis-Related Intestinal Fibrosis by Macrophage Metabolic Reprogramming.” Pharmacological Research 206 (2024): 107277. [CrossRef]
- Yang, Hao, Yu Gan, Shenghai Jiang, Xianchang Zhu, Yang Xia, Dengmei Gong, Xianrang Xie, Yao Gong, Yi Zhang, Qian Lei, Maijian Wang, and Jida Li. “Genomic Alterations in Bacteroides Fragilis Favor Adaptation in Colorectal Cancer Microenvironment.” BMC Genomics 26, no. 1 (2025): 269. [CrossRef]
- Yu, Linda Chia-Hui. “Microbiota Dysbiosis and Barrier Dysfunction in Inflammatory Bowel Disease and Colorectal Cancers: Exploring a Common Ground Hypothesis.” Journal of Biomedical Science 25, no. 1 (2018): 79. [CrossRef]
- van Not, Olivier J., Rik J. Verheijden, Alfonsus J. M. van den Eertwegh, John B. A. G. Haanen, Maureen J. B. Aarts, Franchette W. P. J. van den Berkmortel, Christian U. Blank, Marye J. Boers-Sonderen, Jan-Willem B. de Groot, Geke A. P. Hospers, Anna M. Kamphuis, Ellen Kapiteijn, Anne M. May, Melissa M. de Meza, Djura Piersma, Rozemarijn van Rijn, Marion A. Stevense-den Boer, Astrid A. M. van der Veldt, Gerard Vreugdenhil, Willeke A. M. Blokx, Michel J. M. Wouters, and Karijn P. M. Suijkerbuijk. “Association of Immune-Related Adverse Event Management with Survival in Patients with Advanced Melanoma.” JAMA Oncology 8, no. 12 (2022): 1794-801. [CrossRef]
- Yan, Xiaoqi, Yizong Wang, Fei Wu, Guoren Zhou, Jiannan Shen, and Shaorong Yu. “Comparison of Camrelizumab, Pembrolizumab, Tislelizumab, and Sintilimab as First-Line Treatment in Patients with Non-Small Cell Lung Cancer: A Retrospective Study.” Journal of Thoracic Disease 17, no. 2 (2025): 859-71. [CrossRef]
- Sung, Changhwan, Jinhyeon An, Soohyeon Lee, Jaesoon Park, Kang Seon Lee, Il-Hwan Kim, Ji-Youn Han, Yeon Hee Park, Jee Hyun Kim, Eun Joo Kang, Min Hee Hong, Tae-Yong Kim, Jae Cheol Lee, Jae Lyun Lee, Shinkyo Yoon, Chang-Min Choi, Dae Ho Lee, Changhoon Yoo, Sang-We Kim, Jae Ho Jeong, Seyoung Seo, Sun Young Kim, Sun-Young Kong, Jung Kyoon Choi, and Sook Ryun Park. “Integrative Analysis of Risk Factors for Immune-Related Adverse Events of Checkpoint Blockade Therapy in Cancer.” Nature Cancer 4, no. 6 (2023): 844-59. [CrossRef]
- Chennamadhavuni, Adithya, Laith Abushahin, Ning Jin, Carolyn J. Presley, and Ashish Manne. “Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors.” Frontiers In Immunology 13 (2022): 779691. [CrossRef]
- Halsey, Taylor M., Anusha S. Thomas, Tomo Hayase, Weijie Ma, Hamzah Abu-Sbeih, Baohua Sun, Edwin Roger Parra, Zhi-Dong Jiang, Herbert L. DuPont, Christopher Sanchez, Rawan El-Himri, Alexandria Brown, Ivonne Flores, Lauren McDaniel, Miriam Ortega Turrubiates, Matthew Hensel, Dung Pham, Stephanie S. Watowich, Eiko Hayase, Chia-Chi Chang, Robert R. Jenq, and Yinghong Wang. “Microbiome Alteration Via Fecal Microbiota Transplantation Is Effective for Refractory Immune Checkpoint Inhibitor-Induced Colitis.” Science Translational Medicine 15, no. 700 (2023): eabq4006. [CrossRef]
- Sleiman, Joseph, Wei Wei, Ravi Shah, Muhammad Salman Faisal, Jessica Philpott, and Pauline Funchain. “Incidence of Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis (Imdc) in Patients with Cancer and Preexisting Inflammatory Bowel Disease: A Propensity Score-Matched Retrospective Study.” Journal For Immunotherapy of Cancer 9, no. 6 (2021). [CrossRef]
- Hu, Yingying, Jian Gong, Lifu Zhang, Xiaolin Li, Xina Li, Bin Zhao, and Xin Hai. “Colitis Following the Use of Immune Checkpoint Inhibitors: A Real-World Analysis of Spontaneous Reports Submitted to the Fda Adverse Event Reporting System.” International Immunopharmacology 84 (2020): 106601. [CrossRef]
- Wang, Yucai, Shouhao Zhou, Fang Yang, Xinyue Qi, Xin Wang, Xiaoxiang Guan, Chan Shen, Narjust Duma, Jesus Vera Aguilera, Ashish Chintakuntlawar, Katharine A. Price, Julian R. Molina, Lance C. Pagliaro, Thorvardur R. Halfdanarson, Axel Grothey, Svetomir N. Markovic, Grzegorz S. Nowakowski, Stephen M. Ansell, and Michael L. Wang. “Treatment-Related Adverse Events of Pd-1 and Pd-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis.” JAMA Oncology 5, no. 7 (2019): 1008-19. [CrossRef]
- Schneider, Bryan J., Jarushka Naidoo, Bianca D. Santomasso, Christina Lacchetti, Sherry Adkins, Milan Anadkat, Michael B. Atkins, Kelly J. Brassil, Jeffrey M. Caterino, Ian Chau, Marianne J. Davies, Marc S. Ernstoff, Leslie Fecher, Monalisa Ghosh, Ishmael Jaiyesimi, Jennifer S. Mammen, Aung Naing, Loretta J. Nastoupil, Tanyanika Phillips, Laura D. Porter, Cristina A. Reichner, Carole Seigel, Jung-Min Song, Alexander Spira, Maria Suarez-Almazor, Umang Swami, John A. Thompson, Praveen Vikas, Yinghong Wang, Jeffrey S. Weber, Pauline Funchain, and Kathryn Bollin. “Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: Asco Guideline Update.” Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 39, no. 36 (2021): 4073-126. [CrossRef]
- Haanen, J., M. Obeid, L. Spain, F. Carbonnel, Y. Wang, C. Robert, A. R. Lyon, W. Wick, M. Kostine, S. Peters, K. Jordan, and J. Larkin. “Management of Toxicities from Immunotherapy: Esmo Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up.” Annals of Oncology : Official Journal of the European Society For Medical Oncology 33, no. 12 (2022): 1217-38. [CrossRef]
- Brahmer, Julie R., Hamzah Abu-Sbeih, Paolo Antonio Ascierto, Jill Brufsky, Laura C. Cappelli, Frank B. Cortazar, David E. Gerber, Lamya Hamad, Eric Hansen, Douglas B. Johnson, Mario E. Lacouture, Gregory A. Masters, Jarushka Naidoo, Michele Nanni, Miguel-Angel Perales, Igor Puzanov, Bianca D. Santomasso, Satish P. Shanbhag, Rajeev Sharma, Dimitra Skondra, Jeffrey A. Sosman, Michelle Turner, and Marc S. Ernstoff. “Society for Immunotherapy of Cancer (Sitc) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events.” Journal For Immunotherapy of Cancer 9, no. 6 (2021). [CrossRef]
- Oncology, The Guideline Working Committee of the Chinese Society of Clinical. “, Chinese Society of Clinical Oncology (Csco) Guidelines for the Management of Toxicities Associated with Immune Checkpoint Inhibitors..” People’s Medical Publishing House. : Page 74.
- Machado, Antonio Pizuorno, Abdullah Salim Shaikh, Alice Saji, Malek Shatila, Isabella Glitza Oliva, Yinghong Wang, and Anusha Shirwaikar Thomas. “Outcomes of Budesonide as a Treatment Option for Immune Checkpoint Inhibitor-Related Colitis in Patients with Cancer.” Cancers 16, no. 10 (2024). [CrossRef]
- Gravina, Antonietta Gerarda, Raffaele Pellegrino, Alfonso Esposito, Marina Cipullo, Mario Romeo, Giovanna Palladino, Patrizia Iodice, Alessandro Federico, and Teresa Troiani. “The Jak-Stat Pathway as a Therapeutic Strategy in Cancer Patients with Immune Checkpoint Inhibitor-Induced Colitis: A Narrative Review.” Cancers 16, no. 3 (2024). [CrossRef]
- Harvey, Catriona, Kazi J. Nahar, Janet McKeown, Serigne N. Lo, Sheima Farag, Nadia Yousaf, Kate Young, Liselotte Tas, Aafke Meerveld-Eggink, Christian Blank, Austin Thomas, Jennifer McQuade, Bastian Schilling, Douglas B. Johnson, Roberto Martín Huertas, Ana Arance, Joanna Lee, Lisa Zimmer, Georgina V. Long, Matteo S. Carlino, Yinghong Wang, and Alexander Maxwell Menzies. “Management of Infliximab Refractory Immune Checkpoint Inhibitor Gastrointestinal Toxicity: A Multicenter Case Series.” Journal For Immunotherapy of Cancer 12, no. 1 (2024). [CrossRef]
- Shirwaikar Thomas, Anusha, Seung Eun Lee, Malek Shatila, Enrico N. De Toni, Helga-Paula Török, Najib Ben Khaled, Nicholas Powell, Ryan Weight, David M. Faleck, and Yinghong Wang. “Il12/23 Blockade for Refractory Immune-Mediated Colitis: 2-Center Experience.” The American Journal of Gastroenterology 118, no. 9 (2023): 1679-83. [CrossRef]
- Esfahani, Khashayar, Marie Hudson, and Gerald Batist. “Tofacitinib for Refractory Immune-Related Colitis from Pd-1 Therapy.” The New England Journal of Medicine 382, no. 24 (2020): 2374-75. [CrossRef]
- Holmstroem, Rikke Boedker, Emilie Kristine Dahl, Morten Helms, Henrik Vedel Nielsen, Janne Bayer Andersen, Jacob Tveiten Bjerrum, Inge Marie Svane, Eva Ellebaek, and Jakob Benedict Seidelin. “Tofacitinib and Faecal Microbiota Transplantation in Treating Checkpoint Inhibitor-Induced Enterocolitis: Case Report.” BMJ Open Gastroenterology 9, no. 1 (2022). [CrossRef]
- Kono, Masashi, Yoriaki Komeda, Hisato Kawakami, Satoru Hagiwara, George Tribonias, Kohei Handa, Shunsuke Omoto, Mamoru Takenaka, Hiroshi Kashida, Naoko Tsuji, and Masatoshi Kudo. “Jak Inhibitor Upadacitinib Induces Remission in Refractory Immune-Related Colitis Triggered by Ctla-4 and Pd-1 Inhibitor Combination Therapy in Malignant Pleural Mesothelioma: A Case Report.” Cancer Reports (Hoboken, N.J.) 7, no. 10 (2024): e70032. [CrossRef]
- Elkrief, Arielle, Nicholas R. Waters, Natalie Smith, Angel Dai, John Slingerland, Nathan Aleynick, Binita Febles, Pooja Gogia, Nicholas D. Socci, Melissa Lumish, Paul A. Giardina, Jamie E. Chaft, Juliana Eng, Robert J. Motzer, Robin B. Mendelsohn, Kate A. Markey, Mingqiang Zhuang, Yanyun Li, Zhifan Yang, Travis J. Hollmann, Charles M. Rudin, Marcel R. M. van den Brink, Jinru Shia, Susan DeWolf, Adam J. Schoenfeld, Matthew D. Hellmann, N. Esther Babady, David M. Faleck, and Jonathan U. Peled. “Immune-Related Colitis Is Associated with Fecal Microbial Dysbiosis and Can Be Mitigated by Fecal Microbiota Transplantation.” Cancer Immunology Research 12, no. 3 (2024): 308-21.
- Ju, Mingyi, Jiaojiao Zhang, Zhuoyuan Deng, Minjie Wei, Lianghua Ma, Ting Chen, and Lin Zhao. “Prophylactic Il-23 Blockade Uncouples Efficacy and Toxicity in Dual Ctla-4 and Pd-1 Immunotherapy.” Journal For Immunotherapy of Cancer 12, no. 7 (2024). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).