Submitted:
25 June 2025
Posted:
27 June 2025
You are already at the latest version
Abstract
Keywords:
1. Duchenne Muscular Dystrophy: Epidemiology and Pathophysiology
2. Current and Emerging Treatments
2.1. Corticosteroid Therapy
2.2. Nonsense Suppression Therapy
2.3. Antisense Oligonucleotide Therapy
2.4. Gene Therapy
2.5. Gene Editing Therapy
2.6. HDAC Inhibitor Therapy
2.7. Future Directions for Combination Therapies
3. Diagnostic Approaches in DMD
3.1. From Phenotype to Genotype: The Diagnostic Journey
3.2. Preventive Diagnostics: Prenatal and Preimplantation Testing
4. Conclusions
Acknowledgments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMD | Duchenne Muscular Dystrophy |
| BMD | Becker Muscular Dystrophy |
| HDAC | Histone Deacetylase |
| DAPC | Dystrophin-Associated Protein Complex |
| CK | Creatine Kinase |
| nNOS | neuronal Nitric Oxide Synthase |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| CHMP | Committee for Medicinal Products for Human Use |
| ASO | Antisense Oligonucleotides |
| PMO | Phosphorodiamidate Morpholino Oligomer |
| AAV | Adeno Associated Virus |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| gRNAs | guide RNAs |
| DSB | Double-Stranded Breaks |
| NHEJ | Non-Homologous End Joining |
| iPSC | induced Pluripotent Stem Cells |
| FAPs | Fibro-Adipogenic Progenitors |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| MLPA | Multiplex Ligation-dependent Probe Amplification |
| CGH | Comparative Genomic Hybridization |
| NGS | Next Generation Sequencing |
| cffDNA | cell free fetal DNA |
| NIPT | Non-Invasive Prenatal Testing |
| SGD | Single Gene Disorders |
| RHDO | Relative Haplotype Dosage |
| RMD | Relative Mutation Dosage |
| IVF | In Vitro Fertilisation |
| PGD | Pre-implantation Genetic Diagnosis |
| FISH | Fluorescence In Situ Hybridization |
References
- Mercuri, E.; Bonnemann, C.G.; Muntoni, F. Muscular Dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne Muscular Dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; Van Deutekom, J.C.T.; Fokkema, I.F.; Van Ommen, G.J.B.; Den Dunnen, J.T. Entries in the Leiden Duchenne Muscular Dystrophy Mutation Database: An Overview of Mutation Types and Paradoxical Cases That Confirm the Reading-Frame Rule. Muscle Nerve 2006, 34, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.K.; Korngut, L.; Dykeman, J.; Day, L.; Pringsheim, T.; Jette, N. A Systematic Review and Meta-Analysis on the Epidemiology of Duchenne and Becker Muscular Dystrophy. Neuromuscul. Disord. 2014, 24, 482–491. [Google Scholar] [CrossRef]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Sonnemann, K.J. Biology of the Striated Muscle Dystrophin-Glycoprotein Complex. Int. Rev. Cytol. 2008, 265, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Constantin, B. Dystrophin Complex Functions as a Scaffold for Signalling Proteins. Biochim. Biophys. Acta 2014, 1838, 635–642. [Google Scholar] [CrossRef]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef]
- Mokri, B.; Engel, A.G. Duchenne Dystrophy: Electron Microscopic Findings Pointing to a Basic or Early Abnormality in the Plasma Membrane of the Muscle Fiber. Neurology 1975, 25, 1111–1120. [Google Scholar] [CrossRef]
- Turner, P.R.; Westwood, T.; Regen, C.M.; Steinhardt, R.A. Increased Protein Degradation Results from Elevated Free Calcium Levels Found in Muscle from mdx Mice. Nature 1988, 335, 735–738. [Google Scholar] [CrossRef]
- Sander, M.; Chavoshan, B.; Harris, S.A.; Iannaccone, S.T.; Stull, J.T.; Thomas, G.D.; Victor, R.G. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13818–23. [CrossRef]
- Kim, J.H.; Kwak, H.B.; Thompson, L.V.; Lawler, J.M. Contribution of Oxidative Stress to Pathology in Diaphragm and Limb Muscles with Duchenne Muscular Dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Coletto, L.; Grumati, P.; Bonaldo, P. Misregulation of Autophagy and Protein Degradation Systems in Myopathies and Muscular Dystrophies. J. Cell Sci. 2013, 126, 5325–5333. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Puig, M.; Nagaraju, K.; Hoffman, E.P.; Villalta, S.A.; Rao, V.A.; Wakefield, L.M.; Woodcock, J. Immune-Mediated Pathology in Duchenne Muscular Dystrophy. Sci. Transl. Med. 2015, 7, 299rv294. [Google Scholar] [CrossRef]
- Dumont, N.A.; Wang, Y.X.; von Maltzahn, J.; Pasut, A.; Bentzinger, C.F.; Brun, C.E.; Rudnicki, M.A. Dystrophin Expression in Muscle Stem Cells Regulates Their Polarity and Asymmetric Division. Nat. Med. 2015, 21, 1455–1463. [Google Scholar] [CrossRef]
- Eagle, M.; Bourke, J.; Bullock, R.; Gibson, M.; Mehta, J.; Giddings, D.; Straub, V.; Bushby, K. Managing Duchenne Muscular Dystrophy—The Additive Effect of Spinal Surgery and Home Nocturnal Ventilation in Improving Survival. Neuromuscul. Disord. 2007, 17, 470–475. [Google Scholar] [CrossRef]
- Czifrus, E.; Berlau, D.J. Corticosteroids for the Treatment of Duchenne Muscular Dystrophy: A Safety Review. Expert Opin. Drug Saf. 2024, 23, 1237–1247. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Duong, T.; Joyce, N.C.; Hu, F.; Clemens, P.R.; Hoffman, E.P.; Cnaan, A.; Gordish-Dressman, H. ; CINRG Investigators. Long-Term Effects of Glucocorticoids on Function, Quality of Life, and Survival in Patients with Duchenne Muscular Dystrophy: A Prospective Cohort Study. Lancet 2018, 391, 451–461. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Sajeev, G.; Yao, Z.; McDonnell, E.; Elfring, G.; Souza, M.; Peltz, S.W.; Darras, B.T.; Shieh, P.B.; Cox, D.A.; Landry, J.; Signorovitch, J. ; ACT DMD Study Group and the Tadalafil DMD Study Group. Deflazacort vs Prednisone Treatment for Duchenne Muscular Dystrophy: A Meta-Analysis of Disease Progression Rates in Recent Multicenter Clinical Trials. Muscle Nerve 2020, 61, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Guglieri, M.; Clemens, P.R.; Perlman, S.J.; Smith, E.C.; Horrocks, I.; Finkel, R.S.; Mah, J.K.; Deconinck, N.; Goemans, N.; Haberlova, J.; Straub, V.; Mengle-Gaw, L.J.; Schwartz, B.D.; Harper, A.D.; Shieh, P.B.; De Waele, L.; Castro, D.; Yang, M.L.; Ryan, M.M.; McDonald, C.M.; Tulinius, M.; Webster, R.; McMillan, H.J.; Kuntz, N.L.; Rao, V.K.; Baranello, G.; Spinty, S.; Childs, A.M.; Sbrocchi, A.M.; Selby, K.A.; Monduy, M.; Nevo, Y.; Vilchez-Padilla, J.J.; Nascimento-Osorio, A.; Niks, E.H.; de Groot, I.J.M.; Katsalouli, M.; James, M.K.; van den Anker, J.; Damsker, J.M.; Ahmet, A.; Ward, L.M.; Jaros, M.; Shale, P.; Dang, U.J.; Hoffman, E.P. Efficacy and Safety of Vamorolone vs Placebo and Prednisone Among Boys With Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1005–1014. [Google Scholar] [CrossRef]
- Dang, U.J.; Damsker, J.M.; Guglieri, M.; Clemens, P.R.; Perlman, S.J.; Smith, E.C.; Horrocks, I.; Finkel, R.S.; Mah, J.K.; Deconinck, N.; Goemans, N.M.; Haberlová, J.; Straub, V.; Mengle-Gaw, L.; Schwartz, B.D.; Harper, A.; Shieh, P.B.; De Waele, L.; Castro, D.; Yang, M.L.; Ryan, M.M.; McDonald, C.M.; Tulinius, M.; Webster, R.I.; Mcmillan, H.J.; Kuntz, N.; Rao, V.K.; Baranello, G.; Spinty, S.; Childs, A.M.; Sbrocchi, A.M.; Selby, K.A.; Monduy, M.; Nevo, Y.; Vilchez, J.J.; Nascimento-Osorio, A.; Niks, E.H.; De Groot, I.J.M.; Katsalouli, M.; Van Den Anker, J.N.; Ward, L.M.; Leinonen, M.; D'Alessandro, A.L.; Hoffman, E.P. Efficacy and Safety of Vamorolone over 48 Weeks in Boys with Duchenne Muscular Dystrophy: A Randomized Controlled Trial. Neurology 2024, 102, e208112. [Google Scholar] [CrossRef]
- Keam, S.J. Vamorolone: First Approval. Drugs 2024, 84, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Wong, B.; Barohn, R.; Campbell, C.; Comi, G.P.; Connolly, A.M.; Day, J.W.; Flanigan, K.M.; Goemans, N.; Jones, K.J.; Mercuri, E.; Quinlivan, R.; Renfroe, J.B.; Russman, B.; Ryan, M.M.; Tulinius, M.; Voit, T.; Moore, S.A.; Lee Sweeney, H.; Abresch, R.T.; Coleman, K.L.; Eagle, M.; Florence, J.; Gappmaier, E.; Glanzman, A.M.; Henricson, E.; Barth, J.; Elfring, G.L.; Reha, A.; Spiegel, R.J.; O'donnell, M.W.; Peltz, S.W.; Mcdonald, C.M. ; PTC124-GD-007-DMD STUDY GROUP. Ataluren Treatment of Patients with Nonsense Mutation Dystrophinopathy. Muscle Nerve 2014, 50, 477–487. [Google Scholar] [CrossRef]
- McDonald, C.M.; Campbell, C.; Torricelli, R.E.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Heydemann, P.; Kaminska, A.; Kirschner, J.; Muntoni, F.; Osorio, A.N.; Schara, U.; Sejersen, T.; Shieh, P.B.; Sweeney, H.L.; Topaloglu, H.; Tulinius, M.; Vilchez, J.J.; Voit, T.; Wong, B.; Elfring, G.; Kroger, H.; Luo, X.; McIntosh, J.; Ong, T.; Riebling, P.; Souza, M.; Spiegel, R.J.; Peltz, S.W.; Mercuri, E. ; Clinical Evaluator Training Group; ACT DMD Study Group. Ataluren in Patients with Nonsense Mutation Duchenne Muscular Dystrophy (ACT DMD): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Vlcek, V.; Balabanov, P.; Salmonson, T.; Bakchine, S.; Markey, G.; Weise, M.; Schlosser-Weber, G.; Brohmann, H.; Yerro, C.P.; Mendizabal, M.R.; Stoyanova-Beninska, V.; Hillege, H.L. European Medicines Agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord. 2015 Jan;25(1):5–13. [CrossRef]
- Mercuri, E.; Osorio, A.N.; Muntoni, F.; Buccella, F.; Desguerre, I.; Kirschner, J.; Tulinius, M.; de Resende, M.B.D.; Morgenroth, L.P.; Gordish-Dressman, H.; Johnson, S.; Kristensen, A.; Werner, C.; Trifillis, P.; Henricson, E.K.; McDonald, C.M.; STRIDE and CINRG DNHS investigators. Safety and Effectiveness of Ataluren in Patients with Nonsense Mutation DMD in the STRIDE Registry Compared with the CINRG Duchenne Natural History Study (2015-2022): 2022 Interim Analysis. J. Neurol. 2023, 270, 3896–3913. [Google Scholar] [CrossRef]
- European Medicine Agency. EMA Confirms Recommendation for Non-Renewal of Authorisation of Duchenne Muscular Dystrophy Medicine Translarna. Available online: https://www.ema.europa.eu/en/news/ema-confirms-recommendation-non-renewal-authorisation-duchenne-muscular-dystrophy-medicine-translarna (accessed on 18 June 2025).
- Chwalenia, K.; Wood, M.J.A.; Roberts, T.C. Progress and Prospects in Antisense Oligonucleotide-Mediated Exon Skipping Therapies for Duchenne Muscular Dystrophy. J. Muscle Res. Cell Motil. 2025. [CrossRef] [PubMed]
- Sang, A.; Zhuo, S.; Bochanis, A.; Manautou, J.E.; Bahal, R.; Zhong, X.B.; Rasmussen, T.P. Mechanisms of Action of the US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. BioDrugs 2024, 38, 511–526. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Goemans, N. A Sequel to the Eteplirsen Saga: Eteplirsen is Approved in the United States but Was Not Approved in Europe. Nucleic Acid Ther. 2019, 29, 13–15. [Google Scholar] [CrossRef]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef]
- Lim, K.R.; Maruyama, R.; Yokota, T. Eteplirsen in the Treatment of Duchenne Muscular Dystrophy. Drug Des. Devel. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Krieg, A.M. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017, 27, 1–3. [Google Scholar] [CrossRef]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Anwar, S.; Yokota, T. Golodirsen for Duchenne Muscular Dystrophy. Drugs Today (Barc) 2020, 56, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; Corey, D.R. The 10th Oligonucleotide Therapy Approved: Golodirsen for Duchenne Muscular Dystrophy. Nucleic Acid Ther. 2020, 30, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Mercuri, E.; Straub, V.; Guglieri, M.; Seferian, A.M.; Scoto, M.; Leone, D.; Koenig, E.; Khan, N.; Dugar, A.; Wang, X.; Han, B.; Wang, D.; Muntoni, F.; SKIP-NMD Study Group. Long-Term Safety and Efficacy Data of Golodirsen in Ambulatory Patients with Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping: A First-in-Human, Multicenter, Two-Part, Open-Label, Phase 1/2 Trial. Nucleic Acid Ther. 2022, 32, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Roshmi, R.R.; Yokota, T. Pharmacological Profile of Viltolarsen for the Treatment of Duchenne Muscular Dystrophy: A Japanese Experience. Clin. Pharmacol. 2021, 13, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Roshmi, R.R.; Yokota, T. Viltolarsen for the Treatment of Duchenne Muscular Dystrophy. Drugs Today (Barc) 2019, 55, 627–639. [Google Scholar] [CrossRef]
- Komaki, H.; Nagata, T.; Saito, T.; Masuda, S.; Takeshita, E.; Sasaki, M.; Tachimori, H.; Nakamura, H.; Aoki, Y.; Takeda, S. Systemic Administration of the Antisense Oligonucleotide NS-065/NCNP-01 for Skipping of Exon 53 in Patients with Duchenne Muscular Dystrophy. Sci. Transl. Med. 2018. [CrossRef]
- Komaki, H.; Takeshima, Y.; Matsumura, T.; Ozasa, S.; Funato, M.; Takeshita, E.; Iwata, Y.; Yajima, H.; Egawa, Y.; Toramoto, T.; Tajima, M.; Takeda, S. Viltolarsen in Japanese Duchenne Muscular Dystrophy Patients: A Phase 1/2 Study. Ann. Clin. Transl. Neurol. 2020, 7, 2393–2408. [Google Scholar] [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875–879. [Google Scholar] [CrossRef]
- Assefa, M.; Gepfert, A.; Zaheer, M.; Hum, J.M.; Skinner, B.W. Casimersen (AMONDYS 45™): An Antisense Oligonucleotide for Duchenne Muscular Dystrophy. Biomedicines. 2024 Apr 20;12(4):912. [CrossRef]
- Chwalenia, K.; Wood, M.J.A.; Roberts, T.C. Progress and prospects in antisense oligonucleotide-mediated exon skipping therapies for Duchenne muscular dystrophy. J Muscle Res Cell Motil. 2025 Jan 30. [CrossRef]
- Arechavala-Gomeza, V.; López-Martínez, A.; Aartsma-Rus, A. Antisense RNA therapies for muscular dystrophies. J Neuromuscul Dis. 2025 Mar 27:22143602251324858. [CrossRef]
- Duan, D. Systemic AAV Micro-Dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther. 2018, 26, 2337–2356. [Google Scholar] [CrossRef]
- Happi Mbakam, C.; Tremblay, J.P. Gene Therapy for Duchenne Muscular Dystrophy: An Update on the Latest Clinical Developments. Expert Rev. Neurother. 2023, 23, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Muntoni, F. AAV Gene Therapy for Duchenne Muscular Dystrophy: Lessons Learned from a Phase 3 Trial. Hum. Gene Ther. 2024, 31, 541–543. [Google Scholar] [CrossRef]
- Hoy, S.M. Delandistrogene Moxeparvovec: First Approval. Drugs 2023, 83, 1323–1329. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Proud, C.M.; McDonald, C.M.; Lehman, K.J.; Goedeker, N.L.; Mason, S.; Murphy, A.P.; Guridi, M.; Wang, S.; Reid, C.; Darton, E.; Wandel, C.; Lewis, S.; Malhotra, J.; Griffin, D.A.; Potter, R.A.; Rodino-Klapac, L.R.; Mendell, J.R. Delandistrogene Moxeparvovec Gene Therapy in Ambulatory Patients (Aged ≥4 to <8 Years) with Duchenne Muscular Dystrophy: 1-Year Interim Results from Study SRP-9001-103 (ENDEAVOR). Ann. Neurol. 2023, 94, 955–968. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Expands Approval of Gene Therapy for Patients with Duchenne Muscular Dystrophy. Available online: https://www.fda.gov/news-events/press-announcements/fda-expands-approval-gene-therapy-patients-duchenne-muscular-dystrophy (accessed on 18 June 2025).
- Bhattacharyya, M.; Miller, L.E.; Miller, A.L.; Bhattacharyya, R. The FDA Approval of Delandistrogene Moxeparvovec-Rokl for Duchenne Muscular Dystrophy: A Critical Examination of the Evidence and Regulatory Process. Expert Opin. Biol. Ther. 2024, 24, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef]
- Chemello, F.; Bassel-Duby, R.; Olson, E.N. Correction of Muscular Dystrophies by CRISPR Gene Editing. J. Clin. Invest. 2020, 130, 2766–2776. [Google Scholar] [CrossRef]
- Long, C.; McAnally, J.R.; Shelton, J.M.; Mireault, A.A.; Bassel-Duby, R.; Olson, E.N. Prevention of Muscular Dystrophy in Mice by CRISPR/Cas9-Mediated Editing of Germline DNA. Science 2014, 345, 1184–1188. [Google Scholar] [CrossRef]
- Amoasii, L.; Hildyard, J.C.W.; Li, H.; Sanchez-Ortiz, E.; Mireault, A.; Caballero, D.; Harron, R.; Stathopoulou, T.R.; Massey, C.; Shelton, J.M.; Bassel-Duby, R.; Piercy, R.J.; Olson, E.N. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science. 2018 Oct 5;362(6410):86–91. [CrossRef]
- Moretti, A.; Fonteyne, L.; Giesert, F.; Hoppmann, P.; Meier, A.B.; Bozoglu, T.; Baehr, A.; Schneider, C.M.; Sinnecker, D.; Klett, K.; Fröhlich, T.; Rahman, F.A.; Haufe, T.; Sun, S.; Jurisch, V.; Kessler, B.; Hinkel, R.; Dirschinger, R.; Martens, E.; Jilek, C.; Graf, A.; Krebs, S.; Santamaria, G.; Kurome, M.; Zakhartchenko, V.; Campbell, B.; Voelse, K.; Wolf, A.; Ziegler, T.; Reichert, S.; Lee, S.; Flenkenthaler, F.; Dorn, T.; Jeremias, I.; Blum, H.; Dendorfer, A.; Schnieke, A.; Krause, S.; Walter, M.C.; Klymiuk, N.; Laugwitz, K.L.; Wolf, E.; Wurst, W.; Kupatt, C. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020 Feb;26(2):207–214. [CrossRef]
- Nance, M.E.; Shi, R.; Hakim, C.H.; Wasala, N.B.; Yue, Y.; Pan, X.; Zhang, T.; Robinson, C.A.; Duan, S.X.; Yao, G.; Yang, N.N.; Chen, S.J.; Wagner, K.R.; Gersbach, C.A.; Duan, D. AAV9 Edits Muscle Stem Cells in Normal and Dystrophic Adult Mice. Mol Ther. 2019 Sep 4;27(9):1568–1585. [CrossRef]
- Kwon, J.B.; Ettyreddy, A.R.; Vankara, A.; Bohning, J.D.; Devlin, G.; Hauschka, S.D.; Asokan, A.; Gersbach, C.A. In Vivo Gene Editing of Muscle Stem Cells with Adeno-Associated Viral Vectors in a Mouse Model of Duchenne Muscular Dystrophy. Mol Ther Methods Clin Dev. 2020 Sep 28;19:320–329. [CrossRef]
- Ali, A.; Rahman, M.Y.; Sheikh, D. The Role of CRISPR/Cas9 in Revolutionizing Duchenne's Muscular Dystrophy Treatment: Opportunities and Obstacles. Glob. Med. Genet. 2024, 11, 349–357. [Google Scholar] [CrossRef]
- Dhoke, N.R.; Kim, H.; Azzag, K.; Crist, S.B.; Kiley, J.; Perlingeiro, R.C.R. A Novel CRISPR-Cas9 Strategy to Target DYSTROPHIN Mutations Downstream of Exon 44 in Patient-Specific DMD iPSCs. Cells. 2024 Jun 4;13(11):972. [CrossRef]
- Singh, A.; Irfan, H.; Fatima, E.; Nazir, Z.; Verma, A.; Akilimali, A. Revolutionary Breakthrough: FDA Approves CASGEVY, the First CRISPR/Cas9 Gene Therapy for Sickle Cell Disease. Ann. Med. Surg. Lond. 2024, 86, 4555–4559. [Google Scholar] [CrossRef]
- Lek, A.; Wong, B.; Keeler, A.; et al. Death after High-Dose rAAV9 Gene Therapy in a Patient with Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 2023, 389, 1203–1210. [Google Scholar] [CrossRef]
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016 Jan 28;529(7587):490–5. [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; Virgin, H.W.; Listgarten, J.; Root, D.E. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016 Feb;34(2):184–191. [CrossRef]
- Sandonà, M.; Cavioli, G.; Renzini, A.; Cedola, A.; Gigli, G.; Coletti, D.; McKinsey, T.A.; Moresi, V.; Saccone, V. Histone Deacetylases: Molecular Mechanisms and Therapeutic Implications for Muscular Dystrophies. IJMS 2023, 24, 4306. [Google Scholar] [CrossRef]
- Mozzetta, C.; Sartorelli, V.; Puri, P.L. HDAC Inhibitors as Pharmacological Treatment for Duchenne Muscular Dystrophy: A Discovery Journey From Bench to Patients. Trends Mol. Med. 2024, 30, 278–294. [Google Scholar] [CrossRef]
- Aartsma-Rus, A. Histone Deacetylase Inhibition with Givinostat: A Multi-Targeted Mode of Action with the Potential to Halt the Pathological Cascade of Duchenne Muscular Dystrophy. Front. Cell Dev. Biol. 2025, 12, 1514898. [Google Scholar] [CrossRef] [PubMed]
- Consalvi, S.; Saccone, V.; Mozzetta, C. Histone Deacetylase Inhibitors: A Potential Epigenetic Treatment for Duchenne Muscular Dystrophy. Epigenomics 2014, 6, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Consalvi, S.; Saccone, V.; Giordani, L.; Minetti, G.; Mozzetta, C.; Puri, P.L. Histone Deacetylase Inhibitors in the Treatment of Muscular Dystrophies: Epigenetic Drugs for Genetic Diseases. Mol. Med. 2011, 17, 457–465. [Google Scholar] [CrossRef]
- Mozzetta, C.; Consalvi, S.; Saccone, V.; Tierney, M.; Diamantini, A.; Mitchell, K.J.; Marazzi, G.; Borsellino, G.; Battistini, L.; Sassoon, D.; Sacco, A.; Puri, P.L. Fibroadipogenic Progenitors Mediate the Ability of HDAC Inhibitors to Promote Regeneration in Dystrophic Muscles of Young, But Not Old Mdx Mice. EMBO Mol. Med. 2013, 5, 626–639. [Google Scholar] [CrossRef]
- Saccone, V.; Consalvi, S.; Giordani, L.; Mozzetta, C.; Barozzi, I.; Sandonà, M.; Ryan, T.; Rojas-Muñoz, A.; Madaro, L.; Fasanaro, P.; Borsellino, G.; De Bardi, M.; Frigè, G.; Termanini, A.; Sun, X.; Rossant, J.; Bruneau, B.G.; Mercola, M.; Minucci, S.; Puri, P.L. HDAC-Regulated MyomiRs Control BAF60 Variant Exchange and Direct the Functional Phenotype of Fibro-Adipogenic Progenitors in Dystrophic Muscles. Genes Dev. 2014, 28, 841–857. [Google Scholar] [CrossRef]
- Sandonà, M.; Consalvi, S.; Tucciarone, L.; De Bardi, M.; Scimeca, M.; Angelini, D.F.; Buffa, V.; D'Amico, A.; Bertini, E.S.; Cazzaniga, S.; Bettica, P.; Bouché, M.; Bongiovanni, A.; Puri, P.L.; Saccone, V. HDAC Inhibitors Tune miRNAs in Extracellular Vesicles of Dystrophic Muscle-Resident Mesenchymal Cells. EMBO Rep. 2020, 21, e50863. [Google Scholar] [CrossRef]
- Consalvi, S.; Tucciarone, L.; Macrì, E.; De Bardi, M.; Picozza, M.; Salvatori, I.; Renzini, A.; Valente, S.; Mai, A.; Moresi, V.; Puri, P.L. Determinants of Epigenetic Resistance to HDAC Inhibitors in Dystrophic Fibro-Adipogenic Progenitors. EMBO Rep. 2022, 23, e54721. [Google Scholar] [CrossRef] [PubMed]
- Consalvi, S.; Mozzetta, C.; Bettica, P.; Germani, M.; Fiorentini, F.; Del Bene, F.; Rocchetti, M.; Leoni, F.; Monzani, V.; Mascagni, P.; Puri, P.L.; Saccone, V. Preclinical Studies in the mdx Mouse Model of Duchenne Muscular Dystrophy with the Histone Deacetylase Inhibitor Givinostat. Mol. Med. 2013, 19, 79–87. [Google Scholar] [CrossRef]
- Bettica, P.; Petrini, S.; D'Oria, V.; D'Amico, A.; Catteruccia, M.; Pane, M.; Sivo, S.; Magri, F.; Brajkovic, S.; Messina, S.; Vita, G.L.; Gatti, B.; Moggio, M.; Puri, P.L.; Rocchetti, M.; De Nicolao, G.; Vita, G.; Comi, G.P.; Bertini, E.; Mercuri, E. Histological Effects of Givinostat in Boys with Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2016, 26, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Vilchez, J.J.; Boespflug-Tanguy, O.; Zaidman, C.M.; Mah, J.K.; Goemans, N.; Müller-Felber, W.; Niks, E.H.; Schara-Schmidt, U.; Bertini, E.; Comi, G.P.; Mathews, K.D.; Servais, L.; Vandenborne, K.; Johannsen, J.; Messina, S.; Spinty, S.; McAdam, L.; Selby, K.; Byrne, B.; Laverty, C.G.; Carroll, K.; Zardi, G.; Cazzaniga, S.; Coceani, N.; Bettica, P.; McDonald, C.M. ; EPIDYS Study Group. Safety and Efficacy of Givinostat in Boys with Duchenne Muscular Dystrophy (EPIDYS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Neurol. 2024, 23, 393–403. [Google Scholar] [CrossRef]
- Lamb, Y.N. Givinostat: First Approval. Drugs 2024, 84, 849–856. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Hiller, M.; Jiménez-Gracia, L.; van der Pal, Z.; Balog, J.; Adamzek, K.; Aartsma-Rus, A.; Spitali, P. Premature Termination Codons in the DMD Gene Cause Reduced Local mRNA Synthesis. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 16456–16464. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Hegde, M.; Ben-Omran, T.; Buccella, F.; Ferlini, A.; Gallano, P.; Howell, R.R.; Leturcq, F.; Martin, A.S.; Potulska-Chromik, A.; Saute, J.A.; Schmidt, W.M.; Sejersen, T.; Tuffery-Giraud, S.; Uyguner, Z.O.; Witcomb, L.A.; Yau, S.; Nelson, S.F. Evidence-Based Consensus and Systematic Review on Reducing the Time to Diagnosis of Duchenne Muscular Dystrophy. J Pediatr. 2019 Jan;204:305–313.e14. [CrossRef] [PubMed]
- Mercuri, E.; Pane, M.; Cicala, G.; Brogna, C.; Ciafaloni, E. Detecting Early Signs in Duchenne Muscular Dystrophy: Comprehensive Review and Diagnostic Implications. Front. Pediatr. 2023, 11, 1276144. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The Importance of Genetic Diagnosis for Duchenne Muscular Dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef]
- Nakata, K.C.F.; Pereira, P.P.S.; Riveros, B.S. Creatine Kinase Test Diagnostic Accuracy in Neonatal Screening for Duchenne Muscular Dystrophy: A Systematic Review. Clin. Biochem. 2021, 98, 1–9. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; Myhrvold, C.; Bhattacharyya, R.P.; Livny, J.; Regev, A.; Koonin, E.V.; Hung, D.T.; Sabeti, P.C.; Collins, J.J.; Zhang, F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017 Apr 28;356(6336):438–442. [CrossRef]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; Daringer, N.M.; Bosch, I.; Dudley, D.M.; O'Connor, D.H.; Gehrke, L.; Collins, J.J. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell. 2016 ;165(5):1255–1266. 19 May. [CrossRef]
- Hajian, R.; Balderston, S.; Tran, T.; deBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.Y.; Nokes, J.; Athaiya, M.; Paredes, J.; Peytavi, R.; Goldsmith, B.; Murthy, N.; Conboy, I.M.; Aran, K. Detection of Unamplified Target Genes via CRISPR-Cas9 Immobilized on a Graphene Field-Effect Transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef]
- Abbs, S.; Tuffery-Giraud, S.; Bakker, E.; Ferlini, A.; Sejersen, T.; Mueller, C.R. Best practice guidelines on molecular diagnostics in Duchenne/Becker muscular dystrophies. Neuromuscul Disord. 2010 Jun;20(6):422–7. [CrossRef]
- Bakker, M.; Birnie, E.; Robles de Medina, P.; Sollie, K.M.; Pajkrt, E.; Bilardo, C.M. Total pregnancy loss after chorionic villus sampling and amniocentesis: a cohort study. Ultrasound Obstet Gynecol. 2017 May;49(5):599–606. [CrossRef]
- Allen, S.; Young, E.; Bowns, B. Noninvasive Prenatal Diagnosis for Single Gene Disorders. Curr. Opin. Obstet. Gynecol. 2017, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chiu, R.W.; Chan, K.C.; Gao, Y.; Lau, V.Y.; Zheng, W.; Leung, T.Y.; Foo, C.H.; Xie, B.; Tsui, N.B.; Lun, F.M.; Zee, B.C.; Lau, T.K.; Cantor, C.R.; Lo, Y.M. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20458–63. [CrossRef]
- Parks, M.; Court, S.; Cleary, S.; Clokie, S.; Hewitt, J.; Williams, D.; Cole, T.; MacDonald, F.; Griffiths, M.; Allen, S. Non-Invasive Prenatal Diagnosis of Duchenne and Becker Muscular Dystrophies by Relative Haplotype Dosage. Prenat. Diagn. 2016, 36, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Zaninović, L.; Bašković, M.; Ježek, D.; Bojanac, A.K. Accuracy of Non-Invasive Prenatal Testing for Duchenne Muscular Dystrophy in Families at Risk: A Systematic Review. Diagnostics 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Parikh, F.R.; Athalye, A.S.; Kulkarni, D.K.; Sanap, R.R.; Dhumal, S.B.; Warang, D.J.; Naik, D.J.; Madon, P.F. Evolution and Utility of Preimplantation Genetic Testing for Monogenic Disorders in Assisted Reproduction—A Narrative Review. J. Hum. Reprod. Sci. 2021, 14, 329–339. [Google Scholar] [CrossRef]
- Malcov, M.; Ben-Yosef, D.; Schwartz, T.; Mey-Raz, N.; Azem, F.; Lessing, J.B.; Amit, A.; Yaron, Y. Preimplantation genetic diagnosis (PGD) for Duchenne muscular dystrophy (DMD) by triplex-nested PCR. Prenat Diagn. 2005 Dec;25(13):1200–5. [CrossRef]
- Malmgren, H.; White, I.; Johansson, S.; Levkov, L.; Iwarsson, E.; Fridström, M.; Blennow, E. PGD for dystrophin gene deletions using fluorescence in situ hybridization. Mol Hum Reprod. 2006 May;12(5):353–6. [CrossRef]
- Ren, Z.; Zeng, H.T.; Xu, Y.W.; Zhuang, G.L.; Deng, J.; Zhang, C.; Zhou, C.Q. Preimplantation genetic diagnosis for Duchenne muscular dystrophy by multiple displacement amplification. Fertil Steril. 2009 Feb;91(2):359–64. [CrossRef]
- Mongkolchaipak, S.; Piyamongkol, W.; Piyamongkolx, W. Successful strategy of comprehensive pre-implantation genetic testing for Duchenne muscular dystrophy and chromosome balance using karyomapping and fluorescent PCR. Reproductive BioMedicine Online. 2019 Aug; 39(1):e64–e65. [CrossRef]
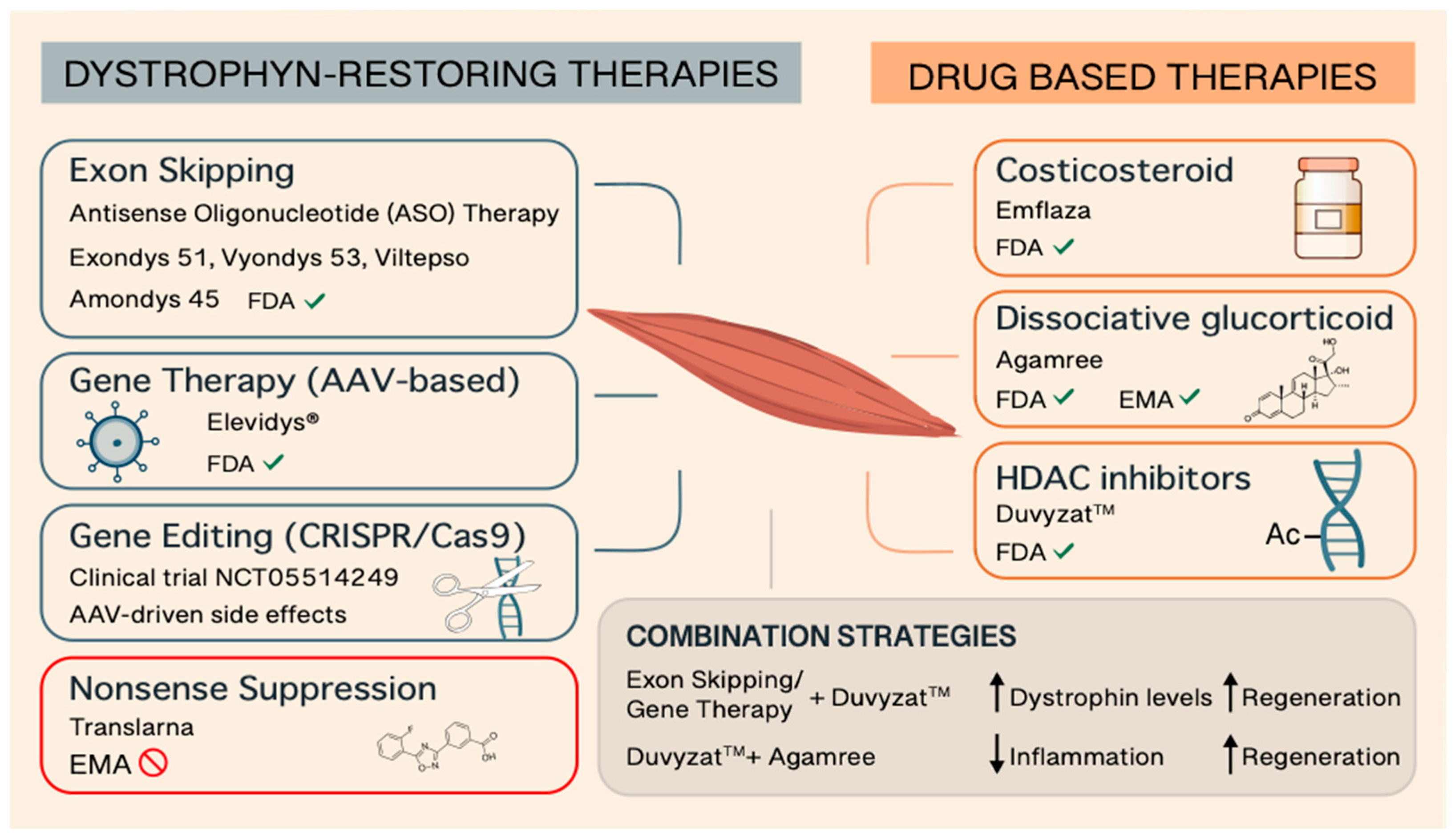
| Brand name | Active Ingredient | Manufacturer | Therapy Type | Target Patients | Approval Year | FDA /EMA | Administration | Additional Notes | References |
| EMFLAZA® | Deflazacort | PTC Therapeutics | Glucocorticoid (Steroid) | ≥2 years old patients | 2016 | Yes/no | Oral | The first FDA-approved corticosteroid treatment for DMD | 17-19 |
| AGAMREE® | Vamorolone | Santhera Pharmaceuticals | Dissociative steroid | ≥2 years old patients | 2023 | Yes/yes | Oral | The only approved medication for DMD in the European Union and the first DMD treatment approved in both the U.S. and E.U. | 20-22 |
| TRANSLARNA™ | Ataluren | PTC Therapeutics | Protein restoration therapy | ≥2 years old ambulatory patients | - | No/non-renewal | Oral | Applies to DMD caused by nonsense mutations by inducing ribosomal readthrough | 23-27 |
| EXONDYS 51™ | Eteplirsen | Sarepta Therapeutics | Exon-skipping (exon 51) | Patients with mutations amenable to exon 51 skipping | 2016 | Yes/no | Weekly IV infusion | First exon-skipping therapy approved for DMD; applies to 14% of DMD patients | 31-33 |
| VYONDYS 53™ | Golodirsen | Sarepta Therapeutics | Exon-skipping (exon 53) | Patients with mutations amenable to exon 53 skipping | 2019 | Yes/no | Weekly IV infusion | Applies to 8-10% of DMD patients | 34-37 |
| VILTEPSO™ | Viltolarsen | NS Pharma | Exon-skipping (exon 53) | Patients with mutations amenable to exon 53 skipping | 2020 | Yes/no | Weekly IV infusion | Applies to 8-10% of DMD patients | 38-41 |
| AMONDYS 45™ | Casimersen | Sarepta Therapeutics | Exon-skipping (exon 45) | Patients with mutations amenable to exon 45 skipping | 2021 | Yes/no | Weekly IV infusion | Applies to 8-9% of DMD patients | 42,43 |
| ELEVIDYS® | Delandistrogene moxeparvovec | Sarepta Therapeutics | Gene therapy (micro-dystrophin) | ≥4 years old 4 ambulatory and non-ambulatory patients | 2023 | Yes/no | Single IV infusion | One-time gene therapy | 48-52 |
| DUVYZAT™ | Givinostat | Italfarmaco S.p.A. | HDAC inhibitor (epigenetic) | ≥6 years old with any dystrophin mutation | 2024 | Yes/no | Oral | First nonsteroidal treatment for DMD approved for broad use; may be used alongside other therapies | 74-79 |
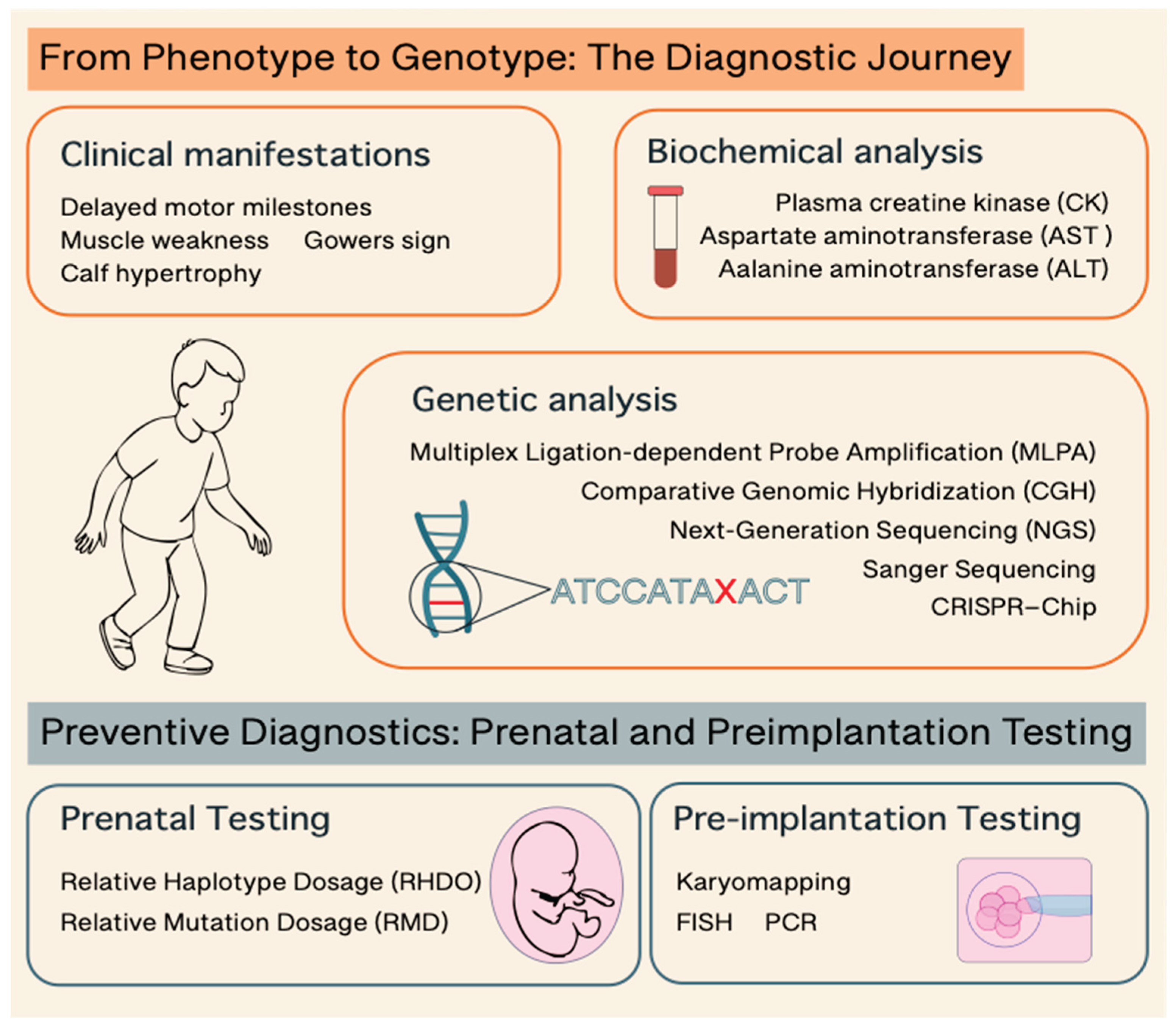
| Diagnostic Method | Invasiveness | Purpose | What It Detects | When It’s Used | Notes | References |
| Creatine Kinase (CK) Test | Non-invasive | Initial screening | Elevated CK (>10x normal) suggests muscle damage | First step in suspected DMD | High CK is common but not specific to DMD | 81-84 |
| Multiplex Ligation-dependent Probe Amplification (MLPA) | Non-invasive | Definitive diagnosis | Detects large deletions/duplications in the DMD gene | Initial genetic test for diagnosis of common DMD mutations | Cannot detect small mutations | 2,83 |
| Next Generation Sequences (NGS) | Non-invasive | Definitive diagnosis | Point mutations, deletions, duplications | Gold standard for diagnosis of all DMD mutations | Most advanced and widely used today | 2,82,83 |
| CRISPR–Chip | Non-invasive | Definitive diagnosis | Common mutations | Not yet available in clinical practice | Rapid (within 15 minutes), and bypass sequence amplification. | 85-87 |
| Muscle Biopsy | Invasive | Definitive diagnosis | Dystrophin expression via immunostaining | Rarely used today; reserved for unclear cases | Confirms lack or absence of dystrophin protein | 2,82 |
| Chorionic villus sampling (CVS)/Amniocentesis | Invasive | Prenatal Genetic Testing | In families with known DMD mutation | For at-risk families | Requires family history or prior diagnosis | 88-89 |
| Relative Haplotype Dosage (RHDO) | Non-invasive | Prenatal Genetic Testing | Mutation in cell-free fetal DNA | For at-risk families | Non suitable for detecting de novo mutations or maternal germline mosaicism. | 93 |
| Relative Mutation Dosage (RMD) | Non-invasive | Prenatal Genetic Testing | Mutation in cell-free fetal DNA | For at-risk families | Non suitable for detecting large deletions or duplications | 93 |
| FISH | Non-invasive | Pre-implantation Testing | Mutation in embryonic cell | For at-risk families using IVF | Requires known familial mutation | 95–97 |
| PCR | Non-invasive | Pre-implantation Testing | Mutation in embryonic cell | For at-risk families using IVF | Requires known familial mutation | 95–97 |
| Karyomapping | Non-invasive | Pre-implantation Testing | Mutation in embryonic cell | For at-risk families using IVF | Faster and broader genetic analysis, including both mutation detection and chromosome balance | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





