Submitted:
20 June 2025
Posted:
23 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Phases of Coagulation Testing
2.1. Pre-Analytical Variables
2.1.1. Specimen Collection
- ✓
- A minimum of 2 patient identifiers: name and date of birth (DOB).
- ✓
- Verbal acknowledgement from patients required by most facilities prior to blood collection.
- ✓
- atients with significant trauma who are not in a state to verify their name or DOB may be referred to as ‘John Doe’; for example, trauma patients, patients suffering from cardiac arrest, patients with dementia– in these cases, the information of patient(s) can be obtained from the relatives or from any previous existing patient history.
- ✓
- Patients with the same last name (e.g., twin newborns) require unique identifiers; twins may be identified as Boy/Girl A and Boy/Girl B.
- ✓
- Phlebotomist affixes label after blood collection, which is then required by the patient to confirm.
- ✓
- 19-22 gauge needles should be used for venipuncture procedure from adult patients; 23-25 gauge needles should be used for pediatric patients or patients with difficult venous access.
- ✓
- Tourniquet application should be less than 1 minute – prolonged application results in hemoconcentration and decreased blood flow, which ultimately affects test results of assays such as Factor VIII, VWF assay, leading to falsely elevated results.
- ✓
- Arterial or intravenous line collections from patient bedside requires flushing of first 10 mL of blood or six dead spaces of the vascular access device (VAD) employing the two-syringe technique generally performed by a nurse.
- ✓
- Blood collection using a butterfly or winged collection set requires use of a discard tube (this can be a blue top tube or any other tube) to remove the air in the tubing of the butterfly needle and fill its dead space, prior to collection in the 3.2% sodium citrate blue top tube. This step prevents inadequate filling of the blue top tube.
- ✓
- Proper filling of blue-top tubes is required, avoiding under or overfilling of tubes, to maintain the 9:1 blood to anticoagulant ratio.
- ✓
- Gentle inversion (5-6 times) after collection is recommended for proper mixing of blood with the sodium citrate anticoagulant.
- ✓
- Patients with elevated hematocrits (>55%) require tubes for blood collection with adjusted volume of sodium citrate.
2.1.2. Specimen Transport and Storage
- ✓
- Specimens should not be transported or stored on ice
- ✓
-
Sample stability is assay specific:
- ○
- Whole blood sample for PT is stable at 18-25oC for 24 hours from time of blood draw [4].
- ○
- For APTT, whole blood sample is stable for 6-8 hours at 18-25oC [4].
- ○
- For heparin monitoring by APTT, whole blood samples are stable for 1 hour at 18-25oC; after 1 hour there is false decrease in APTT values due to heparin neutralization by PF4 (high affinity heparin neutralizing protein). After centrifugation of the whole blood samples, the analysis of the plasma sample needs to be completed within 4 hours of collection [4].
- ○
- Other assays, stability at 18-25oC is defined as shown in the CLSI H21-Ed 6 guideline [4].
- ✓
- Specimens for platelet function tests should be maintained at 18-25oC and should not be transported via pneumatic transport tube systems to ensure sample agitation is kept at a minimum.
- ✓
-
Specimens that cannot be processed within the designated stability hours, should be centrifuged and processed for platelet poor plasma (PPP) and frozen in a -80oC freezer.
- ○
- Frozen specimens should be transported on dry ice to maintain specimen stability and integrity.
- ○
- Storage of frozen specimens at -20oC is allowed for two weeks in a non-frost-free freezer.
- ✓
- Stability of plasma samples after centrifugation and their long-term storage in frozen conditions prior to testing is defined in Appendix D of the CLSI H21-Ed 6 guideline [4].
2.1.3. Specimen Processing
- ✓
- The centrifuge should be maintained at 18-25oC.
- ✓
- ✓
- The centrifuge must be annually verified for yielding proper PPP.
- ✓
- All sample specimens must be centrifuged 2x (double centrifugation) prior to freezing.
- ✓
- If more than one blue top tube is drawn from a single patient, the samples should not be pooled together after processing and prior to freezing.
- ✓
- Use of micropore filters to remove platelets is not recommended – this leads to loss of Factors V, VIII, IX, XII and VWF.
2.1.4. Hemolysis, Icterus, Lipemia (HIL)
2.2. Analytical Variables
- ✓
- Validation of assay calibration (proof of accuracy) and internal quality control ranges (establishment shows proof of assay precision).
- ✓
- Validation of reference intervals and analytical measurement ranges (AMR).
- ✓
- Validation of therapeutic ranges (if applicable; for example – heparin therapeutic range).
- ✓
- Validation of inter-analyzer comparisons; each individual analyzer and test systems require their own evaluation and approval.
- ✓
- Verification of analyzer and IPU/ laboratory Information System (LIS) system.
- ✓
- Additional studies if required for any laboratory developed test (LDT), i.e., analytical sensitivity and specificity, specimen and reagent stability, reagent carryover [15].
- ✓
- All validation studies are summarized and evaluated by the designated laboratory director prior to implementation and reporting of patient results.
- ✓
- Routine maintenance of analyzers and document of all function and error checks.
- ✓
- Manual verification of all critical results and other LIS un-validated test results, for example, a patient sample tested for Lupus screening.
2.2.1. Proof of Accuracy
2.2.2. Proof of Assay Precision
- ✓
- Precision is performed on different assays using a single specimen or Quality control (QC) material; typically, normal and abnormal QC material is assessed.
- ✓
- Studies are designed to assess within-day and between-day variation (random error) around the target/mean value of a QC or calibrator material.
- ✓
- Within-day and between-day precision may be assessed by 2 levels of QC x 5 days x 5 replicates/run x 1 runs/day x 1 instrument [19].
- ✓
- Patient specimens are used for reproducibility of results using normal and abnormal specimens.
- ✓
- Between-day (day-to-day) precision evaluates the effect different operators, laboratory conditions and reagent lots have on results.
- ✓
-
Within-day and between-day data are evaluated by standard deviation (SD) and coefficient of variation in percent (%CV).
- ○
- The %CV gives a measure of dispersion/random error generated by the assay – determines the stability and quality of an assay.
- ○
- If the calculated SD and %CV of the precision data is less than stated by the manufacturer application or insert document, then the laboratory has shown valid proof of assay precision that satisfies the manufacturer’s claim.
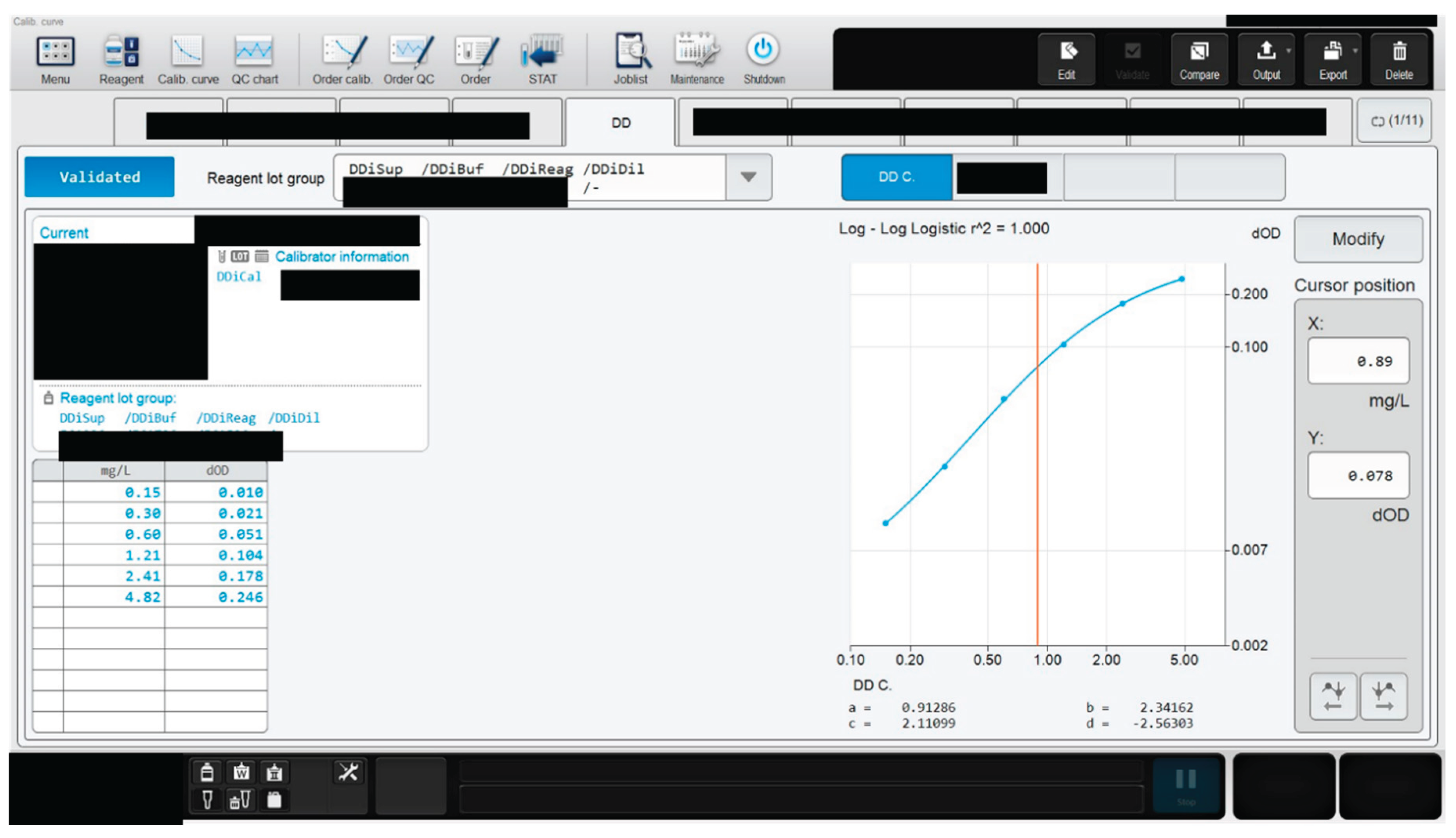
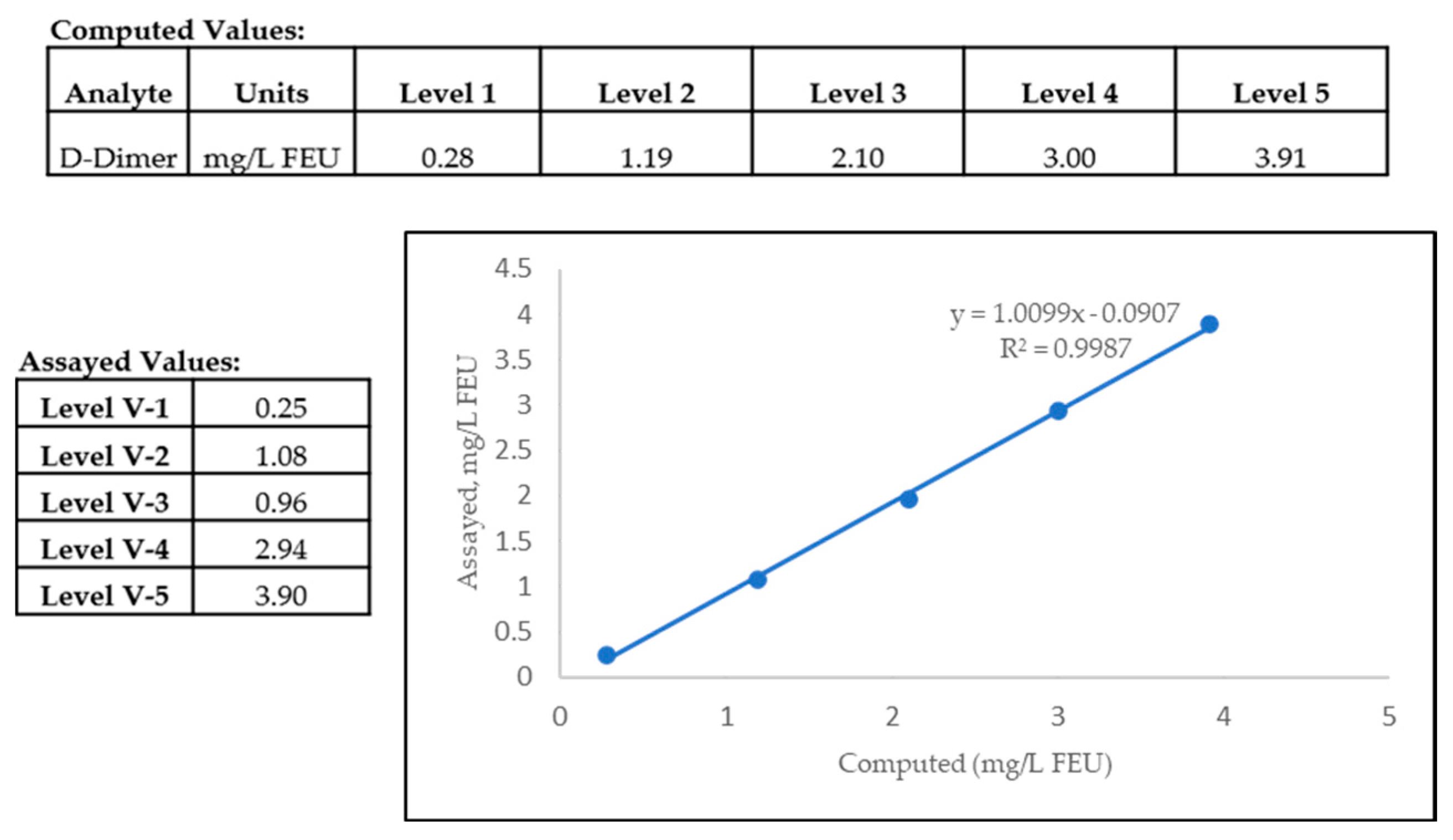
2.2.3. Determination of Assay Linearity
2.2.4. Verification of Analytical Measurement Range (AMR)
2.2.5. Analytical Sensitivity and Specificity
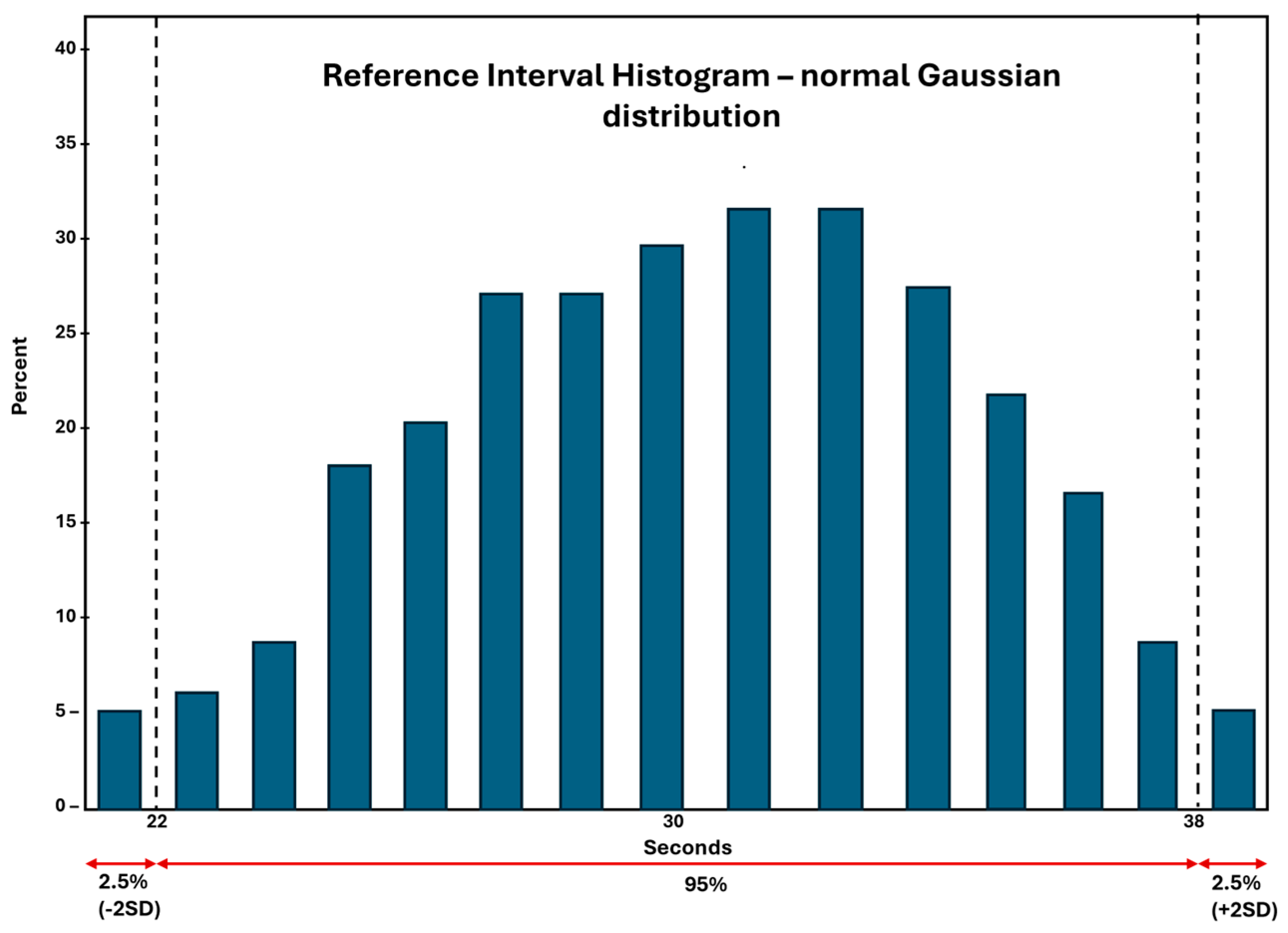
2.2.6. Establishment or Verification of Reference Interval (RI)
- ✓
- Recruitment of healthy donors that are representative of the local population the laboratory is serving.
- ✓
- Informed consent obtained from these healthy individuals that are recruited for the RI studies – the consent form clearly outlines what specimens will be obtained, amount required and how the specimens will be used by the hemostasis laboratory.
- ✓
-
Inclusion and exclusion criteria for the studies are clearly defined by the laboratory.
- ○
- Generally, the hemostasis laboratory will prepare a questionnaire that captures the information of the blood donor to determine the health status of the subject, as well as demographics such as age, gender, ethnicity and if the patient is on any current medications.
- ○
- Individuals such as smokers, and women on drugs/oral contraceptives are likely to be excluded from the healthy donor population.
- ✓
-
An equal number of healthy male and female donors are ideal for RI verification studies – for multiple assay RI verification purposes, multiple blue-top tubes may be collected and frozen at -80oC for later use.
- ○
- To establish RI for a new assay – a minimum of 120 normal healthy donors are required as per CLSI guidelines. This may be difficult, and, in most cases, the laboratory can verify the assay ranges provided in the package insert by recruiting 20-40 consented healthy donors. This process of RI verification is referred to as transference.
- ○
- ✓
- For routine assays such as PT and APTT – hemostasis laboratories may prefer verification of published RI values.
- ✓
- For special coagulation assays – frozen aliquots of normal healthy donor specimens stored at -80oC may be used and can be purchased from third party manufacturers of commercial plasmas to validate the evaluated RI for specific assays [16].
2.2.7. Quality Control in the Hemostasis Laboratory
2.2.7.1. Internal Quality Control and Assessment
- ✓
- Assessing change in the analytical measurement process.
- ✓
-
Detecting a decrease in quality of the calibrated assay/ calibration curve.
- ○
- QC assay keeps in check the calibration verification of an assay.
- ✓
- Confirming reproducibility of testing – precision and accuracy.
- Each month, peer group reports on the hemostasis analyzer controls for routine tests like PT, APTT and fibrinogen are received that provide a general overview of statistics (i.e. Mean, SD and %CV).
- A consistent difference between the laboratory’s Mean and the group’s Mean, or a consistent mean difference over several months of data will be an indicator of possible instrument bias, which would result in instrument performance needing to be verified.
- If the laboratory’s QC limits and target value is within the specified Inter-Laboratory Q.C. Programs limit for acceptability for all parameters, then no action needs to be taken.
- If the laboratory’s QC limits and target value is outside the specified Inter-Laboratory Q.C. Programs limit for acceptability for any parameter, then corrective action is taken by the laboratory.
- %CV is always compared to the previous month %CV and current month’s group %CV. The group %CV is a cumulative of all participating laboratories in the peer review program. If the current month’s %CV has increased from the previous month, but is still lower than the group %CV, then this will not be considered statistically significant. If the current month’s %CV has increased from the previous month and is also higher than the group %CV, this is then investigated by the laboratory.
2.2.7.2. External Quality Control (EQA) and Assessment
- ✓
- Confirm that each test parameter (for example, PT, APTT, FVIII) is performing accurately over a period of time by comparing the data with peer groups across different laboratories – using specimens distributed by EQA, at definite time intervals of each year.
- ✓
- Target values are assigned for different assays, taking into account assay methods, reagents and differences in hemostasis instruments manufactured by different vendors.
- ✓
- EQA specimens are assessed in the same manner as patient specimens.
- ✓
- Submitted results are evaluated against the designated target values – statistical methods are used by the EQA assessment agencies to assign assay performance reports that are distributed to the laboratories.
- ✓
-
Comments are generated for tests that deviate significantly from target values/ allowable limits of error (commonly < or > than ± 2SD).
- ○
- Consecutive failures in proficiency testing results in an assay being taken out of service, necessitating a thorough investigation by the laboratory identifying the root cause of failures, prior to returning it back to the provided test menu.
- ✓
- College of American Pathologists (CAP)
- ✓
- American Proficiency Institute (API)
- ✓
- North American Specialized Coagulation Laboratory Association (NASCOLA) in affiliation with External Quality Control of Diagnostic Assays and Tests (ECAT)
- ✓
- State Health agencies like Wisconsin State Laboratory Hygiene (WSLH) Proficiency testing
2.2.8. Diagnostic Efficacy of an Assay
2.3. Post-Analytical Variables
- ✓
- Turn round time (TAT) – daily TAT reports are checked specifically for all STAT ordered tests and stroke alert patients. In a coagulation laboratory a STAT order of PT and APTT is typically resulted within 15 minutes.
- ✓
- Semi-annual report of all the quality indices measured and observed in the laboratory.
- ✓
- Retention of all patient results that had a critical result reported, for example, a PT/INR result above 4.5 or an APTT result above 100 seconds.
- ✓
- An audit of autoverification rules and assay reference ranges programmed in the laboratory information system (LIS) are performed annually.
- ✓
- Review of all low factor VIII and factor IX results by the medical director, prior to releasing to the ordering physician. The coagulation laboratory keeps a record of these patients as known hemophilia A or hemophilia B patients, as it is likely that these patients will return for future treatments in the same facility.
- ✓
- Review of high INR results (generally greater than INR >8) by the medical director on a routine basis prior to its release to the ordering physician.
3. Integral Processes in a Laboratory to Assure Quality
4. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QA | Quality assurance |
| QC | Quality control |
| QM | Quality management |
| EQA | External quality control and assessment |
| RI | Reference Interval |
| CLSI | Clinical and Laboratory Standards Institute |
| CLIA | Clinical Laboratory Improvement Amendments |
| LDH | Lactate dehydrogenase |
| AT | Antithrombin |
| RPM | Revolutions per minute |
| RCF | Relative centrifugal force |
| AMR | Analytical measurement range |
| LIS | Laboratory Information System |
| HIL | Hemolysis, icterus, lipemia |
| LDT | Laboratory developed test |
| IFU | Instruction for use |
| TAV | Table of assigned values |
| VWF | Von Willebrand factor |
References
- J. Westgard, Basic QC Practices: Training in Statistical Quality Control for Medical Laboratories. 4th Edition ISBN-13 978-1-886958-30-2, Westgard QC, Inc., 2016.
- J. O. Westgard, "A Total Quality-Control Plan with Right-Sized Statistical Quality-Control," vol. 37, pp. 137-150, 2017. [CrossRef]
- M. Plebani, "Quality Indicators to Detect Pre-Analytical Errors in Laboratory Testing," Clin Biochem Rev, vol. 33, pp. 85-88, 2012.
- D. A. Laposata M, "“Pre-pre” and “post-post” analytical error: high-incidence patient safety hazards involving the clinical laboratory," Clin Chem Lab Med, vol. 45, no. 6, pp. 712-719, 2007.
- CLSI, "H21-ED6: Collection, Transport and Processing of Blood Specimens for testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline-," Clinical and Laboratory Standards Institute, 2024.
- CLSI, "H21-ED6-QG: Collection, Handling, Transport and Storage for Hemostasis," Clinical and Laboratory Standards Institute, 2025.
- R. a. M. R. Gosselin, "Preanalytical Variables in Coagulation Testing: Setting the Stae for Accurate results," Semin Thromb Hemost, vol. 45, no. 5, pp. 433-448, 2019. [CrossRef]
- L. G. F. E. Adcock Funk DM, "Quality standards for sample processing, transportation, and storage in hemostasis testing," Semin Thromb Hemost, vol. 38, no. 6, pp. 576-585, 2012.
- G. V. C. T. A. V. S. D’Angela, "Evaluation of the main coagulation tests in the presence of hemolysis in healthy subjects and patients on oral anticoagulant therapy," Int J Lab Hematol, vol. 37, pp. 849-833, 2015.
- D. D. R. e. a. Kitchen S, "International Council for Standardization in Haematology (ICSH) recommendations for processing of blood samples for coagulation testing," Int J Lab Hematol, vol. 43, no. 6, pp. 1272-1283, 2021.
- R. L. D. A. Nagant C, "HIL Interferences on Three Hemostasis Analyzers and Contribution of a Preanalytical Module for Routine Coagulation Assays," Clinical Laboratory, vol. 62, no. 10, pp. 1979-1987, 2016.
- Y. L. L. L. H. A. Parsons L., "Propofol Interference in Coagulation Testing.," J Clin Pathol, vol. 138, p. A285, 2012.
- G. M. D. M. D. F. Feriel J, "Impact of Drugs Used in Intensive Care on Routine Coagulation Testing," Diagnostics (Basel), vol. 15, no. 7, p. 941, 2025.
- R. L. D. A. Nagant C, "HIL Interferences on Three Hemostasis Analyzers and Contribution ofa Preanalytical Module for Routine Coagulation Assays," Clin Lab, vol. 62, no. 10, pp. 1979-1987, 2016.
- D. A. E. M. e. a. Gardiner C, "International Committee for Standardization inHematology (ICSH) Guidance on the Validation ofLaboratory Developed Tests in Haemostasis," Int J Lab Hematol, vol. 0, pp. 1-13, 2025.
- M. a. F. G. Sarkar, "Quality assurance in Hematology and Hemostasis testing," in Rodak’s Hematology: Clinical Principles and Applications, Elsevier, 2024, pp. 18-42.
- CLSI, "H57-A, Protocol for the Evaluation, Validation, and Implementation of Coagulometers; Approved Guideline," Clinical and Laboratory Standards Institute, 2008.
- D. o. H. a. H. S. Centers for Medicare & Medicaid Services (CMS), "Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations Related to Analytes and Acceptable Performance; 42 CFR Part 493 [CMS-3355-F]," 2022.
- CLSI, "CLSI EP15-Ed3-IG1. User Verification of Precision Implementation Guide. 1st ed.," Clinical and Laboratory Standards Institute, 2021.
- W. JO, "Westgard QC," [Online]. Available: https://westgard.com/resources/resources/decision.html. [Accessed 2 6 2025].
- CLSI, "EP17 ED21G:2021 Evaluation of Detection Capability Implementation Guide, 1st Edition," Clinical and Laboratory Standards Institute, 2021.
- CLSI, "EP07-ED3:2018 Interference Testing in Clinical Chemistry, 3rd Edition," Clinical and Laboratory Standards Institute, 2018.
- CLSI, "EP28 A3C: Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd Edition," Clinical and Laboratory Standards Institute, 2010.
- CLSI, "EP28 Ed3-IG: Verification of Reference Intervals in the Medical Laboratory Implementation Guide, 1st Edition," Clinical and Laboratory Standards Institute, 2022.
- V. R. Z. M. e. a. Shikdar S, "International Normalized Ratio: Assessment, Monitoring, and Clinical Implications.," StatPearls Publishing, 2025.
- B. R. C. B. O. P. S. A. Gallus AS, "Consensus guidelines for warfarin therapy. Recommendations from the Australasian Society of Thrombosis and Haemostasis.," Med J Aust, vol. 172, no. 12, pp. 600-605, 2000.
- G. J. J. M. H. J. Brill-Edwards P, "Establishing a therapeutic range for heparin therapy.," Ann Intern Med, vol. 119, no. 2, pp. 104-109, 1993.
- P. S. D. J. e. a. Baker P, "Measurement of heparin, direct oral anti-coagulants and other non-coumarin anti-coagulants and their effects on haemostasis assays: A British Society for Haematology Guideline," B J Haem, vol. 205, no. 4, pp. 1302-1318, 2024.
- W. S. Westgard JO, "Quality control review: implementing a scientifically based quality control system.," Ann Clin Biochem, vol. 53, no. Pt 1, pp. 32-50, 2016.
- PB, "Precision Biologic," Precision Biologic, 2025. [Online]. Available: https://precisionbiologic.com/content/user_files/2021/12/CRYOcheck-Lupus-Controls.pdf. [Accessed 28 5 2025].
- J. I. K. S. W. I. Reilly-Stitt C, "Internal Quality Control in Hemostasis Assays.," Semin Thromb Hemost, vol. 50, no. 8, pp. 1084-1090, 2024.
- F. E. A. D. Bonar R, "Quality in coagulation and haemostasis testing," Biochemia Medica, vol. 20, no. 2, pp. 184-199, 2010.
- P. M. O. P. e. a. Moilanen J, "Performance of Hemochron ACT-LR and ACT+ Test Cuvettes in Monitoring Low to Moderate Heparin Concentrations: An In Vitro Study," J Cardiothoracic Vasc Anesth, vol. 39, no. 2, pp. 447-452, 2025.
- G. T. V. F. Leadbetter NH, "Unique Approach to Quality Assurance in Viscoelastic Testing," J Appl Lab Med, vol. 5, no. 6, pp. 1228-1241, 2020.
- B. R. Favaloro EJ, "An update on quality control for the PFA-100/PFA-200," Platelets, vol. 29, no. 6, pp. 622-627, 2018.
- S. Medicine, "Practical-Haemostasis.com - A practical guide to haemostasis," [Online]. Available: https://practical-haemostasis.com/Platelets/VerifyNow%20Assay.html. [Accessed 5 6 2025].
- M. P. J. I. e. a. Marlar RA, "Consistent accuracy: a goal for thrombosis and hemostasis testing is accomplised using an external quality assurance program," Annals of Blood, vol. 5, no. 11, pp. 1-4, 2020.
- L. A. K. S. Montalvão SAL, "Advantages of external quality assessment-EQA programs," Haemophilia, vol. 28, no. 4, pp. 679-686, 2022.
- K. AG, "Evaluating the Effectiveness of Diagnostic Tests," JAMA, vol. 327, no. 14, pp. 1335-1336, 2022.
- S. P. E. M. Vetter TR, "Diagnostic Testing and Decision-Making: Beauty Is Not Just in the Eye of the Beholder," Anesthesia & Analgesia, vol. 127, no. 4, pp. 1085-1091, 2018.
- L. L. C. H. e. a. Murad MH, "The association of sensitivity and specificity with disease prevalence: analysis of 6909 studies of diagnostic test accuracy," CMAJ, vol. 195, no. 27, pp. 925-931, 2023.
- CLSI, "H59-A: Quantitative D-Dimer for the Exclusion of venous Thromboembolic Disease, 1st Edition," Clinical and Laboratory Standards Institute, 2011.
- W. TE, "Laboratory diagnosis of heparin-induced thrombocytopenia," Int J Lab Hematol, vol. 41, no. Suppl 1, pp. 15-25, 2019.
- E. S. D. T. Westgard JO, "CLIA Final Rules for Quality Systems: Quality Assessment Issues and Answers.," in Clinical Chemistry, vol. 51, 2005, pp. 1911-1912.
- D. o. H. a. H. Centers for Medicare & Medicaid Services, "Code of Federal Regulations, eCFR: 42 CFR 493.1105 Standard: Retention Requirements," 29 May 2025. [Online]. Available: https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-G/part-493/subpart-J/section-493.1105. [Accessed 1 6 2025].
| Patient Variable | Patient Condition |
|---|---|
| Age | Neonatal, pediatric, adult – varying reference ranges; labs may employ a separate pediatric reference range in addition to the normal adult reference range for accurate interpretation of tests results and diagnosis |
| Gender | Male/ Female – coagulation factors vary according to patient gender |
| Health Status | Patients under psychological or physiological stress (for example, after strenuous exercise) may mask or suggest a coagulopathy |
| ABO blood group | Differences may be noted in von Willebrand factor (VWF) and factor VIII levels |
| Time of Blood Collection | Patients on oral anticoagulants, with inflammatory conditions, or post traumatic event – may affect platelet function testing, special coagulation tests like protein C, protein S and lupus anticoagulant testing |
| Order of Draw | Vacutainer tube/ color of tube top/stopper |
|---|---|
| 1 | Blood culture tube – varying colored tube tops |
| 2 | 3.2% Sodium citrate – Light Blue top |
| 3 | Serum tube (glass or plastic) – Red top |
| 4 | SST – Gold or red/black or red top |
| 5 | Heparin (Sodium or Lithium) – Green top |
| 6 | EDTA – Lavender or Pink top |
| 7 | PPT, EDTA with gel – White top |
| 8 | Sodium fluoride – Gray top |
| 9 | Quantiferon – Gold top |
| Assay parameters | Acceptable criteria |
|---|---|
| Prothrombin time/ INR (PT/INR) | Target ± 15% |
| Partial Thromboplastin time (APTT) | Target ± 15% |
| Fibrinogen | Target ± 20% |
| Test (abbreviation) | Level 1 control target | Level 2 control target | Optional/ additional controls and comments |
|---|---|---|---|
| Routine coagulation | Midpoint of normal | Midpoint of therapeutic | High end of therapeutic or abnormal |
| tests (i.e., PT, INR, | reference range | (anticoagulant therapy) range | range |
| APTT, TT). | (high level) | ||
| Fibrinogen | Low end or midpoint of | Midpoint of abnormal (low level) | High end control if thrombosis-risk is |
| normal reference range | range | being assessed. | |
| D-dimer | Low end or midpoint of | Midpoint of abnormal (positive) | High end for quantitative assays, |
| normal (negative) reference | range (positive control) | especially if DIC is being ‘monitored’ | |
| range (negative control) | |||
| Factor assays | Low end or midpoint of | Midpoint of abnormal (low level) | Factor deficient plasma to identify |
| (FII, FV, FVII, FVIII, | normal reference range | range (i.e., 20–40%) | lower assay limit; high-end control |
| FIX, FX, FXI, FXII) | (i.e., 90–100%) | (≥150%) for quantitative assays if | |
| thrombosis-risk is being assessed. | |||
| FXIII | Normal plasma (negative | FXIII deficient plasma (positive | |
| control) | control) | ||
| VWF assays | Low end or midpoint of | Midpoint of abnormal (low level) | VWF deficient plasma to identify lower |
| normal reference range | range (i.e., 20–40%) | assay limit; high-end control (≥150%) for | |
| (i.e., 90–100%) | quantitative assays if thrombosis-risk | ||
| is being assessed; qualitative (Type 2 | |||
| VWD-like) control. | |||
| PC, PS, AT | Low end or midpoint of | Midpoint of abnormal (low level) | |
| normal reference range | range, or heterozygous pattern | ||
| control | |||
| APCR | Low end or midpoint of | Midpoint of abnormal (low level) | |
| normal reference range | range, or heterozygous pattern | ||
| control | |||
| LA | Negative control | Positive control (weak positive | Positive control (moderate or strong |
| near assay cut-off) | positive) |
| Step | Reasons for QC failures |
|---|---|
| 1. Re-test → If re-test still shows failure, then proceed to step 2 |
If results fall above or below the reference limit of ±2 SDs |
| 2. Prepare new control and re-test → If re-test still shows failure, then proceed to step 3 |
If QC run is within reference limits of ±2 SDs, then previous failure may have occurred due to deterioration of initial QC reagent. |
| 3. Prepare fresh reagents and re-test → If re-test still shows failure, then proceed to step 4 |
If QC run is within reference limits of ±2 SDs then the laboratory has identified an issue with the reagent on board the analyzer. At this time, a LOOK-BACK is necessary for the laboratory which is looking back at what point QC deviation beyond acceptable limits occurred with the previous reagent on board the analyzer. |
| 4. Recalibrate instrument → | Recalibration of instrument followed by validation of assay calibration with freshly prepared QC and reagents will be necessary. If recalibration also does not fix QC errors, then the Instrument will need to be taken out of service and may require repair by the manufacturer service engineer. |
| True positive | Assay correctly identifies a disease or condition in those who have the disease or condition. | |
| False positive | Assay incorrectly identifies disease or condition when none is present. | |
| True negative | Assay correctly excludes a disease or condition in those without it. | |
| False negative | Assay incorrectly excludes disease or condition when it is present. | |
| 2x2 table | Healthy subjects | Diseased subjects |
| Assay is negative | True negative | False negative |
| Assay is positive | False positive | True positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





