Introduction
Myalgic encephalomyelitis (also known as chronic fatigue syndrome, or ME/CFS to encompass both) is a debilitating illness typified by extreme, life-altering fatigue, profound exertion intolerance, chronic muscle and neurological pain, and sustained disability. ME/CFS frequently has a post-viral onset, with an estimated 75% of cases emerging after an acute infection [
1]. The condition is usually lifelong, with an estimated recovery rate of less than 10% [
2]. There are currently no FDA-approved therapies or medications to treat the disease. Quality of life estimates among people with ME/CFS are uniformly dismal [
3], with ME/CFS sufferers having lower quality of life than almost any other disease.
The study of post-viral conditions, including ME/CFS, has taken on considerable urgency in the aftermath of the COVID-19 global pandemic. A sizable fraction of individuals who survive acute cases of COVID-19 will develop persistent, long-term symptoms, colloquially known as “long COVID" (LC) [
4]. Comparative analyses of LC and ME/CFS have found that as many as half of all LC cases meet diagnostic criteria for ME/CFS [
5]. Prior to 2020, it was estimated that there were between 1.5-3 million individuals with ME/CFS in the United States [
6]. Following the COVID-19 pandemic, revised estimates put the new number of cases between 5-9 million [
7], reflecting an economic cost of as much as
$362 billion dollars annually, and an incalculable amount of human suffering.
While the term “myalgic encephalomylitis" was first coined in 1956 [
8], the scientific community has not coalesced around a single model of the disease. Instead, different approaches have proliferated, largely in parallel, creating a complex picture of competing theories. These include, but are not limited to: chronic viral persistence [
9], autoimmunity [
10], neuroglial dysfunction [
11], vascular and endothelial dysfunction [
12], metabolic dysfunction [
13], and the largely discredited “functional" or psychosomatic hypothesis [
14]. Here, we propose a complementary perspective: The multi-systemic pathologies observed in ME/CFS result from a dysfunctional upstream “central regulator” that influences multiple systems simultaneously. We propose that the brainstem is the most likely candidate. Further, we propose that in at least some people with ME/CFS, the mechanism of dysfunction may be due to acquired deformation of the brainstem. This central dysfunction then propagates to the rest of the body, likely via vagal signaling, prompting a cascade of dysfunction that results in the diverse and multi-systemic presentation ME/CFS and LC.
Systemic Pathology Suggests a Central Dysfunction
While several different diagnostic criteria for ME/CFS have been proposed over the years, almost all require dysfunction in diverse physiological systems. For example, the Canadian Consensus Criteria (CCC) [
15] requires all of the following core symptoms: severe fatigue, post-exertional malaise (PEM, sometimes referred to as post-exertional neuro-immune exhaustion [
16]), sleep dysfunction, pain, neurological or cognitive dysfunction, and dysfunction of autonomic, neuroendocrine, or immune systems. The Institute of Medicine (IOM) criteria [
8] are similar, requiring a core presentation of the following: sustained, disabling fatigue and post-exertional malaise (including in response to sensory stimulation), as well as sleep dysfunction, and at least one of the following: cognitive impairment and/or orthostatic intolerance. The IOM also lists (but does not require): muscular and neurological pain, immunological symptoms such as inflammation, sore throat, and new-onset allergies, and sensory sensitivities.
The question of how a singular event, typically a viral infection, [
1] could result in such widespread and comprehensive dysfunction is one of the great mysteries of ME/CFS. Here we propose that dysfunction in the brainstem is the most likely source, as the brainstem serves as something of a “master regulator" of diverse physiological systems.
The Brainstem in ME/CFS
Many core features of ME/CFS have been connected to the brainstem, for example, the brainstem has been found to be involved in regulating the sleep/wake cycle and arousal, pain processing and nocioception, inflammation and peripheral immune response, and the autonomic nervous system. Disruption of these systems would account for the cure symptoms required for an ME/CFS diagnosis.
There has been limited direct research into brainstem pathology in ME/CFS, in part due to the brainstem’s inaccessible location. A review of non-invasive neuroimaging studies reports reduced white matter volume, as well as impairments in myelination, in ME/CFS compared to healthy controls [
17]. Structural MRI scans have shown that whole brainstem volume was larger in ME/CFS and Long COVID patients [
18], possibly reflecting inflammation, edema, or deformation. Reduced brainstem perfusion has also been reported [
19]. Functional MRI studies have shown similar patterns, with reductions in signal propagation in the brainstem during a Stroop task being associated with more severe symptoms [
20]. Autopsies of brain tissue from people who died with, or of, ME/CFS have found signs of brainstem involvement, including localized and high levels of enterovirus RNA. [
21].
Perhaps the most compelling evidence that at least a subset of ME/CFS cases are directly caused by brainstem deformation come from a set of well-documented case studies. The first is the case of JW (who is an author on this paper), who describes his experience on a personal website [
22]. Following an acute infection with an unknown virus, JW developed debilitating ME/CFS, which left him bedridden for years. JW discovered that cervical spine traction caused a profoundly reduction in symptoms, including peripheral autonomic dysfunction. He was subsequently diagnosed with craniocervical instability. Following a successful skull-to-C2 spinal fusion, and then a tethered spinal cord surgery six months later, JW experienced sustained, total remission from ME/CFS. The second case, JB [
23], has also documented her experiences online. Her case follows a nearly identical trajectory to JW’s.
In both cases, the fundamental etiology of the ME/CFS was found to be reversible deformation of the brainstem. This was apparently triggered by a loss of structural integrity of the craniocervical junction, due to acquired instability of the craniocervical ligaments and joints. The brainstem deformation prompted a cascade of subsequent energetic, sensory, and immunological effects that manifested as severe ME/CFS. Once this deformation was corrected, via traction or surgical intervention, normal brainstem function was apparently restored.
The question of how representative these case studies are of the broader ME/CFS and LC patient population remains unknown and requires further study. Craniocervical instability is one of several conditions that can cause mechanical deformation of neural tissue. Cervical stenosis is another such condition, with complete ME/CFS remissions are consistent with published case studies of improvement from severe ME/CFS following spinal surgery to correct cervical stenosis in three patients [
24]. In any given patient with ME/CFS or LC, there may be multiple possibilities to consider, as evidenced by the growing literature linking ME/CFS to idiopathic intracranial hypertension (IIH).
Intracranial Hypertension in ME/CFS
There is emerging evidence that ME/CFS may be related to an increase in cerebrospinal fluid (CSF, not to be confused with “CFS”). In a large study [
25] of 205 volunteers with ME/CFS, 55% were found to have indicators of IIH (as evidenced by inflated optic-nerve sheath diameter) and 83% signs of possible IIH. The same study found that, of 125 volunteers who received MRIs, 80% had some kind of obstruction to the cervical spine. An earlier study [
26] of 20 patients with ME/CFS who received lumbar punctures, 25% were found to have diagnosable IIH, and 85% (including all five of the IIH individuals) reported symptomatic relief associated with cerebral-spinal fluid drainage. The same research group also reported a case study [
27] of a woman who had suffered from ME/CFS for many years and reported “life-changing” remission following the placement of bilateral venous stents that allowed CSF to drain. Collectively, these results suggest that a sizable proportion of people with ME/CFS may have a mechanical disruption to the basal brain, either in the form of CCI, IIH, or both.
While the suggestion of a direct link between IIH and ME/CFS is relatively new, it has long been recognized that IIH can produce symptoms that overlap with ME/CFS, including pain, sleep disturbances, light and sound sensitivity, and fatigue [
28], and that sustained intracranial hypertension can cause mechanical deformation and herniation of brain tissue and the cranial nerves [
29].
Brainstem Nuclei and Systemic Immune Regulation
What is the mechanism by which brainstem deformation might trigger the widespread, systemic dysregulation seen in ME/CFS? A recent study [
39] found that the caudal nucleus of the solitary tract (cNST) played a key role in regulating systemic inflammatory response: silencing the cNST caused a significant increase in production and circulation of pro-inflammatory cytokines and a reduction in anti-inflammatory ones. The effect was also reversible: activation of the cNST prompted a global calming of immune activity, suppression of inflammatory cytokine production, and an increase in anti-inflammatory signaling. The authors conclude that the cNST appears to serve as the regulator of a body-brain circuit that regulates global levels of immune activity.
Inflammation, particularly neuroinflammation, has long thought to be a key feature of ME/CFS pathophysiology. Studies have found that levels of inflammation and inflammatory cytokine production correlate with the severity of symptoms in ME/CFS [
40,
41] and inflammation a promising biomarker for long COVID as well [
42,
43]. If the cNST and other brainstem nuclei are damaged or deformed, the result may be a chronic suppression of activity in those regions, leading to an indefinitely extended state of hyper-inflammation. The anatomy and connectivity of the cNST also suggests relevance for other common features of ME/CFS: as part of the dorsal vagal complex, the cNST receives direct inputs from both the peripheral nervous system via the ascending vagus nerve, as well as descending inputs from central nervous systems including the hypothalamus. Dysregulation of the autonomic nervous system implicating vagal dysfunction is a very common feature of ME/CFS [
44].
Connective Tissue Disorder as an Underlying Risk Factor
If the above hypotheses hold and ME/CFS is, at least in some cases, sequelae to a physical injury of the basal brain, then we must ask: By what mechanism might occur following an infection with a virus like COVID-19? One possible mechanism is via damage to connective tissue triggered by the immune response to acute infection.
It has long been recognized that connective tissue disorders, such as hypermobile Ehlers- Danlos syndrome (hEDS), have a significant comorbidity with ME/CFS [
25], and further, the degree of hypermobility often correlates with symptom burden. Hypermobility has also been found to be a risk for long COVID [
45]. This suggests that impaired connective tissue integrity increases the baseline risk and severity of these diseases.
There is evidence that viral and bacterial infections may be associated with new-onset or worsening connective tissue disorders. Two studies reported increased rates of autoimmune connective tissue disorders emerging in the aftermath of the Covid-19 pandemic [
46,
47]. Other viruses, such as the Epstein-Barr Virus (EBV) have also been linked to new-onset autoimmune connective tissue disorders [
48], suggesting that the phenomenon of post-viral, new-onset connective tissue pathologies may be a more general phenomenon. Interestingly, a case study reported a woman whose infection with Bartonella worsened her degree of hypermobility, which resolved following successful treatment with antibiotics [
49].
The acute immune response to infection involves degradation and remodeling of connective tissue throughout the body, typically through the expression of matrix metalloproteinases (MMPs). These enzymes act to degrade collagen, elastin, and other connective proteins, as well as regulating inflammatory processes and cytokine expression [
50]. Curiously, studies of people with hypermobile connective tissue disorders have also found evidence of MMP overproduction, reinforcing the link between immune-mediated loss of connective tissue integrity and post-viral illness [
51]. Mast cell activation, which is very commonly dysregulated in ME/CFS [
12], can also degrade the connective tissue matrix, both through direct release of tryptase enzymes, and via induction of further MPP production [
52,
53].
The Model
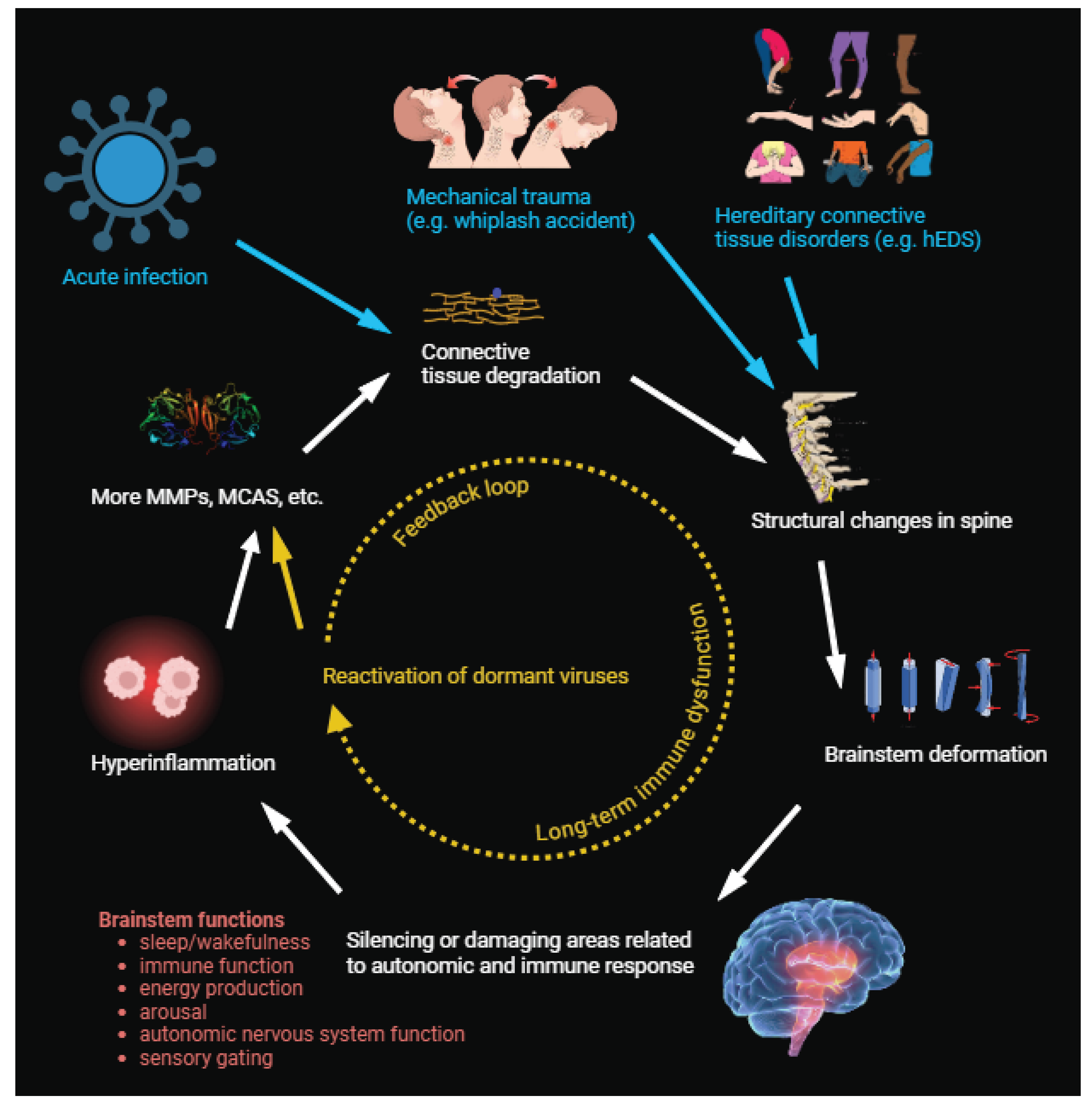
Figure 1.
The basic model, highlighting the potential for feedback loops that further reinforce the initial insult. Mechancial deformation of the brainstem could be triggered by heredetary connective tissue disorders, physical trauma, or acute infection (mediated by connective tissue degradation as part of the normal immune response). This deformation alters brainstem signaling in key regions such as the cNST, which triggers a state of chronic hyperinflammation, leading to further expression of connective-tissue degrading factors, mast cell activation disorders, and latent viral reactivation, which further reinforce the loop.
Figure 1.
The basic model, highlighting the potential for feedback loops that further reinforce the initial insult. Mechancial deformation of the brainstem could be triggered by heredetary connective tissue disorders, physical trauma, or acute infection (mediated by connective tissue degradation as part of the normal immune response). This deformation alters brainstem signaling in key regions such as the cNST, which triggers a state of chronic hyperinflammation, leading to further expression of connective-tissue degrading factors, mast cell activation disorders, and latent viral reactivation, which further reinforce the loop.
Our proposed process begins 1) an infection, such as COVID-19, EBV, etc. 2) The immune response to this infection triggers the normal porcesses of widespread degradation of connective tissue. 3) In a subset of people, this degradation of connective tissue becomes excessive, whether due to a pre-existing lack of tissue integrity, a genetic predisposition, or the specific infectious agent. 4) The loss of connective tissue integrity leads to mechanical deformation of the brainstem by way of neuroantomical pathologies such as CCI, Chiari malformation, intracranial pressure dysregulation, etc., which 5) prompts central regulators to begin sending aberrant signals to the peripheral nervous system via the vagus nerve. 6) Widespread hyperinflammation results in continued degradation of connective tissue or prevents it from healing normally. 7) Over time, this chronic, hyper-inflammatory state produces increasingly profound dysregulation as second-order effects accumulate. These may include typical features of ME/CFS such as reactivation of latent viruses, endothelial dysfunction, etc. The longer these positive feedback loops persist, the more second- and third-order effects can damage or derange the global physiology.
This mechanical basis model suggests a number of readily testable predictions that can be tested both in the clinic and in pre-clinical studies. Continuing epidemiological work on the overlap between brainstem conditions in the vein of [
25] will help solidify our understanding of the correlation between these two different conditions. Studies into the effect of acute infection on the structural integrity of connectivity tissue, particular in those with pre-existing connective tissue disorders, can test specific mechanistic steps in this model. Pre-clinical studies of whether tonic brainstem-induced systemic inflammation (as in [
39]) produces connective tissue remodeling or Ehlers-Danlos-like phenotypes can also probe the link between brainstem function, immune function, and connective tissue structural integrity. Finally, clinical trials of cranio-cervical traction (which helped diagnose JW and JB) or medications that reduce intracranial hypertension (such as acetazolamide) may provide insight into clinically accessible interventions for a family of diseases with almost no existing treatments.
An appealing feature of the mechanical basis hypothesis is that it can be harmonized with other developing theories. For example, both the itaconate shunt hypothesis (ISH) [
54] and the cell danger response hypothesis [
55] propose that post-acute infection syndromes emerge when cells get “stuck" in metabolic states that reduce energy production in response to infection. These hypometabolic states may be adaptive in response to short-term, acute infection, but become disabling when chronic. While promising, both theories are (currently) agnostic as to why the cells become “stuck". The mechanical basis provides a plausible solution: the cells never receive the “safety" signal, as the immune system is trapped in a hyperinflammatory global state, constantly producing danger signals.
Conclusion
In summary, we have proposed a model of how post-acute infection syndromes (ME/CFS, Long COVID, etc) can emerge following immunological insult, explains the high degree of co-morbidity between them and connective tissue disorders, and parsimoniously explains the global, systemic nature of the symptom profile. The mechanical basis model is consistent with other emerging, cell- and molecular-level hypotheses and makes testable predictions at the clinical, pre-clinical, and basic-research levels. Finally, the hypothesis itself is “modular" in the sense that different etiologies may lead to the same underlying brainstem dysfunction: such as cranio-cervical instability, intracranial hyptertension, or other possible etiologies that can impact the brainstem. Our hope is that this will inspire new avenues of research, and ultimately life-changing treatments, for patients suffering from a long-neglected, disabling disease.
Acknowledgments
The authors received no specific funding for this work. We would like to thank the large number of patients living with ME/CFS who have been discussing all of these issues in incredible detail for years: they have been pushing this work forward for years.
References
- Jan Choutka, Viraj Jansari, Mady Hornig, and Akiko Iwasaki. Unexplained post-acute infection syndromes. Nature Medicine, 28(5):911–923, May 2022. Publisher: Nature Publishing Group. [CrossRef]
- Alaa Ghali, Carole Lacout, Jacques-Olivier Fortrat, Karine Depres, Maria Ghali, and Christian Lavigne. Factors Influencing the Prognosis of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics, 12(10):2540, October 2022. [CrossRef]
- Nina L. Muirhead, Jui Vyas, Rachel Ephgrave, Ravinder Singh, and Andrew Y. Finlay. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Impact on Quality of Life (QoL) of Persons with ME/CFS. Medicina, 60(8):1215, August 2024. Number: 8 Publisher: Multidisciplinary Digital Publishing Institute. [CrossRef]
- Hannah E. Davis, Lisa McCorkell, Julia Moore Vogel, and Eric J. Topol. Long COVID: major findings, mechanisms and recommendations. Nature Reviews Microbiology, 21(3):133–146, March 2023. Publisher: Nature Publishing Group.
- Nader Salari, Yassaman Khodayari, Amin Hosseinian-Far, Hosna Zarei, Shabnam Rasoulpoor, Hakimeh Akbari, and Masoud Mohammadi. Global prevalence of chronic fatigue syndrome among long COVID-19 patients: A systematic review and meta-analysis. BioPsychoSocial Medicine, 16:21, October 2022. [CrossRef]
- Ashley R. Valdez, Elizabeth E. Hancock, Seyi Adebayo, David J. Kiernicki, Daniel Proskauer, John R. Attewell, Lucinda Bateman, Alfred DeMaria, Charles W. Lapp, Peter C. Rowe, and Charmian Proskauer. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Frontiers in Pediatrics, 6, January 2019. Publisher: Frontiers. [CrossRef]
- Arthur A. Mirin, Mary E. Dimmock, and Leonard A. Jason. Updated ME/CFS prevalence estimates reflecting post-COVID increases and associated economic costs and funding implications. Fatigue: Biomedicine, Health & Behavior, 10(2):83–93, April 2022. Publisher: Taylor & Francis _eprint: https://doi.org/10.1080/21641846.2022.2062169. [CrossRef]
-
Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press, Washington, D.C., March 2015.
- Maureen R. Hanson. The viral origin of myalgic encephalomyelitis/chronic fatigue syndrome. PLOS Pathogens, 19(8):e1011523, August 2023. [CrossRef]
- Eric Y. Wang, Tianyang Mao, Jon Klein, Yile Dai, John D. Huck, Jillian R. Jaycox, Feimei Liu, Ting Zhou, Benjamin Israelow, Patrick Wong, Andreas Coppi, Carolina Lucas, Julio Silva, Ji Eun Oh, Eric Song, Emily S. Perotti, Neil S. Zheng, Suzanne Fischer, Melissa Campbell, John B. Fournier, Anne L. Wyllie, Chantal B. F. Vogels, Isabel M. Ott, Chaney C. Kalinich, Mary E. Petrone, Anne E. Watkins, Charles Dela Cruz, Shelli F. Farhadian, Wade L. Schulz, Shuangge Ma, Nathan D. Grubaugh, Albert I. Ko, Akiko Iwasaki, and Aaron M. Ring. Diverse functional autoantibodies in patients with COVID-19. Nature, 595(7866):283–288, July 2021. Publisher: Nature Publishing Group.
- Herbert Renz-Polster, Marie-Eve Tremblay, Dorothee Bienzle, and Joachim E. Fischer. The Pathobiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Case for Neuroglial Failure. Frontiers in Cellular Neuroscience, 16:888232, 2022.
- Klaus J. Wirth, Carmen Scheibenbogen, and Friedemann Paul. An attempt to explain the neurological symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Journal of Translational Medicine, 19(1):471, November 2021.
- Robert K. Naviaux, Jane C. Naviaux, Kefeng Li, A. Taylor Bright, William A. Alaynick, Lin Wang, Asha Baxter, Neil Nathan, Wayne Anderson, and Eric Gordon. Metabolic features of chronic fatigue syndrome. Proceedings of the National Academy of Sciences, 113(37):E5472–E5480, September 2016. Publisher: Proceedings of the National Academy of Sciences.
- Todd E. Davenport, Svetlana Blitshteyn, Nicola Clague-Baker, David Davies-Payne, Glenn J. Treisman, and Sarah F. Tyson. Long COVID Is Not a Functional Neurologic Disorder. Journal of Personalized Medicine, 14(8):799, August 2024. Number: 8 Publisher: Multidisciplinary Digital Publishing Institute.
- Bruce M. Carruthers, Anil Kumar Jain, Kenny L. De Meirleir, Daniel L. Peterson, Nancy G. Klimas, A. Martin Lerner, Alison C. Bested, Pierre Flor-Henry, Pradip Joshi, A. C. Peter Powles, Jeffrey A. Sherkey, and Marjorie I. van de Sande. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. Journal Of Chronic Fatigue Syndrome, 11(1):7–115, January 2003. Publisher: Taylor & Francis _eprint: https://doi.org/10.1300/J092v11n01_02. [CrossRef]
- B. M. Carruthers, M. I. van de Sande, K. L. De Meirleir, N. G. Klimas, G. Broderick, T. Mitchell, D. Staines, A. C. P. Powles, N. Speight, R. Vallings, L. Bateman, B. Baumgarten-Austrheim, D. S. Bell, N. Carlo-Stella, J. Chia, A. Darragh, D. Jo, D. Lewis, A. R. Light, S. Marshall-Gradisbik, I. Mena, J. A. Mikovits, K. Miwa, M. Murovska, M. L. Pall, and S. Stevens. Myalgic encephalomyelitis: International Consensus Criteria. Journal of Internal Medicine, 270(4):327–338, 2011. _eprint: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2796.2011.02428.x. [CrossRef]
- Todd Nelson, Lan-Xin Zhang, Hui Guo, Luis Nacul, and Xiaowei Song. Brainstem Abnormalities in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Scoping Review and Evaluation of Magnetic Resonance Imaging Findings. Frontiers in Neurology, 12, December 2021. Publisher: Frontiers.
- Kiran Thapaliya, Sonya Marshall-Gradisnik, Markus Barth, Natalie Eaton-Fitch, and Leighton Barnden. Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients. Frontiers in Neuroscience, 17:1125208, 2023.
- D.C. Costa, C. Tannock, and J. Brostoff. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM: An International Journal of Medicine, 88(11):767–773, November 1995.
- Leighton R Barnden, Zack Y Shan, Donald R Staines, Sonya Marshall-Gradisnik, Kevin Finegan, Timothy Ireland, and Sandeep Bhuta. Intra brainstem connectivity is impaired in chronic fatigue syndrome. NeuroImage: Clinical, 24:102045, January 2019.
- Adam J. O’Neal and Maureen R. Hanson. The Enterovirus Theory of Disease Etiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Critical Review. Frontiers in Medicine, 8:688486, June 2021.
- Jeff Wood. The Mechanical Basis of ME/CFS Craniocervical Instability, CCI, Tethered Cord.
- Cort Johnson. Jennifer Brea’s Amazing ME/CFS Recovering Story: the Spinal Series - Pt. II, May 2019.
- Peter C. Rowe, Colleen L. Marden, Scott Heinlein, and Charles C. Edwards. Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis. Journal of Translational Medicine, 16:21, February 2018.
- Björn Bragée, Anastasios Michos, Brandon Drum, Mikael Fahlgren, Robert Szulkin, and Bo C. Bertilson. Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Frontiers in Neurology, 11:828, 2020.
- Nicholas Higgins, John Pickard, and Andrew Lever. Lumbar puncture, chronic fatigue syndrome and idiopathic intracranial hypertension: a cross-sectional study. JRSM short reports, 4(12):2042533313507920, December 2013.
- Nicholas Higgins, John Pickard, and Andrew Lever. Borderline Intracranial Hypertension Manifesting as Chronic Fatigue Syndrome Treated by Venous Sinus Stenting. Journal of Neurological Surgery Reports, 76(2):e244–e247, November 2015.
- Deborah I. Friedman and Daniel M. Jacobson. Diagnostic criteria for idiopathic intracranial hypertension. Neurology, 59(10):1492–1495, November 2002. Publisher: Wolters Kluwer.
- Aleksey Tadevosyan and Joshua Kornbluth. Brain Herniation and Intracranial Hypertension. Neurologic Clinics, 39(2):293–318, May 2021. Publisher: Elsevier.
- Petra M. Klinge, Abigail McElroy, John E. Donahue, Thomas Brinker, Ziya L. Gokaslan, and Michael D. Beland. Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. Journal of Neurosurgery. Spine, 35(1):18–24, May 2021.
- Fraser C. Henderson, William A. Wilson, Stephen Mott, Alexander Mark, Kristi Schmidt, Joel K. Berry, Alexander Vaccaro, and Edward Benzel. Deformative stress associated with an abnormal clivo-axial angle: A finite element analysis. Surgical Neurology International, 1:30, July 2010.
- Benjamin Langridge, Edward Phillips, and David Choi. Chiari Malformation Type 1: A Systematic Review of Natural History and Conservative Management. World Neurosurgery, 104:213–219, August 2017.
- Vibhor Krishna, Francesco Sammartino, Philip Yee, David Mikulis, Matthew Walker, Gavin Elias, and Mojgan Hodaie. Diffusion tensor imaging assessment of microstructural brainstem integrity in Chiari malformation Type I. Journal of Neurosurgery, 125(5):1112–1119, November 2016.
- Erik Edström, Charlotte Wesslén, Alexander Fletcher-Sandersjöö, Adrian Elmi-Terander, and Ulrika Sandvik. Filum terminale transection in pediatric tethered cord syndrome: a single center, population-based, cohort study of 95 cases. Acta Neurochirurgica, 164(6):1473–1480, June 2022.
- Rick Labuda, Blaise Simplice Talla Nwotchouang, Alaaddin Ibrahimy, Philip A. Allen, John N. Oshinski, Petra Klinge, and Francis Loth. A new hypothesis for the pathophysiology of symptomatic adult Chiari malformation Type I. Medical Hypotheses, 158:110740, January 2022.
- Ryan Pelo, Erin Suttman, Peter C. Fino, Mary M. McFarland, Leland E. Dibble, and Melissa M. Cortez. Autonomic dysfunction and exercise intolerance in concussion: a scoping review. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 33(2):149–163, April 2023.
- Michael D. Freeman, Scott Rosa, David Harshfield, Francis Smith, Robert Bennett, Christopher J. Centeno, Ezriel Kornel, Ake Nystrom, Dan Heffez, and Sean S. Kohles. A case-control study of cerebellar tonsillar ectopia (Chiari) and head/neck trauma (whiplash). Brain Injury, 24(7-8):988–994, 2010.
- David McKean, Umme Sara Zishan, Sarah Billingsley, Shyam S. Swarna, Cormac O’Neill, Monika Banerjee, Safa Siddiqi, Joseph Papanikitas, Sarah Yanny, Richard Hughes, and Tom Meagher. Acquired Chiari malformation following spinal cord injury-a case series. Spinal Cord Series and Cases, 5:65, 2019.
- Hao Jin, Mengtong Li, Eric Jeong, Felipe Castro-Martinez, and Charles S. Zuker. A body–brain circuit that regulates body inflammatory responses. Nature, pages 1–3, May 2024. Publisher: Nature Publishing Group.
- Martin A. Jonsjö, Gunnar L. Olsson, Rikard K. Wicksell, Kjell Alving, Linda Holmström, and Anna Andreasson. The role of low-grade inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology, 113:104578, March 2020.
- Yasuhito Nakatomi, Kei Mizuno, Akira Ishii, Yasuhiro Wada, Masaaki Tanaka, Shusaku Tazawa, Kayo Onoe, Sanae Fukuda, Joji Kawabe, Kazuhiro Takahashi, Yosky Kataoka, Susumu Shiomi, Kouzi Yamaguti, Masaaki Inaba, Hirohiko Kuratsune, and Yasuyoshi Watanabe. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study. Journal of Nuclear Medicine, 55(6):945–950, June 2014. Publisher: Society of Nuclear Medicine Section: Clinical Investigations.
- Felicity Liew, Claudia Efstathiou, Sara Fontanella, Matthew Richardson, Ruth Saunders, Dawid Swieboda, Jasmin K. Sidhu, Stephanie Ascough, Shona C. Moore, Noura Mohamed, Jose Nunag, Clara King, Olivia C. Leavy, Omer Elneima, Hamish J. C. McAuley, Aarti Shikotra, Amisha Singapuri, Marco Sereno, Victoria C. Harris, Linzy Houchen-Wolloff, Neil J. Greening, Nazir I. Lone, Matthew Thorpe, A. A. Roger Thompson, Sarah L. Rowland-Jones, Annemarie B. Docherty, James D. Chalmers, Ling-Pei Ho, Alexander Horsley, Betty Raman, Krisnah Poinasamy, Michael Marks, Onn Min Kon, Luke S. Howard, Daniel G. Wootton, Jennifer K. Quint, Thushan I. de Silva, Antonia Ho, Christopher Chiu, Ewen M. Harrison, William Greenhalf, J. Kenneth Baillie, Malcolm G. Semple, Lance Turtle, Rachael A. Evans, Louise V. Wain, Christopher Brightling, Ryan S. Thwaites, and Peter J. M. Openshaw. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nature Immunology, 25(4):607–621, April 2024. Publisher: Nature Publishing Group.
- Pushpa Tandon, Natalie D. Abrams, Leela Rani Avula, Danielle M. Carrick, Preethi Chander, Rao L. Divi, Johanna T. Dwyer, Gallya Gannot, Nataliya Gordiyenko, Qian Liu, Kyung Moon, Mercy PrabhuDas, Anju Singh, Mulualem E. Tilahun, Merriline M. Satyamitra, Chiayeng Wang, Ronald Warren, and Christina H. Liu. Unraveling Links between Chronic Inflammation and Long COVID: Workshop Report. The Journal of Immunology, 212(4):505–512, February 2024.
- Christof Peter Dr Ziaja, Susanne Young, and Michael Stark. Dysregulation of the autonomic nervous system in ME/CFS and post-COVID syndrome: insights from 48-h heart rate variability monitoring. Journal of the Neurological Sciences, 455, December 2023. Publisher: Elsevier.
- Mei Yang, Brian Logarbo, Jacques Courseault, Nimmi Wickramasuriya, Gregory Bix, and Michele Longo. Long COVID and the Diagnosis of Underlying Hypermobile Ehlers-Danlos Syndrome and Hypermobility Spectrum Disorders (P5-4.014). Neurology, 102(17_supplement_1):2478, April 2024. Publisher: Wolters Kluwer.
- Koushan Kouranloo, Mrinalini Dey, Helen Elwell, and Arvind Nune. A systematic review of the incidence, management and prognosis of new-onset autoimmune connective tissue diseases after COVID-19. Rheumatology International, 43(7):1221–1243, July 2023.
- Sung Ha Lim, Hyun Jeong Ju, Ju Hee Han, Ji Hae Lee, Won-Soo Lee, Jung Min Bae, and Solam Lee. Autoimmune and Autoinflammatory Connective Tissue Disorders Following COVID-19. JAMA Network Open, 6(10):e2336120, October 2023.
- Gunnar Houen and Nicole Hartwig Trier. Epstein-Barr Virus and Systemic Autoimmune Diseases. Frontiers in Immunology, 11, January 2021. Publisher: Frontiers.
- Bobak Robert Mozayeni, Ricardo Guillermo Maggi, Julie Meredith Bradley, and Edward Bealmear Breitschwerdt. Rheumatological presentation of Bartonella koehlerae and Bartonella henselae bacteremias. Medicine, 97(17):e0465, April 2018.
- Sabrina Amar, Lyndsay Smith, and Gregg B. Fields. Matrix metalloproteinase collagenolysis in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1864(11, Part A):1940–1951, November 2017.
- Nicola Chiarelli, Nicoletta Zoppi, Marina Venturini, Daniele Capitanio, Cecilia Gelfi, Marco Ritelli, and Marina Colombi. Matrix Metalloproteinases Inhibition by Doxycycline Rescues Extracellular Matrix Organization and Partly Reverts Myofibroblast Differentiation in Hypermobile Ehlers-Danlos Syndrome Dermal Fibroblasts: A Potential Therapeutic Target? Cells, 10(11):3236, November 2021.
- B. L. Gruber and L. B. Schwartz. The mast cell as an effector of connective tissue degradation: A study of matrix susceptibility to human mast cells. Biochemical and Biophysical Research Communications, 171(3):1272–1278, September 1990.
- Arunasiri Iddamalgoda, Quang Trong Le, Kenichi Ito, Kiyotaka Tanaka, Hiroyuki Kojima, and Hiroshi Kido. Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Archives of Dermatological Research, 300 Suppl 1:S69–76, April 2008.
- Rob Phair. The Itaconate Shunt Hypothesis and ME/CFs: an update. In ME/CFS Research Roadmap Webinar - Physiology, ME/CFS Research Roadmap Seminar Series, February 2024. National Institute of Health.
- Robert Naviuax. ME/CFS and the cell danger response - the quest for a a common denomenator of disease. In ME/CFS Research Roadmap Webinar - Physiology, ME/CFS Research Roadmap Seminar Series, February 2024. National Institute of Health.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).