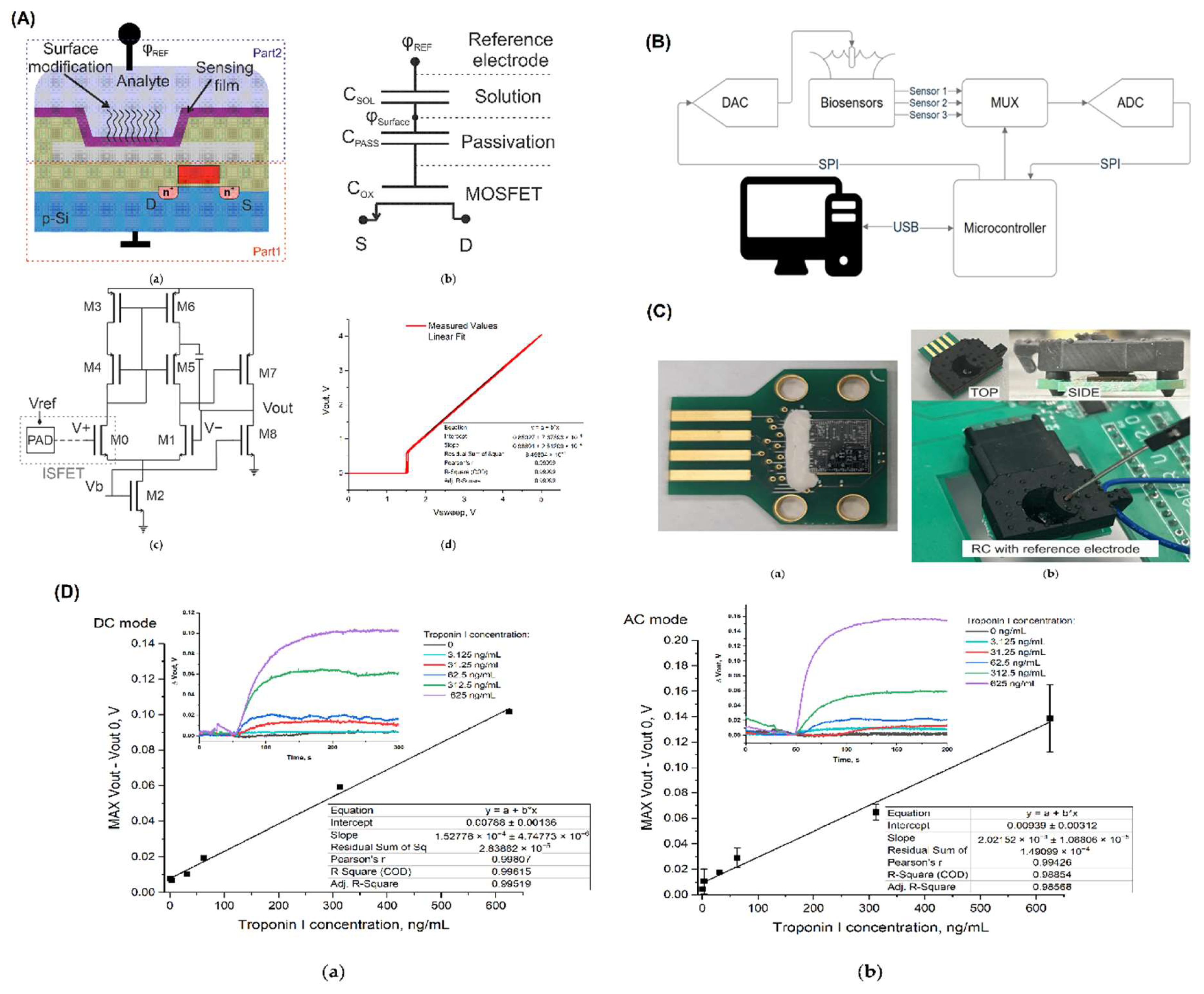
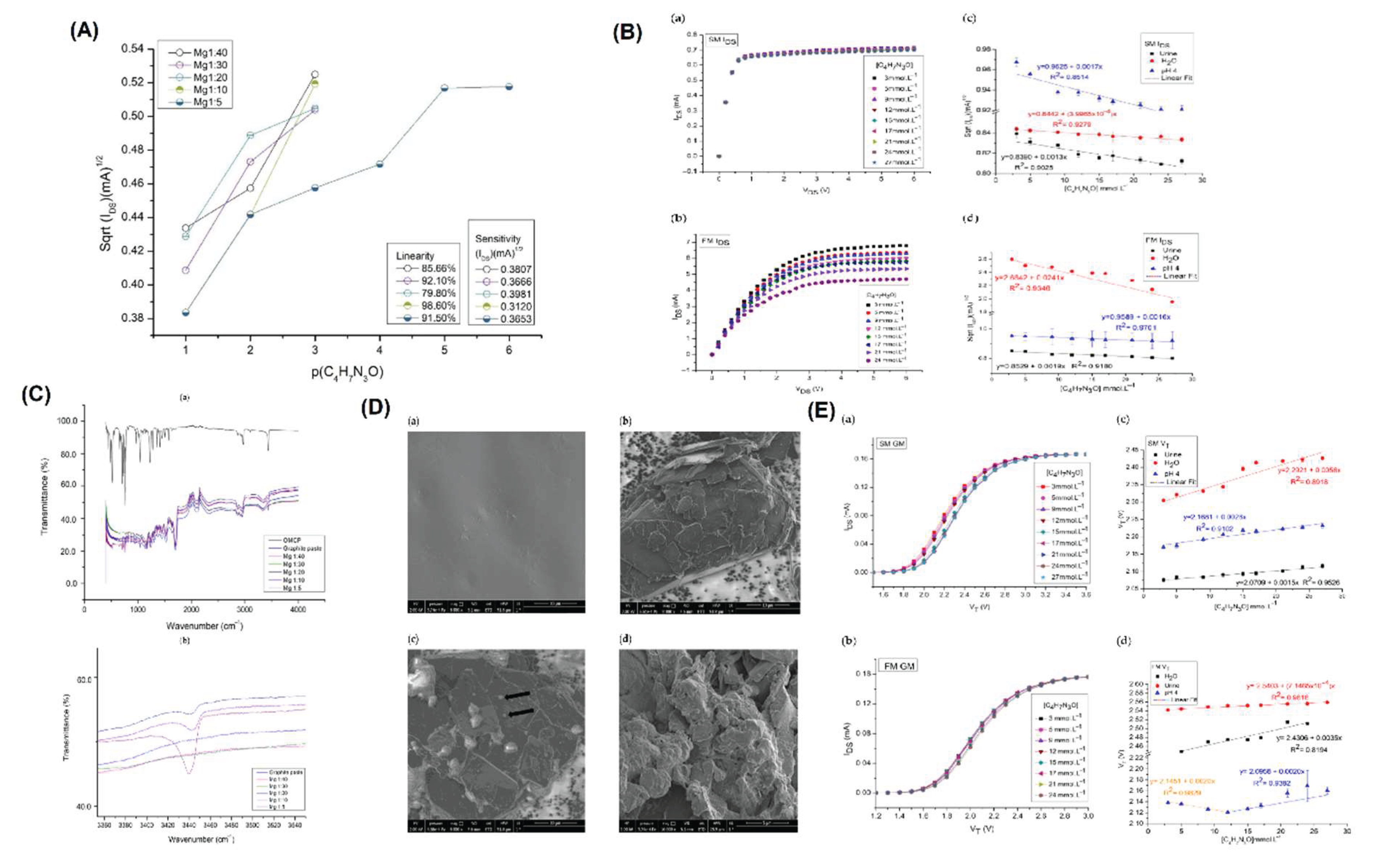
Introduction
The Field Effect Transistor (FET) biosensors has been moving towards being used as a diagnostic tool, particularly for urinary biomarkers detection. The urine is a valuable, non-invasive biofluid, which contains diagnostic molecules, such as uric acid, nucleic acids, proteins and small molecules. However, these biomarkers can be detected using FET-based devices with advantages such as real time, high sensitivity and miniaturization, very important for early disease diagnosis and point of care testing. The importance of such fast and accurate and available diagnostic methods has motivated research into this area.
As demonstrated in many applications of healthcare, FET biosensors are relevant for monitoring of urinary biomarkers associated with metabolic disorders, kidney function, infection, and cancer. FET biosensors are, therefore, a suitable platform for clinical use due to their ability to detect these biomarkers quickly and efficiently.
Despite many existing traditional diagnostic technologies such as immunoassay and chromatography, they are labour extensive and require elaborate instrumentation. On the other hand, FET biosensors use a different method in that biochemical interactions directly output as electrical signals. Biosensing FETs include ion sensitive FETs, extended gate FETs, and electrolyte-gated FETs that work successfully in aqueous and biological environment [
1,
2,
3,
4].
The recent advances in FET design and fabrication consume the efforts of the improvement to perform urinary biomarker detection. Dielectric modulation and nanocavity fabrication helps to increase surface sensitivity [
5,
6,
7]. Device control at the nanoscale is through advanced lithography and precise doping. Flexible sensor platforms come from multi material integration and reconfigurable structures. Improvements in charge transport as well as detection limits can be enabled by tunneling techniques and Schottky barrier engineering [
5,
6,
7]. Through precision fabrication, high interface quality and similar material behaviour are achieved, both critical for stability of biosensor function.
Also, the materials used will affect the performance of FET biosensors. High conductivity and large surface area for biomolecule interaction [
5,
7,
8] are provided by the carbon based nanomaterials. The electronic properties of TMDs can be tuned. Chemical stability is also provided by metal oxides and dielectrics. Much gate control is improved by advanced dielectrics and ferroelectric materials. The electronic functionality and optical properties can be provided in Indium based materials. In spite of this, silicon based and related nanomaterials are still commonly used because they can be fabricated using well developed methods and are compatible [
9,
10,
11].
The literature reviews on FET biosensors for urinary biomarker detection with an emphasis on the specific material and fabrication domains that are applicable. It reviews sensor design for uric acid, nucleic and protein biomarkers and small molecule biomarkers. The review describes recent progress in research, technological issues and future directions in the development of reliable and sensitive FET based platforms for urinary diagnostics.
Domains of FET Innovation
Dielectric Modulation and Nanocavity Fabrication
Metal-Implanted Dielectric Modulated Tunnel Field-Effect Transistor (MI-DMTFET) biosensor is well appreciated for its high sensitivity. This substantive capability owes much to its advanced fabrication means. One of the essential techniques is atomic layer deposition (ALD). This ALD technique, accurately deposits a metal strip above the drain channel junction, improve band lineup, the falls tunneling barrier and enhances ambipolar current [
12]. However, although ALD ensures that metal universality layers will be present, it doesn’t increase where biomolecules may interact to any great extent.To get around this, the Dielectrically Modulated Fully Gate Tunnel Field-Effect Transistor ( DMFGT-FET ) biosensor employs precision etching to produce dual nanocavities. These nanocavities raise the interaction surface area with biomolecules and, enhance the sensitivity of the sensor [
1]. But to do this on a consistent basis across the entire device, so that sensitivity is uniform, remains a problem.The Dielectrically Modulated Electrically Doped Junctionless Tunnel Field-Effect Transistor (DM-ED-JLTFET) biosensor copes with challenge by using sophisticated photolithography and neutral-beam-assisted atomic layer etching. These techniques ensure that only nano-scale features are repeated to keep sensitivity uniform [
2].Another problem is to improve coupling between the gate and channel. The Dielectrically Modulated Dual-Trench Gate-Engineered Metal-Oxide-Semiconductor Field-Effect Transistor (DM DT GE-MOSFET) biosensor resolves this difficulty using a dual-trench gate design and a split transparent indium-doped tin oxide (ITO) outer gate. This design enhances coupling between the gate and channel while leaving less interference, resulting in higher sensitivity and specificity [
13].In a word, these advanced fabrication methods grow more precision down to nanometres of scale, enlarge interaction area with surface bimolecular, and improve the coupling between the gate and channel. This improves both the sensitivity and specificity of the sensors considerably.
Advanced Lithography and Precise Doping Techniques
The FFC-bioTFET sensor achieves high sensitivity and specificity through innovative fabrication techniques. One such technique involves creating cavities in the spacer regions near the source and gate metal. These cavities help modulate fringe fields and the width of the tunneling barrier, which increases the sensor’s sensitivity to biomolecules. However, carving these cavities requires precise engineering, which complicates the fabrication process [
14].The double-gate junctionless tunnel field-effect transistor (JL-TFET) biosensor simplifies the process by eliminating junctions. This approach creates a more uniform electric field and increases sensitivity while also making the fabrication process easier [
15]. However, the integration of nanocavities and the need for advanced doping processes still present challenges.The split gate insulator-controlled Junctionless Field-Effect Transistor (SG-DM-JLFET) further simplifies fabrication by removing the need for sharp doping profiles and complex junctions. This design ensures uniform electrical characteristics and helps maintain consistent sensitivity and specificity across devices [
4]. Despite these improvements, achieving high sensitivity across different biomolecule interactions remains challenging.The Germanium-based Dielectric-Modulated Junctionless Charge Plasma Tunnel Field-Effect Transistor (Ge-DMJ-CPTFET) sensor addresses this by using dielectric modulation and the charge plasma concept to create precise nanogap cavities. This technique eliminates the need for traditional doping, which reduces variability, improves reproducibility, simplifies the fabrication process, and enhances sensor performance [
3].In conclusion, these advancements simplify the fabrication process by eliminating complex junctions and doping, leading to more consistent and precise detection of biomolecules.
Multi-Material Integration and Reconfigurable Structures
Currently, the HM-HD-RFET (Hetero-Material Hetero-Dielectric Reconfigurable Field-Effect Transistor) biosensor is both sensitive and highly specific, provided that the sensor works properly.But due to random dopant fluctuations, performance of the sensor may be unpredictable.The reconfigurable design of FETs eliminates this reliability problem through electrical rather than physical doping. With electrical doping there are no discrete fluctuations to disturb the sensor and make it fail; instead uniform distribution ensures stable and reliable operation [
16].The Bio-RFET sensor goes a step further in standardization. It uses precise manufacturing methods such as atomic hydrogen-assisted molecular beam epitaxy (MBE) or liquid phase deposition (LPD) that provide very uniform and defect-free materials layers; this reduces noise and keeps electrical properties more constant. More uniformity leads to greater sensitivity and reliability [
17].Yet, even with these improvements, it is difficult to control the electrostatic environment.The DM-TMGOS-ZHP-TFET (Dielectric Modulation Triple Metal Gate Oxide Stack Z-shaped Horizontal Pocket Tunnel Field-Effect Transistor) biosensor solves these problems by incorporating both a specially designed three-metal gate and a Z-shaped gate. These features improve electrostatic force control (raising the sensitivity of any sensor) and provide it with greater resilience against external disturbances [
18].The NUTFET-DMS (Non-Uniform Tunnel Field-Effect Transistor Dual Material Source) biosensor uses two types of materials-silicon and germanium-in its non-uniform tunneling FET design. This design improves the efficiency of tunneling and more effectively captures biomolecules. As a result of careful implementation and optimization, this sensor has high sensitivity and specificity. It also overcomes previous difficulties with consistency and effectiveness [
19].In summary, these sensors manage to perform excellently because they can control materials processes very precisely with advanced doping techniques while having intelligent structural designs.
Tunneling Techniques and Schottky Barrier Engineering
The hetero-pH-based stepped oxide gate underlap vertical tunnel field-effect transistor (pHVTFET) biosensor described in this section uses gallium antimony doped layers and stepped oxides with underlapped gates. This will help to control the electrical field better, making the sensor more sensitive to changes in pH [
5]. The Ge-source L-shaped tunnel field-effect transistor (LTFET) BioFET sensor takes a further step toward greater sensitivity. The L-shaped channel and the cavity are carved in the channel and cavity of a flared L-shaped transitional zone to give some gain on a real implementation problem [
8]. Another improvement comes from a biosensor using a double-gate 6H-Silicon Carbide Schottky Barrier FET. Here, a small pit (nanocavity) is dug out under the gates close to the source-channel junction, increasing the spot where interactions take place. This reduces noise and enhances sensing performance [
6]. Finally, the 4H-SiC Schottky Barrier FET with a P+ doping pocket uses advanced growth and doping methods to reduce material defects. It also improves control of the height of the Schottky barrier, enhancing the sensor’s specificity and material quality [
7]. In coclusion, these innovations in sensor structure bring greater leverages over electric field control, material quality, and surface interaction, and this ultimately leads to better performing sensors.
Precision Fabrication for Interface Quality and Material Engineering
By using wet etching and controlled oxidation, the MSM-SiNW (Metal-Semiconductor-Metal Silicon Nano wire) biristor sensor was manufactured. The new technology isn’t without tradition, “These processes make the edges of the nanowires very smooth, reducing surface defects and particularly improving sensor performance”. [
20].This sensor system addresses problems such as maintaining Schottky contacts during metal sputtering.The graphene channel is aligned and its dimensions are controlled using fine lithographic and nanoscale patterning techniques. This offers greater sensitivity, and fewer defects [
21].In the GC-GAA-NWFET (Graded Channel Gate-All-Around Silicon Nanowire Field-Effect Transistor) biosensor, the channel is also graded. This gives better control over the gate, reducing leakage currents and increasing sensitivity. It also provides a gate-all-around structure that provides less noise for biomolecule binding [
22].Finally, the “n-type JL-DM-DG (Junction Less Dielectric Modulated Double-Gate) MOSFET “ Biosensor utilizes Atomic Layer Deposition (ALD) for Hafnium Barium Oxide (BaHfO₃) and Chemical Mechanical Planarization (CMP) to make extremely uniform dielectric layers and a smooth, even surface. This ensures consistent high performance and overcomes the limitations of the earlier processes [
23].In summary, these advances in fabrication reduce defects, increase gate control, and guarantee uniform dielectric layers are beneficial for biosensors for biomolecules.
FET Sensor Typologies
Carbon-Based Nanomaterials
In these biosensors with carbon nanotube (CNT) transistor, electric conduction and surface area all perform well, which not only increases sensitivity but also transforms biological interactions simply into electricity [
24]. However, CNTs can have trouble providing a stable structure for the recognition elements. To address this dilemma, tungsten disulfide (WS2) is used in a field-effect transistor (FET) DNA biosensor. WS2 offers high carrier mobility, a large surface area for DNA attachment, and superior thermal and oxidative stability, guaranteeing reliable performance in a variety of conditions [
25]. But even though WS2 improves stability, it may still not match the current stability and performance levels of graphene-based materials. In a different FET biosensor the trilayer graphene nano-ribbons (TGN) offer higher currents and a better electrical conductivity routine, which in turn results in increased sensitivity and specificity for DNA detection [
26]. And using phosphorodiamidate morpholino oligomers (PMO) to attach to reduced graphene oxide (RGO) in another FET biosensor is able to cut non-specific interactions, thus guaranteeing stable and specific binding and relieving the problems encountered with other materials [
27]. Each material raises conduction, stability or specificity, overcoming the limitations of other materials.
Advanced Dielectrics and Ferroelectric Materials
The sensor in this section is a DC-NC-Delay Accumulated Mode Field Effect Transistor (DC-NC-JAM-FET). It uses hafnium zirconium oxide (HZO) to create a negative capacitance effect. Then the subthreshold slope and the sensitivity to current increases. Thus small changes in biomolecule concentration will be tested precisely by this device [
35]. But although HZO can make it more sensitive, it doesn’t solve the root cause of noise that affects the transmitter’s accuracy. To solve this problem, the RNA field-effect transistor (RNAFET) biosensor uses high-purity HfO2. This material offers a clean and stable surface for DNA immobilization, which reduces non-specific bonding and improves accuracy [
36]. Despite these improvements, problems may still arise with the controlling of the channel potential for RNAFETs. Then the proposed Dual Metal Double Layer Gate-All-Around Nano Wire Field Effect Transistor (DMDL GAA NW-FET) sensor resolves such issues by using high-K dielectric materials that include HfO2 and SiO2. These materials decrease fringing field effects and increase gate capacitance. So channel potential is better regulated, it has greater sensitivity and all-round improved performance overcoming previous challenges [
37]. The benefits stem from the use of HZO and high-k dielectrics materials, improve subthreshold swing, reduce noise and improve biomolecule detection accuracy.
Indium-Based Materials
The design of the biosensor is a Field Effect Transistor (FET) based biosensor using 2D Indium Selenide (InSe) as the channel material. An electron mobility of 300 cm2/Vs and tunable bandgap are provided by InSe, leading to increased sensitivity and specificity of RNA target detection. Nevertheless, InSe is sensitive to Coulomb scattering. This is good in terms of detecting low concentration targets, at the expense of making the sensor more susceptible to noise and interference [
38]. Thus, the extended gate field effect transistor (EG-FET) biosensor based on indium tin oxide are used to solve this issue. High electrical conductivity and stable chemical properties are offered by indium tin oxide (ITO). This insures robust signal transduction and stability with respect to different conditions. However, the ITO-EG-FET could be made to work better if single stranded DNA (ssDNA) probes were immobilized. The coating used is (3Aminopropyl)triethoxysilane/Glutaraldehyde (APTES/GA) [
39]. Based on this work, the Extended Gate Field Effect Transistor (EG-FET) sensor is built based on industrial grade bare ITO suspended onto a polymethyl methacrylate (PMMA) layer. By using this combination, ITO’s conductivity and stability are maintained and the conditions of the loop mediated isothermal amplification (LAMP) reaction are controlled. This is particularly useful to improve the specificity and sensitivity of the sensor by being able to have very precise reaction conditions [
40]. The advantages are due to electrical properties of InSe and ITO and precise environmental control of PMMA based on biosensibility, specificity and stability.
FET Biosensors for Urinary Biomarkers
Uric Acid Sensors
A high performance, acidic pH sensitive, low hysteresis, and low drift rate EGFET biosensor based on NiOx was developed to detect uric acid efficiently and showed pH sensitivity (58.53 mV/pH), selectivity toward interfering substances (glucose, urea), and low hysteresis (1.4 mV) and drift rate (0.30 mV/h) [
41]. Unfortunately, despite its linearity on uric acid detection, the device had slow response time and poor detection limit. To overcome these issues, a uricase/RuO₂ integrated sensing film and EGFET used for working with the resistive divider were suggested through TSMC 180nm CMOS process to obtain the chip level integration. It provided improved sensitivity (12.69 mV/(mg/dL)), response time (8 seconds) and a low detection limit of 0.082 mg/dL; making this system simpler, cost effective and easily incorporated into multi-analyte chip systems [
42]. However, it was lacking in continuous or in vivo useability. This was overcome by employing a MoS₂ based FET sensor with microfluidic integration, and which could be used for real time uric acid monitoring under in vivo like settings. High precision, reversibility and a detection limit (60 nM) were enabled by the use of an atomically thin TMD channel and evaporation free flow environment, making biomolecules, which they predict will be suitable for continuous, miniaturized and reliable biosensing applications in complex biological fluids [
43].The microfluidic integration combined with atomically thin 2D material fabrication enables miniaturized, real-time biosensing with enhanced precision and compatibility in physiological environments.
Nucleic Acid and Protein Biomarkers
As a noninvasive and user friendly early bladder cancer diagnosis strategy, trapping of miRNA in urine by FET based biosensors offers excellent sensitivity, in particular the ability of FET paired biosensors to detect single molecules electrically. Nevertheless, adsorption to non-specific biomolecules is detrimental to their performance in real urine, and the short sensing distance is undesirable due to Debye length effects present in high ionic strength (HSI) fluids. In order to alleviate these restrictions, a catapult-like actuating DNA nanostructured probe was introduced into the FET biosensor that acts to prevent nonspecific adsorption using steric hindrance and can extend upwards when target binding occurs, giving surface potential changes that occur within the Debye length. This design allows sensitive and reliable miRNA detection in undiluted urine at a 10 fM detection limit by discriminating bladder cancer patient from healthy individuals in 20 minutes and without RNA extraction or amplification [
44]. As with other miRNA biosensors, this miRNA biosensor is highly sensitive and specific in complex sample conditions such as urine, but is limited to nucleic acids. In order to address the need for protein biomarker detection in urine, a low cost, label free Si₃N₄-ISFET based immunosensor for human serum albumin (HSA) detection was developed. Through atomic plasma etching and bioconjugation with anti-HSA antibodies coupled with glutaraldehyde, the sensor has high accuracy and precision (detection limit = 5 μg/mL; recovery ~ 100%), and strong correlation with conventional methods (R = 0.99) due to its modification with APTES; the sensor is suitable for simple and reliable urinary microalbumin detection [
45]. As such, the incorporation of DNA nanostructures into FET fabrication is an innovative strategy to extend Debye length limitations to ultrasensitive detection directly in complex urine samples.
Small Molecule Biomarkers
A photocurable membrane based field-effect transistor (FET) sensor was developed to monitor creatinine levels in urine with good linearity, stable performance up to 90 days of storage, and high selectivity to urea interference. As for its practical application, it had been limited by irreversible saturation in the membrane after three days of continuous use [
46]. To overcome these limitations in the lifetime and sensitivity, a more advanced LIG extended gate FET (EG-FET) was developed using dual doping using MnO₂ and gold nanoparticle (NP) to give better conductivity as well as surface functionality. A more damp proof and highly sensitive platform for chronic kidney disease screening has been achieved with this sensor which had an ultralow detection limit of 50 ag/μL and covering a wide range of concentrations [
47]. However, these improvements also left the need for rapid, portable, and recyclable systems that could be applied to broader metabolic monitoring. Thus, in order to address this, a polymer-functionalized graphene FET (P-GFET) based on a covalent glucose binding polymer on a boronic acid, was introduced. The chemisorption selectivity, and high sensitivity (822 μA·cm⁻²·mM⁻¹) and reusability over 20 cycles were attained with reversible deionization by acid treatment. Its portability, real time sensitivity in urine made it a strong candidate for continuous continuous, self sensed health monitoring, which exceeded limitations of earlier fixed use biosensors [
48].The dual-doping laser-induced graphene (LIG) fabrication technique enhances sensitivity, stability, and scalability in next-generation kidney health biosensors.
Figure 2
As the daily health monitoring becomes more and more prevalent, there is a great need for developing simple yet selective biosensor devices for accurate detection of biomarkers in human samples. Thus, a laccase linked mediator was employed in an organic field effect transistor (OFET)-based biosensor to detect dopamine in urine. By using selective recognition and accurate detection with a low detection limit of 0.19 μM and high recovery (97–104%), without pretreatment, a practical urinalysis was confirmed by the design [
49]. The disadvantages of these systems, however, mean it is still difficult to detect extremely low abundance biomarkers such as those responsible for bladder cancer using OFET based platforms. An indium gallium zinc oxide (IGZO) FET biosensor functionalized with single stranded DNA was developed to overcome this with detection limit of 19.8 amol/L. Accurate discrimination between bladder cancer patients and healthy donors can be made based on trace urinary microRNAs using this high performance sensor, which constitutes a major advance for early cancer diagnosis [
50]. Although sensitive, this response was still limited to time scales of fast turnaround for rapid response real time diagnostics. This challenge is addressed by the introduction of a highly sensitive and label free BioFET that can be used for epinephrine detection in urine. This sensor provided rapid response time (0.2 s), wide dynamic range and strong selectivity, using the electrostatic gating, and Schottky barrier effects for improved performance. It is fast, reproducible and stable in complex urine samples, thus serving as a promising platform for point of care diagnostics of macromolecular biomarkers [
51].There is a great potential of using flexible substrates with nanostructured semiconductors, such as BiVO₄ nanofibers, to make biosensing with high sensitivity, rapid response and scalable fabrication.
Table 1
Conclusion
The review has described progress in making FET biosensors suited for urinary biomarker detection based on considerations including material choices, device architectures, and fabrication techniques that allow clinical grade FET biosensors to detect sensitive, non invasive diagnostics. Over the past research, all the FET platforms including EGFETs, ISFETs, OFETs, and recent designs like MoS₂ FETs and IGZO FETs are reported to be compatible with diverse urinary biomarkers: uric acid, creatinine, glucose, dopamine, miRNA, and proteins. Device response, continues to be improved with innovations in dielectric modulation, nanocavity design, precise doping and Schottky barrier engineering and multi material integration as well as interface engineering are provided for stability and reproducibility. The materials are chosen from the list of carbon based nanostructures, transition metal dichalcogenides, indium based oxides and advanced dielectrics to develop FET sensors with improved bio interface performance. Most of these technologies are becoming more reliable at detecting in complex urine samples, paying greater attention to device stability, reusability and fabrication.
Studies in miRNA, creatinine, uric acid, and dopamine detection demonstrate that the platforms of MoS₂ FETs, IGZO FETs, and NiOx-based EGFET are tailored to specific urinary biomarkers using targeted functionalization strategies. Different materials such as BiVO₄, graphene, and RuO₂ showed promising results in the lab; however, there are problems with showing consistent performance, so there is a need for standardized approaches. Overall, FET biosensors are dynamic and evolving urinary diagnostics with good potential to improve with continued interdisciplinary material and engineering research.
Author Contributions
DUR: Written original draft; conducted a survey of the literature; prepared the tables; collected the references; methodology; edited and proofread the final manuscript.
Funding
No funding was provided for the development of this manuscript.
Data Availability Statement
All data relevant to this review are included in the text, references, tables, and figures.
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable.
Acknowledgments
The authors would like to express sincere gratitude to the Shreenivas Deshpande Library at the Indian Institute of Technology (BHU) Varanasi for providing invaluable resources and support.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Harika, P.; Kondavitee, G.S.; Karumuri, S.R.; Lay-Ekuakille, A. High Sensitivity of Dielectrically Modulated Tunnel Field Effect Transistor for Biosensor Applications. IEEE Transactions on NanoBioscience 2024. [Google Scholar] [CrossRef] [PubMed]
- Bind, M.K.; Nigam, K.K. Sensitivity and Non-Ideal Issues Analysis of a Dielectric Modulated Electrically Doped Junctionless TFET-Based Label-Free Biosensor. IEEE Sensors Journal 2024. [Google Scholar] [CrossRef]
- Swati; Kaur, J.; Singh, A.K. Performance investigation of Ge-based dielectric modulated junctionless TFET as a label-free biosensor. Applied Physics A 2024, 130, 133. [Google Scholar] [CrossRef]
- Mandal, R.; Mukherjee, D. Design and Investigation of Split Gate Dielectric Modulated JLFET for Detection of Biological Molecule Using TCAD Simulation. Silicon 2023, 15, 1171–1179. [Google Scholar] [CrossRef]
- Pundir, A.K.S.; Kaur, P.; Burra, S.; Mani, P.; Wadhwa, G. Electrolyte Gated based pH sensing Vertical TFET Biosensor: Design, Simulation and Noise Analysis. Micro and Nanostructures 2024, 207897. [Google Scholar] [CrossRef]
- Rashid, S.; Bashir, F.; Khanday, F.A.; Beigh, M.R. Double gate 6h-silicon carbide schottky barrier fet as dielectrically modulated label free biosensor. Silicon 2023, 15, 3387–3398. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, L.; Su, Q.; Cao, W.; Yang, W.; Wei, X.; Cao, Z.; Yang, Y. A P+ Pocket Doped 4H-SiC Schottky Barrier FET as Highly Sensitive Label-free Biosensor. Micro and Nanostructures 2024, 207931. [Google Scholar] [CrossRef]
- Chakraborti, P.; Biswas, A.; Mallik, A. High sensitivity Ge-source L-shaped tunnel BioFETs for detection of high-K biomolecules. Microsystem Technologies 2022, 28, 2131–2138. [Google Scholar] [CrossRef]
- Wei, S.; Dou, Y.; Yu, Y.; Yang, J.; Yu, F.; Sha, W.; Li, T. A novel biosensor based on a bio-barcode for the detection of Mycobacterium tuberculosis. Analytical Methods 2023, 15, 3683–3691. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wei, J.; Xiao, K.; Chen, Y.; Jiang, Y.-L.; Wan, J. Au Nanoparticles/HfO₂/Fully Depleted Silicon-on-Insulator MOSFET Enabled Rapid Detection of Zeptomole COVID-19 Gene With Electrostatic Enrichment Process. IEEE Transactions on Electron Devices 2023, 70, 1236–1242. [Google Scholar] [CrossRef]
- Yadav, S.; Das, A.; Rewari, S. Dielectrically-modulated GANFET biosensor for label-free detection of DNA and avian influenza virus: proposal and modeling. ECS Journal of Solid State Science and Technology 2024, 13, 047001. [Google Scholar] [CrossRef]
- Hussian, A.; Alkhammash, H.I.; Wani, M.S.; Loan, S.A. Metal Strip Implanted Tunneling Field-Effect Transistor Biosensor as a Label-Free Biosensor. ACS Applied Bio Materials 2024. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; De, A.; Goswami, B.; Shee, S.; Sarkar, S.K. Noise immune dielectric modulated dual trench transparent gate engineered MOSFET as a label free biosensor: proposal and investigation. Journal of Computational Electronics 2021, 20, 2594–2603. [Google Scholar] [CrossRef]
- Chahardah Cherik, I.; Mohammadi, S. Fringe-fields-modulated double-gate tunnel-FET biosensor. Scientific Reports 2024, 14, 168. [Google Scholar] [CrossRef]
- Peesa, R.B.; Panda, D.K. Rapid detection of biomolecules in a junction less tunnel field-effect transistor (JL-TFET) biosensor. Silicon 2022, 14, 1705–1711. [Google Scholar] [CrossRef]
- Biswas, A.; Rajan, C.; Samajdar, D.P. A Novel HM-HD-RFET Biosensor for Label-Free Biomolecule Detection. Journal of Electronic Materials 2022, 51, 6388–6396. [Google Scholar] [CrossRef]
- Biswas, A.; Rajan, C.; Samajdar, D.P. A novel rfet sensor for label-free biomolecule detection. Silicon 2022, 14, 9533–9541. [Google Scholar] [CrossRef]
- Reddy, N.N.; Panda, D.K. Design and investigation of dielectric modulated triple metal gate-oxide-stack Z-shaped gate horizontal pocket TFET device as a label-free biosensor. Journal of Micromechanics and Microengineering 2022, 32, 085001. [Google Scholar] [CrossRef]
- Talukdar, J.; Rawat, G.; Mummaneni, K. Highly sensitivity Non-Uniform Tunnel FET based biosensor using source engineering. Materials Science and Engineering: B 2023, 293, 116455. [Google Scholar] [CrossRef]
- Shaleen; Singh, S.; Kumar, P. Ultrasensitive label-free electrical detection of charged biomolecules using a metal–semiconductor–metal Schottky silicon nanowire biristor. Journal of Computational Electronics 2022, 21, 86–93. [Google Scholar] [CrossRef]
- Dash, S.; Mishra, G.P. Ambipolarity sensitivity investigation using a charge-plasma TFET with graphene channel for biomolecule detection. ECS Journal of Solid State Science and Technology 2024, 13, 011005. [Google Scholar] [CrossRef]
- Ashima; Dhandapani, V.; Raj, B. Design and performance assessment of graded channel gate-all-around silicon nanowire FET for biosensing applications. Silicon 2023, 15, 3535–3542. [Google Scholar] [CrossRef]
- Chowdhury, D.; De, B.P.; Appasani, B.; Singh, N.K.; Kar, R.; Mandal, D.; Bizon, N.; Thounthong, P. A Novel Dielectric Modulated Gate-Stack Double-Gate Metal-Oxide-Semiconductor Field-Effect Transistor-Based Sensor for Detecting Biomolecules. Sensors 2023, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Jiang, L.; Ma, S.; Li, T.; Zhu, Y.; Qiu, R.; Xing, Y.; Yin, F.; Li, Z.; Ye, X. Multi-Body Biomarker Entrapment System: An All-Encompassing Tool for Ultrasensitive Disease Diagnosis and Epidemic Screening. Advanced Materials 2023, 35, 2304119. [Google Scholar] [CrossRef]
- Bahri, M.; Shi, B.; Elaguech, M.A.; Djebbi, K.; Zhou, D.; Liang, L.; Tlili, C.; Wang, D. Tungsten disulfide nanosheet-based field-effect transistor biosensor for DNA hybridization detection. ACS Applied Nano Materials 2022, 5, 5035–5044. [Google Scholar] [CrossRef]
- Rahmani, M. Performance analysis of electrochemical detection platform for DNA hybridization using TGN-based nanobiosensor. ECS Journal of Solid State Science and Technology 2023, 12, 127001. [Google Scholar] [CrossRef]
- Li, K.; Tu, J.; Zhang, Y.; Jin, D.; Li, T.; Li, J.; Ni, W.; Xiao, M.-M.; Zhang, Z.-Y.; Zhang, G.-J. Ultrasensitive detection of exosomal miRNA with PMO-graphene quantum dots-functionalized field-effect transistor biosensor. Iscience 2022, 25. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Atef, M. DNA bases detection via MoS2 field effect transistor with a nanopore: first-principles modeling. Analog Integrated Circuits and Signal Processing 2023, 114, 253–264. [Google Scholar] [CrossRef]
- Goswami, P.P.; Bonam, S.; Jeyaram, K.; Singh, S.G. Device-Physics Realization of ZnO–MWCNT Nanostructure-Based Field-Effect Biosensor for Ultrasensitive Simultaneous Genomic Detection of Foodborne Pathogens. Analytical Chemistry 2023, 95, 14695–14701. [Google Scholar] [CrossRef]
- Hossain, M.M.; Shabbir, B.; Liu, J.; Wan, Z.; Walia, S.; Bao, Q.; Alan, T.; Mokkapati, S. Bisphenol A Detection Using Molybdenum Disulfide (MoS2) Field-Effect Transistor Functionalized with DNA Aptamers. Advanced Materials Technologies 2023, 8, 2201793. [Google Scholar] [CrossRef]
- Majd, S.M.; Mirzapour, F.; Shamsipur, M.; Manouchehri, I.; Babaee, E.; Pashabadi, A.; Moradian, R. Design of a novel aptamer/molecularly imprinted polymer hybrid modified Ag–Au@ Insulin nanoclusters/Au-gate-based MoS2 nanosheet field-effect transistor for attomolar detection of BRCA1 gene. Talanta 2023, 257, 124394. [Google Scholar] [CrossRef] [PubMed]
- Tamersit, K. Dielectric-Modulated Junctionless Carbon Nanotube Field-Effect Transistor as a Label-Free DNA Nanosensor: Achieving Ultra-High Sensitivity in the Band-to-Band Tunneling Regime. IEEE Sensors Journal 2023. [Google Scholar] [CrossRef]
- Ryazantsev, D.; Shustinskiy, M.; Sheshil, A.; Titov, A.; Grudtsov, V.; Vechorko, V.; Kitiashvili, I.; Puchnin, K.; Kuznetsov, A.; Komarova, N. A Portable Readout System for Biomarker Detection with Aptamer-Modified CMOS ISFET Array. Sensors 2024, 24, 3008. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, R.; Kumar, M. Sensitivity enhancement of dual gate FET based biosensor using modulated dielectric for Covid detection. Silicon 2022, 14, 11453–11462. [Google Scholar] [CrossRef]
- Yadav, S.; Rewari, S.; Pandey, R. Physics-based analytical model for trap assisted biosensing in dual cavity negative capacitance junctionless accumulation mode FET. Microelectronics Journal 2024, 143, 106032. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Chen, Q.; Jiang, Y.-L.; Wan, J. Back-Gate Fully-Depleted Silicon-on-Insulator P-Channel Schottky Barrier MOSFET With Ultrahigh Voltage Sensitivity for Label-Free Virus RNA Detection. IEEE Transactions on Instrumentation and Measurement 2024. [Google Scholar] [CrossRef]
- Yadav, S.; Rewari, S. Dual metal dual layer GAA NW–FET (DMDL–GAA–NW–FET) biosensor for label free SARS-CoV-2 detection. Microsystem Technologies 2024, 30, 565–582. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Z.; Wang, S.; Wang, C.; Chu, Y.; Liu, H.; Zhang, Y.; Han, L. A Novel InSe-FET Biosensor based on Carrier-Scattering Regulation Derived from the DNA Probe Assembly-Determined Electrostatic Potential Distribution. Advanced Functional Materials 2023, 33, 2213277. [Google Scholar] [CrossRef]
- Ho, H.-Y.; Kao, W.-S.; Deval, P.; Dai, C.-Y.; Chen, Y.-H.; Yu, M.-L.; Lin, C.-H.; Yu, L.-S. Rapid and sensitive LAMP/CRISPR-powered diagnostics to detect different hepatitis C virus genotypes using an ITO-based EG-FET biosensing platform. Sensors and Actuators B: Chemical 2023, 394, 134278. [Google Scholar] [CrossRef]
- Kao, W.-S.; Yu, L.-S.; Lin, C.-H. Rapid Fluorescence-Free Detection of DNA Loop-Mediated Isothermal Amplification on Bare ITO Surface Under EG-FET Scheme. IEEE Sensors Journal 2023, 23, 13876–13881. [Google Scholar] [CrossRef]
- Pan, T.-M.; Lin, C.-H. High performance NiOx extended-gate field-effect transistor biosensor for detection of uric acid. Journal of The Electrochemical Society 2021, 168, 017511. [Google Scholar] [CrossRef]
- Kuo, P.-Y.; Chen, Y.-Y.; Lai, W.-H.; Chang, C.-H. An extended-gate field-effect transistor applied to resistive divider integrated with the readout circuit using 180nm CMOS process for uric acid detection. IEEE Sensors Journal 2021, 21, 20229–20238. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Waizumi, H.; Takaoka, T.; Wang, Z.; Sainoo, Y.; Al Mamun, M.S.; Ando, A.; Fukuyama, M.; Hibara, A.; Komeda, T. A microfluidic approach for the detection of uric acid through electrical measurement using an atomically thin MoS 2 field-effect transistor. Analyst 2023, 148, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Park, S.; Bang, S.; Kim, H.; Lee, Y.; Park, M.G.; Yoon, S.G.; Jeong, Y.; Kim, H.; Kang, S.H. Catapult-like DNA actuator for highly stable and ultrasensitive detection of urinary miRNA. Chemical Engineering Journal 2025, 159806. [Google Scholar] [CrossRef]
- Saengdee, P.; Thanapitak, S.; Ongwattanakul, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Jeamsaksiri, W.; Promptmas, C. A silicon nitride ion sensitive field effect transistor-based immunosensor for determination of urinary albumin. Electrochemical Science Advances 2022, 2, e2100078. [Google Scholar] [CrossRef]
- Oliveira, D.C.D.B.; Costa, F.H.M.; Beraldo, R.M.; da Silva, J.A.F.; Diniz, J.A. Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine. Sensors (Basel, Switzerland) 2025, 25, 779. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Li, X.; Wei, Z.; Tang, J.; Zhang, M. Laser-induced multi-doped graphene extended-gate field-effect transistor sensor for enhanced detection of cystatin C. Talanta 2025, 282, 127039. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Zhao, X.; Wang, H.; Li, F.; Liu, S.; Pan, Y. A fully integrated graphene-polymer field-effect transistor biosensing device for on-site detection of glucose in human urine. Materials Today Chemistry 2022, 23, 100635. [Google Scholar] [CrossRef]
- Ohshiro, K.; Sasaki, Y.; Minami, T. An extended-gate-type organic transistor-based enzymatic sensor for dopamine detection in human urine. Talanta Open 2023, 7, 100190. [Google Scholar] [CrossRef]
- Guo, J.; Shen, R.; Shen, X.; Zeng, B.; Yang, N.; Liang, H.; Yang, Y.; Yuan, Q. Construction of high stability indium gallium zinc oxide transistor biosensors for reliable detection of bladder cancer-associated microRNA. Chinese Chemical Letters 2022, 33, 979–982. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. BiVO 4 nanofiber-based field-effect transistors for detection of epinephrine/adrenaline hormones. Materials Chemistry Frontiers 2021, 5, 8281–8289. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).