Submitted:
22 May 2025
Posted:
22 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
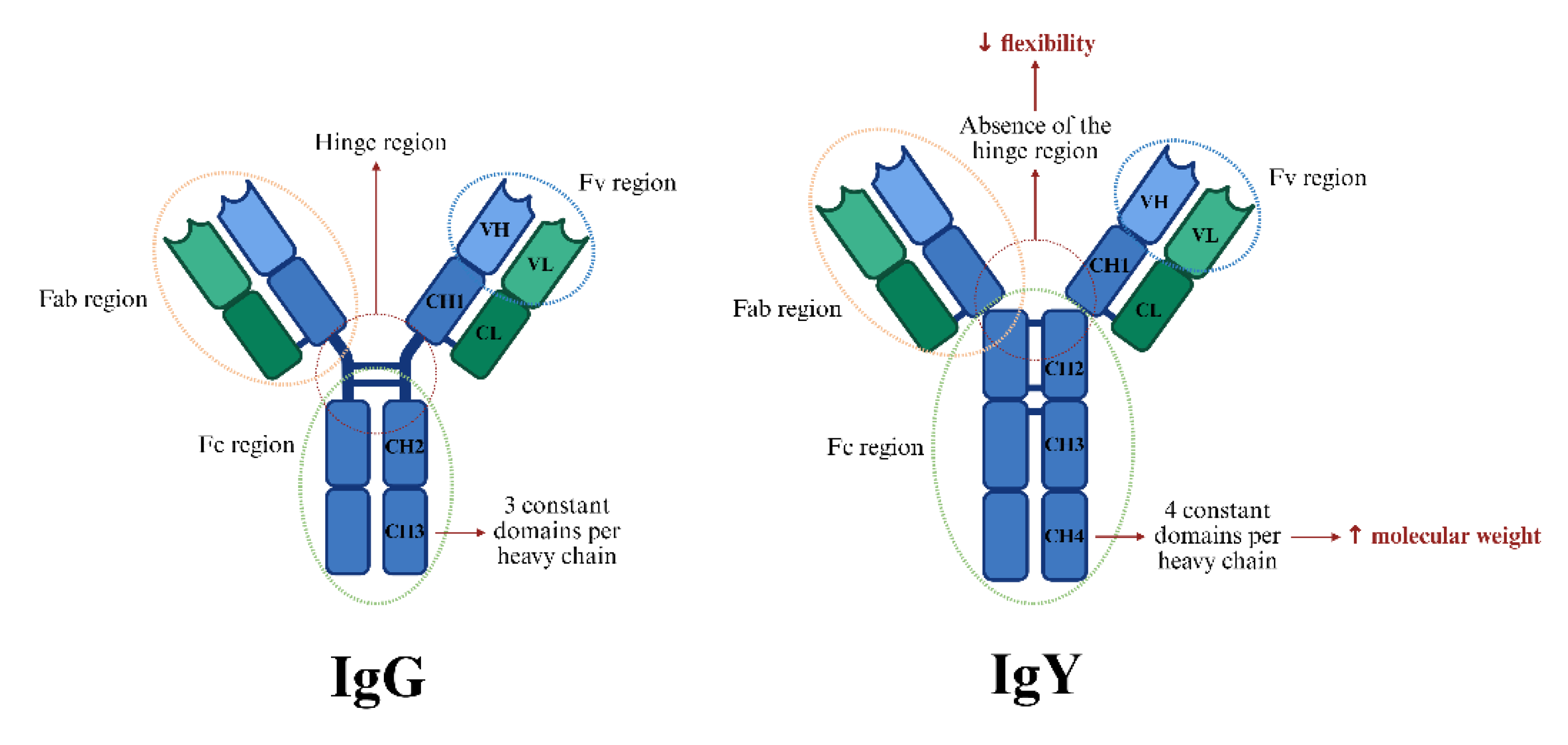
2. Structure and Function of IgY
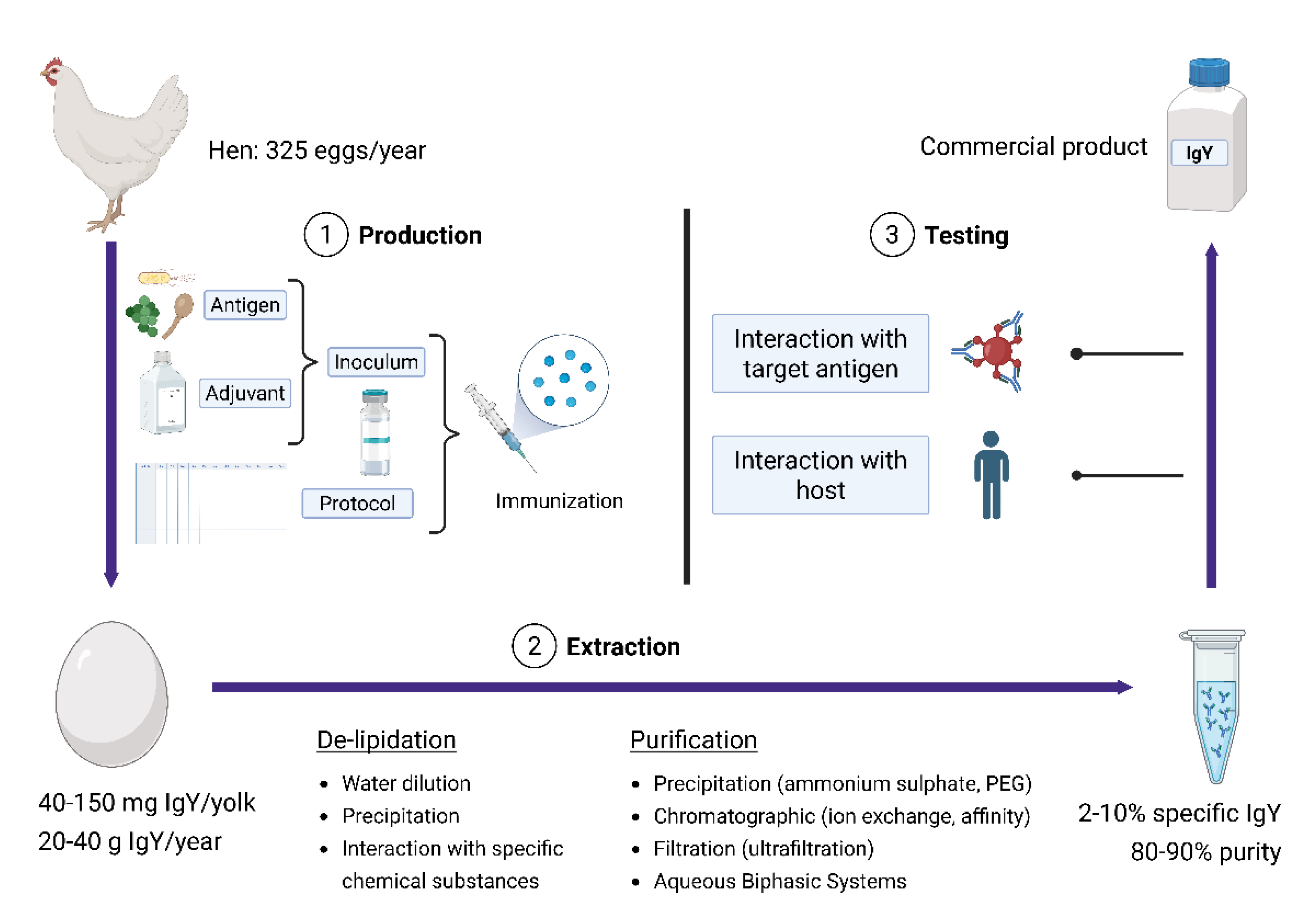
3. Immunoglobulin Y Production and Extraction
3.1. Immunization Protocol
3.2. Extraction and Purification of IgY
3.3. Evaluation of IgY
3.4. Stability and Routes of Administration for IgY Products
4. Biomedical Applications of IgY
4.1. Avian Antibodies in Diagnostic
4.2. The Role of IgY in Prevention and Therapy
4.3. Clinical Trials Using IgY
5. Advantages and Limitations of Using IgY Compared to IgG
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IgY | Immunoglobulin Y |
| IgG | Immunoglobulin G |
| Fc | Fragment crystallizable |
| Fab | Fragment antigen-binding |
| Fv | Variable fragment |
| VH | Variable region of the heavy chain |
| VL | Variable region of the light chain |
| CL | Constant region of the light chain |
| CH | Constant region of the heavy chain |
| FcRY | Fc receptor for IgY (avian neonatal Fc receptor) |
| ECVAM | European Centre for the Validation of Alternative Methods |
| WSF | Water-soluble filtrate |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| HPLC | High-performance liquid chromatography |
| BCA | Bicinchoninic acid assay |
| ELISA | Enzyme-linked immunosorbent assay |
| ETEC | Enterotoxigenic Escherichia coli |
| LTB | Heat-labile toxin subunit B |
| OmpW | Outer membrane protein W |
| CtxB | Cholera toxin subunit B |
| HPDLF | Human periodontal ligament fibroblast |
| DH | Dentin hypersensitivity |
| CFU | Colony-forming unit |
| VN | Virus neutralization |
| HAMA | Human anti-mouse antibodies |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| SpCas9 | Streptococcus pyogenes Cas9 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| NCT | ClinicalTrials.gov identifier number |
| PA | Pseudomonas aeruginosa |
| AI | Avian influenza |
| IBDV | Infectious Bursal Disease Virus |
| ZEN | Zearalenone |
| BCoV | Bovine Coronavirus |
| NCD | Neonatal Calf Diarrhea |
References
- Langel, S.N.; Blasi, M.; Permar, S.R. Maternal Immune Protection against Infectious Diseases. Cell Host Microbe 2022, 30, 660–674. [Google Scholar] [CrossRef]
- Niewiesk, S. Maternal Antibodies: Clinical Significance, Mechanism of Interference with Immune Responses, and Possible Vaccination Strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef]
- Okamoto, M.; Sasaki, R.; Ikeda, K.; Doi, K.; Tatsumi, F.; Oshima, K.; Kojima, T.; Mizushima, S.; Ikegami, K.; Yoshimura, T.; et al. FcRY Is a Key Molecule Controlling Maternal Blood IgY Transfer to Yolks during Egg Development in Avian Species. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.O.P.T.; Guedes, M.I.F. Egg Yolk Antibodies (IgY) and Their Applications in Human and Veterinary Health: A Review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Leslie, G.A.; Clem, L.W. Phylogen of Immunoglobulin Structure and Function. 3. Immunoglobulins of the Chicken. J. Exp. Med. 1969, 130, 1337–1352. [Google Scholar] [CrossRef]
- Schade, R.; Staak, C.; Hendriksen, C.; Erhard, M.; Hugl, H.; Koch, G.; Larsson, A.; Pollmann, W.; van Regenmortel, M.; Rijke, E.; et al. The Production of Avian (Egg Yolk) Antibodies: IgY: The Report and Recommendations of ECVAM Workshop 211,2. Altern. Lab. Anim. 1996, 24, 925–934. [Google Scholar] [CrossRef]
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Structure of a Typical Antibody Molecule. In Immunobiology: The Immune System in Health and Disease. 5th edition; Garland Science, 2001.
- Hersh, R.T.; Kubo, R.T.; Leslie, G.A.; Benedict, A.A. Molecular Weights of Chicken, Pheasant, and Quail IgG Immunoglobulins. Immunochemistry 1969, 6, 762–765. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Vassilakopoulou, V.; Livaniou, E. IgY Technology: Methods for Developing and Evaluating Avian Immunoglobulins for the in Vitro Detection of Biomolecules. World J. Methodol. 2021, 11, 243–262. [Google Scholar] [CrossRef]
- Gandhi, S.; Alshehri, S.M. Molecular Stability of the Rabbit and Chicken Egg Yolk Immunoglobulins. Front. Biosci. Elite Ed. 2021, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Härtle, S.; Magor, K.E.; Göbel, T.W.; Davison, F.; Kaspers, B. Chapter 5 - Structure and Evolution of Avian Immunoglobulins. In Avian Immunology (Third Edition); Kaspers, B., Schat, K.A., Göbel, T.W., Vervelde, L., Eds.; Academic Press: Boston, 2022; pp. 101–119 ISBN 978-0-12-818708-1. [Google Scholar]
- Gallagher, J.S.; Voss, E.W. Immune Precipitation of Purified Chicken Antibody at Low pH. Immunochemistry 1970, 7, 771–785. [Google Scholar] [CrossRef]
- Higgins, D.A. Precipitating Antibodies of the Duck (Anas Platyrhynchos). Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 135–144. [Google Scholar] [CrossRef]
- Spillner, E.; Braren, I.; Greunke, K.; Seismann, H.; Blank, S.; du Plessis, D. Avian IgY Antibodies and Their Recombinant Equivalents in Research, Diagnostics and Therapy. Biologicals 2012, 40, 313–322. [Google Scholar] [CrossRef]
- Lee, W.; Syed Atif, A.; Tan, S.C.; Leow, C.H. Insights into the Chicken IgY with Emphasis on the Generation and Applications of Chicken Recombinant Monoclonal Antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef]
- Karlsson, M.; Kollberg, H.; Larsson, A. Chicken IgY: Utilizing the Evolutionary Advantage. Worlds Poult. Sci. J. 2004, 60, 341–348. [Google Scholar] [CrossRef]
- IgY-Technology: Production and Application of Egg Yolk Antibodies: Basic Knowledge for a Successful Practice; Zhang, X.-Y., Vieira-Pires, R.S., Morgan, P.M., Schade, R., Eds.; Springer International Publishing: Cham, 2021; ISBN 978-3-030-72686-7. [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Carlander, D.; Larsson, A. Avian Antibodies Can Eliminate Interference Due To Complement Activation In ELISA. Ups. J. Med. Sci. 2001, 106, 189–195. [Google Scholar] [CrossRef]
- Leiva, C.L.; Gallardo, M.J.; Casanova, N.; Terzolo, H.; Chacana, P. IgY-Technology (Egg Yolk Antibodies) in Human Medicine: A Review of Patents and Clinical Trials. Int. Immunopharmacol. 2020, 81, 106269. [Google Scholar] [CrossRef]
- Gassmann, M.; Thömmes, P.; Weiser, T.; Hübscher, U. Efficient Production of Chicken Egg Yolk Antibodies against a Conserved Mammalian Protein. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1990, 4, 2528–2532. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhao, R.; Li, H.; Gao, P.; Jin, C.; Fang, C.; Liu, Y.; Hei, A.; Zhou, J.; et al. Development of a Novel Recombinant Full-Length IgY Monoclonal Antibody against Human Thymidine Kinase 1 for Automatic Chemiluminescence Analysis on a Sandwich Biotin-Streptavidin Platform for Early Tumour Discovery. J. Immunol. Res. 2023, 2023, 7612566. [Google Scholar] [CrossRef]
- Li, X.; He, P.; Yu, L.; He, Q.; Jia, C.; Yang, H.; Lu, M.; Wei, X.; Zhao, S. Production and Characteristics of a Novel Chicken Egg Yolk Antibody (IgY) against Periodontitis-Associated Pathogens. J. Oral Microbiol. 12, 1831374. [CrossRef]
- Nilsson, E.; Stålberg, J.; Larsson, A. IgY Stability in Eggs Stored at Room Temperature or at +4°C. Br. Poult. Sci. 2012, 53, 42–46. [Google Scholar] [CrossRef]
- Shimizu, M.; Nagashima, H.; Hashimoto, K.; Suzuki, T. Egg Yolk Antibody (Ig Y) Stability in Aqueous Solution with High Sugar Concentrations. J. Food Sci. 1994, 59, 763–765. [Google Scholar] [CrossRef]
- Schade, R.; Gutierrez Calzado, E.J.; Sarmiento, R.; Chacana, P.; Porankiewicz-Asplund, J.; Horacio Raúl, T. Chicken Egg Yolk Antibodies (IgY-Technology): A Review of Progress in Production and Use in Research and Human and Veterinary Medicine. Altern. Lab. Anim. ATLA 2005, 33, 129–154. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Soejoedono, R.D.; Wibawan, I.W.; Afdhal, A. Biological and Immunogenicity Property of IgY Anti S. Mutans ComD. Open Dent. J. 2016, 10, 308–314. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Behrouz, B.; Mousavi Gargari, S.L. Polyclonal Anti-Whole Cell IgY Passive Immunotherapy Shields against P. Aeruginosa-Induced Acute Pneumonia and Burn Wound Infections in Murine Models. Sci. Rep. 2024, 14, 405. [Google Scholar] [CrossRef]
- Bok, M.; Vega, C.G.; Castells, M.; Colina, R.; Wigdorovitz, A.; Parreño, V. Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves. Viruses 2023, 15, 708. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, H.; Lei, H.; Zhang, L.; Zhang, Y.; Shen, X.; Xu, B.; Zhu, J.; Xiao, W.; Zheng, J.; et al. Preparation of Anti-Zearalenone IgY and Development of an Indirect Competitive ELISA Method for the Measurement of Zearalenone in Post-Fermented Tea. Foods 2023, 12, 4478. [Google Scholar] [CrossRef]
- de Souza, P.C.; Corrêa, A.E. do N.; Gameiro, J.G.; de Oliveira Júnior, A.G.; Panagio, L.A.; Venancio, E.J.; Almeida, R.S. Production of IgY against Iron Permease Ftr1 from Candida Albicans and Evaluation of Its Antifungal Activity Using Galleria Mellonella as a Model of Systemic Infection. Microb. Pathog. 2023, 181, 106166. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.; Nazarian, S.; Rouhi, S.; Hosseini, S.E.; Fathi, J. Production of Egg Yolk Antibody (IgY) against a Chimeric Protein Containing IpaD, StxB, and TolC Antigens from Shigella: An Investigation of Its Prophylactic Effects against Shiga Toxin (Stx) and Shigella Dysenteriae in Vitro and in Vivo. Heliyon 2024, 10, e26361. [Google Scholar] [CrossRef]
- Hoseini, H.S.N.; Ahmadi, T.S.; Gargari, S.L.M.; Nazarian, S. IgY-Mediated Protection against Vibrio Cholerae Infection: Efficacy of Avian Antibodies Targeting a Chimeric Recombinant Protein. BioImpacts 2024. [Google Scholar] [CrossRef]
- Mohammadkhani, F.; Mousavi Gargari, S.L.; Nazarian, S.; Mafi, M. Protective Effects of Anti-CfaB-EtpA-LTB IgY Antibody against Adherence and Toxicity of Enterotoxigenic Escherichia Coli (ETEC). J. Appl. Microbiol. 2023, 134, lxad013. [Google Scholar] [CrossRef]
- Hedrick, E.D.; Matulka, R.A.; Conboy-Schmidt, L.; May, K.A. Evaluation of Anti-Fel d 1 IgY Ingredient for Pet Food on Growth Performance in Kittens. Front. Vet. Sci. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Kpordze, S.W.; Mobegi, V.A.; Kikuvi, G.M.; Gikunju, J.K.; Setsoafia Saba, C.K.; Moshe, J.; Kimotho, J.H. Generation of Chicken-Based IgY Polyclonal Antibodies against Dendroaspis Polylepis and Preclinical Evaluation of Envenomation-Neutralizing Efficacy Vis-à-Vis Selected Commercial Antivenoms. Toxicon X 2024, 23, 100201. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Alturaiki, W.; Dawoud, T.; El-Tayeb, M.; Elbadawi, Y.; Moussa, I. Production of Anti-Camel IgY for Diagnosis of Infectious Diseases Affecting Camels Located in Kingdom of Saudi Arabia. J. King Saud Univ. - Sci. 2023, 35, 102421. [Google Scholar] [CrossRef]
- TETİK, K.; SAREYYÜPOĞLU, B. Production, Purification, and Specificity Serologically Determination of Immunoglobulin-Y (IgY) from Chicken Eggs against Clostridium Tetani Toxoid. Turk. J. Vet. Anim. Sci. 2024, 48, 165–173. [Google Scholar] [CrossRef]
- Li, Y.; Huang, B.; Sun, S.; Liu, N.; Li, Y.; Lan, M.; Wang, X.; Zhang, Y.; Wu, A.; Yang, S.; et al. Immunoprotection Effects of Chicken Egg Yolk Immunoglobulins (IgY) against Aeromonas Veronii Infection in Sinocyclocheilus Grahami. Aquaculture 2023, 563, 738935. [Google Scholar] [CrossRef]
- Shoushtari, M.; Shooshtari, A.B.; Asadi, S.; Karami, Y.; Honari, M.; Alizadeh, G.A.; Zeinoddini, M.; Fathi, J. Production of Egg Yolk Antibody (IgY) Against Vibrio Cholerae O1: Protective Effect in Mice. Avicenna J. Med. Biotechnol. 2023, 15, 239–244. [Google Scholar] [CrossRef]
- Su, H.; Wei, K.; Zhao, M.; Li, X.; Zhang, Y. Research Note: A Novel Method for Preparation of Egg Yolk Immunoglobulin Y against Porphyromonas Gingivalis. Poult. Sci. 2023, 102, 102863. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Bogari, N.M.; Al-Allaf, F.A.; Ekram, S.N.; Athar, M.; Dannoun, A.; Schubert, T.; Syed, S.N.; Youssef, A.-R.; Alqahtani, M.; et al. Anti-E. Coli Immunoglobulin Yolk (IgY): Reduction of Pathogen Receptors and Inflammation Factors Could Be Caused by Decrease in E. Coli Load. Heliyon 2023, 9, e13876. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Endri, H.; Huang, Y.; Pan, X.; Qiu, S.; Cao, Y.; Gu, W.; Du, J.; Wang, L.; et al. Protective Effect of Chicken Egg Yolk Immunoglobulin (IgY) against Spiroplasma Eriocheiris Infection in Chinese Mitten Crab. Aquaculture 2023, 572, 739488. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.; Cui, P.; Chen, J.; Chao, J.; Wu, X.; Lu, J.; Zhang, X.; Xu, G.; Liu, Y. Differential Polyvalent Passive Immune Protection of Egg Yolk Antibodies (IgY) against Live and Inactivated Vibrio Fluvialis in Fish. Fish Shellfish Immunol. 2024, 151, 109751. [Google Scholar] [CrossRef]
- León, E.; Ortiz, V.; Pérez, A.; Téllez, J.; Díaz, G.J.; Ramírez H, M.H.; Contreras R, L.E. Anti-SpCas9 IgY Polyclonal Antibodies Production for CRISPR Research Use. ACS Omega 2023, 8, 33809–33818. [Google Scholar] [CrossRef] [PubMed]
- Montini, M.P.O.; Fernandes, E.V.; Ferraro, A.C.N. dos S.; Almeida, M.A.; da Silva, F.C.; Venancio, E.J. Effects of Inoculation Route and Dose on Production and Avidity of IgY Antibodies. Food Agric. Immunol. 2018, 29, 306–315. [Google Scholar] [CrossRef]
- Emam, M.; Mehrabani-Yeganeh, H.; Barjesteh, N.; Nikbakht, G.; Thompson-Crispi, K.; Charkhkar, S.; Mallard, B. The Influence of Genetic Background versus Commercial Breeding Programs on Chicken Immunocompetence. Poult. Sci. 2014, 93, 77–84. [Google Scholar] [CrossRef]
- Forte, C.; Moscati, L.; Acuti, G.; Mugnai, C.; Franciosini, M.P.; Costarelli, S.; Cobellis, G.; Trabalza-Marinucci, M. Effects of Dietary Lactobacillus Acidophilus and Bacillus Subtilis on Laying Performance, Egg Quality, Blood Biochemistry and Immune Response of Organic Laying Hens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 977–987. [Google Scholar] [CrossRef]
- Meethenkul (PDF) Comparison of Immunoglobulin Y Antibody Production in New and Spent Laying Hens. ResearchGate 2024. [CrossRef]
- Erhard, M.H.; Özpinar, H.; Bilal, T.; Abbas, Y.; Kutay, C.; Eseceli, H.; Stangassinger, M. The Humoral Immune Response and the Productivity of Laying Hens Kept on the Ground or in Cages. Altern. Lab. Anim. 2000, 28, 699–705. [Google Scholar] [CrossRef]
- He, J.-X.; Thirumalai, D.; Schade, R.; Zhang, X.-Y. Chronobiological Studies of Chicken IgY: Monitoring of Infradian, Circadian and Ultradian Rhythms of IgY in Blood and Yolk of Chickens. Vet. Immunol. Immunopathol. 2014, 160, 266–272. [Google Scholar] [CrossRef]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, B.L.B.; Nogueira, C.P.; Venancio, E.J. IgY Antibodies from Birds: A Review on Affinity and Avidity. Animals 2023, 13, 3130. [Google Scholar] [CrossRef] [PubMed]
- Marcq, C.; Marlier, D.; Beckers, Y. Improving Adjuvant Systems for Polyclonal Egg Yolk Antibody (IgY) Production in Laying Hens in Terms of Productivity and Animal Welfare. Vet. Immunol. Immunopathol. 2015, 165, 54–63. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.; Guo, E.; Zhou, P.; Xu, D.; Qi, Z.; Deng, L. The Preparation and Antibacterial Effect of Egg Yolk Immunoglobulin (IgY) against the Outer Membrane Proteins of Vibrio Parahaemolyticus. J. Sci. Food Agric. 2019, 99, 2565–2571. [Google Scholar] [CrossRef]
- Tirado, J.; Caza, J.; Tufiño, A.; Pardo, H.; Muñoz, D.; Manjunatha, B. DEVELOPMENT, PURIFICATION, AND CHARACTERIZATION OF IGY ANTIBODIES AGAINST VITELLOGENIN OF THE "VIEJA COLORADA” FISH (CICHLASOMA FESTAE), A SPECIES NATIVE TO THE GUAYAS RIVER, ECUADOR. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e3159–e3159. [Google Scholar] [CrossRef]
- Felegary, A.; Nazarian, S.; Zafarmand-Samarin, M.; Sadeghi, D.; Fathi, J.; Samiei-Abianeh, H. Evaluation of the Prophylactic Effect of Egg Yolk Antibody (IgY) Produced against the Recombinant Protein Containing IpaD, IpaB, StxB, and VirG Proteins from Shigella. Mol. Immunol. 2024, 173, 53–60. [Google Scholar] [CrossRef]
- Schön, J.; Aebischer, A.; Halwe, N.J.; Ulrich, L.; Hoffmann, D.; Reiche, S.; Beer, M.; Grund, C. Evaluation of SARS-CoV-2-Specific IgY Antibodies: Production, Reactivity, and Neutralizing Capability against Virus Variants. Int. J. Mol. Sci. 2024, 25, 7976. [Google Scholar] [CrossRef]
- Kubo, N.; Nishii, M.; Osada-Oka, M.; Hatta, H. A Comparative Study on Egg Yolk IgY Production with Different Adjuvants and Their Inhibitory Effects on Staphylococcus Aureus. J. Poult. Sci. 2021, 58, 192–199. [Google Scholar] [CrossRef]
- Rizkiantino, R.; Wibawan, I.W.T.; Pasaribu, F.H.; Soejoedono, R.D.; Poetri, O.N.; Arnafia, W.; Sasi, K.D.; Reisinta, D. THE POTENTIAL OF ADJUVANT AGAINST PRODUCTION OF ANTISTREPTOCOCCAL IMMUNOGLOBULIN Y (IGY) IN AQUACULTURE. J. Kedokt. Hewan - Indones. J. Vet. Sci. 2020, 14. [Google Scholar] [CrossRef]
- Sun, B.-Y.; Kou, H.-Y.; Jian, P.-Y.; Kong, L.-J.; Fang, J.; Meng, P.-K.; Wu, K.; Yang, C.-G.; Yang, G.; Song, X.-H. Protective Effects of Egg Yolk Immunoglobulins (IgY) against CyHV-2 Infection in Gibel Carp (Carassius Gibelio). Aquaculture 2023, 569, 739371. [Google Scholar] [CrossRef]
- Amro, W.A.; Al-Qaisi, W.; Al-Razem, F. Production and Purification of IgY Antibodies from Chicken Egg Yolk. J. Genet. Eng. Biotechnol. 2018, 16, 99–103. [Google Scholar] [CrossRef]
- Marcq, C.; Théwis, A.; Portetelle, D.; Beckers, Y. Refinement of the Production of Antigen-Specific Hen Egg Yolk Antibodies (IgY) Intended for Passive Dietary Immunization in Animals. A Review. BASE 2013. [Google Scholar]
- Santos, J.S.; Araújo, I.C.S.; Lacerda, M.J.R.; Andrade, M.A.; Café, M.B.; Leandro, N.S.M.; Stringhini, J.H. Effects of Broiler Breeder Age on Immune System Development of Progeny. R Bras Zootec 2022, 51. [Google Scholar] [CrossRef]
- Leenaars, M.; Hendriksen, C.F.M. Critical Steps in the Production of Polyclonal and Monoclonal Antibodies: Evaluation and Recommendations. ILAR J. 2005, 46, 269–279. [Google Scholar] [CrossRef]
- Artman, C.; Idegwu, N.; Brumfield, K.D.; Lai, K.; Hauta, S.; Falzarano, D.; Parreño, V.; Yuan, L.; Geyer, J.D.; Goepp, J.G. Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection. Viruses 2022, 14, 2371. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Dąbrowska, A.; Bobak, Ł.; Szołtysik, M. Egg Yolk Proteins and Peptides with Biological Activity. Postępy Hig. Med. Dośw. 2014, 68, 1524–1529. [Google Scholar] [CrossRef]
- Ahn, D.; Kim, S.; Shu, H. Effect of Egg Size and Strain and Age of Hens on the Solids Content of Chicken Eggs. Poult. Sci. 1997, 76, 914–919. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, F.; Yang, Y.; Xiong, C.; Xu, M.; Tu, Y. Gelation Behavior of Egg Yolk under Physical and Chemical Induction: A Review. Food Chem. 2021, 355, 129569. [Google Scholar] [CrossRef]
- De Meulenaer, B.; Huyghebaert, A. Isolation and Purification of Chicken Egg Yolk Immunoglobulins: A Review. Food Agric. Immunol. 2001, 13, 275–288. [Google Scholar] [CrossRef]
- Akita, E.M.; Nakai, S. Immunoglobulins from Egg Yolk: Isolation and Purification. J. Food Sci. 1992, 57, 629–634. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Šimůnek, J.; Hudecek, J.; Stiborova, M. Optimized Protocol of Chicken Antibody (IgY) Purification Providing Electrophoretically Homogenous Preparations. Int. J. Electrochem. Sci. 2013, 8, 113–124. [Google Scholar] [CrossRef]
- Polson, A.; von Wechmar, M.B.; van Regenmortel, M.H. Isolation of Viral IgY Antibodies from Yolks of Immunized Hens. Immunol. Commun. 1980, 9, 475–493. [Google Scholar] [CrossRef]
- Ko, K.Y.; Ahn, D.U. Preparation of Immunoglobulin Y from Egg Yolk Using Ammonium Sulfate Precipitation and Ion Exchange Chromatography. Poult. Sci. 2007, 86, 400–407. [Google Scholar] [CrossRef]
- Kubo, N.; Nishii, M.; Osada-Oka, M.; Hatta, H. A Comparative Study on Egg Yolk IgY Production with Different Adjuvants and Their Inhibitory Effects on Staphylococcus Aureus. J. Poult. Sci. 2021, 58, 192–199. [Google Scholar] [CrossRef]
- Tan, S.H.; Mohamedali, A.; Kapur, A.; Lukjanenko, L.; Baker, M.S. A Novel, Cost-Effective and Efficient Chicken Egg IgY Purification Procedure. J. Immunol. Methods 2012, 380, 73–76. [Google Scholar] [CrossRef]
- Asemota, H.; Curtello, S.; Vaillant, A.; Mohammed, W.; Vuma, S.; AV, C.; Kurhade, A.; Kissoon, S.; Smikle, M.; Wisdom, B.; et al. Purification of Avian IgY with Trichloroacetic Acid (TCA). J Chromatogr. Separat Tech. 2013, 4. [Google Scholar] [CrossRef]
- McLaren, R.D.; Prosser, C.G.; Grieve, R.C.J.; Borissenko, M. The Use of Caprylic Acid for the Extraction of the Immunoglobulin Fraction from Egg Yolk of Chickens Immunised with Ovine α-Lactalbumin. J. Immunol. Methods 1994, 177, 175–184. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Chapter Seven - Salting out of Proteins Using Ammonium Sulfate Precipitation. In Methods in Enzymology; Lorsch, J., Ed.; Laboratory Methods in Enzymology: Protein Part C; Academic Press, 2014; Vol. 541, pp. 85–94. [CrossRef]
- Deignan, T.; Kelly, J.; Alwan, A.; O’Farrelly, C. Comparative Analysis of Methods of Purification of Egg Yolk Immunoglobulin. Food Agric. Immunol. 2000, 12, 77–85. [Google Scholar] [CrossRef]
- Radwan, F.; Elshemy, A.; Toaleb, N.; Bastamy, M.; Amer, M. SEPARATION AND PURIFICATION OF CHICKEN IgY WITH ITS FIELD EFFICACY IN CONTROLLING AVIAN INFLUENZA IN MUSCOVY DUCKS. 2023. [CrossRef]
- Indhuprakash, S.T.; S, P.; C, D.R.; Thirumalai, D. Efficacy Evaluation of Anti-DEC-IgY against Antibiotic-Resistant Diarrhoeagenic Escherichia Coli. J. Med. Microbiol. 2024, 73, 001801. [Google Scholar] [CrossRef]
- O’Donnell, K.L.; Espinosa, D.A.; Puerta-Guardo, H.; Biering, S.B.; Warnes, C.M.; Schiltz, J.; Nilles, M.L.; Li, J.; Harris, E.; Bradley, D.S. Avian Anti-NS1 IgY Antibodies Neutralize Dengue Virus Infection and Protect against Lethal Dengue Virus Challenge. Antiviral Res. 2020, 183, 104923. [Google Scholar] [CrossRef]
- Tong, H.-F.; Lin, D.-Q.; Pan, Y.; Yao, S.-J. A New Purification Process for Goose Immunoglobulin IgY(ΔFc) with Hydrophobic Charge-Induction Chromatography. Biochem. Eng. J. 2011, 56, 205–211. [Google Scholar] [CrossRef]
- Łupicka-Słowik, A.; Walczak, M.; Grzywa, R.; Bobrek, K.; Łęcka, M.; Boivin, S.; Gaweł, A.; Stefaniak, T.; Oleksyszyn, J.; Sieńczyk, M. Generation and Application of Polyclonal Igy Antibodies Specific for Full-Length and Nicked Prostate-Specific Antigen. Bioanalysis 2014, 6, 3197–3213. [Google Scholar] [CrossRef]
- Xia, W.; Lu, H.; Li, Y.; Cao, J.; Zhou, X.; Zhang, X.; Xia, X.; Sun, H. Purification of Chicken IgY by Binding Capture Using Elastin-like Polypeptide-Tagged Immunoglobulin-Binding Domain of Streptococcal Protein G. Vet. Immunol. Immunopathol. 2017, 192, 13–19. [Google Scholar] [CrossRef]
- Vicente, F.A.; Castro, L.S.; Mondal, D.; Coutinho, J.A.P.; Tavares, A.P.M.; Ventura, S.P.M.; Freire, M.G. Purification of Immunoglobulin Y from Egg Yolk Using Thermoresponsive Aqueous Micellar Two-Phase Systems Comprising Ionic Liquids. Sep. Purif. Technol. 2022, 288, 120589. [Google Scholar] [CrossRef]
- Al-Qaoud, K.M.; Obeidat, Y.M.; Al-Omari, T.; Okour, M.; Al-Omari, M.M.; Ahmad, M.I.; Alshadfan, R.; Rawashdeh, A.M. The Development of an Electrochemical Immunosensor Utilizing Chicken IgY Anti-Spike Antibody for the Detection of SARS-CoV-2. Sci. Rep. 2024, 14, 748. [Google Scholar] [CrossRef]
- Sabahi, S.; Mortazavi, S.A.; Nassiri, M.; Ghazvini, K.; Shahidi, F.; Abbasi, A. Production of Functional Ice Cream Fortified by Immunoglobulin Y against Escherichia Coli O157:H7 and Helicobacter Pylori. BIOINTERFACE Res. Appl. Chem. 2023, 13, 188. [Google Scholar] [CrossRef]
- Eriksson, M.; Nylén, S.; Grönvik, K.-O. Passive Immunization of Mice with IgY Anti-H5N1 Protects against Experimental Influenza Virus Infection and Allows Development of Protective Immunity. Vaccine 2024. [Google Scholar] [CrossRef]
- Pauly, D.; Chacana, P.A.; Calzado, E.G.; Brembs, B.; Schade, R. IgY Technology: Extraction of Chicken Antibodies from Egg Yolk by Polyethylene Glycol (PEG) Precipitation. J. Vis. Exp. JoVE 2011, 3084. [Google Scholar] [CrossRef]
- Shimelis, E. Extraction, Purification and Invitro Challenging of IgY of Commercial Chicken Eggs with the Newcastle Disease. Biomed. J. Sci. Tech. Res. 2022, 45. [Google Scholar] [CrossRef]
- Goldring, J.P.D.; Coetzer, T.H.T. Isolation of Chicken Immunoglobulins (IgY) from Egg Yolk. BAMBEd 2003, 31, 185–187. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Simunek, J.; Hudecek, J.; Stiborova, M. Optimized Protocol of Chicken Antibody (IgY) Purification Providing Electrophoretically Homogenous Preparations. Int. J. Electrochem. Sci. 2013, 8, 113–124. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Z.; Zhang, W.; Huang, H.; Wang, J.; Hei, A.; Cong, Y.; Xu, J. Preparation and characterization of chicken anti-human B7-H4 IgY polyclonal antibody. Chin. J. Cell. Mol. Immunol. 2024, 40, 257–266. [Google Scholar]
- El-Kafrawy, S.A.; Abbas, A.T.; Oelkrug, C.; Tahoon, M.; Ezzat, S.; Zumla, A.; Azhar, E.I. IgY Antibodies: The Promising Potential to Overcome Antibiotic Resistance. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Hakalehto, E. Chicken IgY Antibodies Provide Mucosal Barrier against SARS-CoV-2 Virus and Other Pathogens. Isr. Med. Assoc. J. 2021, 23, 208–211. [Google Scholar]
- Frumkin, L.R.; Lucas, M.; Scribner, C.L.; Ortega-Heinly, N.; Rogers, J.; Yin, G.; Hallam, T.J.; Yam, A.; Bedard, K.; Begley, R.; et al. Egg-Derived Anti-SARS-CoV-2 Immunoglobulin Y (IgY) With Broad Variant Activity as Intranasal Prophylaxis Against COVID-19. Front. Immunol. 2022, 13, 899617. [Google Scholar] [CrossRef]
- Otterbeck, A.; Skorup, P.; Hanslin, K.; Larsson, A.; Stålberg, J.; Hjelmqvist, H.; Lipcsey, M. Intravenous Anti-P. Aeruginosa IgY-Antibodies Do Not Decrease Pulmonary Bacterial Concentrations in a Porcine Model of Ventilator-Associated Pneumonia. Innate Immun. 2022, 28, 224–234. [Google Scholar] [CrossRef]
- Rahman, S.; Nguyen, S.V.; Jr, F.C.I.; Umeda, K.; Kodama, Y. Oral Passive IgY-Based Immunotherapeutics. Hum. Vaccines Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef]
- Kollberg, H.; Carlander, D.; Olesen, H.; Wejåker, P.-E.; Johannesson, M.; Larsson, A. Oral Administration of Specific Yolk Antibodies (IgY) May Prevent Pseudomonas Aeruginosa Infections in Patients with Cystic Fibrosis: A Phase I Feasibility Study. Pediatr. Pulmonol. 2003, 35, 433–440. [Google Scholar] [CrossRef]
- Yokoyama, H.; Peralta, R.C.; Sendo, S.; Ikemori, Y.; Kodama, Y. Detection of Passage and Absorption of Chicken Egg Yolk Immunoglobulins in the Gastrointestinal Tract of Pigs by Use of Enzyme-Linked Immunosorbent Assay and Fluorescent Antibody Testing. Am. J. Vet. Res. 1993, 54, 867–872. [Google Scholar] [CrossRef]
- Guo, R.; Wu, S.; Guan, L.; Xie, Y.; Yang, X.; Li, Z.; Zhang, Z. Dietary Multivalent Anti-Helicobacter Pylori Immunoglobulin Y Significantly Increase the H. Pylori Eradication and Improve the Clinical Symptoms in Patients. Helicobacter 2021, 26, e12843. [Google Scholar] [CrossRef]
- Panigrahi, A.; Pattanaik, S.; Mohammad, N.; Sahoo, S.N.; Patnaik, S.; Sarangi, D. Effect of Passive Immunotherapeutics (IgY) on S. Mutans in Caries Active Children 8-11 Years of Age. J. Pharm. Bioallied Sci. 2024, 16, S4448. [Google Scholar] [CrossRef]
- Eriksson, M.; Nylén, S.; Grönvik, K.-O. Passive Immunization of Mice with IgY Anti-H5N1 Protects against Experimental Influenza Virus Infection and Allows Development of Protective Immunity. Vaccine 2024, S0264-410X(24)00796-5. [CrossRef]
- Shen, H.; Cai, Y.; Zhang, H.; Wu, J.; Ye, L.; Yang, P.; Lin, X.; Jiang, S.; Liao, M. Anti-SARS-CoV-2 IgY Isolated from Egg Yolks of Hens Immunized with Inactivated SARS-CoV-2 for Immunoprophylaxis of COVID-19. Virol. Sin. 2021, 36, 1080–1082. [Google Scholar] [CrossRef]
- Wongso, H.; Mahendra, I.; Arnafia, W.; Idar, I.; Yusuf, M.; Achmad, A.; Holik, H.A.; Kurniawan, A.; Halimah, I.; Sriyani, M.E.; et al. Preclinical Evaluation of Chicken Egg Yolk Antibody (IgY) Anti-RBD Spike SARS-CoV-2—A Candidate for Passive Immunization against COVID-19. Vaccines 2022, 10, 128. [Google Scholar] [CrossRef]
- Agurto-Arteaga, A.; Poma-Acevedo, A.; Rios-Matos, D.; Choque-Guevara, R.; Montesinos-Millán, R.; Montalván, Á.; Isasi-Rivas, G.; Cauna-Orocollo, Y.; Cauti-Mendoza, M. de G.; Pérez-Martínez, N.; et al. Preclinical Assessment of IgY Antibodies Against Recombinant SARS-CoV-2 RBD Protein for Prophylaxis and Post-Infection Treatment of COVID-19. Front. Immunol. 2022, 13, 881604. [Google Scholar] [CrossRef] [PubMed]
- Madai, M.; Hanna, D.; Hetényi, R.; Földes, F.; Lanszki, Z.; Zana, B.; Somogyi, B.; Papp, H.; Kuczmog, A.; Faragó-Sipos, O.; et al. Evaluating the Protective Role of Intranasally Administered Avian-Derived IgY Against SARS-CoV-2 in Syrian Hamster Models. Vaccines 2024, 12, 1422. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh-Dehaghani, A.; Towhidi, A.; Zhandi, M.; Mojgani, N.; Fouladi-Nashta, A. Combined Effect of Probiotics and Specific Immunoglobulin Y Directed against Escherichia Coli on Growth Performance, Diarrhea Incidence, and Immune System in Calves. Animal 2021, 15, 100124. [Google Scholar] [CrossRef]
- Qandoos P27 | The Efficacy of Maternally Delivered Immunoglobulin (Y) against Some Gut Pathogens in Chickens. J. Vet. Pharmacol. Ther. 2023, 46, 98–98. [CrossRef]
- Soltani, N.; Rahimi, S.; Khaki, P.; Karimi Torshizi, M.A.; Eskandari, B.; Grimes, J. Efficacy of Hyperimmunized Egg Yolk Antibodies (IgY) against Campylobacter Jejuni: In Vitro and In Vivo Evaluations. Poult. Sci. 2025, 104, 104718. [Google Scholar] [CrossRef]
- Ul Abadeen, Z.; Javed, M.T.; Rizvi, F.; Rahman, S.U. Salutary Effects of Anti-Clostridium Perfringens Type A Egg Yolk Antibodies (IgY) on Growth Performance and Hemato-Biochemical Parameters in Experimentally Infected Broiler Chicken. Pak. Vet. J. 2021, 41, 562–566. [Google Scholar] [CrossRef]
- Matulka, R.A.; Thompson, L.; Corley, D. Multi-Level Safety Studies of Anti Fel d 1 IgY Ingredient in Cat Food. Front. Vet. Sci. 2020, 6, 477. [Google Scholar] [CrossRef]
- Kota, R.K.; Reddy, P.N.; Sreerama, K. Application of IgY Antibodies against Staphylococcal Protein A (SpA) of Staphylococcus Aureus for Detection and Prophylactic Functions. Appl. Microbiol. Biotechnol. 2020, 104, 9387–9398. [Google Scholar] [CrossRef]
- Vansofla, A.N.; Nazarian, S.; Kordbache, E.; Fathi, J. An IgG/IgY Sandwich-ELISA for the Detection of Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia Coli. Gene Rep. 2021, 23, 101099. [Google Scholar] [CrossRef]
- Bayat, M.; Khabiri, A.; Hemati, B. Development of IgY-Based Sandwich ELISA as a Robust Tool for Rapid Detection and Discrimination of Toxigenic Vibrio Cholerae. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4032531. [Google Scholar] [CrossRef]
- Ge, S.; Wu, R.; Zhou, T.; Liu, X.; Zhu, J.; Zhang, X. Specific Anti-SARS-CoV-2 S1 IgY-scFv Is a Promising Tool for Recognition of the Virus. AMB Express 2022, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiao, H.; Lin, M.; Zhou, J.; Xuan, Q.; Cui, X.; Zhao, S. Preparation and Evaluation of IgY against Human Papillomavirus. J. Virol. Methods 2025, 334, 115115. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Faria, L.S. de; Sousa, J.E.N. de; Borges, I.P.; Ribeiro, R.P.; Bueno, L.L.; Ávila, V.M.R.; Júnior, Á.F.; Costa-Cruz, J.M. Anti-Ascaris Suum Immunoglobulin Y as a Novel Biotechnological Tool for the Diagnosis of Human Ascariasis. J. Helminthol. 2020, 94, e71. [Google Scholar] [CrossRef]
- Cakir-Koc, R.; Budama-Kilinc, Y.; Ustun, E.; Babur, C. Conjugation and Characterization of Latex Particles with Toxoplasma Gondii-Specific Immunoglobulin Y Antibodies for Diagnostic Aim and Evaluation Efficiency in In Vitro Culture. J. Equine Vet. Sci. 2020, 92, 103145. [Google Scholar] [CrossRef]
- Cakir-Koc, R. Production of Anti-SAG1 IgY Antibody against Toxoplasma Gondii Parasites and Evaluation of Antibody Activity by ELISA Method. Parasitol. Res. 2016, 115, 2947–2952. [Google Scholar] [CrossRef]
- Teimoori, S.; Arimatsu, Y.; Laha, T.; Kaewkes, S.; Sereerak, P.; Sripa, M.; Tangkawattana, S.; Brindley, P.J.; Sripa, B. Chicken IgY-Based Coproantigen Capture ELISA for Diagnosis of Human Opisthorchiasis. Parasitol. Int. 2017, 66, 443–447. [Google Scholar] [CrossRef]
- Prasertsincharoen, N.; Metheenukul, P. Production of Immunoglobulin Y against Dog Erythrocyte Antigen (DEA) 1.1 from Egg Yolk. Thai J. Vet. Med. 2020, 50, 381–387. [Google Scholar] [CrossRef]
- Syahruni, S.; Hartati, Y.W.; Yusuf, M.; Kusumawardani, S.; Wibawan, I.W.T.; Arnafia, W.; Sibit, G.; Subroto, T. Development of Lateral Flow Assay Based on Anti-IBDV IgY for the Rapid Detection of Gumboro Disease in Poultry. J. Virol. Methods 2021, 291, 114065. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.C.; Eto, S.F.; Claudiano, G. da S.; Marcusso, P.F.; Ramos-Espinoza, F.C.; Engracia de Moraes, J.R. Production and Use of Immunoglobulin Y in the Diagnosis of Haemorrhagic Septicemia Caused by Aeromonas Hydrophila in Piaractus Mesopotamicus. Aquac. Res. 2022, 53, 2930–2936. [Google Scholar] [CrossRef]
- Alves, C.F.; Notário, A.F.O.; Correia, L.I.V.; Ferreira, K.N.; Araujo, K.C.L.; Taketomi, E.A.; Souza, G.R.L.; Medeiros, E.S.; Cunha-Júnior, J.P.; Alves, R.P.; et al. A Novel Electrochemical Immunosensor Based on Anti-IgY and PLA/PEG Nanofibrous Mats Used for Allergens Detection in Farinaceous Products. J. Electrochem. Soc. 2023, 170, 037509. [Google Scholar] [CrossRef]
- Tu, Y.-Y.; Ma, C.-Y.; Ho, S.-B.; Chen, C.-C.; Chang, H.-M. Afffinity Measurement of Lactoferrin (LF)-Anti-LF Immunoglobulin in Yolk (IgY) Complexes by Competitive Indirect Enzyme-Linked Immunosorbent Assay (CI-ELISA). J. Food Drug Anal. 2020, 14. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Xu, L.; Ge, S.; Wang, J.; Zhang, X. Generation and Evaluation of Anti-Mouse IgG IgY as Secondary Antibody. Prep. Biochem. Biotechnol. 2020, 50, 788–793. [Google Scholar] [CrossRef]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Tabll, A.A.; Hassan, A.M.; Iwata-Yoshikawa, N.; Nagata, N.; Azhar, E.I. Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model. Vaccines 2020, 8, 634. [Google Scholar] [CrossRef]
- Bentes, G.A.; Lanzarini, N.M.; Guimarães, J.R.; Heinemann, M.B.; Volotão, E. de M.; da Silva, A. dos S.; Heneine, L.G.D.; de Oliveira, J.M.; Pinto, M.A. Production and Evaluation of Chicken Egg Yolk Immunoglobulin (IgY) against Human and Simian Rotaviruses. Viruses 2022, 14, 1995. [Google Scholar] [CrossRef] [PubMed]
- Bentes, G.A.; Guimarães, J.R.; Volotão, E. de M.; Lanzarini, N.M.; da Silva, A. dos S.; Gardinali, N.R.; Marchevsky, R.S.; Leite, J.P.G.; de Oliveira, J.M.; Pinto, M.A. Passive Immunotherapy of Cynomolgus Monkeys with Anti-Rotavirus IgY. Pharmaceutics 2024, 16, 1149. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, H.; Lin, M.; Zhou, J.; Xuan, Q.; Cui, X.; Zhao, S. Preparation and Evaluation of IgY against Human Papillomavirus. J. Virol. Methods 2025, 334, 115115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Y.; Li, Y.; Wang, X.; Liu, Y.; Tian, D.; Jia, X.; Gong, R.; Liu, W.; Yang, L. IgY Antibodies against Ebola Virus Possess Post-Exposure Protection in a Murine Pseudovirus Challenge Model and Excellent Thermostability. PLoS Negl. Trop. Dis. 2021, 15, e0008403. [Google Scholar] [CrossRef]
- Hao, N.; Liu, B.; Zhao, M.; Lu, M.; Chen, F.; Kang, J.; Tang, X.; Zhang, Y.; Dang, C. Real-World Evidence of a Novel Tetravalent Immunoglobulin Y Effectiveness and Safety in Patients with the Refractory Helicobacter Pylori Infection. BMC Infect. Dis. 2024, 24, 647. [Google Scholar] [CrossRef]
- Han, S.; Wen, Y.; Yang, F.; He, P. Chicken Egg Yolk Antibody (IgY) Protects Mice Against Enterotoxigenic Escherichia Coli Infection Through Improving Intestinal Health and Immune Response. Front. Cell. Infect. Microbiol. 2021, 11, 662710. [Google Scholar] [CrossRef]
- Capotă, R.; Iliescu, A.C.B.; Năstasă, V.; Minea, B.; Sliwa, D.C.; Mareș, M. Hyperimmune Egg-Based IgY-Rich Formulations as Adjuvant Therapy in a Murine Model of Urinary Tract Infection. J. Glob. Antimicrob. Resist. 2024, 39, 48. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Mousavi Gargari, S.L.; Talei, D. Anti-Flagellin IgY Antibodies Protect against Pseudomonas Aeruginosa Infection in Both Acute Pneumonia and Burn Wound Murine Models in a Non-Type-Specific Mode. Mol. Immunol. 2021, 136, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, F.; Behrouz, B.; Ranjbar, M.; Mousavi Gargari, S.L. Immunotherapy with IgY Antibodies toward Outer Membrane Protein F Protects Burned Mice against Pseudomonas Aeruginosa Infection. J. Immunol. Res. 2020, 2020, 7840631. [Google Scholar] [CrossRef]
- Sanches, R.F.; dos Santos Ferraro, A.C.N.; Marroni, F.E.C.; Venancio, E.J. Synergistic Activity between Beta-Lactams and Igy Antibodies against Pseudomonas Aeruginosa in Vitro. Mol. Immunol. 2022, 148, 1–5. [Google Scholar] [CrossRef]
- Thomsen, K.; Christophersen, L.; Lerche, C.J.; Holmgaard, D.B.; Calum, H.; Høiby, N.; Moser, C. Azithromycin Potentiates Avian IgY Effect against Pseudomonas Aeruginosa in a Murine Pulmonary Infection Model. Int. J. Antimicrob. Agents 2021, 57, 106213. [Google Scholar] [CrossRef] [PubMed]
- Wannigama, D.L.; Hurst, C.; Monk, P.N.; Abe, S.; Chatsuwan, T.; Stick, S.M.; Kicic, A. Novel Inhale Treatment of IgY Immunoglobulin Combination with Macrocyclic Peptide Eliminate Multidrug-Resistant Pseudomonas Aeruginosa Pulmonary Infections. RESPIROLOGY 2023, 28, 6–6. [Google Scholar]
- Zamani, K.; Irajian, G.; Zahedi Bialvaei, A.; Zahraei Salehi, T.; Khormali, M.; Vosough, A.; Masjedian Jazi, F. Passive Immunization with Anti- Chimeric Protein PilQ/PilA –DSL Region IgY Does Not Protect against Mortality Associated with Pseudomonas Aeruginosa Sepsis in a Rabbit Model. Mol. Immunol. 2022, 141, 258–264. [Google Scholar] [CrossRef]
- Zafarmand-Samarin, M.; Nazarian, S.; Aghaie, S.M.; Sadeghi, D.; Samiei-Abianeh, H.; Felegary, A. Therapeutic Efficacy of SipD/LptD-Specific IgY Entrapped in Alginate Nanoparticles against Salmonella Typhimurium Infection. Heliyon 2024, 10, e39650. [Google Scholar] [CrossRef]
- Cabré, N.; Hartmann, P.; Llorente, C.; Kouno, T.; Wang, Y.; Zeng, S.; Kim, H.Y.; Zhang, X.; Kisseleva, T.; Iyer, S.; et al. Immunoglobulin Y Antibodies against Cytolysin Reduce Ethanol-Induced Liver Disease in Mice. Hepatol. Baltim. Md 2023, 78, 295–306. [Google Scholar] [CrossRef]
- Mary, D.J.; Mathew, M.G. Effect of Streptococcal IgY on Quantity of Streptococcus Mutans in High Caries Risk Children. J. Pharm. Res. Int. 2020, 6–11. [Google Scholar] [CrossRef]
- Li, X.; He, P.; Yu, L.; He, Q.; Jia, C.; Yang, H.; Lu, M.; Wei, X.; Zhao, S. Production and Characteristics of a Novel Chicken Egg Yolk Antibody (IgY) against Periodontitis-Associated Pathogens. J. Oral Microbiol. 2020, 12, 1831374. [Google Scholar] [CrossRef]
- Sindhu, B.U.; Praveen, S.; Sandeep, J.N.; Avinash, J.L.; Rajiv, N.P.; Rudraswamy, S. Evaluation of Antimicrobial Efficacy of Immunoglobulin Y (IgY) against Periodontal Biofilm. J. Oral Maxillofac. Pathol. JOMFP 2023, 27, 499–506. [Google Scholar] [CrossRef]
- Yan, Y.; Guan, Y.; Luo, L.; Lu, B.; Chen, F.; Jiang, B. Effects of Immunoglobulin Y-Loaded Amorphous Calcium Phosphate on Dentinal Tubules Occlusion and Antibacterial Activity. Front. Bioeng. Biotechnol. 2022, 10, 921336. [Google Scholar] [CrossRef] [PubMed]
- Mares, M.; Iliescu, A.C.B.; Minea, B.; Capota, R.; Sliwa, D.C.; Nastasa, V. Hyperimmune Egg-Based IgY-Rich Formulations as Adjuvant Therapy in a Murine Model of Vaginal Candidiasis. J. Glob. Antimicrob. Resist. 2024, 39, 63. [Google Scholar] [CrossRef]
- Jin, Y.; Lv, H.; Wang, M.; Cho, C.-S.; Shin, J.; Cui, L.; Yan, C. Effect of Microencapsulation of Egg Yolk Immunoglobulin Y by Sodium Alginate/Chitosan/Sodium Alginate on the Growth Performance, Serum Parameters, and Intestinal Health of Broiler Chickens. Anim. Biosci. 2023, 36, 1241–1251. [Google Scholar] [CrossRef]
- Radwan, F.M.; EL-Shemy, A.; Bosila, M.M.; Bastamy, M.A.; Amer, M.M. Evaluation of The Efficacy of Purified Egg Yolk Immunoglobulin (IgY) in Preventing and Controlling Newcastle Disease Virus Infection in Broiler Chickens. Egypt. J. Vet. Sci. 2024, 55, 931–943. [Google Scholar] [CrossRef]
- Yang, D.; Mai, K.; Zhou, Q.; Zhu, Y.; Xing, J.; Luo, C.; Liu, S.; Zhou, Q.; Huang, W.; Luo, J.; et al. The Protective Efficacy of Specific Egg Yolk Immunoglobulin Y(IgY) against Riemerella Anatipestifer Infections. Vet. Microbiol. 2020, 243, 108642. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Xu, L.; Li, B.; Zhong, F.; Liu, X.; Zhang, X. Canine Parvovirus Is Diagnosed and Neutralized by Chicken IgY-scFv Generated against the Virus Capsid Protein. Vet. Res. 2020, 51, 110. [Google Scholar] [CrossRef]
- Bousquet, J.; Gherasim, A.; de Blay, F.; Mathieu-Dupas, E.; Batot, G.; Laune, D.; Sousa-Pinto, B.; Zuberbier, T.; Pham-Thi, N.; Group, M. study Proof-of-Concept Study of Anti-Fel d 1 IgY Antibodies in Cat Food Using the MASK-Air® App. Clin. Transl. Allergy 2024, 14, e12353. [Google Scholar] [CrossRef]
- Bustos, C.P.; Leiva, C.L.; Gambarotta, M.; Guida, N.; Chacana, P.A. In Vitro Inhibitory Activity of IgY Antibodies Against Salmonella Ser. Newport Isolated from Horses. J. Equine Vet. Sci. 2021, 103, 103657. [Google Scholar] [CrossRef]
- Czoska, P.; Tarsalewska, K.; Ponichtera, M.; Rybicka, M.; Sowa-Rogozinska, N.; Sominka-Pierzchlewicz, H.; Stodolna, A.; Ogonowska, P.; Kosciuk, A.; Glosnicka, R.; et al. Growth-Inhibitory Effect of Chicken Egg Yolk Polyclonal Antibodies (IgY) on Zoonotic Pathogens Campylobacter Jejuni, Salmonella Spp. and Escherichia Coli, In Vitro. Int. J. Mol. Sci. 2025, 26, 1040. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Yan, L.; Liu, W.; Shi, H.; Lu, Y.; Liu, X. Protective Effects of Egg Yolk Immunoglobulins (IgY) on Juvenile Groupers (Epinephelus Fuscoguttatus × Epinephelus Lanceolatus) with Red-Spotted Grouper Nervous Necrosis Virus Infection. Aquaculture 2021, 545, 737218. [Google Scholar] [CrossRef]
- Liang, Z.; Ning, Y.; Cao, J.; Liu, S.; Liang, X.; Peng, X.; Huang, Y.; Wei, J.; Xiao, S.; Qin, Q.; et al. The Protective Effect of Specific Yolk Antibody against Nervous Necrosis Virus Infection in Mandarin Fish(Siniperca Chuatsi). Fish Shellfish Immunol. 2024, 155, 109996. [Google Scholar] [CrossRef] [PubMed]
- Setthawong, P.; Yamkasem, J.; Khemthong, M.; Tattiyapong, P.; Metheenukul, P.; Prasertsincharoen, N.; Lertwanakarn, T.; Thengchaisri, N.; Surachetpong, W. Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays. Viruses 2025, 17, 448. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Pedrosa-Gerasmio, I.R.; Alenton, R.R.R.; Nozaki, R.; Kondo, H.; Hirono, I. Anti-PirA-like Toxin Immunoglobulin (IgY) in Feeds Passively Immunizes Shrimp against Acute Hepatopancreatic Necrosis Disease. J. Fish Dis. 2019, 42, 1125–1132. [Google Scholar] [CrossRef]
- James, T.; Rahiman KM ,Mujeeb; Sebastian ,Denoj; S ,Suja Rani; and MD, D. The Combinational Effect of Avian IgY Antibodies and Broad-Spectrum Antibiotics (Ciprofloxacin and Chloramphenicol) in Vitro Reduces the Incidence of Aeromonas Hydrophila. J. Appl. Aquac. 2025, 1–16. [CrossRef]
- Xu, L.; Che, J.; Xu, Y.; Chen, Y.; Li, Y.; Murtaza, B.; Wang, L.; Zhang, M.; Li, X. Oral Administration of Microencapsulated Egg Yolk Immunoglobulin (IgY) in Turbot (Scophthalmus Maximus) to Combat against Edwardsiella Tarda 2CDM001 Infections. Fish Shellfish Immunol. 2020, 106, 609–620. [Google Scholar] [CrossRef]
- Soto-Dávila, M.; Rodríguez-Cornejo, T.; Benito, V.W.; Rodríguez-Ramos, T.; Mahoney, G.; Supinski, R.; Heath, G.; Dang, X.; Valle, F.M.; Hurtado, C.; et al. Innate and Adaptive Immune Response of Rainbow Trout (Oncorhynchus Mykiss) Naturally Infected with Yersinia Ruckeri. Fish Shellfish Immunol. 2024, 151, 109742. [Google Scholar] [CrossRef] [PubMed]
- Gopal, G.; Antonysamy ,Michael; Visaga Ambi ,Senthil; and Thirumalai, D. Development of Chicken Egg Yolk Antibodies (IgY) against Venom of Cobra (Naja Naja) and Krait (Bungarus Caeruleus) and a Study on Its Neutralization Potential. Toxin Rev. 2024, 43, 484–504. [CrossRef]
- Nguyen, V.T.; Vu, T.T.H.; Hoang, V.T.; Nguyen, T.V.; Lam, T.D.; Nguyen, K.C.; Than, V.T.; Le, V.P. Immunological Properties of the Chicken Egg Yolk (IgY) Antibodies against Vietnamese Cobra Naja Naja Venom. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012012. [Google Scholar] [CrossRef]
- Duan, H.; He, Q.; Zhou, B.; Wang, W.; Li, B.; Zhang, Y.; Deng, Q.; Zhang, Y.; Yu, X. Anti-Trimeresurus Albolabris Venom IgY Antibodies: Preparation, Purification and Neutralization Efficacy. J. Venom. Anim. Toxins Trop. Dis. 2016, 22, 23. [Google Scholar] [CrossRef]
- Leiva, C.L.; Cangelosi, A.; Mariconda, V.; Farace, M.; Geoghegan, P.; Brero, L.; Fernández-Miyakawa, M.; Chacana, P. IgY-Based Antivenom against Bothrops Alternatus: Production and Neutralization Efficacy. Toxicon 2019, 163, 84–92. [Google Scholar] [CrossRef]
- Alvarez, A.; Montero, Y.; Jiménez, E.; Zerpa, N.; Parrilla, P.; Malavé, C. IgY Antibodies Anti-Tityus Caripitensis Venom: Purification and Neutralization Efficacy. Toxicon Off. J. Int. Soc. Toxinology 2013, 74. [Google Scholar] [CrossRef] [PubMed]
- Leiva, C.L.; Geoghegan, P.; Lammer, M.; Cangelosi, A.; Mariconda, V.; Celi, A.B.; Brero, M.L.; Chacana, P. In Vivo Neutralization of Bee Venom Lethality by IgY Antibodies. Mol. Immunol. 2021, 135, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.V.; Nguyen, M.T.H.; Tran, B.C.; Ho, M.T.Q.; Umeda, K.; Rahman, S. Evaluation of Lozenges Containing Egg Yolk Antibody against Porphyromonas Gingivalis Gingipains as an Adjunct to Conventional Non-Surgical Therapy in Periodontitis Patients: A Randomized Controlled Clinical Trial. J. Periodontol. 2018, 89, 1334–1339. [Google Scholar] [CrossRef]
- Nilsson, E.; Larsson, A.; Olesen, H.V.; Wejåker, P.-E.; Kollberg, H. Good Effect of IgY against Pseudomonas Aeruginosa Infections in Cystic Fibrosis Patients. Pediatr. Pulmonol. 2008, 43, 892–899. [Google Scholar] [CrossRef]
- Immunoglobulin IgY Pseudomonas A Clinical Trial for Cystic Fibrosis Treatment | FP7. Available online: https://cordis.europa.eu/project/id/261095/reporting (accessed on 24 April 2025).
- Liu, S.T.H.; Mirceta, M.; Lin, G.; Anderson, D.M.; Broomes, T.; Jen, A.; Abid, A.; Reich, D.; Hall, C.; Aberg, J.A. Safety, Tolerability, and Pharmacokinetics of Anti-SARS-CoV-2 Immunoglobulin Intravenous (Human) Investigational Product (COVID-HIGIV) in Healthy Adults: A Randomized, Controlled, Double-Blinded, Phase 1 Study. Antimicrob. Agents Chemother. 2023, 67, e01514-22. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Egg Yolk Antibodies for Passive Immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef]
- Yamaki, K.; Ohta, K.; Kobayashi, N.; Morita, I.; Kiguchi, Y.; Oyama, H.; Ito, K.; Nanbo, A.; Oh-oka, H.; Koyama, Y.; et al. Purification of Emu IgY for Therapeutic and Diagnostic Use Based on Monoclonal Secondary Antibodies Specific to Emu IgY. Biol. Pharm. Bull. 2022, 45, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Z.; Shahsavar, S.K.; Keikha, M.; Ghazvini, K. Chicken Immunoglobulin (IgY) as an Alternative Treatment for Bacterial Infections, Emphasizing Advantages, Disadvantages and Mechanisms. J. Microbiol. Infect. Dis. 2024, 14, 95–95. [Google Scholar] [CrossRef]
- Nastasa, V.; Minea, B.; Pasca, A.-S.; Bostanaru-Iliescu, A.-C.; Stefan, A.-E.; Gologan, D.; Capota, R.; Foia, L.-G.; Mares, M. Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 8701. [Google Scholar] [CrossRef]
| Factor | Recommendation |
|---|---|
| Adjuvant | Freund’s incomplete adjuvant, Specol, lipopeptide (Pam₃-Cys-Ser-[Lys]₄; 250 µg) |
| Antigen dose | 10 ng–1 mg (preferably 10–100 µg) |
| Injection site | Intramuscular (field studies; young laboratory chickens) Subcutaneous (older laboratory chickens) |
| Injection volume | < 1 ml |
| Injection frequency | 2–3 times; boosters during laying period |
| Vaccination interval | 4–8 weeks |
| Use of chickens | Entire laying period (about 1 year) |
| Study overview | Condition/Goal | Intervention/ Treatment | Study completion |
|---|---|---|---|
| Evaluation of IgY Antibody Effectiveness in Supportive Therapy of Periodontitis Patients [171] Study Id Number: NCT02705885 |
Chronic Periodontitis | IgY antibodies against Porphyromonas gingivalis gingipains as dietary supplement | 2015-03 |
| Phase I and II: Post Marketing Study of Anti-pseudomonas IgY in Prevention of Recurrence of Pseudomonas Aeruginosa Infections Infections in Cystic Fibrosis (CF) Patients [172] Study Id Number: NCT00633191 |
Cystic fibrosis. Infection with Pseudomonas aeruginosa |
Anti-pseudomonas IgY gargle | 2012-12 |
| Phase III Study to Evaluate Clinical Efficacy and Safety of Avian Polyclonal Anti-Pseudomonas Antibodies (IgY) in Prevention of Recurrence of Pseudomonas Aeruginosa Infection in Cystic Fibrosis Patients [173] Study Id Number: NCT01455675 |
Cystic fibrosis | Avian polyclonal anti-pseudomonas antibodies (IgY) | 2017-06 |
| Phase I: Assessing the Safety of an IGY Supplement on the Gut Microbiome Study Id Number: NCT06702280 |
Healthy | Dietary Supplement: IgY supplement | Estimated 2025-10 |
| Phase I and II: A Randomized, Double-blind, Placebo-controlled, Parallel Dose Ranging Study on the Influence of IgY Max on Inflammatory Markers and the Gut Microbiome Study Id Number: NCT02972463 |
Healthy | Immunoglobulin Y | 2018-01 |
| A Phase 1 Study in Healthy Participants to Evaluate the Safety, Tolerability, and Pharmacokinetics of Single -Ascending and Multiple Doses of an Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Chicken Egg Antibody (IgY) [174] Study Id Number: NCT04567810 |
COVID19 | anti-SARS-CoV-2 IgY | 2020-12 |
| Evaluation of a Health Food Supplement Containing Anti-Helicobacter Pylori Urease IgY Antibody on Patients with Chronic Gastritis in Hanoi, Vietnam Study Id Number: NCT02721355 |
Chronic Gastritis Caused by Helicobacter Pylori | GastimunHP | 2016-06 |
| Randomized, Double-blind, Placebo-controlled Exploratory Trial to Investigate Efficacy and Safety of IGN-ES001 in Patients with Chronic Widespread Pain with or Without Fibromyalgia Study Id Number: NCT03058224 |
Chronic Widespread Pain Fibromyalgia |
Polyclonal avian immunoglobulin IgY containing specific IgY against E. coli F18ab and S. typhimurium in partially delipidated avian egg yolk powder | 2017-12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





