Submitted:
01 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Polyphenols
- a)
- Classification of Polyphenols and Sources of Polyphenols Dietary Sources
- b)
- General Biological Activities of Polyphenols Relevant to Antiviral Activity
3. Common Antiviral Polyphenols
4. Mechanisms of Antiviral Action of Polyphenols
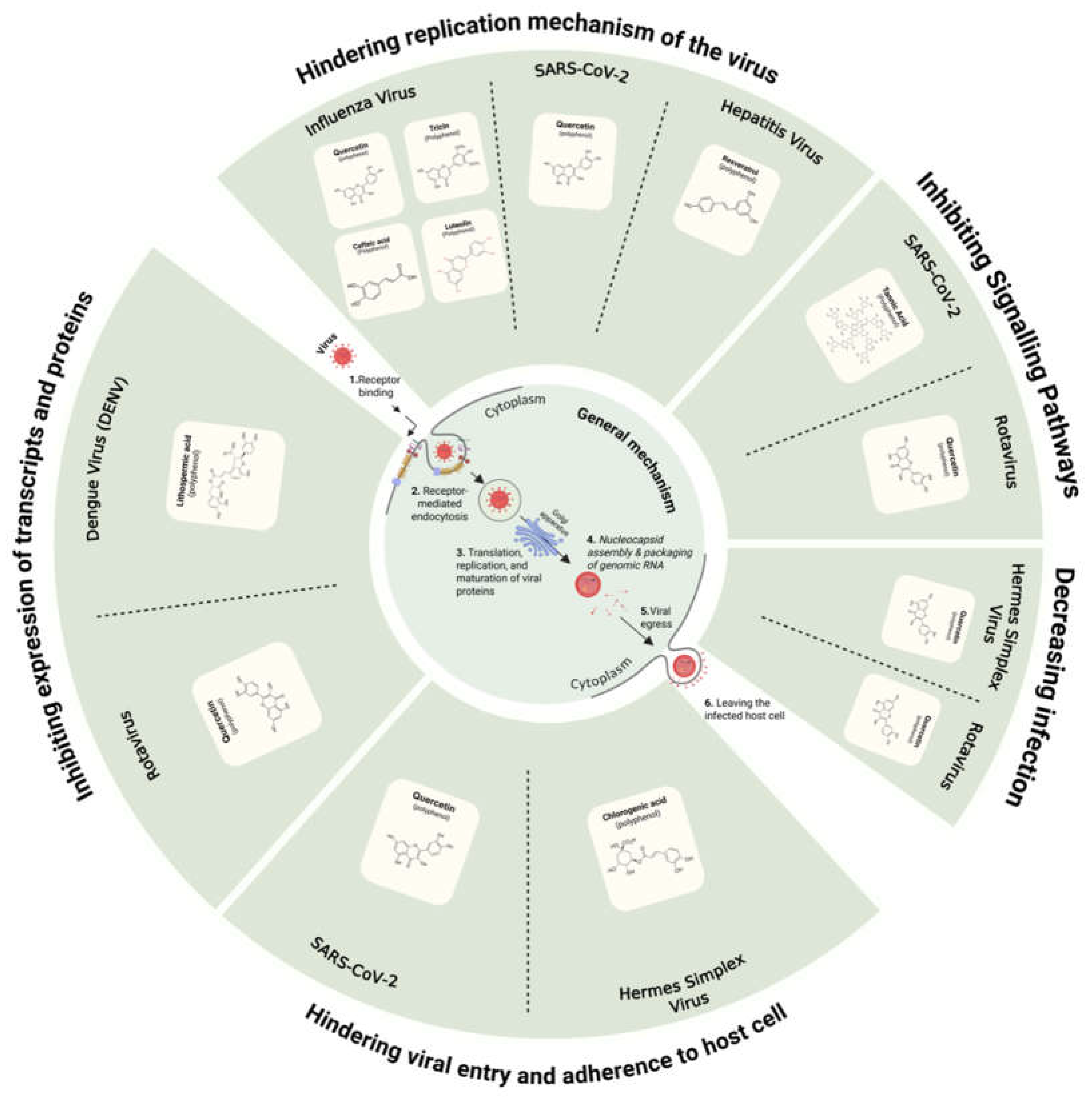
- i)
- SARS-CoV-2
- ii)
- Influenza Virus
- iii)
- Hepatitis Virus
- iv)
- Herpes Simplex Virus
- v)
- Dengue Virus (DENV)
- vi)
- Rotavirus
| Polyphenols | Against | Mechanisms of Antiviral Action | References |
|---|---|---|---|
| Resveratrol | Hepatitis B virus | -Resveratrol alleviates oxidative stress and inhibits Hepatitis B virus replication. | [143] |
| -Exifone - Benserazide hydrochloride) |
SARS-CoV-2 | -These polyphenols impede the 3CLpro protease activity vital for SARS-CoV-2 replication. | [43] |
| Curcumin | Hepatitis B virus | -It triggers a cell-type-specific response in hepatoma cell lines. -It prevents an adaptive cellular optimization that enhances replication of the hepatitis B virus. |
[87] |
| Epigallocatechin-3-Gallate | Mayaro virus | -It shows antiviral activity against Mayaro virus. | [86] |
| Ajwa date extract | Herpes Simplex virus type 1 | -It protects cells by preventing virus uptake to host cells. | [122] |
| Silibinin | Influenza A virus (H1N1) | -It inhibits Influenza A virus infection with its strong immunomodulatory properties and reduces dose-dependently lung tissue damage. | [124] |
| Polyphenol rich Spiraea extracts | Influenza A virus (H1N1) | -It shows highly antiviral effect. | [125] |
| Tannic acid | SARS-CoV-2 | -It prevents the virus uptake to cells by regulating the proteins. -It exhibits an antioxidant role in ROS that is caused by viral infection. |
[123] |
| Lithospermic acid | Dengue virus | -It inhibits viral replication by binding Envelope Protein and Non-Structural Protein 3 which are important for viral uptake, at the onset of infection. | [136] |
| Pongamia pinnata L. seed-derived karanjin | Newcastle disease virus | - It enhances antiviral responses and influences glucose metabolism. - It reduces Newcastle disease virus replication. |
[6] |
| Polygonum aviculare extract | Murine norovirus | - It efficiently inactivates norovirus and prevents the infection. | [148] |
| Arachis hypogaea L. extract | Dengue virus | -Tegument ethanolic extract from A. hypogaea could be an antiviral agent against dengue virus 2 infections. |
[146] |
| -Resveratrol -Quercetin |
Herpes Simplex Virus type 1 | -It reduced viral infectivity. - It showed significant potential for virus suppression. |
[144] |
| Kalanchoe daigremontiana extract | Human herpesvirus type 1 | - It inhibits viral infection and treats an already present infection. | [1] |
| Curcumin | SARS-CoV-2 | - It exhibits anti-inflammatory effects in low-symptom individuals who have SARS-CoV-2 infection. | [149] |
| Polyphenol rich Ilex paraguariensis extract | Hepatitis B virus | - Its antiviral phenolic compounds exhibit potential therapeutic efficacy. | [150] |
| Quercetin | SARS-CoV-2 | -It inhibits viral replication processes by preserving syncytium formation. | [132] |
| Curcumin containing film spray | SARS-CoV-2 and Influenza infection | -Its ability to inhibit inflammation and apoptosis in alveolar epithelial cells, adjust macrophage polarization and protect alveolar epithelial cell integrity. | [74] |
| Polyphenol rich sugarcane extract | Influenza A virus | -It shows antiviral effects against viral strains in vitro. | [116] |
| Plant extracts of Caesalpinia spinosa and Petiveria alliacea | Mouse coronavirus MHV-A59 | - They continue or increase the exposure of calreticulin on the surface, which is induced during infection. | [2] |
| Nonvolatile polyphenols | SARS-CoV-2 | -It exhibits an inhibitory effect on the viral infection. | [129] |
| Catechin | Dengue virus | -It inhibits Dengue Virus replication. | [151] |
| Opuntia ficus-indica peel | Rotavirus | -It exhibits antioxidant, antimicrobial and anticancer properties. | [152] |
| Cranberry pomace extract | Zika and Dengue virus | -They exhibit nutraceutical antiviral effects on Zika and Dengue virus. | [153] |
| Quercetin | Influenza A virus (H1N1) | -It exhibits clinical therapeutic agent treatment against Influenza A virus infection. | [24] |
| -Catechin -Epicatechin |
Hepatitis B virus | - They particularly inhibit the viral antigen surface and show antiviral effect. | [141] |
| Peucedanum japonicum (Sacna) | Influenza A and B virus | - It inhibits the viral replication of both types of influenza infection. | [34] |
| Polyphenol- rich plant extracts | Zika virus | -They exhibit inhibitory activity against Zika virus. | [35] |
| Polydatin | Influenza A virus | -Its treatment reduces IL-6 cytokine production by correcting its anti-inflammatory properties during the viral infection. | [25] |
| Brazilin, theaflavin-3,3’-digallate, and curcumin | SARS-CoV-2 pseudo-virions | -It exhibits multiple anti-SARS-CoV-2 activities. | [128] |
| Epigallocatechin-3-Gallate | Human Papillomavirus | -It has anti-viral activity by targeting the E6 and E7 proteins. | [17] |
| Catechin | Influenza A virus | -It reduces viral infection in mice by absorption on the pharyngeal mucosa. | [5] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chodkowski, M.; Nowak, S.; Janicka, M.; Sobczak, M.; Granica, S.; Bańbura, M.W.; Krzyzowska, M.; Cymerys, J. In Vitro Antiviral Activity of Kalanchoe Daigremontiana Extract against Human Herpesvirus Type 1. IJMS 2024, 25, 7507. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K.; Arévalo, C.; Lasso, P.; Carlosama, C.; Urueña, C.; Fiorentino, S.; Barreto, A. Plant Extracts Modulate Cellular Stress to Inhibit Replication of Mouse Coronavirus MHV-A59. Heliyon 2024, 10, e23403. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Gerardi, C.; Uberti-Foppa, C.; Lopalco, L. Can Natural Polyphenols Help in Reducing Cytokine Storm in COVID-19 Patients? Molecules 2020, 25, 5888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Zhang, M.; Han, F.; Liao, W.; Duan, X. Natural Polyphenols for Drug Delivery and Tissue Engineering Construction: A Review. European Journal of Medicinal Chemistry 2024, 266, 116141. [Google Scholar] [CrossRef]
- Onishi, S.; Mori, T.; Kanbara, H.; Habe, T.; Ota, N.; Kurebayashi, Y.; Suzuki, T. Green Tea Catechins Adsorbed on the Murine Pharyngeal Mucosa Reduce Influenza A Virus Infection. Journal of Functional Foods 2020, 68, 103894. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Rangan, L. Pongamia Pinnata L. Seed-Derived Karanjin as Prominent Antiviral Agent against Newcastle Disease Virus. Virology 2024, 600, 110272. [Google Scholar] [CrossRef] [PubMed]
- Food Bioactives and Health; Galanakis, C.M., Ed.; Springer International Publishing: Cham, 2021; ISBN 978-3-030-57468-0. [Google Scholar]
- Sun, X.; Ye, H.; Liu, J.; Wu, L.; Lin, D.; Yu, Y.; Gao, F. Assessment of Anti-Diabetic Activity of Peanut Shell Polyphenol Extracts. J. Zhejiang Univ. Sci. B 2018, 19, 764–775. [Google Scholar] [CrossRef]
- Science and Engineering of Polyphenols: Fundamentals and Industrial Scale Applications, 1st ed.; Verma, C., Ed.; Wiley, 2024; ISBN 978-1-394-20390-1. [Google Scholar]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef]
- Goldberg, D. Critical Reviews in Clinical Laboratory Sciences. Critical Reviews in Clinical Laboratory Sciences 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. IJMS 2022, 23, 15482. [Google Scholar] [CrossRef]
- Tago, R.; Yamauchi, S.; Maruyama, M.; Akiyama, K.; Sugahara, T.; Kishida, T.; Koba, Y. Structure-Antibacterial Activity Relationship for 9- O,9′- O -Demethyl (+)-Virgatusin. Bioscience, Biotechnology, and Biochemistry 2008, 72, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. Journal of the Saudi Society of Agricultural Sciences 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Ciesek, S.; Von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The Green Tea Polyphenol, Epigallocatechin-3-Gallate, Inhibits Hepatitis C Virus Entry. Hepatology 2010, 54, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The Major Constituent of Green Tea, Epigallocatechin-3-Gallate (EGCG), Inhibits the Growth of HPV18-Infected Keratinocytes by Stimulating Proteasomal Turnover of the E6 and E7 Oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus Synthetic Drugs; Beliefs and Facts. J Nephropharmacol 2015, 4, 27–30. [Google Scholar]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. Journal of Traditional and Complementary Medicine 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Mulay, A.; Konda, B.; Garcia, G.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 Infection of Primary Human Lung Epithelium for COVID-19 Modeling and Drug Discovery. Cell Reports 2021, 35, 109055. [Google Scholar] [CrossRef]
- Terliesner, N.; Unterwalder, N.; Edelmann, A.; Corman, V.; Knaust, A.; Rosenfeld, L.; Gratopp, A.; Ringe, H.; Martin, L.; Von Bernuth, H.; et al. Viral Infections in Hospitalized Children in Germany during the COVID-19 Pandemic: Association with Non-Pharmaceutical Interventions. Front. Pediatr. 2022, 10, 935483. [Google Scholar] [CrossRef]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral Activity of Green Tea and Black Tea Polyphenols in Prophylaxis and Treatment of COVID-19: A Review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Zeng, Y.-T.; Lin, C.-J.; Harroun, S.G.; Anand, A.; Chang, L.; Wu, C.-J.; Lin, H.-J.; Huang, C.-C. Partial Carbonization of Quercetin Boosts the Antiviral Activity against H1N1 Influenza A Virus. Journal of Colloid and Interface Science 2022, 622, 481–493. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef]
- Siew, Z.Y.; Asudas, E.; Khoo, C.T.; Cho, G.H.; Voon, K.; Fang, C.-M. Fighting Nature with Nature: Antiviral Compounds That Target Retroviruses. Arch Microbiol 2024, 206, 130. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, D.-H. 5,7-Dihydroxy-6-Methoxy-Flavonoids Eliminate HIV-1 D3-Transfected Cytoprotective Macrophages by Inhibiting the PI3K/Akt Signaling Pathway: Dihydroxymethoxyflavonoids Eliminate HIV1-Transfected Macrophages. Phytother. Res. 2015, 29, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J Nutr Sci 2016, 5, e47. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discovery Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Saini, R.; Ali, M.I.; Pant, M.; Warghane, A. Current Status of Potential Antiviral Drugs Derived from Plant, Marine,and Microbial Sources. AIA 2024, 22, e090124225414. [Google Scholar] [CrossRef]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Cantos-Villar, E. Sulfur Free Red Wines through the Use of Grapevine Shoots: Impact on the Wine Quality. Food Chemistry 2018, 243, 453–460. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A Rapid Quantification of Stilbene Content in Wine by Ultra-High Pressure Liquid Chromatography – Mass Spectrometry. Food Control 2020, 108, 106821. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia Melanocarpa and Its Components Demonstrate Antiviral Activity against Influenza Viruses. Biochemical and Biophysical Research Communications 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, R.; Morimoto, R.; Horio, Y.; Sumitani, H.; Isegawa, Y. Inhibition of Influenza Virus Replication by Apiaceae Plants, with Special Reference to Peucedanum Japonicum (Sacna) Constituents. Journal of Ethnopharmacology 2022, 292, 115243. [Google Scholar] [CrossRef] [PubMed]
- Santos Pereira, R.; Vasconcelos Costa, V.; Luiz Menezes Gomes, G.; Rodrigues Valadares Campana, P.; Maia De Pádua, R.; Barbosa, M.; Oki, Y.; Heiden, G.; Fernandes, G.W.; Menezes De Oliveira, D.; et al. Anti-Zika Virus Activity of Plant Extracts Containing Polyphenols and Triterpenes on Vero CCL-81 and Human Neuroblastoma SH-SY5Y Cells. Chemistry & Biodiversity 2022, 19, e202100842. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Lévèques, A.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Li, H.; Schwab, M.; Eichelbaum, M.; Williamson, G. Intestinal Absorption, Metabolism, and Excretion of (–)-Epicatechin in Healthy Humans Assessed by Using an Intestinal Perfusion Technique. The American Journal of Clinical Nutrition 2013, 98, 924–933. [Google Scholar] [CrossRef]
- De Freitas Queiroz Barros, H.D.; Maróstica Junior, M.R. Phenolic Compound Bioavailability Using In Vitro and In Vivo Models. In Bioactive Compounds; Elsevier, 2019; pp. 113–126. ISBN 978-0-12-814774-0. [Google Scholar]
- Ozkan, G.; Ceyhan, T.; Çatalkaya, G.; Rajan, L.; Ullah, H.; Daglia, M.; Capanoglu, E. Encapsulated Phenolic Compounds: Clinical Efficacy of a Novel Delivery Method. Phytochem Rev 2024, 23, 781–819. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Gold Nanoparticles in Nanomedicine: Unique Properties and Therapeutic Potential. Nanomaterials 2024, 14, 1854. [Google Scholar] [CrossRef]
- Duman, H.; Akdaşçi, E.; Eker, F.; Bechelany, M.; Karav, S. Gold Nanoparticles: Multifunctional Properties, Synthesis, and Future Prospects. Nanomaterials 2024, 14, 1805. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Applied Sciences 2024, 14, 9183. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.-Y.; Zhou, S.-H.; Cheng, G.; Wei, R.-F.; Zhou, Y.-M.; Zhang, Y.; Xie, T.-L.; Zhang, L. The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China. IJMS 2024, 25, 8624. [Google Scholar] [CrossRef]
- Lu, J.; Tang, Y.; Li, H.; Chen, X.; Qin, P.; Xu, J.; Li, W.; Chen, L. Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds. IJMS 2025, 26, 2243. [Google Scholar] [CrossRef]
- Chen, C.-Y. Tannic Acids and Proanthocyanidins in Tea Inhibit SARS-CoV-2 Variants Infection. Am J Cancer Res 2024, 14, 2555–2569. [Google Scholar] [CrossRef] [PubMed]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Milić, A.; Šumić, Z.; Žunić, D.; Adamović, B.; Ilin, Ž. Biostimulants Improve Bulb Yield, Concomitantly Affecting the Total Phenolics, Flavonoids, and Antioxidant Capacity of Onion (Allium Cepa). Horticulturae 2024, 10, 391. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of Light Intensity on Celery Growth and Flavonoid Synthesis. Front. Plant Sci. 2024, 14, 1326218. [Google Scholar] [CrossRef]
- Foschi, M.; Marsili, L.; Luciani, I.; Gornati, G.; Scappaticci, C.; Ruggieri, F.; D’Archivio, A.A.; Biancolillo, A. Optimization of the Cold Water Extraction Method for High-Value Bioactive Compounds from Chamomile (Matricaria Chamomilla L.) Flower Heads Through Chemometrics. Molecules 2024, 29, 4925. [Google Scholar] [CrossRef] [PubMed]
- Curtasu, M.V.; Nørskov, N.P. Quantitative Distribution of Flavan-3-Ols, Procyanidins, Flavonols, Flavanone and Salicylic Acid in Five Varieties of Organic Winter Dormant Salix Spp. by LC-MS/MS. Heliyon 2024, 10, e25129. [Google Scholar] [CrossRef]
- Arora, B.; Lather, V.; Pathalingappa, M.B.; Walia, R. Enhancement of Aqueous Solubility of Hesperidin and Naringenin Utilizing Hydrotropic Solubilization Technique: Characterization and in Vitro Evaluation. Journal of Asian Natural Products Research 2024, 26, 1207–1218. [Google Scholar] [CrossRef]
- Mohammed, H.; Abdullah, A.S.; AL-Mozie’l, M.S.G. Protection Effect of Soy Isoflavones (Genistein and Daidzein) on Hematologic Parameters in Acute Kidney Injury. acopen 2024, 9. [Google Scholar] [CrossRef]
- Jiang, N.; Gomez, L.; Grotewold, E. Extraction and Quantification of Total Anthocyanins, Determination of Anthocyanidin Core Structures, and Characterization of Specific Anthocyanins from Maize. Cold Spring Harb Protoc 2024, protocols;pdb.prot108577v1. [Google Scholar] [CrossRef]
- Abdullah, Z.L.; Mohammed, R.K. The Study of the Antibacterial Effect of Anthocyanin Pigment Extracted From Red Cabbage (Brassica Oleracea Var. Capitata f. Rubra) and Red Radish Peels (Raphanus Sativus. Var. Sativus). IOP Conf. Ser.: Earth Environ. Sci. 2024, 1371, 052089. [Google Scholar] [CrossRef]
- Hariri, M.; Amirkalali, B.; Gholami, A. Effects of Purified Anthocyanins Supplementation on Serum Concentration of Inflammatory Mediators: A Systematic Review and Dose–Response Meta-analysis on Randomized Clinical Trials. Phytotherapy Research 2024, 38, 1494–1508. [Google Scholar] [CrossRef]
- Bo, S.; Chang, S.K.; Chen, Y.; Sheng, Z.; Jiang, Y.; Yang, B. The Structure Characteristics, Biosynthesis and Health Benefits of Naturally Occurring Rare Flavonoids. Critical Reviews in Food Science and Nutrition 2024, 64, 2490–2512. [Google Scholar] [CrossRef] [PubMed]
- Arzuk, E.; Armağan, G. Genistein and Daidzein Induce Ferroptosis in MDA-MB-231 Cells. Journal of Pharmacy and Pharmacology 2024, 76, 1599–1608. [Google Scholar] [CrossRef]
- Mehrabi, M.; Esmaeili, S.; Ezati, M.; Abassi, M.; Rasouli, H.; Nazari, D.; Adibi, H.; Khodarahmi, R. Antioxidant and Glycohydrolase Inhibitory Behavior of Curcumin-Based Compounds: Synthesis and Evaluation of Anti-Diabetic Properties in Vitro. Bioorganic Chemistry 2021, 110, 104720. [Google Scholar] [CrossRef]
- Lassouane, N.; Aïd, F.; Quinet, M.; Lutts, S. Phenolic Acids and Flavonoids Classes in Acacia Arabica (Lam) Willd. Seedling during Water Stress and Subsequent Re-Hydration. Plant Soil 2024, 496, 449–471. [Google Scholar] [CrossRef]
- Kika, J.; Jakubczyk, K.; Ligenza, A.; Maciejewska-Markiewicz, D.; Szymczykowska, K.; Janda-Milczarek, K. Matcha Green Tea: Chemical Composition, Phenolic Acids, Caffeine and Fatty Acid Profile. Foods 2024, 13, 1167. [Google Scholar] [CrossRef] [PubMed]
- Özel, H.B.; Baş Topcu, K.S.; Dere, S.; Genç, N.; Kisa, D. In Vitro and in Silico Based Assessment of Biological Activity of Endemic Allium Species: LC-MS/MS Analysis of Onions. Food Bioscience 2024, 59, 104209. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A Systematic UHPLC Q-ToF MS Approach for the Characterization of Bioactive Compounds from Freeze-Dried Red Goji Berries (L. Barbarum L.) Grown in Serbia: Phenolic Compounds and Phenylamides. Food Chemistry 2024, 456, 140044. [Google Scholar] [CrossRef] [PubMed]
- AboelAinin, M.A.; El-Ashmony, R.M.S.; Tantawy, I.A.A.; Mohamed, H.S.; Galal, A.A. Acetic Acid and Hydrogen Peroxide Improved Defense-Related Biochemical Responses of Onion Bulbs to Black Mold Rot Caused by Aspergillus Niger L. International Journal of Vegetable Science 2024, 30, 411–428. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Lu, X.; Liu, Z.; Sun, S.; Guo, H.; Tian, W.; Li, Z.; Wang, L.; Li, L.; et al. Dynamic Changes in Polyphenols in Fruit Development of Red Flesh Apple ‘Hongxun 2. ’ Horticulturae 2024, 10, 1125. [Google Scholar] [CrossRef]
- Ceylan, F.D.; Günal-Köroğlu, D.; Saricaoglu, B.; Ozkan, G.; Capanoglu, E.; Calina, D.; Sharifi-Rad, J. Anticancer Potential of Hydroxycinnamic Acids: Mechanisms, Bioavailability, and Therapeutic Applications. Naunyn-Schmiedeberg’s Arch Pharmacol 2025, 398, 469–495. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum Lycopersicum L.). Food Chemistry 2024, 438, 137863. [Google Scholar] [CrossRef] [PubMed]
- Beilankouhi, S.; Pourfarzad, A.; Ghanbarzadeh, B.; Rasouli, M.; Hamishekar, H. Identification of Polyphenol Composition in Grape ( Vitis Vinifera Cv. Bidaneh Sefid) Stem Using Green Extraction Methods and LC–MS/MS Analysis. Food Science & Nutrition 2024, 12, 6789–6798. [Google Scholar] [CrossRef]
- Hanzouli, F.; Daldoul, S.; Zemni, H.; Boubakri, H.; Vincenzi, S.; Mliki, A.; Gargouri, M. Stilbene Production as Part of Drought Adaptation Mechanisms in Cultivated Grapevine ( Vitis Vinifera L.) Roots Modulates Antioxidant Status. Plant Biol J 2025, 27, 102–115. [Google Scholar] [CrossRef]
- Gołąbek-Grenda, A.; Juzwa, W.; Kaczmarek, M.; Olejnik, A. Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages. Nutrients 2024, 16, 3483. [Google Scholar] [CrossRef]
- D’Amico, E.; Cinquini, C.; Petrini, M.; Barone, A.; Iezzi, G.; D’Ercole, S.; De Filippis, B.; Pierfelice, T.V. The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites 2024, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, Z.; Song, Z.; Li, Y.; Shim, Y.Y.; Reaney, M.J.T.; Lee, Y.Y.; Wang, Y.; Zhang, N. Bioconversion of Lignans in Flaxseed Cake by Fermented Tofu Microbiota and Isolation of Enterococcus Faecium Strain ZB26 Responsible for Converting Secoisolariciresinol Diglucoside to Enterodiol. Food Chemistry 2024, 457, 140077. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Liu, B.; Li, Y.; Wang, M.; Sun, Q. Lignan Intake and Type 2 Diabetes Incidence Among US Men and Women. JAMA Netw Open 2024, 7, e2426367. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.O. Seed Quality and Relative Lignan Profiles of Sesame Prospected from Northern Ghana. Heliyon 2024, 10, e39108. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.-W.; Sung, J.; Kim, Y. Optimal Extraction Conditions and Quantification of Lignan Phytoestrogens in Cereal Grains Using Targeted LC-MS/MS. Front. Nutr. 2024, 11, 1409309. [Google Scholar] [CrossRef]
- Oguz, M.C. Stimulating Endogenous Hormone Content by Plant Extracts: Increased in Vitro Regeneration of Flax (Linum Usitatissimum) Cultivars. J. Crop Sci. Biotechnol. 2025, 28, 93–105. [Google Scholar] [CrossRef]
- Nittayananta, W.; Lerdsamran, H.; Chutiwitoonchai, N.; Promsong, A.; Srichana, T.; Netsomboon, K.; Prasertsopon, J.; Kerdto, J. A Novel Film Spray Containing Curcumin Inhibits SARS-CoV-2 and Influenza Virus Infection and Enhances Mucosal Immunity. Virol J 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, N.M.; Punia, J.; Jat, S.; Pawar, S.D.; Devi, N.; Radhakrishnanand, P.; Murty, U.S.; Saini, A.; Sethi, K.K.; Kumar, P. Purification and Characterization of Pure Curcumin, Desmethoxycurcumin, and Bisdemethoxycurcumin from North-East India Lakadong Turmeric (Curcuma Longa). Journal of Chromatography A 2023, 1708, 464358. [Google Scholar] [CrossRef] [PubMed]
- Idowu-Adebayo, F.; Fogliano, V.; Linnemann, A. Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health. Foods 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A. Tannin Content in Vitis Species Red Wines Quantified Using Three Analytical Methods. Molecules 2021, 26, 4923. [Google Scholar] [CrossRef]
- Rouxinol, M.I.; Martins, M.R.; Murta, G.C.; Mota Barroso, J.; Rato, A.E. Quality Assessment of Red Wine Grapes through NIR Spectroscopy. Agronomy 2022, 12, 637. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Buruleanu, C.L.; (Tudorache), M.N.; Dulama, I.D.; Stirbescu, R.M.; Bucurica, I.A.; Stanescu, S.G.; Banica, A.L. Polyphenolic Screening and the Antioxidant Activity of Grape Pomace Extracts of Romanian White and Red Grape Varieties. Antioxidants 2024, 13, 1133. [Google Scholar] [CrossRef]
- Stannard, H.; Koszalka, P.; Deshpande, N.; Desjardins, Y.; Baz, M. Pre-Clinical Evaluation of the Antiviral Activity of Epigalocatechin-3-Gallate, a Component of Green Tea, against Influenza A(H1N1)Pdm Viruses. Viruses 2023, 15, 2447. [Google Scholar] [CrossRef]
- Yuna, L.; Bo-Gyeong, Y.; Fengjia, C.; Eui-Baek, B.; Ha-Yeon, S. Fermented Codonopsis Lanceolata Root Extract Exhibits Anti-Viral Effects against Influenza A Infection by Inhibiting Neuraminidase Activity and Inflammatory Responses. Food Bioscience 2024, 61, 104617. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; Da Cunha Lima, B.F.; Rodrigues, B.M.; et al. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci Rep 2024, 14, 10696. [Google Scholar] [CrossRef]
- Rajendrasozhan, S. Antioxidant, Antibacterial and Antiviral Effect of the Combination of Ginger and Garlic Extracts. Bioinformation 2024, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Thomasi, R.M.D.O.; Teixeira, T.R.; Lopes, G.F.M.; Mendonça, S.C.; Gomes, B.A.; Leitão, S.G.; Oliveira, T.A.D.; Fonseca, S.T.D.D.; Taranto, A.G.; Ferreira, J.M.S.; et al. Antiviral Activity of Flavonoids from Bauhinia Holophylla Leaves against Zika Virus. Microbiology Research 2024, 15, 582–597. [Google Scholar] [CrossRef]
- Da Conceição, P.J.P.; Ayusso, G.M.; Carvalho, T.; Duarte Lima, M.L.; Marinho, M.D.S.; Moraes, F.R.; Galán-Jurado, P.E.; González-Santamaría, J.; Bittar, C.; Zhang, B.; et al. In Vitro Evaluation of the Antiviral Activity of Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) Against Mayaro Virus. Viruses 2025, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, M.M.; Fuentes, P.; Chiappetta, D.; Flichman, D.M. Contrasting Effect of Curcumin on Hepatitis B Virus Replication According to the Hepatoma Cell Line. Pathogens 2025, 14, 203. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase Inhibitory Activities of Flavonols Isolated from Rhodiola Rosea Roots and Their in Vitro Anti-Influenza Viral Activities. Bioorganic & Medicinal Chemistry 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Kim, Y.; Narayanan, S.; Chang, K.-O. Inhibition of Influenza Virus Replication by Plant-Derived Isoquercetin. Antiviral Research 2010, 88, 227–235. [Google Scholar] [CrossRef]
- Ochnik, M.; Franz, D.; Sobczyński, M.; Naporowski, P.; Banach, M.; Orzechowska, B.; Sochocka, M. Inhibition of Human Respiratory Influenza A Virus and Human Betacoronavirus-1 by the Blend of Double-Standardized Extracts of Aronia Melanocarpa (Michx.) Elliot and Sambucus Nigra L. Pharmaceuticals 2022, 15, 619. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Rho, M.-C.; Kim, S.-J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia Cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The Green Tea Molecule EGCG Inhibits Zika Virus Entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Naderi, M.; Salavatiha, Z.; Gogoi, U.; Mohebbi, A. An Overview of Anti-Hepatitis B Virus Flavonoids and Their Mechanisms of Action. Front. Cell. Infect. Microbiol. 2024, 14, 1356003. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yu, Z.-Y.; Chen, Y.-C.; Hung, S.-L. Effects of Epigallocatechin-3-Gallate and Acyclovir on Herpes Simplex Virus Type 1 Infection in Oral Epithelial Cells. Journal of the Formosan Medical Association 2021, 120, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hao, M.; Yang, M.; Guo, H.; Rayman, M.P.; Zhang, X.; Zhang, J. Influence of EGCG Oxidation on Inhibitory Activity against the SARS-CoV-2 Main Protease. International Journal of Biological Macromolecules 2024, 274, 133451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Li, Q.-S.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef]
- Setyawati, I.; Setiawan, A.G.; Nemchinova, M.; Vidilaseris, K. The Potential Inhibitory Mechanism of EGCG against the Chikungunya Virus Targeting Non-Structural Protein 2 through Molecular Dynamics Simulation. Sci Rep 2024, 14, 29797. [Google Scholar] [CrossRef]
- Chowdhury, P.; Sahuc, M.-E.; Rouillé, Y.; Rivière, C.; Bonneau, N.; Vandeputte, A.; Brodin, P.; Goswami, M.; Bandyopadhyay, T.; Dubuisson, J.; et al. Theaflavins, Polyphenols of Black Tea, Inhibit Entry of Hepatitis C Virus in Cell Culture. PLoS ONE 2018, 13, e0198226. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, R.; Hagedorn, C.H. Tannic Acid Inhibits Hepatitis C Virus Entry into Huh7.5 Cells. PLoS ONE 2015, 10, e0131358. [Google Scholar] [CrossRef]
- Hesari, A.; Ghasemi, F.; Salarinia, R.; Biglari, H.; Tabar Molla Hassan, A.; Abdoli, V.; Mirzaei, H. Effects of Curcumin on NF-κB, AP-1, and Wnt/Β-catenin Signaling Pathway in Hepatitis B Virus Infection. J of Cellular Biochemistry 2018, 119, 7898–7904. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Extract Diminishes Atherogenic Risk in Type 2 Diabetes Mellitus Patients With Obesity 2024.
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive Compounds from Pomegranate Peels - Biological Properties, Structure–Function Relationships, Health Benefits and Food Applications – A Comprehensive Review. Journal of Functional Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Sundararajan, A.; Ganapathy, R.; Huan, L.; Dunlap, J.R.; Webby, R.J.; Kotwal, G.J.; Sangster, M.Y. Influenza Virus Variation in Susceptibility to Inactivation by Pomegranate Polyphenols Is Determined by Envelope Glycoproteins. Antiviral Research 2010, 88, 1–9. [Google Scholar] [CrossRef]
- Kwon, E.-B.; Kim, Y.S.; Han, S.M.; Kim, S.-G.; Choi, J.-G. The Protective Effect of Tilia Amurensis Honey on Influenza A Virus Infection through Stimulation of Interferon-Mediated IFITM3 Signaling. Biomedicine & Pharmacotherapy 2022, 153, 113259. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic Potential of Resveratrol against Emerging Respiratory Viral Infections. Pharmacology & Therapeutics 2020, 214, 107613. [Google Scholar] [CrossRef]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol Inhibits Epstein Barr Virus Lytic Cycle in Burkitt’s Lymphoma Cells by Affecting Multiple Molecular Targets. Antiviral Research 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Yiu, C.-Y.; Chen, S.-Y.; Chang, L.-K.; Chiu, Y.-F.; Lin, T.-P. Inhibitory Effects of Resveratrol on the Epstein-Barr Virus Lytic Cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Hsu, H.-Y.; Chen, Y.-J.; Chou, S.-P.; Huang, S.-Y.; Cheng, Y.-J.; Lin, S.-F.; Chang, Y.; Tsai, C.-H.; et al. Luteolin Inhibits Epstein-Barr Virus Lytic Reactivation by Repressing the Promoter Activities of Immediate-Early Genes. Antiviral Research 2016, 132, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.; Hosoda, A.; Morishita, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Taniguchi, H. Synthesis of Novel Polyphenols Consisted of Ferulic and Gallic Acids, and Their Inhibitory Effects on Phorbol Ester-Induced Epstein–Barr Virus Activation and Superoxide Generation. Bioorganic & Medicinal Chemistry 2002, 10, 1069–1075. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Liu, Z.; Le, Z.; Nie, T.; Qiao, D.; Su, Y.; Mai, H.; Chen, Y.; Liu, L. Therapeutic Nanovaccines Sensitize EBV-Associated Tumors to Checkpoint Blockade Therapy. Biomaterials 2020, 255, 120158. [Google Scholar] [CrossRef]
- Beik, A.; Joukar, S.; Najafipour, H. A Review on Plants and Herbal Components with Antiarrhythmic Activities and Their Interaction with Current Cardiac Drugs. Journal of Traditional and Complementary Medicine 2020, 10, 275–287. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Zhou, G.; Zhu, Z.; Cui, Y.; Pu, R. Antiviral Activities of Resveratrol against Rotavirus in Vitro and in Vivo. Phytomedicine 2020, 77, 153230. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; De Paula Castro, T.L.; Gerba, C.P. In Vitro Antiviral Effect of Mexican and Brazilian Propolis and Phenolic Compounds against Human Coronavirus 229E. International Journal of Environmental Health Research 2023, 33, 1591–1603. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali, *!!! REPLACE !!!*; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential Use of Polyphenols in the Battle against COVID-19. Current Opinion in Food Science 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Carrera Montoya, J.; Fritzlar, S.; Flavel, M.; Londrigan, S.L.; Mackenzie, J.M. Polyphenol Rich Sugarcane Extract (PRSE) Has Potential Antiviral Activity against Influenza A Virus in Vitro. Virology 2024, 590, 109969. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. [Google Scholar] [CrossRef]
- Valdiviezo-Campos, J.E.; Rodriguez-Aredo, C.D.; Ruiz-Reyes, S.G.; Venegas-Casanova, E.A.; Bussmann, R.W.; Ganoza-Yupanqui, M.L. Identification of Polyphenols by UPLC-MS/MS and Their Potential in Silico Antiviral Activity from Medicinal Plants in Trujillo, Peru. J Pharm Pharmacogn Res 2024, 12, 323–347. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Krylova, N.V.; Iunikhina, O.V.; Kalinovskiy, A.I.; Shchelkanov, M.Y.; Mishchenko, N.P.; Aminin, D.L.; et al. Polyphenols from Maackia Amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. IJMS 2024, 25, 4142. [Google Scholar] [CrossRef]
- Okumuş, N.; Erdoğmuş, S.F.; Doğan, H.H.; Altintaş, Ö.E.; Çelik, S.; Duman, R.; Ünlü, Ü. Anti HSV-1 Activity of Cistus Laurifolius and Development of Antiviral Herbal Lip Balm. Rev. Bras. Farmacogn. 2024, 34, 625–636. [Google Scholar] [CrossRef]
- Zima, K.; Khaidakov, B.; Sochocka, M.; Ochnik, M.; Lemke, K.; Kowalczyk, P. Exploring the Potency of Polyphenol-Rich Blend from Lonicera Caerulea Var. Kamtschatica Sevast., Aronia Melanocarpa, and Echinacea Purpurea: Promising Anti-Inflammatory, Antioxidant, and Antiviral Properties. Heliyon 2024, 10, e35630. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, A.K.; Maghrabi, N.A.; Alrehili, O.M.; Alharbi, A.S.; Alsihli, R.S.; Alharthe, A.M.; Albladi, R.S.; Alosaimi, K.A.; Albadrani, B.M.; Miski, S.F.; et al. Ajwa Date Extract ( Phoenix Dactylifera L. ): Phytochemical Analysis, Antiviral Activity against Herpes Simplex Virus-I and Coxsackie B4 Virus, and in Silico Study. SMJ 2025, 46, 26–35. [Google Scholar] [CrossRef]
- Augustus, A.R.; Radhakrishnan, Y.; Bhaskar, J.P.; Ramamurthi, S.; Shunmugiah, K.P. Tannic Acid Modulates SARS-CoV-2 Pathogenesis by Curbing Key Host Receptors and Oxidative Stress. Toxicology in Vitro 2025, 103, 105971. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ghorbani, M.; Shamsizadeh, F.; Namdari, H.; Salimi, V.; Rezaei, F. Effects and Mechanisms of Silibinin on Influenza A/H1N1 Pathogenesis in a Mouse Model. Journal of Tropical Medicine 2025, 2025, 6618423. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Esaulkova, Y.L.; Ilyina, P.A.; Zarubaev, V.V.; Sheikin, V.V.; Petruk, A.A.; Rubtsova, E.D.; Veklich, T.N. Antiviral Potential of Spiraea Extracts (Prepared by Repercolation) Against Influenza A (H1N1) Virus. Foods 2024, 13, 4008. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Ochiai, H.; Sakai, S.; Nakajima, K.; Terasawa, K. Inhibitory Effect of Ferulic Acid and Isoferulic Acid on Murine Interleukin-8 Production in Response to Influenza Virus Infections in Vitro and in Vivo. Planta Med 1995, 61, 221–226. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Y.-X.; Liu, A.-L.; Wang, H.-D.; Wang, Y.-L.; Du, G.-H. Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles Speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions. PLoS ONE 2021, 16, e0253489. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, I.; Jamsransuren, D.; Hashida, K.; Matsuda, S.; Ogawa, H.; Takeda, Y. An Abies Extract Containing Nonvolatile Polyphenols Shows Virucidal Activity against SARS-CoV-2 That Is Enhanced in Increased pH Conditions. Pathogens 2023, 12, 1093. [Google Scholar] [CrossRef]
- Chen, C.; Yu, X.; Kuo, C.; Min, J.; Chen, S.; Ma, L.; Liu, K.; Guo, R. Overview of Antiviral Drug Candidates Targeting Coronaviral 3C-like Main Proteases. The FEBS Journal 2021, 288, 5089–5121. [Google Scholar] [CrossRef]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of Covid-19. Top Curr Chem (Z) 2021, 379, 40. [Google Scholar] [CrossRef]
- Roy, A.V.; Chan, M.; Banadyga, L.; He, S.; Zhu, W.; Chrétien, M.; Mbikay, M. Quercetin Inhibits SARS-CoV-2 Infection and Prevents Syncytium Formation by Cells Co-Expressing the Viral Spike Protein and Human ACE2. Virol J 2024, 21, 29. [Google Scholar] [CrossRef]
- Jang, M.; Park, Y.-I.; Cha, Y.-E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea Polyphenols EGCG and Theaflavin Inhibit the Activity of SARS-CoV-2 3CL-Protease In Vitro. Evidence-Based Complementary and Alternative Medicine 2020, 2020, 5630838. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological Effects and Mechanisms of Tannic Acid. Biomedicine & Pharmacotherapy 2022, 154, 113561. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, X.; Meng, Y.; Liao, F.; Li, Z.; Zheng, W.; Wang, W.; Dai, W.; Zhang, S.; Li, G. Lithospermic Acid Inhibits Dengue Virus Infection through Binding with Envelope Proteins. Microbial Pathogenesis 2024, 197, 107055. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.; Kim, C.; Yoon, S.-W.; Jeon, S.; Kweon, M.-N.; Seong, B.-L.; Seo, S.-U.; Jang, Y.H. Antiviral Activity of the Water Extract and Ethanol Extract of Sorbus Commixta against Influenza A Virus in Vitro. Heliyon 2024, 10, e39049. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Palese, P. Influenza Virus. In Clinical Virology; Richman, D.D., Whitley, R.J., Hayden, F.G., Eds.; Wiley, 2009; pp. 943–976. ISBN 978-1-68367-407-8. [Google Scholar]
- Influenza Virus. Transfus Med Hemother 2008, 35, 42–49. [CrossRef] [PubMed]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral Activity of Chlorogenic Acid against Influenza A (H1N1/H3N2) Virus and Its Inhibition of Neuraminidase. Sci Rep 2017, 7, 45723. [Google Scholar] [CrossRef]
- Parvez, M.; Al-Dosari, M.; Abdelwahid, M.; Alqahtani, A.; Alanzi, A. Novel Anti-hepatitis B Virus-active Catechin and Epicatechin from Rhus Tripartita. Exp Ther Med 2022, 23, 398. [Google Scholar] [CrossRef]
- Pan, P.; Li, J.; Lin, W.; Long, G. Effects of Resveratrol on Hepatitis B Virus Replication: In Vitro and in Vivo Experiments. Intervirology 2022, 65, 206–214. [Google Scholar] [CrossRef]
- Yang, G.; Li, T.; Zhang, X.; Wang, L.; Li, T.; Zhang, X. Resveratrol Mitigates Oxidative Stress and Suppresses HBV Replication via Modulation of the SIRT1-Nrf2 Pathway in Liver Cells. Future Virology 2025, 20, 83–92. [Google Scholar] [CrossRef]
- Zangooie, S.; Ghanbari, R.; Jalilian, F.A.; Mahmoudvand, S.; Teimoori, A. Antiviral Potential of Phenolic Compounds against HSV-1: In-Vitro Study. Antiviral Therapy 2024, 29, 13596535241271589. [Google Scholar] [CrossRef]
- Malavige, G.N.; Fernando, S.; Fernando, D.J.; Seneviratne, S.L. Dengue Viral Infections. Postgraduate Medical Journal 2004, 80, 588–601. [Google Scholar] [CrossRef]
- Menis Candela, F.; Soria, E.A.; Moliva, M.V.; Suárez Perrone, A.; Reinoso, E.B.; Giordano, W.; Sabini, M.C. Anti-DENV-2 Activity of Ethanolic Extracts from Arachis Hypogaea L.: Peanut Skin as a Relevant Resource of Bioactive Compounds against Dengue Virus. Plants 2024, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat Rev Dis Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Han, J.-H.; Lee, N.; Choi, S.-W.; Yoo, M.; Yun, S.-I.; Chang, H.-J. Antiviral Activity of Polygonum Aviculare Extract against Murine Norovirus as Norovirus Surrogate and Its Application in Model Food. LWT 2024, 211, 116887. [Google Scholar] [CrossRef]
- Kishimoto, A.; Komiyama, M.; Wada, H.; Satoh-Asahara, N.; Yamakage, H.; Ajiro, Y.; Aoyama, H.; Katsuura, Y.; Imaizumi, A.; Hashimoto, T.; et al. Efficacy of Highly Bioavailable Oral Curcumin in Asymptomatic or Mild COVID-19 Patients: A Double-Blind, Randomized, Placebo-Controlled Trial. J Health Popul Nutr 2024, 43, 93. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K. Antiviral Flavonoids and Polyphenols Driven Novel Anti-HBV Efficacy of Ilex Paraguariensis. Arch Med Sci 2024. [Google Scholar] [CrossRef]
- Yi, B.; Chew, B.X.Z.; Chen, H.; Lee, R.C.H.; Fong, Y.D.; Chin, W.X.; Mok, C.K.; Chu, J.J.H. Antiviral Activity of Catechin against Dengue Virus Infection. Viruses 2023, 15, 1377. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical Screening and Characterization of the Antioxidant, Anti-Proliferative and Antibacterial Effects of Different Extracts of Opuntia Ficus-Indica Peel. Journal of King Saud University - Science 2022, 34, 102216. [Google Scholar] [CrossRef]
- Tamkutė, L.; Haddad, J.G.; Diotel, N.; Desprès, P.; Venskutonis, P.R.; El Kalamouni, C. Cranberry Pomace Extract Exerts Antiviral Activity against Zika and Dengue Virus at Safe Doses for Adult Zebrafish. Viruses 2022, 14, 1101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





