Submitted:
22 April 2025
Posted:
23 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
3. The Heritability of BMI
4. The Gut Microbiome and the Metagenome
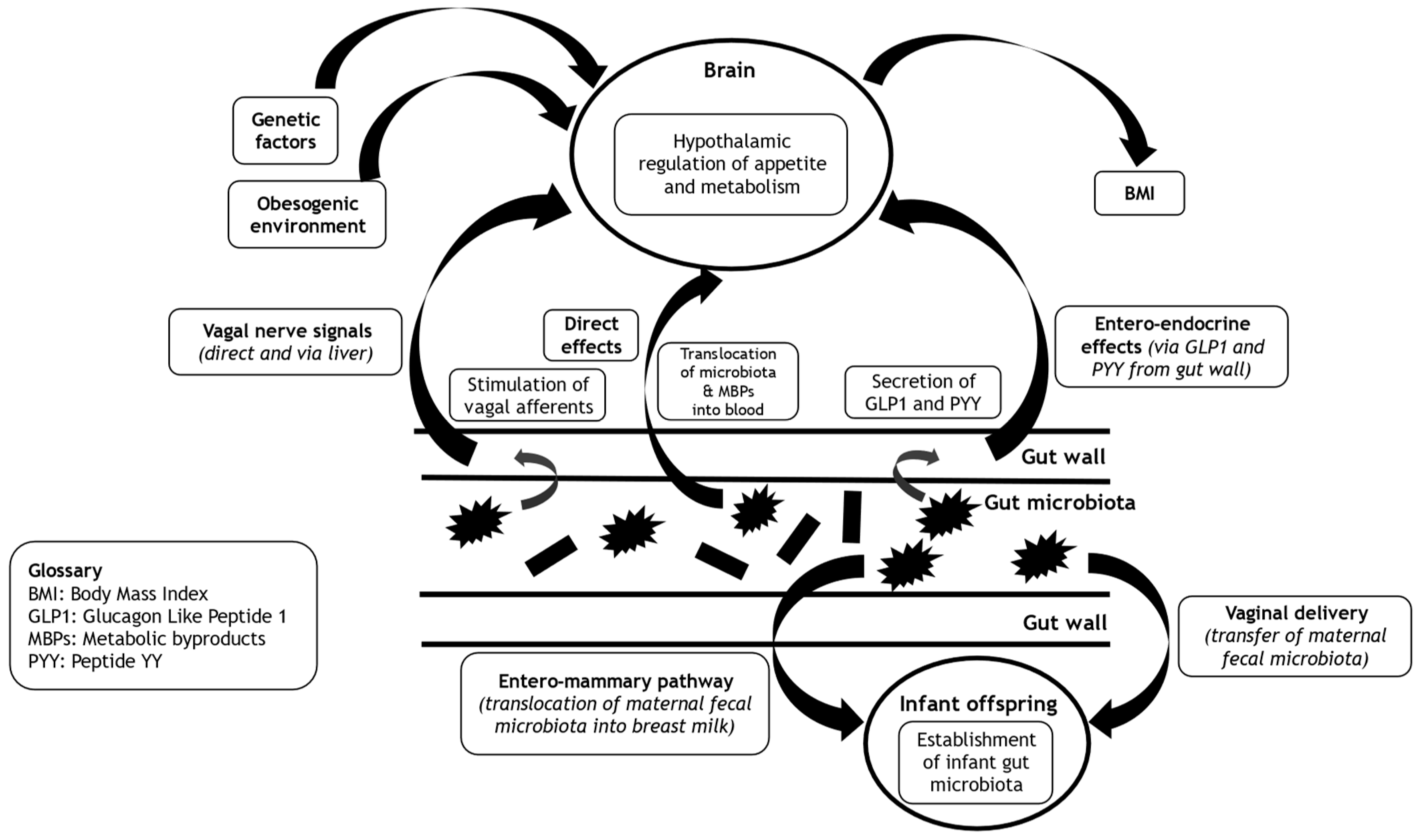
5. Interlinks Between the Gut Microbiome and Central Appetitive and Metabolic Control
5.1. Entero-Endocrine Pathway
5.2. Autonomic Pathway
5.3. Neuro-Humeral Pathway
6. Heritability of the Gut Microbiome
6.1. Vaginal Delivery in the Establishment of the Gut Microbiome of the Newborn
6.2. Breast Feeding as a Means of Seeding the Gut Microbiome of Early Infancy
6.3. Shared Food Environment of Offspring and Parents and the Gut Microbiome
7. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Factsheet 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 December 2024).
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. Journal of the American Association of Nurse Practitioners. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and polycystic ovary syndrome. Clin Endocrinol [Oxf]. 2021, 95, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M. Why are women with polycystic ovary syndrome obese? Br Med Bull. 2022, 143, 4–15. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. Metabolic-Associated Fatty Liver Disease and Insulin Resistance: A Review of Complex Interlinks. Metabolites. 2023, 13, 757. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. The link between polycystic ovary syndrome and both Type 1 and Type 2 diabetes mellitus: what do we know today? Womens Health [Lond]. 2012, 8, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgraduate medicine. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Peeters, A.; Barendregt, J.; Willekens, F.; Mackenbach, J.; Al Mamun, A.; Bonneux, L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Annals of internal medicine. 2003, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M. Use of healthcare services and expenditure in the US in 2025: The effect of obesity and morbid obesity. PLoS One. 2018, 13, e0206703. [Google Scholar] [CrossRef]
- Nigatu, Y.T.; van de Ven, H.A.; van der Klink, J.J.; Brouwer, S.; Reijneveld, S.A.; Bultmann, U. Overweight, obesity and work functioning: the role of working-time arrangements. Appl Ergon. 2016, 52, 128–134. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol Metab Clin North Am. 2016, 45, 677–688. [Google Scholar] [CrossRef]
- Westbury, S.; Oyebode, O.; van Rens, T.; Barber, T.M. Obesity Stigma: Causes, Consequences, and Potential Solutions. Curr Obes Rep. 2023, 12, 10–23. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. Dietary and Lifestyle Strategies for Obesity. Nutrients. 2024, 16, 2714. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wu, Z.; Cao, W.; Lv, J.; Yu, C.; Huang, T.; et al. Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age. Nutrients. 2022, 15, 164. [Google Scholar] [CrossRef]
- Chodick, G.; Simchoni, M.; Jensen, B.W.; Derazne, E.; Pinhas-Hamiel, O.; Landau, R.; et al. Heritability of Body Mass Index Among Familial Generations. JAMA Netw Open. 2024, 7, e2419029. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C. Genetics of Obesity: What We Have Learned Over Decades of Research. Obesity [Silver Spring]. 2021, 29, 802–820. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Abraham, A.; Yaghootkar, H. Identifying obesity subtypes: A review of studies utilising clinical biomarkers and genetic data. Diabet Med. 2023, 40, e15226. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013, 40, 28–39. [Google Scholar]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015, 6, 7502. [Google Scholar] [CrossRef]
- Dong, S.S.; Guo, Y.; Yang, T.L. Addressing the Missing Heritability Problem With the Help of Regulatory Features. Evol Bioinform Online. 2019, 15, 1176934319860861. [Google Scholar] [CrossRef]
- Barber, T.M.; Valsamakis, G.; Mastorakos, G.; Hanson, P.; Kyrou, I.; Randeva, H.S.; et al. Dietary Influences on the Microbiota-Gut-Brain Axis. Int J Mol Sci. 2021, 22, 3502. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O. Metabolic-Associated Fatty Liver Disease and the Gut Microbiota. Endocrinol Metab Clin North Am. 2023, 52, 485–496. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: mom matters. Trends in molecular medicine. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J Clin Invest. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; de, V.o.s.EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012, 70 (Suppl 1), S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioessays. 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A. 2008, 105, 2193–2197. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Oduro-Donkor, D.; Turner, M.C.; Farnaud, S.; Renshaw, D.; Kyrou, I.; Hanson, P.; et al. Modification of fecal microbiota as a mediator of effective weight loss and metabolic benefits following bariatric surgery. Expert Rev Endocrinol Metab. 2020, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. The metagenomics worldwide research. Curr Genet. 2017, 63, 819–829. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Chassaing, B.; Raja, S.M.; Lewis, J.D.; Srinivasan, S.; Gewirtz, A.T. Colonic microbiota encroachment correlates with dysglycemia in humans. Cellular and molecular gastroenterology and hepatology. 2017, 4, 205–221. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients. 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients. 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Goehler, L.E.; Gaykema, R.P.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain, behavior, and immunity. 2005, 19, 334–344. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the national academy of sciences. 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Bishop, C.A.; Wittmuss, M.; Machate, T.; Schifelbein, T.; Schulze, M.B.; et al. Effect of Microbial Status on Hepatic Odd-Chain Fatty Acids Is Diet-Dependent. Nutrients. 2021, 13, 1546. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Seyssel, K.; Chiu, S.; Pan, P.H.; Lin, S.Y.; Stanley, E.; et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Sci Rep. 2017, 7, 44845. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of lipid research. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences. 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.; Stains, J.; Coveleskie, K.; et al. Surgically induced changes in gut microbiome and hedonic eating as related to weight loss: preliminary findings in obese women undergoing bariatric surgery. Psychosomatic medicine. 2017, 79, 880. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.T.; Kampe, J.; Castaneda, T.R.; Vahl, T.; D'Alessio, D.A.; Kruthaupt, T.; et al. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab. 2007, 92, 583–588. [Google Scholar] [CrossRef]
- Weickert, M.O.; Spranger, J.; Holst, J.J.; Otto, B.; Koebnick, C.; Mohlig, M.; et al. Wheat-fibre-induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br J Nutr. 2006, 96, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Ruan, M.; Huang, F.; Yang, L.; Xu, T.; et al. Prebiotic inulin controls Th17 cells mediated central nervous system autoimmunity through modulating the gut microbiota and short chain fatty acids. Gut Microbes. 2024, 16, 2402547. [Google Scholar] [CrossRef]
- Kabisch, S.; Weickert, M.O.; Pfeiffer, A.F.H. The role of cereal soluble fiber in the beneficial modulation of glycometabolic gastrointestinal hormones. Crit Rev Food Sci Nutr. 2024, 64, 4331–4347. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Arafat, A.M.; Blaut, M.; Alpert, C.; Becker, N.; Leupelt, V.; et al. Changes in dominant groups of the gut microbiota do not explain cereal-fiber induced improvement of whole-body insulin sensitivity. Nutr Metab [Lond]. 2011, 8, 90. [Google Scholar] [CrossRef]
- Weickert, M.O. High fiber intake, dietary protein, and prevention of type 2 diabetes. Expert Rev Endocrinol Metab. 2018, 13, 223–224. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007, 167, 956–965. [Google Scholar] [CrossRef]
- de Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; van, D.a.m.RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef]
- Isken, F.; Klaus, S.; Osterhoff, M.; Pfeiffer, A.F.; Weickert, M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J Nutr Biochem. 2010, 21, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Track, N.S.; Cawkwell, M.E.; Chin, B.C.; Chiu, S.S.; Haberer, S.A.; Honey, C.R. Guar gum consumption in adolescent and adult rats: short- and long-term metabolic effects. Can J Physiol Pharmacol. 1985, 63, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Russo, S.; Mingrone, G. Incretin hormones, obesity and gut microbiota. Peptides. 2024, 178, 171216. [Google Scholar] [CrossRef]
- Statham, L.; Pelling, M.; Hanson, P.; Kyrou, I.; Randeva, H.; Barber, T.M. Designer GLP1 poly-agonist peptides in the management of diabesity. Expert Rev Endocrinol Metab. 2023, 18, 231–240. [Google Scholar] [CrossRef]
- Rudovich, N.N.; Nikiforova, V.J.; Otto, B.; Pivovarova, O.; Gogebakan, O.; Erban, A.; et al. Metabolomic linkage reveals functional interaction between glucose-dependent insulinotropic polypeptide and ghrelin in humans. Am J Physiol Endocrinol Metab. 2011, 301, E608–17. [Google Scholar] [CrossRef]
- Isken, F.; Weickert, M.O.; Tschop, M.H.; Nogueiras, R.; Mohlig, M.; Abdelrahman, A.; et al. Metabolic effects of diets differing in glycaemic index depend on age and endogenous glucose-dependent insulinotrophic polypeptide in mice. Diabetologia. 2009, 52, 2159–2168. [Google Scholar] [CrossRef]
- Ruban, A.; Ashrafian, H.; Teare, J.P. The EndoBarrier: Duodenal-Jejunal Bypass Liner for Diabetes and Weight Loss. Gastroenterol Res Pract. 2018, 2018, 7823182. [Google Scholar] [CrossRef]
- Isken, F.; Pfeiffer, A.F.; Nogueiras, R.; Osterhoff, M.A.; Ristow, M.; Thorens, B.; et al. Deficiency of glucose-dependent insulinotropic polypeptide receptor prevents ovariectomy-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008, 295, E350–5. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. The Gut-Liver Axis in the Control of Energy Metabolism and Food Intake in Animals. Annu Rev Anim Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011, 23, 255–264, e119. [Google Scholar] [CrossRef]
- Neufeld, K.A.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Shaban, H.; O'Connor, R.; Ovsepian, S.V.; Dinan, T.G.; Cryan, J.F.; Schellekens, H. Electrophysiological approaches to unravel the neurobiological basis of appetite and satiety: use of the multielectrode array as a screening strategy. Drug Discov Today. 2017, 22, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef]
- Korpela, K. Impact of Delivery Mode on Infant Gut Microbiota. Ann Nutr Metab. 2021, 77, 11–19. [Google Scholar] [CrossRef]
- Andersen, V.; Moller, S.; Jensen, P.B.; Moller, F.T.; Green, A. Caesarean Delivery and Risk of Chronic Inflammatory Diseases [Inflammatory Bowel Disease, Rheumatoid Arthritis, Coeliac Disease, and Diabetes Mellitus]: A Population Based Registry Study of 2,699,479 Births in Denmark During 1973-2016. Clin Epidemiol. 2020, 12, 287–293. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Nagpal, R.; Yamashiro, Y. Gut Microbiota Composition in Healthy Japanese Infants and Young Adults Born by C-Section. Ann Nutr Metab. 2018, 73 (Suppl 3), 4–11. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.Y.; Soh, S.E.; Lee, Y.K.; Kwek, K.Y.C.; Holbrook, J.D.; Van der Beek, E.M.; et al. Ratio of Klebsiella/Bifidobacterium in early life correlates with later development of paediatric allergy. Benef Microbes. 2017, 8, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef]
- Korpela, K.; Costea, P.; Coelho, L.P.; Kandels-Lewis, S.; Willemsen, G.; Boomsma, D.I.; et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018, 28, 561–568. [Google Scholar] [CrossRef]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients. 2021, 13, 3094. [Google Scholar] [CrossRef]
- Martin, R.; Langa, S.; Reviriego, C.; Jiminez, E.; Marin, M.L.; Xaus, J.; et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr Clin Pract. 2017, 32, 354–364. [Google Scholar] [CrossRef]
- Togo, A.; Dufour, J.C.; Lagier, J.C.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of human breast and milk microbiota: a systematic review. Future Microbiol. 2019, 14, 623–641. [Google Scholar] [CrossRef]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems. 2017, 2, e00164–16. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.J.; et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS One. 2020, 15, e0219633. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schutte, U.M.; Beck, D.L.; Abdo, Z.; et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O'Callaghan, T.F.; O'Shea, C.A.; Dempsey, E.M.; O'Toole, P.W.; et al. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl Environ Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef]
- Kim, K.U.; Kim, W.H.; Jeong, C.H.; Yi, D.Y.; Min, H. More than Nutrition: Therapeutic Potential of Breast Milk-Derived Exosomes in Cancer. Int J Mol Sci. 2020, 21, 7327. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp Mol Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef]
- Bryant, W.A.; Stentz, R.; Le Gall, G.; Sternberg, M.J.E.; Carding, S.R.; Wilhelm, T. In Silico Analysis of the Small Molecule Content of Outer Membrane Vesicles Produced by Bacteroides thetaiotaomicron Indicates an Extensive Metabolic Link between Microbe and Host. Front Microbiol. 2017, 8, 2440. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Faith, J.J.; McNulty, N.P.; Rey, F.E.; Gordon, J.I. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science. 2011, 333, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Simoes, C.D.; Maukonen, J.; Kaprio, J.; Rissanen, A.; Pietilainen, K.H.; Saarela, M. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr. 2013, 143, 417–423. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.G.; Worsley, A.; Liem, D.G. Parents' food choice motives and their associations with children's food preferences. Public Health Nutr. 2015, 18, 1018–1027. [Google Scholar] [CrossRef]
- Kral, T.V.; Rauh, E.M. Eating behaviors of children in the context of their family environment. Physiol Behav. 2010, 100, 567–573. [Google Scholar] [CrossRef]
- Smoczek, M.; Vital, M.; Wedekind, D.; Basic, M.; Zschemisch, N.H.; Pieper, D.H.; et al. A combination of genetics and microbiota influences the severity of the obesity phenotype in diet-induced obesity. Sci Rep. 2020, 10, 6118. [Google Scholar] [CrossRef] [PubMed]
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci [Lond]. 2016, 130, 943–986. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clinical endoscopy. 2019, 52, 137. [Google Scholar] [CrossRef]
- Lee, P.; Yacyshyn, B.R.; Yacyshyn, M.B. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant [FMT]. Diabetes Obes Metab. 2019, 21, 479–490. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojarvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619 e6. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O'Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients. 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




