1. Introduction
Actinomyces odontolyticus is a Gram-positive, facultatively anaerobic bacterium of the genus Actinomyces. It is a normal inhabitant of the mucous membranes of the oral cavity, upper respiratory tract and gastrointestinal tract of humans. However, when the barrier function of the body is compromised, this microorganism can become pathogenic, causing various infectious processes, including abscesses, bacteremia and soft tissue infections [
1,
2].
Infections associated with A.odontolyticus are relatively rare. They include liver abscesses, empyemas and inflammatory diseases of the cervicofacial region. These infections most commonly develop after surgery, trauma, or in the setting of a patient's compromised immune response [
2].
Diagnosis of infections caused by
A. odontolyticus is difficult because clinical manifestations are nonspecific and the bacterium grows slowly on standard nutrient media. Long-term culturing, mass spectrometry (MALDI-TOF) and molecular genetic studies are recommended for accurate identification of the pathogen [
3].
The present clinical case demonstrates the development of a soft tissue infection of a postoperative wound caused by A.odontolyticus, which emphasizes the importance of including this microorganism in the differential diagnosis in inflammatory processes in surgical patients.
2. Case Presentation
A 55-year-old female patient was admitted to the emergency room of the Karaganda Medical University Clinic in Karaganda, Kazakhstan, with complaints of intense stabbing pains in the right side of the abdomen, primarily in the right iliac region, dry mouth, and a temperature rise to 37.5°C. The pain began three days before admission, initially in the abdominal area without a clear localization, and then it moved to the right iliac region. The patient has no history of surgical interventions or chronic diseases.
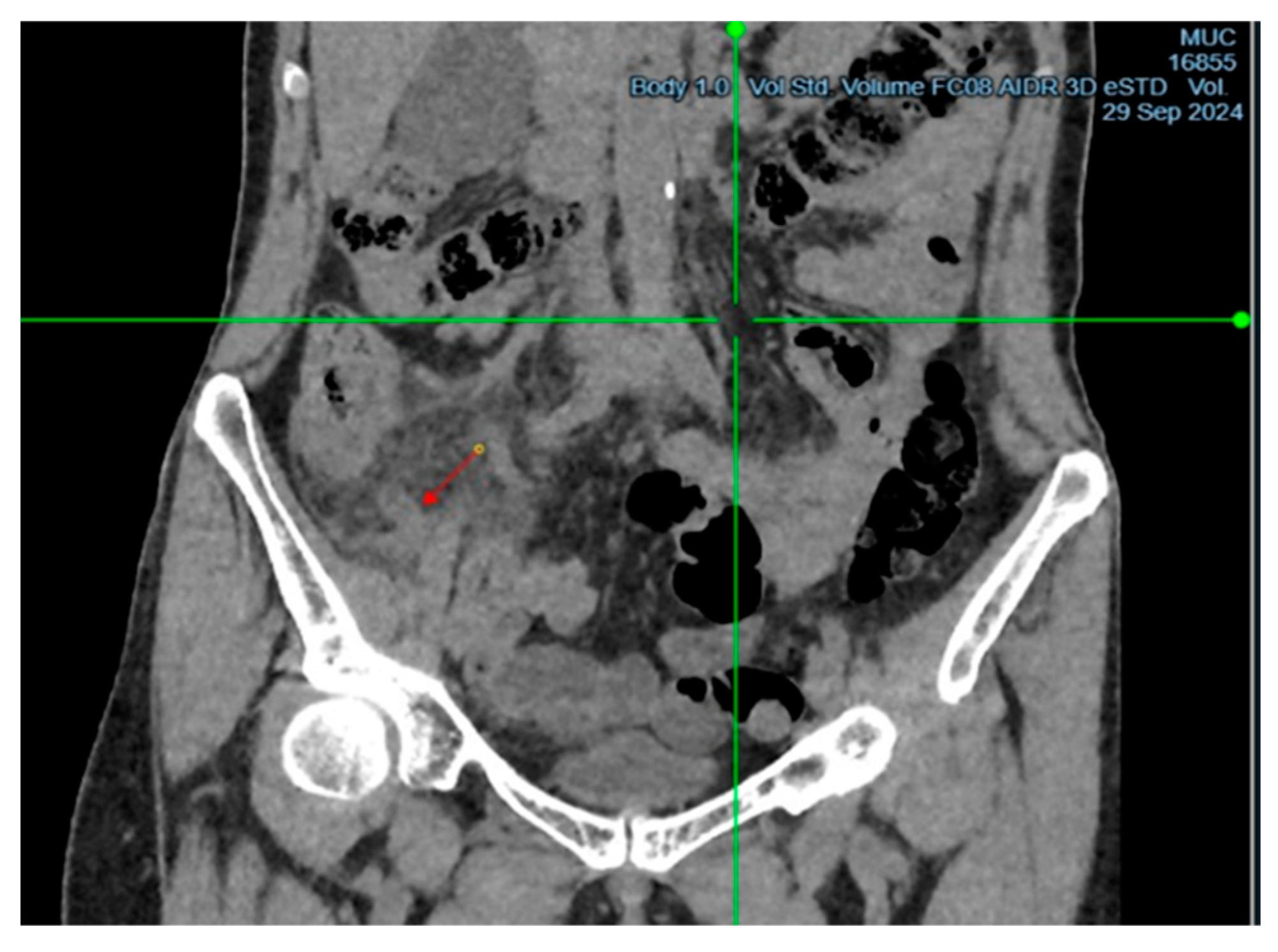
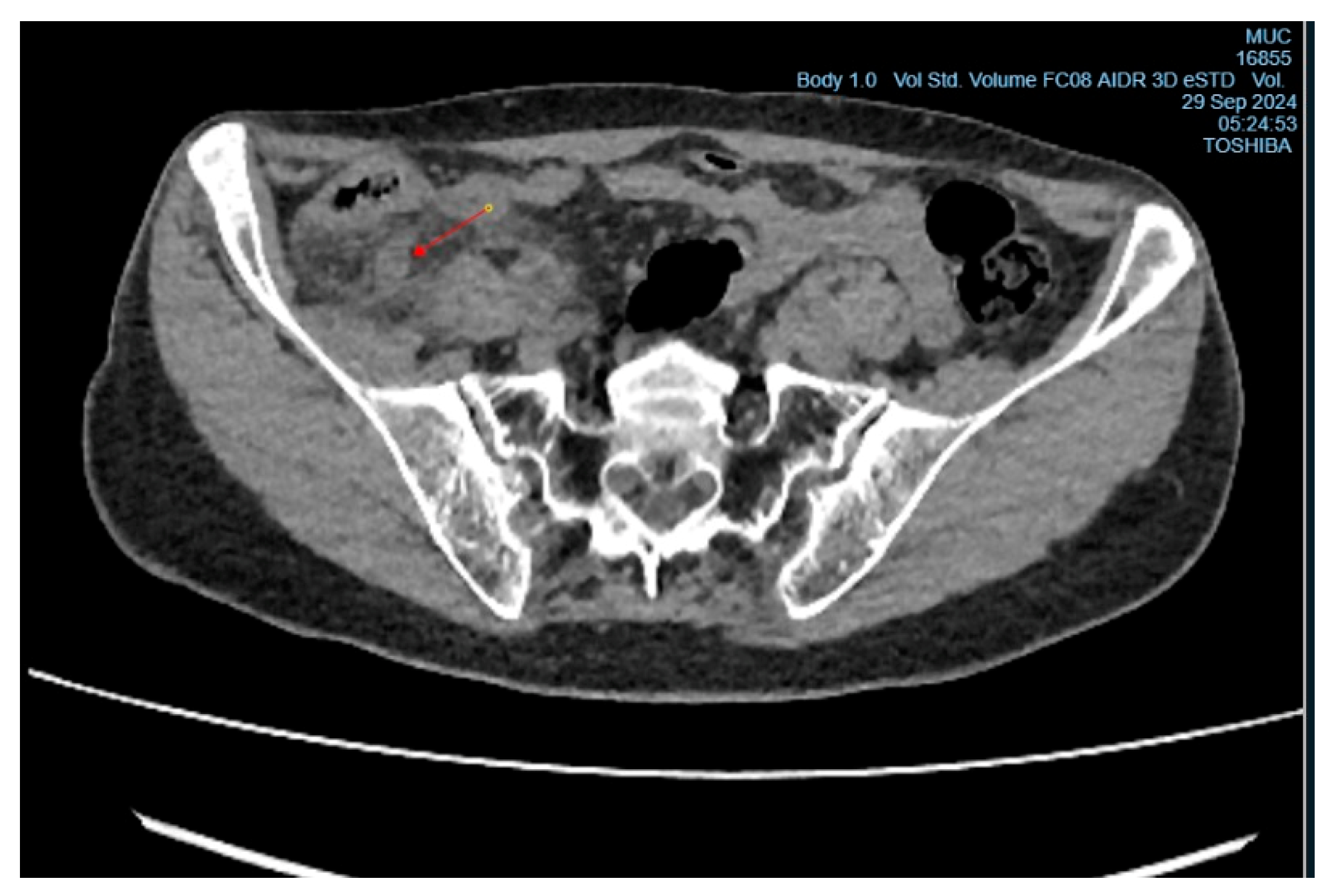
Upon examination, the patient showed tenderness on palpation in the right iliac region, with positive signs of Sitkovsky, Rovsing, and Bartomé-Michelson. Laboratory tests revealed an elevated level of C-reactive protein (6 mg/L, reference range <5 mg/L), while the white blood cell count was within normal limits. A computed tomography (CT) scan of the abdominal organs showed signs of acute appendicitis (
Figure 1 and
Figure 2). The patient underwent surgery – an open appendectomy through an oblique incision according to Volkovich-Dyakonov. Intraoperatively, the diagnosis of acute phlegmonous appendicitis was confirmed.
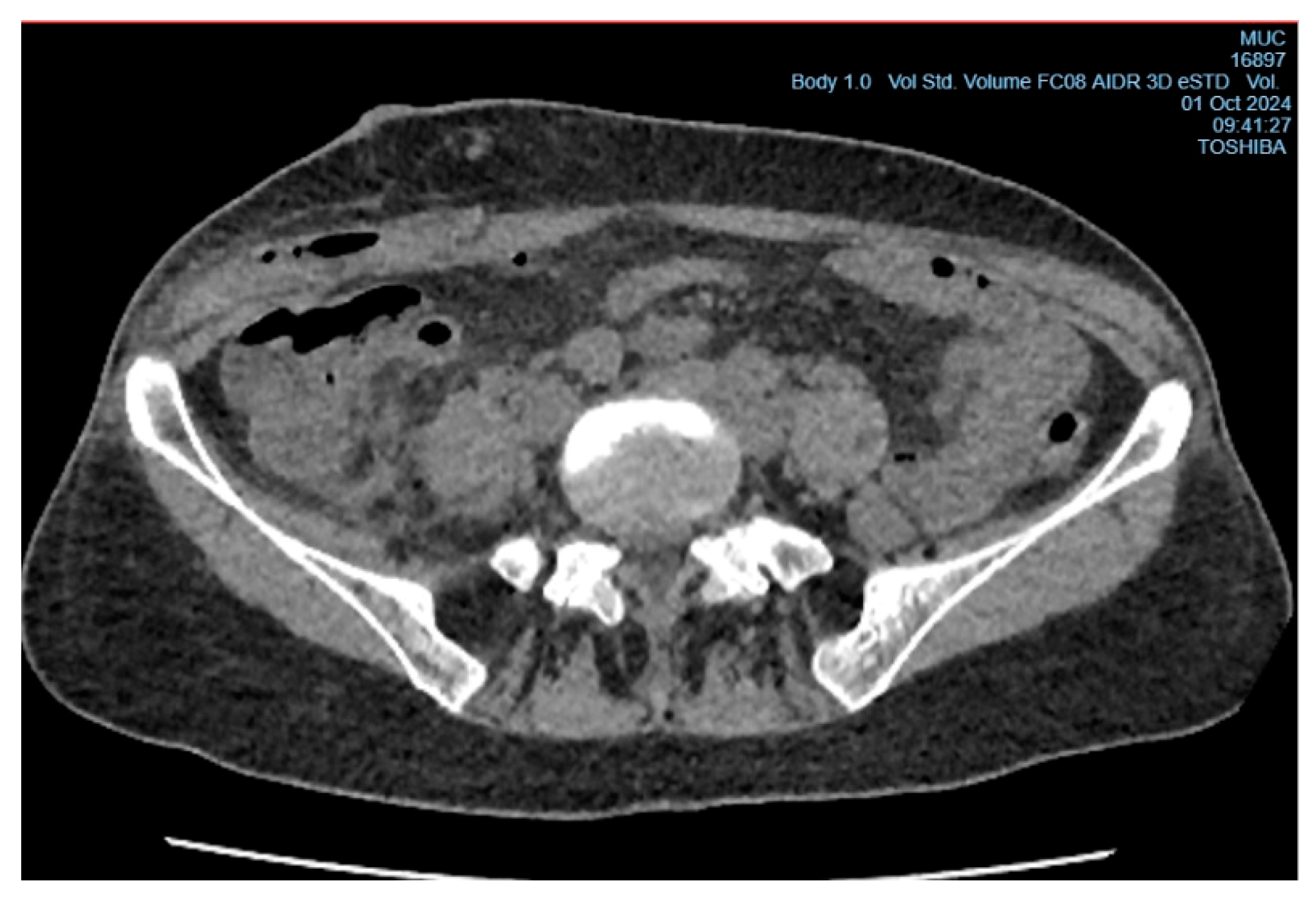
In the early postoperative period, the patient had a persistent elevated body temperature of 37.5°C–38.0°C, localized tenderness at the site of the surgical wound, but no local hyperemia. Leukocytosis was observed (11.2×10⁹/L, reference range from 4 to 9×10⁹/L) with an increase in neutrophils (neutrophils - 78%, reference range from 35% to 70%, band neutrophils - 4%, reference range from 1% to 5%). Despite antibacterial therapy (Cef III, 1.0 gram intramuscularly twice daily), no clinical improvement was observed, raising suspicion of a subgaleal abscess of the postoperative wound (
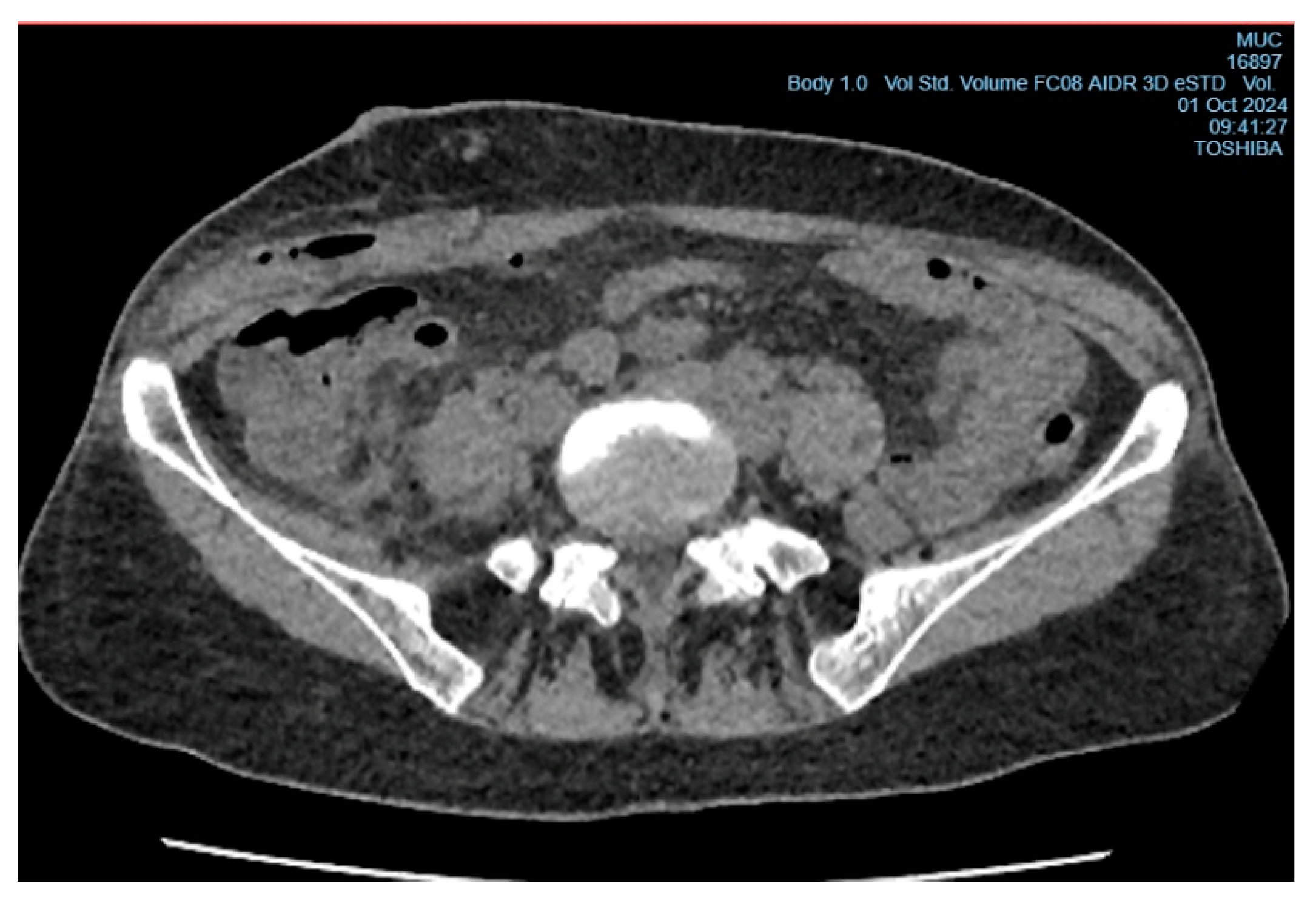
Figure 3). On the 6th day after surgery, the patient underwent wound revision under local anesthesia with 0.5% Novocain solution (30 ml). During the procedure, a collection of pus under the aponeurosis of the external oblique abdominal muscle was diagnosed, and the abscess cavity was drained (
Figure 4). A sample for microbiological analysis was collected and sent to the research laboratory of the Karaganda Medical University.
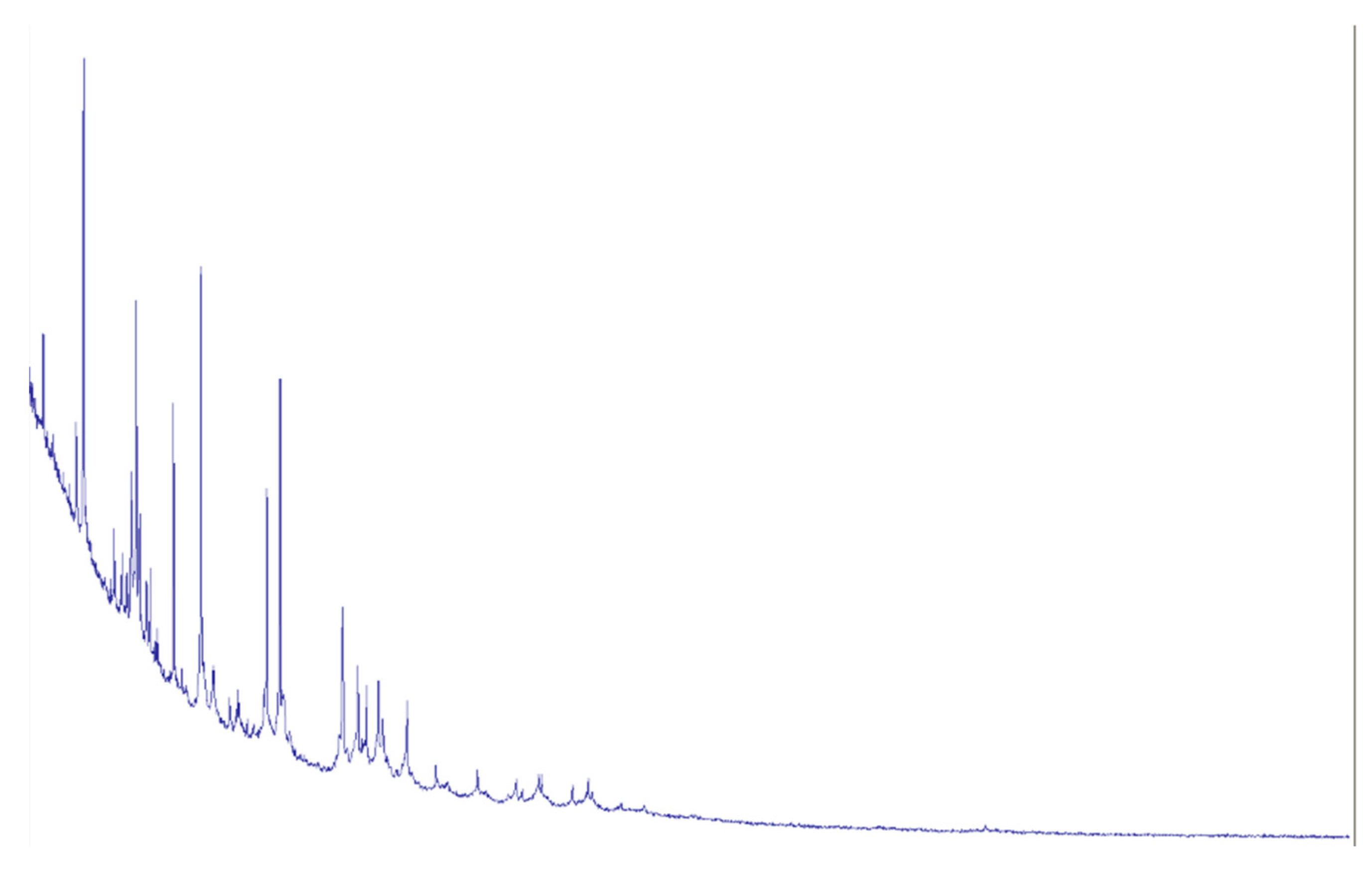
The material was cultured on blood agar according to standard microbiological procedures. After incubation, colonies were found that, based on morphological characteristics, were consistent with the Actinomyces genus, with a colony-forming unit (CFU) count of 10^5. MALDI-TOF mass spectrometry was used for precise microorganism identification, which confirmed the presence of
Actinomyces odontolyticus (
Figure 5). Antibiotic susceptibility testing was also conducted, allowing for the selection of an effective antibacterial treatment.
A. odontolyticus showed sensitivity to penicillin, amoxicillin, cefoxitin, ceftriaxone, imipenem, fusidic acid, tetracycline, levofloxacin, and linezolid, but was resistant to clindamycin and azithromycin.
In accordance with the results of sensitivity testing to antibacterial drugs, the patient was prescribed ceftriaxone in a dose of 1.0 g intramuscularly 2 times a day, daily dressings with antiseptic solutions. On the background of the treatment positive dynamics was noted: the main complaints regressed, body temperature normalized. In the control analyses the level of leukocytes was 7×109/l. The postoperative wound was cleared of purulent discharge, edema and hyperemia regressed, the postoperative wound was tightened by secondary tension, with active growth of granulation tissue. The patient was discharged in satisfactory condition. Bacteriologic culture was repeatedly taken from the area of the postoperative wound, as a result of which A. odontolyticus was not identified, indicating a positive effect of treatment and complete elimination of this pathogen.
3. Discussion
Abdominal actinomycosis is a rare disease caused by facultatively anaerobic, Gram-positive bacilli of the genus Actinomyces, which normally inhabit the oral cavity, nasopharynx, gastrointestinal tract, skin, and genitourinary tract [
3]. However, these microorganisms can become pathogenic when the barrier function of mucous membranes is compromised, for example, due to surgical interventions. Abdominal actinomycosis usually develops after invasive procedures or due to abdominal infection such as appendicitis [
4]. In some cases of actinomycosis affecting the abdomen, Actinomyces can cause pericarditis or liver damage, with
A. odontolyticus being found in liver abscesses [
5].
In our case, the patient developed actinomycosis after appendectomy, manifested by a subgaleal abscess in the right iliac region. It should be noted that this disease is predominantly diagnosed in immunocompromised patients.
Screening of the material obtained from the postoperative abscess revealed the presence of colonies morphologically corresponding to representatives of the genus
Actinomyces. The diagnosis was confirmed using MALDI-TOF mass spectrometry, which made it possible to accurately identify
Actinomyces odontolyticus. Traditional biochemical tests for the identification of suspected
Actinomyces species can be applied to most of them, but often there is an indifferent growth in test media, which leads to false negative results and low reproducibility [
6]. In this regard, given the limited accuracy of conventional biochemical methods, alternative methods with high accuracy, such as MALDI-TOF mass spectrometry, which is widely used in clinical laboratories, have been increasingly used recently [
7]. In one comparative study, MALDI-TOF correctly identified 97% of 32 strains to the species level, while a commercially available biochemical kit achieved only 33% success [
8].
Positive dynamics was observed against the background of antibacterial therapy with ceftriaxone: the patient's clinical condition improved. It should be noted that sensitivity testing of anaerobic bacteria is rarely performed in clinical microbiological laboratories. However, this is often not a particular problem for Actinomyces species, since they usually do not show resistance to beta-lactam drugs, and being gram-positive, they are also sensitive to vancomycin [
9].
Thus, actinomycosis remains a significant diagnostic problem in clinical practice. In this case, bacterial translocation on the background of acute phlegmonous appendicitis probably caused the formation of a subgaleal abscess caused by A. odontolyticus.
4. Conclusions
This case is the first example of abdominal actinomycosis caused by A. odontolyticus reported in the Republic of Kazakhstan. He emphasizes the critical importance of early laboratory diagnosis of microorganisms and their spectrum of antibiotic sensitivity for the appointment of timely and effective treatment. The use of modern diagnostic methods, such as MALDI-TOF mass spectrometry, makes it possible to identify pathogens and conduct effective therapy.
Author Contributions
Conceptualization, N.A., R.B. and N.U.; methodology, R.B., N.U., A.L.; validation, N. A., R. B..; formal analysis, N. U., A.T.; investigation, R. B., N.U., A.L., D.E.; resources, N.A., A.L., and N.U.; data curation, N.A., A.L., R.B., A.T..; writing—original draft preparation, N.U. and A. T.; writing – review and editing, R.B., N.U., A.L. and A. T.; visualization, N. A.; supervision, N.A., R.B., A.L.; project administration, N.A., D.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
For this case study, a decision from the Bioethics Committee was not required. The authors were granted permission by the Head of the University Clinic of the Karaganda Medical University in Karaganda to conduct this study, using information from the medical records of the hospitalized patient with the provision that no personal data of the patient would be used.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MALDI-TOF |
Matrix Assisted Laser Desorption/Ionization - time-of-flight analyzer |
| CT |
Computer tomography |
| CFU |
Colony-forming unit |
References
- Paulino S, Duran M, Allena N, Sosa F, Singhal R. Understanding Actinomyces Odontolyticus: A Rare Culprit of Bacteremia. Cureus. 2024 Aug 3;16(8):e66086. [CrossRef] [PubMed] [PubMed Central]
- Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015 Apr;28(2):419-42. [CrossRef] [PubMed] [PubMed Central]
- Sharma S, Hashmi MF, Valentino III DJ. Actinomycosis. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482151/.
- Wong VK, Turmezei TD, Weston VC. 2016. Actinomycosis. BMJ 343:d6099. [CrossRef]
- Chao CT, Liao CH, Lai CC, Hsueh PR. 2017. Liver abscess due to Actinomyces odontolyticus in an immunocompetent patient. Infection 39:77–79. [CrossRef]
- Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015 Apr;28(2):419-42. [CrossRef] [PubMed] [PubMed Central]
- Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. 2019. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49:543–551. [CrossRef]
- Ng LS, Sim JH, Eng LC, Menon S, Tan TY. 2018. Comparison of phenotypic methods and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for the identification of aero-tolerant Actinomyces spp. isolated from soft-tissue infections. Eur J Clin Microbiol Infect Dis 31:1749–1752.
- De Simeis D, Serra S. Actinomycetes: A Never-Ending Source of Bioactive Compounds-An Overview on Antibiotics Production. Antibiotics (Basel). 2021 Apr 22;10(5):483. [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).