3.5. Heat Capacity Differences for the Adjusting the Fusion Enthalpies of Diols from Tfus to 298.15 K
The enthalpy of fusion (or melting enthalpy), , is usually measured at the melting (or fusion) temperature and this enthalpy and temperature are considered as constants specific for the compound. Is there any difference between notation melting and fusion? As a matter of fact, melting and fusion are closely related, but have subtle differences: melting refers specifically to the phase transition of a solid that turns into a liquid when heated to its melting point. Fusion is a broader term that can refer to melting, but also includes other processes in which materials combine, such as ‘nuclear fusion’ (in which atomic nuclei fuse to form a heavier nucleus and release energy). In physics and materials science, the term ‘fusion’ is sometimes used interchangeably with ‘melting’, but it has a broader application than just phase transitions. Melting is therefore a special type of fusion, but fusion can mean more than just melting. Nevertheless, melting and fusion can be considered equivalent in thermochemistry.
The fusion enthalpy (or melting enthalpy),
, was not always adjusted to the reference temperature
T = 298.15 K. However, such an adjustment is essential for thermochemical calculations, since according to the textbook, the consistency of the energetics of the phase transition can be determined using a general equation:
whereby all properties have to be adjusted to a common temperature (e.g., to the reference temperature
T = 298.15 K in this work). According to the Chickos method, the temperature adjustment of the fusion enthalpy is usually carried out using Equation (4), whereby the heat capacity difference is estimated according to Equation (7). However, since the heat capacities of the liquid and crystalline phases are significantly overestimated in the original parameterisation of the Chickos’s method, the
also follows this trend (see
Table 6, column 6).
To eliminate the consequences of overestimates, the corrected values of
from
Table S7, column 7 and the corrected values of
from
Table S8, column 4 were used to calculate the reliable
values (see
Table 6, column 4), which were recommended for temperature adjustment of the fusion enthalpy of diols from
Tfus to 298.15 K. For practical reasons, it is also useful to derive a correlation between
and the heat capacity of the liquid phase. Using the data in columns 2 and 4 of
Table 6, the following equation was developed:
This equation can be useful for a quick appraisal of values and the resulting adjustment of the fusion enthalpies to the reference temperature T = 298.15 K.
3.6. Are the Literature Experimental Data on Energetics of Phase Transition of the Long-Chained Diols Reliable?
Experimental studies on the thermochemistry of diols are very limited, especially for the long-chain species. Systematic studies on the energetics of phase transitions for α,ω-alkanediols have been described mainly in a few papers by the Sabbah group [
20] and the Della Gatta group [
12,
13]. In the Sabbah group, the molar heat capacities in the gas phase at 298.15 K and
Tav were calculated from the group increments tabulated by Benson [
24]. The enthalpy differences
H(
T) -
H(298.15 K) of the condensed phase were measured with a Tian-Calvet calorimeter using the drop method. In the Della Gatta group, the molar enthalpy functions for the gas phase were calculated using the Benson group additivity scheme [
24] and for the liquid phase, the molar enthalpy functions were also estimated using the Missenard’s empirical additive method [
25]. Since the approaches used in the literature for temperature adjustments of the enthalpies of vaporisation are not identical, it makes sense to apply the heat capacity differences evaluated in this work in order to obtain an appropriate comparison and analysis of the available experimental results.
Equation (15) is a generally useful tool for establishing the consistency of the energetics of the liquid-vapor, solid-vapor and solid-liquid phase transitions. Admittedly, for these three phase transitions, it is more advantageous to compare and analyse the values of the enthalpies of vaporisation, since this thermochemical property obeys the rules of group additivity and correlates successfully with boiling temperatures, the number of carbon atoms in a molecule, etc. The compilation of the enthalpies of vaporisation of the short-chained α,ω-alkanediols is given in
Table 4. The compilation of the enthalpies of vaporisation of the long-chained α,ω-alkanediols is given in
Table 7.
Some of the results given in this table were measured directly above the melting temperature and these enthalpies of vaporisation
were adjusted from
to
T = 298.15 K using the
–values from
Table 2, column 7. Other results given in this table were measured below the melting temperature and the corresponding sublimation enthalpies
were first adjusted from
to
T = 298.15 K (see
Table S11) using the
from
Table 5, column 7. After that, the fusion enthalpies,
, available for these diols were also adjusted to
T = 298.15 K (see
Table S12) using the values (
Table 6, column 4). Finally, the enthalpies of vaporisation
(298.15 K) available in the literature for 1,6-hexanediol, 1,8-octanediol and 1,10-decanediol [
20] were calculated according to Equation (15) and given in
Table 7 for comparison.
If the sufficient quantity and good consistency of the available enthalpies of vaporisation of the short-chain α,ω-alkanediols were observed in
Table 4, the data for the long-chain species (with the exception of 1,6-hexanediol) are rather confusing (see
Table 7). Even when the enthalpies of vaporisation were uniformly adjusted to
T = 298.15 K, the unusual differences between the corresponding homologous data observed in the literature are rather questionable. In fact, the comparison of the vaporisation enthalpies in
Table 7 for the sequence of 1,8-octanediol
= 106.2 ± 2.0 kJ mol
−1), 1,9-nonanediol
= 113.6 ± 2.1 kJ mol
−1) and 1,10-decanediol
= 111.0±2.0 kJ mol
−1, calorimetrically measured in the Sabbah group [
20], make it clear that there is no reason or specific interaction that makes the enthalpy of vaporisation of 1,9-nonanediol higher than that of 1,10-decanediol.
The set of vaporisation enthalpies of α,ω-alkanedios measured by the Della Gatta group [
12,
13] measured using a combination of the torsion-effusion and Knudsen methods is more problematic. The results of the adjustment of the experimental enthalpies of vaporisation,
, of α,ω-alkanediols [
12,
13] from (
Tav) to the reference temperature
T = 298.15 K are shown in
Table 8.
This table shows that
reported by Della Gatta group [
12,
13] for the series of diols from 1,11-undecanediol to 1,16-hexadecanediol are practically the same within the limits of experimental uncertainties. Such an anomaly cannot be meaningfully explained and could only be attributed to possible experimental problems in the investigation of the diols. The general trends of the
chain length dependencies for the diol series measured in the Sabbah and Della Gatta groups are shown in
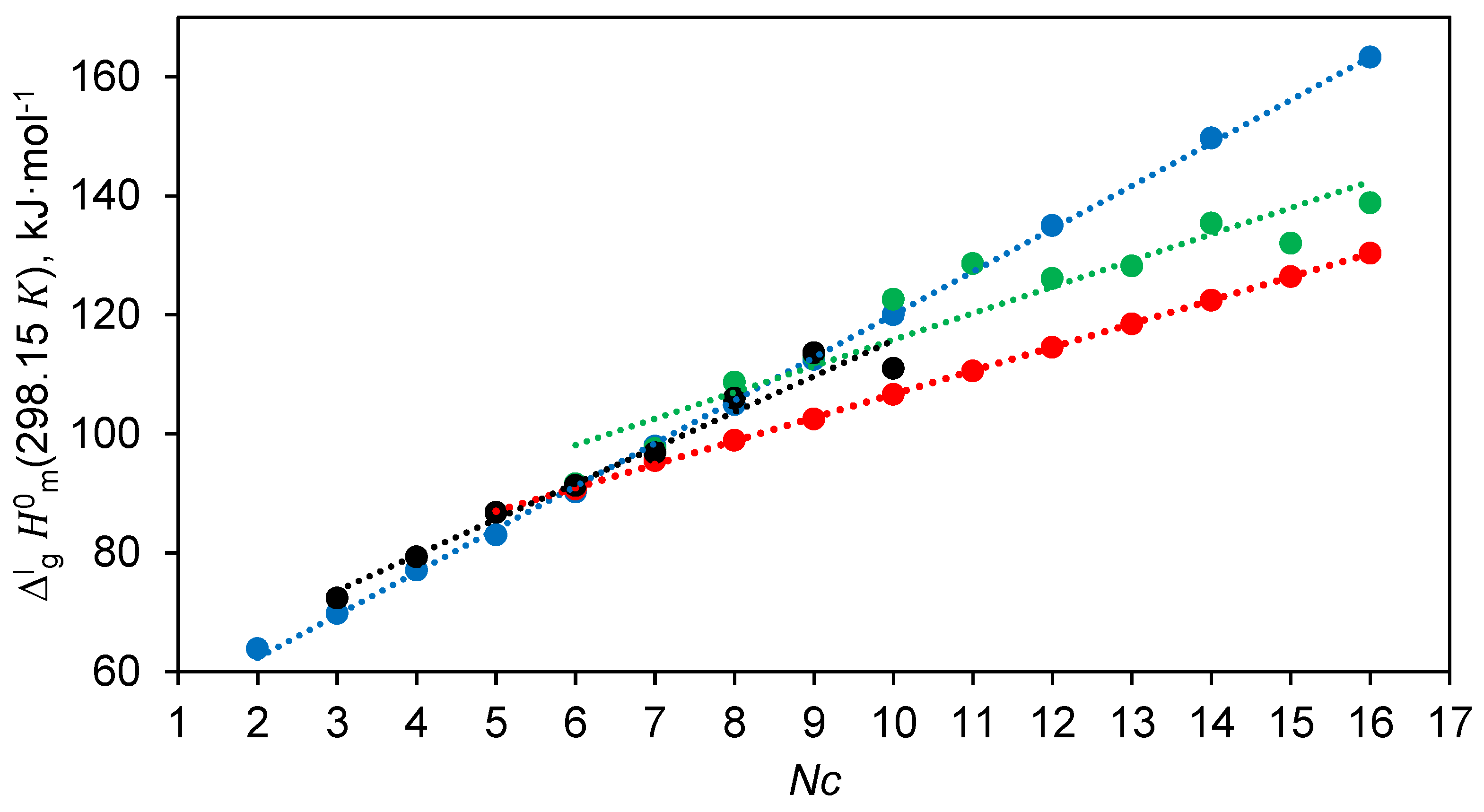
Figure 1.
The following linear correlations were obtained from the numerical results compiled in
Table 7, column 4:
The significantly different slopes of these correlations are further evidence of the internal inconsistencies of the experimental data sets. This inconsistency seems to have been overlooked by the Chickos group, as they used the data for the long-chain diols from [
12,
13,
20] (see
Table 7) as standards for the correlation gas chromatography (CGC) method.
The CGC method used by the Chickos group is based on measurements of the retention times
tR of the series of “target” compounds, which are generally structural parent molecules [
26]. In the first step, the mixture of “target“ compounds and “standards” (similarly shaped compounds with reliable enthalpies of vaporisation) are injected simultaneously into a gas chromatographic column and their retention times recorded at different temperatures.
The slope of the dependence
(
Tm) =
f (1/T) provides the transfer enthalpy,
(
Tm), of the “target” from the liquid phase of the column to the gas phase.
Tm is the average temperature of the GC experiment. In the second step, the transfer enthalpies,
(
Tm), of the standards are correlated with their known enthalpies of vaporisation,
(298.15 K). The unknown enthalpies of vaporisation of the “target” substances are then derived from this correlation. The CGC has proven to be a reliable method for the evaluation of the enthalpy of vaporisation, provided that a sufficient number of suitable standards with reliable enthalpies of vaporisation are available [
27].
The original
values derived by Chickos et al. [
26] were based primarily on the correlations with the calorimetric data reported by Sabbah et al. [
20]. Consequently, their correlation with chain length does not differ significantly from those shown in Equation (17):
The most surprising thing about this correlation is that all diols from 1,2-ethanediol to 1,16-hexadecanediol lie on the flawless straight line (see
Figure 1). At the same time, it is known that 1,2-ethanediol, 1,3-propanediol and 1,4-butanediol exhibit pronounced intramolecular hydrogen bonding [
28], and this peculiarity should influence the vaporisation energetics and the resulting decrease in enthalpy of vaporisation, as a consequence [
29].
Another unusual aspect that can be seen from Equations (17) and (19) is that the slope of both dependencies is around 7 kJ mol
-1, suggesting that the contribution of the CH
2 fragment to the enthalpies of vaporisation of diols is extremely high compared to the contribution of CH
2 from 4.4 to 5.0 kJ mol
-1 in the most homologous series of organic compounds (see
Table S13) and even for the ionic liquid with CH
2 = 3.9 ± 0.2 kJ mol
-1 [
30].
All these issues led to additional experimental work, and the liquid samples of 1,6-hexanediol and 1,10-decanediol were deliberately measured using the transpiration method in this work. The latter method has already been successfully used to measure the vapor pressure of the short-chained diols 1,2-ethanediol [
22], 1,3-propanediol [
31] and 1,4-butanediol [
32]. These results were used in
Section 3.3.3 to determine reliable values for the heat capacity differences (see
Table S9). The decisive advantage of this method is that the measurements can be carried out close to
T = 298.15 K. Furthermore, the residual water content typical for diols is removed from the sample in the preconditioning experiment. In addition, the sample is held in a saturator under a supply of the inert gas (nitrogen) during the transpiration experiment. In order to gain experience with long-chain diols, 1,6-hexanediol was measured (see
Table S5), and the enthalpy of vaporisation from the transpiration measurements agreed very well with the results of other methods (see
Table 7 and
Table S10).
The results of the transpiration experiment with 1,10-decanediol are shown in
Table S5 and the final value
= 106.6 ± 0.9 kJ mol
-1 was compared with the results of other methods in
Table 7. Our new value is perfectly consistent with those derived from the very good quality ebulliometric result 106.6±6.7 kJ mol
-1 [
11], but it is far away from the vaporisation enthalpies determined by the Sabbah group (111.0±2.0 kJ mol
-1, Table 11) and the Della Gatta group (122.6±5.0 kJ mol
-1 [
12]), and consequently also differs from the enthalpy of vaporisation (120.0±0.6 kJ mol
-1) of the CGC method of the Chickos group [
26]. In order to find possible independent support for our new or previous results, we collected numerous boiling points under reduced pressure from the literature (see
Table S14). These are usually given by organic chemists as a constant value during distillation after synthesis and are necessary to characterise the compound produced. These vapor pressure data typically cover a wide range from atmospheric boiling point down to pressures attainable with rotary vane vacuum pumps commonly used in chemical laboratories. In our experience [
33], these reduced pressures, when treated as a function of temperature, usually provide a rough but reasonable trend for a compound of interest (significant details are given in ESI).
The approximation of the boiling points (BP) found for 1,10-decanediol (see
Table S14) yields
= 106.2 ± 6.2 kJ mol
-1 (see
Table 7), which is in excellent agreement with our transpiration result. The approximation of the boiling points found for 1,6-hexanediol (see
Table S14) yields
= 89.5 ± 3.4 kJ mol
-1 (see
Table 7), which is in good agreement with other results collected for comparison in this table. This good performance with the BP method prompted the collection of the available boiling points for the diols with a chain length between six and ten (see
Table S14) in order to evaluate the dependence on the chain length and possibly understand why the previous Eqs. (17) and (18) are so different. The treatment of the BP data yielded the following results (see
Table 7):
= 95.5 ± 3.9 kJ mol
-1 for 1,7-heptanediol,
= 98.9±4.1 kJ mol
-1 for 1,8-octanediol, and
= 102.5±5.2 kJ mol
-1 for 1,9-nonanediol.
The BP result for 1,7-heptanediol is about 2.5 kJ mol
-1 lower than previous values (see
Table 7). For 1,8-octanediol and 1,9-nonanediol, the BP results are significantly (by 10 kJ mol
-1) lower than the previous results obtained by the groups of Sabbah and Della Gatta. In such an ambiguous situation, we decided to reassess the CGC results reported by Chickos et al. [
26] using their original transfer enthalpies. However, instead of the original standards published by Sabbah [
20] and Della Gatta [
12,
13], which Chickos [
26] used in his work, we used the evaluated
from
Table 4 and
Table 7. The algorithm of the revision is shown in
Table S15 and the final results are listed in
Table 7 for comparison. It is not unexpected, but now the revised enthalpies of vaporisation
derived in this way are hardly distinguishable from the carefully evaluated results in
Table 4 and the results obtained in this work using the experimental transpiration method and the empirical BP method. This perfect match resolves our doubts about the selection of the “true” enthalpies of vaporisation
for the long-chain α,ω-alkanediols. It is now obvious that the enthalpies of vaporisation for 1,7-heptanediol, 1,8-octanediol, 1,9-nonanediol and 1,10-decanediol, measured by the Sabbah and Della Gatta groups, should be considered questionable (they are given in brackets in
Table 7).
The enthalpies of vaporisation of 1,5-pentanediol (
Table 4) evaluated in this work and the values for 1,6-hexanediol, 1,7-heptanediol, 1,8-octanediol, 1,9-nonanediol and 1,10-decanediol (see
Table 7) show a good linear dependence on the chain length
NC (see
Figure 1):
This dependence reproduces the selected for correlation values of the long-chain diols C5 – C10 within ± 1.0 kJ mol
-1 (see
Table 7 and
Table S16) and can be recommended for calculating the
values for the α,ω-alkanediols with the longer chain. It turned out that the enthalpies of vaporisation of the first three representatives of the α,ω-alkanediols are completely outside the correlation represented by Equation (20): 1,2-ethanediol with −9.5 kJ mol
-1, 1,3-propanediol with −7.6 kJ mol
-1 and 1,4-butanediol with −3.9 kJ mol
-1 (see
Table S16). These significant stabilisations of the short-chain diols are due to the relatively strong contribution of the intramolecular hydrogen bond, which is a special feature of these molecules.