Submitted:
14 April 2025
Posted:
15 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Agrochemicals in Sri Lanka
1.2. Heavy Metals in Agrochemicals and Their Effects on Human Health
1.3. Copper and Nickel as Heavy Metals
1.4. Removal of Heavy Metals from Contaminated Water
1.4.1. Biosorbents
1.5. Hydrogels as Adsorbents for Heavy Metals and More
1.6. Synthesis of Hydrogels via Crosslinking
1.6.1. Alginate Based Hydrogels
1.7. Pomegranate Peel as a Heavy Metal Adsorbent
1.8. Flame Atomic Absorption Spectroscopy (FAAS)
1.9. Project Aims
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Organic Pomegranate Peel Powder (PPP)
2.3. Synthesis of Sodium Alginate- Organic Pomegranate Peel Hydrogels (SA-PP-H (org))
2.4. Characterization
2.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Diameter Size
2.4.4. Water Uptake Capacity
2.4.5. Effect of Pomegranate Peel Concentration on Water Uptake Capacity
2.5. Batch Adsorption Tests
2.5.1. Effect of Contact Time
2.5.2. Effect of Initial pH
2.5.3. Effect of Hydrogel Amount
3. Results
3.1. Characterization
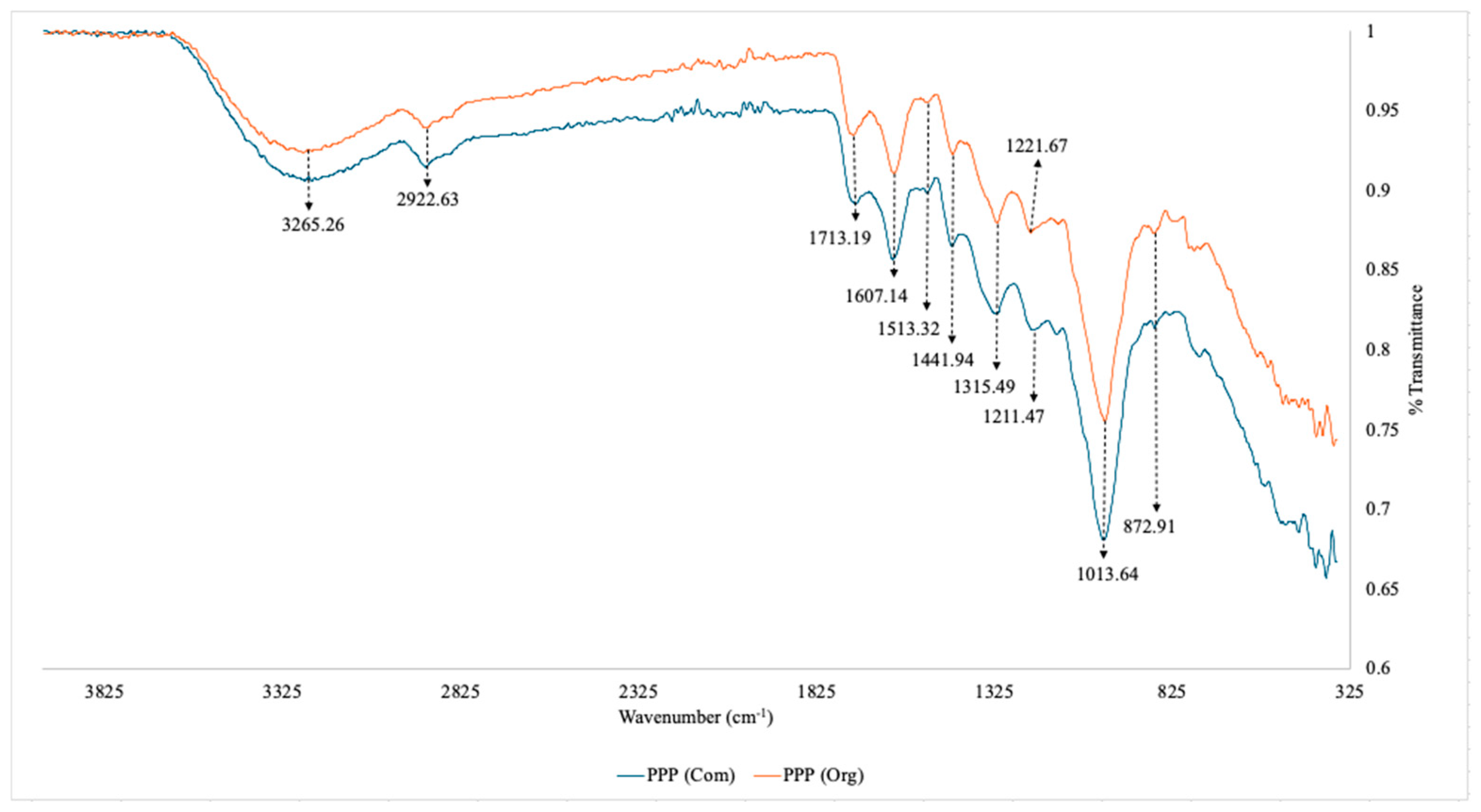
3.1.1. FTIR for Commercial vs Organic Pomegranate Peel Powder
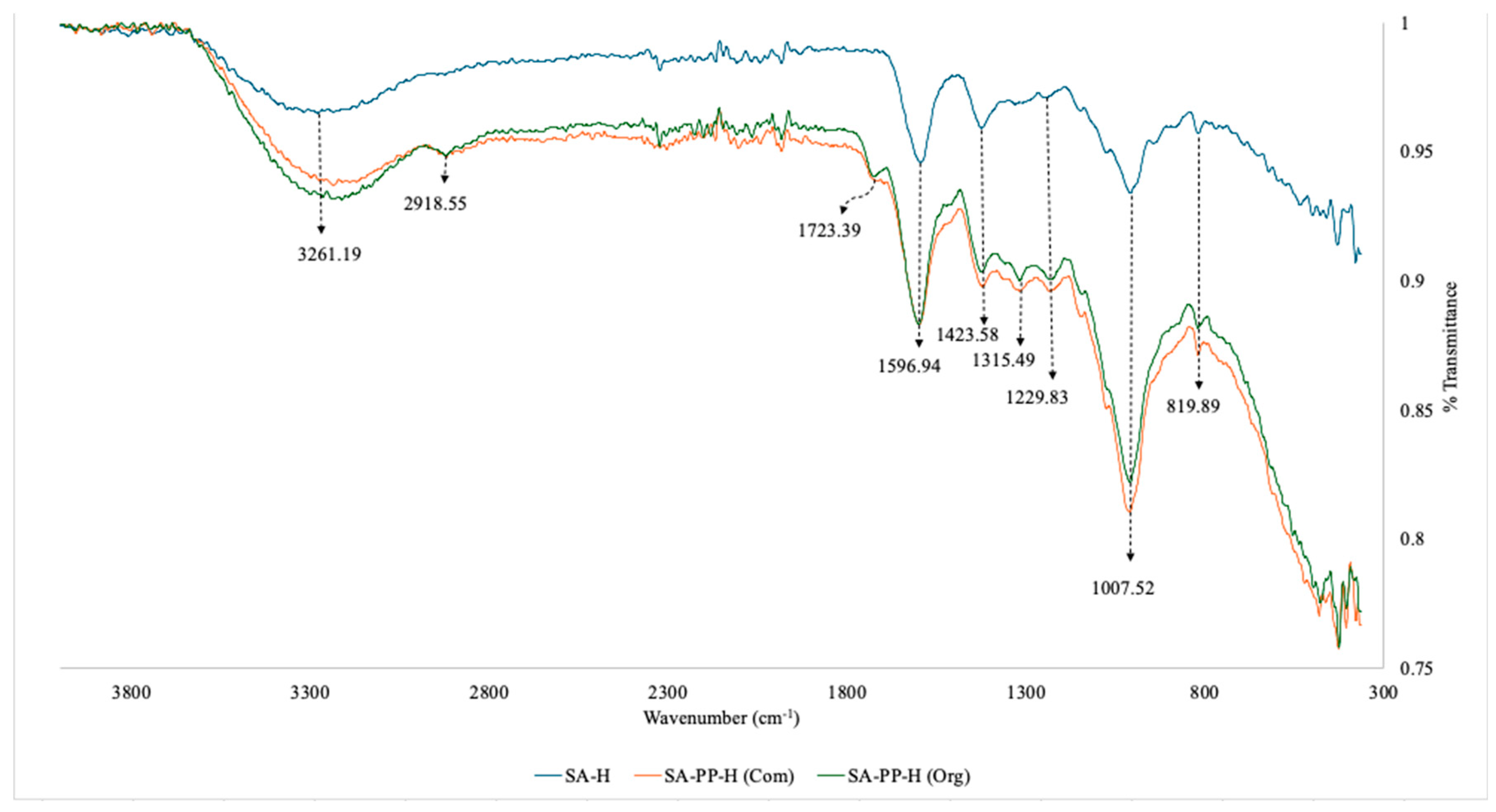
3.1.2. FTIR for SA-H, SA-PP-H (com) and SA-PP-H (org)
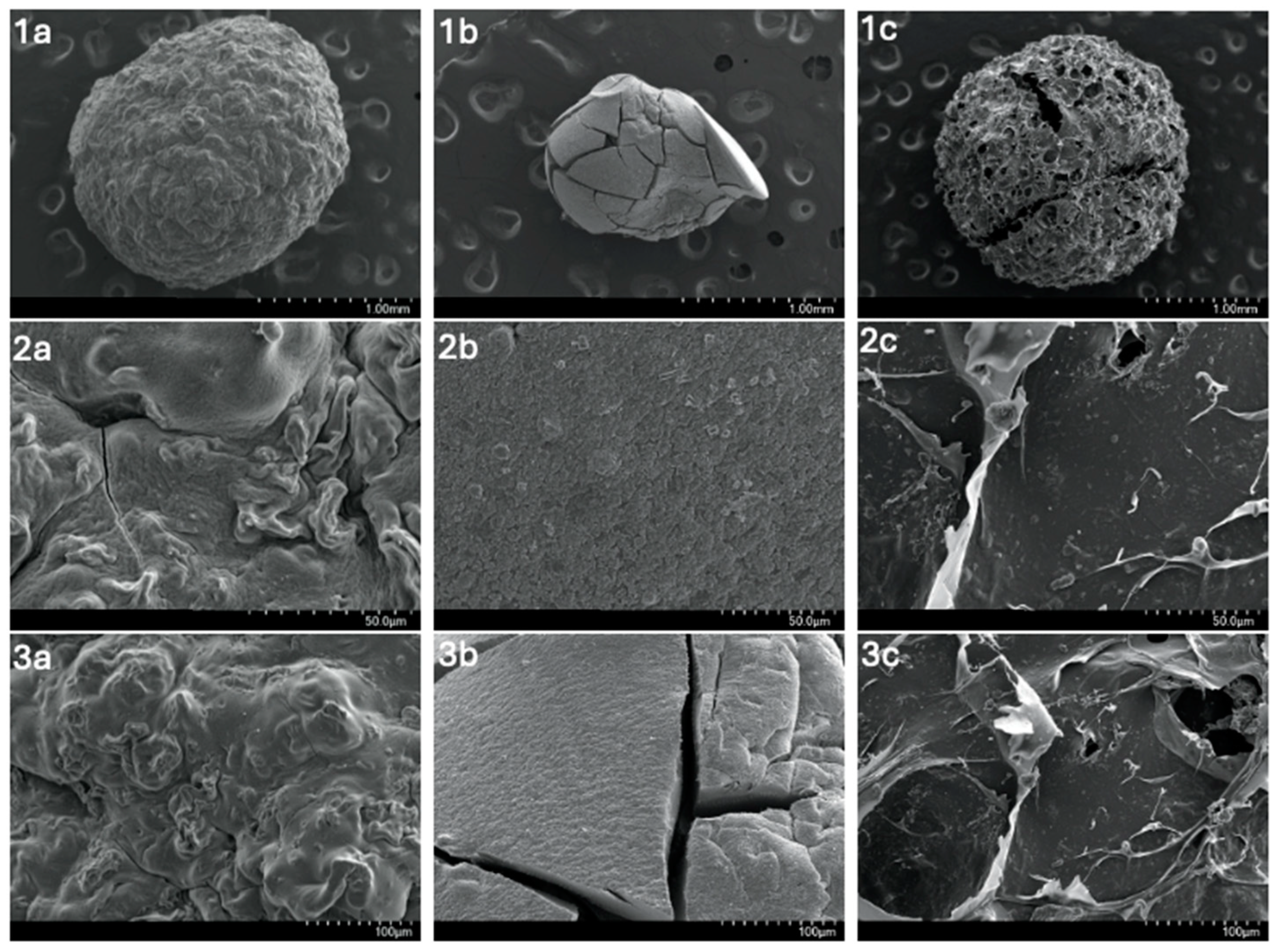
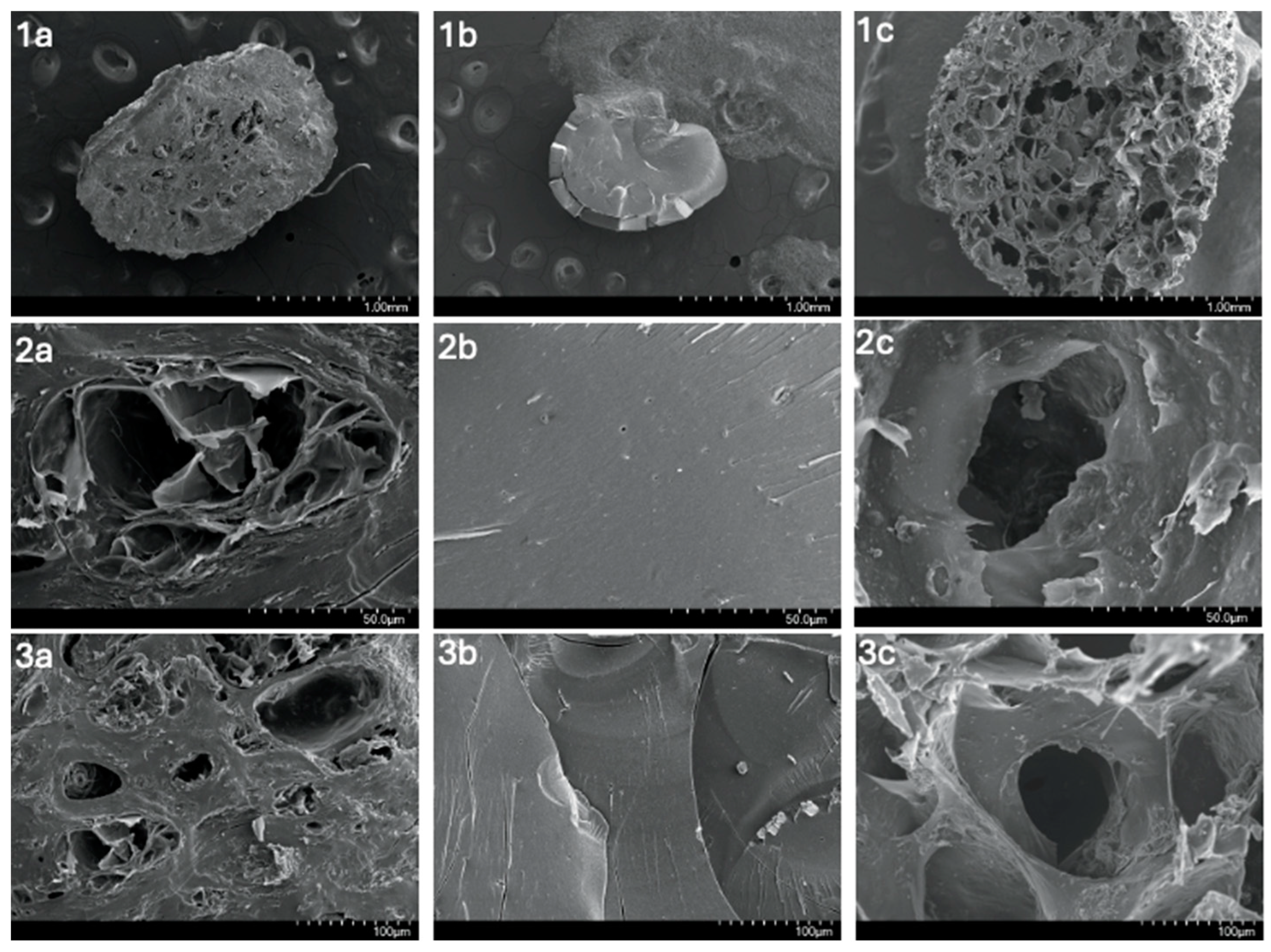
3.1.3. SEM
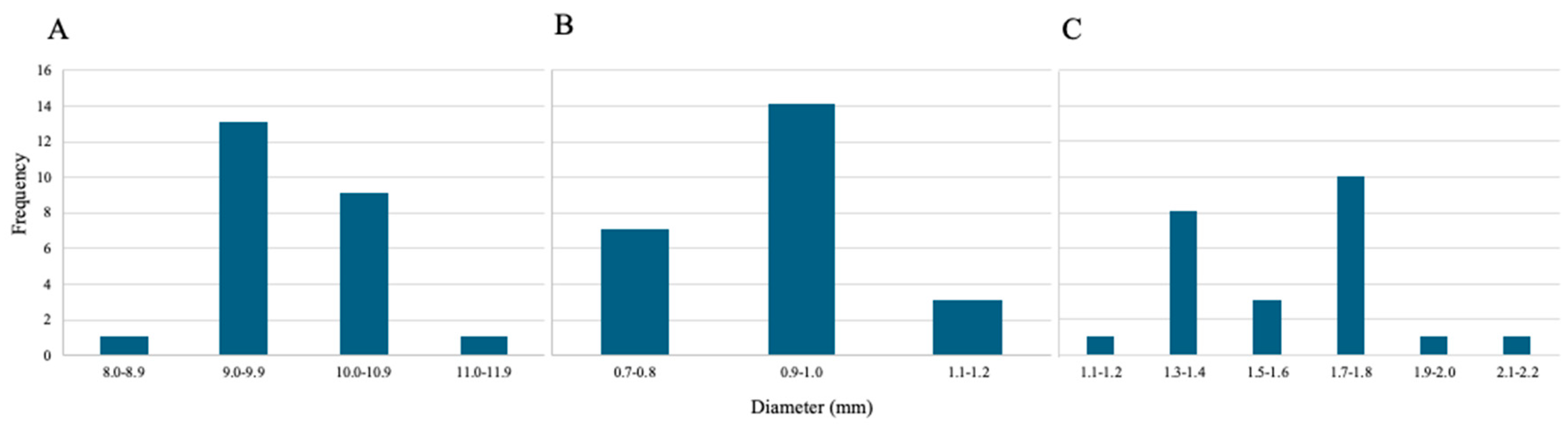
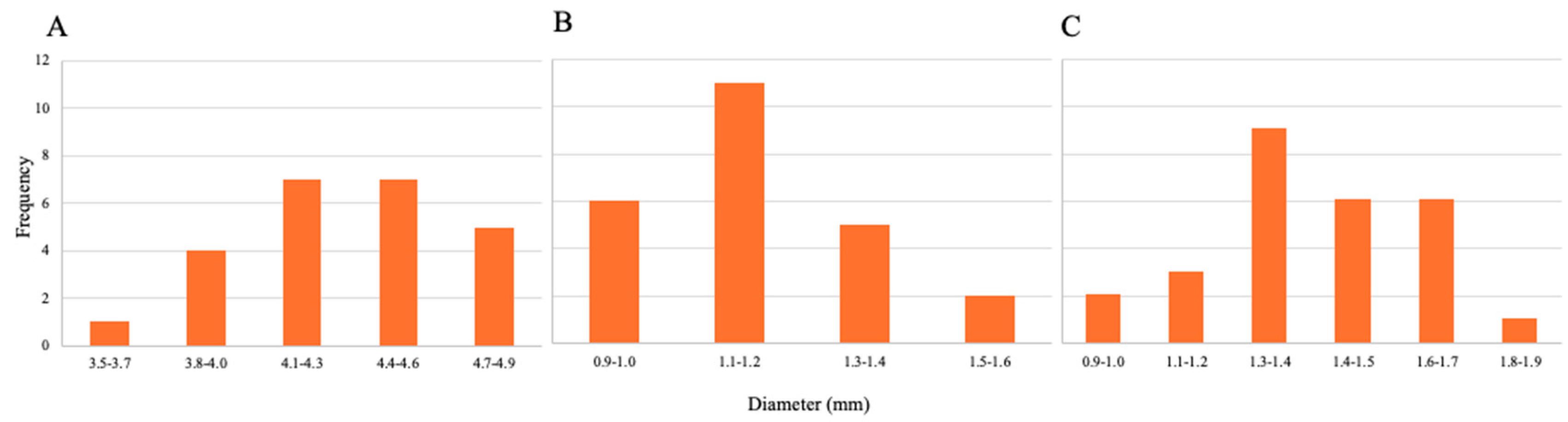
3.1.4. Diameter Size
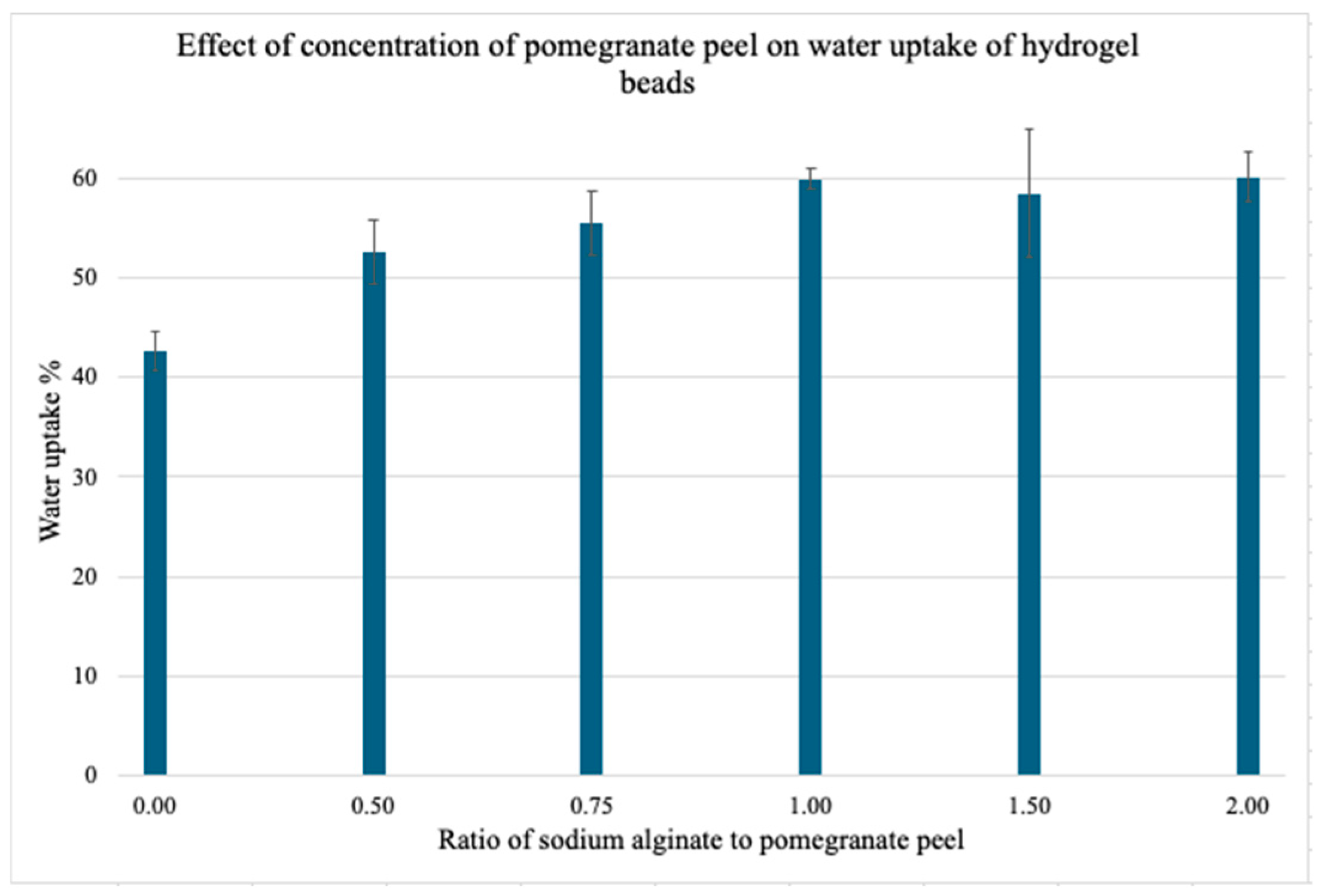
3.1.5. Effect of Pomegranate Peel Powder Concentration on Water Uptake Capacity
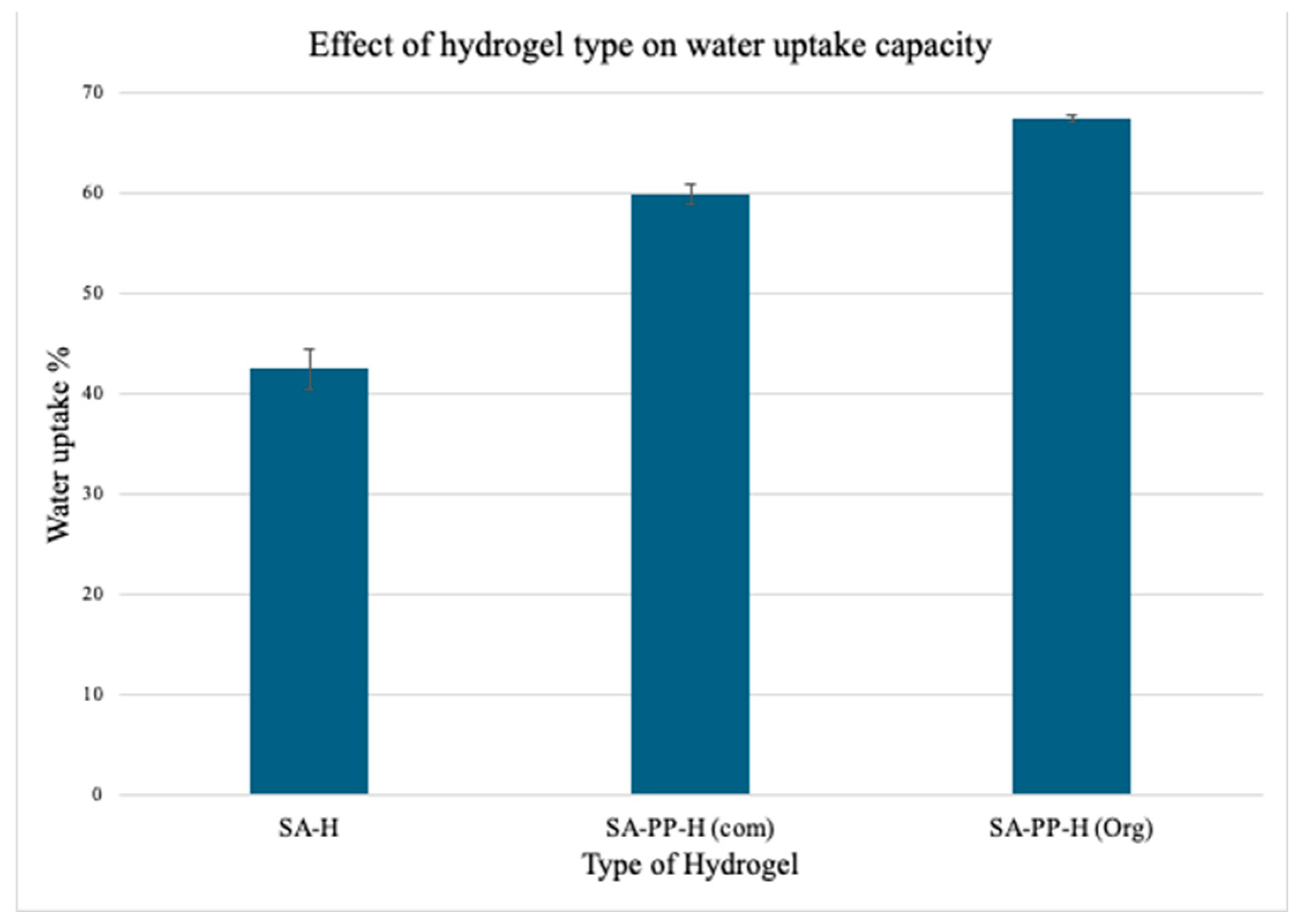
3.1.5. Water Uptake Capacity of SA-H, SA-PP-H (com) and SA-PP-H (org)
3.2. Batch Adsorption Tests for Copper and Nickel
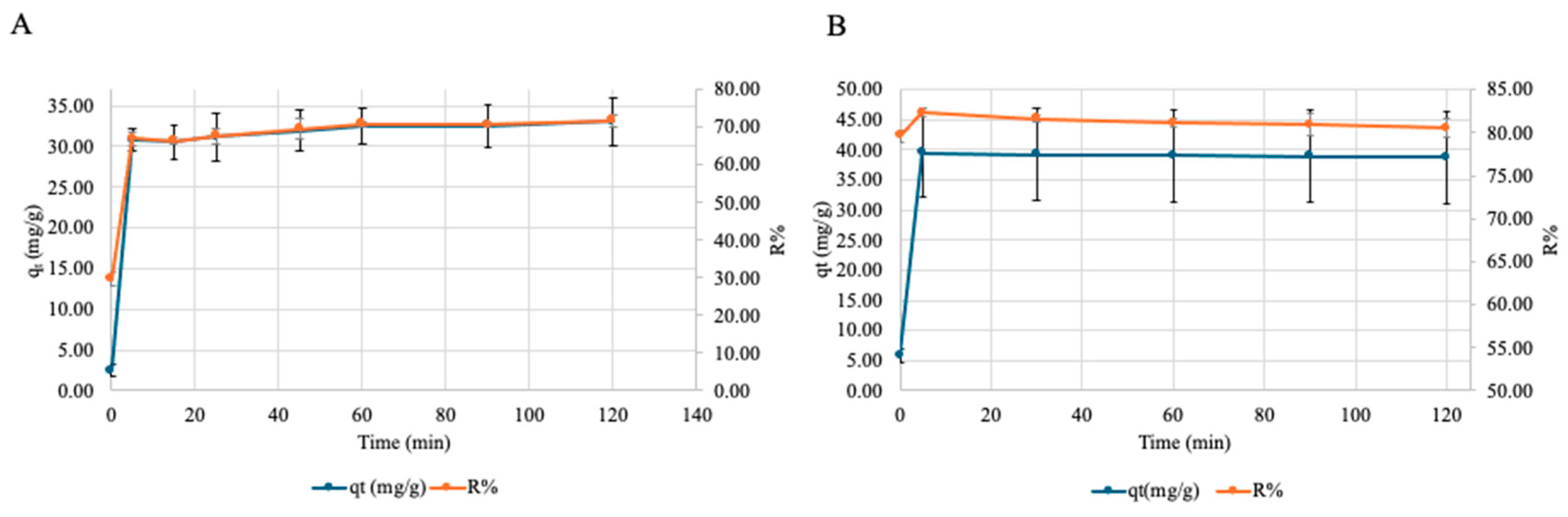
3.2.1. Effect of Contact Time on Cu and Ni Adsorption
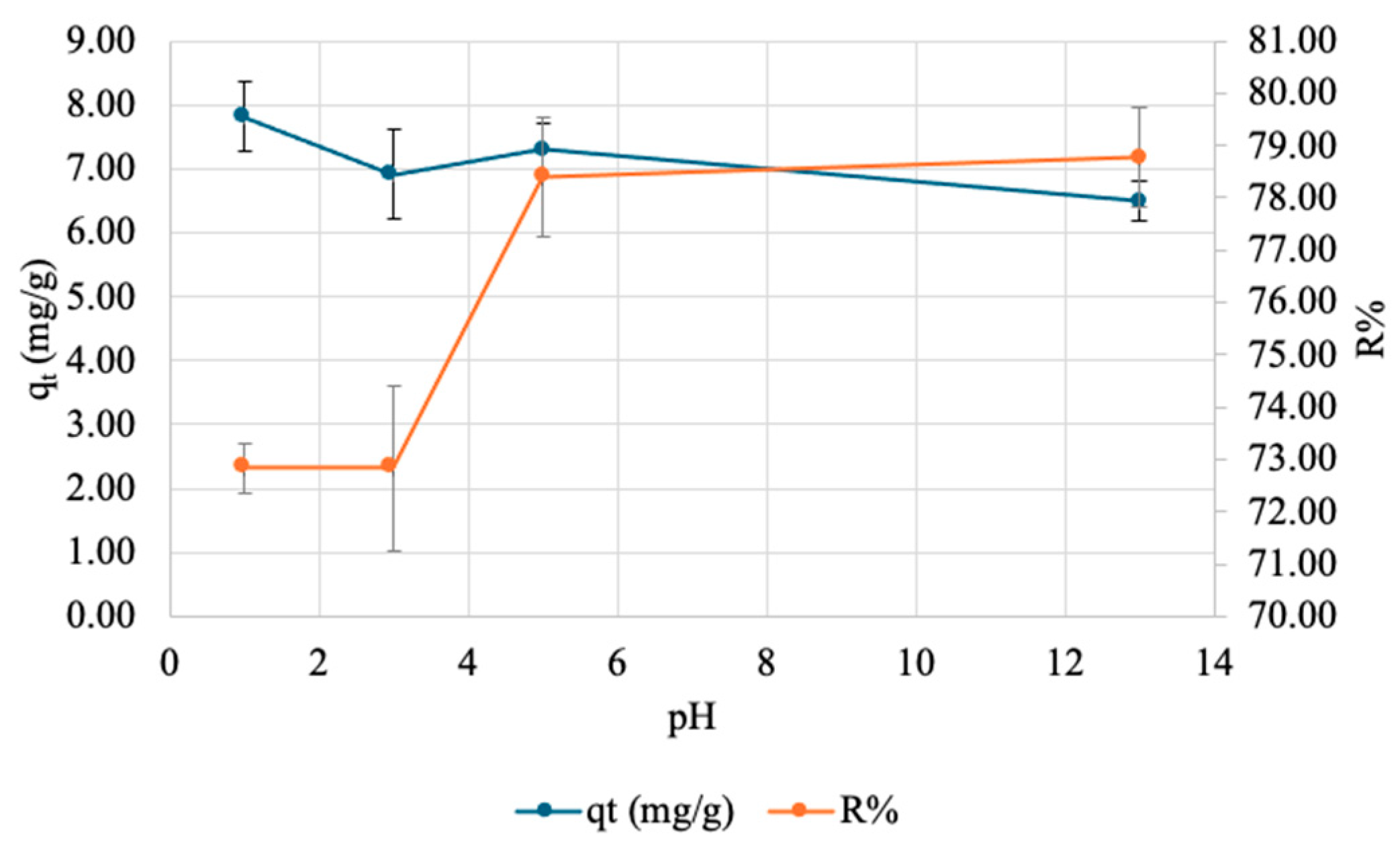
3.2.3. Effect of pH on Adsorption of Copper onto SA-PP-H (org)
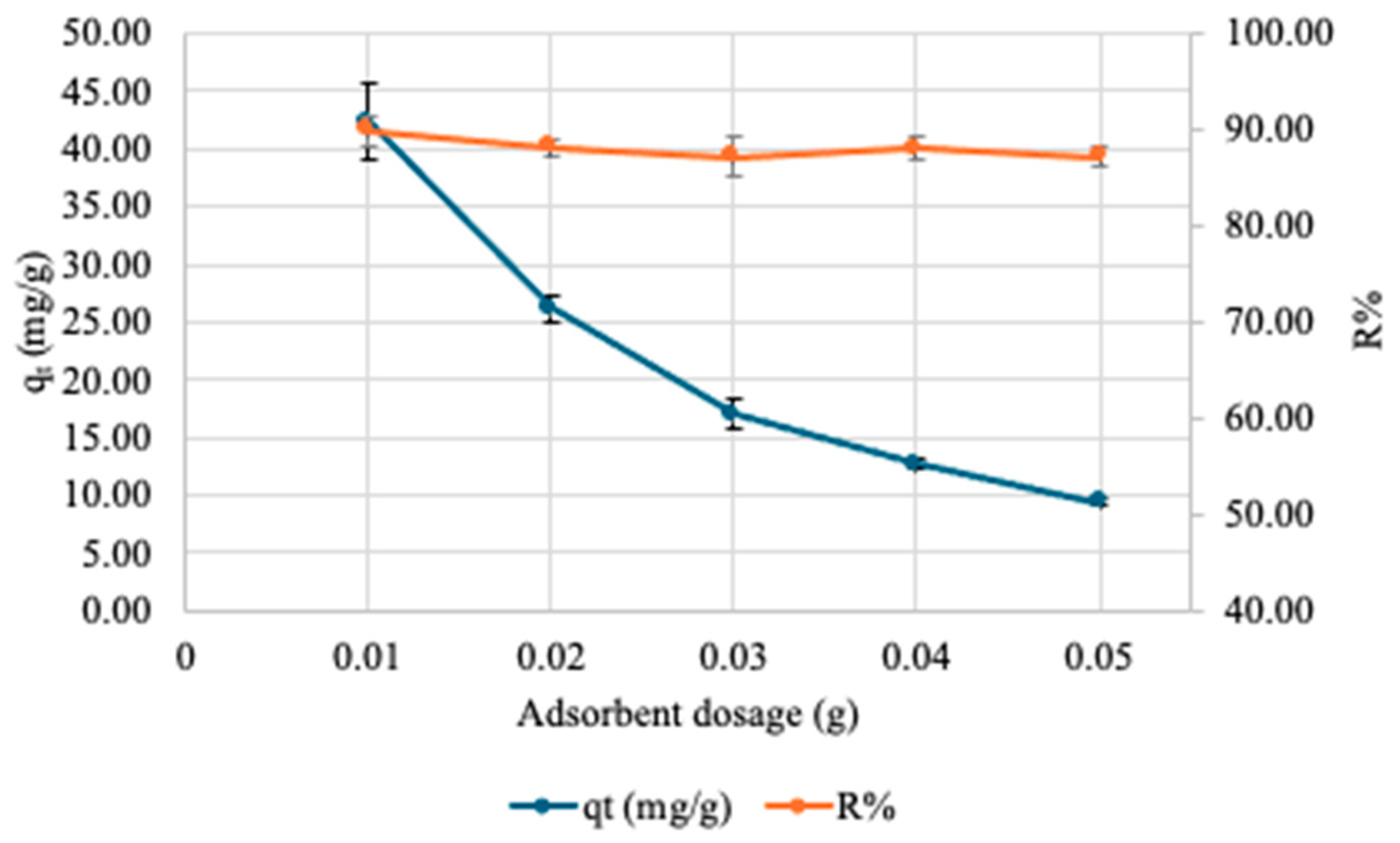
3.2.2. Effect of Adsorbate Amount on Adsorption of Cu onto SA-PP-H (org)
4. Discussion
4.1. Evaluation of Hydrogel Efficiency
4.2. Comparison to Existing Adsorbents
4.3. Future Directions
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Devi, P. I.; Manjula, M.; Bhavani, R. V. Agrochemicals, Environment, and Human Health. Annual Review of Environment and Resources 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Sumudumali, R. G. I.; Jayawardana, J. M. C. K.; Piyathilake, I. D. U. H.; Randika, J. L. P. C.; Udayakumara, E. P. N.; Gunatilake, S. K.; Malavipathirana, S. What Drives the Pesticide User Practices among Farmers in Tropical Regions? A Case Study in Sri Lanka. Environ Monit Assess 2021, 193(12), 860. [Google Scholar] [CrossRef]
- Tchounwou, P. B.; Yedjou, C. G.; Patlolla, A. K.; Sutton, D. J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Naccarato, A.; Vommaro, M. L.; Amico, D.; Sprovieri, F.; Pirrone, N.; Tagarelli, A.; Giglio, A. Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio Molitor. Toxics 2023, 11(6), 499. [Google Scholar] [CrossRef]
- Bernardini, G.; Spinelli, O.; Vismara, C.; Presutti, C.; Bolzacchini, E.; Orlandi, M.; Settimi, R. Evaluation of the Developmental Toxicity of the Pesticide Mcpa and Its Contaminants Phenol and Chlorocresol. Environmental Toxicology and Chemistry 1996, 15(5), 754–760. [Google Scholar] [CrossRef]
- The WHO Recommended Classification of Pesticides by Hazard and guidelines to classification, 2019 edition. https://www.who.int/publications/i/item/9789240005662 (accessed 2024-12-08).
- Priyashantha, A. K. H.; Mahendranathan, C. Heavy Metal Contamination and Accumulation in Groundwater and Food Crops in Sri Lanka: A Review. Vingnanam J Sci 2019, 14(2), 7. [Google Scholar] [CrossRef]
- Ding, C.; Chen, J.; Zhu, F.; Chai, L.; Lin, Z.; Zhang, K.; Shi, Y. Biological Toxicity of Heavy Metal(Loid)s in Natural Environments: From Microbes to Humans. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Indika, S.; Wei, Y.; Cooray, T.; Ritigala, T.; Jinadasa, K. B. S. N.; Weragoda, S. K.; Weerasooriya, R. Groundwater-Based Drinking Water Supply in Sri Lanka: Status and Perspectives. Water 2022, 14(9), 1428. [Google Scholar] [CrossRef]
- Coelho, F. C.; Squitti, R.; Ventriglia, M.; Cerchiaro, G.; Daher, J. P.; Rocha, J. G.; Rongioletti, M. C. A.; Moonen, A.-C. Agricultural Use of Copper and Its Link to Alzheimer’s Disease. Biomolecules 2020, 10(6), 897. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M. S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int J Environ Res Public Health 2020, 17(3), 679. [Google Scholar] [CrossRef]
- Qasem, N. A. A.; Mohammed, R. H.; Lawal, D. U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. npj Clean Water 2021, 4(1), 1–15. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and Challenges in Adsorption-Based Wastewater Remediation: A Comprehensive Review. Heliyon 2024, 10(9), e29573. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Wen, J. Study on the Harm of Waste Activated Carbon and Novel Regeneration Technology of It. 2021, 769, 022047. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120(15), 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Alsaka, L.; Alsaka, L.; Altaee, A.; Zaidi, S. J.; Zhou, J.; Kazwini, T. A Review of Hydrogel Application in Wastewater Purification. Separations 2025, 12(2), 51. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Q.; Lin, S.; Li, J. Water: The Soul of Hydrogels. Progress in Materials Science 2025, 148, 101378. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-Based Hydrogels Mediated Biomedical Applications: A Review. International Journal of Biological Macromolecules 2024, 279, 135019. [Google Scholar] [CrossRef]
- Djemaa, I. B.; Auguste, S.; Drenckhan-Andreatta, W.; Andrieux, S. Hydrogel Foams from Liquid Foam Templates: Properties and Optimisation. Advances in Colloid and Interface Science 2021, 294, 102478. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. International Journal of Biological Macromolecules 2023, 239, 124275. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Abbaz, A.; Arris, S.; Viscusi, G.; Ayat, A.; Aissaoui, H.; Boumezough, Y. Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies. Gels 2023, 9(11), 916. [Google Scholar] [CrossRef]
- Savić Gajić, I. M.; Savić, I. M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15(12), 2592. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K. Z.; Ahmed, M. M.; Akhdhar, A.; Sulaiman, M. G. M.; Khan, Z. A. Recent Advances in Alginate-Based Adsorbents for Heavy Metal Retention from Water: A Review. Desalination and Water Treatment 2022, 272, 50–74. [Google Scholar] [CrossRef]
- Jayakody, M. M.; Vanniarachchy, M. P. G.; Wijesekara, I. Composition Analysis of Selected Sri Lankan Seaweeds. Journal of Tropical Forestry and Environment 2019, 9(2). [Google Scholar] [CrossRef]
- Poudel, B. R.; Ale, D. S.; Aryal, R. L.; Ghimire, K. N.; Gautam, S. K.; Paudyal, H.; Pokhrel, M. R. Zirconium Modified Pomegranate Peel for Efficient Removal of Arsenite from Water. BIBECHANA 2022, 19(1–2), 1–13. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural Waste Peels as Versatile Biomass for Water Purification – A Review. Chemical Engineering Journal 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Preparation of Hydrogel Beads Based on Sodium Alginate and Aqueous Extract from Pomegranate Peel and Its Cyanobacteria Removal Performance. Environment Protection Engineering 2018, 44, 159–167. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Cano-Lamadrid, M.; Hernández, F.; Carbonell-Barrachina, Á. A.; Calín-Sánchez, Á. Comparison of Fresh and Commercial Pomegranate Juices from Mollar de Elche Cultivar Grown under Conventional or Organic Farming Practices. 2015, 34–44. [Google Scholar] [CrossRef]
- Bulska, E.; Ruszczyńska, A. Analytical Techniques for Trace Element Determination. Physical Sciences Reviews 2017, 2(5). [Google Scholar] [CrossRef]
- Hill, S. J.; Fisher, A. S. Atomic Absorption, Methods and Instrumentation*. In Encyclopedia of Spectroscopy and Spectrometry (Second Edition); Lindon, J. C., Ed.; Academic Press: Oxford, 1999; pp. 46–53. [Google Scholar] [CrossRef]
- Bagherian, G.; Arab Chamjangali, M.; Shariati Evari, H.; Ashrafi, M. Determination of Copper(II) by Flame Atomic Absorption Spectrometry after Its Perconcentration by a Highly Selective and Environmentally Friendly Dispersive Liquid–Liquid Microextraction Technique. Journal of Analytical Science and Technology 2019, 10(1), 1–11. [Google Scholar] [CrossRef]
- Escudero, L. A.; Blanchet, A. J.; Sombra, L. L.; Salonia, J. A.; Gasquez, J. A. Determination of the Total and Extractable Fraction of Ni in Lake Sediments and Natural Waters of San Luis (Argentina) by FAAS Using a Simple Solid Phase Extraction System. Microchemical Journal 2014, 116, 92–97. [Google Scholar] [CrossRef]
- Salih, S. I.; Oleiwi, J. K.; Mohamed, A. S. INVESTIGATION OF MECHANICAL PROPERTIES OF PMMA COMPOSITE REINFORCED WITH DIFFERENT TYPES OF NATURAL POWDERS. 2018, 13(22). [Google Scholar]
- Hashem, A.; Aniagor, C. O.; Fikry, M.; Taha, G. M.; Badawy, S. M. Characterization and Adsorption of Raw Pomegranate Peel Powder for Lead (II) Ions Removal. J Mater Cycles Waste Manag 2023, 25(4), 2087–2100. [Google Scholar] [CrossRef]
- Ben-Ali, S. Application of Raw and Modified Pomegranate Peel for Wastewater Treatment: A Literature Overview and Analysis. International Journal of Chemical Engineering 2021, 2021(1), 8840907. [Google Scholar] [CrossRef]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR Spectroscopy of the Atmosphere. I. Principles and Methods. Applied Spectroscopy Reviews 2004, 39(3), 295–363. [Google Scholar] [CrossRef]
- Magangana, T. P.; Makunga, N. P.; Fawole, O. A.; Opara, U. L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica Granatum L.) Peel Waste: A Review. Molecules 2020, 25(20), 4690. [Google Scholar] [CrossRef]
- Baines, D. 1 - Defining the Term ‘Natural’ in the Context of Food Products. In Natural food additives, ingredients and flavourings; Elsevier Ltd, 2012; pp. 1–22. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D. J.; Han, Y. Drying Cellulose Nanofibrils: In Search of a Suitable Method. Cellulose 2012, 19(1), 91–102. [Google Scholar] [CrossRef]
- Tenea, A.-G.; Dinu, C.; Rus, P. A.; Ionescu, I. A.; Gheorghe, S.; Iancu, V. I.; Vasile, G. G.; Pascu, L. F.; Chiriac, F. L. Exploring Adsorption Dynamics of Heavy Metals onto Varied Commercial Microplastic Substrates: Isothermal Models and Kinetics Analysis. Heliyon 2024, 10(15), e35364. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de La Fuente, E.; Franco, J. M.; Gallegos, C. The Importance of Understanding the Freezing Step and Its Impact on Freeze-Drying Process Performance. Journal of Pharmaceutical Sciences 2019, 108(4), 1378–1395. [Google Scholar] [CrossRef]
- Baniasadi, H.; Fathi, Z.; Lizundia, E.; Cruz, C. D.; Abidnejad, R.; Fazeli, M.; Tammela, P.; Kontturi, E.; Lipponen, J.; Niskanen, J. Development and Characterization of Pomegranate Peel Extract-Infused Carboxymethyl Cellulose Composite Films for Functional, Sustainable Food Packaging. Food Hydrocolloids 2025, 158, 110525. [Google Scholar] [CrossRef]
- Shivakumara, L. R.; Demappa, T. Synthesis and Swelling Behavior of Sodium Alginate/Poly(Vinyl Alcohol) Hydrogels. Turk J Pharm Sci 2019, 16(3), 252–260. [Google Scholar] [CrossRef] [PubMed]
- El-Ashtoukhy, E.-S. Z.; Amin, N. K.; Abdelwahab, O. Removal of Lead (II) and Copper (II) from Aqueous Solution Using Pomegranate Peel as a New Adsorbent. Desalination 2008, 223(1–3), 162–173. [Google Scholar] [CrossRef]
- Parsons, S.; Poyntz-Wright, O.; Kent, A.; McManus, M. C. Green Chemistry for Stainless Steel Corrosion Resistance: Life Cycle Assessment of Citric Acid versus Nitric Acid Passivation. Materials Today Sustainability 2019, 3–4, 100005. [Google Scholar] [CrossRef]
- Safranin O Dye content = 85 477-73-6. https://www.sigmaaldrich.com/CA/en/product/sial/s2255?srsltid=AfmBOoq82HdBgKZj2ICNSP2DMJxitDgs0TeOyEH5k6Gj9DAOEjtL0NKm (accessed 2025-01-26).
- Copper - Copper. https://www.sigmaaldrich.com/CA/en/substance/copper63557440508 (accessed 2025-01-26).
- Natarajan, R.; Saikia, K.; Ponnusamy, S. K.; Rathankumar, A. K.; Rajendran, D. S.; Venkataraman, S.; Tannani, D. B.; Arvind, V.; Somanna, T.; Banerjee, K.; Mohideen, N.; Vaidyanathan, V. K. Understanding the Factors Affecting Adsorption of Pharmaceuticals on Different Adsorbents – A Critical Literature Update. Chemosphere 2022, 287(Part 1). [Google Scholar] [CrossRef]
- Fan, Y.; Lan, H.; Qi, Z.; Liu, R.; Hu, C. Removal of Nickel and Copper Ions in Strongly Acidic Conditions by In-Situ Formed Amyloid Fibrils. Chemosphere 2022, 297(Complete). [Google Scholar] [CrossRef]
- Saadi, W.; Othman, M.; Souissi-Najar, S.; Ouederni, A. COPPER ADSORPTION ONTO POMEGRANATE PEEL ACTIVATED CARBON AS A NEW ADSORBENT. Cellulose Chemistry and Technology 2023, 57. [Google Scholar] [CrossRef]
- Saeed, H.; Chaudhry, F. S.; Rehman, S.; Rashid, Z.; Ijaz, A.; Awan, J. A. Removal of Toxic Metallic Ions Cr(VI), Cu(II), Ni(II), Co(II) and Cd(II) from Waste Water Effluents of Tanneries by Using Punica Granatum (Pomgranate) Membrane. Iranica Journal of Energy & Environment 2016, 7(1), 52–57. [Google Scholar] [CrossRef]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B. M.; Saccone, S.; Mazzei, V.; Brundo, M. V. Toxic Effects of Zinc Chloride on the Bone Development in Danio Rerio (Hamilton, 1822). Front Physiol 2016, 7, 153. [Google Scholar] [CrossRef]
- Nashira, A.; Istiqomah, I.; Putri, A. N.; Badari, M. F. Carbon Footprint Analysis on Phosphoric Acid Production Using Wet Process at PT Petrokimia Gresik. E3S Web of Conf. 2024, 517, 08005. [Google Scholar] [CrossRef]
- Abbasi, Z.; Alikarami, M.; Homafar, A. Adsorption Study on Pomegranate Peel: Removal of Ni 2+ and Co 2+ from Aqueous Solution. Inorganic CHEMISTRY 2013. [Google Scholar]
- Nickel - Nickel. https://www.sigmaaldrich.com/CA/en/substance/nickel58697440020 (accessed 2025-02-15).
- Bhatnagar, A.; Minocha, A. K. Biosorption Optimization of Nickel Removal from Water Using Punica Granatum Peel Waste. Colloids and surfaces, B, Biointerfaces 2010, 76(2), 544–548. [Google Scholar] [CrossRef] [PubMed]
- Cuppett, J.; Duncan, S.; Dietrich, A. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chemical senses 2006, 31, 689–697. [Google Scholar] [CrossRef] [PubMed]

| Hydrogel type | Appearance | |
| Colour (wet, dry, lyophilized) | Shape | |
| SA-H | White, off-white, off white | Circular |
| SA-PP-H (com) | Dark brown, black, black | Circular |
| SA-PP-H (org) | Light brown, light brown, light brown | Circular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





