Submitted:
11 April 2025
Posted:
14 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
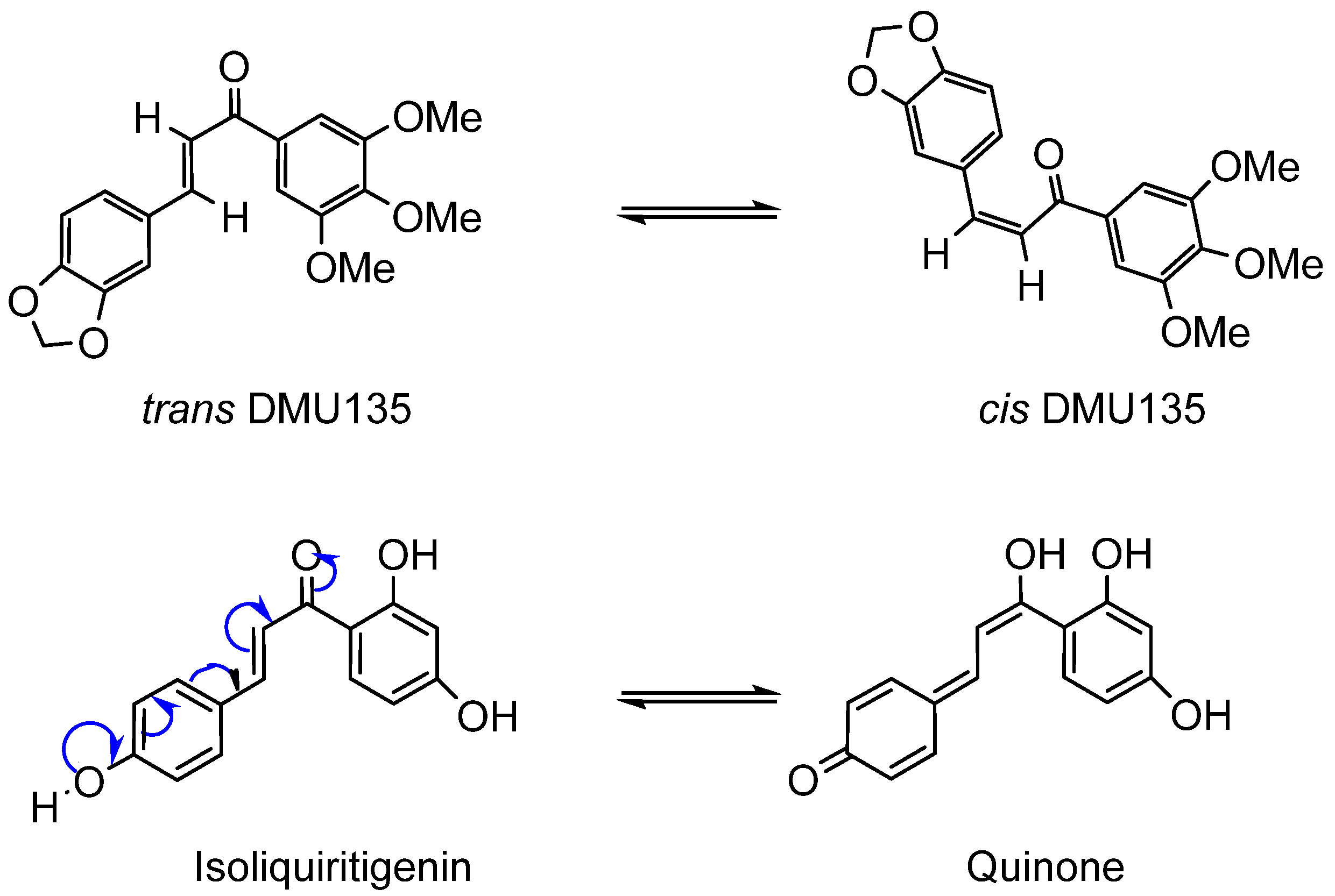
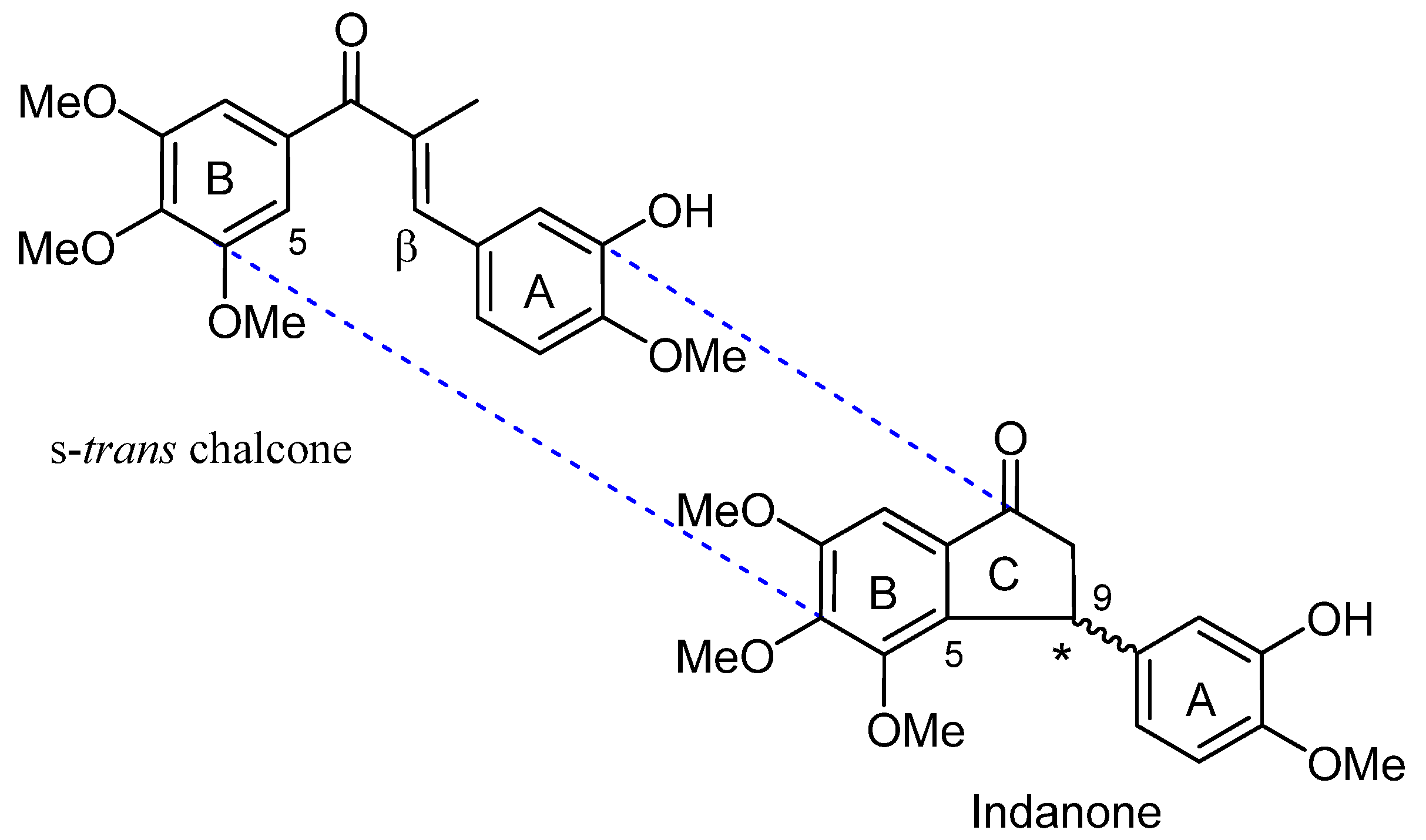
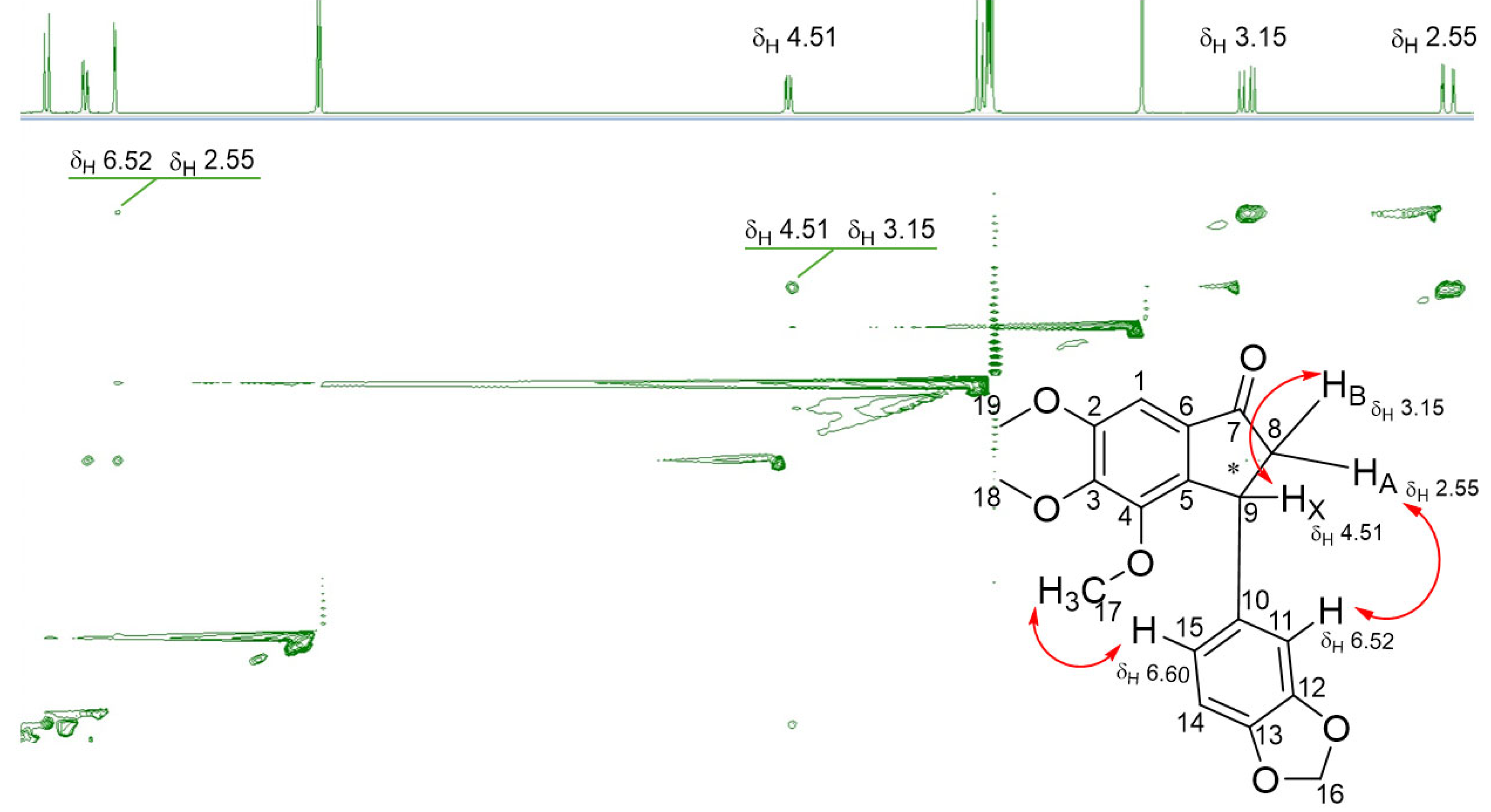
2. Results and Discussion
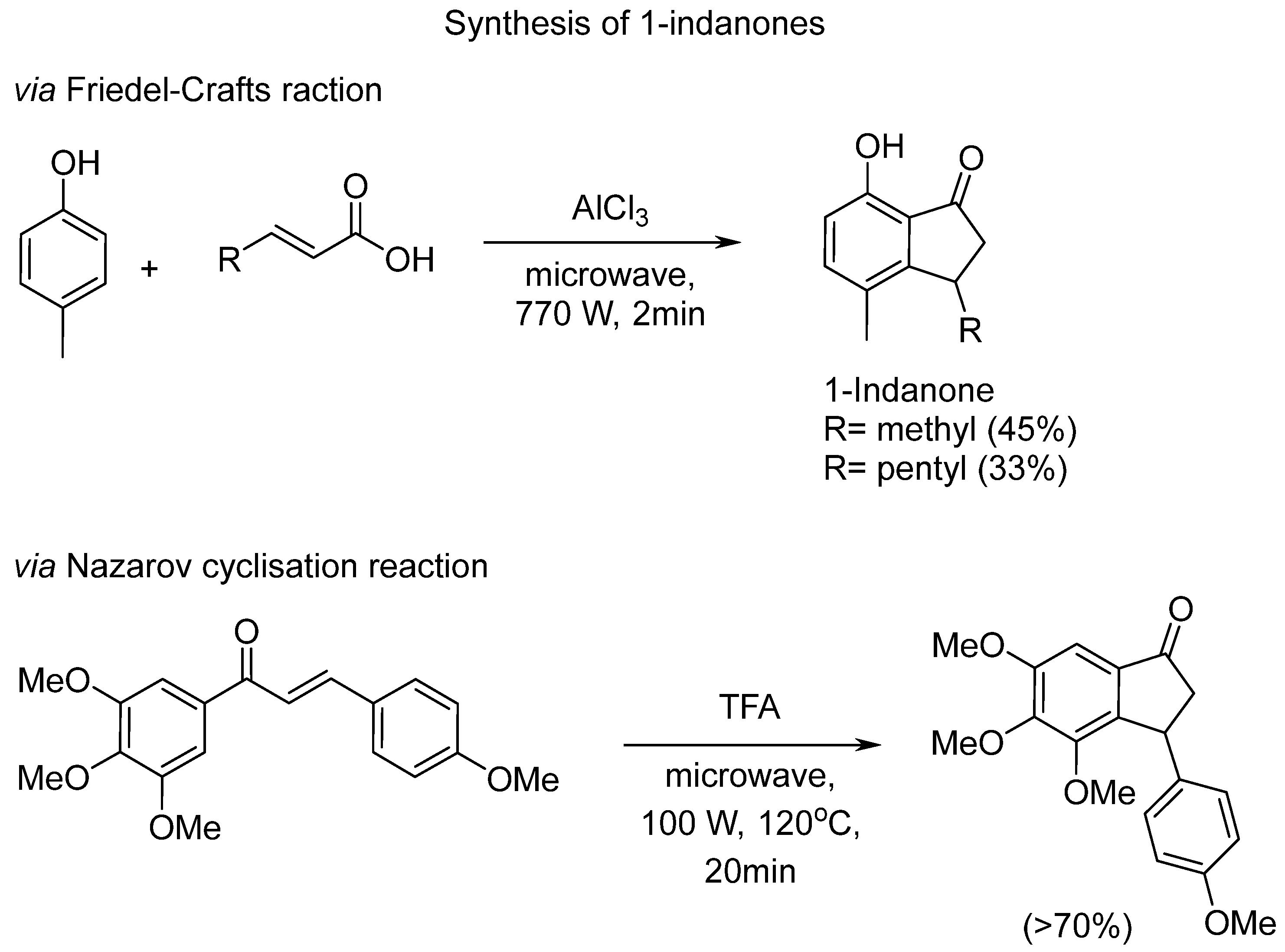
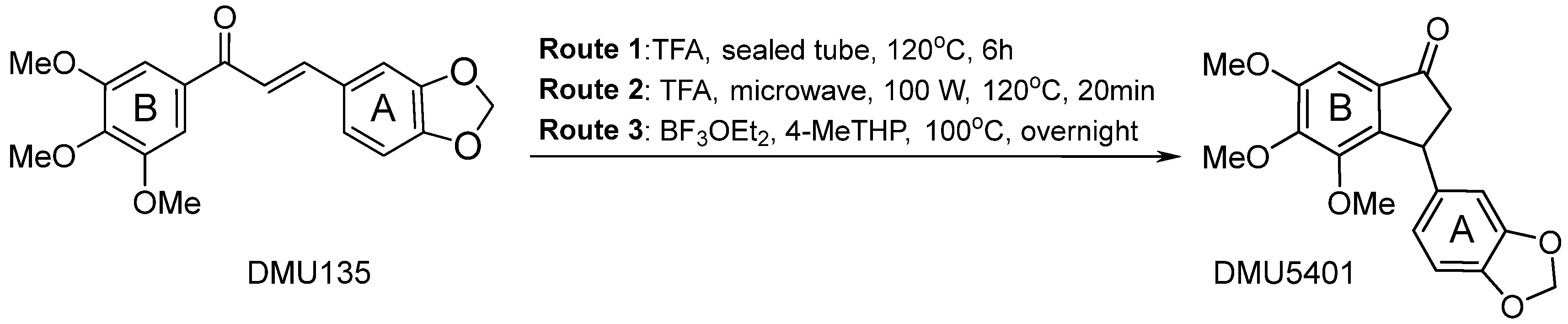
4. Materials and Methods
General Chemistry Information
General Procedure to the Preparation of Chalcone DMU135
Procedures to the Preparation of Indanone DMU5401
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziafor, K.; Ruparelia, K.; Moulds, B.; Zloh, M.; Parish, T.; Brucoli, F. Design and Synthesis of Pyridyl and 2-Hydroxyphenyl Chalcones with Antitubercular Activity. Molecules. 2024, 29, 4539. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C., Zhang, W., Sheng, C., Zhang, W., Xing, C.,; Miao. Chalcone: A Privileged Structure in Medicinal Chemistry. Chemical Reviews 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, D.; Nikolic, D.; Zhu, D.; Pezzuto, J.M.; van Breemen, R.B. In vitro metabolism of isoliquiritigenin by human liver microsomes. Drug metabolism and disposition 2008, 36, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Nishino, T.; Inoue, H.; Nagata, N.; Satomi, Y.; Nishino, H.; Shibata, S. Antitumorigenic activities of chalcones (II). Photo-isomerization of chalcones and the correlation with their biological activities. Biological and Pharmaceutical Bulletin 1997, 20, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Kuo, P.L.; Lin, C.C. Isoliquiritigenin induces apoptosis and cell cycle arrest through p53-dependent pathway in Hep G2 cells. Life Sciences 2005, 77, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Sale, S.; Tunstall, R.G.; Ruparelia, K.C.; Butler, P.C.; Potter, G.A.; Steward, W.P.; Gescher, A.J. Effects of the potential chemopreventive agent DMU-135 on adenoma development in the Apc Min+ mouse. Investigational new drugs. 2006, 459–64. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorganic & Medicinal Chemistry Letters 1998, 1051–1056. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. European Journal of Medicinal Chemistry 2017, 138, 182–198. [Google Scholar] [CrossRef] [PubMed]
- da Camera e Silva, E.; Figueroa-Villar, J.D.; de Aguiar, A.P. Preparation of 1-indanones with conventional heating versus microwaves. Synthetic Communications, 2002, 32, 3193–3198. [Google Scholar] [CrossRef]
- Muckensturm, B.; Diyani, F. An improved preparation of 7-hydroxyindan-1-ones. Journal of Chemical Research-S 1995, 442–443. [Google Scholar] [CrossRef]
- Turek, M.; Szczęsna, D.; Koprowski, M.; Bałczewski, P. Synthesis of 1-indanones with a broad range of biological activity. Beilstein Journal of Organic Chemistry 2017, 13, 451–494. [Google Scholar] [CrossRef] [PubMed]
- Prakasham, A.P.; Saxena, A.K.; Luqman, S.; Chanda, D.; Kaur, T.; Gupta, A.; Yadav, D.K.; Chanotiya, C.S.; Shanker, K.; Khan, F.; Negi, A.S. Synthesis and anticancer activity of 2-benzylidene indanones through inhibiting tubulin polymerization. Bioorganic & Medicinal Chemistry 2012, 20, 3049–3057. [Google Scholar] [CrossRef]
- Lawrence N.J., Armitage E.S.M., Greedy B., Cook D., Ducki S., McGown A.T. The synthesis of indanones related to combretastatin A-4 via microwave-assisted Nazarov cyclization of chalcones. Tetrahedron letters 2006, 47, 1637–1640. [Google Scholar] [CrossRef]
- Gu, X.H.; Yu, H.; Jacobson, A.E.; Rothman, R.B.; Dersch, C.M.; George, C.; Flippen-Anderson, J.L.; Rice, K.C. Design, synthesis, and monoamine transporter binding site affinities of methoxy derivatives of indatraline. Journal of medicinal chemistry. 2000, 43, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi S., Tamura T., Yoshimoto S., Kawakami T., Masuyama A. 4-Methyltetrahydropyran (4-MeTHP): Application as an Organic Reaction Solvent. Chemistry–An Asian Journal 2019, 14, 3921–3937. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, K.C.; Zeka, K.; Ijaz, T.; Ankrett, D.N.; Wilsher, N.E.; Butler, P.C.; Tan, H.L.; Lodhi, S.; Bhambra, A.S.; Potter, G.A.; Arroo, R.R. The synthesis of chalcones as anticancer prodrugs and their bioactivation in CYP1 expressing breast cancer cells. Medicinal Chemistry 2018, 14, 322–332. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




