Submitted:
11 April 2025
Posted:
15 April 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Results
2.1. Characterization of Antibodies and Epitope Peptides
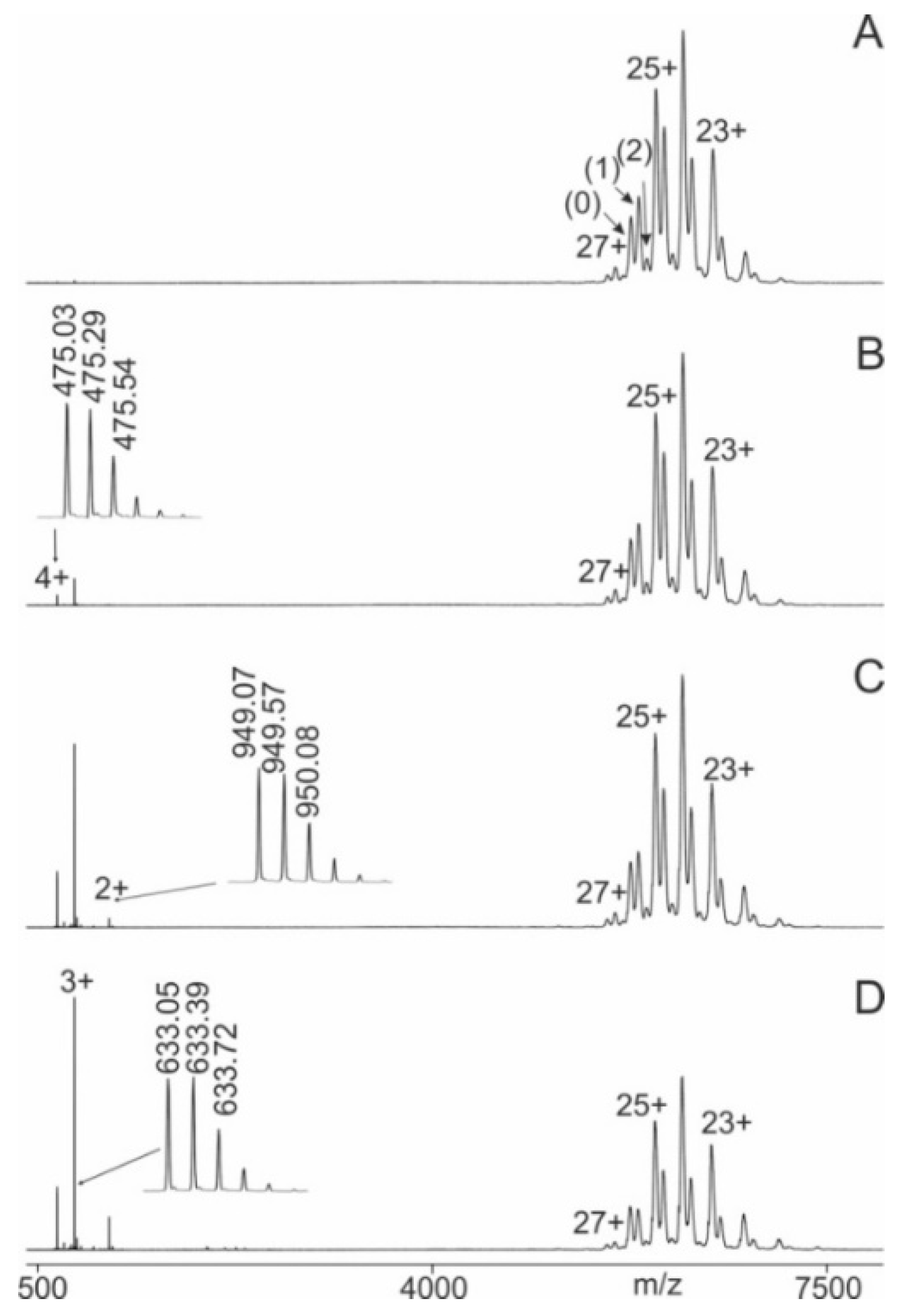
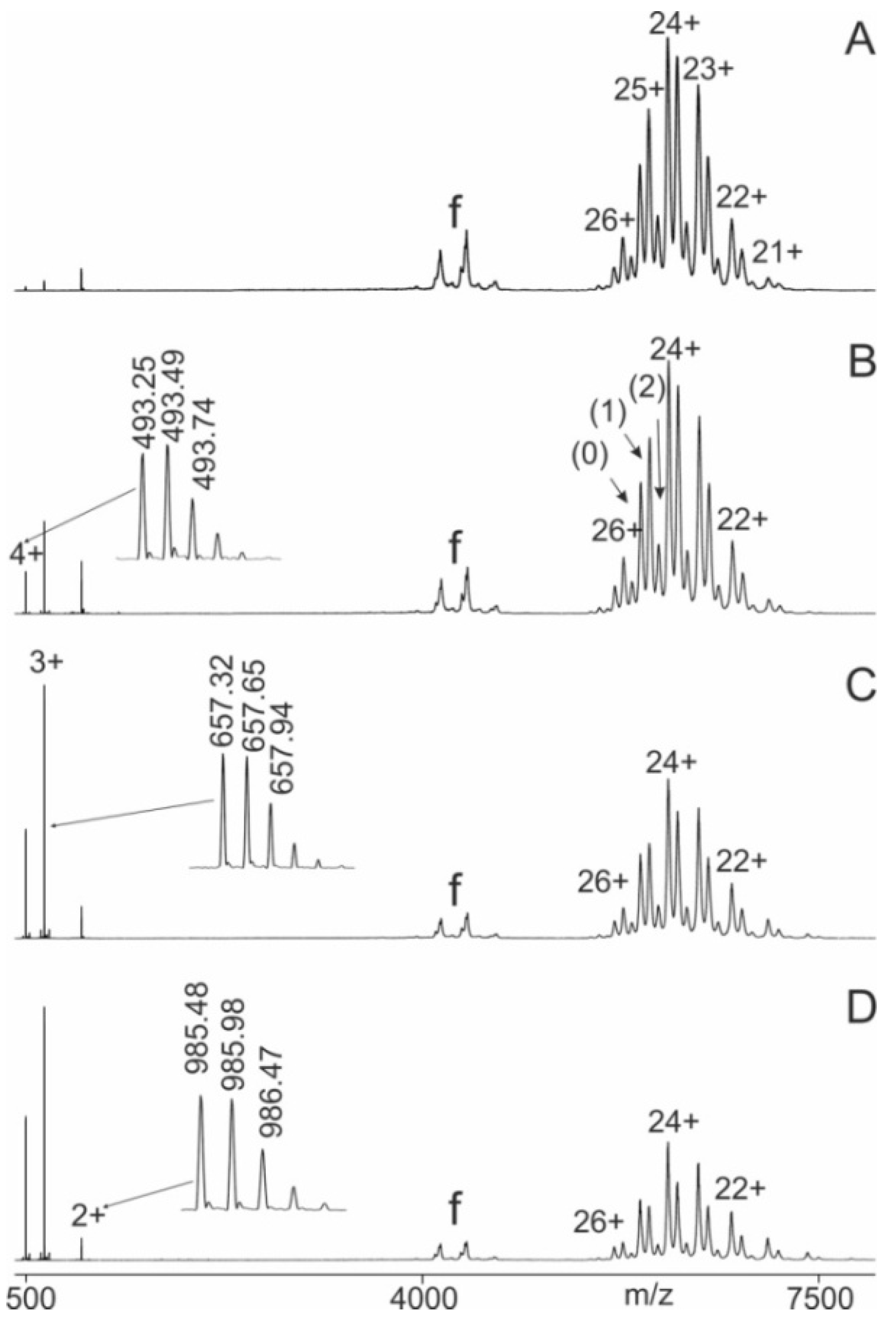
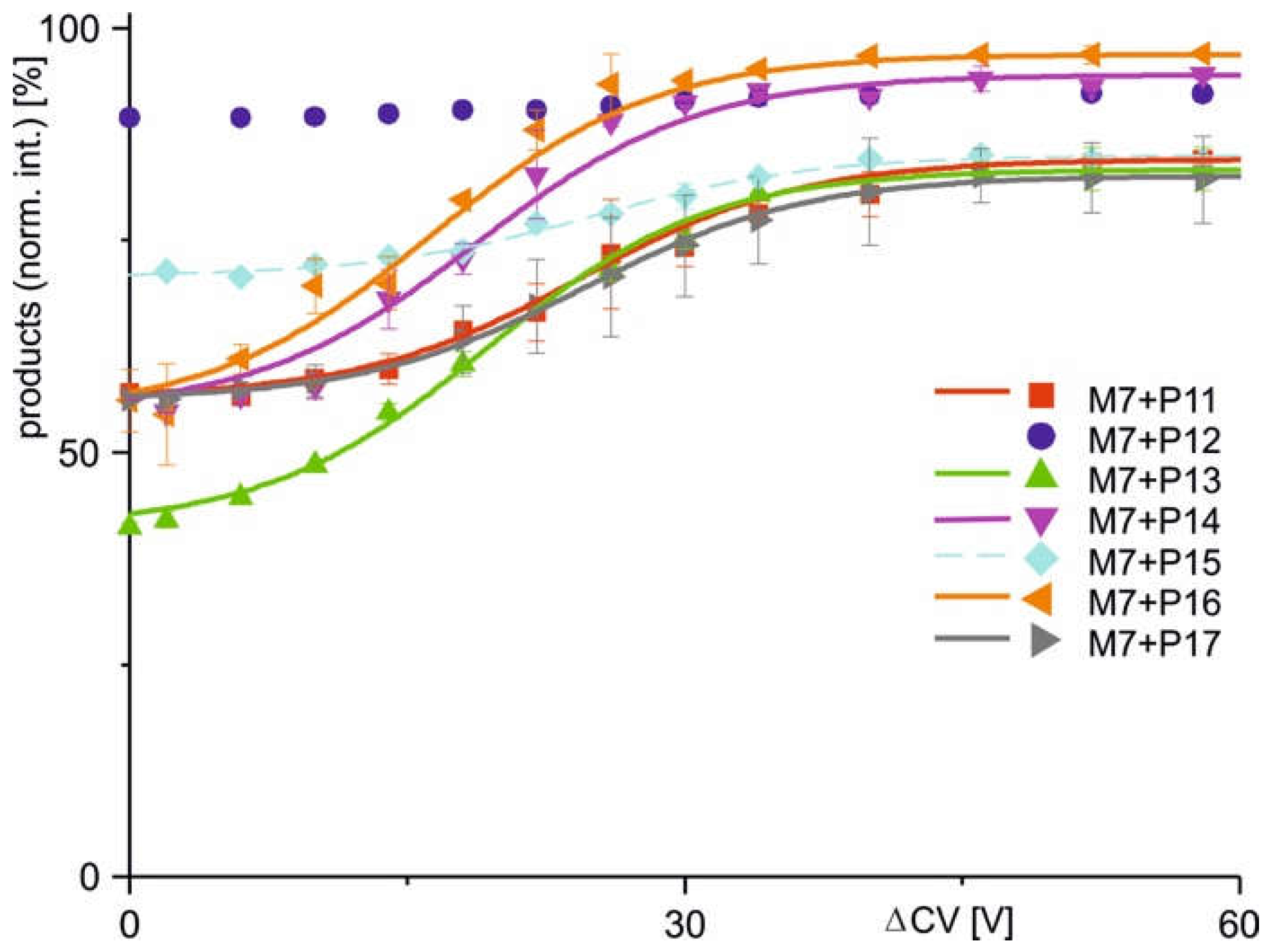
2.2. Binding Strength Analysis by ITEM-FOUR Mass Spectrometry
2.3. Binding Motif Deduction
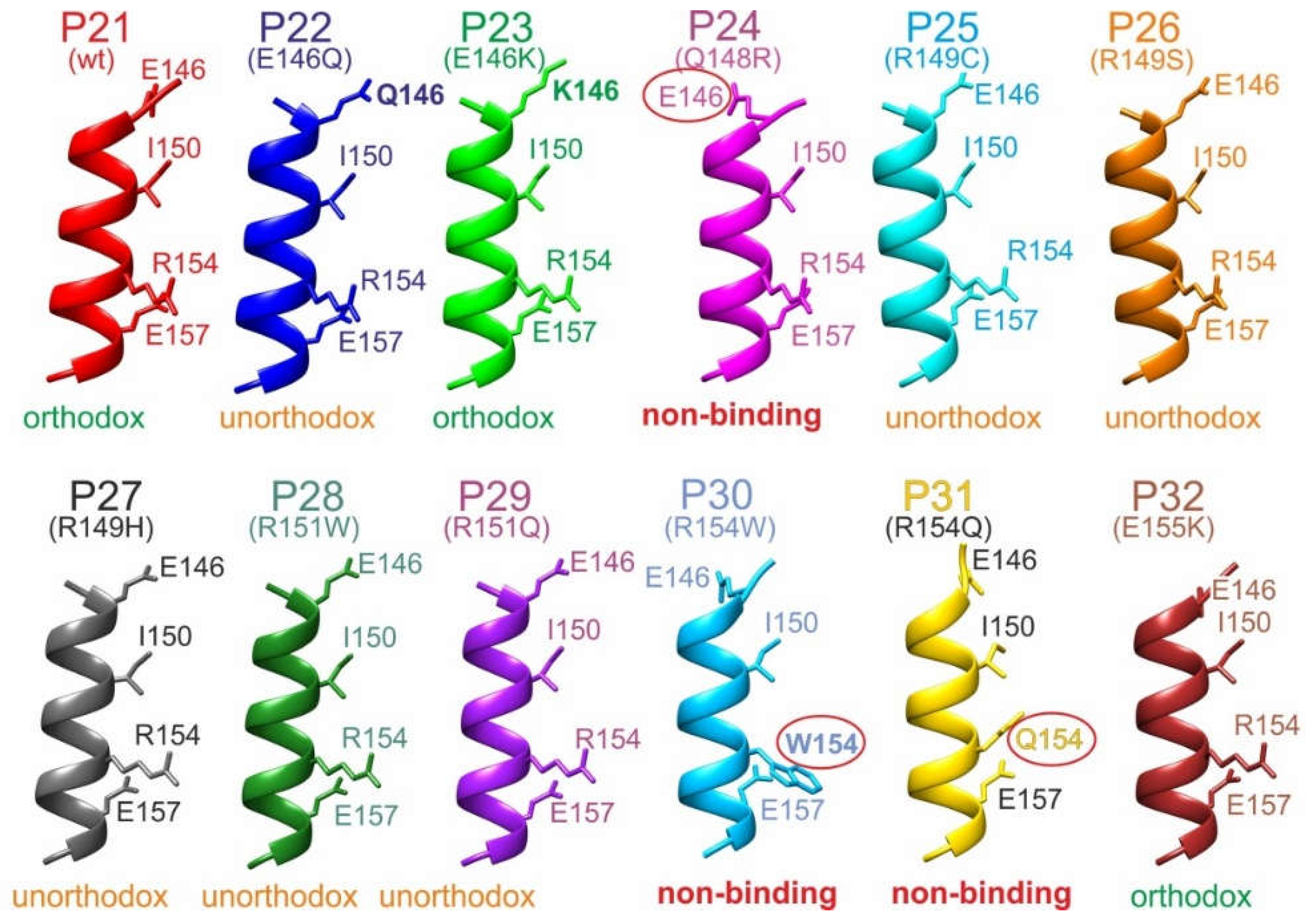
3. Discussion
4. Materials and Methods
4.1. Preparation of Solutions with Peptides, Antibodies, and Antibody – Peptide Complexes
4.2. Protein and Peptide Concentration Determination
4.3. SDS-PAGE Analysis of the hcTnT Antigen
4.4. Western Blot Analysis of the Anti-hcTnT Antibodies
4.5. Mass Calibration of Mass Spectrometry Instruments
4.6. Preparation of nanoESI–MS Emitters, Filling and Mounting
4.7. Q-ToF 2 Instrument Settings and Data Acquisition
4.8. Synapt G2S Instrument Settings and Data Acquisition
4.9. ITEM-FOUR Spectral Data Analysis
4.10. ITEM-FOUR Calculations of Apparent Kinetic and Apparent Thermodynamic Values
4.11. Molecular Modeling of Protein and Peptide Structures
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Å | Ångström |
| ACC | American College of Cardiology |
| antiTNFα | anti-tumor necrosis factor alpha |
| BSA | bovine serum albumin |
| cSNP | coding single nucleotide polymorphism |
| cTnT | cardiac troponin T |
| DCM | dilative cardiomyopathy |
| dbSNP | single nucleotide polymorphism database |
| ELISA | enzyme-linked immunosorbent assay |
| ESC | European Society for Cardiology |
| ESI | electrospray-ionization |
| HCM | hypertrophic cardiomyopathy |
| hcTNT | human cardiac troponin T |
| hs | high sensitive |
| ITEM | Intact Transition Epitope Mapping |
| ITEM FOUR | Intact Transition Epitope Mapping - Force Differences between Original and Unusual Residues |
| kDa | kiloDalton |
| MI | myocardial infarction |
| m/z | mass to charge ratio |
| nanoESI-MS | nano electrospray ionization-mass spectrometry |
| PBS | phosphate buffered saline |
| POC | point-of-care |
| PRIDE | PRoteomics IDEntification |
| PVDF | poly vinyliden fluoride |
| Q-ToF | quadrupole-time of flight |
| RCM | restrictive cardiomyopathy |
| rpm | revolutions per minute |
| RIPA | radio immunoprecipitation assay |
| SDC | sodium deoxy cholate |
| SDS | sodium dodecyl sulfate |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SNP | single nucleotide polymorphism |
| tif | tagged image file |
| Tn | troponin |
| TnI | troponin I |
| TNNT2 | troponin T gene |
| TnT | troponin T |
| TRIS | tris (hydroxyl methyl) amino methane |
| ∆CV | voltage difference in the collision cell |
| 3D | three-dimensional |
References
- Dwivedi, S.; Purohit, P.; Misra, R.; Pareek, P.; Goel, A.; Khattri, S.; Pant, K.K.; Misra, S.; Sharma, P. Diseases and Molecular Diagnostics: A Step Closer to Precision Medicine. Indian J Clin Biochem 2017, 32, 374–398. [Google Scholar] [CrossRef]
- Jeremias, A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vascular Health and Risk Management 2010, 691. [Google Scholar] [CrossRef]
- Mingels, A.M.; Mills, N.L.; Mueller, C. Cardiac troponin T and I: back to basics. Eur Heart J Acute Cardiovasc Care 2023, 12, 631–632. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Januzzi, J.L., Jr. Clinical applications of highly sensitive troponin assays. Cardiol Rev 2010, 18, 12–19. [Google Scholar] [CrossRef]
- Mair, J.; Hammarsten, O. Potential analytical interferences in cardiac troponin immunoassays. Journal of Laboratory and Precision Medicine 2023, 8, 12–12. [Google Scholar] [CrossRef]
- von Meyer, A.; Albert, G.; Kunzelmann, S.; Rank, C.; Zerback, R.; Imdahl, R. Evaluating the performance of an updated high-sensitivity troponin T assay with increased tolerance to biotin. Clin Chem Lab Med 2021, 59, 591–597. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G. Genetic polymorphisms of human cardiac troponins as an unrecognized challenge for diagnosing myocardial injury. Int J Cardiol 2014, 171, 467–470. [Google Scholar] [CrossRef]
- Antontseva, E.V.; Degtyareva, A.O.; Korbolina, E.E.; Damarov, I.S.; Merkulova, T.I. Human-genome single nucleotide polymorphisms affecting transcription factor binding and their role in pathogenesis. Vavilovskii Zhurnal Genet Selektsii 2023, 27, 662–675. [Google Scholar] [CrossRef]
- Manolio, T.A.; Brooks, L.D.; Collins, F.S. A HapMap harvest of insights into the genetics of common disease. J Clin Invest 2008, 118, 1590–1605. [Google Scholar] [CrossRef]
- Alwi, Z.B. The Use of SNPs in Pharmacogenomics Studies. Malays J Med Sci 2005, 12, 4–12. [Google Scholar]
- Li, X.; Luo, R.; Gu, H.; Deng, Y.; Xu, X.; Wu, X.; Hua, W. Cardiac troponin T (TNNT2) mutations in chinese dilated cardiomyopathy patients. Biomed Res Int 2014, 2014, 907360. [Google Scholar] [CrossRef]
- Myers, M.C.; Wang, S.; Zhong, Y.; Maruyama, S.; Bueno, C.; Bastien, A.; Fazeli, M.S.; Golchin, N. Prevalence of Genetically Associated Dilated Cardiomyopathy: A Systematic Literature Review and Meta-Analysis. Cardiology Research 2024, 15, 233–245. [Google Scholar] [CrossRef]
- Glavaški, M.; Velicki, L.; Vučinić, N. Hypertrophic Cardiomyopathy: Genetic Foundations, Outcomes, Interconnections, and Their Modifiers. Medicina 2023, 59, 1424. [Google Scholar] [CrossRef]
- Fitzgerald, R.L.; Hollander, J.E.; Peacock, W.F.; Limkakeng, A.T.; Breitenbeck, N.; Rivers, E.J.; Ziegler, A.; Laimighofer, M.; deFilippi, C. The 99th percentile upper reference limit for the 5th generation cardiac troponin T assay in the United States. Clin Chim Acta 2020, 504, 172–179. [Google Scholar] [CrossRef]
- Macht, M.; Fiedler, W.; Kürzinger, K.; Przybylski, M. Mass spectrometric mapping of protein epitope structures of myocardial infarct markers myoglobin and troponin T. Biochemistry 1996, 35, 15633–15639. [Google Scholar] [CrossRef]
- Röwer, C.; Ortmann, C.; Neamtu, A.; El-Kased, R.F.; Glocker, M.O. Intact Transition Epitope Mapping-Force Differences between Original and Unusual Residues (ITEM-FOUR). Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Yefremova, Y.; Danquah, B.D.; Opuni, K.F.; El-Kased, R.; Koy, C.; Glocker, M.O. Mass spectrometric characterization of protein structures and protein complexes in condensed and gas phase. Eur J Mass Spectrom (Chichester) 2017, 23, 445–459. [Google Scholar] [CrossRef]
- Danquah, B.D.; Opuni, K.F.M.; Roewer, C.; Koy, C.; Glocker, M.O. Mass Spectrometric Analysis of Antibody-Epitope Peptide Complex Dissociation: Theoretical Concept and Practical Procedure of Binding Strength Characterization. Molecules 2020, 25, 4776. [Google Scholar] [CrossRef]
- Morris, C.B.; Poland, J.C.; May, J.C.; McLean, J.A. Fundamentals of Ion Mobility-Mass Spectrometry for the Analysis of Biomolecules. Methods Mol Biol 2020, 2084, 1–31. [Google Scholar] [CrossRef]
- Nibbering, N.M.M. The role of mass spectrometric methods in ionic reaction mechanistic studies. International Journal of Mass Spectrometry 2000, 200, 27–42. [Google Scholar] [CrossRef]
- Mehmood, S.; Allison, T.M.; Robinson, C.V. Mass spectrometry of protein complexes: from origins to applications. Annu Rev Phys Chem 2015, 66, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Christ, P.; Rutzinger, S.; Seidel, W.; Uchaikin, S.; Pro, F.; Koy, C.; Glocker, M.O. High detection sensitivity achieved with cryogenic detectors in combination with matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Eur J Mass Spectrom (Chichester) 2004, 10, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Scherf, M.; Danquah, B.D.; Koy, C.; Lorenz, P.; Steinbeck, F.; Neamtu, A.; Thiesen, H.J.; Glocker, M.O. Epitope Fine Mapping by Mass Spectrometry: Investigations of Immune Complexes Consisting of Monoclonal Anti-HpTGEKP Antibody and Zinc Finger Protein Linker Phospho-Hexapeptides. ChemBioChem 2022, 23. [Google Scholar] [CrossRef]
- Hager-Braun, C.; Tomer, K.B. Determination of protein-derived epitopes by mass spectrometry. Expert Review of Proteomics 2005, 2, 745–756. [Google Scholar] [CrossRef]
- Röwer, C.; Ortmann, C.; Neamtu, A.; El-Kased, R.F.; Glocker, M.O. Intact Transition Epitope Mapping—Force Differences between Original and Unusual Residues (ITEM-FOUR). Biomolecules 2023, 13, 187. [Google Scholar] [CrossRef]
- Al-Majdoub, M.; Koy, C.; Lorenz, P.; Thiesen, H.J.; Glocker, M.O. Mass spectrometric and peptide chip characterization of an assembled epitope: analysis of a polyclonal antibody model serum directed against the Sjøgren/systemic lupus erythematosus autoantigen TRIM21. Journal of Mass Spectrometry 2013, 48, 651–659. [Google Scholar] [CrossRef]
- Al-Majdoub, M.; Opuni, K.; Koy, C.; Glocker, M. Facile fabrication and instant application of miniaturized antibody-decorated affinity columns for higher-order structure and functional characterization of TRIM21 epitope peptides. Anal. Chem. (Washington, DC, U. S.) 2013, 85, 10479–10487. [Google Scholar] [CrossRef]
- Opuni, K.F.; Al-Majdoub, M.; Yefremova, Y.; El-Kased, R.F.; Koy, C.; Glocker, M.O. Mass spectrometric epitope mapping. Mass Spectrom. Rev 2018, 37, 229–241. [Google Scholar] [CrossRef]
- Hoffmüller, U.; Knaute, T.; Hahn, M.; Höhne, W.; Schneider-Mergener, J.; Kramer, A. Evolutionary transition pathways for changing peptide ligand specificity and structure. Embo j 2000, 19, 4866–4874. [Google Scholar] [CrossRef]
- Yefremova, Y.; Opuni, K.F.-M.; Danquah, B.D.; Thiesen, H.-J.; Glocker, M.O. Intact Transition Epitope Mapping (ITEM). J. Am. Soc. Mass Spectrom. 2017, 28, 1612–1622. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.L.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic Cardiomyopathy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Li, Q.; Wang, Q.; Zhou, X.; Chen, J.; Chen, X.; Bellou, A.; Zhuang, J.; Lei, L. Cardiomyopathy: pathogenesis and therapeutic interventions. MedComm (2020) 2024, 5, e772. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Luo, R.; Gu, H.; Zhang, C.; Zhang, Y.; Hui, R.; Wu, X.; Hua, W. TNNT2 gene polymorphisms are associated with susceptibility to idiopathic dilated cardiomyopathy in the Han Chinese population. Biomed Res Int 2013, 2013, 201372. [Google Scholar] [CrossRef]
- Rani, D.S.; Dhandapany, P.S.; Nallari, P.; Narasimhan, C.; Thangaraj, K. A novel arginine to tryptophan (R144W) mutation in troponin T (cTnT) gene in an indian multigenerational family with dilated cardiomyopathy (FDCM). PLoS One 2014, 9, e101451. [Google Scholar] [CrossRef]
- ESC Guidelines for the management of cardiomyopathies. 2023.
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149. [Google Scholar] [CrossRef]
- Kockum, I.; Huang, J.; Stridh, P. Overview of Genotyping Technologies and Methods. Curr Protoc 2023, 3, e727. [Google Scholar] [CrossRef]
- Grant, P.; Langlois, S.; Lynd, L.D.; Austin, J.C.; Elliott, A.M. Out-of-pocket and private pay in clinical genetic testing: A scoping review. Clin Genet 2021, 100, 504–521. [Google Scholar] [CrossRef]
- Dhawan, A.P. Collaborative Paradigm of Preventive, Personalized, and Precision Medicine With Point-of-Care Technologies. IEEE J Transl Eng Health Med 2016, 4, 2800908. [Google Scholar] [CrossRef]
- Glocker, M.O.; Guthke, R.; Kekow, J.; Thiesen, H.J. Rheumatoid arthritis, a complex multifactorial disease: on the way toward individualized medicine. Med Res Rev 2006, 26, 63–87. [Google Scholar] [CrossRef]
- Koy, C.; Opuni, K.F.M.; Danquah, B.D.; Neamtu, A.; Glocker, M.O. Mass Spectrometric and Bio-Computational Binding Strength Analysis of Multiply Charged RNAse S Gas-Phase Complexes Obtained by Electrospray Ionization from Varying In-Solution Equilibrium Conditions. Int. J. Mol. Sci. 2021, 22, 10183. [Google Scholar] [CrossRef]
- Danquah, B.D. , Yefremova, Yelena, Opuni Kwabena F.M., Röwer, Claudia, Koy, Cornelia, Glocker, Michael O. Intact Transition Epitope Mapping - Thermodynamic Weak-force Order (ITEM - TWO). J Proteomics 2019, 212, 103572. [Google Scholar] [CrossRef]
- Opuni, K.F.M.; Koy, C.; Russ, M.; Reepmeyer, M.; Danquah, B.D.; Weresow, M.; Alef, A.; Lorenz, P.; Thiesen, H.J.; Glocker, M.O. ITEM-THREE analysis of a monoclonal anti-malaria antibody reveals its assembled epitope on the pfMSP119 antigen. J Biol Chem 2020, 295, 14987–14997. [Google Scholar] [CrossRef] [PubMed]
- Heitner, J.C. , Koy, C., Kreutzer, M., Gerber, B., Reimer, T., Glocker, M.O. Differentiation of HELLP patients from healthy pregnant women by proteome analysis–on the way towards a clinical marker set. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 840, 10–19. 2006, 840, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Scherf, M.; Danquah, B.D.; Koy, C.; Lorenz, P.; Steinbeck, F.; Neamtu, A.; Thiesen, H.-J.; Glocker, M.O. Epitope fine mapping by mass spectrometry: Investigations of immune complexes consisting of monoclonal anti-HpTGEKP antibody and zinc finger protein linker phospho-hexapeptides. Chembiochem 2022, 23, e202200390. [Google Scholar] [CrossRef]
- Kang, D.-H.; Gho, Y.-S.; Suh, M.-K.; Kang, C.-H. Highly sensitive and fast protein detection with coomassie brilliant blue in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Bull Korean Chem Soc. 2002, 23, 1511–1512. [Google Scholar] [CrossRef]
- Lorenz, P.; Koczan, D.; Thiesen, H.-J. Transcriptional Repression Mediated by the KRAB Domain of the Human C2H2 Zinc Finger Protein Kox1/ZNF10 Does Not Require Histone Deacetylation. Biol. Chem. 2001, 382, 637–644. [Google Scholar] [CrossRef]
- Kyhse-Andersen, J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polycrylamide to nitrocellulose. J Biochem Biophys Methods. 1984, 10, 203–209. [Google Scholar] [CrossRef]
- Eickner, T. Anreicherung und Charakterisierung von TRIM28-Interaktionspartnern mit massenspektrometrischen Methoden. Ph.D. Dissertation, Rostock, Proteom-Zentrum, Rostock, 2012. [Google Scholar]
- Born, N.; Thiesen, H.-J.; Lorenz, P. The B-Subdomain of the Xenopus laevis XFIN KRAB-AB Domain Is Responsible for Its Weaker Transcriptional Repressor Activity Compared to Human ZNF10/Kox1. PLoS One. 2014, 9, e87609. [Google Scholar] [CrossRef]
- Al Chiblak, M.; Steinbeck, F.; Thiesen, H.-J.; Lorenz, P. DUF3669, a “domain of unknown function” within ZNF746 and ZNF777, oligomerizes and contributes to transcriptional repression. BMC Mol Cell Biol. 2019, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Scherf, M.; Koy, C.; Röwer, C.; Neamtu, A.; Glocker, M.O. Characterization of Phosphorylation-Dependent Antibody Binding to Cancer-Mutated Linkers of C2H2 Zinc Finger Proteins by Intact Transition Epitope Mapping-Thermodynamic Weak-Force Order Analysis. J Am Soc Mass Spectrom 2023, 34, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Danquah, B.D.; Röwer, C.; Opuni, K.M.; El-Kased, R.; Frommholz, D.; Illges, H.; Koy, C.; Glocker, M.O. Intact Transition Epitope Mapping–Targeted High-Energy Rupture of Extracted Epitopes (ITEM-THREE)*[S]. Mol. Cell. Proteomics 2019, 18, 1543–1555. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Bannister, D.; Popovic, B.; Sridharan, S.; Giannotta, F.; Filée, P.; Yilmaz, N.; Minter, R. Epitope mapping and key amino acid identification of anti-CD22 immunotoxin CAT-8015 using hybrid β-lactamase display. Protein Engineering, Design and Selection 2010, 24, 351–360. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tufféry, P. Improved PEP-FOLD Approach for Peptide and Miniprotein Structure Prediction. J Chem Theory Comput 2014, 10, 4745–4758. [Google Scholar] [CrossRef]
- Thévenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tufféry, P. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 2012, 40, W288–293. [Google Scholar] [CrossRef]
- Linnebacher, M.; Lorenz, P.; Koy, C.; Jahnke, A.; Born, N.; Steinbeck, F.; Wollbold, J.; Latzkow, T.; Thiesen, H.J.; Glocker, M.O. Clonality characterization of natural epitope-specific antibodies against the tumor-related antigen topoisomerase IIa by peptide chip and proteome analysis: a pilot study with colorectal carcinoma patient samples. Anal Bioanal Chem 2012, 403, 227–238. [Google Scholar] [CrossRef]

| peptide no. (wt or SAP) a,b) | amino acid sequence (wt or SAP) a,b) | SNP entry c) | SNP c) | cardiomyopathy association c) | atom no. | Mr (mono) d) | MM (exp.) e) |
|---|---|---|---|---|---|---|---|
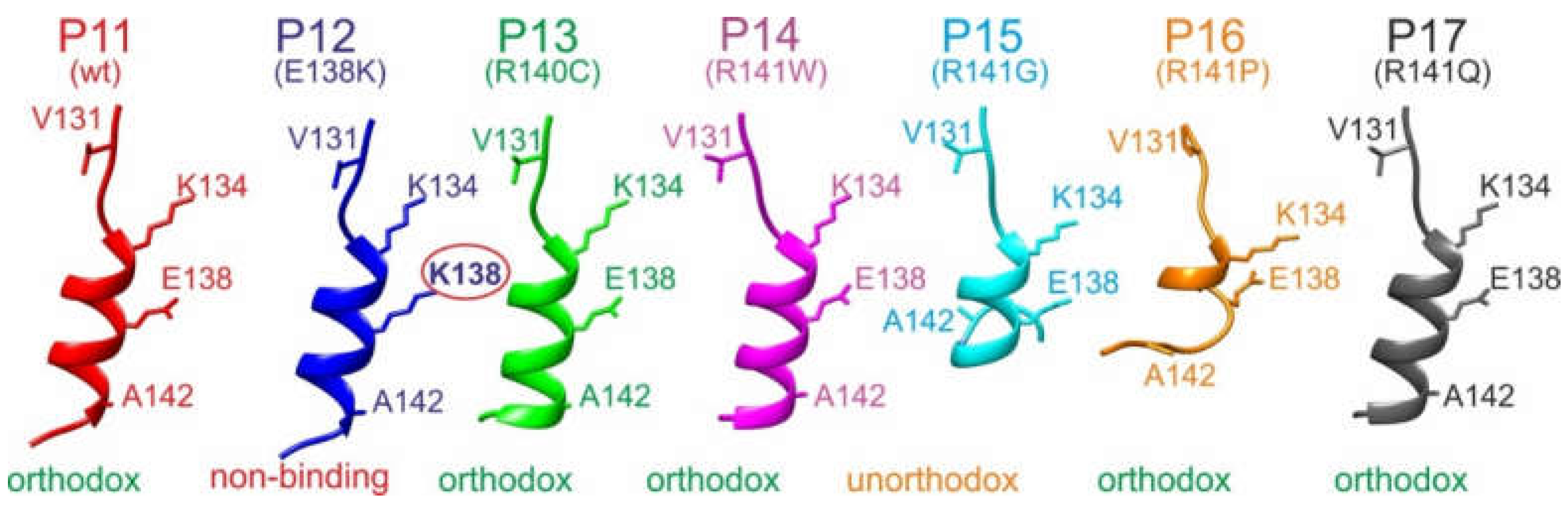
| 11 (wt) | LVSLKDRIERRRAER | n.a. | n.a. | n.a. | 278 | 1896.14 | 1896.91 |
| 12 (E138K) | LVSLKDRIKRRRAER | rs 730881100 | G>A | hypertrophic | 283 | 1896.27 | 1896.44 |
| 13 (R140C) | LVSLKDRIERCRAER | rs 397516463 | C>T | hypertrophic / familial restrictive | 264 | 1842.03 | 1842.09 |
| 14 (R141W) | LVSLKDRIERRWAER | rs 74315380 | C>T | dilated | 279 | 1926.11 | 1926.36 |
| 15 (R141G) | LVSLKDRIERRGAER | rs 74315380 | C>G | not provided | 262 | 1797.07 | 1797.19 |
| 16 (R141P) | LVSLKDRIERRPAER | rs 397516464 | G>C | dilated | 269 | 1837.08 | 1837.43 |
| 17 (R141Q) | LVSLKDRIERRQAER | rs 397516464 | G>A | dilated | 272 | 1868.09 | 1868.18 |
| 21 (wt) | AEQQRIRNEREKERQ | n.a. | n.a. | n.a. | 274 | 1969.04 | 1969.67 |
| 22 (E146Q) | AQQQRIRNEREKERQ | rs 371142225 | G>C | dilated | 275 | 1968.07 | 1968.78 |
| 23 (E146K) | AKQQRIRNEREKERQ | rs 371142225 | G>A | dilated | 279 | 1968.10 | 1969.02 |
| 24 (Q148R) | AEQRRIRNEREKERQ | rs 730880232 | A>G | not provided | 280 | 1997.08 | 1997.43 |
| 25 (R149C) | AEQQCIRNEREKERQ | rs 397516465 | C>T | familial dilated | 261 | 1914.94 | 1915.46 |
| 26 (R149S) | AEQQSIRNEREKERQ | rs 397516465 | C>A | not provided | 262 | 1899.96 | 1899.56 |
| 27 (R149H) | AEQQHIRNEREKERQ | rs 397516466 | G>A | dilated | 268 | 1950.00 | 1951.24 |
| 28 (R151W) | AEQQRIWNEREKERQ | rs 74315379 | C>T | dilated | 275 | 1999.03 | 2000.02 |
| 29 (R151Q) | AEQQRIQNEREKERQ | rs 730881101 | G>A | familial restrictive | 268 | 1941.00 | 1941.99 |
| 30 (R154W) | AEQQRIRNEWEKERQ | rs 483352832 | C>T | dilated | 275 | 1999.03 | 2000.95 |
| 31 (R154Q) | AEQQRIRNEQEKERQ | rs 745632066 | G>A | familial restrictive | 268 | 1941.00 | 1941.90 |
| 32 (E155K) | AEQQRIRNERKKERQ | rs 984218824 | G>A | familial restrictive | 279 | 1968.10 | 1969.03 |
| complex a) | peptide sequence | mean charge ± SD. b,c) | initial[%] b,c,d) | final[%] b,c,e) | Δ[% pts] | ∆CV50[V] b) | dx[V] b) | slope[%/V] b) | R2 b,c) |
|---|---|---|---|---|---|---|---|---|---|
| M7+P11 | LVSLKDIERRRAER | 24.1 ± 0.23 | 56.43 | 84.36 | 27.93 | 25.27 | 6.69 | 1.04 | 0.995 |
| M7+P12 | LVSLKDIKRRRAER | 24.5 ± 0.67 | 89.38 | 92.28 | 2.90 | n.a. | n.a. | n.a. | n.a. |
| M7+P13 | LVSLKDIERCRAER | 24.3 ± 0.29 | 40.36 | 83.40 | 43.04 | 23.30 | 6.55 | 1.64 | 0.999 |
| M7+P14 | LVSLKDIERRWAER | 24.1 ± 0.22 | 54.37 | 93.86 | 39.49 | 18.02 | 4.33 | 2.28 | 0.997 |
| M7+P15 | LVSLKDIERRGAER | 24.1 ± 0.01 | 70.69 | 84.98 | 14.29 | 24.89 | 6.09 | 0.59 | 0.995 |
| M7+P16 | LVSLKDIERRPAER | 24.2 ± 0.15 | 55.34 | 96.90 | 41.56 | 15.83 | 5.05 | 2.06 | 0.993 |
| M7+P17 | LVSLKDIERRQAER | 24.0 ± 0.01 | 56.18 | 82.69 | 26.51 | 24.61 | 6.46 | 1.03 | 0.999 |
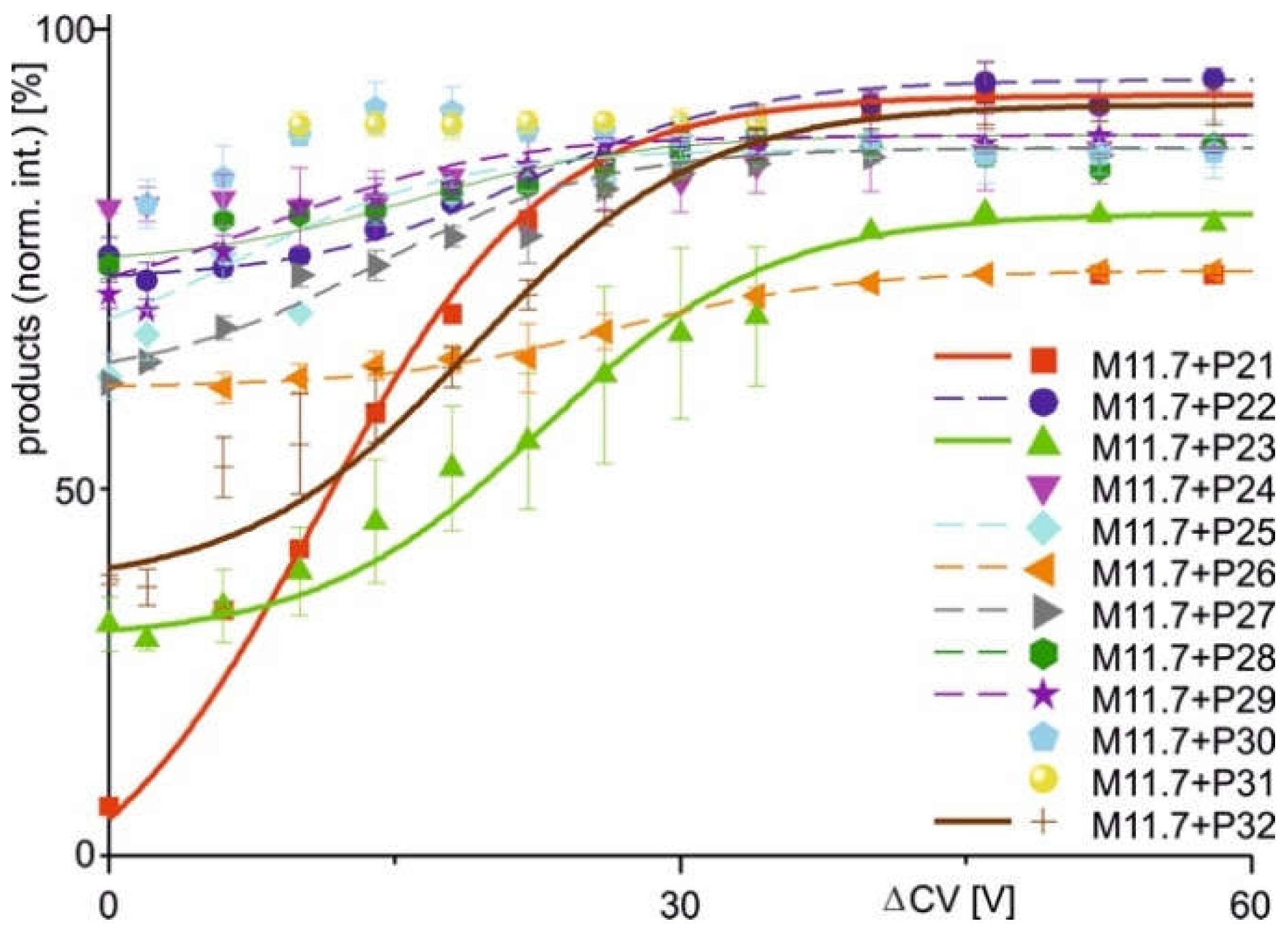
| M11.7+P21 | AEQQRIRNEREKERQ | 23.6 ± 0.11 | 56.91 | 96.29 | 39.38 | 12.77 | 5.77 | 1.71 | 0.996 |
| M11.7+P22 | AQQQRIRNEREKERQ | 23.7 ± 0.10 | 87.08 | 97.12 | 10.04 | 25.18 | 7.70 | 0.33 | 0.970 |
| M11.7+P23 | AKQQRIRNEREKERQ | 23.3 ± 0.01 | 67.00 | 89.77 | 22.77 | 22.47 | 7.29 | 0.78 | 0.994 |
| M11.7+P24 | AEQRRIRNEREKERQ | 23.3 ± 0.06 | 90.25 | 93.57 | 3.32 | n.a. | n.a. | n.a. | n.a. |
| M11.7+P25 | AEQQCIRNEREKERQ | 23.2 ± 0.17 | 80.75 | 93.33 | 12.58 | 10.06 | 6.00 | 0.52 | 0.962 |
| M11.7+P26 | AEQQSIRNEREKERQ | 22.9 ± 0.00 | 80.48 | 86.48 | 6.00 | 26.67 | 6.49 | 0.25 | 0.996 |
| M11.7+P27 | AEQQHIRNEREKERQ | 23.1 ± 0.07 | 80.22 | 93.49 | 13.27 | 14.04 | 7.60 | 0.44 | 0.987 |
| M11.7+P28 | AEQQRIWNEREKERQ | 23.4 ± 0.01 | 86.91 | 93.90 | 6.99 | 13.80 | 6.90 | 0.25 | 0.955 |
| M11.7+P29 | AEQQRIQNEREKERQ | 23.2 ± 0.15 | 84.39 | 93.92 | 9.53 | 9.96 | 5.17 | 0.46 | 0.981 |
| M11.7+P30 | AEQQRIRNEWEKERQ | 23.5 ± 0.09 | 90.48 | 94.03 | 3.55 | n.a. | n.a. | n.a. | n.a. |
| M11.7+P31 | AEQQRIRNEQEKERQ | 23.2 ± 0.02 | 93.60 | 93.39 | -0.21 | n.a. | n.a. | n.a. | n.a. |
| M11.7+P32 | AEQQRIRNERKKERQ | 23.1 ± 0.06 | 69.09 | 96.03 | 26.94 | 17.63 | 7.32 | 0.92 | 0.992 |
| complex a) | peptide sequence | [1/s] | b,c)[Ø] | [kJ/mol] | [kJ/mol] | [kJ/mol] | binding type |
|---|---|---|---|---|---|---|---|
| M7+P11 | LVSLKDIERRRAER | 3,69∙1012 | 0.59 | 1.28 | 40.25 | 38.96 | orthodox |
| M7+P12 | LVSLKDIKRRRAER | n.a. | n.a. | n.a. | n.a. | n.a. | non-binding |
| M7+P13 | LVSLKDIERCRAER | 1.72∙1012 | 0.28 | 3.17 | 55.16 | 51.99 | orthodox |
| M7+P14 | LVSLKDIERRWAER | 1.75∙1012 | 0.28 | 3.13 | 92.09 | 88.96 | orthodox |
| M7+P15 | LVSLKDIERRGAER | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M7+P16 | LVSLKDIERRPAER | 2.95∙1012 | 0.48 | 1.84 | 85.61 | 83.77 | orthodox |
| M7+P17 | LVSLKDIERRQAER | 3.82∙1012 | 0.62 | 1.14 | 38.06 | 36.92 | orthodox |
| M11.7+P21 | AEQQRIRNEREKERQ | 5.70∙1012 | 0.92 | 0.21 | 72.53 | 72.32 | orthodox |
| M11.7+P22 | AQQQRIRNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P23 | AKQQRIRNEREKERQ | 7.33∙1012 | 1.18 | -0.42 | 37.41 | 37.81 | orthodox |
| M11.7+P24 | AEQRRIRNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | non-binding |
| M11.7+P25 | AEQQCIRNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P26 | AEQQSIRNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P27 | AEQQHIRNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P28 | AEQQRIWNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P29 | AEQQRIQNEREKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | unorthodox |
| M11.7+P30 | AEQQRIRNEWEKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | non-binding |
| M11.7+P31 | AEQQRIRNEQEKERQ | n.a. | n.a. | n.a. | n.a. | n.a. | non-binding |
| M11.7+P32 | AEQQRIRNERKKERQ | 8.85∙1012 | 1.43 | -0.88 | 50.35 | 51.23 | orthodox |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





