Submitted:
08 April 2025
Posted:
11 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Catalyst Characterization
3. Experimental
3.1. Materials and Methods
3.1.1. Materials
3.1.2. Catalysts Preparation
3.1.3. Experimental Setup and Procedure for Catalysts’ Testing in Glycerol Carbonate Synthesis
4. Results and Discussions
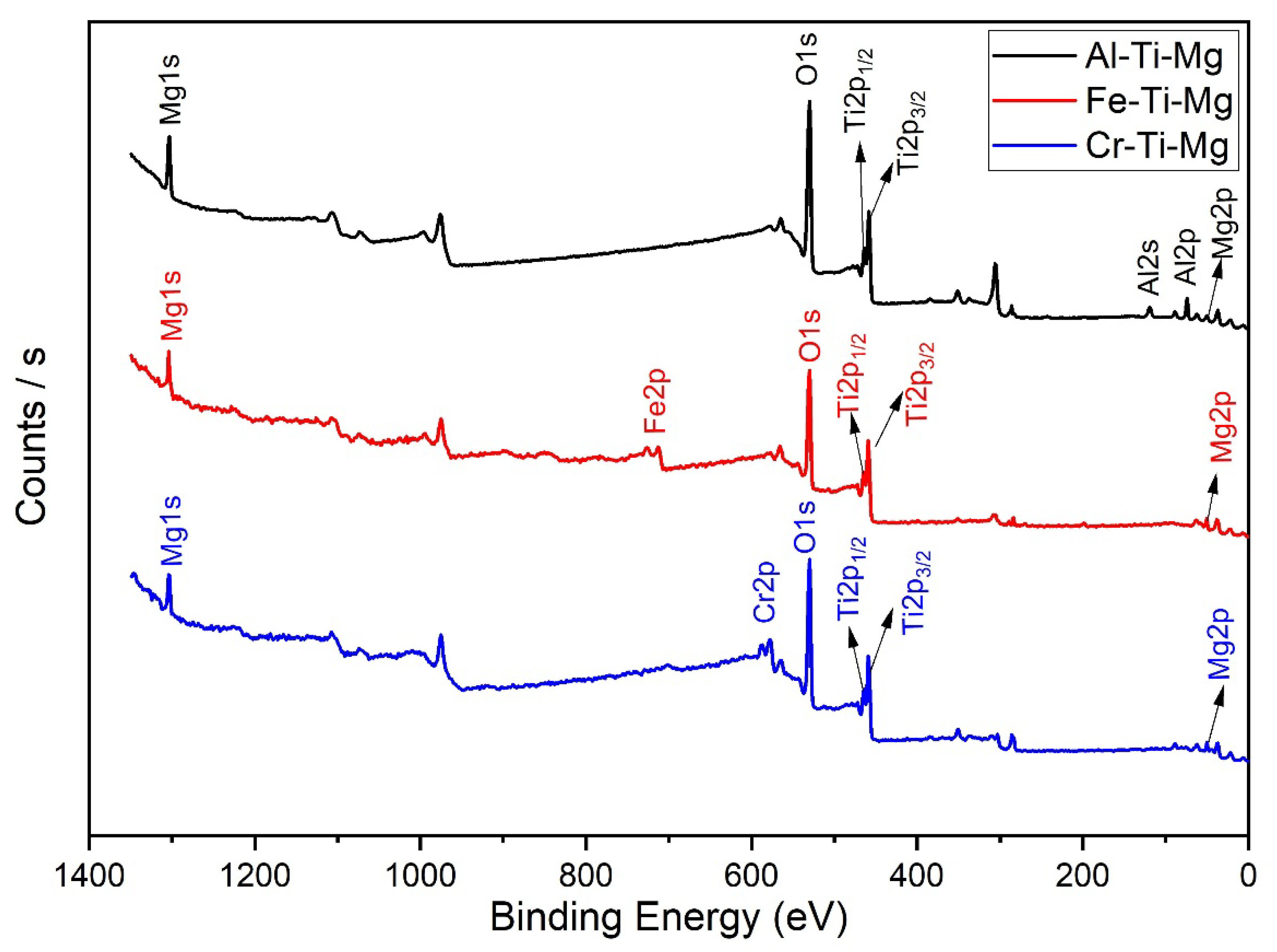
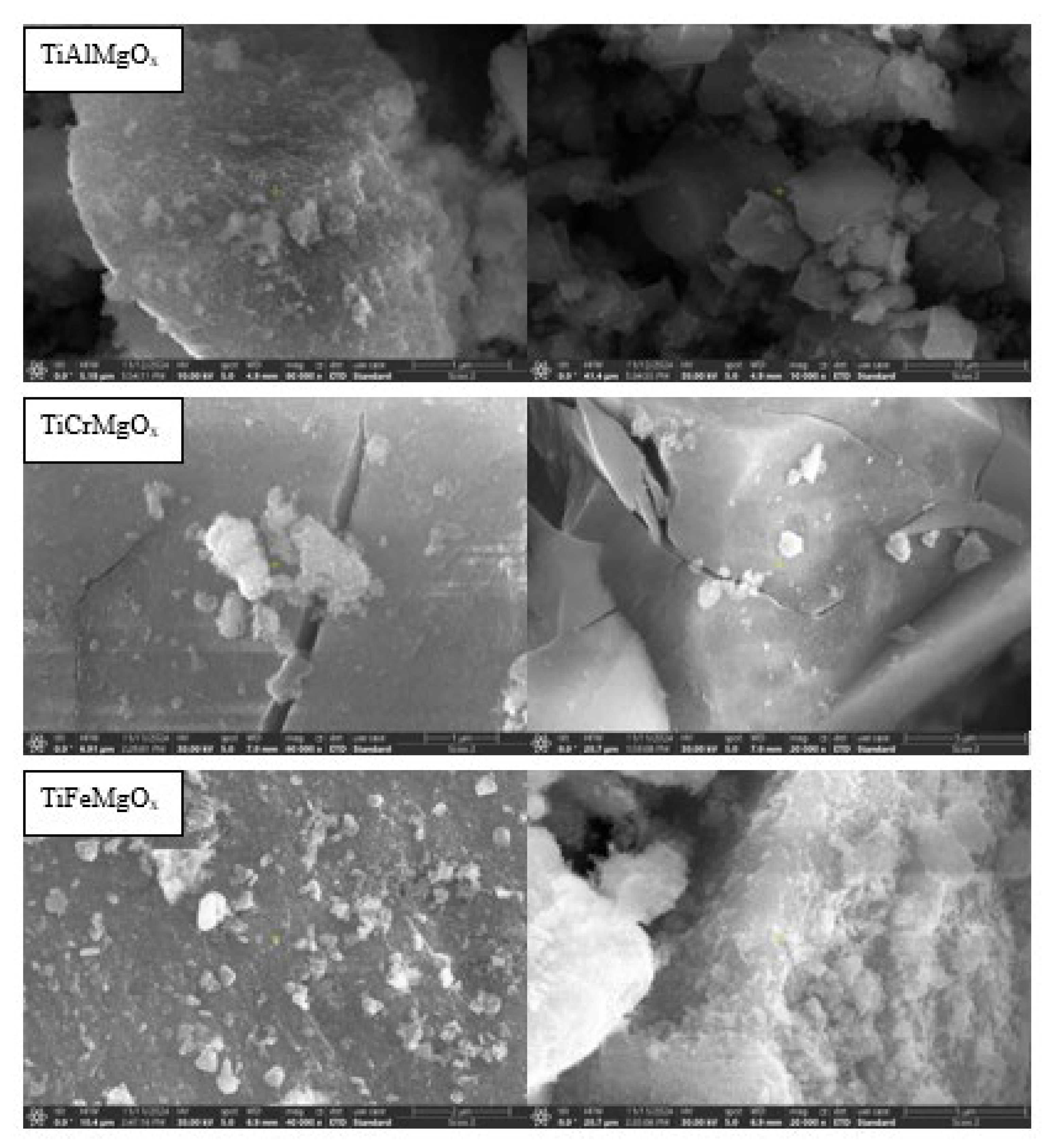
4.1. Catalyst Characterization
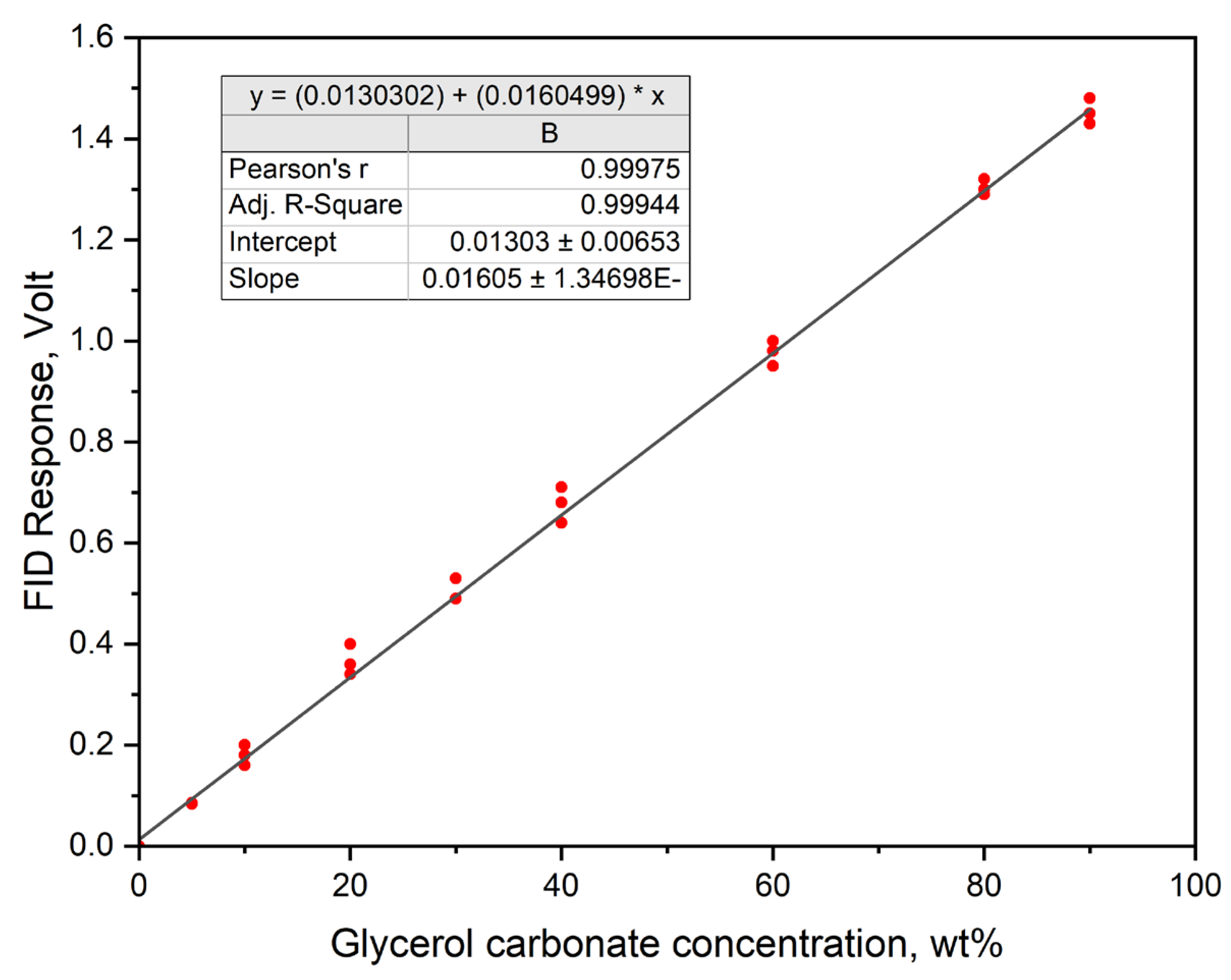
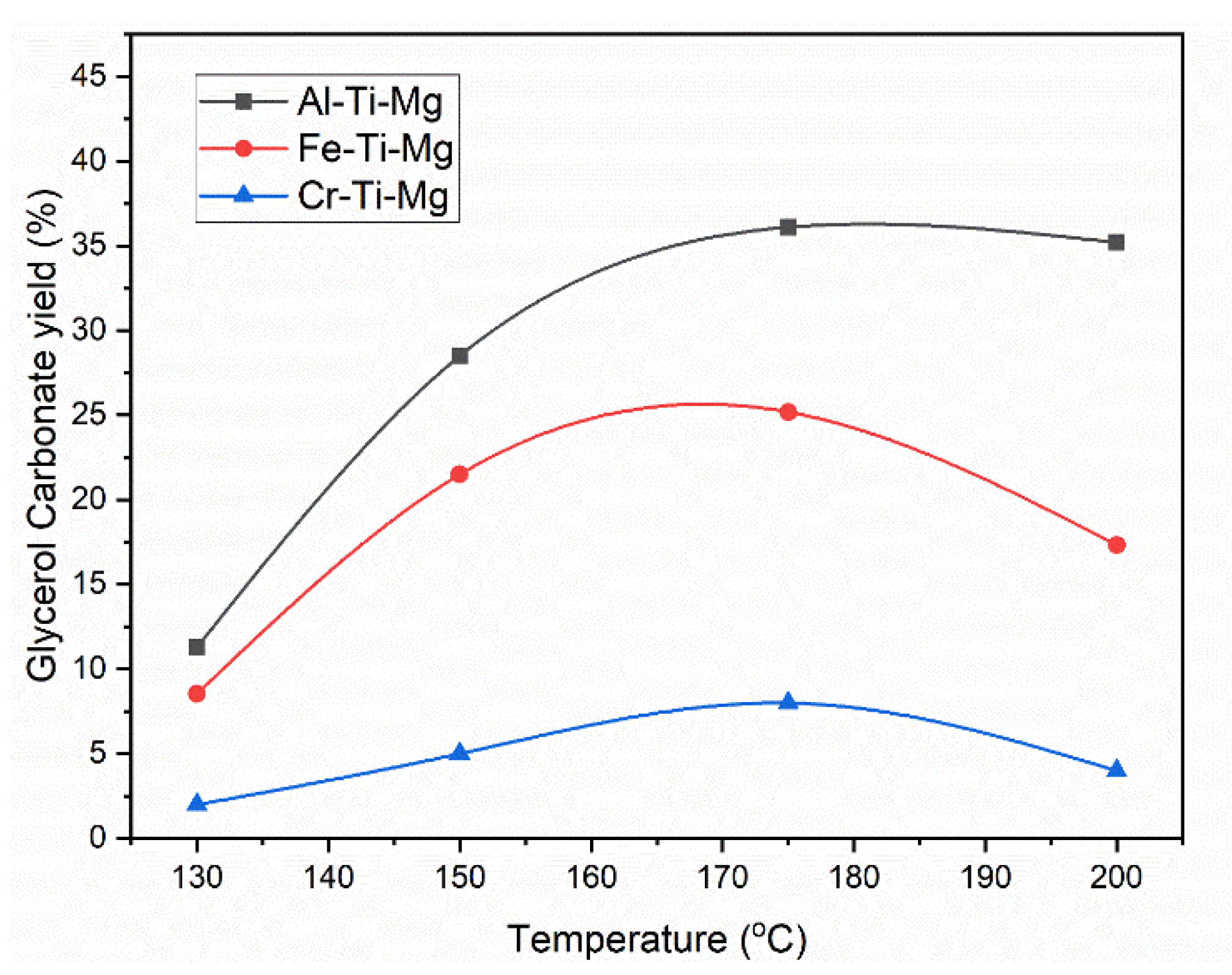
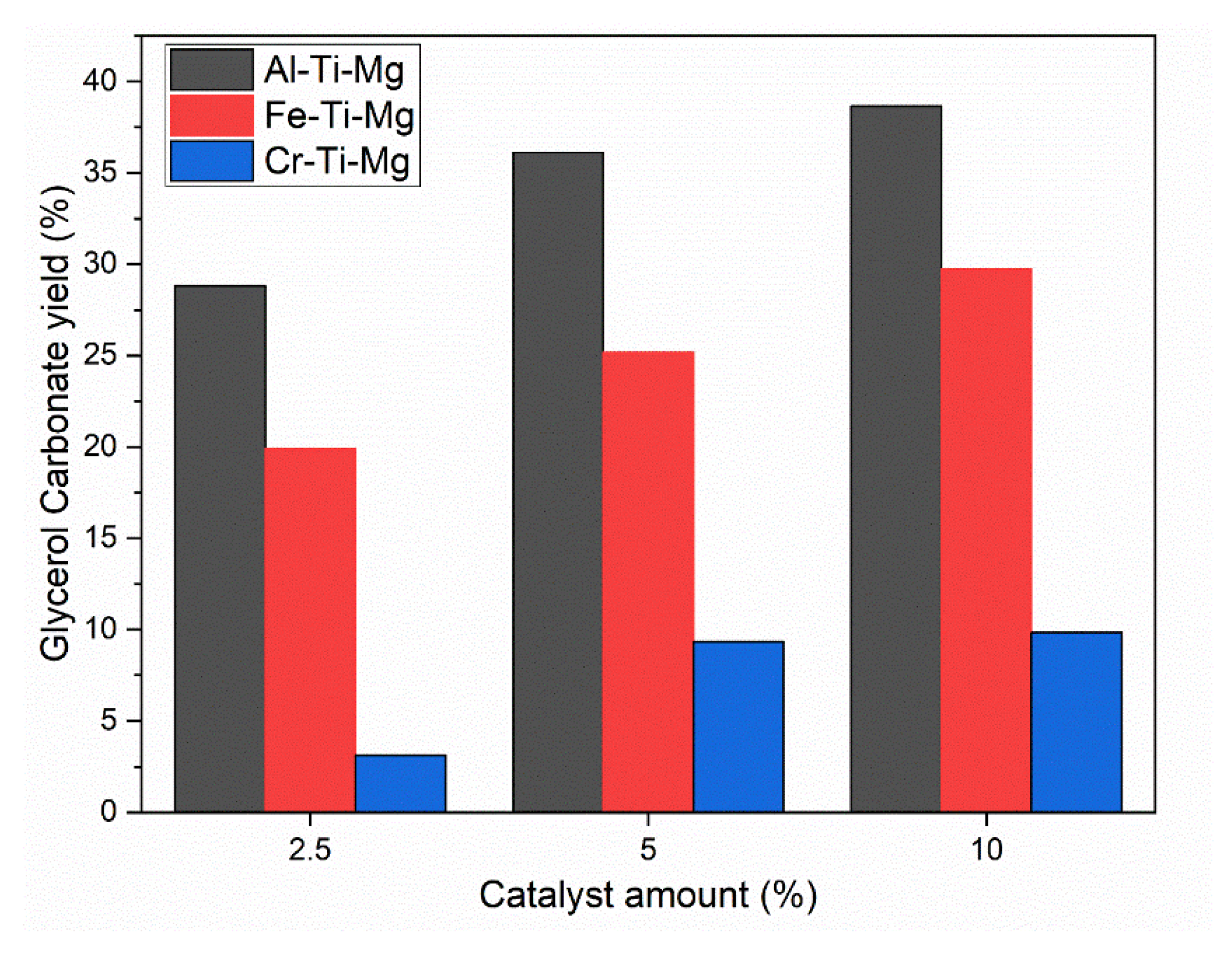
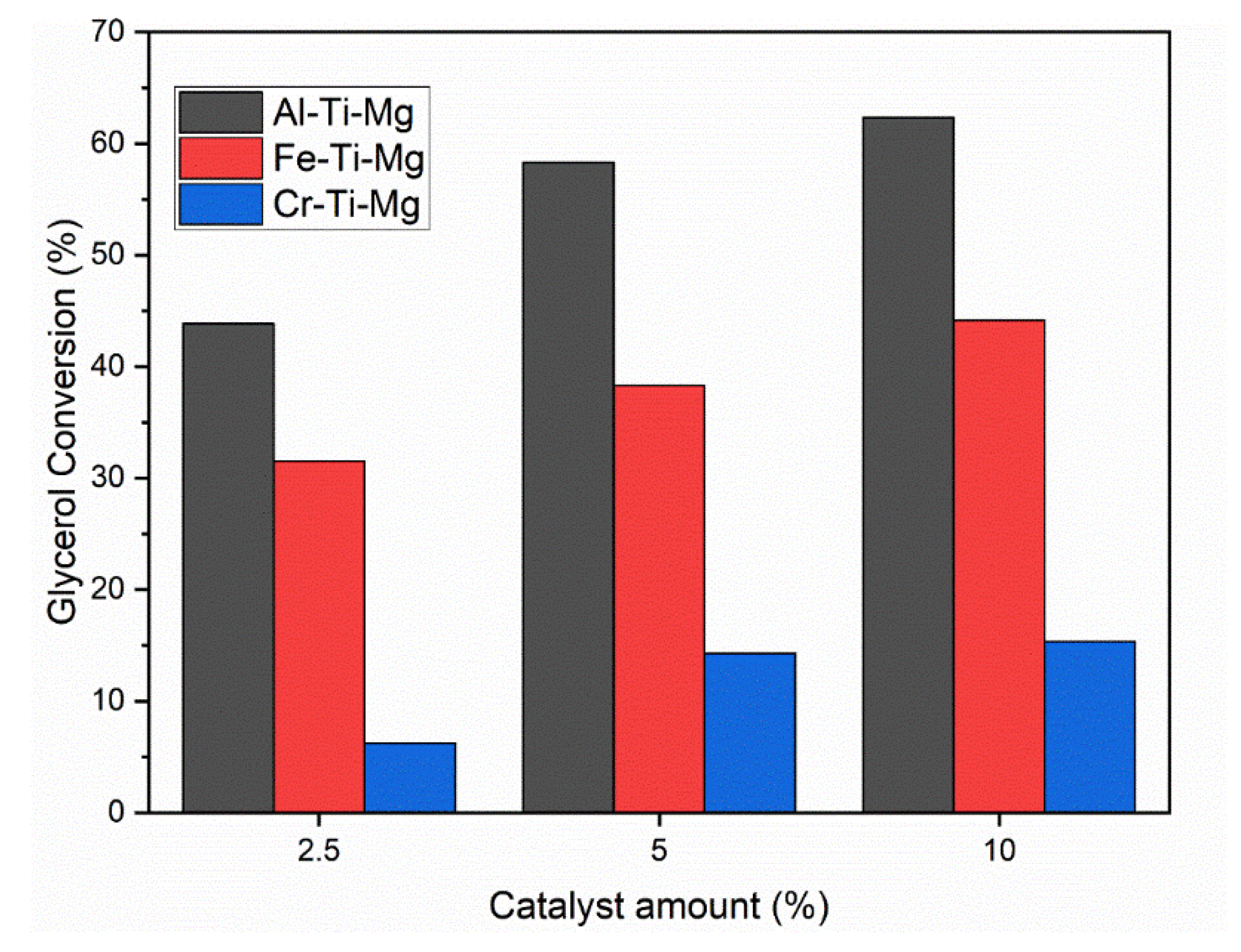
4.2. Catalysts Effect on Glycerol Carbonate Synthesis
5. Conclusions
References
- Global Energy Review 2025. Glob. Energy Rev. 2025 2025. [CrossRef]
- Vasquez, W. V.; Hernández, D.M.; del Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical Carbon Dioxide Extraction of Oil and Minor Lipid Compounds of Cake Byproduct from Brazil Nut (Bertholletia Excelsa) Beverage Production. J. Supercrit. Fluids 2021, 171, 105188. [CrossRef]
- IEA Putting CO 2 to Use. Energy Rep. 2019, 86.
- Lukato, S.; Kasozi, G.N.; Naziriwo, B.; Tebandeke, E. Glycerol Carbonylation with CO2 to Form Glycerol Carbonate: A Review of Recent Developments and Challenges. Curr. Res. Green Sustain. Chem. 2021, 4, 100199. [CrossRef]
- Gao, Z.; Xiang, M.; He, M.; Zhou, W.; Chen, J.; Lu, J.; Wu, Z.; Su, Y. Transformation of CO2 with Glycerol to Glycerol Carbonate over ETS-10 Zeolite-Based Catalyst. Molecules 2023, 28, 1–13. [CrossRef]
- Inrirai, P.; Keogh, J.; Centeno-Pedrazo, A.; Artioli, N.; Manyar, H. Recent Advances in Processes and Catalysts for Glycerol Carbonate Production via Direct and Indirect Use of CO2. J. CO2 Util. 2024, 80, 102693. [CrossRef]
- Ke, Y.H.; Xu, H.; Wang, X.; Liu, H.; Yuan, H. Production of Glycerol Carbonate by Coupling Glycerol and CO2 over Various Metal Oxide Catalyst. J. CO2 Util. 2024, 83. [CrossRef]
- Al-Kurdhani, J.M.H.; Wang, H. The Synthesis of Glycerol Carbonate from Glycerol and Carbon Dioxide over Supported CuO-Based Nanoparticle Catalyst. Molecules 2023, 28, 1–18. [CrossRef]
- Ozorio, L.P.; Mota, C.J.A. Direct Carbonation of Glycerol with CO2 Catalyzed by Metal Oxides. ChemPhysChem 2017, 18, 3260–3265. [CrossRef]
- Li, H.; Gao, D.; Gao, P.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The Synthesis of Glycerol Carbonate from Glycerol and CO2 over La2O2CO3-ZnO Catalysts. Catal. Sci. Technol. 2013, 3, 2801–2809. [CrossRef]
- Manuscript, A. Nanoscale; ISBN 9117225450.
- Liu, J.; Li, Y.; Liu, H.; He, D. Transformation of CO2 and Glycerol to Glycerol Carbonate over CeO2–ZrO2 Solid Solution —— Effect of Zr Doping. Biomass and Bioenergy 2018, 118, 74–83. [CrossRef]
- Li, H.; Jiao, X.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Zhang, B. Synthesis of Glycerol Carbonate by Direct Carbonylation of Glycerol with CO 2 over Solid Catalysts Derived from Zn/Al/La and Zn/Al/La/M (M = Li, Mg and Zr) Hydrotalcites. Catal. Sci. Technol. 2015, 5, 989–1005. [CrossRef]
- Xu, L.; Yang, Q.; Hu, L.; Wang, D.; Peng, Y.; Shao, Z.; Lu, C. Insights over Titanium Modified FeMgO x Catalysts for Selective Catalytic Reduction of NO x with NH 3 : 2019, 14–18.
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A Review on the Performance of Glycerol Carbonate Production via Catalytic Transesterification: Effects of Influencing Parameters. Energy Convers. Manag. 2014, 88, 484–497. [CrossRef]
- Selvamani, T.; Anandan, S.; Asiri, A.M.; Maruthamuthu, P.; Ashokkumar, M. Preparation of MgTi2O5 Nanoparticles for Sonophotocatalytic Degradation of Triphenylmethane Dyes. Ultrason. Sonochem. 2021, 75, 105585. [CrossRef]
- Ehsan, M.A.; Naeem, R.; McKee, V.; Hakeem, A.S.; Mazhar, M. MgTi2O5 Thin Films from Single Molecular Precursor for Photoelectrochemical Water Splitting. Sol. Energy Mater. Sol. Cells 2017, 161, 328–337. [CrossRef]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art. Titan. Dioxide - Mater. a Sustain. Environ. 2018. [CrossRef]
- Umar, A.; Harraz, F.A.; Ibrahim, A.A.; Almas, T.; Kumar, R.; Al-Assiri, M.S.; Baskoutas, S. Iron-Doped Titanium Dioxide Nanoparticles as Potential Scaffold for Hydrazine Chemical Sensor Applications. Coatings 2020, 10, 1–13. [CrossRef]
- Veiko, V.P.; Karlagina, Y.Y.; Samokhvalov, A.A.; Polyakov, D.S.; Manokhin, S.S.; Radaev, M.M.; Odintsova, G. V.; Gornushkin, I.B. Surface Structuring and Reverse Deposition of Nanoporous Titanium Oxides by Laser Ablation of Titanium in Air. Plasma Chem. Plasma Process. 2022, 42, 923–937. [CrossRef]
- Cai, Y.; Shi, Q.; Wang, M.; Lv, X.; Cheng, Y.; Huang, W. Synthesis of Nanoscale Lambda-Ti3O5 via a PEG Assisted Sol-Gel Method. J. Alloys Compd. 2020, 848, 156585. [CrossRef]
- Rani, N.; Chahal, S.; Chauhan, A.S.; Kumar, P.; Shukla, R.; Singh, S.K. X-Ray Analysis of MgO Nanoparticles by Modified Scherer’s Williamson-Hall and Size-Strain Method. Mater. Today Proc. 2019, 12, 543–548. [CrossRef]
- Al-hadeethi, Y.; Sayyed, M.I. E Ff Ect of Gd 2 O 3 on the Radiation Shielding Characteristics Of. Ceram. Int. 2020, 185, 0–1.
- Karan, P.; Chakraborty, R. E-Waste Derived Silica-Alumina for Eco-Friendly and Inexpensive Mg-Al-Ti Photocatalyst towards Glycerol Carbonate (Electrolyte) Synthesis: Process Optimization and LCA. Waste Manag. 2022, 140, 213–224. [CrossRef]
- Xing, Y.; Shen, X.; Niu, Q.; Duan, H.; Tang, C.; Tao, B.; Chen, S.; Shangguan, Q.; Feng, B.; Yu, H.; et al. Thermally and Chemically Stable Fe/Mg-Layered Double Oxides-Biochar for Enhanced Polystyrene Nanoplastic Adsorption and Sustainable Recycling. Chem. Eng. J. 2025, 508, 160918. [CrossRef]
- Liu, J.; Zang, P.; Liu, X.; Mi, J.; Wang, Y.; Zhang, G.; Chen, J.; Zhang, Y.; Li, J. A Novel Highly Active Catalyst Form CuFeMg Layered Double Oxides for the Selective Catalytic Reduction of NO by CO. Fuel 2022, 317, 123469. [CrossRef]
- Zainuri, M. Hematite from Natural Iron Stones as Microwave Absorbing Material on X-Band Frequency Ranges. IOP Conf. Ser. Mater. Sci. Eng. 2017, 196. [CrossRef]
- Gibot, P. Centimetric-Sized Chromium (III) Oxide Object Synthesized by Means of the Carbon Template Replication. Ceramics 2020, 3, 92–100. [CrossRef]
- Mallesham, B.; Rangaswamy, A.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Solvent-Free Production of Glycerol Carbonate from Bioglycerol with Urea Over Nanostructured Promoted SnO2 Catalysts. Catal. Letters 2020, 150, 3626–3641. [CrossRef]
- Kloprogge, J.T. X-Ray Photoelectron Spectroscopy (XPS) Study of Layered Double Hydroxides with Different Exchangeable Anions. Appl. Sci. 2025, 15. [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.H. In Situ Growth of Mg-Al Hydrotalcite Conversion Film on AZ31 Magnesium Alloy. Corros. Sci. 2011, 53, 3281–3288. [CrossRef]
- Suzuki, Y.; Shinoda, Y. Magnesium Dititanate (MgTi2O5) with Pseudobrookite Structure: A Review. Sci. Technol. Adv. Mater. 2011, 12. [CrossRef]
- Jayanthi, S.; Kutty, T.R.N. Microwave Dielectric Properties of Mg4Al2Ti9O25 Ceramics. Mater. Lett. 2008, 62, 556–560. [CrossRef]
- Akaogi, M.; Ishii, T.; Yamaura, K. Post-Spinel-Type AB2O4 High-Pressure Phases in Geochemistry and Materials Science. Commun. Chem. 2024, 7. [CrossRef]
- Pilania, G.; Kocevski, V.; Valdez, J.A.; Kreller, C.R.; Uberuaga, B.P. Prediction of Structure and Cation Ordering in an Ordered Normal-Inverse Double Spinel. Commun. Mater. 2020, 1, 1–11. [CrossRef]
- Liu, R.; Conradie, J.; Erasmus, E. Comparison of X-Ray Photoelectron Spectroscopy Multiplet Splitting of Cr 2p Peaks from Chromium Tris(β-Diketonates) with Chemical Effects. J. Electron Spectros. Relat. Phenomena 2016, 206, 46–51. [CrossRef]
- Lee, H.; Aytuna, Z.T.; Bhardwaj, A.; Wilhelm, M.; Khan, L.; May, B.; Mueller, D.N.; Mathur, S. Controlling Degree of Inversion in MgFe2O4 Spinel Films Grown in External Magnetic Fields. Adv. Eng. Mater. 2023, 25, 2–9. [CrossRef]
- Ishii, T.; Miyajima, N.; Sinmyo, R.; Kojitani, H.; Mori, D.; Inaguma, Y.; Akaogi, M. Discovery of New-Structured Post-Spinel MgFe2O4: Crystal Structure and High-Pressure Phase Relations. Geophys. Res. Lett. 2020, 47, 1–9. [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.L. Hierarchically Structured Porous Materials: Synthesis Strategies and Applications in Energy Storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [CrossRef]
- Perego, C.; Millinib, R. Porous Materials in Catalysis: Challenges for Mesoporous Materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [CrossRef]
- Granados-Reyes, J.; Salagre, P.; Cesteros, Y. CaAl-Layered Double Hydroxides as Active Catalysts for the Transesterification of Glycerol to Glycerol Carbonate. Appl. Clay Sci. 2016, 132–133, 216–222. [CrossRef]
- Wang, D.; Zhu, Q.; Xing, Z.; Fang, L. Control of Chloride Ion Corrosion by MgAlOx/MgAlFeOx in the Process of Chloride Deicing. Environ. Sci. Pollut. Res. 2022, 29, 9269–9281. [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [CrossRef]
- CHEN, K.; ZHANG, T.; CHEN, X.; HE, Y.; LIANG, X. Model Construction of Micro-Pores in Shale: A Case Study of Silurian Longmaxi Formation Shale in Dianqianbei Area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [CrossRef]
- Yadav, G.D.; Chandan, P.A. A Green Process for Glycerol Valorization to Glycerol Carbonateover Heterogeneous Hydrotalcite Catalyst. Catal. Today 2014, 237, 47–53. [CrossRef]
- Manuscript, A. Catalysis Science & Echnology. 2020. [CrossRef]
- Tsuzuki, R.; Ichikawa, K.; Kase, M. [CONTRIBUTION FROM THE RESEARCH AND DEVELOPMENT DIVISIOK OF DAINIPPON PRINTING INK MFG New Reactions of Organic Isocyanates. I. Reaction with Alkylene Carbonates;.
- Galadima, A.; Muraza, O. Sustainable Production of Glycerol Carbonate from By-Product in Biodiesel Plant. Waste and Biomass Valorization 2017, 8, 141–152.
- Rousseau, J.; Rousseau, C.; Lynikaite, B.; Šačkus, A.; de Leon, C.; Rollin, P.; Tatibouët, A. Tosylated Glycerol Carbonate, a Versatile Bis-Electrophile to Access New Functionalized Glycidol Derivatives. Tetrahedron 2009, 65, 8571–8581. [CrossRef]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.M.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [CrossRef]
- Benoit, M.; Brissonnet, Y.; Guélou, E.; De-Oliveira-Vigier, K.; Barrault, J.; Jérôme, F. Acid-Catalyzed Dehydration of Fructose and Inulin with Glycerol or Glycerol Carbonate as Renewably Sourced Co-Solvent. ChemSusChem 2010, 3, 1304–1309. [CrossRef]
- Ursin, C.; Hansen, C.M.; Van Dyk, J.W.; Jensen, P.O.; Christensen, I.J.; Ebbehoej, J. Permeability of Commercial Solvents Through Living Human Skin. Am. Ind. Hyg. Assoc. J. 1995, 56, 651–660. [CrossRef]
- Tudorache, M.; Protesescu, L.; Coman, S.; Parvulescu, V.I. Efficient Bio-Conversion of Glycerol to Glycerol Carbonate Catalyzed by Lipase Extracted from Aspergillus Niger. Green Chem. 2012, 14, 478–482. [CrossRef]
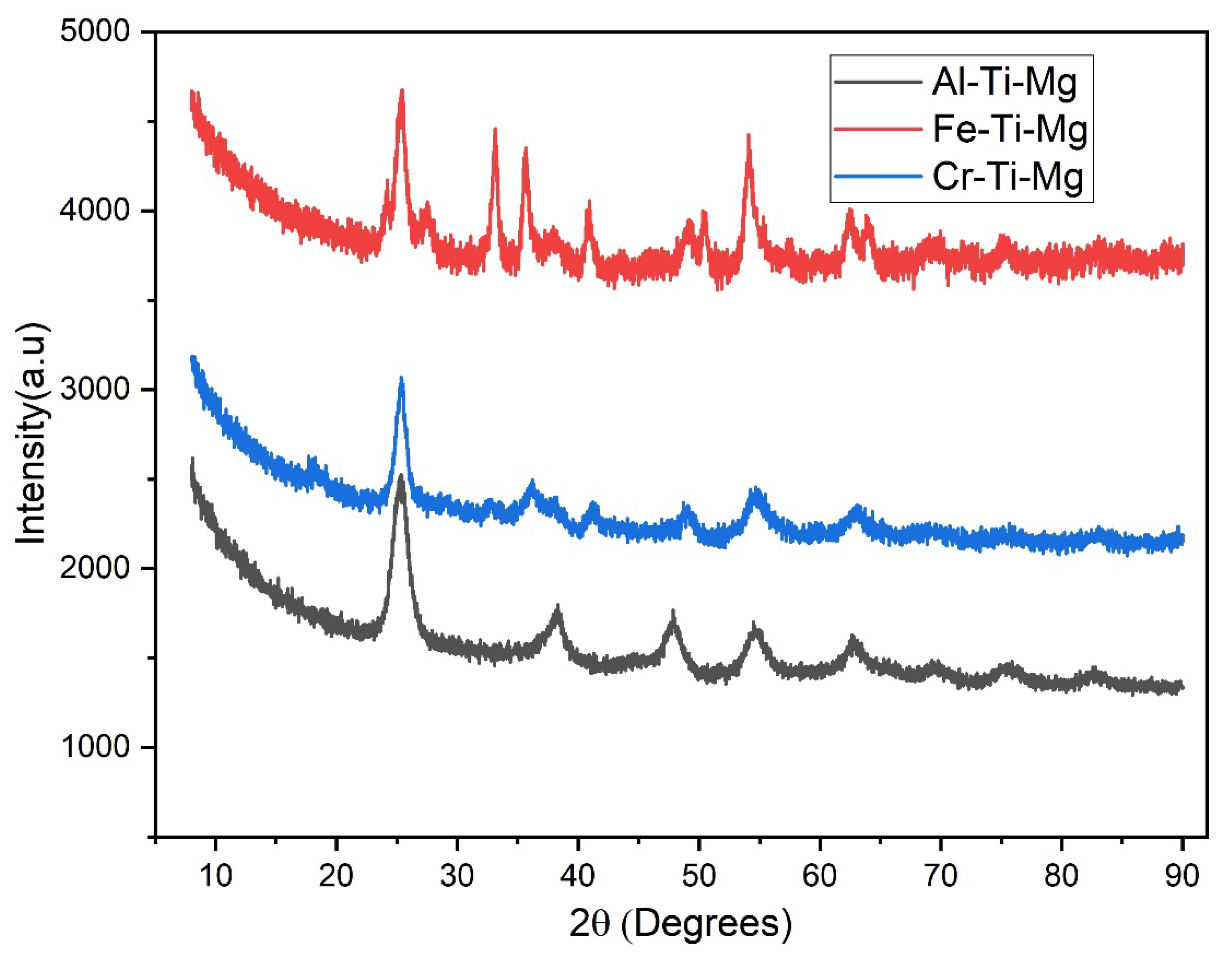
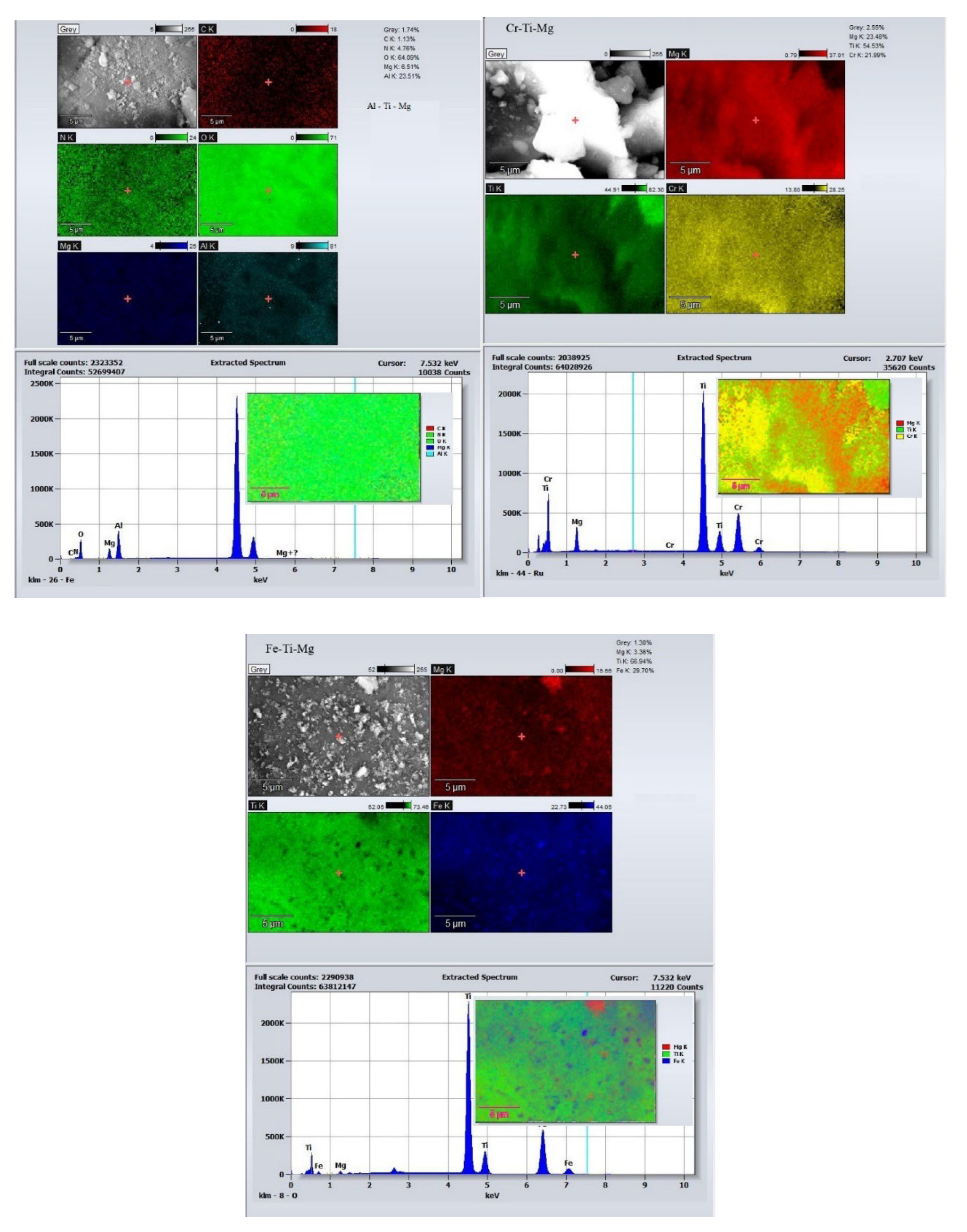

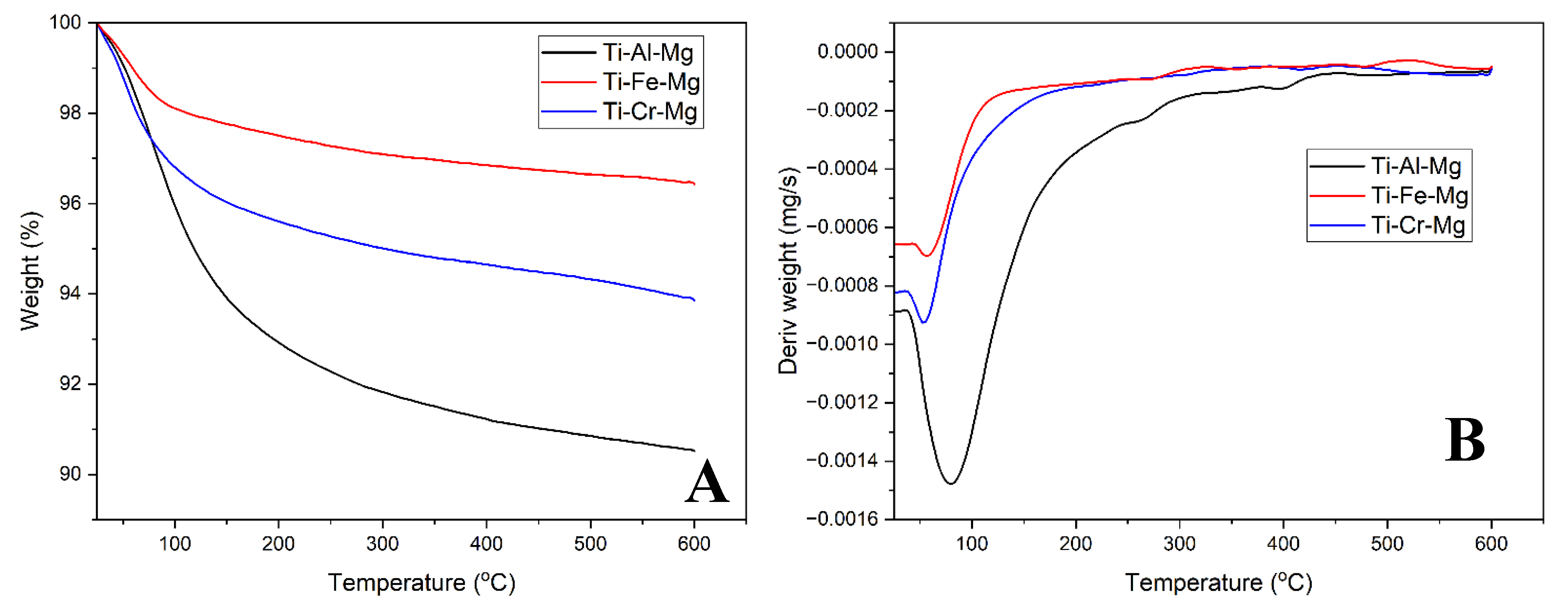

| 2θ | Ti-Al-Mg | Ti-Fe-Mg | Ti-Cr-Mg | |||
|---|---|---|---|---|---|---|
| Oxide type | Crystallite size (nm) | Oxide type | Crystallite size (nm) | Oxide type | Crystallite size (nm) | |
| 25.37 | MgTi2O4 | 0.087 | MgTi2O4 | 0.143 | MgTi2O4 | 0.116 |
| 33.27 | - | - | Fe2O3 | 0.249 | - | - |
| 36.17 | - | - | - | - | Cr2MgO4 | 0.092 |
| 38.25 | TiO2 | 0.062 | TiO2 | 0.276 | TiO2 | 0.074 |
| 41.23 | - | - | - | - | Cr2O3 | 0.135 |
| 47.45 | MgAl2Ti3O10 | 0.108 | - | - | - | - |
| 48.80 | - | - | Ti3O5 | 0.268 | Ti3O5 | 0.149 |
| 54.65 | TiO2 | 0.088 | TiO2 | 0.224 | TiO2 | 0.086 |
| 62.75 | MgO | 0.090 | MgO | 0.172 | MgO | 0.106 |
| 64.10 | - | - | MgFe2O4 | 0.237 | - | - |
| Catalysts | BET surface area (m2/g) | Pore volume (cm3/g) | Average pore width (nm) |
|---|---|---|---|
| Ti-Al-Mg | 119.43 | 0.345 | 6.81 |
| Ti-Cr-Mg | 54.47 | 0.193 | 7.12 |
| Ti-Fe-Mg | 28.20 | 0.166 | 10.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





