Introduction
Repair of descending thoracic aortic aneurysms (DTAA) by endovascular treatment (TEVAR) is increasingly becoming a preferred alternative to open surgery with aortic stent graft (SG) insertion. Post-procedural medical and radiological follow-up after TEVAR is crucial to monitor for adverse aortic remodeling. Indeed, the principle of TEVAR leaves diseased aorta in place, potentially leading to disease progression both at the aneurysm and at the proximal and distal neck, where the aortic wall interfaces with the SG. Each aortic SG has unique characteristics, such as rigidity and conformability, which impose new constraints on the diseased aortic wall. Despite technological advancements improving SG flexibility, durability [
1], a significant number of patients still experience adverse aortic remodeling during follow-up[
2].
Radiological monitoring primarily focuses on aortic diameter evolution and SG integrity to detect material defects. However, resents studies suggest that advanced parameters beyond diameter analysis can provide valuable insights for detecting early adverse aortic remodeling [
3,
4,
5].These parameters offer a more complex assessment of aortic morphology, enabling earlier identification of patients at risk for complications compared to standard diameter-based follow-up.
This paper quantifies the evolution of 3D morphological changes of the aorta over the course of follow-up after TEVAR, which might help predict adverse events associated with SG. We present a clinical case of a patient treated with TEVAR for a thoracic aortic aneurysm, followed for 10 years with CTA. The patient developed type IIIa and IIIb endoleaks. Advanced evaluation was performed using EndoSize software (Therenva, Rennes-France) [
5], providing insights for improved long-term follow-up.
This article, therefore, aims to evaluate, through a narrative review of the literature and the presentation of our clinical case, whether it would be relevant to integrate geometric calculations into the long-term follow-up of patients treated with TEVAR to improve the detection of complications, such as late stent graft migrations.
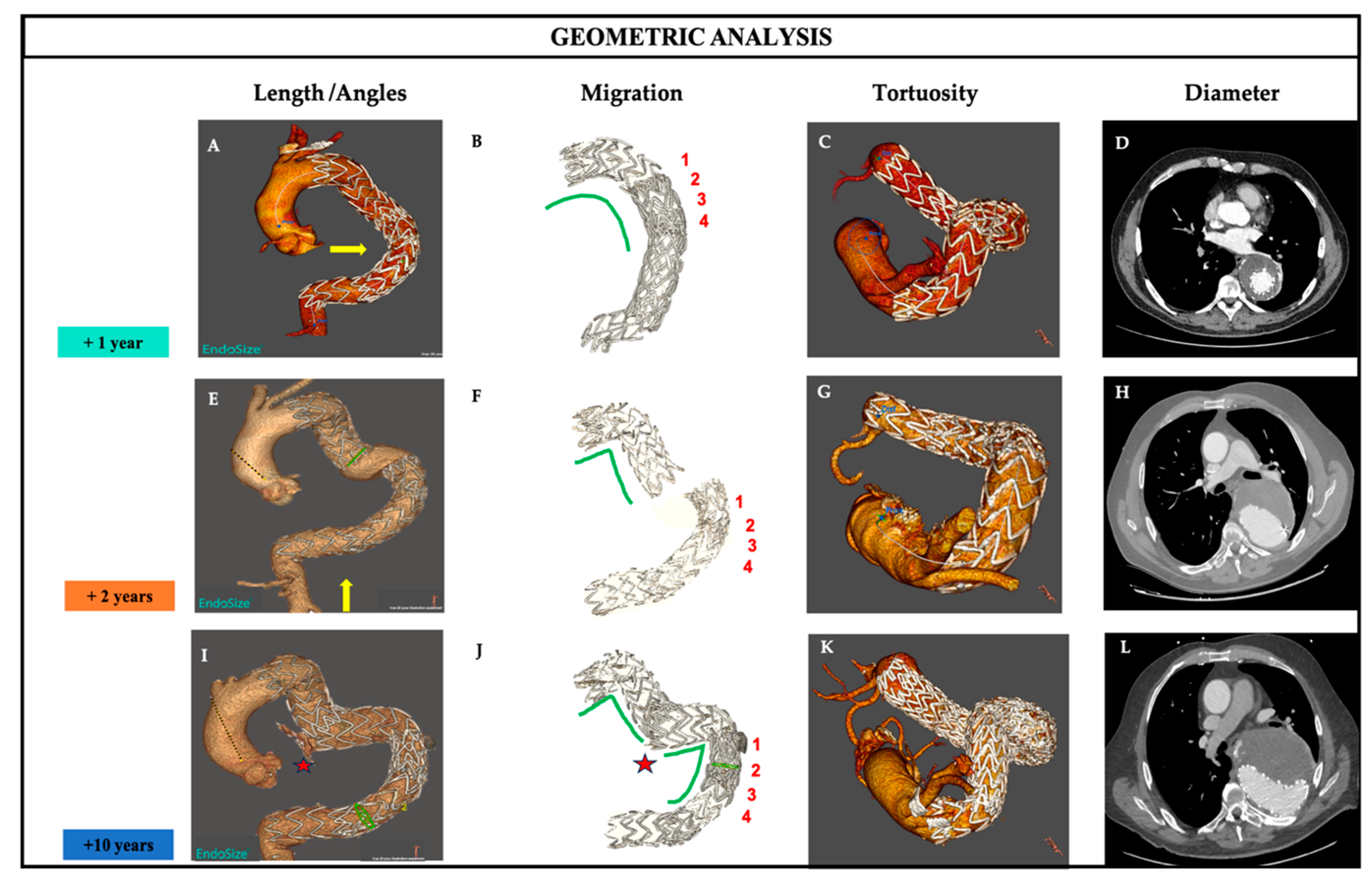
Clinical Case Presentation A 52-year-old male patient was admitted with an extensive descending thoracic aortic aneurysm (DTAA) measuring 73 mm in maximal diameter. A multidisciplinary team decided a cervical debranching procedure (left axillary artery was bypassed from left common carotid artery) followed TEVAR using three Valiant thoracic SG (Medtronic, Santa Rosa, California, USA). The TEVAR procedure was successfully performed, and CTA 12 months later (+1Y) showed no evidence of an endoleak and a regression of the aortic sac (
Figure 2D). However, 24 months (+2Y) post-TEVAR, CTA follow up revealed a type IIIa endoleak caused by the disconnection of two SG (
Figure 2E-F). This required the deployment of an additional stent graft to exclude the aneurysm between the two disconnected SG elements. One hundred thirty-two months after this secondary intervention, the patient presented with acute aortic syndrome accompanied by posterior chest pain. CTA revealed an enlargement of the thoracic aneurysm, now measuring 110 mm in maximal diameter due to a type IIIb endoleak and impending rupture (
Figure 2L). Surgical treatment was complicated by the elongation of the descending thoracic aorta and severe tortuosity of the visceral aorta. A multidisciplinary team opted to use a double guidewire technique to reduce aortic tortuosity during stent graft progression and employed a low-profile TEVAR device. The patient provided informed consent for all procedures, and the ethics committee at our institute approved the use of anonymized image data for this study.
Definitions
Stent migration was defined, in accordance with current guidelines, as a displacement of the stent > 10 mm relative to a primary anatomical landmark [
6]. Type III endoleaks are classified into two subtypes: type IIIA endoleak is a leak between graft components, and type IIIB endoleak originates from a structural defect within the endograft, such as fabric fracture or tear. Migration-related morbidity was defined as of the following: aortic rupture, type I/III endoleaks (ELs), or aneurysm sac expansion. As proposed by Chen et al.[
7] the tortuosity index was used to assess aortic configuration, especially elongation. It is determined by dividing the curved distance along a centerline by the straight spacing between proximal and distal landing zones. In this study, the distance between most proximal and most distal complete stent circumference was used for determination of the tortuosity index. Aortic elongation during follow-up was defined as an increase of centerline-measured aortic length between left carotid commune artery (LCCA) and celiac trunk (CT).
Image Analysis
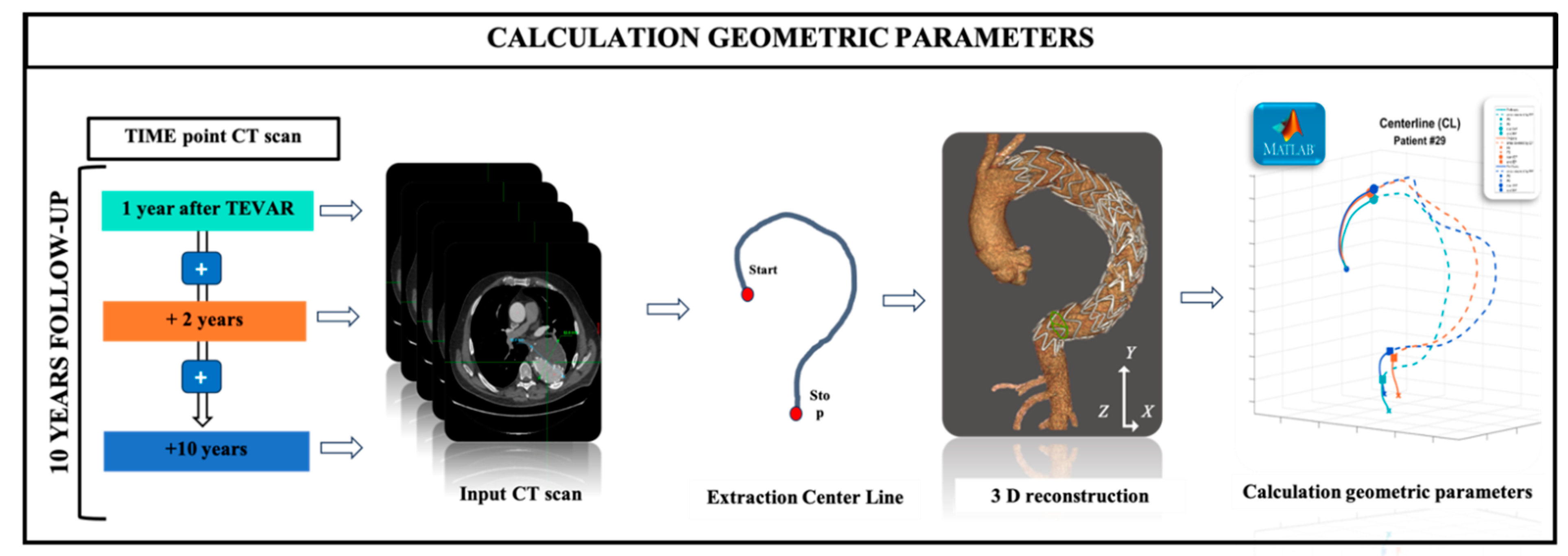
The analysis was performed using the EndoSize software (Therenva, France), a 3D sizing tool optimized for use on a conventional personal computer (
Figure 1). EndoSize was applied to three sets of medical images, clearly demostrating morphological changes of the SG at different timeframes (+1Y, +2Y and +10Y). Each set of medical images was obtained from contrast-enhanced CT scan acquired using a multi-detector 64-row scanner (REVO EVO, General Electric Healthcare, Buc, France) after contrast media injection, following standard parameters. The in-plane resolution was 0.650 mm and the slice thickness was 3.0 mm.
A semi-automatic segmentation process, requiring three user-defined key points, was used to extract the vascular and bony structures. This process allowed the computation of a 3D vessel description scheme, reformatted CT slices, and vessel lumen contours Specifically, the proximal and distal ends of the thoracic aorta from sinotubular junction to celiac trunk, were manually selected, enabling the automatic generation of a 3D aortic centerline (CL). All sizing data were exported for analysis. CL coordinates were recorded at 1-mm interval in R&D tables. These coordinates were further analyzed using custom-developed MATLAB applications.
The study parameters included the calculation of the centerline length (L), angulation (A) and tortuosity (T) along the centerline, as well as the measurement of the migrated distance of the distal end of the centerline of the thoracic SG. The maximal aortic diameter was assessed perpendicular to the CL, including aortic thrombus and aortic wall. With this modeling system, we performed the segmentation and center line extraction process for each time point.
The segmentation was performed both for the native arterial lumen and the SG.
Results
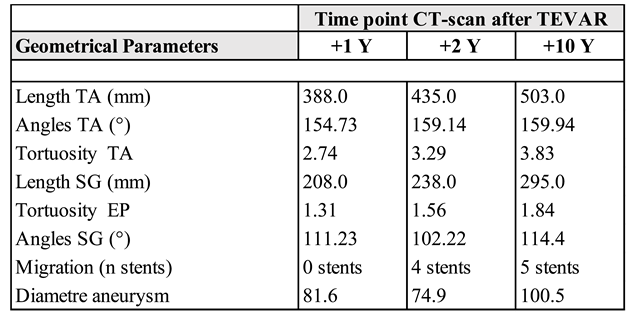
The center lines were extracted at the three time points. The total length of the SG at +1 year after TEVAR is 208 mm. However, the length of the covered aorta after migration of the distal SG showed a significant increase at two years and ten years of follow-up: 238.0 mm at two years (14.4% increase from 1 year) and 295.0 mm at ten years (23.95% increase from 2 years). Subsequently, the thoracic aorta lengthened progressively since the first intervention: from 388.0 mm at 1 year after TEVAR to 435.0 mm at 2 years (12.11% increase) and to 503.0 mm at 10 years (15.63% increase from 2 years), corresponding to a 30% total increase from 1 year.
-
b.
Migration
We evaluated the overlap length by considering the number of stents. In
Figure 1 E, we observed four stent displacements, with one additional stent-graft inserted at 2 years.
-
c.
Angles
The angulation of the entire endograft, and the overall aortic angulation of the whole thoracic aorta were also assessed and calculated between planes perpendicular to the centerline (CL). We observed a gradual increase from 154.73° to 159.94° over 10 years of follow-up for the thoracic aorta, corresponding to an increase of 3%. Meanwhile, the angulation of the stent graft showed a change from 111.23° to 114.4°, reflecting a 2.8% increase. This indicates that while there was a slight increase in the angulation of the thoracic aorta and the stent graft, the overall aortic angulation did not significantly change in this patient, suggesting that the healthy sections of the aorta, both proximal and distal, remained stable.
-
d.
Tortuosity
The tortuosity index is calculated by dividing the centerline distance between two zones by the direct distance. We observed an increase from 2.74 to 3.83, corresponding to an increase of nearly 40%, which indicates that the aorta becomes more twisted or curved over time. This supports the observed increase in length and the slight change in the 'direct/straight' distance between the proximal and distal segments.
-
e.
Diameter
Maximal aortic diameter was assessed perpendicular to the centerline (CL) and included the aortic thrombus and aortic wall. It was automatically computed by the post-processing tool and corrected, if necessary, by the reader. The chronological change in the diameters is shown in
Table 1: they were 81.6 mm, 74.9 mm, and 100.5 mm (after 1, 2, and 10 years post-TEVAR), corresponding to an increase of 23%.
Figure 2.
Long-term follow-up of aortic morphology and Stent-Graft (SG) behavior. One year after TEVAR (+1Y). The aortic diameter decreased to 47 mm with a normal overlapping zone and the absence of tortuosity in the descending thoracic aorta (A.B.C.D). Two years after implantation (+2Y). Stents-graft separation in the overlapping zone resulting in type IIIa endoleak (E.F.), descending thoracic aorta begins to show significant tortuosity (G) and diameter increase (H). Ten years after implantation (+ 10 Y) the development of a type IIIb endoleak with impending rupture (red star in I and J), high tortuosity along the centerline (K) and significant aneurysm growth in L.
Figure 2.
Long-term follow-up of aortic morphology and Stent-Graft (SG) behavior. One year after TEVAR (+1Y). The aortic diameter decreased to 47 mm with a normal overlapping zone and the absence of tortuosity in the descending thoracic aorta (A.B.C.D). Two years after implantation (+2Y). Stents-graft separation in the overlapping zone resulting in type IIIa endoleak (E.F.), descending thoracic aorta begins to show significant tortuosity (G) and diameter increase (H). Ten years after implantation (+ 10 Y) the development of a type IIIb endoleak with impending rupture (red star in I and J), high tortuosity along the centerline (K) and significant aneurysm growth in L.
Table 1.
Total length, tortuosity and angles of the Stent Graft, the distance of the migration and diameter of aneurism at 3 time points.
Table 1.
Total length, tortuosity and angles of the Stent Graft, the distance of the migration and diameter of aneurism at 3 time points.
Discussion
Aortic elongation had a significant influence on the occurrence of late migration of thoracic endografts, thus presenting an independent risk factor for graft migration. Aortic elongation, associated with increased tortuosity, can generate traction forces on the endograft, leading to migration, loss of landing and overlapping zones, as well as type III endoleaks and graft separation [
8]. This phenomenon is particularly visible in patients with large thoracic aortic aneurysms (TAA) involving a long aortic segment, as observed in our patient. Morales et al.[
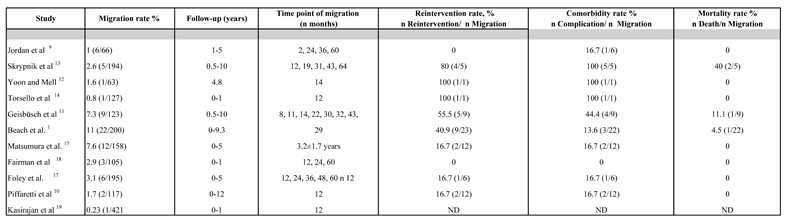
9] emphasized that endografts generally conform to the outer curvature of the aorta or aneurysm. Therefore, in cases of aortic expansion or elongation after TEVAR, additional endograft length is necessary. This can lead to the graft being pulled out of the landing or overlapping zones toward the outer curvature, causing type III endoleaks, as seen in our case. Our results indicate that pronounced tortuosity of the thoracic aortic segment is associated with complications such as endoleaks. Measurements using EndoSize software coupled with MATLAB allowed us to quantify the geometric parameters, revealing that they are key determinants in long-term follow-up, as much as the aortic diameter. To further support our clinical case that type III endoleaks are a relevant pathogenetic factor in the development of rupture and, in particular, enlargement of the sac and drastic changes in the structure of the stent-graft, we conducted an extensive literature search in MEDLINE (via PubMed), Web of Science, and the Cochrane Central Register of Controlled Trials for articles published up to May 2024. No language restrictions were applied, and reference lists of all included studies were manually searched for other potentially eligible studies. We identified only 11 articles, all involving patients treated by TEVAR for thoracic aortic aneurysms with type III endoleaks in the follow-up (
Table 2) [
1,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
The
Table 2 illustrates critical data on stent migration rates, reintervention rates, comorbidity, and mortality following TEVAR. A significant focus is placed on the role of stent-graft (SG) fatigue over time as a contributor to type III endoleaks, migration, and subsequent changes in the aortic geometry, all of which carry serious clinical implications. SGs used in TEVAR are subjected to continuous mechanical stress due to the dynamic movement of the aorta, driven by blood flow, cardiac cycles, and respiratory motion. Over time, this repetitive stress can leads to material fatigue of the stent components, which plays a pivotal role in stent migration. Short-term studies, such as those Jordan et al. [
10] and Fairman et al. [
18] reported low migration rates of 1% and 2.9%, respectively, over follow-up period of 1-5 years. Similary, Piffaretti et al. [
11] documented a 1.7% migration rate after 12 months, and Kasirajan et al. [
19] reported a 0.23% migration rate within the same period. These findings suggest that stent migration is relatively uncommon in the short term. In contrast, longer-term studies reveal a progressively higher risk of stent migration due to fatigue-related mechanical failures in the stent graft (SG) structure. Beach et al. [
1] observed the highest migration rate at 11%, and and Geisbüsch et al.[
12], reported a rates of 7.3% over follow-up periods of up to 10 years. These studies highlight how stent fatigue is closely associated with structural weakening of stent grafts, leading to fracture or elongation that compromises the fixation of the endograft to the aortic wall. Over time, this mechanical stress contributes significantly to migration and associated complications. These findings underscore the need for long-term monitoring of patients undergoing TEVAR and further research into materials and designs that can better withstand prolonged mechanical stress[
20]. Type III endoleaks result from component separation or fabric tears in the endograft, which often occur as a result of stent migration. These leaks are especially dangerous because they allow blood flow back into the aneurysm sac, as seen in our clinical case, thereby increasing the risk of aneurysm expansion and rupture. Studies such as Skrypnik et al.[
14] and Yoon & Mell [
13] demonstrate high reintervention rates for migration cases (80% and 100%, respectively), underscoring the importance of early detection and intervention to prevent catastrophic outcomes like type III endoleaks. Migration often leads to geometric malalignment of stent components, creating gaps that allow blood to flow into the excluded aneurysmal sac. This emphasizes the critical relationship between stent migration and type III endoleak formation. Changes in aortic geometry following endograft placement significantly contribute to the risk of complications. The aorta undergoes dynamic remodeling over time, which can exacerbate mechanical stress on the stent graft, particularly in cases of increased tortuosity. For instance, Beach et al. [
1] reported the highest migration rate at 11% with a significant proportion of these cases requiring reintervention (40.9%) and experiencing complications (13.6%). These finding highlight how changes in aortic curvature and tortuosity impose excessive mechanical stress on the SGs, pontentially leading to fatigue failure and endoleak formation [
21] . In our view, aortic tortuosity is a key factor influencing stent graft behavior. Increased tortuosity alters flow dynamics and amplifies mechanical strain on the graft, contributing to both fatigue failure and migration. This is evident in cases where progressive changes in aortic geometry and stent migration were observed over extended follow-up periods of 5-10 years. The interplay of stent fatigue, migration, and remodeling underscores the necessity of long-term monitoring and adaptive strategies in the management of TEVAR patients.
Table 2.
Migration of Stent-graft with related reinterventions, morbidity and mortality.
Table 2.
Migration of Stent-graft with related reinterventions, morbidity and mortality.
Conclusion
Our clinical case study provides compelling evidence that stent graft (SG) migration leads to significant aortic geometric changes, which are crucial determinants of SG durability. Migration-induced aortic remodeling is associated with severe complications, such as aneurysm rupture, due to re-pressurization of the sac and subsequent risks of endoleaks. Furthermore, changes in aortic geometry, including elongation and increased tortuosity, complicate reinterventions by making navigation through the aorta significantly more challenging. This highlights the importance of preventive strategies and early detection of migration to avoid such undesirable consequences. The literature reviewed further supports the need for timely detection and management of SG migration to mitigate risks of type III endoleaks, aneurysm expansion, and rupture. Migration rates tend to increase over time, emphasizing the role of fatigue-related mechanical failures in stent materials. High rates of reintervention in cases of migration demonstrate the clinical impact of this complication and the necessity of proactive follow-up strategies.
Future advancements in stent materials, including fatigue-resistant alloys and more flexible designs, alongside innovations in anchoring techniques, may help reduce the incidence of migration and improve the long-term outcomes of thoracic endovascular aortic repair (TEVAR). Additionally, integrating advanced imaging technologies and computational modeling into routine follow-up may allow for earlier detection of migration or other complications, paving the way for more personalized and preventive care in patients undergoing TEVAR.
References
- Beach JM, Kuramochi Y, Brier C, Roselli EE, Eagleton MJ. Durable outcomes of thoracic endovascular aortic repair with Zenith TX1 and TX2 devices. J Vasc Surg. mai 2017;65(5):1287-96.
- Ricotta, JJ. Endoleak management and postoperative surveillance following endovascular repair of thoracic aortic aneurysms. J Vasc Surg. oct 2010;52(4 Suppl):91S-9S.
- De Masi, M. Thoracic Aorta Remodeling after TEVAR: Monitoring Morphological Parameters to Predict Unfavorable Evolution. J Surg [Internet]. 7 juill 2023 [cité 19 mars 2024];8(12). Available online: https://www.gavinpublishers.com/article/view/thoracic-aorta-remodeling-after-tevar--monitoring-
morphological-parameters-to-predict-unfavorable-evolution.
- Grassi V, Trimarchi S, Weaver F, De Beaufort HWL, Azzizzadeh A, Upchurch Jr GR, et al. Endovascular repair of descending thoracic aortic aneurysms—a mid-term report from the Global Registry for Endovascular Aortic Treatment (GREAT). European Journal of Cardio-Thoracic Surgery [Internet]. 24 janv 2022 [cité 10 déc 2023];61(2):357-64. Available online: https://academic.oup.com/ejcts/article/61/2/357/6352543.
- De León Ayala IA, Cheng YT, Chen SW, Chu SY, Nan YY, Liu KS. Outcomes of type Ia endoleaks after endovascular repair of the proximal aorta. J Thorac Cardiovasc Surg. juin 2022;163(6):2012-2021.e6.
- Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL, Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. oct 2010;52(4):1022-33, 1033.e15.
- Chen CK, Chou HP, Chang YY, Shih CC. Elongation of the Aorta after Thoracic Endovascular Aortic Repair: A longitudinal study. IJERPH [Internet]. 13 févr 2020 [cité 25 mars 2024];17(4):1205. Available online: https://www.mdpi.com/1660-4601/17/4/1205.
- Hassoun HT, Mitchell RS, Makaroun MS, Whiting AJ, Cardeira KR, Matsumura JS. Aortic neck morphology after endovascular repair of descending thoracic aortic aneurysms. Journal of Vascular Surgery [Internet]. janv 2006 [cité 26 janv 2025];43(1):26-31. Available online: https://linkinghub.elsevier.com/retrieve/pii/S074152140501726X.
- Morales JP, Greenberg RK, Morales CA, Cury M, Hernandez AV, Lyden SP, et al. Thoracic aortic lesions treated with the Zenith TX1 and TX2 thoracic devices: Intermediate- and long-term outcomes. Journal of Vascular Surgery [Internet]. juill 2008 [cité 26 janv 2025];48(1):54-63. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0741521408002796.
- Jordan WD, Desai N, Letter AJ, Matsumura JS. Long-term outcomes of the conformable TAG thoracic endoprosthesis in a prospective multicenter trial. J Vasc Surg. nov 2021;74(5):1491-8.
- Piffaretti G, Negri S, Ferraro S, Bossi M, Rivolta N, Fontana F, et al. Delayed graft dislocation after thoracic aortic endovascular repair. Kathmandu Univ Med J (KUMJ). 2014;12(46):97-100.
- Geisbüsch P, Skrypnik D, Ante M, Trojan M, Bruckner T, Rengier F, et al. Endograft migration after thoracic endovascular aortic repair. J Vasc Surg. mai 2019;69(5):1387-94.
- Yoon WJ, Mell MW. Outcome comparison of thoracic endovascular aortic repair performed outside versus inside proximal landing zone length recommendation. J Vasc Surg. déc 2020;72(6):1883-90.
- Skrypnik D, Bischoff MS, Meisenbacher K, Kronsteiner DB, Böckler D. A 10-Year Single-Center Experience With the GORE TAG Conformable Thoracic Stent Graft in the Treatment of Thoracic Aortic Disease. J Endovasc Ther. juin 2022;29(3):370-80.
- Torsello GF, Argyriou A, Stavroulakis K, Bosiers MJ, Austermann M, Torsello GB, et al. One-Year Results From the SURPASS Observational Registry of the CTAG Stent-Graft With the Active Control System. J Endovasc Ther. juin 2020;27(3):421-7.
- Matsumura JS, Melissano G, Cambria RP, Dake MD, Mehta S, Svensson LG, et al. Five-year results of thoracic endovascular aortic repair with the Zenith TX2. J Vasc Surg. juill 2014;60(1):1-10.
- Foley PJ, Criado FJ, Farber MA, Kwolek CJ, Mehta M, White RA, et al. Results with the Talent thoracic stent graft in the VALOR trial. J Vasc Surg. nov 2012;56(5):1214-1221.e1.
- Fairman AS, Beck AW, Malas MB, Goodney PP, Osborne NH, Schermerhorn ML, et al. Reinterventions in the modern era of thoracic endovascular aortic repair. J Vasc Surg. févr 2020;71(2):408-22.
- Kasirajan K, Morasch MD, Makaroun MS. Sex-based outcomes after endovascular repair of thoracic aortic aneurysms. Journal of Vascular Surgery [Internet]. sept 2011 [cité 18 oct 2024];54(3):669-76. 0741. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0741521411004447.
- Torsello GF, Inchingolo M, Austermann M, Torsello GB, Panuccio G, Bisdas T. Durability of a low-profile stent graft for thoracic endovascular aneurysm repair. Journal of Vascular Surgery [Internet]. déc 2017 [cité 18 oct 2024];66(6):1638-43. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0741521417311503.
- De Masi M, Guivier-Curien C, Cortaredona S, Omnes V, Bal L, Muselier B, et al. The Value of Aortic Volume and Intraluminal Thrombus Quantification for Predicting Aortic Events after Endovascular Thoracic Aneurysm Repair. J Clin Med. 18 mai 2024;13(10):2981.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).