1. Introduction
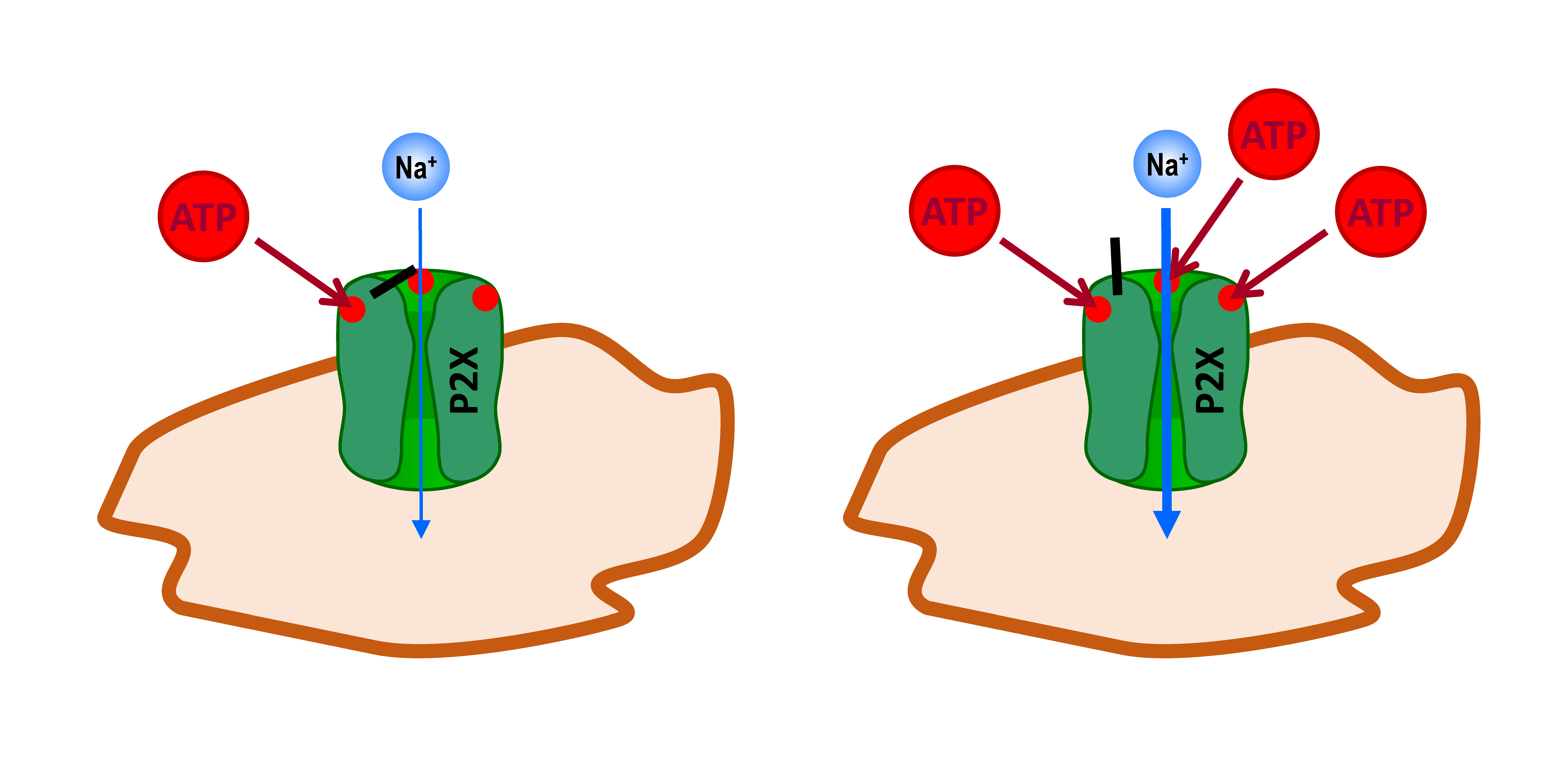
The P2X7 receptor (P2X7R) is a non-selective cation channel predominantly expressed in cells of the immune and inflammatory systems. Like other P2X subunits, except P2X6 [
1,
2,
3,
4], P2X7 subunits efficiently assemble as homotrimers of three identical membrane-spanning subunits [
5]. The P2X7R is activated by extracellular ATP, which is released from stressed or dying cells under pathophysiological conditions such as coagulation, inflammation and cell death. Extracellular ATP acts as a Danger-Associated Molecular Patterns (DAMPs) [
6,
7], signaling through the P2X7R to modulate immune responses.
High extracellular ATP concentrations are required for P2X7R activation for two reasons: (i) under physiological conditions, released ATP is largely complexed by millimolar extracellular Ca
2+ and Mg
2+, and (ii) the genuine P2X7R agonist, free-ATP (ATP
4-), has a relatively low affinity, with an EC
50 of ~100 µM in Ca
2+- and Mg
2+-free media [
8,
9]. In contrast, in divalent-free solutions, the EC
50 values of P2X1, P2X2, P2X3, and P2X4 receptors range only between 1.2 and 2.0 µM ATP, which corresponds to the fully ionized form ATP
4- as divalents cations were absent in these experiments [
10]. For clarity and consistency, we explicitly use ATP
4- when referring to the free ATP concentration and ATP when referring to the total ATP concentration [
11]. Since the extracellular solution in voltage clamp experiments was always divalent cation-free, in the “Results” section, we refer consistently to the agonist ATP
4- simply as ATP.
The P2X7R is formed by three identical membrane-spanning subunits with large extracellular and intracellular domains [
12]. The ectodomain contains three ATP binding sites, one at each subunit interface, which are located in the large extracellular domain at the interface between two adjacent subunits [
4,
12]. The binding pocket is formed by the head, upper body and left flipper of one subunit, and the lower body and dorsal fin of the adjacent subunit. Key residues involved in the coordination of ATP in a U-shaped configuration in the rat P2X7 receptor include the positively charged residues K64, K66, and K311, as well as T189, which interacts with the adenine moiety, the β-phosphate group with N292, and R294 [
4,
12]. These residues are conserved across the P2X family [
13]. K64 plays a crucial role in ATP binding for the P2X7 receptor by being centrally located among the three phosphate groups, forming hydrogen bonds with all of them, which is essential for ATP activation.
Although apparently structurally identical, we have previously observed that the three ATP binding sites of the human P2X7R (hP2X7R) contribute differentially to activation [
14]. High and low affinity functional ATP activation sites were identified with dissociation constants of 10 and 300 µM ATP
4-, respectively. The high affinity activation site leads to an activation of approximately 10% of the maximum. For a full activation of the hP2X7R, at least two ATP
4- molecules must bind with a K
D value of 300 µM ATP
4-, as measured by single-channel recording [
15].
To gain better insight into the putative sequential activation of rP2X7R through stepwise ATP binding at its three ATP activation sites, we generated genetically concatenated trimeric rP2X7R constructs with zero (7-7-7) to three (7ko-7ko-7ko) ATP binding site knockouts. Using long, flexible linkers, we observed negligible proteolytically cleaved side products from concatenated rP2X7 trimers, but not from concatenated rP2X7 dimers. AlphaFold2 modeling revealed only minimal effects of the introduced linkers on the overall structure of the trimeric rP2X7 concatamers. Following expression in X. laevis oocytes, two-microelectrode voltage-clamp recordings on concatenated rP2X7 trimers revealed that the binding of a single ATP4- molecule is sufficient to partially activate the P2X7R. When all three ATP4- binding sites are occupied, allosteric effects result in distinct functional activation states.
2. Materials and Methods
2.1. Reagents
Standard chemicals and molecular biology reagents were obtained from Merck (Darmstadt, Germany) and New England Biolabs (Schwalbach, Germany), at analytical or higher grade, unless otherwise specified.
2.2. Cloning of rP2X7 Concatamers
The rat P2X7 receptor (rP2X7R) was first identified and cloned as a permeabilizing receptor [
16]. Based on this sequence, we cloned the rP2X7R cDNA from total rat brain RNA using RT-PCR into the pNKS2 vector, as previously described [
17,
18]. rP2X7 concatamers were generated using a previously described strategy [
19]. Briefly, NcoI and BspHI restriction sites were inserted at the 5’ and 3’ ends of the rP2X7R coding region, respectively, using the QuikChange site-directed mutagenesis protocol [
20] with Phusion high-fidelity DNA polymerase and DpnI restriction endonuclease (both from New England BioLabs, Schwalbach, Germany).
To enable protein purification, the sequence encoding a double repeat of the Strep-tagII (WSHPQFEK), also known as Twin-Strep-tag or SIII tag [
21] was inserted following codon 4 (alanine) of the rP2X7 to generate
S3rP2X7 (see cartoon
Figure 1). The codon for asparagine (N) in the original Strep-tagII sequence (NWSHPQFEK) was omitted to avoid creating an N-glycosylation site that is utilized when luminally exposed [
22]. Additionally, to abolish ATP-dependent activation, a K
64A mutation was introduced to generate
S3rP2X7
ko. The crucial role of basic residues in ATP-dependent activation was first demonstrated for the rP2X1 receptor, where the K68A mutation resulted in a non-functional but plasma membrane-expressed channel with a 1,800-fold reduced ATP potency [
23]. In the P2X7R, K64 is the residue homologous to K68 in rP2X1.
All DNA constructs were designed and analyzed using Vector NTI Deluxe software. Introduced mutations were verified by comparing both the band patterns on agarose gels, generated by digestion with selected restriction enzymes, and commercial DNA sequencing results (Eurofins Genomics, Ebersberg, Germany) to the in silico predictions made using Vector NTI predictions. Exact the same modular assembly strategy was used to clone the concatenated S3rP2X4 homotrimer.
Full-length coding sequences, including appropriate flanking sequences, were excised using HindIII and BspHI and ligated in-frame between the HindIII and NcoI sites of the parental rP2X7 plasmids to generate concatenated S3rP2X7 and S3rP2X7ko homo- and heteromultimers. Note that NcoI and BspHI sites can be ligated together, but the resulting hybrid cannot be cleaved at the ligated sites by either enzyme. This principle enables a modular assembly approach to generate S3rP2X7 and S3rP2X7ko-containing concatamers in any desired order.
cRNA was synthesized as previously described in detail [
24] including co-transcriptional incorporation of the anti-reverse cap analog (ARCA Cap Analog, m
27,3’-OGp
3G; NU-855; Jena Bioscience, Germany) to ensure the correct orientation at the ATG start codon of the cRNA [
25]. Polyadenylation, which enhances cRNA translation in
X. laevis oocytes [
26], was co-transcriptionally encoded by the pNKS2 vector used [
17]. The quality of the cRNA was assessed by ethidium bromide-stained agarose gel electrophoresis and spectrophotometric determination of concentration and the 260/280 nm absorbance ratio, which ranged from 2.00 to 2.04, indicating minimal protein contamination.
2.3. Electrophysiological Characterization of rP2X7 Concatamers by Two-Electrode Voltage Clamp
Collagenase-defolliculated
X. laevis oocytes injected with cRNAs encoding the indicated rP2X7 constructs were cultured for 2-3 days at 21°C [
18]. To ensure comparable amplitudes of ionic currents, the cRNA of the rP2X7 concatamer 7-7-7 (without knockout) was diluted 1:10 to a final concentration of approximately 0.1 µg/µl in voltage-clamp experiments.
2.4. Biochemical Visualization of the Intactness of Plasma Membrane-Bound P2X7 Concatamers
S3-affinity-tagged rP2X7R subunits were expressed in
X. laevis oocytes by cRNA injection as monomers, concatenated dimers, or concatenated trimers. On day 3 post-injection, the cell surface of intact oocytes was covalently labeled with membrane-impermeable fluorescent LI-Cor IRDye 800CW as previously described [
27]. A digitonin extract (1% digitonin) of the oocytes was prepared [
26], from which the indicated proteins were purified by Strep-Tactin chromatography [
5]. The purified proteins were then denatured by incubation with 0.2% SDS and 10 mM DTT at 37°C in a Coomassie-fre sample buffer and resolved on a clear SDS-PAGE gradient gel. Coomassie was avoided because it would strongly suppress fluorescence signals. Instead, fluorescence scanning of the wet SDS-PAGE gel using a Bio-Rad ChemiDoc MP visualized the 800CW-stained plasma membrane-bound proteins in the near-infrared channel and the covalently blue-stained Precision Plus Protein Standards (Bio-Rad) in the red channel. The intensity of the 800CW-stained bands was quantified using Bio-Rad Image Lab software.
2.5. Two-Electrode Voltage-Clamp Recording (TEVC)
The voltage clamp protocol was as described previously [
18]. In brief, microelectrodes filled with 3 M KCl, with resistances of 0.8–1.6 MΩ, were impaled into oocytes superfused with oocyte Ringer's solution (ORi: 100 mM NaCl, 2.5 mM KCl, 1 mM CaCl
2, 1 mM MgCl
2, 5 mM HEPES, pH 7.4). Currents were recorded at room temperature using an OC-725C oocyte clamp amplifier (Warner Instruments, Hamden, CT, USA). The signals were filtered at 100 Hz and sampled at 85 Hz, with a holding potential of -40 mV. Switching between the different bathing solutions was achieved within <1 s by a set of computer-controlled magnetic valves using a modified U-tube technique [
24].
Measurements of the rP2X7R-dependent currents were performed in bathing solutions consisting of 100 mM NaCl, 2.5 mM KCl, and 5 mM HEPES, pH 7.4. This Ca2+-free solution was supplemented with 0.1 mM flufenamic acid to block the conductance evoked by external divalent cation removal. To test for rP2X7R-dependent changes of the cell membrane conductance, the Ca2+-free solution was replaced by the same solution with added ATP.
The data were stored and analyzed on a personal computer. For ion current recording and analysis, a custom software system developed in our department was used (Superpatch 2000, SP-Analyzer by T. Böhm). The SigmaPlot program (SPSS) was used for non-linear approximations and graphical representations of the data. Statistical data were expressed as mean ± SEM and analyzed via one-way ANOVA. The statistical significance of the differences between means was tested using multiple t-tests with Bonferroni correction, performed using the SigmaPlot program. Significance was set at p < 0.05.
2.6. Alphafold2 Structure Predictions of Concatenated and Non-Concatenated rP2X7 Homotrimers
To potentially detect gross structure disturbances caused by the introduced 126-residue intersubunit linker and Strep-tag sequences, the full amino acid sequence of the 1911-residue rP2X7 7-7-7 concatamer was run on AlphaFold2 ColabFold v1.5.5 with the following settings: Template mode: none; MSA mode: mmseq2_unifre_env; Pair mode: unpaired-paired; Advanced settings: Model type: alphafold2_multimer_v3; Number of recycles: 6; Recycle early stop tolerance: 0.5; Relax max iterations: 200; Pairing strategy: greedy [
28]. The models obtained were then visualized via their PDB files using PyMOL (Schrödinger LLC). For a direct comparison, we used the same settings to generate an AlphaFold2 model of the non-concatenated rP2X7 protein, despite the availability of cryo-EM structures [
4,
12].
2.7. Positioning of the Alphafold2 Models of the rP2X7 and 7-7-7 Concatamer in the Membrane
The membrane boundaries of the Alphafold2 modeled structures of the rP2X7 7-7-7 concatamer (total length 1,911 residues) and the wild-type non-concatenated rP2X7 homotrimer (total length 1,785 residues) were determined by uploading the PDB files to the PPM2.0 Web Server (
https://opm.phar.umich.edu/ppm_server2_cgopm), which is part of the OPM (Orientations of Proteins in Membranes) server (
https://opm.phar.umich.edu/), using the following conditions: “Membrane type: Mammalian plasma membrane,” “Allow curvature” set to “Yes”, and topology with the: “N-terminus in” [
29]. The resulting PDB files, which included the membrane boundaries, were then downloaded and visualized using PyMOL 3 (Schrödinger LLC).
2.8. Comparison of the Alphafold2-Modeled Structures of the rP2X7 and 7-7-7 with PyMOL 3
To compare the structures of the two AlphaFold2-modeled proteins, we superimposed their structures in PyMOL 3 using the align command to visualize and quantify their spatial alignment. The root mean square deviation (RMSD) was calculated to determine the average distance between corresponding atoms in the two structures, serving as a measure of their similarity. The structures were visualized in a cartoon to illustrate the secondary structure and spatial arrangement of the proteins and color-coded to highlight differences in conformation or the position of amino acids.
3. Results
3.1. Evaluation of the Proteoloytic Stability of Homodimeric and Homotrimeric rP2X7 Concatamers
The N- and C-terminal ends of P2X receptors are generally located intracellularly, allowing the expression of covalently linked subunit concatamers through genetic N- to C-terminal linkage. Based on our previous observation that rP2X1 concatamers can be proteolytically unstable when expressed in
X. laevis oocytes [
19], we genetically linked the rP2X7 subunits N- to C-terminally with long GGS-linkers to improve concatamer stability by increasing flexibility. We also included intervening “Strep-double tags” to enable their purification.
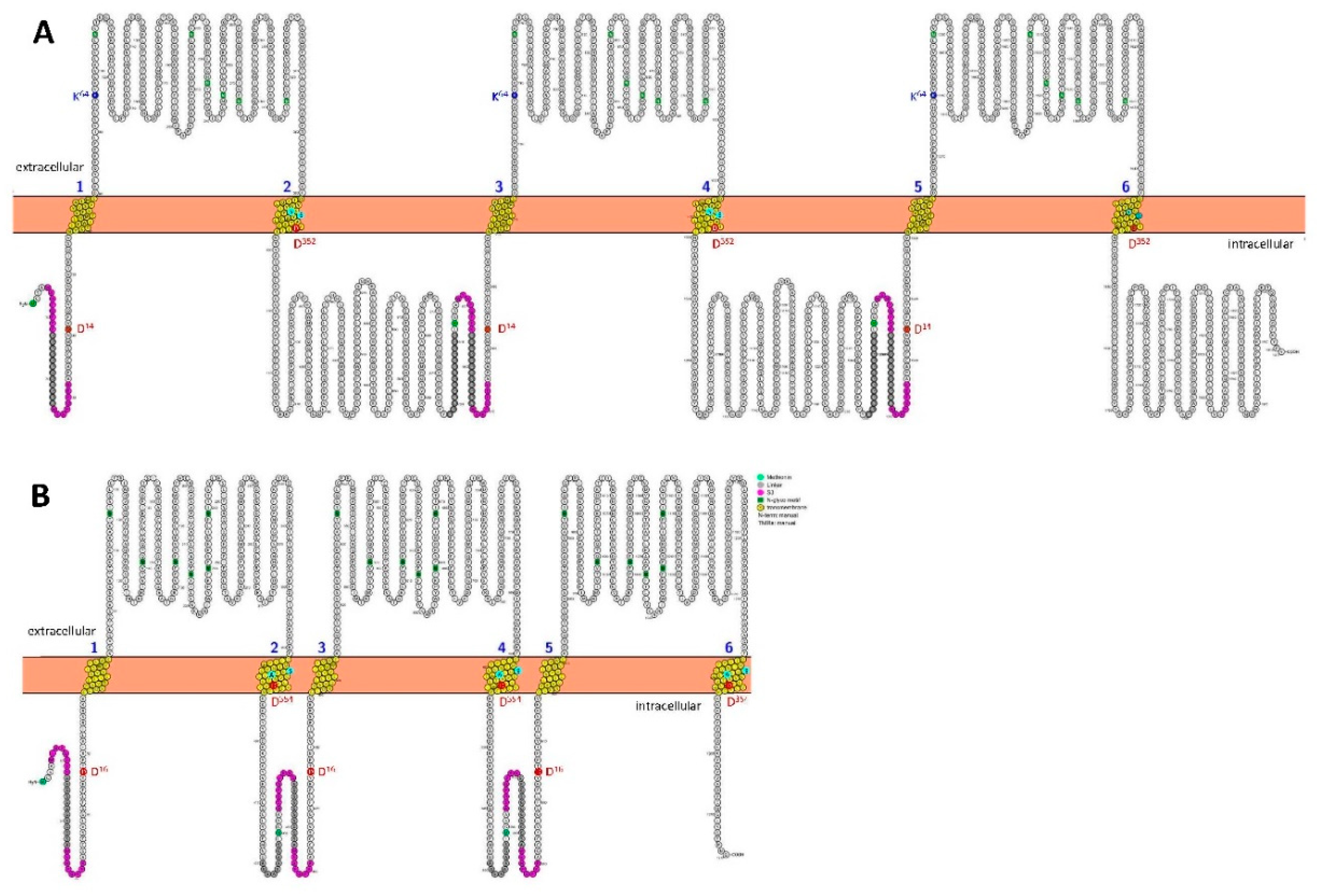
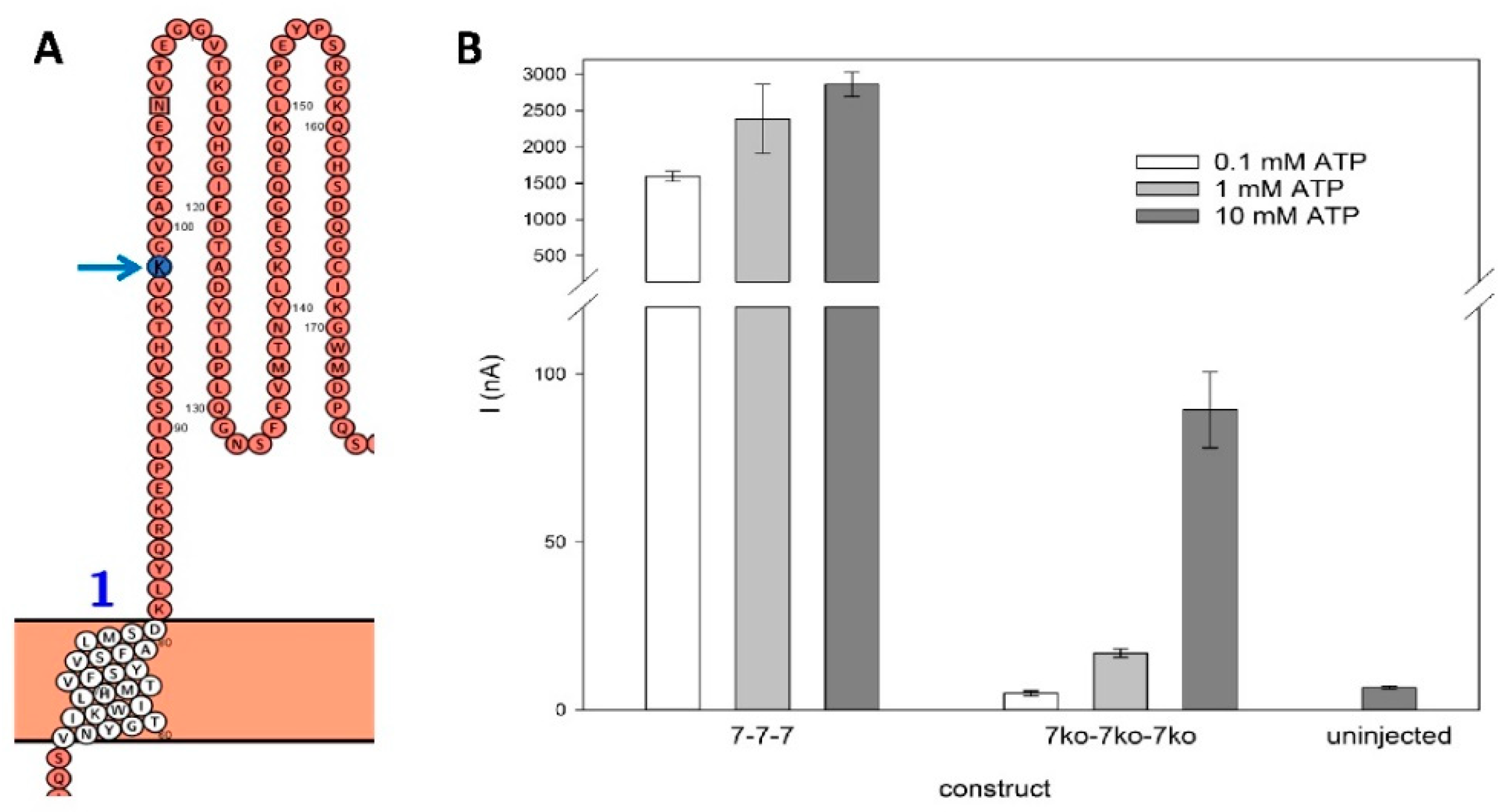
Figure 1 illustrates the transmembrane folding of the wild-type (wt) homotrimeric
S3rP2X7 and
S3rP2X4 concatamers, generated using the online tool Protter [
30], available at (
https://wlab.ethz.ch/protter). The residues equivalent to the K64 residue, highlighted in blue in the ectodomain of each rP2X7 subunit, are completely conserved across the P2X family. Mutation of this lysine residue to alanine abolishes ATP-dependent activation, as first demonstrated for the corresponding rP2X1R
K68A mutant [
23].
Figure 1.
Transmembrane topology of homotrimeric rP2X7 and rP2X4 concatamers. Membrane-embedded residues are shown in yellow, N-glycosylation sites in green, the S3 tag sequences (WSHPQFEK) are colored magenta, and the long and flexible GGS linker sequences are shown in grey. (
A) rP2X7: The cation-selectivity determining residues D14 and D352 are shown in red (D41 and D379 in concatamer numbering), the gating residues S339 and S342 in cyan (S366 and S369 in concatamer numbering), and the ATP-binding residue K64 (knocked out by mutation to alanine singly, doubly, or up to triply in the 7
ko-7
ko-7
ko concatamer) in blue (K96 in concatamer numbering). (
B) rP2X4: Residues homologous to those in rP2X7 in rP2X4 are D16, D354 (D47 and D385 in concatamer numbering), and the putative gating residues S341 and A344 in cyan (S372 and S375 in concatamer numbering). The figures were generated using the open-source tool Protter (
https://wlab.ethz.ch/protter/start).
Figure 1.
Transmembrane topology of homotrimeric rP2X7 and rP2X4 concatamers. Membrane-embedded residues are shown in yellow, N-glycosylation sites in green, the S3 tag sequences (WSHPQFEK) are colored magenta, and the long and flexible GGS linker sequences are shown in grey. (
A) rP2X7: The cation-selectivity determining residues D14 and D352 are shown in red (D41 and D379 in concatamer numbering), the gating residues S339 and S342 in cyan (S366 and S369 in concatamer numbering), and the ATP-binding residue K64 (knocked out by mutation to alanine singly, doubly, or up to triply in the 7
ko-7
ko-7
ko concatamer) in blue (K96 in concatamer numbering). (
B) rP2X4: Residues homologous to those in rP2X7 in rP2X4 are D16, D354 (D47 and D385 in concatamer numbering), and the putative gating residues S341 and A344 in cyan (S372 and S375 in concatamer numbering). The figures were generated using the open-source tool Protter (
https://wlab.ethz.ch/protter/start).
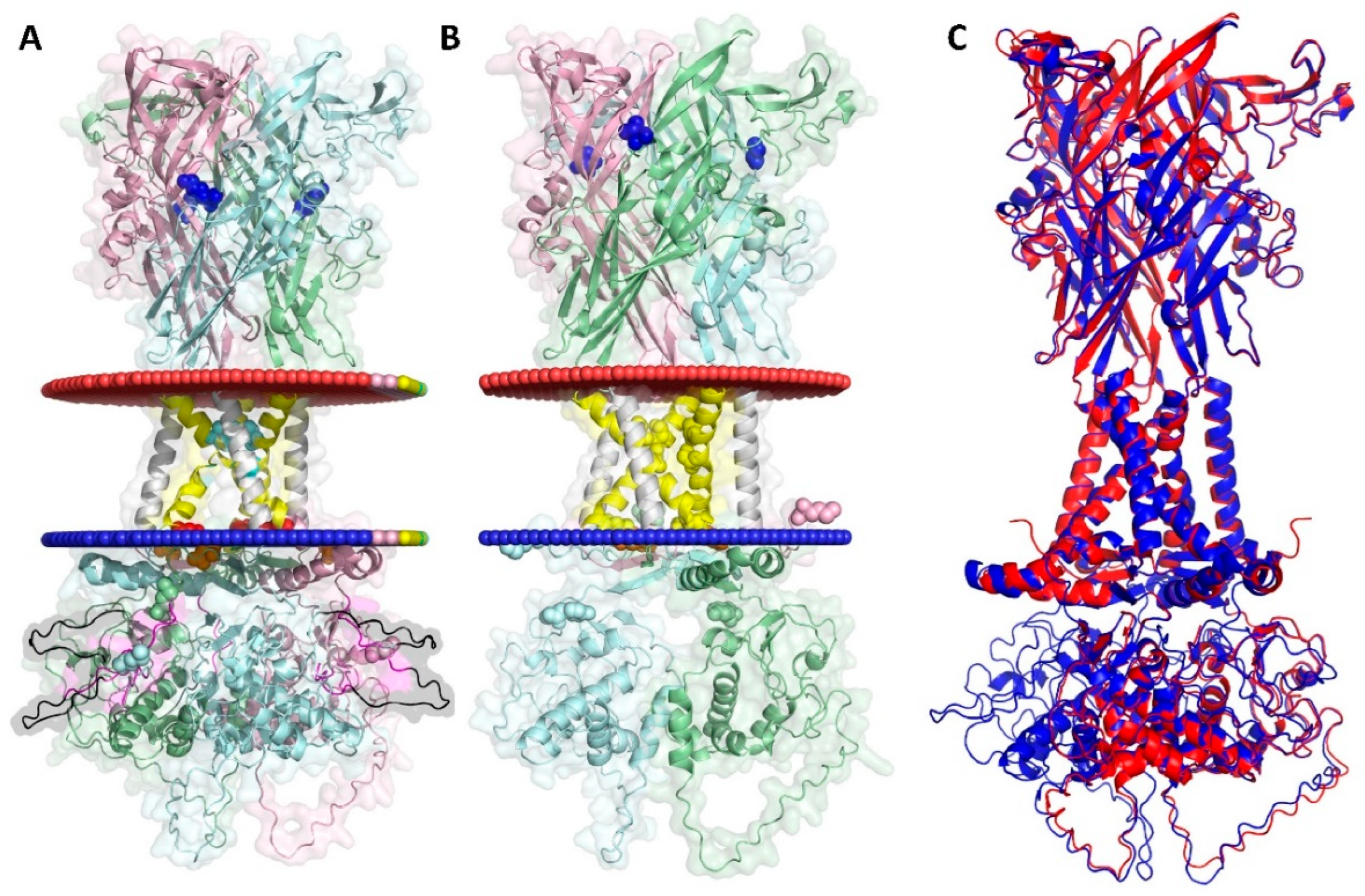
Figure 2A shows the full-length AlphaFold2-modeled structure of the 7-7-7 concatenated homotrimer, comprising 585 residues per monomer, connected by GGS linkers and Strep tags highlighted in the cytoplasmic domain (for the exact sequences, see
Figure 1A).
Figure 2B depicts a non-concatenated rP2X7R AlphaFold2-modeled homotrimer, consisting of the same 585 genuine rP2X7R residues per monomer, but lacking the cytoplasmic GGS linkers and Strep tags present in the concatamer in
Figure 2A.
To visualize the potential impact of the engineered linker and Strep-tag sequences on the overall structure, we superimposed both structures using PyMOL 3 (
Figure 2C). As a measure of their similarity, we calculated the root mean square deviation (RMSD), which quantifies the average distance between corresponding atoms in the two structures [
31]. The RMSD of 0.481 Å between the two structures indicates a high degree of structural similarity, suggesting that the linkers and Strep tags do not significantly alter the overall structure of the concatamer. However, the linkers may still influence protein stability and flexibility, potentially affecting biological activity.
In addition, to verify their biochemical integrity, we expressed homodimeric and homotrimeric rP2X7 concatamers by cRNA injection in
X. laevis oocytes for three days, purified them by StrepTactin chromatography, and resolved them by SDS-PAGE. The concatameric constructs, including the single, double and triple (7
ko-7
ko-7
ko) knockouts
o, were detected at the
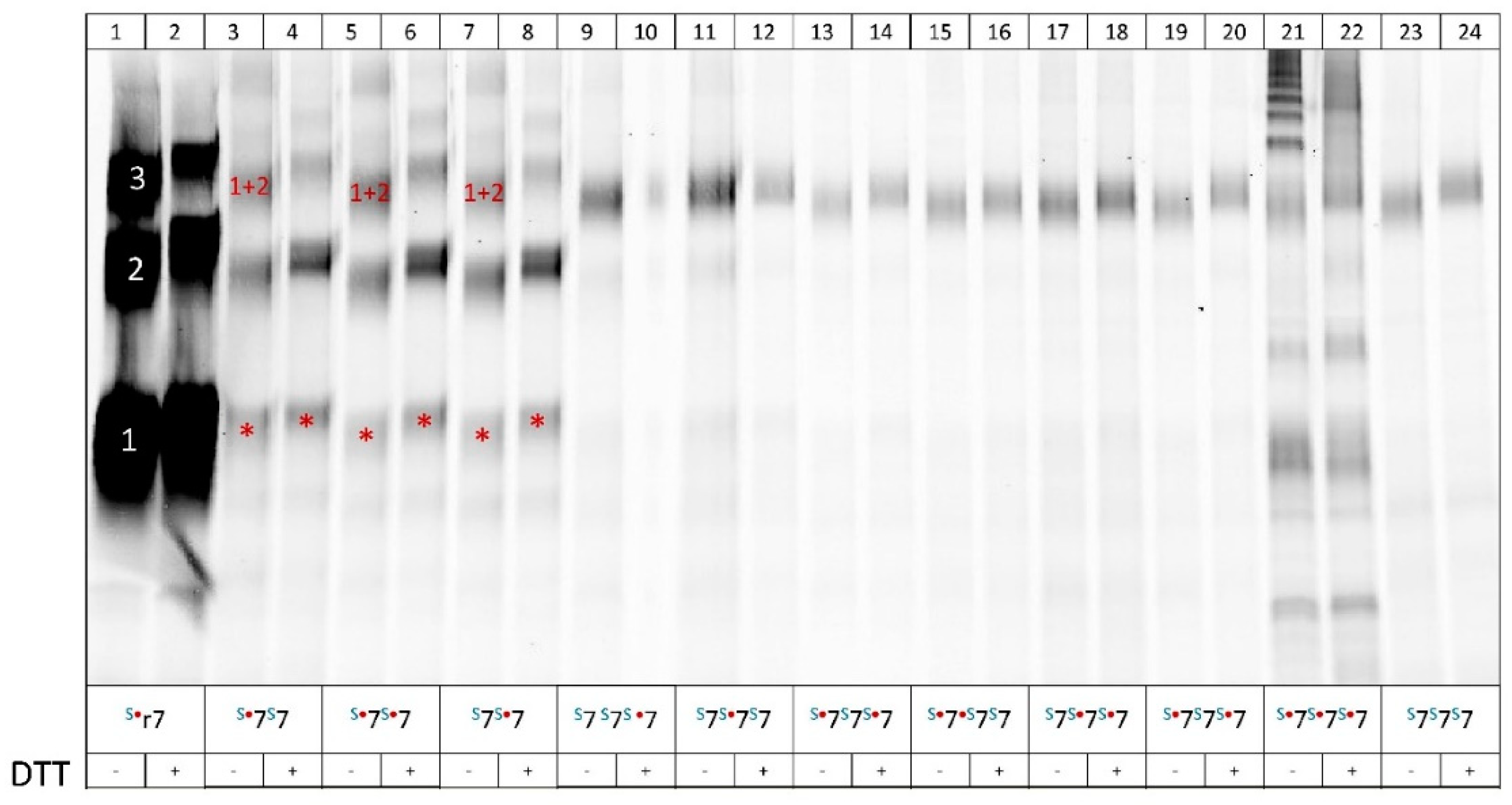
oocyte's plasma membrane three days after injection of the corresponding cRNAs, albeit at a significantly lower level (
Figure 3, lanes 3-24) compared to the parental monomers (
Figure 3, lanes 1-2). The expression level was not significantly influenced by the K64A knock-out mutation.
The positive result is that the concatenated rP2X7 homotrimers migrated virtually completely as homotrimers (
Figure 3, lanes 3-24). This view was further substantiated by quantitative scanning of the IR800 dye covalently bound to the rP2X7 concatamers expressed in the plasma membrane, which revealed little to no cleavage products migrating as a dimer or monomer (Supplementary
Figure 3).
However, significant amounts of monomeric cleavage products were detected when homodimeric concatamers were expressed (
Figure 3, lanes 3-8). The expression of homodimers resulted in obvious proteolytic cleavage of a fraction of the expressed homodimers, as evident from the significant amounts of apparently rP2X7 monomers that are absent when concatenated homotrimers are expressed (
Figure 3, lanes 9-20 and 23-24; see also the quantitative evaluation of
Figure 3 in the Supplementary
Figure 1). A likely explanation is that the monomers are part of a frustrated assembly as a pseudo-tetramer, consisting of two dimeric rP2X7 concatamers, which results in ER-associated degradation [
32] of the subunit not integrated into the trimeric structure, akin to a “leave-one-out” scenario. The final “heterotrimeric” construct (labeled in Fig.3 as 1-2) is exported to the plasma membrane, where it is labeled by the membrane-impermeable IR800 dye. Most likely, also the visible concatenated homodimers and the monomer (labeled with an asterisk) also existed as pseudo-homotrimers before they were purified and partially denatured by SDS. Taken together, misassembly due to a non-native structure, here a tetramer formed of two dimers), combined with ER-associated degradation can lead to a significant misinterpretation of functional experiments when not combined with appropriate biochemical controls.
3.2. Electrophysiological Characterization of Knockouts of Individual ATP Binding Sites in Trimeric rP2X7 Concatamers
The localization of the K64A mutation, which knocks out ATP binding sites in the rP2X7 ectodomain, is schematically illustrated in
Figure 4A. The drastic reduction in ATP-induced currents for the triple knockout, by a factor of approximately 0.03 across all tested ATP concentrations (
Figure 4B), indicates a significant decrease in the ATP affinity of the mutated agonist binding sites.
Although the detailed effect of the knockouts on single-channel current and channel open probability is not known, the similar physical expression of all concatameric constructs (
Figure 3) is reflected by comparable ATP-induced currents when the cRNA of the 7
wt-7
wt-7
wt concatamer was diluted 1:10 for injection into
X. laevis oocytes (
Figure 5D). Typical time courses of ATP-induced rP2X7 concatamer-mediated currents are shown in
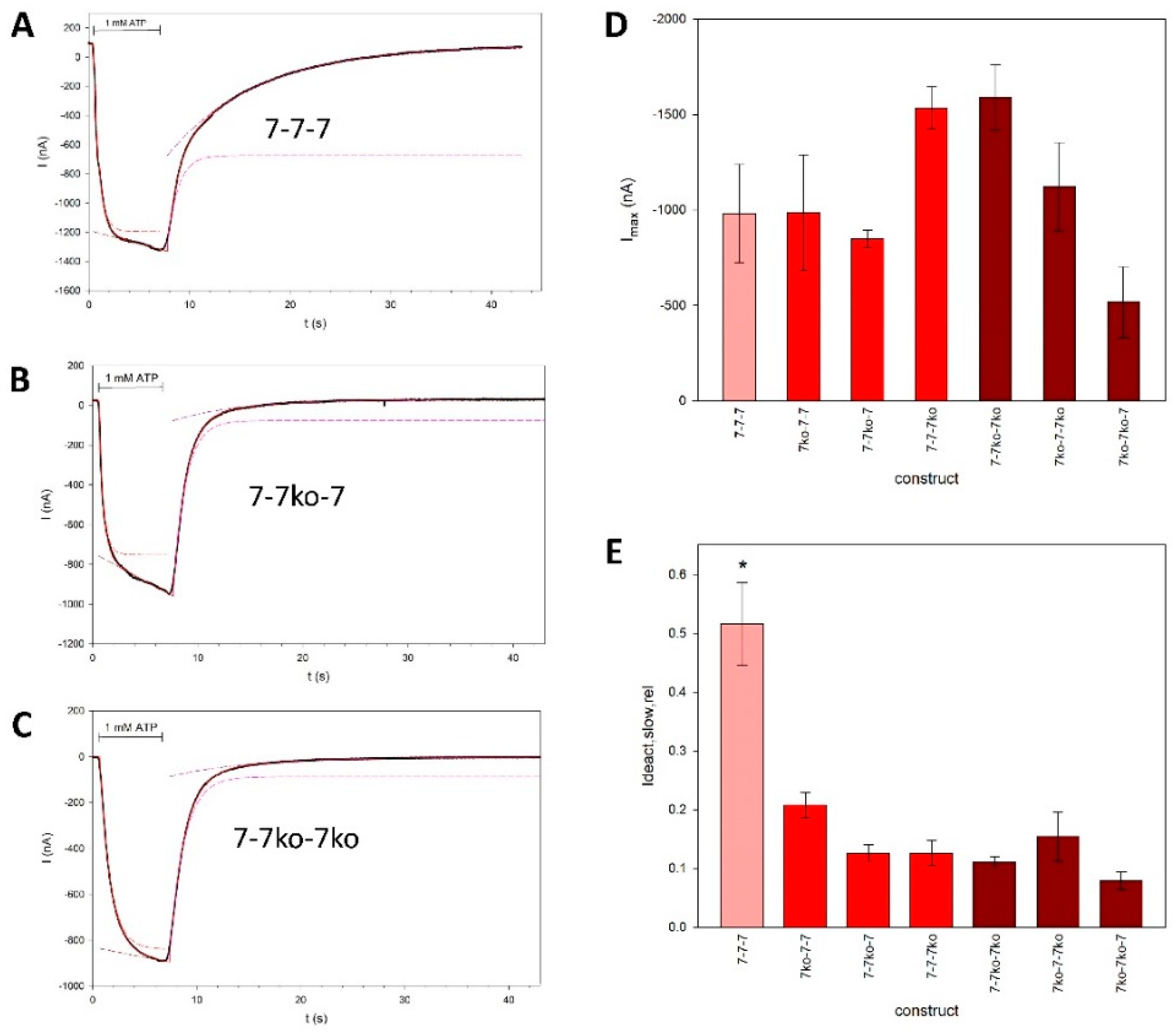
Figure 5A-C. The best fit was always achieved by a model with the sum of two exponentially saturating components for activation and two exponentially decreasing current components for deactivation of the current (eqs. 1 and 2, see legend).
The main difference between the "wild-type" concatamer 7
wt-7
wt-7
wt and the shown knockout constructs is the presence of a large, slowly deactivating current component in addition to the fast component (
Figure 5A). This suggests the dissociation of bound ATP from binding sites with high and low affinity, respectively, indicating the existence of functionally different ATP activation sites. However, the slowly deactivating component is strongly reduced in all knockout concatamers (
Figure 5E). The detailed fitted parameters are shown in
Figure 6.
To gain further insight into the function of the ATP activation sites, we constructed concentration-response curves for the activation of ATP-dependent whole-cell ion currents for all concatameric constructs. We then attempted to approximate these curves using different Hill plots based on eqs. 4 and 5. The models resulting from these equations provided the best fits, yielding the highest correlation coefficients and lowest sums of squares of errors [
33].
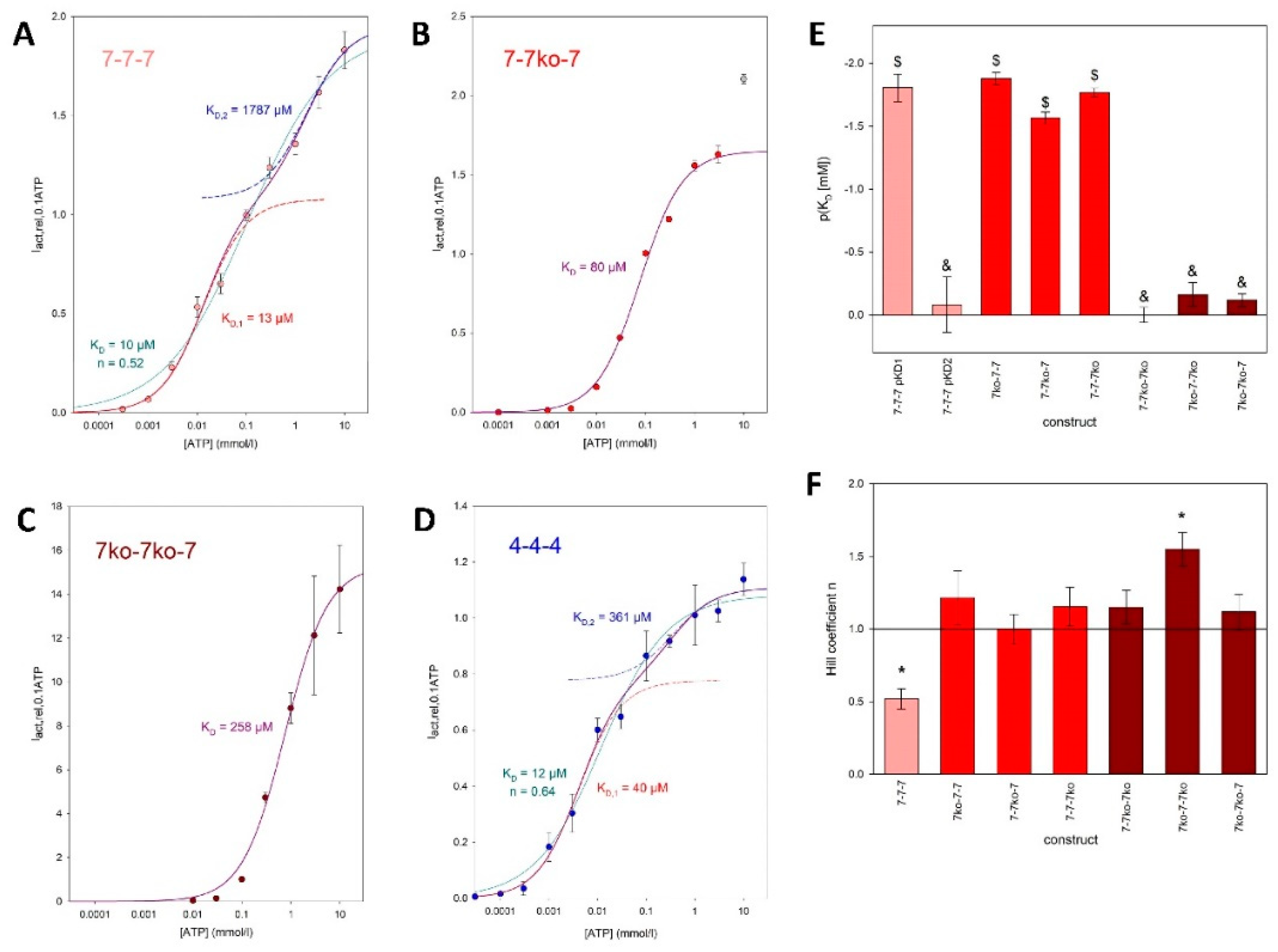
The curves for the concatamers consisting either of rP2X7 subunits (
Figure 7A) or P2X4 subunits (
Figure 7D) display a biphasic behavior, again indicating functionally different ATP activation sites. Approximation of these data using a single Hill plot resulted in Hill coefficients significantly lower than unity, indicating negative cooperativity between the ATP binding sites for these concatamers (
Figure 7A and F). Only the sum of two Hill functions resulted in the best fit for the 7-7-7 concatamer, yielding functional apparent K
D values of about 10 and 1800 µM. In contrast, the concentration-response curves for the concatamers with one (see, for example,
Figure 7B) or two (
Figure 7C) ATP binding site knockouts were best fit with single Hill curves. The K
D values of the concentration-response curves for the single knockout constructs were statistically equivalent to the value for the high-affinity ATP binding site of the 7-7-7 concatamer.
Although the single knockout concatamers contain two intact ATP binding sites, the approximation by a Hill function with a free Hill coefficient (n) yielded values not significantly different from 1 (
Figure 7F). This indicates the absence of cooperativity between the remaining two activation sites. The concentration dependency of the inward current amplitudes of the double knockout concatamers was also best fit by a single Hill curve, as expected for constructs with only one functional ATP binding site remaining. The corresponding K
D values were equal to the K
D value of the low-affinity ATP binding site of the 7-7-7 concatamer, with the exception of the 7
ko-7-7
ko concatamer, where fitting with a Hill function with a free Hill coefficient (eq. 5) resulted in a Hill coefficient significantly larger than 1 (
Figure 7E).
4. Discussion
4.1. Homotrimeric rP2X7 Concatamers, in Contrast to Their Homodimeric Precursors, Are Proteolytically Stable
We have previously observed that P2X1 concatamers not only reduces overall stability, but also results in the appearance of small byproducts that we interpreted as unwanted cleavage products [
19]. Significant proteolytic cleavage is not generally observed with P2X concatmers, as concatenated P2X2 receptor subunits resulted in only negligible, if any, formation of dimers Stelmashenko, 2012 26031 /id. To remove possible constraints by linking the ends of P2X7 subunits together, we inserted very long and flexible linkers combined with multiple Strep Affinity tags (see
Figure 1) that allowed us to purify not only intact concatamers, but also shorter possible cleavage products. Mol Pharmacol. 2012 Oct;82(4):760–766. doi: 10.1124/mol.112.080903
4.2. Binding of One ATP Is Sufficient to Open the rP2X7 Channel Pore
The number of ATP molecules required to open a single P2X receptor channel remains a subject of intense research and discussion [
15,
34,
35,
36,
37,
38,
39,
40,
41,
42]. Structure models of P2X receptors show apo-closed and fully open states for P2X4R [
43], P2X3R [
44], and P2X7 [
12], including calculated simulations of symmetrical pore opening. This aligns with P2X kinetic models deduced from voltage clamp measurements [
34,
37,
38].
However, our findings and those of Gusic et al. [
41] indicate that binding of one ATP molecule is sufficient to open the rP2X7 ion channel, suggesting an asymmetric pore opening mechanism. Analysis of P2X receptor activation time course and its ATP concentration dependency has led to the conclusion that binding of just one or two ATP molecules can induce P2X receptor-dependent ion currents [
35,
36,
40,
42]. Our previous detailed investigation of the kinetics of single hP2X7 receptor channels resulted in a model where binding of two ATP molecules is necessary and sufficient to fully open the ion channel [
15]. Given the existence of three identical ATP activation sites, it can therefore be hypothesized that the binding of the first ATP molecule either does not lead to any channel opening or generates a very small current due to incomplete channel pore formation, which may have escaped detection in single-channel recordings.
The structure of the cytoplasmic ballast of the rP2X7R, which contains the N- and C-terminal ends of the subunits [
12], might be altered by concatamerization due to the covalent connection of N- and C-termini from different subunits, thereby potentially altering the channel kinetics. However, the typical biexponential activation and deactivation time courses of the wt P2X7R channels are preserved in the concatamers (see Figs. 3 and 4). Furthermore, structural models of the closed and open hP2X7R ion channel indicate that the structure of the cytoplasmic ballast, including its intracellular side windows crucial for cation selectivity, remains largely unchanged between open and closed channel pores [
45]. This is further confirmed by structural alignments of our AlphaFold2 3D models of the wt and concatenated rP2X7R , which reveal only minor conformational changes due to concatamerization, as verified by the low RMSD value of 0.481 Å (see
Figure 2). An RMSD value below 1 Å indicates very high similarity between two structures, supporting the conclusion that concatamerization had only minor effects on the overall structure [
46]. In contrast, the combination of concatamerization and the use of K
64A knockout subunits affects the activation kinetics.
Unlike the experiments reported by the Benndorf group [
41], some of our knockout constructs exhibit a slow desensitization characterized by a positive I
act,slow,6s,rel current, rather than the slowly exponentially growing inward current observed for the wild-type rP2X7R and the 7
wt-7
wt-7
wt concatamer (see
Figure 6C). Since the activation of P2X7Rs measured in outside-out patches at saturating ATP concentrations occurs within 20 ms (rP2X7R [
41]) or 10 ms (hP2X7R [
15]), the activation measured in our whole-cell experiments is strongly influenced by the slower solution change compared to patch measurements. The cause of the slow P2X7R-dependent current activation in whole-cell current measurements remains unclear [
6] and deserves further investigation.
4.3. Functionally Distinct Activation Sites at P2X Receptors
A symmetrical opening of P2XR channels after binding of all three ATP molecules to the structurally equal binding sites would result in a dependence of the ion current on ATP concentration that could be described by a single Hill function. However, this was not the case for the concentration dependency of concatamerized rat P2X7 and P2X4 receptors, as shown here. Instead, approximation by a single Hill function resulted in low correlations and Hill coefficients smaller than 1 (
Figure 7A, D and F). The assumption of functionally different activation sites is supported by the clearly biphasic deactivation of the ATP-dependent currents observed for the 7
wt-7
wt-7
wt concatamer, which is absent for the rP2X7 concatamers with ATP binding site knockouts (
Figure 5).
Biphasic ATP concentration dependencies, which required approximation by the sum of two Hill functions, have already been reported for human P2X7 receptors [
14,
47] and mouse P2X7 receptors [
48,
49]. An explanation for the differing affinities of the three structurally equal P2X7R activation sites is the assumption of negative cooperativity between these sites. This would imply that the binding of the first and possibly also the second ATP molecule has an allosteric effect on the third binding site, thereby reducing its binding affinity, as also shown here (
Figure 7). In accordance with this, the concentration dependencies of the rP2X7R concatamers with two intact ATP activation sites were approximated by single Hill functions with K
D values indistinguishable from the high-affinity K
D value of the 7-7-7 concatamer (
Figure 7E).
As a logical conclusion from this argument, one would expect that concatamers with only one intact ATP binding site would become activated at low ATP concentrations too, i.e., low K
D values. However, this is not the case (
Figure 7). Instead, these concatamers have K
D values similar to the low-affinity K
D of the 7-7-7 concatamer. The cause of this remains unclear. Either certain allosteric effects of the two mutated ATP binding sites lead to a low affinity of the remaining intact activation site, or strong positive cooperativity of two ATP bindings at the unmodified P2X7R increases the functional ATP affinity. However, the approximated Hill coefficients near 1 for the single mutation concatamers (7
ko-7-7, 7-7
ko-7, and 7-7-7
ko) argue against strong cooperativity.
On the other hand, the Hill coefficient alone appears to be a weak argument for making a statement about cooperativity [
50]. For example, the Hill coefficient greater than 1 for the 7
ko-7
wt-7
ko concatamer, which has only one intact activation site, is similar to what was found for a 2
ko-2
ko-2
wt P2X2 concatamer [
42]. Recent reports have demonstrated that, depending on experimental conditions, Hill coefficients near 1 are observed for all P2X7 concatamers [
41]. For concatamerized P2X2 receptors, Hill coefficients range between 1 and 2 [
38,
51], are greater than 2 [
34,
52,
53], or indicate negative cooperativity for ATP binding but positive cooperativity for the subsequent structural change [
40]. This variability highlights the uncertainty of deriving the number of ATP bonds required for channel activation from the fitted Hill coefficients.
Negative cooperativity between ATP binding sites, associated with functionally distinct activation sites, is presumably a property of P2X receptors other than the P2X7R too, as exemplified by the need for two Hill functions to fit the ATP concentration dependency also of rP2X4 receptors, as shown here (
Figure 7D). This phenomenon may have been overlooked in studies that did not investigate concentration dependencies up to 10 mM ATP
4-, especially for P2X receptors with apparently higher ATP
4- affinity than the P2X7 receptor [
40,
51,
54,
55,
56]. Another possibility is that either the high of the low ATP affinity component may be too small to become evident in voltage clamp experiments.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
F.M. and G.S. contributed to conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing-original draft and writing-reviewing & editing. M.B. and S.H.Y. contributed to investigation.
Funding
F.M. and G.S. thank the Deutsche Forschungsgemeinschaft for financial support through grants MA 1581/15-2, SCHM 536/9-2, and SCHM 536/12-1.
Institutional Review Board Statement
The procedures for maintenance of the frogs and their ovariectomy were approved by the local animal welfare committees (Halle, Germany, reference no. Az. 203.42502-2-1493 MLU, and Düsseldorf, Germany, reference no. 8.87-51.05.20.10.131 for experiments performed in Halle and Aachen, respectively) in compliance with EC Directive 86/609/EEC for animal experiments.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aschrafi, A.; Sadtler, S.; Niculescu, C.; Rettinger, J.; Schmalzing, G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J. Mol. Biol. 2004, 342, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Bäumert, H.G.; Rettinger, J.; Eichele, A.; Lambrecht, G.; Mutschler, E.; Schmalzing, G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998, 17, 3016–3028. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Rettinger, J.; Büttner, C.; Eichele, A.; Lambrecht, G.; Schmalzing, G. Evolving view of quaternary structures of ligand-gated ion channels. Prog. Brain Res. 1999, 120, 61–80. [Google Scholar] [CrossRef]
- Oken, A.C.; Lisi, N.E.; Krishnamurthy, I.; McCarthy, A.E.; Godsey, M.H.; Glasfeld, A.; Mansoor, S.E. High-affinity agonism at the P2X7 receptor is mediated by three residues outside the orthosteric pocket. Nat. Commun. 2024, 15, 6662. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Woltersdorf, R.; Boldt, W.; Schmitz, S.; Braam, U.; Schmalzing, G.; Markwardt, F. The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J. Biol. Chem. 2008, 283, 25725–25734. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The elusive P2X7 macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signaling, DAMPs, and inflammation. Am. J. Physiol. 2020, 318, C832–C835. [Google Scholar] [CrossRef]
- Klapperstück, M.; Büttner, C.; Böhm, T.; Schmalzing, G.; Markwardt, F. Characteristics of P2X7 receptors from human B lymphocytes expressed in Xenopus oocytes. Biochim. Biophys. Acta 2000, 1467, 444–456. [Google Scholar] [CrossRef]
- Markwardt F.; Löhn M.; Böhm T.; Klapperstück M. Purinoceptor-operated cationic channels in human B lymphocytes. J. Physiol. (Lond. ) 1997, 498, 143-151. [CrossRef]
- Li, M.; Silberberg, S.D.; Swartz, K.J. Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+. Proc. Natl. Acad. Sci. USA 2013, 110, E3455–E3463. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Banerjee, R.; Marinelli, F.; Silberberg, S.; Faraldo-Gomez, J.D.; Hattori, M.; Swartz, K.J. Molecular mechanisms of human P2X3 receptor channel activation and modulation by divalent cation bound ATP. eLife 2019, 8, e47060. [Google Scholar] [CrossRef]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 structures reveal how palmitoylation prevents channel desensitization. Cell 2019, 179, 659–670. [Google Scholar] [CrossRef]
- Kawate, T. P2X receptor activation. Adv. Exp. Med. Biol. 2017, 1051, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Klapperstück, M.; Büttner, C.; Schmalzing, G.; Markwardt, F. Functional evidence of distinct ATP activation sites at the human P2X7 receptor. J. Physiol. (Lond. ) 2001, 534, 25-35. [CrossRef]
- Riedel, T.; Lozinsky, I.; Schmalzing, G.; Markwardt, F. Kinetics of P2X7 receptor-operated single channels currents. Biophys. J. 2007, 92, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Gloor, S.; Pongs, O.; Schmalzing, G. A vector for the synthesis of cRNAs encoding Myc epitope-tagged proteins in Xenopus laevis oocytes. Gene 1995, 160, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Prudic, K.; Pippel, A.; Klapperstück, M.; Braam, U.; Müller, C.E.; Schmalzing, G.; Markwardt, F. Interaction of purinergic P2X4 and P2X7 receptor subunits. Front. Pharmacol. 2017, 8, 860. [Google Scholar] [CrossRef]
- Nicke, A.; Rettinger, J.; Schmalzing, G. Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol. Pharmacol. 2003, 63, 243–252. [Google Scholar] [CrossRef]
- Braman, J.; Papworth, C.; Greener, A. Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol. Biol. 1996, 57, 31–44. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Basic, M.; Varjosalo, M.; Mäkinen, K. One-step purification of twin-strep-tagged proteins and their complexes on strep-tactin resin cross-linked with bis(sulfosuccinimidyl) suberate (BS3). J. Vis. Exp. 2014, 86, e51536. [Google Scholar] [CrossRef]
- Pult, F.; Fallah, G.; Braam, U.; Detro-Dassen, S.; Niculescu, C.; Laube, B.; Schmalzing, G. Robust post-translocational N-glycosylation at the extreme C-terminus of membrane and secreted proteins in Xenopus laevis oocytes and HEK293 cells. Glycobiology 2011, 21, 1147–1160. [Google Scholar] [CrossRef]
- Ennion, S.; Hagan, S.; Evans, R.J. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J. Biol. Chem. 2000, 275, 29361–29367. [Google Scholar] [CrossRef] [PubMed]
- Schmalzing G, Markwardt F. Established protocols for cRNA expression and voltage-clamp characterization of the P2X7 receptor in Xenopus laevis oocytes. In: Nicke A (editor). The P2X7 receptor: Methods and protocols. New York: Humana Press, 2022; 157-192.
- Grudzien-Nogalska, E.; Stepinski, J.; Jemielity, J.; Zuberek, J.; Stolarski, R.; Rhoads, R.E.; Darzynkiewicz, E. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007, 431, 203–227. [Google Scholar] [CrossRef]
- Schmalzing, G.; Kröner, S.; Schachner, M.; Gloor, S. The adhesion molecule on glia (AMOG/b2) and a1 subunits assemble to functional sodium pumps in Xenopus oocytes. J. Biol. Chem. 1992, 267, 20212–20216. [Google Scholar] [CrossRef]
- Fallah G.; Römer T.; Braam U.; Detro-Dassen S.; Markwardt F.; Schmalzing G. TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol. Cell. Proteomics 2011, 10, M110.004697. [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; Bridgland, A.; Meyer, C.; Kohl, S.A.A.; Ballard, A.J.; Cowie, A.; Romera-Paredes, B.; Nikolov, S.; Jain, R.; Adler, J.; Back, T.; Petersen, S.; Reiman, D.; Clancy, E.; Zielinski, M.; Steinegger, M.; Pacholska, M.; Berghammer, T.; Bodenstein, S.; Silver, D.; Vinyals, O.; Senior, A.W.; Kavukcuoglu, K.; Kohli, P.; Hassabis, D. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Omasits, U.; Ahrens, C.H.; Muller, S.; Wollscheid, B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]
- Kabsch, W. A solution for the best rotation to relate two sets of vectors. Acta Cryst. A 1976, A32, 922–923. [Google Scholar] [CrossRef]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef]
- Horn, R. Statistical methods for model discrimination: Application to gating kinetics and permeation of the acetylcholine receptor channel. Biophys. J. 1987, 51, 255–263. [Google Scholar] [CrossRef]
- Ding, S.H.; Sachs, F. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 1999, 113, 695–719. [Google Scholar] [CrossRef]
- Karoly, R.; Mike, A.; Illes, P.; Gerevich, Z. The unusual state-dependent affinity of P2X3 receptors can be explained by an allosteric two-open-state model. Mol. Pharmacol. 2007, 73, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Khadra, A.; Li, S.; Tomic, M.; Sherman, A.; Stojilkovic, S.S. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J. Neurosci. 2010, 30, 14213–14224. [Google Scholar] [CrossRef]
- Browne, L.E.; Cao, L.; Broomhead, H.E.; Bragg, L.; Wilkinson, W.J.; North, R.A. P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat. Neurosci. 2011, 14, 17–18. [Google Scholar] [CrossRef]
- Keceli, B.; Kubo, Y. Signal transmission within the P2X2 trimeric receptor. J. Gen. Physiol. 2014, 143, 761–782. [Google Scholar] [CrossRef]
- Schmid, R.; Evans, R.J. ATP-gated P2X receptor channels: Molecular insights into functional roles. Annu. Rev. Physiol. 2018, 81, 43–62. [Google Scholar] [CrossRef]
- Sattler, C.; Eick, T.; Hummert, S.; Schulz, E.; Schmauder, R.; Schweinitz, A.; Unzeitig, C.; Schwede, F.; Benndorf, K. Unravelling the intricate cooperativity of subunit gating in P2X2 ion channels. Sci. Rep. 2020, 10, 21751. [Google Scholar] [CrossRef] [PubMed]
- Gusic, M.; Benndorf, K.; Sattler, C. Dissecting activation steps in P2X7 receptors. Biochem. Biophys. Res. Commun. 2021, 569, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Stelmashenko, O.; Lalo, U.; Yang, Y.; Bragg, L.; North, R.A.; Compan, V. Activation of trimeric P2X2 receptors by fewer than three ATP molecules. Mol. Pharmacol. 2012, 82, 760–766. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- Mansoor, S.E.; Lu, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef]
- Markwardt, F.; Schön, E.C.; Raycheva, M.; Malisetty, A.; Hawro, Y.S.; Berthold, M.; Schmalzing, G. Two serial filters control P2X7 cation selectivity, Ser342 in the central pore and lateral acidic residues at the cytoplasmic interface. PNAS Nexus 2024, 3, ae349. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Chen, F.; Saven, J.G.; Roos, D.S.; Babbitt, P.C.; Sali, A. Evolutionary constraints on structural similarity in orthologs and paralogs. Protein Sci. 2009, 18, 1306–1315. [Google Scholar] [CrossRef]
- Flittiger, B.; Klapperstück, M.; Schmalzing, G.; Markwardt, F. Effects of protons on macroscopic and single-channel currents mediated by the human P2X7 receptor. Biochim. Biophys. Acta Biomembranes 2010, 1798, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, M.P.; Reiss, M.; Anand, P.K.; Treede, I.; Holzer, D.; Hoffmann, E.; Klapperstueck, M.; Steinberg, T.H.; Markwardt, F.; Griffiths, G. Sphingosine-1-phosphate receptors stimulate macrophage plasma-membrane actin assembly via ADP release, ATP synthesis and P2X7R activation. J. Cell Sci. 2009, 122, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Schmalzing, G.; Müller, C.E.; Markwardt, F. Dissection of P2X4 and P2X7 receptor current components in BV-2 microglia. Int. J. Mol. Sci. 2020, 21, 8489. [Google Scholar] [CrossRef]
- Santillan, M. On the use of the Hill functions in mathematical models of gene regulatory networks. Math. Model. Nat. Phenom. 2008, 3, 85–97. [Google Scholar] [CrossRef]
- Khakh, B.S.; Bao, X.R.; Labarca, C.; Lester, H.A. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat. Neurosci. 1999, 2, 322–330. [Google Scholar] [CrossRef]
- Clyne, J.D.; Brown, T.C.; Hume, R.I. Expression level dependent changes in the properties of P2X2 receptors. Neuropharmacology 2003, 44, 403–412. [Google Scholar] [CrossRef]
- Li, M.; Chang, T.H.; Silberberg, S.D.; Swartz, K.J. Gating the pore of P2X receptor channels. Nat. Neurosci. 2008, 11, 883–887. [Google Scholar] [CrossRef]
- Jiang, L.H.; Rassendren, F.; Spelta, V.; Surprenant, A.; North, R.A. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X2 receptor. J. Biol. Chem. 2001, 276, 14902–14908. [Google Scholar] [CrossRef]
- Clyne, J.D.; LaPointe, L.D.; Hume, R.I. The role of histidine residues in modulation of the rat P2X2 purineceptor by zinc and pH. J. Physiol. (Lond. ) 2002, 539, 347-359. [CrossRef]
- Yan, Z.H.; Liang, Z.D.; Tomic, M.; Obsil, T.; Stojilkovic, S.S. Molecular determinants of the agonist binding domain of a P2X receptor channel. Mol. Pharmacol. 2005, 67, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Lomize, A.L.; Todd, S.C.; Pogozheva, I.D. Spatial arrangement of proteins in planar and curved membranes by PPM 3.0. Protein Sci. 2022, 31, 209–220. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).