Submitted:
20 March 2025
Posted:
20 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
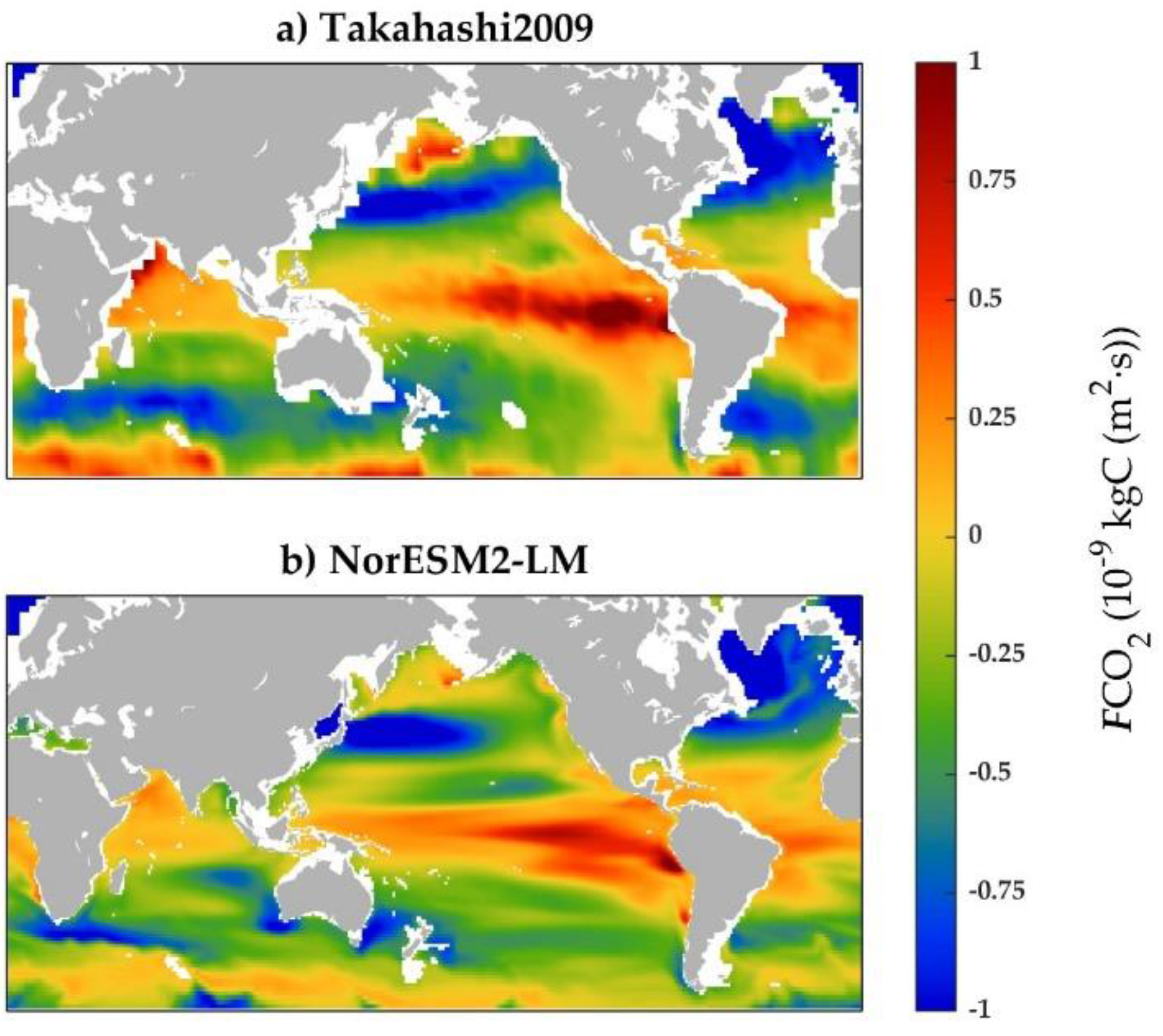
3.1. Performance of the model simulation
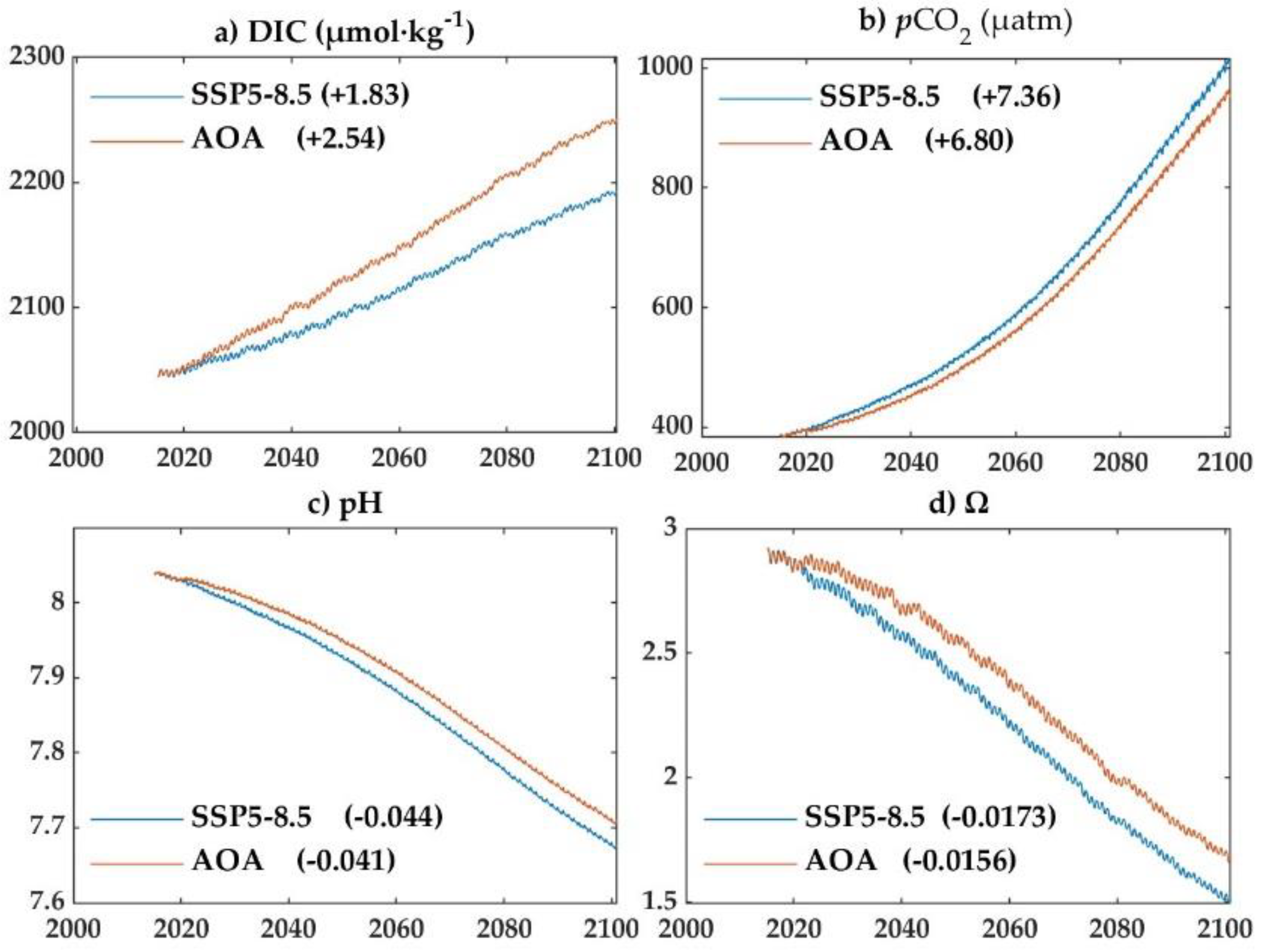
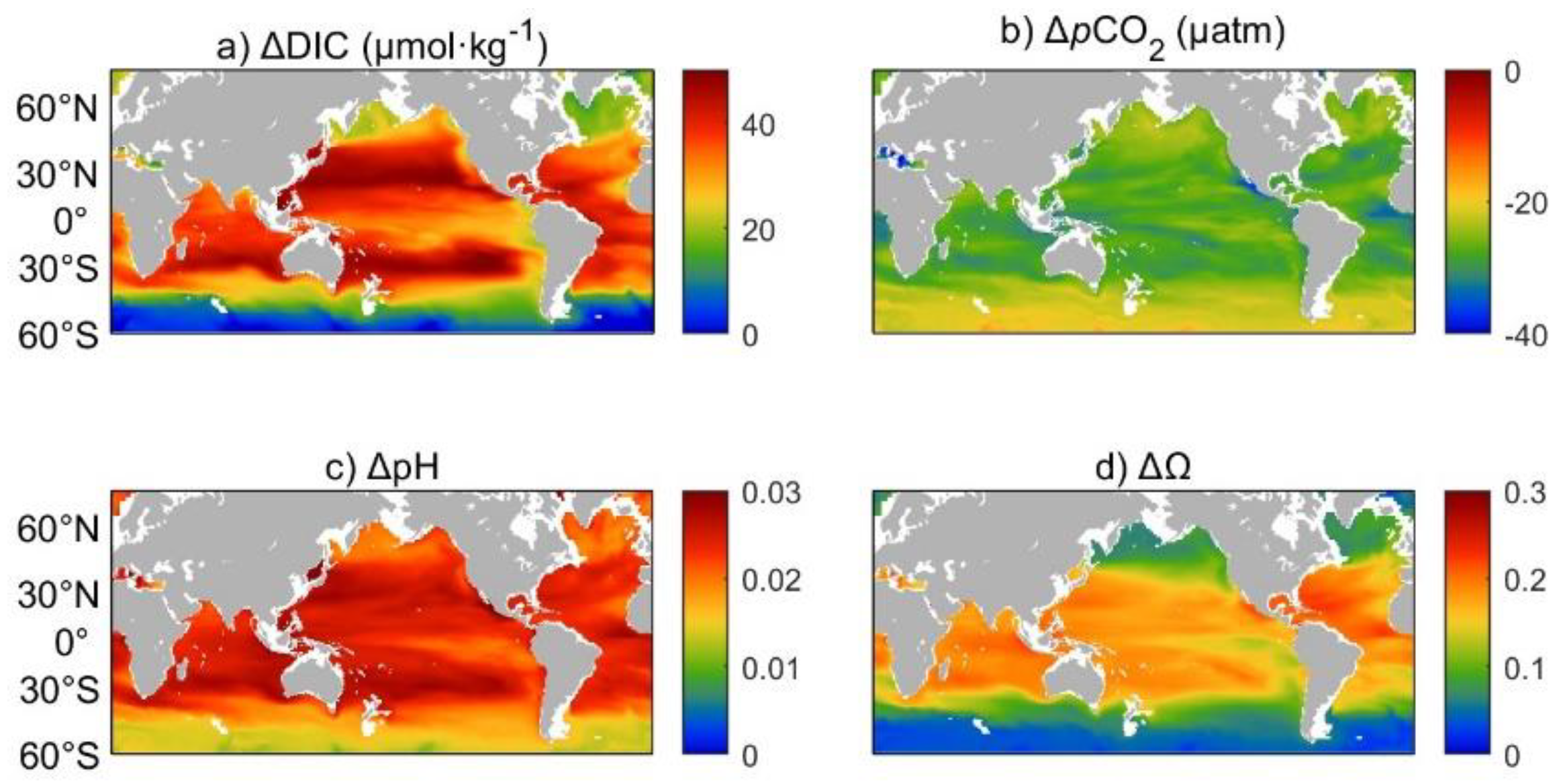
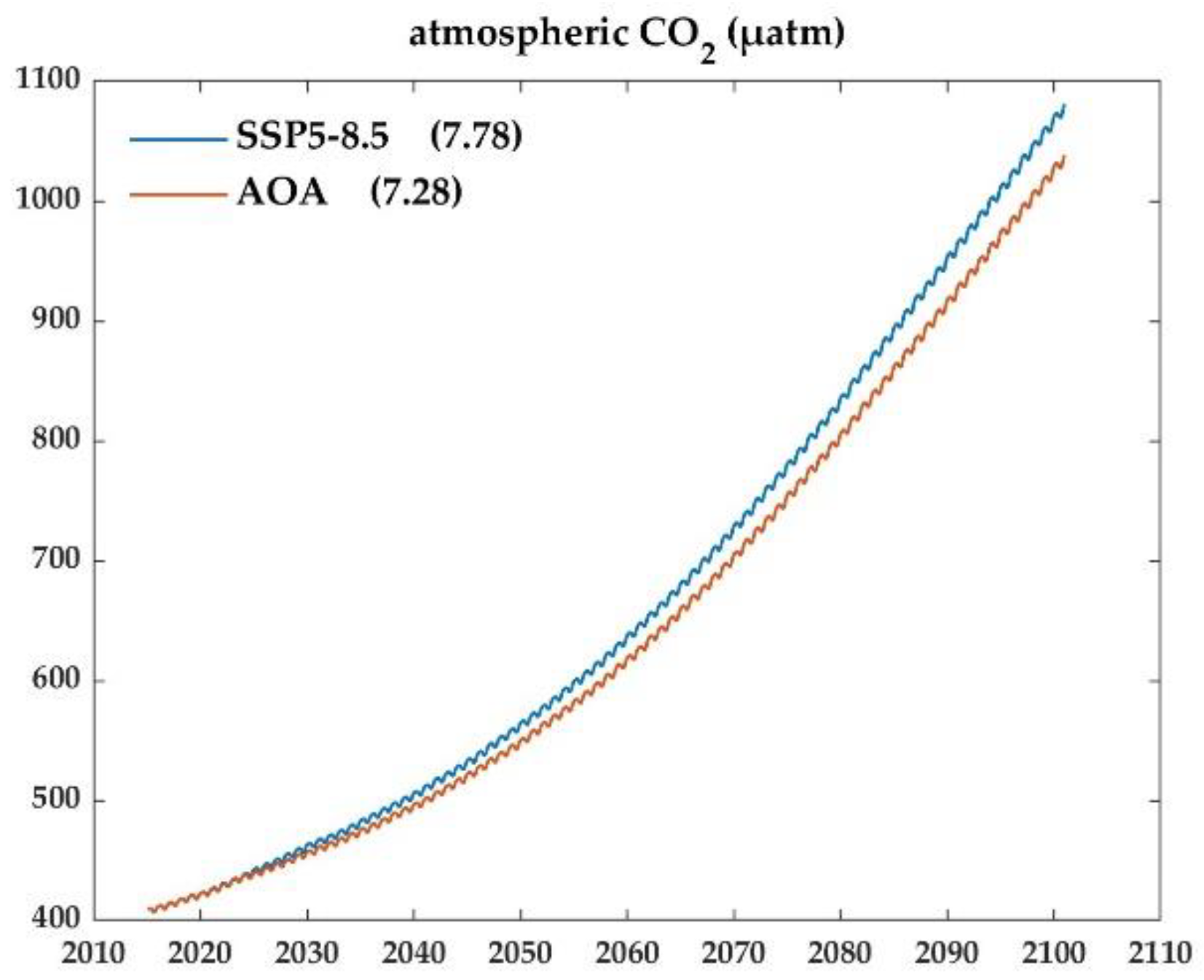
3.2. Variations of the marine carbon system during AOA
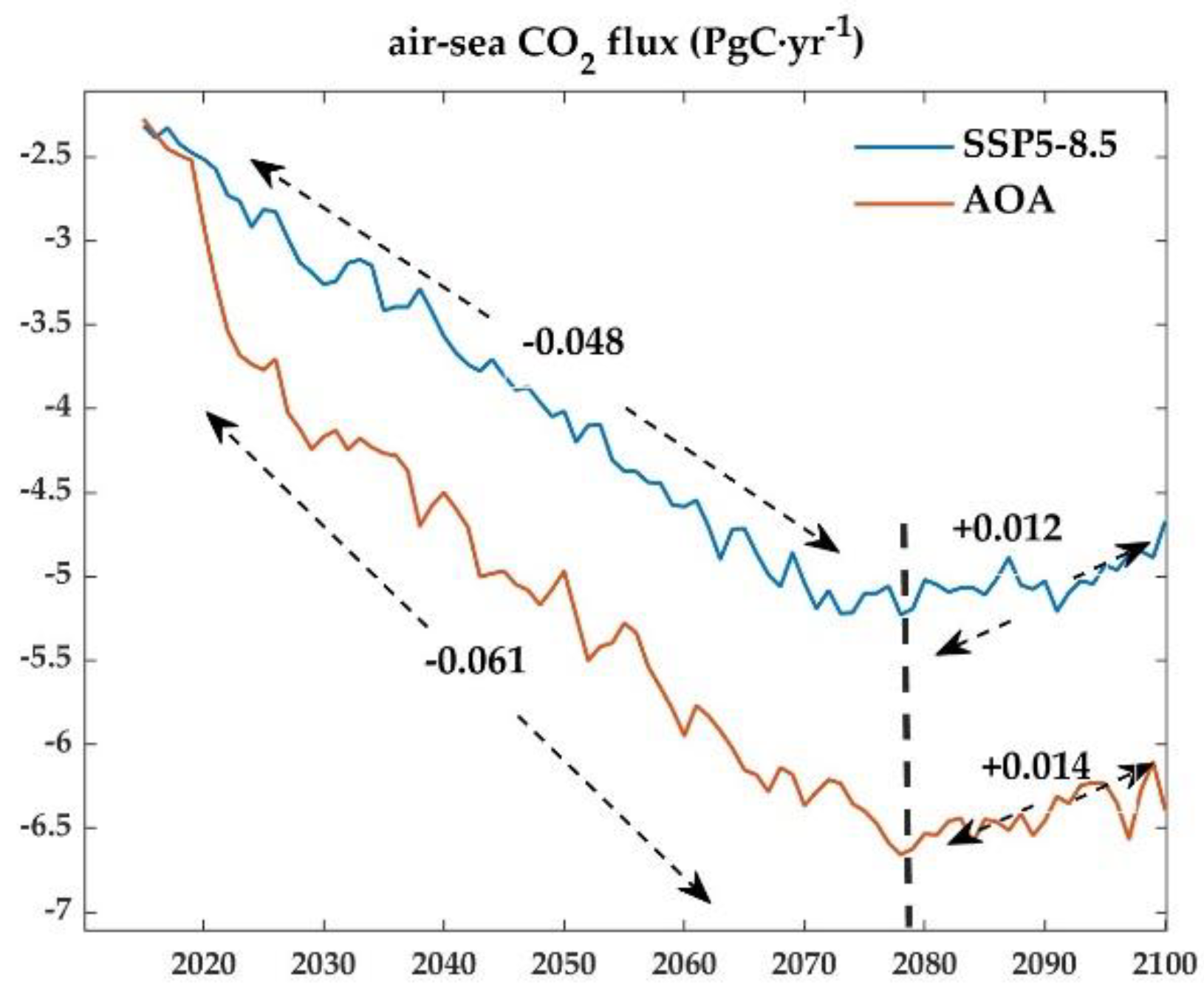
3.3. Effect of AOA on air-sea CO2 exchange flux
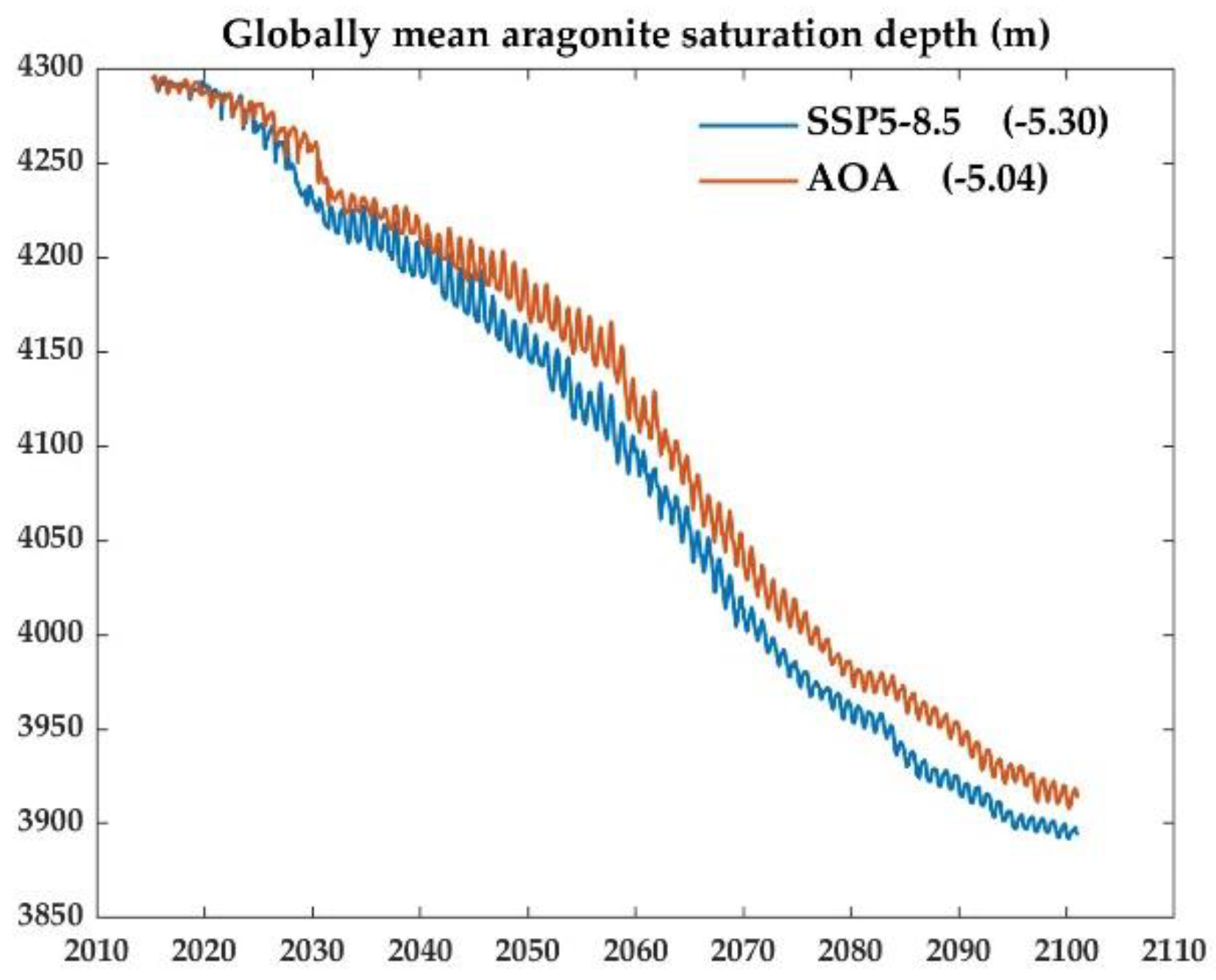
3.4. Effects of AOA into the ocean interior, take Ω for an example
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Core Writing Team. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, H. Lee., J. Romero., Eds.; IPCC: Geneva, Switzeland, pp. 4.
- Zhou, M., Tyka, M.D., Ho, D.T., Yankovsky, E., Bachman, S., Nicholas, T., Karspeck, A.R., Long, M.C. Mapping the global variation in the efficiency of ocean alkalinity enhancement for carbon dioxide removal. Nat. Clim. Chang. 2024, 1–7. [CrossRef]
- Friedlingstein, P., O’Sullivan, M., Jones, M.W., Andrew, R.M., Bakker, D.C.E., Hauck, J., Landschützer, P., Le Quéré, C., Luijkx, I.T., Peters, G.P., Peters, W., Pongratz, J., Schwingshackl, C., Sitch, S., Canadell, J.G., Ciais, P., Jackson, R.B., Alin, S.R., Anthoni, P., Barbero, L., Bates, N.R., Becker, M., Bellouin, N., Decharme, B., Bopp, L., Brasika, I.B.M., Cadule, P., Chamberlain, M.A., Chandra, N., Chau, T.-T.-T., Chevallier, F., Chini, L.P., Cronin, M., Dou, X., Enyo, K., Evans, W., Falk, S., Feely, R.A., Feng, L., Ford, D.J., Gasser, T., Ghattas, J., Gkritzalis, T., Grassi, G., Gregor, L., Gruber, N., Gürses, Ö., Harris, I., Hefner, M., Heinke, J., Houghton, R.A., Hurtt, G.C., Iida, Y., Ilyina, T., Jacobson, A.R., Jain, A., Jarníková, T., Jersild, A., Jiang, F., Jin, Z., Joos, F., Kato, E., Keeling, R.F., Kennedy, D., Klein Goldewijk, K., Knauer, J., Korsbakken, J.I., Körtzinger, A., Lan, X., Lefèvre, N., Li, H., Liu, J., Liu, Z., Ma, L., Marland, G., Mayot, N., McGuire, P.C., McKinley, G.A., Meyer, G., Morgan, E.J., Munro, D.R., Nakaoka, S.-I., Niwa, Y., O’Brien, K.M., Olsen, A., Omar, A.M., Ono, T., Paulsen, M., Pierrot, D., Pocock, K., Poulter, B., Powis, C.M., Rehder, G., Resplandy, L., Robertson, E., Rödenbeck, C., Rosan, T.M., Schwinger, J., Séférian, R., Smallman, T.L., Smith, S.M., Sospedra-Alfonso, R., Sun, Q., Sutton, A.J., Sweeney, C., Takao, S., Tans, P.P., Tian, H., Tilbrook, B., Tsujino, H., Tubiello, F., van der Werf, G.R., van Ooijen, E., Wanninkhof, R., Watanabe, M., Wimart-Rousseau, C., Yang, D., Yang, X., Yuan, W., Yue, X., Zaehle, S., Zeng, J., Zheng, B. Global Carbon Budget 2023. Earth Sys. Sci. Data 2023, 15, 5301–5369.
- Gruber, N., Bakker, D.C.E., DeVries, T., Gregor, L., Hauck, J., Landschützer, P., McKinley, G.A., Müller, J.D. Trends and variability in the ocean carbon sink. Nat Rev Earth Environ 2023, 4, 119–134. [CrossRef]
- Zeebe, R.E. History of Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification. Ann. Rev. Ear. Plane. Sci 2012, 40, 141–165.
- Wolf-Gladrow, D.A., Zeebe, R.E., Klaas, C., Körtzinger, A., Dickson, A.G. Total alkalinity: The explicit conservative expression and its application to biogeochemical processes. Mar. Chem. 2007, 106, 287–300. [CrossRef]
- Köhler, P., Abrams, J.F., Völker, C., Hauck, J., Wolf-Gladrow, D.A. Geoengineering impact of open ocean dissolution of olivine on atmospheric CO2, surface ocean pH and marine biology. Environ. Res. Lett. 2013, 8, 014009.
- Renforth, P., Henderson, G. Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics 2017, 55, 636–674. [CrossRef]
- Feng, E.Y., Koeve, W., Keller, D.P., Oschlies, A. Model-Based Assessment of the CO2 Sequestration Potential of Coastal Ocean Alkalinization. Earth’s Future 2017, 5, 1252–1266. [CrossRef]
- Ilyina, T., Wolf-Gladrow, D., Munhoven, G., Heinze, C. Assessing the potential of calcium-based artificial ocean alkalinization to mitigate rising atmospheric CO2 and ocean acidification. Geophy. Res Lett 2013, 40, 5909–5914. [CrossRef]
- Feng, E.Y., Keller, D.P., Koeve, W., Oschlies, A. Could artificial ocean alkalinization protect tropical coral ecosystems from ocean acidification? Environ. Res. Lett. 2016, 11, 074008. [CrossRef]
- Keller, D.P., Feng, E.Y., Oschlies, A. Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario. Nat. Comm. 2014. 5, 3304. [CrossRef]
- Zhou, M., Tyka, M.D., Ho, D.T., Yankovsky, E., Bachman, S., Nicholas, T., Karspeck, A.R., Long, M.C. Mapping the global variation in the efficiency of ocean alkalinity enhancement for carbon dioxide removal. Nat. Clim. Chang. 2024, 1–7. [CrossRef]
- Tollefson, J. Start-ups are adding antacids to the ocean to slow global warming. Will it work? Nature 2023, 618, 902–904. [CrossRef]
- Xin, X., Goldenberg, S.U., Taucher, J., Stuhr, A., Arístegui, J., Riebesell, U. Resilience of Phytoplankton and Microzooplankton Communities under Ocean Alkalinity Enhancement in the Oligotrophic Ocean. Environ. Sci. Technol 2024, https://doi.org/10.1021/acs.est.4c09838. [CrossRef]
- Cai, W.-J., Jiao, N. Wastewater alkalinity addition as a novel approach for ocean negative carbon emissions. The Innovation 2022, 3, 100272. [CrossRef]
- González, M.F., Ilyina, T., Sonntag, S., Schmidt, H. Enhanced Rates of Regional Warming and Ocean Acidification After Termination of Large-Scale Ocean Alkalinization. Geophy. Res. Lett. 2018, 45, 7120–7129. [CrossRef]
- Burt, D.J., Fröb, F., Ilyina, T. The Sensitivity of the Marine Carbonate System to Regional Ocean Alkalinity Enhancement. Front. Clim. 2021, 3, 624075. [CrossRef]
- Bach, L.T., Ferderer, A.J., LaRoche, J., Schulz, K.G. Technical note: Ocean Alkalinity Enhancement Pelagic Impact Intercomparison Project (OAEPIIP). Biogeosci. 2024, 21, 3665–3676. [CrossRef]
- Seland, Ø., Bentsen, M., Olivié, D., Toniazzo, T., Gjermundsen, A., Graff, L.S., Debernard, J.B., Gupta, A.K., He, Y.-C., Kirkevåg, A., Schwinger, J., Tjiputra, J., Aas, K.S., Bethke, I., Fan, Y., Griesfeller, J., Grini, A., Guo, C., Ilicak, M., Karset, I.H.H., Landgren, O., Liakka, J., Moseid, K.O., Nummelin, A., Spensberger, C., Tang, H., Zhang, Z., Heinze, C., Iversen, T., Schulz, M. Overview of the Norwegian Earth System Model (NorESM2) and key climate response of CMIP6 DECK, historical, and scenario simulations. Geoscientific Model Development 2020, 13, 6165–6200. [CrossRef]
- Tjiputra, J.F., Roelandt, C., Bentsen, M., Lawrence, D.M., Lorentzen, T., Schwinger, J., Seland, Ø., Heinze, C. Evaluation of the carbon cycle components in the Norwegian Earth System Model (NorESM). Geoscientific Model Development 2020, 6, 301–325. [CrossRef]
- Hunke E. C., W. H. Lipscomb, A. K. Turner, N. Jeffery, and Scott Elliott. CICE: The Los Alamos Sea Ice Model. Documentation and Software User’s Manual. 2015, Version 5.1. T-3 Fluid Dynamics Group, Los Alamos National Laboratory, Tech. Rep. LA-CC-06-012.
- Lawrence, D.M., Fisher, R.A., Koven, C.D., Oleson, K.W., Swenson, S.C., Bonan, G., Collier, N., Ghimire, B., Kampenhout, L. van, Kennedy, D., Kluzek, E., Lawrence, P.J., Li, F., Li, H., Lombardozzi, D., Riley, W.J., Sacks, W.J., Shi, M., Vertenstein, M., Wieder, W.R., Xu, C., Ali, A.A., Badger, A.M., Bisht, G., Broeke, M. van den, Brunke, M.A., Burns, S.P., Buzan, J., Clark, M., Craig, A., Dahlin, K., Drewniak, B., Fisher, J.B., Flanner, M., Fox, A.M., Gentine, P., Hoffman, F., Keppel-Aleks, G., Knox, R., Kumar, S., Lenaerts, J., Leung, L.R., Lipscomb, W.H., Lu, Y., Pandey, A., Pelletier, J.D., Perket, J., Randerson, J.T., Ricciuto, D.M., Sanderson, B.M., Slater, A., Subin, Z.M., Tang, J., Thomas, R.Q., Martin, M.V., Zeng, X. The Community Land Model Version 5: Description of New Features, Benchmarking, and Impact of Forcing Uncertainty. J. Adv. Mod. Ear. Sys. 2019, 11, 4245–4287. [CrossRef]
- Eyring, V., Bony, S., Meehl, G.A., Senior, C.A., Stevens, B., Stouffer, R.J., Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Mod. Dev. 2016, 9, 1937–1958. [CrossRef]
- Keller, D.P., Lenton, A., Scott, V., Vaughan, N.E., Bauer, N., Ji, D., Jones, C.D., Kravitz, B., Muri, H., Zickfeld, K. The Carbon Dioxide Removal Model Intercomparison Project (CDRMIP): rationale and experimental protocol for CMIP6. Geosci. Mod. Dev. 2018, 11, 1133–1160. [CrossRef]
- Takahashi, T., Sutherland, S.C., Wanninkhof, R., Sweeney, C., Feely, R.A., Chipman, D.W., Hales, B., Friederich, G., Chavez, F., Sabine, C., Watson, A., Bakker, D.C.E., Schuster, U., Metzl, N., Yoshikawa-Inoue, H., Ishii, M., Midorikawa, T., Nojiri, Y., Körtzinger, A., Steinhoff, T., Hoppema, M., Olafsson, J., Arnarson, T.S., Tilbrook, B., Johannessen, T., Olsen, A., Bellerby, R., Wong, C.S., Delille, B., Bates, N.R., de Baar, H.J.W. Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep Sea Res. II: Topical Studies in Oceanography 2009, 56, 554–577. [CrossRef]
- Takahashi, T., Sutherland, S.C., Sweeney, C., Poisson, A., Metzl, N., Tilbrook, B., Bates, N., Wanninkhof, R., Feely, R.A., Sabine, C., Olafsson, J., Nojiri, Y. Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Res. Part II: Topical Studies in Oceanography 2002, 49, 1601–1622.
- Qu, B., Song, J., Li, X., Yuan, H., Zhang, K., Xu, S. Global air-sea CO2 exchange flux since 1980s: results from CMIP6 Earth System Models. J. Ocean. Limnol. 2022, 40, 1417–1436. [CrossRef]
- Meinshausen, M., Nicholls, Z.R.J., Lewis, J., Gidden, M.J., Vogel, E., Freund, M., Beyerle, U., Gessner, C., Nauels, A., Bauer, N., Canadell, J.G., Daniel, J.S., John, A., Krummel, P.B., Luderer, G., Meinshausen, N., Montzka, S.A., Rayner, P.J., Reimann, S., Smith, S.J., van den Berg, M., Velders, G.J.M., Vollmer, M.K., Wang, R.H.J. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Mod. Dev. 2020, 13, 3571–3605. [CrossRef]
| CMIP6 Experiment ID | Simulation description | Run time |
|---|---|---|
| esm-his | CO2-emission-driven historical scenario | 1850-2014 |
| esm-ssp585 | CO2-emission-driven SSP5-8.5 scenario | 2015-2100 |
| esm-ssp585-ocn-alk | SSP5-8.5 scenario with 0.14 Pmol yr−1 alkalinity added | 2015-2100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




